Abstract
Deficits in the domains of attention and response inhibition are central to many psychiatric disorders. As such, animal models of disorders purporting to replicate these behavioral deficits first require tests that can accurately assess the behaviors with high fidelity. The gold-standard clinical test of attention and response inhibition is the continuous performance test (CPT). Although there are a number of CPTs, all share the premise of responding to target stimuli and inhibiting from responding to non-target stimuli. The recently developed rodent five-choice CPT (5C-CPT) requires similar behavioral responses, enabling signal detection parameter calculations. With demonstrable feasibility for rodent testing, the 5C-CPT permits/facilitates: (1) delineation of neural mechanisms underlying these behaviors; (2) multifactorial analyses of the complex interplay between genetic and environmental manipulations relevant to psychiatric disorders; and hence (3) development of novel targeted treatments. All data to date indicate that the rodent 5C-CPT described here has direct translatability to clinical CPTs, producing equivalent measures of behavior in experimental animals to those assessed in humans. The 5C-CPT task provides an important tool toward delineating these mechanisms and developing treatments. However, it is also complex, with long training times and nuances requiring a thorough understanding before utilization. This unit will enable researchers to avoid potential missteps, greatly increasing the likelihood of success.
Keywords: attention, vigilance, impulsivity, cross-species
INTRODUCTION
Impaired attention and response disinhibition are key cognitive deficits that have been broadly identified across a number of neuropsychiatric illnesses including bipolar disorder, schizophrenia, obsessive-compulsive disorder, attention deficit hyperactivity disorder, Huntington’s disease, Alzheimer’s disease, and autism, among others. Given the pervasive nature of pathology within these domains of function, the paucity of efficacious pro-cognitive treatments is a longstanding barrier impeding positive treatment outcomes for patients suffering from these illnesses. Within these domains, treatment development will rely on accurate, high-fidelity measurement of behaviors in model animals and humans.
The gold-standard clinical test of attention and response disinhibition is the continuous performance test (CPT). Several CPTs (e.g., CPT-AX, MacDonald, 2008; X-CPT, Beck et al., 1956; Conners CPT, Conners, 1985) exist and they share the same premise, wherein subjects must respond to target stimuli and be inhibited from responding to non-target stimuli. For rodent studies, the difficulty has been the lack of directly comparable tests with the same requirements. The five-choice serial reaction time task (5CSRTT) has long been used to study sustained attention and waiting impulsivity in rodents (UNIT 8.5H; Humby et al., 2005). It does not have non-target trials, however, and thus does not require response inhibition. The five-choice CPT (5C-CPT) was designed to address this problem and was built on the 5CSRTT platform due to the latter test’s worldwide use. In addition to a target stimulus (a singly illuminated aperture), the 5C-CPT incorporates discrete, infrequent trials in which animals are required to withhold from performing the prepotent response. That is, they must avoid nose poking the illuminated target aperture when the additional four apertures (non-target trials) are illuminated. The inclusion of this competing behavior enables the dissociation of target and false-alarm (responses to infrequent non-target trials) responses. Hence, consistent with human CPTs, the 5C-CPT enables the calculation of bona fide signal detection parameters to assess sensitivity and response bias as measures of vigilance (attention) and response motivation. Construct validity has been verified in a number of studies reporting consistent cross-species (1) deficits resulting from 36-hr sleep deprivation (van Enkhuizen et al., 2014), (2) involvement of parietal cortex across human fMRI and rodent lesion studies (McKenna et al., 2013), and (3) decrements in vigilance across time (Young et al., 2009, 2013b). Further, the 5C-CPT incorporates a variable intertrial interval (ITI) between trial initiation and stimulus onset that minimizes the animal’s ability to effectively time the stimulus presentation, dissociating premature responding from temporal strategies. 5C-CPT training can also act as an easily shapeable platform for within-cohort multidimensional behavioral analyses, such as probabilistic reward learning, progressive ratio breakpoint, or rodent gambling tasks (Young et al., 2015).
The following protocols describe the procedures involved in 5C-CPT training (see Basic Protocols 1 and 2) as well as the extended session, reduced event, or distracter condition challenges (see Basic Protocol 3) that can be used to tease apart subtle differences in the domains of attention, response inhibition, and impulsivity. Additionally, details are provided for calculating signal detection parameters from the data to provide high-fidelity cross-species relevant measures within domains.
All experimental procedures described here were approved by the Animal Care and Use Committee of the University of California San Diego.
BASIC PROTOCOL 1
HANDLING, FOOD RESTRICTION, AND OPERANT CHAMBER HABITUATION
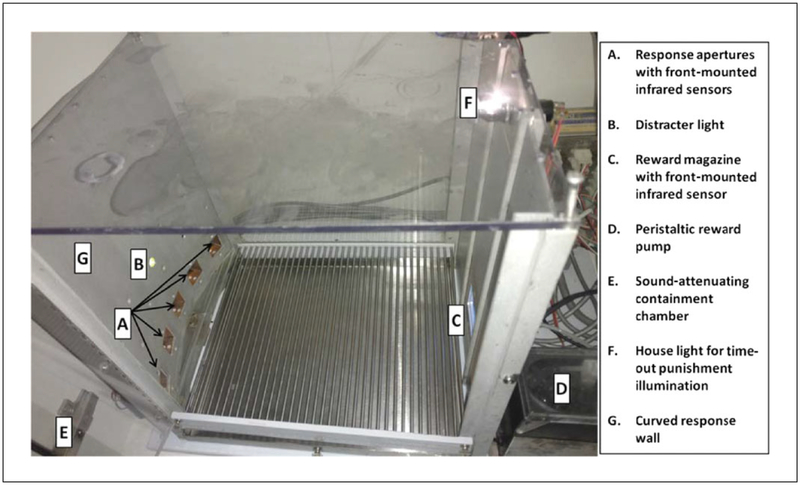
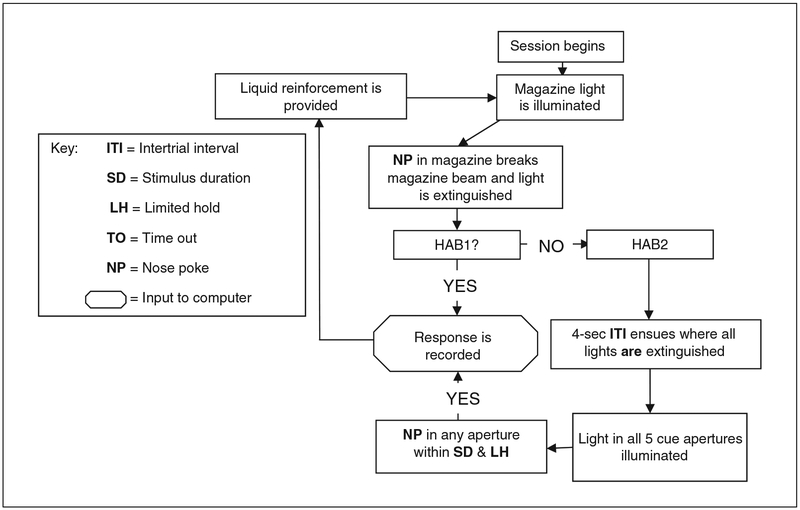
These pre-training procedures are conducted to prevent neophobic aversion and promote reward seeking and consumption, associating responses to illuminated apertures with reward. The protocol below describes environmental habituation, fixed-ratio reward habituation (HAB1), and fixed-ratio response training (HAB2). In HAB1, the animals learn to nose poke in the reward magazine. In HAB2, they learn to nose poke in any one of the five lit apertures. The apparatus used for testing is shown in Figure 9.56.1, and a flowchart summary of the behavioral program is shown in Figure 9.56.2.
Figure 9.56.1.
Setup of a 5C-CPT testing chamber.
Figure 9.56.2.
Flow diagram of HAB1 and HAB2 sessions during 5CSRTT training. During HAB training, animals are required to nose poke in the reward magazine (HAB1) or any of the five response apertures after initiating the trial by leaving the reward magazine (HAB2) to receive reward.
It is important to introduce any new behavioral procedures or stimuli gradually. In addition, reward-seeking behavior can be maximized by utilizing mild food restriction, which also equalizes motivation across individuals. By following these procedures, stress in experimental animals associated with the initiation of training can be minimized, thereby maximizing their motivation to participate in the task.
Materials
Rodent species of interest (see Critical Parameters; Table 9.56.1)
Table 9.56.1.
Verified Rat and Mouse Strains for the 5C-CPT
| Species | Strain | Identifier/reference |
|---|---|---|
| Rats | Long-Evans | RRID:RGD_2308852 |
| Inbred mice | C57BL/6J | RRID:IMSR_JAX:000664 |
| C57BL/6NJ | RRID:IMSR_JAX:005304 | |
| DBA/2A | RRID:IMSR_JAX:000671 | |
| NIHBL(S) | ||
| 129S1/SvImJ | RRID:IMSR_JAX:002448 | |
| In-house mutant mice | D4 heterozygotes (DRD4) Dopamine transporter (DAT) hypomorphs (DAT-HY) |
Young et al. (2011) |
| Specificity Protein 4 (SP4) hypomorphs | Young et al. (2011) |
Strawberry milkshake powder (e.g., Nestle Nesquik)
Skim milk
Means for rodent identification, e.g., numbered ear tags, ear punches, tattoos, microchip transponders
Appropriate rodent housing facilities (see step 1 and Critical Parameters), including:
Standard Allentown Microvent cages with HEPA filtration
Rodent chow pellets (e.g., Harlan Teklad 8460) suitable for wire cage top hoppers
Sipper tube bottles
Nestlets, paper towels, chew blocks
Cart and drape for transporting animals
Digital small animal scale (e.g., Ohaus CS200)
Apparatus:
Five-Hole Nose Poke Wall Chambers for rat (25 × 25 × 25 mm; Med Associates, product no. MED-NP5L-B1; see Fig. 9.56.1)
Power Control and Interface (PCI) Operating Package (Med Associates, MED-SYST-16)
Windows XP computer (or higher capability) with PCI card installed
Med-PC software package (Med Associates, SOF-735)
Smart Control Interface (Med Associates, DIG-716P2; one per chamber)
Med-PC executable behavioral programs (see Fig. 9.56.2)
Fluid reward delivery system:
Peristaltic pump for liquid reward (Lafayette, model 80204M)
Small plastic cup ([223c]50 ml) for each behavioral chamber (to hold [2265]2 ml Nesquik reward per animal throughout the day)
Cleaning materials:
Large lint-free tissues (e.g., Kimtech Kimwipes)
60% (v/v) ethanol
Disinfectant spray (e.g., Airx 44)
Personal protection equipment:
N-95 Mask
Disposable gloves
Lab coat or disposable coveralls
Perform environmental and handling habituation, identification, and weight Regulation
-
1
If animals have been acquired from an outside vendor, allow a minimum of 5 days before conducting any experimental procedures. During this period, house animals as follows:
House all animals in AAALAC-accredited vivarium facilities under IACUC-approved conditions for ambient temperature and humidity.
House mice three to four per cage or rats two per cage in standard Allentown Microvent cages with HEPA filtration.
Keep animals under a reversed 12-hr light cycle, with all testing occurring during the active (dark) phase (7 am to 7 pm).
Provide food and water ad libitum.
Provide environmental enrichment (nestlets, paper towels, chew blocks)
If internally raised animals are used, do not begin testing until they reach 20 g.
-
2
Uniquely identify each animal according to institutional standards.
These protocols generally utilize numbered ear tags, although other approved methods would likely suffice, provided the chance of misidentifying an animal is minimized. -
3
Expose mice to strawberry milkshake in their home cages 24 hr prior to initiating food restriction to prevent neophobia when it is introduced during testing. Dissolve 7 g milkshake powder in 100 ml skim milk and place [223c]150–200 ml in a water bottle in the home cage overnight (in addition to regular food and water). Remove milkshake within 24 hr.
If pellets are to be used instead of milkshake, provide ten pellets per mouse at this time. -
4
During the animals’ active (dark) period, transport them to the testing facilities to be weighed on 3 consecutive days. If animals must be transported between vivarium and testing facilities, load them onto a cart under a dark covering drape.
The mean free feeding weight for each animal will be calculated from these three daily values.The animals will be under a reversed light cycle, meaning they will normally be trained during the dark period. As most experimental rodent species are nocturnal, it is important to minimize exposure to light during the active period.Maintaining a consistency of timing, procedure, and location will establish occasion setting and circadian cues that will help the animal to habituate to procedures more rapidly. -
5
At the start of food restriction, remove most food from the home cage, leaving ~2 g food for each mouse.
If larger pellets are used, it is important to break them into three to four separate pieces to minimize any potential fighting or food hoarding. -
6
Continue daily weighing and feeding until target weight (85% free feeding weight) is achieved. Record weight regularly and adjust daily food provisions accordingly throughout training, as defined by institutional guidelines.
Food quantity should be adjusted to ensure gradual weight loss not exceeding 5% per day. Once target weight is achieved, food provisions should be adjusted to maintain weight at target values. Weighing should coincide with regular training to ensure adequate monitoring of animal weight and health. -
7
Assign each mouse to a behavioral chamber.
The chamber specified as “for rat” by the manufacturer is used for mice as well.Mice should be trained in the same chamber throughout all procedures to minimize introduction of unique exogenous stimuli from day-to-day exposure. Experimental groups should be counterbalanced across behavioral chambers and, if multiple runs are to be used, across the testing cycle, as time of day can affect performance (Porter et al., 2016). -
8
Transport mice to testing facilities.
-
9
Mix enough milkshake to fill each reward retention cup. Insert the intake tubing of the peristaltic pump into the cup and attach the output to the spigot of the operant chamber reward. Run the peristaltic pump to ensure flow of milkshake into the reward well.
Excess milkshake should be used to ensure that it does not run out in the middle of training. Generally, 300 ml milkshake per day is sufficient for training ~90 mice, with excess remaining. Milkshake should be mixed well to ensure that undissolved powder does not produce clogs in peristaltic tubing. -
10
Weigh mice before placing them into the assigned chamber.
This weight should be compared to free feeding average to estimate the chow provision per cage.
Perform fixed-ratio reward habituation (HAB1)
-
11
Initiate HAB1 program (see Fig. 9.56.2).
Animals are permitted 20 min to complete a maximum of 80 trials. Each trial consists of reward delivery (0.03 ml milkshake) and magazine (reward aperture) illumination. The reward aperture light is then turned off if the rodent enters for reward consumption. After 15 sec from previous delivery, the reward aperture is again illuminated and reward delivered. The primary outcome is the total count of reward aperture entries per 15 sec (maximum 80 in 20 min). This training session continues for a minimum of 3 days, until the animal performs a minimum of 60 entries per day for 2 consecutive days. -
12
Remove mice from chambers and return to home cages. Inspect the reward aperture in each chamber to ensure consumption of reward. Wipe away any unconsumed milkshake.
The latter step will not be needed as mice become accustomed to the chamber and retrieving the reward. -
13
Return mice to housing facilities and distribute the proper food provision to each cage.
-
14
At the conclusion of all daily training, discard any remaining milkshake, flush peristaltic tubing with warm water, and clear remaining water from tubing.
-
15
Clean all animal waste from floor trays of operant chambers and wipe floor trays and chamber walls with 60% ethanol daily.
Depending on usage, waste trays and floor grating should be removed regularly (e.g., once per week for 8 runs per day) and washed with soap and warm water, and the chamber should be wiped clean and disinfected. In addition, reward spigots should be thoroughly cleaned and flushed on the same schedule. -
16
Repeat steps 8–15 until each group of mice has been trained to criterion: a minimum of 60 entries per day for 2 consecutive days.
Perform fixed-ratio response training (HAB2)
-
17
Repeat steps 8–10.
-
18
Initiate HAB2 program (see Fig. 9.56.2).
Animals are permitted 30 min to complete a maximum of 150 trials. Mice initiate a trial by nose poking in the reward aperture. Then, 4 sec after the mouse leaves the reward aperture, the cue lights are illuminated in all five nose poke apertures, located on a curved wall opposite the reward aperture (see Fig. 9.56.1). Animals are required to nose poke in any one of the five illuminated apertures to receive the milkshake reward. Response in any of the five apertures results in all five being extinguished simultaneously, the reward aperture is illuminated, and the milkshake delivered. Once the animal collects the reward and leaves the reward aperture, the 4-sec intertrial interval (ITI) begins before the cycle is repeated. -
19
Repeat steps 13–16 for a minimum of three sessions and then until mice meet criterion: a minimum of 70 responses over 2 consecutive days.
-
20
To avoid overtraining, once each mouse meets criterion, place it on a restrictive training schedule (only 2 days training per week) while the other mice continue.
Once all animals have achieved criterion for HAB2, they can immediately proceed to 10-sec 5CSRTT training (see Basic Protocol 2) on the subsequent training day.
BASIC PROTOCOL 2
5CSRTT-TO-5C-CPT SHAPING
Once mice achieve HAB2 criteria, they begin training to respond to target trials. This version is essentially consistent with the 5CSRTT training. During this stage, mice are trained to nose poke in only the one aperture that is illuminated in a given trial. The rodent is given plenty of time initially, and then the behavior is accelerated over successive stages to make the behavior prepotent. During this initial training, the ITI is held consistent at 4 sec. Once the animal is responding reliably at a 2-sec stimulus duration, the ITI is made variable (3–7 sec). Once responding is again consistent, non-target trials are included, where all five nose poke apertures are illuminated, requiring inhibition from responding. This protocol carries over largely from daily procedures already described, and therefore focuses on evaluating stable performance at each successive stage of training. All materials are described in Basic Protocol 1.
Perform 10-sec 5CSRTT training
-
1
Transport animals to testing facilities and prepare operant chambers and reward delivery apparatuses as described (see Basic Protocol 1, steps 8–10).
-
2
Weigh each animal and place into the appropriate operant chamber. Use the recorded weights to calculate the appropriate food provisions.
-
3
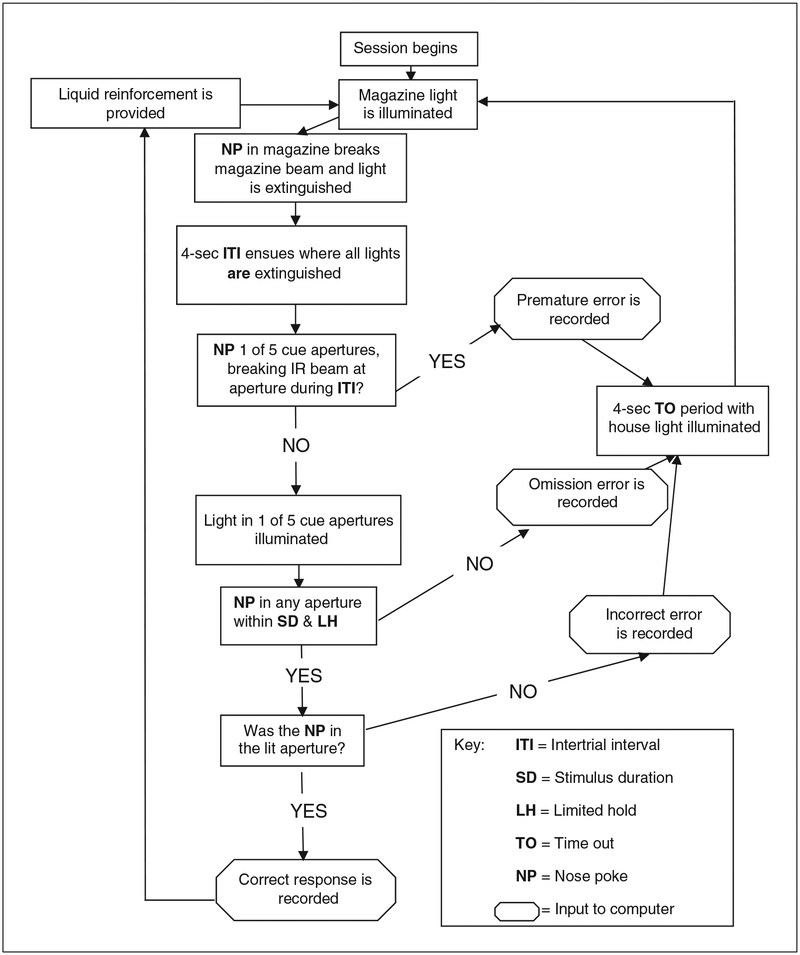
Initiate 10-sec 5CSRTT program (see Fig. 9.56.3).
Animals are permitted 30 min daily to perform a maximum of 120 trials. At this stage, mice will initiate a trial by performing a nose poke in the reward aperture. Then, 4 sec after the animal leaves the reward aperture, a cue light will be illuminated in one of the five nose poke response apertures. This light will remain illuminated for 10 sec or until a nose poke is performed. Nose pokes into the correct response aperture are rewarded with strawberry milkshake in the reward aperture. Nose pokes into any of the unlit response apertures, failing to perform a nose poke within 10 sec, or nose poking before a light is illuminated will result in illumination of the house light for a 4-sec time out, after which a new trial can be initiated. -
4
At the end of the session, remove animals from operant chambers and return them to their home cages. Repeat steps 2–3 for each animal until all animals have been tested.
-
5
Return all animals to housing facilities and distribute appropriate food provisions.
-
6
Clean operant chambers as described (see Basic Protocol 1, steps 14–15).
-
7
Repeat steps 1–6 daily until all animals have been trained to criterion: a minimum of 30 correct trials with mean overall correct response latency <5 sec for two consecutive sessions.
Figure 9.56.3.
Flow diagram of a 5CSRTT session. During 5CSRTT training, stimulus duration is decreased from 10 to 2 sec in 2-sec increments according to each animal’s performance to criteria. The number of training sessions is dependent on the animals’ performance at each stage.
Perform 5CSRTT training with decreasing stimulus duration
-
8
Perform training as in steps 1c, this time using an 8-sec 5CSRTT program (see Fig. 9.56.3).
The program is identical to the 10-sec program, except that the cue light will remain illuminated for the appropriate time (e.g., 8 sec), and failure to perform a nose poke within that time will result in illumination of the house light. -
9
Repeat 8-sec program daily until all animals have been trained to criterion: a minimum of 30 correct trials with mean overall correct response latency <4 sec for two consecutive sessions.
-
10
Perform training using a 4-sec 5CSRTT program. Repeat daily until all animals have been trained to criterion: a minimum of 30 correct trials with mean overall correct response latency <2 sec for two consecutive sessions.
-
11
Perform training using a 2-sec 5CSRTT program. Repeat daily until all animals have been trained to criterion: a minimum of 30 correct trials with mean overall correct response latency <1.5 sec for two consecutive sessions.
Perform 2-sec 5CSRTT training with variable ITI
-
12
Perform training using a 2-sec 5CSRTT variable ITI program (see Fig. 9.56.3).
The program is identical to the 2-sec 5CSRTT program, except that the time until the cue light is illuminated (previously, 4 sec after the mouse leaves the reward aperture) will be varied between 3 to 7 sec. -
13
Repeat daily for a minimum of 5 training days, ensuring performance reaches >30 correct responses for two consecutive sessions.
Speed of response is less important at this stage, provided the mice are responding within the 2-sec window. If performance stays below 30 correct responses for >10 training sessions (very rare), mice can be moved back to step 10.
Perform training with frequent (highly salient) non-target trials
-
14
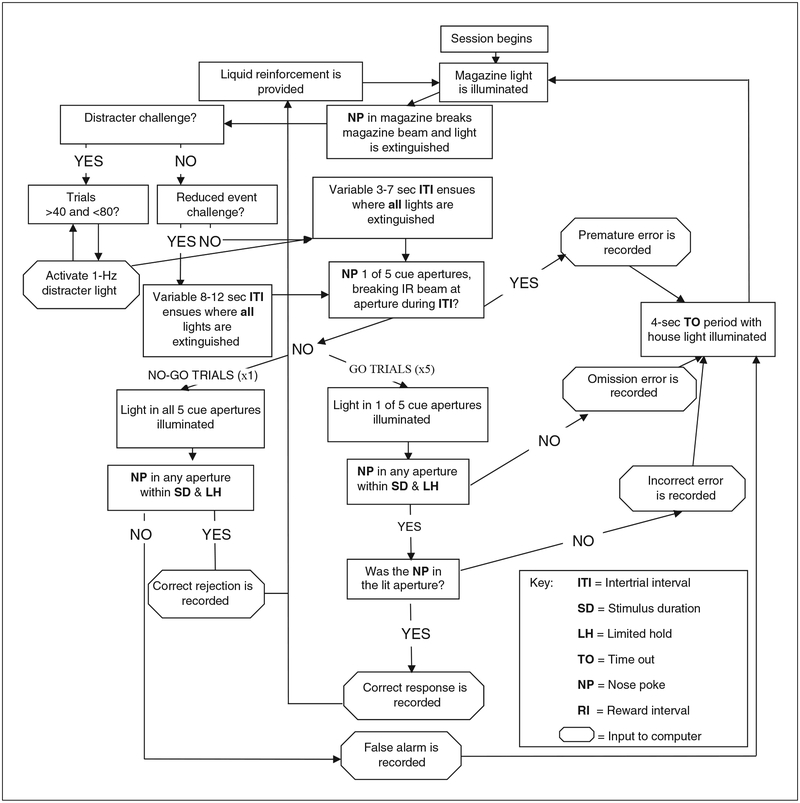
Perform training using a program for 5C-CPT with frequent non-target trials (see Fig. 9.56.4).
At this stage, in two-thirds of trials, one of the five nose poke response apertures will be illuminated 3–7 sec after the mouse leaves the reward aperture, and a nose poke in the correct aperture will be rewarded, exactly as in step 11. In the remaining one-third of trials, all five response apertures will be illuminated 3–7 sec after the mouse leaves the reward aperture. All lights will remain illuminated for 2 sec or until a nose poke is performed. If no nose poke is performed (inhibit the prepotent response), the trial is rewarded by delivering milkshake to the reward aperture. A nose poke in any response aperture during the 2-sec interval (false alarm) will result in illumination of the house light for a 4-sec time out. -
15
Repeat daily until all animals have been trained to criterion: a minimum of 30 correct trials with mean overall correct response latency <1.5 sec and <50% false alarm rate for two consecutive sessions.
An alternative to <50% false alarms is a positive sensitivity index (SI >0.05; see Fig. 9.56.5 for derivation of formulas) for three consecutive sessions.
Figure 9.56.4.
Flow diagram of regular and challenge sessions in the 5C-CPT. Food reinforcement is provided initially, then following every correct response. The inputs relayed to the computer are used in the calculations of performance measures (see Fig. 9.56.5 and Table 9.56.2).
Figure 9.56.5.
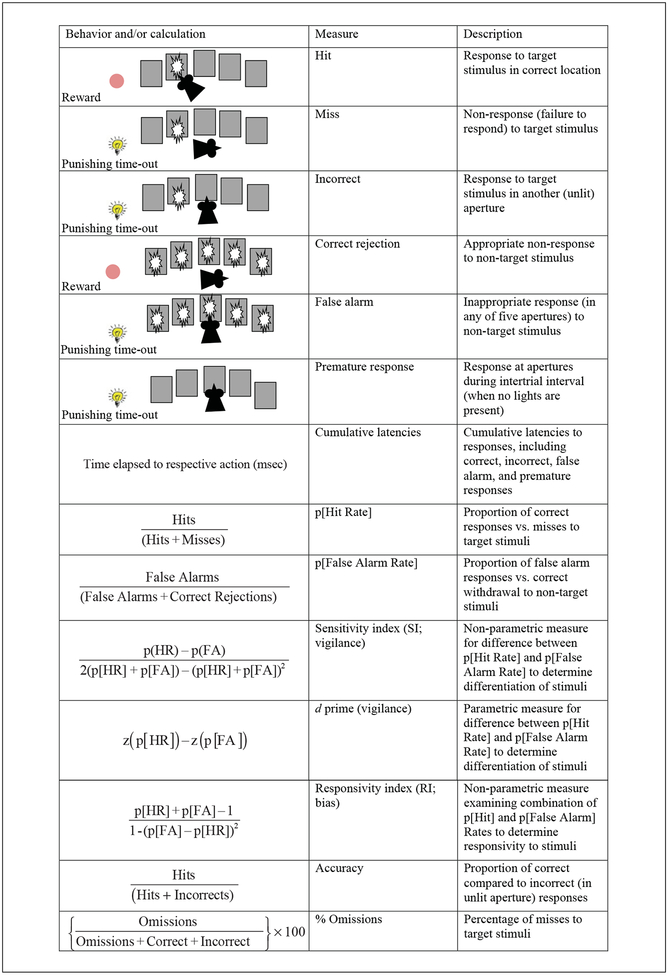
Key dependent measures of the 5C-CPT with schematics of trial outcomes or relevant equations used to calculate the dependent variable, when applicable.
Perform 5C-CPT training with infrequent non-target trials
-
16
Perform training using a program for 5C-CPT with infrequent non-target trials.
The final stage is carried out as in the previous stage, except that the ratio of target to non-target trials is changed: a single aperture will be illuminated in five out of six trials, and all apertures will be illuminated in the remaining trial. -
17
Repeat daily until all mice have been trained to criterion: a minimum of 30 correct trials with mean overall correct response latency <1.5 sec and <50% false alarm rate for two consecutive sessions.
Again, an alternative to <50% false alarms is a positive sensitivity index (>0.05) for three consecutive sessions.At this point, mice can proceed to stability assessment (step 18) and/or challenges (see Basic Protocol 3). -
18
To assess stability of performance, train all mice for five consecutive training sessions. Analyze performance measures (Fig. 9.56.5) from the latter four training sessions using a within-session analysis of variance (normally distributed) or Friedman test (non-normally distributed) with session day as a within-subjects factor.
No measure should result in a main effect of session, ensuring that performance is not improving over time, prior to any challenges being introduced.
BASIC PROTOCOL 3
5C-CPT CHALLENGES
Once animals are fully trained in the 5C-CPT (120 trials or 30 min long with a 2-sec stimulus duration), challenge conditions can be implemented to assess distinct cognitive domains. Trial stimuli and the required operant responses are unmodified compared to 5C-CPT variable ITI standard sessions. Modifications in session or ITI length, stimulus duration, or presentation of distracter stimuli enable assessment of distinct domains of vigilance, cognitive control, and response inhibition.
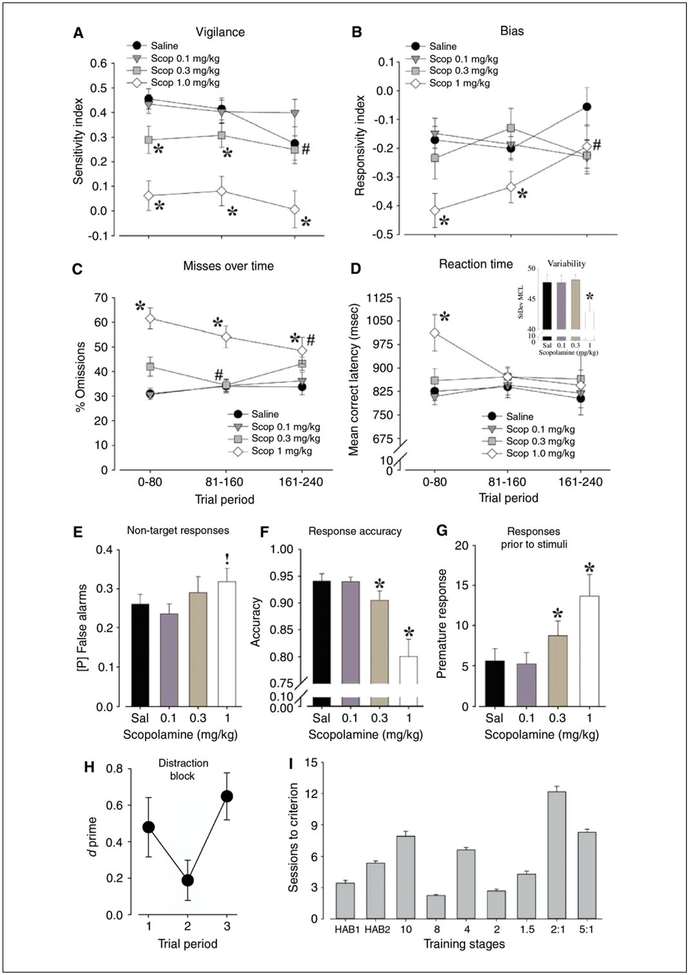
An extended-session 5C-CPT can be utilized to assess vigilance decrements by extending testing beyond that of a typical training session. Whereas a normal 5C-CPT training session lasts up to 120 trials, extended-session CPTs last up to 250 trials. Decreased responsivity as measured by RI is indicative of waning participation over the course of the extended session. In contrast, a decrement in SI (which is more consistently observed) indicates waning discrimination between target and non-target trails (Fig. 9.56.6A). Hence, a vigilance decrement is consistently observed in human CPTs and is exaggerated in psychiatric populations (e.g., schizophrenics). In addition, by comparing patterns between SI and RI, the vigilance decrement can be determined to result not from waning motivation or satiety, but from deficiency in target/non-target discrimination (Young et al., 2009).
Figure 9.56.6.
Anticipated results from 5C-CPT experiments. (A–G) Example data from an extended-session 5C-CPT challenge. Mice received one of three doses of the muscarinic acetylcholine receptor antagonist scopolamine. Scopolamine induced a dose-dependent decrease in sensitivity index (A), an initial decrease in responsivity (B-D), diminished accuracy (F), and increased premature responses (G). Data from Young et al. (2013b). (H) In a distracter challenge, a 1-Hz flashing light is presented above the five-hole array during the middle third of the block of trials (trial period 2), and the performance of mice in response to distraction is assessed. Typically, poorer performance is observed during such distraction. Data from Young et al. (2015). (I) Example of training data. C57BL/6J mice (n 58) were trained on HAB1, HAB2, and then 5CSRTT for 10, 8, 4, and 2 sec, followed by training = using 2–to–1 and 5–to–1 target to non-target stimulus ratios. The initial 10-sec 5CSRTT takes some time, given that previously a response in any lit hole would result in a reward (HAB2). The 2–to–1 and 5-to-1 training also takes some time, given the need to learn to inhibit the prepotent (target trial) response. *p < 0.05, !p < 0.1 compared with vehicle-treated mice; #p < 0.05 compared with trial period 1.
A reduced-event 5C-CPT can be utilized to best assess differences in waiting impulsivity. Whereas the standard 5C-CPT has a varied ITI of 3–7 sec, this challenge extends the average ITI to 8–12 sec, placing additional demand on the animal to wait for stimulus onset and maintain attention for a longer period between trials. This challenge is commonly used in 5CSRTT studies (Young et al., 2011; Barnes et al., 2012).
The distracter condition 5C-CPT is utilized to assess differences in distractibility to extraneous stimuli. This challenge consists of three 40-trial blocks that proceed identically to the 5C-CPT training session. On the second 40-trial block, a never-before-illuminated light, centrally oriented above the five response nose pokes, begins flashing continuously at a rate of 1 Hz. Distraction can be assayed by comparing performance before (block 1) and after (block 3) exposure to the distracter stimulus (see Fig. 9.56.6H; Young et al., 2015).
Transport animals to testing facilities and prepare operant chambers and reward delivery apparatuses as described (see Basic Protocol 1, steps 8–10).
Weigh each animal and place them into the appropriate operant chamber. Use the recorded weights to calculate the appropriate food provisions.
Initiate 5C-CPT extended session, reduced event, or distracter condition challenge program (see Fig. 9.56.4).
Remove animals from operant chambers and return to their home cages. Repeat steps 2–3 for each animal until all animals have been tested.
Return all animals to housing facilities and distribute appropriate food provisions.
Clean operant chambers as described (see Basic Protocol 1, steps 14–15).
COMMENTARY
Background Information
The 5C-CPT was developed to improve the cross-species translational fidelity of rodent assays of sustained attention, vigilance, and response inhibition. It was developed given the need for tasks with cross-species validity for clinical tests (Geyer et al., 2012; Moore et al., 2013; Young and Geyer, 2015; Young and Markou, 2015), and is generally regarded as the gold-standard test of vigilance and response inhibition (Riccio et al., 2001). Such tests are needed given that functional outcomes in psychiatric disorders such as schizophrenia and bipolar disorder have been repeatedly correlated with cognitive performance, particularly in the domain of attention (Green et al., 2008; Goldberg and Chengappa, 2009). Yet, despite great effort, procognitive treatments have repeatedly fallen short of effectively mitigating these symptoms (Sarter, 2004; Floresco et al., 2005). This limited efficacy may be the result of an overreliance on “fast and dirty” cognitive tasks that, while experimentally expedient, lack the necessary degree of translational validity to bridge the gap from rodent to human (Sarter, 2004). Hence, greater cross-species fidelity of testing is urgently required.
One method for improving fidelity of cross-species behavioral assays is to minimize overgeneralization of ethologically relevant behaviors measured in rodents to those quantified in humans. This approach is most effectively achieved by designing tasks that can produce dependent variables that measure equivalent behaviors across species, as described by the National Institute of Mental Health (NIMH)–sponsored CNTRICS initiative (Barch and Carter, 2008; Dudchenko et al., 2013; Gilmour et al., 2013; Lustig et al., 2013; Moore et al., 2013; Young et al., 2013a) as well as Research Domain Criteria initiatives (Sarter, 2004; Young and Geyer, 2015; Young and Markou, 2015; Cope et al., 2016). The 5CSRTT was originally designed to replicate Leonard’s choice reaction time, not CPTs. Although the 5CSRTT has been widely used to measure sustained attention, it falls short of clinical CPT-like testing, because it lacks the need for inhibition of responding. The 5C-CPT incorporates a non-target trial, requiring the withholding of responding on infrequent trials in which all five nose-poke apertures are illuminated. Hence, the 5C-CPT enables bona fide calculation of signal detection parameters that have been long validated in human research. Although the additional investment in animal training is not trivial, this important addition is worthwhile in facilitating high-fidelity, cross-species translational research.
A further advantage of the 5C-CPT lies in its minimization of timing strategies utilized by rodents, which have been observed to confound measures of motoric/waiting impulsivity in the 5CSRRT. The premise that premature responses reflect motoric impulsivity has been questioned following performance comparisons between rats and mice (Fletcher et al., 2007; Young et al., 2013a; Cope et al., 2016). It has been suggested that premature responses could reflect an improper use of a temporal mediating strategy (Spratt et al., 2001). If this were the case, experimental manipulations that interfere with timing ability could be erroneously quantified as a change in motoric impulsivity (Cope et al., 2016). To minimize the use of this strategy, the 5C-CPT incorporates a variable ITI, enabling the attribution of motor impulsivity that is more likely independent of the animals’ capacity for temporal perception. In addition, the 5C-CPT quantifies response inhibition to non-target trials (consistent with human CPTs).
Critical Parameters
Reward choice
This protocol is designed to be conducted using strawberry milkshake as the positive reinforcer. The reasons for selecting this reinforcer are three-fold: (1) mice in particular can consume large quantities without satiation interfering with potential interpretations of performance; (2) rodents find this reinforcer highly palatable based on their progressive ratio breakpoint efforts to acquire this vs. other reinforcers (e.g., sucrose solution or solid reinforcers); (3) the olfactory cue provided by milkshake enables additional cue-driven behavior manipulated by the experimenter to encourage task engagement (see Troubleshooting). It is possible to conduct these experiments with solid food reinforcers, but the large number of trials conducted in the task challenges will no longer be possible in mice.
Animal selection
This protocol has been validated for use in various mouse (Young et al., 2009, 2011) and rat (Barnes et al., 2012) strains, but not all have yet been tested. Although adult rodents (>P60) are typically used, adolescent (<P60) and aged rodents may still be used with a few caveats. Motor abilities should not be impaired in any way that would prevent the animal from effectively performing a nose poke within task requirements. In addition, food restriction in young animals should still allow for growth. This could also become a concern for animals with cranial implants that could bump against the reward or response nose pokes. Further, the animal’s abilities to sense and perceive the stimuli presented them should not be impaired.
Animal housing and testing environment
Outside of relevant experimental manipulations, animals should always be tested and trained during their active cycle. Because this is the dark cycle for most laboratory rodent species, care should be taken to minimize exposure to extraneous ambient white light. Disruption of circadian patterns can critically undermine the attention and vigilance this task intends to measure. Ambient red light can be used to illuminate the testing room so that experimenters can carry out procedures.
Chamber testing and maintenance
Care should be taken to test each nose poke daily prior to training/testing. Often, it will not be obvious that infrared beams are not functioning properly unless they are tested. Checking before testing is preferable to finding out later that animals have performed an entire session with faulty equipment.
Troubleshooting
Training rodents in the 5CSRTT is relatively straightforward, and many groups have adopted this task worldwide. Nonetheless, some troubleshooting points remain, and additional concerns are raised when transitioning to the 5C-CPT.
HAB1
Rodents pick this aspect up very quickly. It is important to check the reward aperture to ensure that the rodent is drinking the milkshake. If an animal is drinking all the milkshake, but not scoring >60 responses, it is still possible to move them to the next stage (HAB2), as the primary outcome is to ensure they drink milkshake from the reward aperture.
HAB2
Very rarely (in perhaps one to five of 60 rodents), an animal will not readily acquire nose poking for a reward. When such an animal is identified and has low scores (<10) for 5 days, while all others are increasing in score (>10), that animal can be encouraged to respond by using a cotton-tipped applicator to bait the five apertures behind the infrared beam at the start of each training session. This technique can be utilized for several days to encourage responding.
Single-light target training
At this stage, animals have moved from responding to any aperture to having to respond to one only. Overall response rates will drop moving into this task, and should be monitored to ensure the animals continue to respond (>5 responses, either correct or incorrect). If responses drop below 5 for 3 consecutive days, rodents can be put back into HAB2 to in crease responding. This is rarely required, but possible.
Moving to faster stimulus durations
Rodents (mice in particular) may not move fast enough to achieve criterion at a faster stimulus duration (e.g., attain <2 sec mean correct latency with a 4-sec stimulus duration). If they are responding well (i.e., above criterion) for 6 consecutive days, they can be ‘forced’ to the faster stimulus duration.
Five-light non-target training
At first, a 2:1 target:non-target trial ratio is utilized to maximize the saliency of the non-target stimuli. Although mice have been trained in an initial 5:1 ratio, it was discovered that the majority of rats could not learn at this rate (Barnes et al., 2012), and so a 2:1 ratio was used. It was then discovered that mice also rapidly acquire the non-target trial using this technique. It has since become standard to start with 2:1 ratio, moving to a 5:1 once acquired. It is also possible that rodents will not learn using the 2:1 contingency (>0.6 pFA). In this case, a 1:1 ratio can be employed to maximize non-target saliency.
Statistical Analyses
In addition to numerous trial-outcome variables, two primary index variables are calculated from the behavioral data to assess performance in the 5C-CPT. To evaluate responses to target stimuli, the probability of hit rate (pHR) is calculated (Fig. 9.56.5), representing the likelihood of responding or missing target stimuli. The probability of false alarms (pFA) is equal to the probability of making an incorrect hole-poke response on non-target trials (false alarm) compared to total correct rejections (CR) of non-target stimuli (response inhibition) and false alarms (Fig. 9.56.5). Hence, the ability of animals to perform the task is based on the difference between pHR and pFA, because to maximize performance rodents should always respond to target trials and be inhibited from responding to non-target trials. Signal detection theory can be used to calculate the difference between these two variable outcomes. With a few trials, non-parametric statistics should be used to calculate this difference, referred to as the sensitivity index (SI; Fig. 9.56.5). In addition, because pHR and pFA can also vary as a function of an animal’s individual tendency to respond (higher response rates equal higher pHR and pFA, and vice versa), SI can be compared against an additional index variable, the responsivity index (RI), to determine response bias (tendency to respond). Theoretically, if differences are observed in SI but not RI, then the difference is more likely to be due to cognitive capabilities of distinguishing between target and non-target trials vs. changes in motivation. The 5C-CPT benefits from having a measure pFA, so these calculations can be conducted, whereas the 5CSRTT must rely on interpretation of omissions, which could reflect attention or motivation. With sufficient trial numbers, parametric statistics can also be used to calculate the difference between pHR and pFA using d prime, which is consistently used in human CPT studies. As with SI, d prime provides a calculation of the degree of discrimination between target and non-target trials, but does so parametrically. All of the variables described can be calculated using the formulas shown in Figure 9.56.5.
If an animal never reaches criterion, it should be excluded from all statistical analyses. This is very rare, however. The animals will almost always reach criterion performance given enough attempts.
Anticipated Results
Typical results are taken from an extended session challenge (Young et al., 2013b), a distracter challenge (Young et al., 2015), and training from a group of C57BL/6J mice (n = 58; see Fig. 9.56.6). In this study, mice were given one of three doses of the muscarinic acetylcholine receptor antagonist scopolamine to assess similarities in attentional dysfunction to that seen in humans with schizophrenia performing the human 5C-CPT. Mice treated with saline exhibit a vigilance decrement, as measured by SI in the final block of trials on the extended session 5C-CPT. However, mice treated with 1.0 mg/kg scopolamine exhibit impaired attention throughout the session (Fig. 9.56.6A). High dose scopolamine (1.0 mg/kg) also induced an initial decrease in responsivity in these animals, as evidenced by negative RI, increased omissions, and slowed reaction time in the first trial block. This impairment normalized over the subsequent two trial blocks (Fig. 9.56.6B–D). Over the whole session, scopolamine increased false-alarm responses (Fig. 9.56.6E), decreased accuracy (Fig. 9.56.6F), and increased premature responses (Fig. 9.56.6G) in a dose-dependent fashion. This treatment recapitulated deficits witnessed in patients with schizophrenia, including decreased SI and increased omissions, demonstrating its efficacy as a cross-species platform for comparison of animal models to the psychiatric population. In addition, flashing a 1-Hz light stimulus above the five-hole array reduces performance of mice during the 5C-CPT compared to when no distractions are present (Fig. 9.56.5H).
Time Considerations
An experienced investigator can complete this training procedure in a single cohort of animals within 4 months; the maximum time required to execute this protocol is 6 months. HAB1 and HAB2 can be completed within 2 weeks, with the remaining time devoted to shaping and speeding of 5CSRTT→5C-CPT behavior.
Table 9.56.2.
Explanation of the Signal Detection Variables SI and RI in Terms of Interpretation of Each Animal’s Behavior in the 5C-CPT
| Sensitivity index (SI) | Responsivity index (RI) | Hit rate | False alarm rate | Explanation of performance |
|---|---|---|---|---|
| +1 | 0 | 1.0 | 0.0 | Optimal: Accurate response to every target stimulus and inhibition of response to all non-target stimuli |
| 0 | +1 | 1.0 | 1.0 | Chance level: Response to every stimulus (target and non-target) |
| 0 | −1 | 0.0 | 0.0 | Chance level: Non-response to every stimulus (target and non-target) |
| −1 | 0 | 0.0 | 1.0 | Rule reversed: Inhibition of response to all target stimuli and response to all non-target stimuli |
Acknowledgments
This work has been supported by numerous collaborators over the years, including preclinical (Drs. Geyer, Neil, Barnes, Burne, Harms, Turner, Stanford, Porter, Pillidge) and clinical (Drs. Eyler, Asgaard, Risbrough, Ache-son, Perry, Minassian, and Light) researchers. This work was originally supported by an NIMH R21 grant, and has most recently been supported by NIH grants (R01-MH071916, R01-MH104344, UH2-MH109168) and the Veteran’s Administration Mental Illness Research, Education, and Clinical Centers (VISN 22).
Literature Cited
- Barch DM and Carter CS 2008. Measurement issues in the use of cognitive neuroscience tasks in drug development for impaired cognition in schizophrenia: A report of the Second Consensus Building Conference of the CNTRICS Initiative. Schizophr Bull 34(4):613–618. doi: 10.1093/schbul/sbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, and Neill JC 2012. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-choice continuous performance test (5C-CPT) when the attentional load was increased. Neuropharmacology 62:1432–1441. doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LH, Bransome ED Jr., Mirsky AF, Rosvold HE, and Sarason I 1956. A continuous performance test of brain damage. J. Consult. Psychol 20:343–350. doi: 10.1037/h0039381. [DOI] [PubMed] [Google Scholar]
- Conners CK 1985. The computerized continuous performance test. Psychopharmacol. Bull 21:891–892. [PubMed] [Google Scholar]
- Cope ZA, Halberstadt AL, van Enkhuizen J, Flynn AD, Breier M, Swerdlow NR, Geyer MA, and Young JW 2016. Premature responses in the five-choice serial reaction time task reflect rodents’ temporal strategies: Evidence from no-light and pharmacological challenges. Psychopharmacology (Berl) 233: 3513–3525. doi: 10.1007/s00213-016-4389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, and Baxter MG 2013. Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci. Biobehav. Rev 37:2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, and Higgins GA 2007. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Geyer MA, Gold LH, and Grace AA 2005. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr. Bull 31:888–894. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Olivier B, Joels M, and Kahn RS 2012. From antipsychotic to anti-schizophrenia drugs: Role of animal models. Trends Pharmacol. Sci 33:515–521. doi: 10.1016/j.tips.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour G, Arguello A, Bari A, Brown VJ, Carter C, Floresco SB, Jentsch DJ, Tait DS, Young JW, and Robbins TW 2013. Measuring the construct of executive control in schizophrenia: Defining and validating translational animal paradigms for discovery research. Neurosci. Biobehav. Rev 37:2125–2140. doi: 10.1016/j.neubiorev.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Goldberg JF and Chengappa KN 2009. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord 2:123–137. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Kern RS, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Seidman LJ, Stover E, and Marder SR 2008. Functional co-primary measures for clinical trials in schizophrenia: Results from the MATRICS Psychometric and Standardization Study. Am. J. Psychiatry 165:221–228. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson L, and Dawson G 2005. Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr. Protoc. Neurosci 31:8.5H.1–8.5H.15. doi: 10.1002/0471142301.ns0805hs31. [DOI] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, and Robbins TW 2013. CNTRICS final animal model task selection: Control of attention. Neurosci. Biobehav. Rev 37:2099–2110. doi: 10.1016/j.neubiorev.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW 3rd. 2008. Building a clinically relevant cognitive task: Case study of the AX paradigm. Schizophr. Bull 34:619–628. doi: 10.1093/schbul/sbn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Young JW, Dawes SE, Asgaard GL, and Eyler LT 2013. Bridging the bench to bedside gap: Validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res 212:183–191. doi: 10.1016/j.pscychresns.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Moore H, Geyer MA, Carter CS, and Barch DM 2013. Harnessing cognitive neuroscience to develop new treatments for improving cognition in schizophrenia: CNTRICS selected cognitive paradigms for animal models. Neurosci. Biobehav. Rev 37:2087–2091. doi: 10.1016/j.neubiorev.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AJ, Pillidge K, Stanford SC, and Young JW 2016. Differences in the performance of NK1R−/− (‘knockout’) and wild-type mice in the 5-Choice Continuous Performance Test. Behav. Brain Res 298:268–277. doi: 10.1016/j.bbr.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, and Lowe P 2001. Effects of stimulants on the continuous performance test (CPT): Implications for CPT use and interpretation. J. Neuropsychiatry Clin. Neurosci 13(3):326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Sarter M 2004. Animal cognition: Defining the issues. Neurosci. Biobehav. Rev 28:645–650. doi: 10.1016/j.neubiorev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Spratt C, McQuatt NE, Sharkey J, Kelly JS, and Marston HM 2001. Comparison of rats and mice in a serial reaction task. British Neuroscience Association, U.K., Abstracts 16. [Google Scholar]
- van Enkhuizen J, Acheson D, Risbrough V, Drummond S, Geyer MA, and Young JW 2014. Sleep deprivation impairs performance in the 5-choice continuous performance test: Similarities between humans and mice. Behav. Brain Res 261:40–48. doi: 10.1016/j.bbr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW and Geyer MA 2015. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J. Psychopharmacol 29:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW and Markou A 2015. Translational rodent paradigms to investigate neuromechanisms underlying behaviors relevant to amotivation and altered reward processing in schizophrenia. Schizophr. Bull 41:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, and Geyer MA 2009. The 5-choice continuous performance test: Evidence for a translational test of vigilance for mice. PLoS One 4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, and Geyer MA 2011. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav. Brain Res 222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Jentsch JD, Bussey TJ, Wallace TL, and Hutcheson DM 2013a. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci. Biobehav. Rev 37:2181–2193. doi: 10.1016/j.neubiorev.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, and Light GA 2013b. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl. Psychiatry 3:e324. doi: 10.1038/tp.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kamenski ME, Higa KK, Light GA, Geyer MA, and Zhou X 2015. GlyT-1 inhibition attenuates attentional but not learning or motivational deficits of the Sp4 hypomorphic mouse model relevant to psychiatric disorders. Neuropsychopharmacology 40:2715–2726. doi: 10.1038/npp.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]