Abstract
Background.
Rising alpha-fetoprotein (AFP) is a potential marker of worse prognosis after liver transplant (LT) for hepatocellular carcinoma (HCC), but prior studies relied on only 2 data points and were imprecise in assessing AFP slope. The aim of this study was to examine the association between AFP slope and post-LT HCC recurrence, with AFP slope estimated from multiple data points over time.
Methods.
Our cohort included 336 patients undergoing LT with Model for End Stage Liver Disease exception for HCC within Milan criteria from 2003 to 2013. Most (98%) had pre-LT locoregional therapy. AFP slope was estimated by fitting a regression line to the AFP levels over time.
Results.
The 1- and 5-year post-LT survivals were 94% and 77% and 1- and 5-year recurrence-free probabilities were 95% and 86%, respectively. In univariate analysis, HCC recurrence was significantly associated with microvascular invasion (hazard ratio [HR], 13.1; P<0.001), tumor grade (HR, 1.8; P<0.001), pathologic stage >Milan criteria (HR, 8.9; P<0.001), 3 tumor nodules (HR, 5.5; P=0.002), AFP slope greater than 7.5 ng/mL per month (HR, 3.9; P=0.005), and female sex (HR, 2.3; P=0.01). In multivariable analysis of factors known before LT, 3 tumor nodules (HR, 7.6; P<0.001), female sex (HR, 2.5; P=0.01), and AFP slope >7.5 (HR, 3.0; P=0.03) were significantly associated with HCC recurrence. AFP slope greater than 7.5 was also associated with microvascular invasion (odds ratio, 6.8; P=0.008).
Conclusions.
AFP slope increasing greater than 7.5 ng/mL per month despite locoregional therapy is associated with post-LT HCC recurrence and may serve as a surrogate for microvascular invasion. These findings support incorporating changes in the AFP into candidate selection for LT.
Hepatocellular carcinoma (HCC) is a major health burden in the United States with nearly 1% being diagnosed with liver or intrahepatic bile duct cancer at some point during their lifetime.1 The rate of new diagnoses of primary liver cancer has increased in recent decades, from 4.5 cases per 100 000 people in 1992 to 8.7 cases in 2011.1 Liver transplantation (LT) provides an opportunity for cure in patients with HCC fulfilling Milan criteria (one lesion < 5 cm or 2 to 3 lesions < 3 cm).2 For these patients, the 5-year post-LT survival rate of 70% to 75% approaches that for patients without HCC. More than 20 years after the establishment of the Milan criteria, there is still debate around optimizing LT selection criteria for HCC given that more than 15% of the patients meeting the Milan criteria before LTstill develop HCC recurrence3 with significant adverse impact on posttransplant survival.4,5
Growing evidence suggests additional factors (known before LT) exist beyond size and number of tumors that predict HCC recurrence after LT. A history of ablative therapy before transplant,6 donors ≥ 60 year old and from a nonlocal share distribution7 and short wait time (less than 120 days)8 were recently identified as predictors of HCC recurrence. Alpha-fetoprotein (AFP) level before LT also appears to be a surrogate marker of microvascular invasion and predictor of post-LTsurvival.6 A recent report from our institution suggested that AFP >1000 ng/mL at LT was a significant predictor of HCC recurrence9 and both a French multi-center study10 and an analysis of the Scientific Registry of Transplant Recipients also showed that high AFP levels were associated with poor post-LT outcomes.11
Similar to an elevated AFP at the time of LT, rising AFP while awaiting LT has also been suggested to be a marker of poor prognosis after LT for HCC. However, prior studies relied on only 2 AFP data points and were imprecise in assessing the AFP trend. These studies also did not adequately account for fluctuations of AFP commonly seen in chronic liver disease.12–16 Moreover, the slope threshold at which the recurrence can be predicted varied from 1 study to another. The primary aim of this study was to examine the association between AFP slope and post-LT HCC recurrence in patients awaiting LT, with AFP slope more precisely estimated from multiple data points over time.
MATERIALS AND METHODS
Patient Population
The study population included patients aged 18 years or older receiving Model for End Stage Liver Disease (MELD) exception for T2 (1 nodule, 2–5 cm; 2–3 nodules, all less than 3 cm) HCC who received LT from January 2003 to February 2013 at a single center. Only patients meeting T2 criteria were considered, whereas additional patients who underwent LT after HCC downstaging and patients who underwent LT according to extended criteria beyond the Milan criteria during this period were excluded from this analysis. Among the 375 patients initially evaluated, 39 patients with less than 3 AFP values identified in their medical charts were excluded from the study. The final cohort consisted of the remaining 336 patients. The minimal duration of follow-up after LT was 1 year. This study protocol was approved by our institutional review board.
Diagnosis of HCC and Pretransplant Locoregional Therapy
The diagnosis of HCC was based on radiographic characteristics, including arterial phase enhancement and portal venous phase washout for lesions measuring at least 1 cm. Nodules not demonstrating these features were considered indeterminate for HCC and were followed up by computed tomography or magnetic resonance imaging every 3 months. Percutaneous biopsy was not routinely performed for the purpose of diagnosing HCC. Nodules <1 cm were not considered HCC. At our center since 2004, all HCC cases were discussed in a weekly tumor board to formulate decisions regarding locoregional therapy (LRT). We applied LRT, with repetitive interventions if needed, to induce complete necrosis of all tumor nodules if possible before LT. Follow-up cross-sectional imaging for all HCC patients awaiting LT is performed 1 month after each LRT and at least every 3 months.
AFP Slope
AFP slope was estimated by fitting an ordinary least squares regression line through all reported AFP levels from the time of HCC diagnosis for each patient over time. At our center, patients have AFP measured at the time of initial listing with MELD exception, 1 month after each LRT and at least every 3 months while awaiting LT. To determine the best slope threshold predicting HCC recurrence, we tested AFP slope cutoffs used previously in the literature (15 ng/mL per month14,15) and other potential cutoffs including 0, 5, 7.5, and 10 ng/mL per month. We evaluated these cutoffs by univariate Cox proportional hazards regression and compared Akaike information criterion (AIC) between models.
Histopathologic Analysis
Explant histopathologic features evaluated in all patients included histologic grade of differentiation based on the Edmondson and Steiner criteria (grade 1, well differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated), and the presence or absence of microvascular or macrovascular invasion.17 Explant tumor staging in this study was based on the size and number of only viable tumors.
Statistical Analysis
The primary outcome of this study was risk of HCC recurrence. The variables collected included demographic data (age, sex, and race), tumor number at time of listing, etiology of liver disease, date of HCC diagnosis, date of listing, number of LRT before transplant, date of LT, all available AFP measurements from time of HCC diagnosis to LT and explant histology (tumor size, number, and differentiation, and presence or absence of microvascular invasion). Patient characteristics were described with frequencies (proportions) and medians (interquartile ranges [IQR]), as appropriate.
We estimated post-LT patient and recurrence-free survival and 95% confidence intervals (CI) using the Kaplan-Meier method. For recurrence-free probability, only HCC recurrences were counted as an event whereas deaths were censored if the patient had not experienced HCC recurrence. We also provided results of survival without recurrence when both were counted as events. Survival comparisons by AFP slope were assessed with the long-rank test. We evaluated the association between HCC recurrence and patient characteristics using Cox proportional hazards regression and estimated hazard ratios (HR) and 95% CI for risk of recurrence. Variables known before LT with a P value less than 0.1 in univariate analysis were assessed in the multivariable model. The final multivariable model was selected by backward elimination (P<0.05), and AFP slope was added to the model as the primary predictor of interest. To compare AFP slope to static AFP at LT, we modeled AFP slope and AFP at LT in 2 separate models both adjusted for number of tumor nodules and sex. For each model, we calculated the overall C-index, a measure of model discrimination (the ability of a model to correctly classify patients into events and nonevents)18 and the bootstrap 95% CI.
We evaluated factors associated with microvascular invasion using logistic regression to estimate odds ratios (OR) and 95% CI for probability of microvascular invasion. Factors known before transplant with a univariate P value less than 0.1 were assessed in the multivariable model which was selected using backward elimination (P<0.05). Statistical analyses were completed in SAS v9.4 (Cary, NC).
RESULTS
Baseline Characteristics
The baseline characteristics of the 336 patients are summarized in Table 1. The majority of the 336 patients were men (76.7 %), 42.3% were Caucasian and 33.0 % were Asian. The median age was 58 years old. The most common etiologies of liver disease were Hepatitis C and B, accounting for 86.6 % of all patients. The median baseline AFP was 15.5 ng/mL (IQR, 5–72). Most patients (76.2%) had a single tumor at the time of HCC diagnosis and 98.2% of patients underwent LRT before transplant.
TABLE 1.
Baseline characteristics of the study population (n = 336)
| Characteristics | Values |
|---|---|
| Age: median (range), y | 58 (21–77) |
| Male/female (%) | 258/78 (76.8%/23.2%) |
| Race | |
| White | 142 (42.3%) |
| Asian | 111 (33%) |
| Hispanic | 52 (15.5%) |
| African American | 18 (5.4%) |
| Other | 13 (3.9%) |
| Liver disease (n, %) | |
| HCV | 198 (58.9%) |
| HBV | 93 (27.7%) |
| Others | 45 (13.4%) |
| HCC number; mean diameter of largest lesion (cm) ±SD | |
| 1 | 256 (76.2%); 3.0 ± 0.8 |
| 2 | 64 (19%); 2.2 ± 0.5 |
| 3 | 16 (4.8%); 2.3 ± 0.6 |
| Types of LRT* | |
| TACE only | 193 (57.4%) |
| RFA only | 50 (14.9%) |
| RFA + TACE | 82 (24.4%) |
| Other | 5 (1.5%) |
| No. LRT | |
| 0 | 6 (1.8%) |
| 1 | 164 (48.8%) |
| 2 | 87 (25.9%) |
| 3 | 51 (15.2%) |
| >3 | 28 (8.3%) |
HCV, hepatitis C; HBV, hepatitis B; SD, standard deviation; RFA, radiofrequency ablation.
Locoregional treatment.
AFP Slope and Pretransplant LRT
During a median of 9.8 months (IQR, 6.4–13.5) between HCC diagnosis and LT, a median of 5 (IQR, 4–8) AFP measurements where reported per patient. The median AFP slope per month was −0.15 ng/mL (IQR, −1.48 to 0.17). In our cohort, 149 patients (44.3%) had normal AFP (<20 ng/mL) throughout their time on the waiting list and 23 patients (6.8%) had an AFP slope greater than 7.5 ng/mL per month. Among these 23 patients, 43.5% were female (P=0.02 compared with 21.7% female with AFP slope <7.5 ng/mL per month), 87.0% had viral hepatitis (P=0.20), and 78.3% had a single HCC at listing (P=0.53). The median AFP at LT was 8.3 (IQR, 4–39).
Most patients had LRT before LT (98.2%). The median number of treatments was 1 (IQR, 1–2). Transarterial chemoembolization (TACE) was performed at least 1 time in 81.8% of patients, and radiofrequency ablation was performed at least 1 time in 39.3% of the patients.
Explant Histological Characteristics
Explant histopathological characteristics are summarized in Table 2. Twenty-three (6.9%) of the 336 patients had vascular invasion on explant. Pretransplant LRT resulted in complete necrosis of the tumor in 138 patients (41.1%). Well or moderately differentiated tumors were present in 72 (21.4%) and 105 (32.2%) patients, respectively. Only 21 (6.3%) patients had poorly differentiated tumors, and 53 (15.8%) patients had tumor understaging by pre-LT imaging with viable tumor burden beyond Milan criteria on explant.
TABLE 2.
Explant histological tumor characteristics
| Characteristic | Value, n (%) |
|---|---|
| Microvascular invasion | |
| Yes | 23 (6.9) |
| No | 313 (93.1) |
| Pathological tumor stage | |
| Respecting Milan criteria | 283 (84.2) |
| Beyond Milan criteria | 53 (15.8) |
| Histological tumor grade | |
| Complete necrosis | 138 (41.1) |
| Well differentiated | 72 (21.4) |
| Moderately differentiated | 105 (32.2) |
| Poorly differentiated | 21 (6.3%) |
Predictors of HCC Recurrence
In univariate analysis, HCC recurrence was associated with microvascular invasion (HR, 13.1; 95% CI, 6.8–25.2; P < 0.001), explant pathologic stage outside of Milan criteria (HR, 8.9; 95% CI, 3.9–20.6; P < 0.001), presence of 3 nodules (HR, 5.4; 95% CI, 2.2–13.4; P = 0.002), AFP, female sex (HR, 2.3; 95% CI, 1.2–4.3; P = 0.01) and explant tumor histologic grade (HR, 1.8; 95% CI, 1.3–2.5; P < 0.001). When considering the AIC with AFP slope cutoffs of 0, 5, 7.5, 10 and 15 ng/mL per month tested, 7.5 and 10 had similar results. We used the 7.5 cutoff because this included a larger group of patients at risk. With this cutoff, AFP slope greater than 7.5 ng/mL per month (HR 3.0, 95% CI 1.3–7.2, P = 0.005) was predictive of HCC recurrence, when compared with the group with a slope between −7.5 and 0 ng/mL per month. No significant association was detected between HCC recurrence and age, race/ethnicity, etiology of liver disease, time from diagnosis to LT or number of LRT, donor age, and cold ischemia time (Table 3).
TABLE 3.
Univariate analysis of predictors of HCC recurrence
| Predictor | HR (95% Cl) | P |
|---|---|---|
| No. lesions | ||
| 2 (vs 1) | 1.4 (0.6–3.0) | 0.41 |
| 3 (vs 1) | 5.5(2.2–13.4) | <0.001 |
| Days from HOC to LT | 1.0 (1.0–1.0) | 0.49 |
| No. LRT | 1.2 (0.9–1.5) | 0.13 |
| Cold ischemia time (per 1 h increase from 8 h | 0.95 (0.87–1.05) | 0.31 |
| Donor age (≥ 60 y vs < 60 y) | 0.99 (0.41–2.39) | 0.98 |
| Microvascular invasion | 13.1 (6.8–25.2) | <0.001 |
| Explant grade (as continuous variable) | 1.8 (1.3–2.5) | <0.001 |
| Pathologic stage outside Milan | 8.9 (3.9–20.6) | <0.001 |
| Age at HCC diagnosis | 1.01 (1.0–1.1) | 0.47 |
| Sex (female) | 2.3 (1.2–4.3) | 0.01 |
| Diagnosis of chronic liver disease | ||
| HBV (vs HCV) | 1.1 (0.5–2.2) | 0.85 |
| Other (vs HCV) | 1.9 (0.9–4.1) | 0.11 |
| Race (vs white) | ||
| African | 1.4 (0.4–4.9) | 0.56 |
| Asian | 0.7 (0.3–1.4) | 0.26 |
| Hispanic | 0.7 (0.3–1.8) | 0.50 |
| Others | 0.00 | 0.99 |
| AFP slope (vs −7.5 to 0) | ||
| <−7.5 | 1.8 (0.7–4.4) | 0.21 |
| 0–7.5 | 1.9 (0.9–4.1) | 0.08 |
| >7.5 | 3.9(1.5–10.2) | 0.005 |
Given that explant pathology cannot be used to improve patient selection before LT, we focused on factors known before LT in the multivariable analysis. Factors associated with HCC recurrence in this multivariable model included 3 tumor nodules (HR, 7.6; 95% CI, 3.0–19.6; P < 0.001), female sex (HR, 2.5; 95% CI, 1.2–5.0; P = 0.01) and AFP slope greater than 7.5 ng/mL per month (HR, 3.0; 95% CI, 1.1–8.1; P = 0.03) (Table 4).
TABLE 4.
Multivariate analysis of predictors of HCC recurrence (pre-LT variables)
| Predictor | HR (95% Cl) | P |
|---|---|---|
| 3 tumor nodules | 7.6(3.0–19.6) | <0.001 |
| AFP slope >7.5 ng/mL per month | 3.0(1.1–8.1) | 0.03 |
| Female sex | 2.5(1.2–5.0) | 0.01 |
On univariate logistic regression analysis, patients with 3 tumors at listing were more likely to be beyond Milan on explant (P=0.005), have microvascular invasion (P=0.008), and either moderate (P=0.02) or poor tumor grade (P=0.03). The presence of 3 tumors at listing was also associated with AFP at transplant both as a continuous variable (P=0.04) and at cutoffs greater than 400 ng/mL (P=0.001) and greater than 500 ng/mL (P<0.001).
Additional Evaluations of AFP/AFP Slope in Predicting HCC Recurrence
The C-statistic for the multivariable model including AFP slope was 0.72 (95% CI, 0.65–0.79) compared with 0.67 (95% CI, 0.59–0.75) for the model including static AFP at LT adjusted for the same variables. We also performed additional analysis and found that the AIC and HR/P value for AFP slope greater than 7.5 ng/mL per month predicted HCC recurrence better than did static AFP both as a continuous variable and at all tested cutoffs (Table 5).
TABLE 5.
Univariate analysis of AFP kinetics as predictors of post-LT HCC recurrence
| AFP evaluation | Univariate HR (95% CI) | P | AIC |
|---|---|---|---|
| Static AFP at LT | 1.00 (1.00–1.00) | 0.04 | 431 |
| AFP (continuous) | 2.61 (0.93–7.35) | 0.07 | 431 |
| AFP > 400 | 2.23 (0.69–7.25) | 0.18 | 432 |
| AFP > 500 | |||
| AFP slope (continuous) | 1.00 (1.00–1.00) | 0.10 | 432 |
| AFP slope > 7.5 ng/mL per month | 2.90 (1.21–6.92) | 0.02 | 429 |
To fully examine the association between AFP and post-LT HCC recurrence, we tested different static AFP and AFP slope categories to determine the optimal predictor of recurrence. Specifically, we tested the relationship between HCC recurrence and maximal AFP on the waitlist, AFP at transplant, AFP slope as a continuous variable and AFP slope using only 2 data points (last minus first and last minus second to last). Of these, the only model which performed as well as AFP slope based on all available AFP values (ie, similar AIC) was last AFP minus second to last AFP. On univariate analysis, last AFP minus second to last AFP greater than 10 ng/mL per month (n=37) had a HR of 5.25 (95% CI, 2.65–10.39; P<0.001) compared with those with an AFP less than 10 ng/mL per month for predicting HCC recurrence. On multivariable analysis, this association remained (HR, 3.76; 95% CI, 1.82–7.76; P<0.001) with female sex and 3 HCC lesions at listing additional significant predictors of recurrence.
Association Between AFP Slope and Explant Findings
Of all variables tested, microvascular invasion was most strongly associated with HCC recurrence. However, presence or absence of microvascular invasion is unknown before LT. Therefore, we analyzed the association between pre-LT factors and presence of microvascular invasion. Among the covariates known before LT in the multivariable model, only AFP slope greater than 7.5 ng/mL per month (OR, 6.8; 95% CI, 1.6–28.7; P = 0.008) and 3 tumor nodules (OR, 5.8; 95% CI, 1.5–21.5; P = 0.009) were predictive of microvascular invasion. Among the 23 patients with AFP slope greater than 7.5 ng/mL per month, 17.4%(n=4) had microvascular invasion as opposed to 6.1%(n=19/313) with AFP slope less than 7.5 ng/mL per month (P=0.04). Additionally, 21.7% (n=5) had poorly differentiated tumors as opposed to 5.1% (n=16/313) with AFP slope less than 7.5 ng/mL per month (P<0.001) and 30.4% had explant tumor burden beyond Milan criteria compared with 14.7% with AFP slope less than 7.5 ng/mL per month (P=0.004).
Overall Survival and Recurrence-Free Probabilities
The median post-LT follow-up time was 4.0 years (IQR, 2.0–6.2). There were 72 (21.4%) deaths and 40 (11.9%) HCC recurrences. Characteristics between those with and without HCC recurrence are shown in Table 6. The Kaplan-Meier 1- and 5-year post-LT patient survival was 94.0% (95% CI, 90.8–96.1) and 77.0% (95% CI, 71.3–81.7), respectively. The 1- and 5-year recurrence-free probabilities were 95.4% (95% CI, 92.4–97.2) and 86.2% (95% CI, 81.5–89.8), respectively. HCC recurrence occurred in only 10.9% (n=34/313) with an AFP slope less than 7.5 ng/mL per month versus 26.1% (n=6/23, P=0.04) in those with an AFP slope greater than 7.5 ng/mL per month. When stratified by AFP slope, 1- and 5-year recurrence-free probabilities were 95.7% (95% CI, 92.7–97.5) and 87.4% (95% CI, 82.6–90.9) for patients with an AFP slope of 7.5 ng/mL or less per month compared with 91.3% (95% CI, 69.5–97.8) and 69.8% (95% CI, 44.5–85.3) for patients with AFP slope greater than 7.5 ng/mL per month (P = 0.01) (Figure 1). The overall 1- and 5-year survivals without recurrence were 94.0 (95% CI, 90.8–96.1) and 74.1 (95% CI, 68.2–79.0). When stratified by AFP slope, 1- and 5-year survivals without recurrence were 94.8 (95% CI, 91.7–96.8) and 75.3 (95% CI, 69.2–80.3) for patients with an AFP slope of 7.5 ng/mL or less per month compared with 82.4 (95% CI 59.6–93.0) and 59.5 (95% CI 34.2–77.8) for patients with AFP slope greater than 7.5 ng/mL per month (P = 0.02).
TABLE 6.
Clinical characteristics between patients with (n=40) and without HCC recurrence (n=296)
| Characteristics | HCC recurrence, n = 40 | No HCC recurrence, n = 296 | P |
|---|---|---|---|
| Median age (IQR) | 59.4 (55.4–62.1) | 57.9 (53.2–62.9) | 0.21 |
| Male (%) | 25 (62.5%) | 233 (78.7%) | 0.02 |
| Liver disease (%) | |||
| HCV | 20 (50.0%) | 178 (60.1%) | 0.18 |
| HBV | 11 (27.5%) | 82 (27.7%) | |
| Other | 9 (22.5%) | 36 (12.2%) | |
| HCC number at listing | |||
| 1 | 25 (62.5%) | 231 (78.0%) | 0.003 |
| 2 | 9 (22.5%) | 55 (18.6%) | |
| 3 | 6 (15.0%) | 10 (3.4%) | |
| Type of LRT received | |||
| TACE | 34 (85.0%) | 241 (81.4%) | 0.58 |
| RFA | 21 (52.5%) | 111 (37.5%) | 0.07 |
| Median number of LRT received (IQR) | 2 (1–2) | 1 (1–2) | 0.06 |
| Median AFP at diagnosis (ng/mL) (IQR) | 30.0 (5–108) | 13.0 (5–66) | 0.13 |
| AFP always <20 ng/mL | 13 (32.5%) | 137 (46.3%) | 0.10 |
| Median AFP at transplant (IQR) | 31.1 (5–144) | 8.0 (4–32) | 0.007 |
| Median AFP slope (ng/mL per month) | −0.03 (−1.07–0.84) | −0.16 (−1.60–0.11) | 0.15 |
| Explant tumor grade | |||
| No viable tumor | 9 (22.5%) | 129 (43.6%) | 0.002 |
| Well differentiated | 5 (12.5%) | 67 (22.6%) | |
| Moderately diffentiated | 22 (55.0%) | 83 (28.0%) | |
| Poorly differentiated | 4 (10.0%) | 17 (5.7%) | |
| Microvascular invasion | 14 (35.0%) | 9 (3.0%) | <0.001 |
| Explant tumor stage beyond Milan | 18 (45.0%) | 35 (11.8%) | <0.001 |
FIGURE 1.
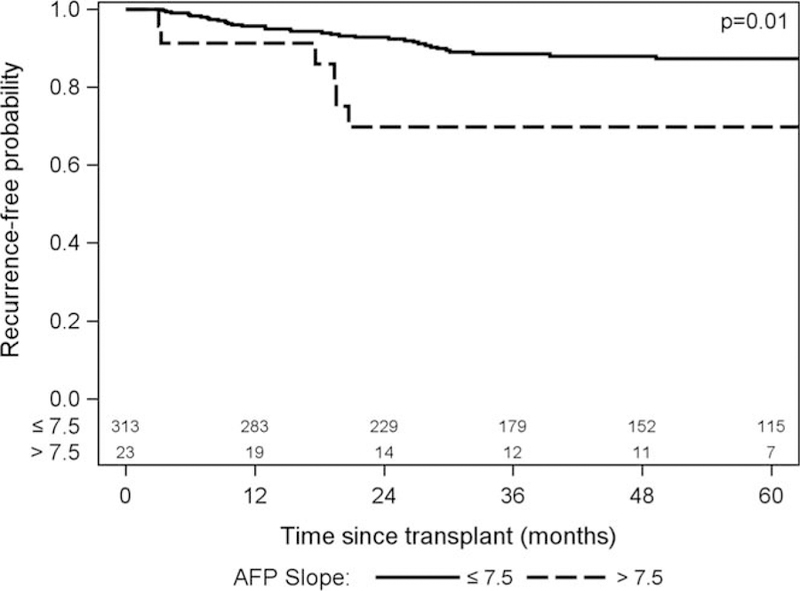
Recurrence-free probability.
DISCUSSION
In the ongoing efforts to refine current selection criteria and to further improve outcomes after LT for HCC, we are still searching for pretransplant tumor characteristics beyond size and number that can serve as surrogates for tumor aggressiveness and predict the development of HCC recurrence after LT. Adding AFP to the patient selection process has been proposed,10,11 but it remains unclear the best way to incorporate this. As rising AFP is believed to be an ominous sign of tumor progression, we examined the association between AFP slope and post-LT HCC recurrence, with AFP slope estimated from multiple data points over time. We found that AFP slope >7.5 ng/mL per month despite LRT for HCC within Milan criteria predicts post-LT HCC recurrence, with an associated HR of 3.0. Further, the c-index for the multivariable model was higher (0.72) using AFP slope >7.5 to predict post-LT recurrence than using the static AFP value before LT (0.67).
There have been several studies on the role of AFP slope before LT as a predictor of post-LT outcomes. A small Canadian study by Han et al12 evaluated 48 LT recipients with HCC both within and beyond Milan criteria and found worse 1 year recurrence-free survival in patients with an AFP slope greater than 50 ng/mL per month (40% vs 90%, P < 0.001). A second Canadian study by Dumitra et al13 including 144 patients showed that a rising AFP slope >3 ng/mL per month was the best predictor of microvascular invasion and HCC recurrence. A third study by Vibert et al14 included 290 French patients with HCC who received LT from 1985 to 2005 but only used the lowest and highest AFP values to calculate AFP slope rather than accounting for all AFP values. Using only these 2 values, patients with AFP slope >15 ng/mL per month had a worse overall survival. More recently, Lai et al15 reported post-LT outcomes of 306 patients within Milan criteria and 116 patients outside Milan criteria from 6 European centers, with specific focus on AFP slope. The authors calculated the AFP slope using only the AFP at listing and AFP at LT with a median time of 6 months between these 2 data points. In their multivariate analysis, AFP slope greater than 15 ng/mL per month was predictive of survival with a HR of 5.4. However, by relying only on 2 data points for calculating the AFP slope in these last 2 studies, the authors likely did not sufficiently account for the fluctuations in the AFP levels frequently seen in chronic liver disease, especially in patients with chronic hepatitis C. In addition, using listing AFP in these studies would not account for the effects of LRT on AFP when performed before LT listing. Lastly, in a study by Lai et al from Brussels, 124 patients transplanted for HCC were evaluated and a “delta-slope,” calculated with 3 AFP data points, was used to predict outcomes after LT listing. AFP slope >15 ng/mL per month was a significant predictor of intention-to-treat death and HCC recurrence after LT.16
To the best of our knowledge, the present study is the largest to date on the influence of AFP dynamics in HCC patients within Milan criteria before LT. In our study, we used all available AFP values from the time of HCC diagnosis to LT to construct the best fitting AFP slope. The median AFP slope was −0.15 ng/mL per month, likely reflecting the effects of LRT. With a median of 5 AFP values per patient, we captured the fluctuations in AFP. Unlike prior studies suggesting and AFP slope of 15 ng/mL per month as the best cutoff, we found 7.5 ng/mL per month to be most predictive of HCC recurrence, with a 3-time increased risk compared to those with a decreasing AFP slope. Moreover, an AFP slope greater than 7.5 ng/mL per month was also associated with microvascular invasion, which is a strong predictor of HCC recurrence.
AFP slope has potentially important clinical implications, both as a measure of response (or lack of response) to LRT and as a prognostic marker for post-LT outcomes. The AFP slope may not be relevant when the waitlist time is very short. However, the recent implementation by the United Network for Organ Sharing of a mandatory waiting period of at least 6 months before receiving MELD exception points for HCC will result in increased waitlist times across the country, giving extra importance to the AFP slope while on the waiting list. At our center, HCC patients with an AFP greater than 1000 ng/mL are not eligible for LT unless there is a significant decrease in the AFP to < 500 ng/mL with LRT.9 Based on the results of the present study, rising AFP slope may serve as an additional exclusion criterion for LT. Regardless of the absolute AFP value at baseline, our study suggests that an AFP slope > 7.5 ng/mL per month is associated with a higher risk for HCC recurrence. While these findings require confirmation, one strategy to consider for these patients with an AFP slope greater than 7.5 ng/mL per month is to place LT (including live donor LT) on hold until we observe a decrease in the AFP over time with additional LRT.
Apart from AFP slope greater than 7.5 ng/mL per month, the presence of 3 tumors, when compared to a solitary lesion, was associated with a higher risk for post-LT HCC recurrence. In a previous study by Mehta et al,19 patients with 3 lesions within Milan criteria had increased risk of waitlist drop-out compared with those with a single lesion, usually due to tumor progression. Patients with 3 tumors within Milan criteria at initial diagnosis may have more aggressive tumor biology leading to worse waitlist and post-LT outcomes, as evidenced by the association between 3 tumors and elevated AFP at LT, explant tumor beyond Milan, microvascular invasion, and both moderate and poor tumor grade. These results further highlight the importance of an observation period for evaluating tumor progression and change in the AFP, in particular for those with 3 tumor nodules, to avoid transplanting very aggressive tumors that pertain poor outcomes after LT. Female sex was also found in this study to be significantly associated with tumor recurrence after LT. The reason for the sex difference in tumor recurrence risk is unknown. Interesting, a recent analysis of United Network for Organ Sharing data showed a nearly 3-time higher risk of HCC recurrence in women (OR, 4.2; P<0.001) than men (OR, 1.5; P=0.02) among patients with AFP at LT between 101 and 500 ng/dL.20
The strengths of the study include its large sample size and the multiple AFP values used to construct the AFP slope. This study, however, is limited by its retrospective design and a lack of information on response to LRT as a possible predictor of recurrence. Response to LRT was significantly associated with HCC recurrence after LT in 2 recently published studies.15,21 Kim et al showed that HCC recurrence rate for patients with tumor size greater than 3 cm and no response to TACE was 35.8%, compared with 1.9% for patients with tumor size of 3 cm or less and response to TACE. In the European multicenter study published by Lai et al, radiological tumor progression according to modifed Response Evaluation Criteria in Solid Tumors (mRECIST) was a significant predictor of HCC recurrence. Evaluation of response to LRT is difficult in the present study because of the prolonged waitlist time at our center, during which multiple LRT were applied at different time points in many of our patients. Our study included only patients receiving MELD exception for T2 HCC with relatively low median AFP of 15.5 at listing and 8.3 at LT. We included only patients meeting T2 criteria to improve generalizability of our results that could be applied to centers not routinely using down-staging and extended criteria. Consequently, additional studies are needed to evaluate the significance of AFP slope in patients with tumor burden beyond Milan criteria.
In summary, rising AFP with slope greater than 7.5 ng/mL per month in HCC patients within Milan criteria awaiting LT predicts an increased risk of post-LT HCC recurrence and may serve as a surrogate for microvascular invasion. These findings support incorporating changes in the AFP into candidate selection while on the LT waiting list.
Footnotes
J.-M.G. participated in research design, performance of the research, data analysis, and writing of the article. N.M. participated in research design, performance of the research, data analysis, participated in the writing of the article. J.L.D. participated in research design, participated in data analysis, participated in the writing of the article. J.P.R. participated in the writing of the article. F.Y.Y. participated in research design, participated in the performance of the research, participated in data analysis, participated in the writing of the article.
This work was supported in part by the UCSF Liver Center (P30 DK026743).
REFERENCES
- 1.SEER Stat Fact Sheets: Liver and Intrahepatic Bile Duct Cancer (Accessed May 25, 2016, at http://seer.cancer.gov/statfacts/html/livibd.html).
- 2.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 3.Parfitt JR, Marotta P, Alghamdi M, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl 2007;13:543–551. [DOI] [PubMed] [Google Scholar]
- 4.Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534–540. [DOI] [PubMed] [Google Scholar]
- 5.Kornberg A, Kupper B, Tannapfel A, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol 2010;36:275–280. [DOI] [PubMed] [Google Scholar]
- 6.Samoylova ML, Dodge JL, Vittinghoff E, et al. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transpl 2013;19:1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagefi PA, Dodge JL, Yao FY, et al. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2015;21:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samoylova ML, Dodge JL, Yao FY, et al. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2014;20:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20: 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–994. [DOI] [PubMed] [Google Scholar]
- 11.Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients Database. Hepatology 2009;49:832–838. [DOI] [PubMed] [Google Scholar]
- 12.Han K, Tzimas GN, Barkun JS, et al. Preoperative alpha-fetoprotein slope is predictive of hepatocellular carcinoma recurrence after liver transplantation. Can J Gastroenterol 2007;21:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumitra TC, Dumitra S, Metrakos PP, et al. Pretransplantation α-fetoprotein slope and milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation 2013;95:228–233. [DOI] [PubMed] [Google Scholar]
- 14.Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010;10:129–137. [DOI] [PubMed] [Google Scholar]
- 15.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl 2013;19:1108–1118. [DOI] [PubMed] [Google Scholar]
- 16.Lai Q, Inostroza M, Rico Juri JM, et al. Delta-slope of alpha-fetoprotein improves the ability to select liver transplant patients with hepatocellular cancer. HPB (Oxford) 2015;17:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 19.Mehta N, Dodge JL, Goel A, et al. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar M, Dodge J, Roberts JP, et al. Increased hepatocellular carcinoma cecurrence in women compared to men with high alpha fetoprotein at liver transplant. Ann Hepatol 2016;15:545–549. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DJ, Clark PJ, Heimbach J, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant 2014;14:1383–1390. [DOI] [PubMed] [Google Scholar]


