Abstract
The wake-promoting drug modafinil is frequently used off-label to improve cognition in psychiatric and academic populations alike. The domain-specific attentional benefits of modafinil have yet to be quantified objectively in healthy human volunteers using tasks validated for comparison across species. Further, given that modafinil is a low-affinity inhibitor for the dopamine and norepinephrine transporters (DAT/NET respectively) it is unclear if any effects are attributable to a non-specific increase in arousal, a feature of many catecholamine reuptake inhibitors (e.g., cocaine, amphetamine). These experiments were designed to test for domain-specific enhancement of attention and cognitive control by modafinil (200 and 400 mg) in healthy volunteers using the 5-choice continuous performance task (5CCPT) and Wisconsin Card Sort Task (WCST). An additional cross-species assessment of arousal and hyperactivity was performed in this group and in mice (3.2, 10, or 32 mg/kg) using species-specific versions of the behavioral pattern monitor (BPM). Modafinil significantly enhanced attention (d prime) in humans performing the 5C-CPT at doses that did not affect WCST performance or induce hyperactivity in the BPM. In mice, only the highest dose elicited increased activity in the BPM. These results indicate that modafinil produces domain-specific enhancement of attention in humans not driven by hyperarousal, unlike other drugs in this class, and higher equivalent doses were required for hyperarousal in mice. Further, these data support the utility of using the 5C-CPT across species to more precisely determine the mechanism(s) underlying the pro-cognitive effects of modafinil and potentially other pharmacological treatments.
Keywords: Attention, Stimulant, Healthy, Activity, Mice, Continuous performance task, Cognitive control
1. Introduction
Cognitive deficits, particularly in the domains of attention and cognitive control, are key features of multiple psychiatric illnesses, e.g., schizophrenia (SCZ), bipolar disorder (BD), and attention deficit hyperactivity disorder (ADHD). Traditional treatments such as methylphenidate have been considered as therapeutic agents in the treatment of impaired attention and cognitive control. There has been longstanding reticence to use these drugs for individuals with SCZ and BD however, since they can exacerbate many of their symptoms (Chiarello and Cole, 1987). Further, given the mechanism of action of stimulants in potently blocking or reversing the dopa-mine transporter (DAT), these drugs carry a high potential for abuse (Volkow and Swanson, 2003). The lack of pro-cognitive pharmacotherapies with low abuse potential stands as a critical treatment gap for individuals suffering from these illnesses (Fusar-Poli et al., 2015; Geddes and Miklowitz, 2013; Lindenmayer et al., 2013).
Modafinil, a low potency inhibitor of the DAT and norepinephrine transporter (NET), is a Federal Drug Administration-approved compound that was developed to increase wakefulness in the treatment of narcolepsy (Madras et al., 2006; Volkow et al., 2009). This drug however, is increasingly used off-label to remediate deficient attention and cognitive control in psychiatric patients (Minzenberg and Carter, 2008), as well as a cognition-enhancing aid in academic institutions (Sahakian and Morein-Zamir, 2015). Certainly, there are myriad findings reporting modafinil-induced improved working memory, cognitive control, and sustained attention in healthy sleep-deprived volunteers, psychiatric populations, and rodents (Sahakian and Morein-Zamir, 2015). Improved cognition in non-sleep deprived healthy adults has been rarely observed however (Baranski et al., 2004; Battleday and Brem, 2015; Muller et al., 2004), except at the highest levels of difficulty in working memory, or in higher order planning and decision-making tasks (Battleday and Brem, 2015; Muller et al., 2013; Turner et al., 2003). Modafinil-induced improvements in the attentional domain have yet to be observed in healthy adults, which contrasts with what has been seen with high-potency DAT inhibitors such as amphetamine (Linssen et al., 2014; Smith and Farah, 2011).
In addition to improving attention, high-potency DAT inhibitors such as amphetamine also increase arousal (Bensadoun et al., 2004; Berridge, 2006; Kalivas and Volkow, 2005), an effect linked to its abuse potential (Sahakian and Morein-Zamir, 2015). In both healthy human volunteers and mice, D-amphetamine increases activity in the behavioral pattern monitor (BPM) at clinically relevant doses (Minassian et al., 2016). Similarly, modafinil increases activity in the mouse BPM (Young et al., 2011a) and increases motivation in mice as measured by progressive ratio breakpoint (Young and Geyer, 2010) at similar doses (32 mg/kg). It would therefore be important to separate the potential effect of modafinil on attention from its effects on arousal. In terms of feedback-related decision-making, amphetamine increased safe lever choice preference (preference for low risk, low reward option), while the more selective DAT inhibitor GBR12909 worsened performance, and modafinil had no effect in a mouse consolidation-dependent Gambling Task (van Enkhuizen et al., 2013b). This suggests a separation of the effect of modafinil from other DAT inhibitors in a risk-based decision-making task. The effect of modafinil on various psychiatric-related behaviors, however, remains unclear. The use of cross-species tasks would enable future studies to investigate mechanism-related effects of modafinil in rodents using neuroscience tools not available for testing in humans.
In an attempt to parse these potential domains of effect, we assessed the effects of clinically relevant doses of modafinil in healthy, non-sleep deprived human volunteers in the five-choice continuous performance task (5C-CPT), Wisconsin Card Sort Task (WCST), and the human BPM. These tests are validated for the assessment of attention and cognitive control (Cope et al., 2016; Lustig et al., 2013), and arousal and exploration (Minassian et al., 2011; Perry et al., 2009; van Enkhuizen et al., 2013a), respectively. We also determined the effects of modafinil on the arousal and exploratory behavior of male and female mice at doses that are directly comparable to those used in human testing. Given the separation of effects for more selective DAT inhibitors, we hypothesized that modafinil would improve attention without inducing hyperarousal.
2. Material and methods
2.1. Healthy volunteer studies
2.1.1. Volunteer recruitment
Procedures were approved by The University of California San Diego (UCSD) School of Medicine’s institutional review board. Using online advertisements and flyers posted throughout the community, 61 male and female participants were recruited according to the following inclusion criteria: 1) Ages 18–35; 2) In good general health; 3) No lifetime history of an axis I or axis II disorder; 4) No first-degree relative with a history of psychotic or mood disorder; and 5) No specific contraindications or previous adverse reactions to amphetamine. Exclusion criteria included the following: 1) Clinically significant electrocardiogram or physical exam determined by the study physician; 2). Women with a positive serum HCG pregnancy test or who are lactating; 3) History of alcohol or substance (e.g., sedative-hypnotics, cannabis, stimulants, opioids, cocaine, hallucinogens) abuse or dependence within the last 30 days, or a positive urine toxicology screen for illegal substances completed on study entrance. Nicotine abuse or dependence was not an exclusion criterion; 4) Current severe, systemic medical illness that may compromise cognitive functioning or serious cardiac disease; and 5) Current or history of neurological disorder such as seizures or stroke, Parkinson’s disease, dementia, or a history of head injury with loss of consciousness for at least 15 min. Data from a subset of the placebo group has been published previously (Minassian et al., 2016), but recruitment and consenting procedures were identical and overlapping for participants in the placebo group.
2.1.2. Randomization and drug treatment
Participants meeting all inclusion/exclusion criteria were randomized, double blind, into one of three groups: placebo, 200, or 400 mg modafinil. During the consenting process, volunteers were told they could receive either amphetamine, caffeine, modafinil or placebo to limit expectations of drug effects, as this investigation was part of a larger study designed to assess the effects of stimulants on cognition and behavior.
2.1.3. 5 Choice continuous performance task
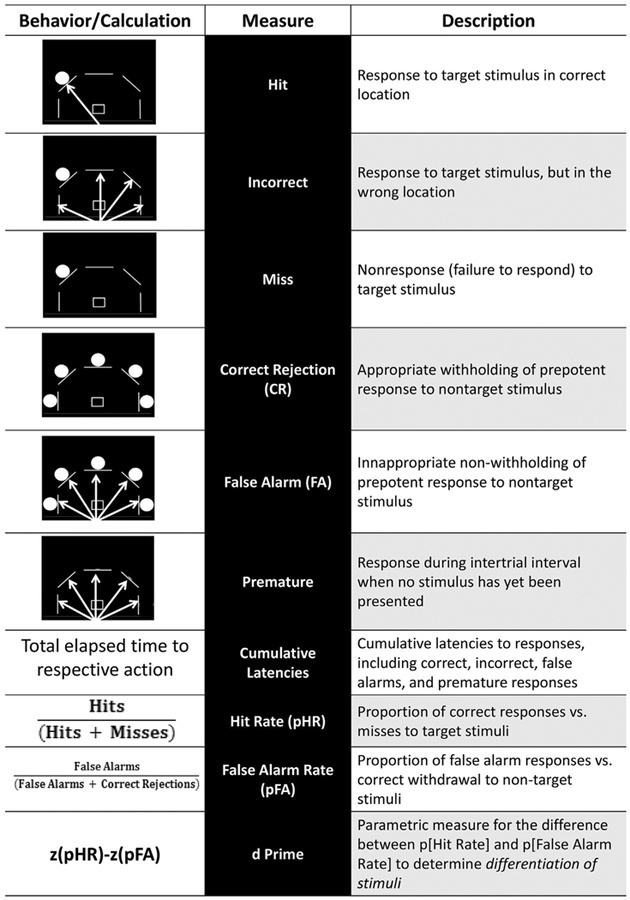
Procedures for the human version of the 5C-CPT have been described in detail (McKenna et al., 2013; van Enkhuizen et al., 2014; Young et al., 2013). In brief, participants were positioned 60 cm away from a 56 cm computer monitor. A spring-mounted analog joystick was provided to the participant to record responses using their dominant hand. This joystick automatically returned to center when released following a response. Participants were forewarned that 5 white lines (3 cm) in an arc would appear on the black background. If a single white dot (2 cm diameter) appeared behind any of the lines, they were instructed to move the joystick in the corresponding direction (target). If dots appeared behind all lines, they were instructed to avoid responding on the joystick (non-target). See Table 1 for descriptions of each trial type and explanation of outcome variables. Before performing 5C-CPT, participants were allowed one 12-trial practice session (10 target and 2 non-target trials, randomly presented). The full task consisted of 270 trials, 225 target and 45 non-target, presented pseudo-randomly to ensure no more than three consecutive presentations of the same trial type. This high ratio of target:non-target trial types engendered prepotent responses relevant to the cognitive control aspect of 5C-CPT performance (Young et al., 2016). To reduce temporal predictability of stimulus presentation, 5C-CPT trials were separated by a variable intertrial interval (ITI; 0.5, 1, or 1.5 s) following presentation of the stimulus on the previous trial. ITI length was chosen pseudo-randomly to ensure that no more than three of one specific ITI occurred consecutively. Response outcomes were recorded according to criteria in Table 1, including hits, misses, false alarms (FA), and correct rejections (CR) that were used to calculate hit rate (HR), false alarm rate (FAR), and signal detection variables of d-prime (d’, signal detection).
Table 1. A description of behavior, calculations, and interpretation used to quantify effects in the 5C-CPT.
White arrows in diagrams indicate possible joystick movements for the behavior being demonstrated.
|
2.1.4. Behavioral pattern monitor
The procedures and testing room for the human behavioral pattern monitor (BPM) have been described in detail previously (Henry et al., 2010, 2011, 2013a, 2013b, Minassian et al., 2010; Minassian et al., 2016; Perry et al., 2009; Young et al., 2007). Before entering the BPM room, patients were fitted with an ambulatory monitoring device (Vivometrics, Ventura, CA) worn around the torso to quantify motor activity. Participants then entered the BPM room, where they were asked to wait for 15 min without any instructions other than to wait as the experimenter prepares a separate task. Videos of the participants’ activity in the room were sampled at 30 frames per second were stored on a computer in an adjacent room for analysis. Participants were notified during the informed consent process that they might be videotaped during a part of their examination, but were not specifically told when videotaping would occur.
To assess motor activity, mean acceleration in digital units was derived for each of the three 5-min time periods of the 15-min BPM session. Object interactions were quantified manually by trained raters blind to group condition. We quantified the total number of object interactions, defined as deliberate physical contact with a novel object with any part of the body, e.g., hand or foot.
To assess spatial patterns of behavior, digitized video images were subjected to frame-by-frame analysis with proprietary software (TopScan 1.0; Clever Systems Inc, Washington, DC) to generate x-y coordinates of participants within a 720 by 480-pixel grid. The x-y data were initially processed with a low-pass Butterworth filter to remove instrumental noise. Spatial d (i.e., dimensionality), measured between values of 1 and 2, indicates the extent to which a subject travels in a straight line (close to 1) or adopts a meandering path, such as very localized, circumscribed movements (close to 2). This measure is calculated by plotting successive x-y coordinates of the path traveled against varying lengths of measuring resolutions. Distance traveled is plotted against the number of movement counts using a double-logarithmic plot, with the slope of this line of fit being used to calculate spatial d (Geyer et al., 1986; Paulus and Geyer, 1991, 1993). Values at either end of this range may indicate perseverative behavior (Perry et al., 2009; Young et al., 2010). Activity counts, defined as the number of discrete instances of movement or the smallest measured change in x-y coordinates, were also derived.
Acceleration, object interaction, activity counts, and spatial d values greater than 3 standard deviations from the grand mean were considered outliers; these data were not included in the statistical analyses (4 subjects). Spatial d was not calculated for 2 subjects due to a software error. Group differences were tested using analyses of variance (ANOVA), with treatment (placebo, 200 mg modafinil, 400 mg modafinil) as the between-subjects measures. Effect sizes were calculated with partial eta-squared. Statistical analyses were conducted with SPSS version 22.0 (IBM Corporation 2013).
2.1.5. Wisconsin card sorting task
Participants performed a computer version of the WCST-64 card version (Heaton, 1993). The WCST is a well-established measure of executive function, designed to assess deficits in rule attainment and cognitive set shifting linked to frontal cortex pathology (Goldberg and Miller, 1986; Perry and Braff, 1998). The WCST requires participants to sort cards based on three perceptual dimensions (color, shape, and number), and provides feedback to allow the subject to identify the correct matching rule. After a specific number of correct responses, the card sorting category changes. Failure to abandon the previous sorting rule when it has been explicitly changed is associated with prefrontal dysfunction and perseverative behavior, a tendency to engage in maladaptive repetitive responses (Perry and Braff, 1998). Dependent measures include: (1) total number of errors, (2) perseverative errors, and (3) number of categories completed. Error scores for the task are converted to T scores corrected for age and education, where a higher score indicates better performance on the measure. Results for two of the 62 volunteers were incorrectly recorded and subsequently excluded from the analysis.
2.2. Mouse studies
2.2.1. Behavioral pattern monitor (BPM)
Sixty-three C57BL/6J mice (31 female, 32 male) were obtained from Jackson Laboratories and tested at approximately 4 months of age. One month prior, these mice had previously been utilized for an unrelated study where they received a single intraperitoneal injection of either 0.56 mg/kg mecamylamine or saline immediately before a single 5-min session of the forced swim task. No other testing or experimental manipulation took place in the intervening period between these two experiments. Throughout all procedures, these mice were housed 4 per cage in a reverse 12-h light-cycle (lights off at 8:00 a.m.) room in a UCSD-operated vivarium with food (Harlan 8604, Madison, WI, USA) and water available ad-libitum, except during the BPM test. Animals were transported to the testing room by 10:00 a.m. and allowed to acclimate for 1 h prior to testing, which took place between 11:00 a.m. and 5:00 p.m. over two consecutive days. Male mice were tested on day 1 and females on day 2. All testing procedures were approved by the UCSD Institutional Care and Use Committee, and all facilities met federal and state requirements for animal care as approved by the American Association for Accreditation of Laboratory Animal Care.
Methods and testing equipment for the mouse BPM have been described in detail previously (Geyer et al., 1986; Minassian et al., 2016; Perry et al., 2009; Risbrough et al., 2006; Young et al., 2010). Mice were tested in eight BPM chambers; each chamber consisting of a 30.5 × 60 × 38-cm arena designed to detect horizontal and vertical activity, as well as nosepoking. Mice were placed in the upper left corner of the chamber at the beginning of a session which was immediately initiated.
Primary dependent measures were total activity counts, hole-pokes, rearing, and spatial d. Data were analyzed using ANOVA, with between-subjects factors of sex and treatment. Dependant variables were collapsed across sex where no main effect of sex was observed. Data were analyzed using Biomedical Data Programs software (Statistical Solutions Inc., Saugus, MA, USA) with an alpha level of 0.05.
2.2.2. Drug preparation
Modafinil (Sigma Aldrich, St. Louis, MO, USA) was dissolved in warm (60°C) homogenized vehicle (1% methylcellulose and 5% Tween 80 in saline) and sonicated for 1 h at room temperature to produce the 32 mg/kg dose, from which 10.1 and 3.2 mg/kg doses were created. These doses were chosen for compatibility (in terms of mg/kg vs. human study) and with previous evidence of treatment-induced hyperarousal and increased wakefulness in mice (Willie et al., 2005). In the absence of a thorough and specific cross-species pharmacokinetic analysis of modafinil, we used dose conversion guidelines from Nair and Jacob (2016) to guide equivalent dosing in mice. Specifically, we divided the human dose (mg/kg, based on average bodyweight of participants) by a conversion factor of 0.081. The dose calculated came to roughly 33 mg/kg. Based on our own data, 32 mg/kg is sufficient to induce hyperactivity in the BPM (Young et al., 2011a) leading us to believe this estimated dose was higher than the dose required to improve 5C-CPT performance in our participants. Given that these guidelines were not specific to modafinil and based solely on human bodyweight in our studies resulted in doses of 3.2 mg/kg (for 200 mg), we therefore included two half-log doses lower starting from 32 mg/kg (10.1 and 3.2 mg/kg). Mice received one of three doses of modafinil or vehicle via intraperitoneal injection (10 ml/kg) immediately prior to the start of testing.
3. Results
3.1. Demographics and treatment randomization
Demographic information and treatment group sample sizes are reported in Table 2. Although a greater number of female participants participated in this study, the gender difference did not reach statistical significance (Pearson χ2(1,62) = 1.61, p = 0.20). There were more females than males in the modafinil groups; this difference approached statistical significance (Fisher’s Exact Test (2,62) = 4.43, p = 0.11). Participants did not differ significantly in terms of age (F(2,61) = 1.32, n.s.) or education (F(2,61)<1, n.s.), although Body Mass Index (BMI) was higher in participants in the 200 mg modafinil group compared to placebo (F(2,55) = 4.89, p < 0.05).
Table 2. Demographic information for subjects given placebo (n=33), modafinil 200 mg (n=14) and modafinil 400 mg (n=15).
Groups differed only in Body Mass Index (placebo < 200 mg modafinil, * = main effect of group). No other significant differences were found for any other variable analyzed.
| Variable | Placebo | 200 mg Modafinil | 400 mg Modafinil |
|---|---|---|---|
| Sex | |||
| Male | 18 | 4 | 4 |
| Female | 15 | 10 | 11 |
| Mean (SD) age in years | 23.4 (4.2) | 24.6 (4.0) | 25.5 (5.3) |
| Mean (SD) years of Education | 14.8 (1.8) | 14.9 (2.1) | 14.7 (1.4) |
| Mean (SD) Body Mass Index * (kg/m2) | 22.7 (3.0) | 26.7 (5.2) | 24.8 (4.2) |
3.2. Modafinil improved attention in healthy volunteers
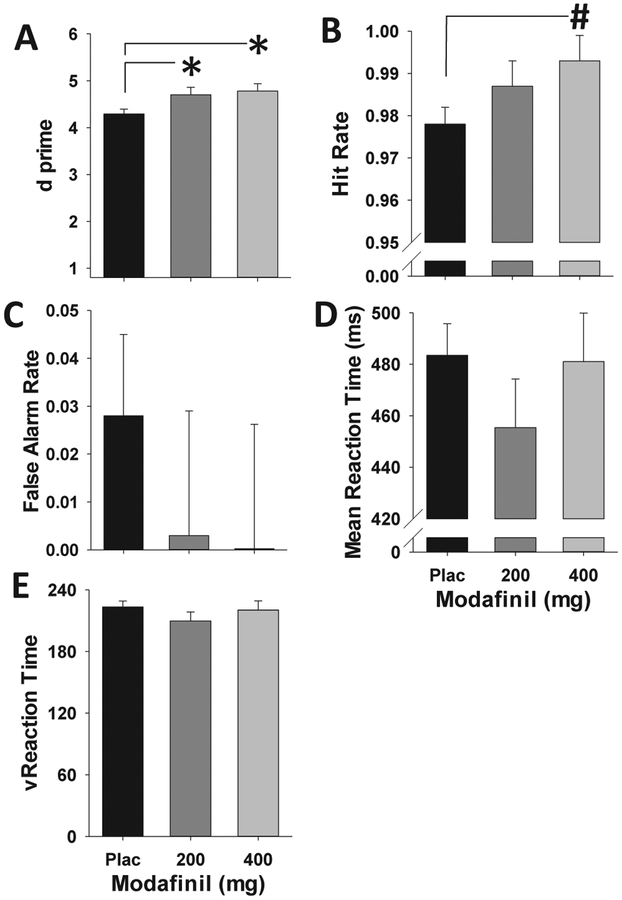
Modafinil significantly improved attention as measured by d prime, (F(2,58) = 4.4, p < 0.05, partial η2 = 0.18; Fig. 1A). Post-hoc analyses revealed that this enhancement was found with both doses of modafinil compared to placebo (p < 0.05). This improvement was driven by a strong trend towards increased hit rate, representing improved target detection (F(2,58) = 2.8, p = 0.07, partial η2 = 0.019; Fig. 1B) in modafinil-compared to placebo-treated individuals (p < 0.05). Modafinil did not significantly alter false alarm rate, mean reaction time, or reaction time variability (Fs(2,58)<1, n.s., Fig. 1C–F). No significant main effects or interactions with gender were evident for any of these variables.
Fig. 1. Modafinil Improves Attention as Measured by the 5C-CPT.
D prime is significantly increased compared to placebo (Plac) by the 200 and 400 mg doses of modafinil (A). This modafinil-induced enhancement of signal detection was driven by a strong trend toward improved hit rate (target detection) in these groups (B). No significant effects of modafinil were observed in terms of false alarm rate (C), mean reaction time (D), or reaction time variability (E), at the doses tested. Data presented as mean + SEM, * denotes p < 0.05 vs. Plac. # denotes p < 0.1 vs. Plac.
3.3. Modafinil did not produce hyperactivity in healthy volunteers at pro-cognitive doses
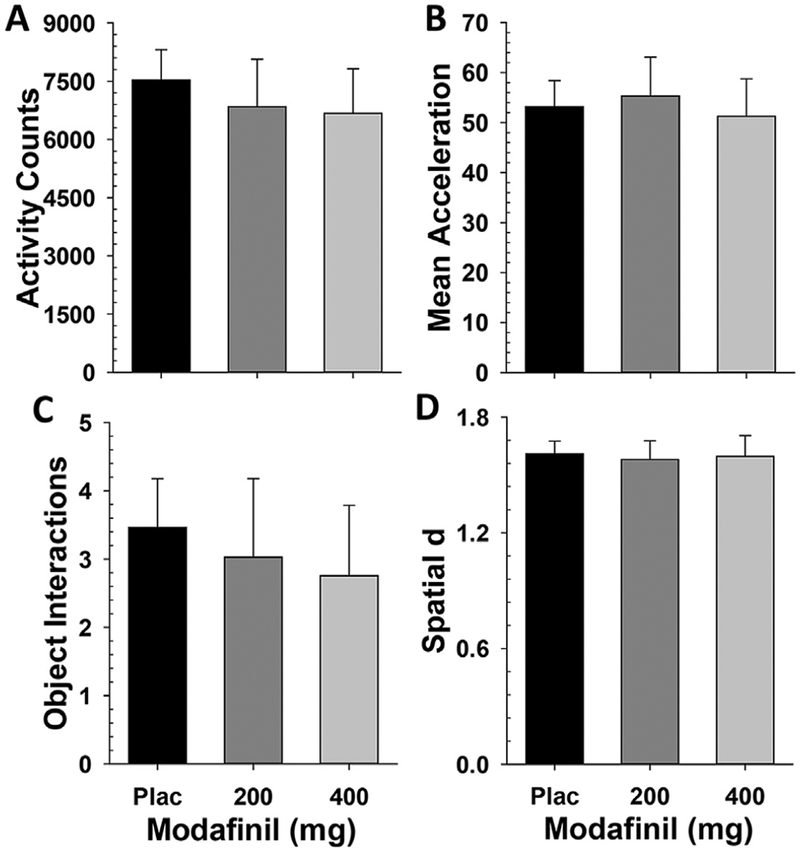
Modafinil did not significantly alter any measure of activity in the human BPM as measured by activity counts, acceleration, specific object interactions, or spatial d (Fs(2,57)<1, ns, Fig. 2A–D).
Fig. 2. Modafinil does not induce hyperactivity in the human BPM at doses efficacious to improve attention.
In humans, modafinil did not produce significant increases in locomotor activity measured by total activity counts (A), acceleration (B), or specific object interactions (C), nor did it affect spatial d (D). Data presented as mean + SEM.
Because BMI was higher in the 200-mg modafinil group compared to placebo, the human BPM analyses were repeated with BMI as a covariate. There was no main effect of BMI nor did any of the activity measures reach or approach statistical significance.
3.4. Modafinil did not effect WCST performance
Modafinil did not significantly modify any measure of cognitive control or flexibility measured by the WCST (Fs(2, 54)≤1.95, ns, Table 3). No significant sex by drug interactions were observed on any measure (Fs(2, 54)≤1.88, ns).
Table 3.
Modafinil does not significantly alter performance of the WCST.
| Measure | Mean (SEM) | F | p-value |
|---|---|---|---|
| Total Correct | 0.73 | 0.49 | |
| Placebo | 48.3 (1.8) | ||
| 200 mg Modafinil | 45.4 (3.1) | ||
| 400 mg Modafinil | 46.6 (3.3) | ||
| Total Errors | 0.75 | 0.48 | |
| Placebo | 15.6 (1.8) | ||
| 200 mg Modafinil | 18.6 (3.1) | ||
| 400 mg Modafinil | 17.4 (3.3) | ||
| % Perseverative Responses | 0.91 | 0.41 | |
| Placebo | 37.4 (1.8) | ||
| 200 mg Modafinil | 28.3 (8.8) | ||
| 400 mg Modafinil | 44.6 (3.6) | ||
| % Perseverative Errors | 1.04 | 0.36 | |
| Placebo | 35.2 (4.6) | ||
| 200 mg Modafinil | 27.9 (7.8) | ||
| 400 mg Modafinil | 45.1 (8.4) | ||
| % Nonperseverative Errors | 0.09 | 0.91 | |
| Placebo | 50.6 (5.2) | ||
| 200 mg Modafinil | 50.4 (8.8) | ||
| 400 mg Modafinil | 54.3 (9.4) | ||
| Categories Completed | 0.24 | 0.79 | |
| Placebo | 3.5 (0.3) | ||
| 200 mg Modafinil | 3.3 (0.4) | ||
| 400 mg Modafinil | 3.6 (0.5) | ||
| Trials to Complete 1st Category | 1.95 | 0.15 | |
| Placebo | 14.2 (2.3) | ||
| 200 mg Modafinil | 19.5 (3.8) | ||
| 400 mg Modafinil | 21.3(4.1) | ||
3.5. Modafinil produced hyperactivity only at high doses in mice
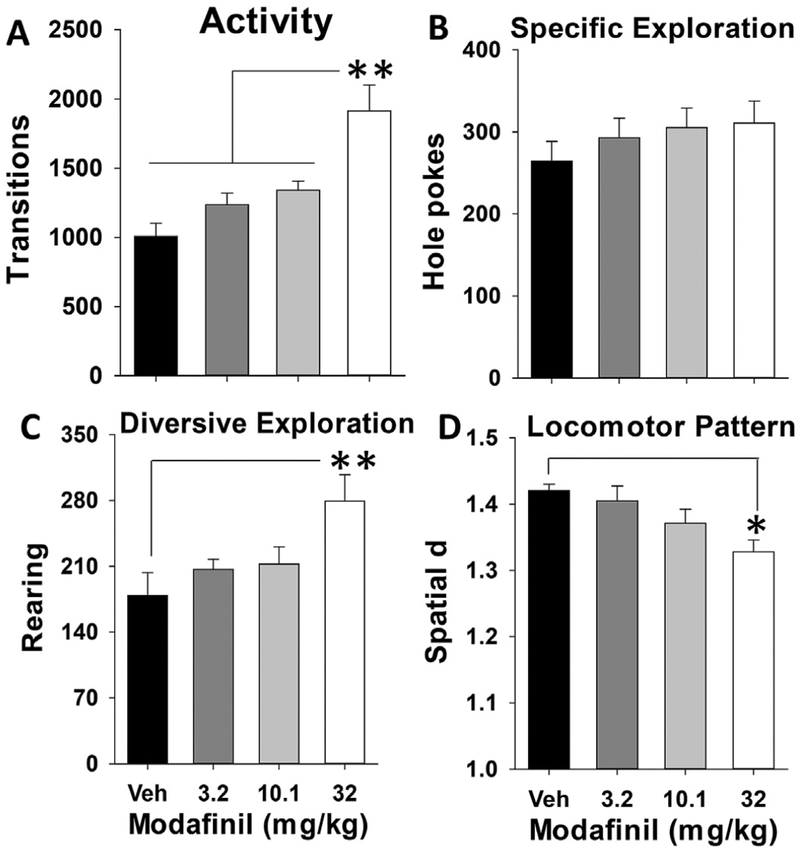
Modafinil significantly increased activity (BPM transitions, F(3,55) = 10.2, p < 0.001, partial η2 = 0.36; Fig. 3A). Post-hoc analyses revealed that only the 32 mg/kg modafinil significantly increased transitions compared with vehicle and all other groups (p < 0.05). Modafinil had no significant effect on holepoking (F(3,55)<1, ns, Fig. 3B). Modafinil also significantly increased exploration (rearing, F(3,55) = 4.7, p < 0.01, partial η2 = 0.20; Fig. 3C) and decreased spatial d (F(3,55) = 4.0, p < 0.05, partial η2 = 0.18; Fig. 3D), again selectively at the 32 mg/kg dose. Main effects of sex were only observed on rearing (F(3,55) = 10.4, p < 0.01; partial η2 = 0.16), with male mice exhibiting significantly more rearing than females. No significant sex by drug interactions were observed on any measure.
Fig. 3. Modafinil induces hyperactivity in mice at high doses.
Modafinil dose dependently increased horizontal activity (A) at 32 mg/kg compared to all other doses. No significant changes in specific exploration (hole poking) (B) were seen at any dose of modafinil. Diversive exploration (rearing) (C) was significantly increased compared to control at the highest dose. The 32 mg/kg dose also significantly decreased circumscribed, meandering locomotor patterns as measured by spatial d (D). Data presented as mean + SEM, * denotes p < 0.05 vs. vehicle (Veh), ** denotes p < 0.01 vs. Veh.
4. Discussion
Here, we report that modafinil improved 5C-CPT performance. Further, enhancement of attention/cognitive control was achieved at doses that did not induce hyperarousal in either healthy volunteers or mice. Therefore, it appears that the pro-cognitive effects of modafinil may be domain-specific, occurring independently from the levels of arousal induced by similar drugs, supporting its use as a pro-cognitive pharmacotherapy with a likely limited abuse potential.
Modafinil significantly improved attention in healthy volunteers as measured by d prime. This modafinil-induced improvement arose from a strong trend of modafinil increasing target detection (hit rate). Importantly, this increased target detection was accompanied by a reduced false alarm rate (though non-significant). Hence, modafinil-induced increased target detection was not driven by increased overall responsivity to stimuli, as supported by no change in reaction times. The human BPM and WCST data provide further evidence that the modafinil-induced improvement in attention (via target detection) was domain-specific, given that 5C-CPT improvement did not result from hyperarousal or preservative behavior in these volunteers. Cross-species testing in mice was able to replicate previously reported hyperactivity induced by high doses of modafinil (Young et al., 2011a), and demonstrate that this effect was observed irrespective of sex. Moreover, this modafinil-induced hyperactivity was not observed at lower doses, which may better represent the doses used in humans. Overall, these data indicate pro-cognitive properties of modafinil within the domain of attention and cognitive control at therapeutic doses that do not induce hyperactivity in healthy volunteers.
To our knowledge, these data represent the first evidence that modafinil can enhance baseline attention of healthy adults. Previously, improvements in cognitive function have been limited to studies involving tasks requiring exceptionally high cognitive demand (Muller et al., 2013), tasks unable to dissociate attention from other domains (Randall et al., 2005), or with participants experiencing sleep deprivation (Baranski et al., 2004; Muller et al., 2004), or psychiatric impairment (Minzenberg and Carter, 2008). Consistent with previous set-shifting-based tasks (Turner et al., 2003), modafinil did not affect WCST performance in healthy subjects. The 5C-CPT improvements highlighted in this investigation may therefore indicate highly domain-specific efficacy to improve attention and cognitive control of modafinil in improving baseline cognitive performance. The mechanism of action underlying this effect remains to be determined however. The pro-cognitive effects of modafinil may arise from its ability to facilitate middle frequency cortical oscillations (theta, alpha, beta) acutely (Minzenberg et al., 2014a, 2014b), or higher frequency cognitive-control related activity (gamma) with sustained treatment (Minzenberg et al., 2016). Further, McKenna and colleagues (McKenna et al., 2013) identified numerous regions underlying 5C-CPT performance using fMRI. They identified task-specific activation of premotor cortex, inferior parietal lobe, basal ganglia, and thalamus during target trials. In addition, activation in inferior frontal cortex, premotor cortex, presupplementary motor area, and inferior parietal lobe were observed during non-target trials. Given that this task is available for use in rodents (Barnes et al., 2012a, b; Young et al., 2009), future investigation will assess regional and/or neurochemical contributions to distinct aspects of 5C-CPT task performance to determine where/how modafinil improves attention. Identification of these targets, which have remained elusive to date, could provide key insights leading to the development of more targeted pro-cognitive therapeutics.
Given its limited affinity for DAT and NET (Volkow et al., 2009), the primary sites of action for classical stimulants that induce arousal states such as cocaine or amphetamine, it was necessary to determine whether these pro-cognitive effects were simply due to hyperarousal in the volunteers. At the pro-cognitive doses in healthy human volunteers, we found no evidence of hyperarousal in the BPM. Based on the weight of the human volunteers, the 200 and 400 mg doses equated to around 3.2–5.0 mg/kg. When assessed in mice, this dose (3.2 mg/kg) also did not affect activity in the BPM. Higher doses (32 mg/kg) were required to induce measurable arousal in both male and female mice, consistent with our earlier studies in males only (Young et al., 2011a). Ideally comparisons could be made at doses that achieve comparable peak plasma concentrations across species, although plasma concentrations and exact scaling parameters at doses <32 mg/kg have not been determined in C57Bl-6 mice to our knowledge. Given that modafinil is approved for treatment of narcolepsy, its therapeutic doses are thought to be comparable to wake-inducing (reducing non-REM sleep, increasing wakefulness) behavior in mice, observed in doses as low as 10 mg/kg (Willie et al., 2005). This dose resulted in modest but non-significant arousal effects in the BPM. While the current studies are not sensitive to rule out modest increases in arousal, these findings do support a lack of hyperarousal at these low doses in mice and in humans. Alternatively, calculations based on potential metabolism differences between mice and humans (Nair and Jacob, 2016) estimated the 400 mg dose (which improved 5C-CPT performance in humans), would be equivalent to 33 mg/kg in mice, the dose that induced hyperactivity in the mouse BPM here and in previous reports (Young et al., 2011a). It is therefore unlikely, that properties of modafinil conform to these general guidelines given the comparable wake-promoting effects described in mice at doses as low as 10 mg/kg (Willie et al., 2005). Similar scaling properties in the BPM have also been reported for amphetamine. When only accounting for cross-species differences in metabolism, doses of amphetamine producing hyperactivity in mice are insufficient to induce hyperactivity in humans (Minassian et al., 2016). Taken together, these data support the conclusion that the efficacy of modafinil as a cognitive enhancer is not attributable to a significant increase in overall arousal and, when tested in rodents, doses from 3 to 32 mg/kg should be investigated.
The potential pro-cognitive effects of modafinil are unlikely to be limited to one domain, (Minzenberg and Carter, 2008; Sahakian and Morein-Zamir, 2015), hence others remain to be tested. For example, although accurate performance in 5C-CPT was never explicitly rewarded, ongoing performance monitoring by the volunteer could imbue a degree of feedback-related reward, another behavior highly susceptible to reduction or inhibition of catecholamine reuptake (van Enkhuizen et al., 2013b; Young et al., 2011c; Zeeb et al., 2009). Hence, investigating feedback-related behavior would be beneficial. For example, the Iowa Gambling Task is another available cross-species task that measures feedback-related behavior during risky decision-making. Such tasks performed in humans and mice could be utilized to test whether the effects observed in the 5C-CPT were due to enhanced feedback-related processing of information in the task itself. In addition, using this cross-species task would further enable delineation of the mechanism(s) of action of modafinil (Young et al., 2011a). If modafinil were found to increase reward-related feedback processing, concerns regarding abuse potential could increase and hamper the possible therapeutic use of modafinil as a cognitive enhancer. Additional known modafinil sites of action that could produce cognitive enhancement include effects on serotonin (Tanganelli et al., 1992), histamine (Scammell et al., 2000), orexin (Willie et al., 2005), GABA, or glutamate signaling (Ferraro et al., 1999). Studies assessing their potential role in these effects are required in the future, with the availability of the rodent 5C-CPT making such studies possible.
These findings are limited by significant differences in the gender makeup of the treatment groups. Overall, a greater number of females participated in the human studies and treatment randomization resulted in fewer males in the two modafinil groups compared to placebo. As a result, this study was likely underpowered to detect drug by gender interactions, increasing the probability of type II error in the BPM experiment if a sexually dimorphic response to modafinil exists. If this were the case, any heightened response to modafinil in males in the BPM could have been statistically blunted overall by the low participation of this group. Given a review of the literature, there is little evidence currently to suggest a sexually dimorphic response to modafinil. Further, no sexually dimorphic response was observed in mice where statistical power was not an issue. Thus, while further study will be required to fully rule out this possibility, we do not find convincing evidence to suspect prominent gender differences in the present study.
5. Conclusions
In summary, it is clear that modafinil effectively enhances cognitive ability within the domain of attention and cognitive control in healthy adult volunteers. Further, this pro-cognitive effect is domain-specific insofar as it is achieved at doses that did not result in hyperactivity. Although the precise mechanism underlying this effect remains to be determined, the availability of rat and mouse 5C-CPTs (Barnes et al., 2012a, b; Young et al., 2015; Young et al., 2011b) enables the use of neuroscience techniques to determine underlying mechanisms. Furthermore, given that patients with schizophrenia exhibit deficient 5C-CPT performance (Young et al., 2013), the present findings support the suggestion that modafinil may remediate such a deficit.
Acknowledgements
We thank all of the participants who volunteered for these studies, which were made possible by funding from NIMH grants R01MH104344–03, UH2MH109168e01, R01 MH071916, and T32MH018399–30, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. They have full control of all primary data. The authors thank Dr. Harriet De Wit for her assistance in designing this study, and Mahalah Buell, Elise Winbrock, and Karen Kloezeman for their contributions to data collection and analysis.
Footnotes
Disclosure of biomedical financial interests and potential conflicts of interest
Dr. Geyer has received consulting compensation from Lundbeck, Omeros, Otsuka, and Sunovion, and holds an equity interest in San Diego Instruments. Dr. Young has received funding from Cerca Insights and Lundbeck Ltd, and has received consulting compensation for Amgen, and honoraria from Arena Pharmaceuticals and Sunovion. Drs. Cope, Perry, Minassian, and MacQueen, plus Ms. Kreitner and Milienne-Petiot report no biomedical financial interests or potential conflict of interest.
References
- Baranski JV, Pigeau R, Dinich P, Jacobs I, 2004. Effects of modafinil on cognitive and meta-cognitive performance. Hum. Psychopharmacol 19, 323–332. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC, 2012a. D(1) receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacol. Berl 220, 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC, 2012b. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology 62, 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battleday RM, Brem AK, 2015. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur. Neuropsychopharmacol 25, 1865–1881. [DOI] [PubMed] [Google Scholar]
- Bensadoun JC, Brooks SP, Dunnett SB, 2004. Free operant and discrete trial performance of mice in the nine-hole box apparatus: validation using amphetamine and scopolamine. Psychopharmacol. Berl 174, 396–405. [DOI] [PubMed] [Google Scholar]
- Berridge CW, 2006. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology 31, 2332–2340. [DOI] [PubMed] [Google Scholar]
- Chiarello RJ, Cole JO, 1987. The use of psychostimulants in general psychiatry. A reconsideration. Arch. Gen. Psychiatry 44, 286–295. [DOI] [PubMed] [Google Scholar]
- Cope ZA, Halberstadt AL, van Enkhuizen J, Flynn AD, Breier M, Swerdlow NR, Geyer MA, Young JW, 2016. Premature responses in the five-choice serial reaction time task reflect rodents’ temporal strategies: evidence from no-light and pharmacological challenges. Psychopharmacol. Berl 233, 3513–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O’Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K, 1999. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology 20, 346–356. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P, 2015. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr. Bull 41, 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Miklowitz DJ, 2013. Treatment of bipolar disorder. Lancet 381,1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL, 1986. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol. Biochem. Behav 25, 277–288. [DOI] [PubMed] [Google Scholar]
- Goldberg JO, Miller HR, 1986. Performance of psychiatric inpatients and intellectually deficient individuals on a task that assesses the validity of memory complaints. J. Clin. Psychol 42, 792–795. [DOI] [PubMed] [Google Scholar]
- Heaton RK, 1993. WCST: Computer Version-2 Research Edition Manual. Psychological Assessment Resources, Odessa. [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A, Translational Methamphetamine A.R.C.G., 2013a. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav. Brain Res. 236, 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, Geyer MA, Perry W, 2013b. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. J. Affect Disord. 150, 948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W, Translational Methamphetamine A.R.C.G., 2011. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacol. Berl 215, 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W, 2010. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci. Biobehav Rev. 34, 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND, 2005. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Nasrallah H, Pucci M, James S, Citrome L, 2013. A systematic review of psychostimulant treatment of negative symptoms of schizophrenia: challenges and therapeutic opportunities. Schizophr. Res 147, 241–252. [DOI] [PubMed] [Google Scholar]
- Linssen AM, Sambeth A, Vuurman EF, Riedel WJ, 2014. Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int. J. Neuropsychopharmacol 17, 961–977. [DOI] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW, 2013. CNTRICS final animal model task selection: control of attention. Neurosci. Biobehav Rev. 37, 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ, 2006. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J. Pharmacol. Exp. Ther 319, 561–569. [DOI] [PubMed] [Google Scholar]
- McKenna BS, Young JW, Dawes SE, Asgaard GL, Eyler LT, 2013. Bridging the bench to bedside gap: validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res. 212, 183–191. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W, 2010. The quantitative assessment of motor activity in mania and schizophrenia. J. Affect Disord. 120, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W, 2011. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS One 6, e24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Young JW, Cope ZA, Henry BL, Geyer MA, Perry W, 2016. Amphetamine increases activity but not exploration in humans and mice. Psychopharmacol. Berl 233, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS, 2008. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33, 1477–1502. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Gomes GC, Yoon JH, Watrous AJ, Geng J, Firl AJ, Carter CS, 2014a. Modafinil augments oscillatory power in middle frequencies during rule selection. Psychophysiology 51, 510–519. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH, Cheng Y, Carter CS, 2014b. Modafinil effects on middle-frequency oscillatory power during rule selection in schizophrenia. Neuropsychopharmacology 39, 3018–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH, Cheng Y, Carter CS, 2016. Sustained modafinil treatment effects on control-related gamma oscillatory power in schizophrenia. Neuropsychopharmacology 41, 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ, 2013. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology 64, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steffenhagen N, Regenthal R, Bublak P, 2004. Effects of modafinil on working memory processes in humans. Psychopharmacol. Berl 177, 161–169. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, 1991. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 15, 903–919. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, 1993. Three independent factors characterize spontaneous rat motor activity. Behav. Brain Res. 53, 11–20. [DOI] [PubMed] [Google Scholar]
- Perry W, Braff DL, 1998. A multimethod approach to assessing perseverations in schizophrenia patients. Schizophr. Res 33, 69–77. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA, 2009. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch. Gen. Psychiatry 66, 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, File SE, 2005. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J. Clin. Psychopharmacol 25, 175–179. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA, 2006. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology 31, 2349–2358. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morein-Zamir S, 2015. Pharmacological cognitive enhancement: treatment of neuropsychiatric disorders and lifestyle use by healthy people. Lancet Psychiatry 2, 357–362. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB, 2000. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J. Neurosci 20, 8620–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Farah MJ, 2011. Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol. Bull 137, 717–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanganelli S, Fuxe K, Ferraro L, Janson AM, Bianchi C, 1992. Inhibitory effects of the psychoactive drug modafinil on gamma-aminobutyric acid outflow from the cerebral cortex of the awake freely moving Guinea-pig. Possible involvement of 5-hydroxytryptamine mechanisms. Naunyn Schmiedeb. Arch. Pharmacol 345, 461–465. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ, 2003. Cognitive enhancing effects of modafinil in healthy volunteers. Psycho-pharmacol. Berl 165, 260–269. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Acheson D, Risbrough V, Drummond S, Geyer MA, Young JW, 2014. Sleep deprivation impairs performance in the 5-choice continuous performance test: similarities between humans and mice. Behav. Brain Res. 261, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW, 2013a. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int. J. Neuropsychopharmacol 16, 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW, 2013b. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task: relevance to mania. Psychopharmacol. Berl 225, 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K, 2009. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA 301, 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, 2003. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am. J. Psychiatry 160, 1909–1918. [DOI] [PubMed] [Google Scholar]
- Willie JT, Renthal W, Chemelli RM, Miller MS, Scammell TE, Yanagisawa M, Sinton CM, 2005. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience 130, 983–995. [DOI] [PubMed] [Google Scholar]
- Young JW, Bismark AW, Sun Y, Zhang W, McIlwain M, Grootendorst I, Light GA, 2016. Neurophysiological characterization of attentional performance dysfunction in schizophrenia patients in a reverse-translated task. Neuropsychopharmacology 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, 2010. Action of modafinil–increased motivation via the dopamine transporter inhibition and D1 receptors? Biol. Psychiatry 67, 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, Light GA, 2013. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl. Psychiatry 3, e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA, 2010. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol. Biochem. Behav 96, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kamenski ME, Higa KK, Light GA, Geyer MA, Zhou X, 2015. GlyT-1 inhibition attenuates attentional but not learning or motivational deficits of the Sp4 hypomorphic mouse model relevant to psychiatric disorders. Neuropsychopharmacology 40, 2715–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA, 2011a. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology 36, 1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA, 2009. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One 4, e4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W, 2007. A reverse-translational approach to bipolar disorder: rodent and human studies in the behavioral pattern monitor. Neurosci. Biobehav Rev. 31, 882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA, 2011b. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: separating response inhibition from premature responding. Behav. Brain Res. 222, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA, 2011c. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J. Psychopharmacol 25, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA, 2009. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34, 2329–2343. [DOI] [PubMed] [Google Scholar]