Abstract
Thyroid hormones are critical for normal brain development. This study examined autism spectrum disorders (ASD) and thyroid stimulating hormone (TSH) levels measured in mid-pregnancy maternal serum and infant blood after birth. Three groups of children born in Orange County, CA in 2000–2001were identified: ASD (n = 78), developmental delay (n = 45), and general population controls (GP) (n = 149). Samples were retrieved from prenatal and newborn screening specimen archives. Adjusted logistic regression models showed inverse associations between ASD and log transformed TSH levels in maternal serum samples (ASD vs. GP: OR [95 % CI] 0.33 [0.12–0.91], Early Onset ASD vs. GP: 0.31 [0.10–0.98]). Results for thyroid levels in newborn blood samples were similar though not significant (ASD vs. GP:0.61 [0.18–2.04]).
Keywords: Thyroid, Thyroid stimulating hormone, Autism, Pregnancy, Developmental delay
Introduction
Autism spectrum disorders (ASD) are neurodevelopmental disorders that include autism, Asperger syndrome, and pervasive developmental disorder, not otherwise specified (PDD-NOS). Individuals with ASD may demonstrate impairments in social interaction and communication, and restricted, stereotyped interests and behaviors (American Psychiatric Association 2000; Lord et al. 2000). The currently estimated prevalence of ASD in the United States is 1 in 68 (CDC 2014). The etiology of ASD is not well understood, but based on family and twin studies, there is a clear genetic contribution (Szatmari et al. 1998; Nordenbæk et al. 2013; Posthuma and Polderman 2013). However, several other studies have strongly suggested that environmental factors are also likely involved in ASD etiology (Lawler et al. 2004). In a twin study conducted by Hallmayer et al. 2011, lack of complete ASD concordance among monozygotic twins and higher than expected ASD concordance among dizygotic twins indicated a significant contribution from a shared environment to ASD etiology. Additionally, a growing body of evidence linking ASD and non-genetic factors such as prenatal maternal antidepressant use (Croen et al. 2011), infections (Atladóttir et al. 2010; Zerbo et al. 2013), proximity to air pollution (Volk et al. 2013) and low periconceptional folic acid intake (Surén et al. 2013) has emerged as well. Research has shown that prenatal and early-life are the critical windows of vulnerability to neurodevelopmental disruption (Beversdorf et al. 2005; Rodier 2004).
Thyroid hormones are critical for normal human brain development (Morreale de Escobar et al. 2004). During the prenatal period, the fetus is solely dependent on maternal thyroid hormones until gestational week 12–14, and then partially dependent until birth. Animal studies have shown that thyroid hormones are involved in the formation of the hippocampus, and cytoarchitecture of the somatosensory cortex (Lavado-Autric et al. 2003), and that deficiency of thyroid hormone during certain periods of brain development can lead to brain defects (Bernal et al. 2003). The mechanism by which thyroid hormones may influence brain development is thought to be by influencing cell migration, cortical layer development, and cell differentiation. Differentiation of oligodendrocytes, astrocytes, and microglia have all been linked to thyroid hormone levels, and indirectly, thyroid hormones influence myelination and gene expression (Bernal et al. 2003). Maternal thyroid dysfunction in pregnant women has been linked to neurological deficits in offspring (Williams 2008; Morreale de Escobar et al. 2004; Haddow et al. 1999).
The importance of thyroid hormones for normal brain development has resulted in almost universal newborn screening for hypothyroidism. Thyroid stimulating hormone (TSH) is one measure used as an indicator of thyroid function. TSH and thyroid hormone levels (T3, known as triiodothyronine, and T4, known as thyroxine) are linked via a negative regulatory feedback loop. Increased production of TSH is often triggered by low thyroid hormone levels. According to the National Academy of Clinical Biochemistry (NACB), TSH levels steadily decrease from infancy to adulthood, with a normal range at birth between 1.3 and 19 lIU/mL and at adulthood between 0.4 and 2.5 lIU/mL (Baloch et al. 2003; Baskin et al. 2002).
A limited number of studies have examined the potential association between thyroid function and ASD. One study found that family history of autoimmune thyroid disease was associated with ASD with developmental regression (Molloy et al. 2006) while another study showed no differences between thyroid hormone levels measured in children already diagnosed with autism compared with typically developing controls (Cohen et al. 1980). In an animal model of neonatal hypothyroidism, changes in brain were suggestive of the neuropathology seen in individuals with autism (Sadamatsu et al. 2006). Only two studies have examined thyroid concentrations at birth in relation to ASD status; one found that very low T4 levels (\3rd percentile) in children was associated with higher ASD risk (Hoshiko et al. 2011), while the other found no association between neonatal T4 levels and ASD or any other neurological condition (Soldin et al. 2003). In the Generation R study of pregnancy, severe early gestation maternal hypothyroxinemia was associated with autistic symptoms at age 6 (Román et al. 2013).
To take advantage of an existing newborn screening database and prenatal screening specimen archive, we conducted a case–control study to investigate the potential association between the risk of autism spectrum disorders and TSH levels measured in maternal serum from mid- pregnancy and in newborn blood spots. We hypothesized that high TSH levels would be associated with increased risk of ASD.
Methods
Subjects
Study participants were from the Early Markers for Autism (EMA) study, a population-based, nested case–control study described in detail elsewhere (Croen et al. 2008a, b). EMA utilizes archived maternal mid-pregnancy and neonatal blood specimens from the same mother-baby pairs to examine early biomarkers and their relationship with autism spectrum disorders. Women were eligible for EMA if they participated in the prenatal expanded alphafetoprotein screening program (XAFP) in Orange County, California, and delivered a live born infant from July 2000 to September 2001. Three groups of children born to women in the cohort were identified: children with ASD, children with developmental delay or intellectual disability (DD), and general population controls (GP). Children with ASD or DD were recruited from one of the 21 Regional Centers (RC) contracted by the California Department of Developmental Services (DDS), a statewide service delivery system that coordinates the diagnosis and provision of services for individuals with developmental disabilities. Eligibility is determined on the basis of diagnostic parameters without financial or citizenship stipulations. GP controls were randomly sampled from the birth certificate files at a 2:1 ratio after excluding all past or current DDS/RC clients. Controls were frequency matched to ASD cases by gender, birth month, and birth year. At the time of case and control selection, children were between 3 and 4 years old. The institutional review boards of the California Department of Public Health and Kaiser Permanente Northern California approved the study protocol and methods.
Diagnostic Verification
Following a protocol developed by the Metropolitan Atlanta Developmental Disabilities Surveillance Program (Yeargin-Allsopp et al. 2003), expert medical record abstractors reviewed and collected detailed diagnostic and clinical data from RC records for all children receiving services for ASD or DD. Following expert clinical review of abstracted data, children with ASD were categorized based on disease onset type and cognitive status using DSM-IV criteria. Early Onset ASD, ASD with regression, or unknown onset type was determined using information from parental report or clinical observations recorded in the RC medical record. “Early onset ASD” was defined by no statement of loss of social and/or language skills, or early and sustained delays or plateauing of skills without actual loss. “ASD with Regression” was defined as clear loss of previously acquired language and/or social skills. Determination of cognitive status was based on composite scores from standardized cognitive and functional tests (DD defined as Mullen Scales of Early Learning and Vineland Adaptive Behavior Scales composite score \70; no DD defined as all scores [70 or some scores \70 and others [70; unknown: no standardized scores in chart). The final analytic sample consisted of 78 children with ASD, 45 children with DD, and 149 GP controls (Table 1).
Table 1.
EMA demographic characteristics
| ASD (N = 78) | DD (N = 45) | GP (N = 149) | Autism versus GP p valuea | Autism versus DD p valuea | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Child’s sex | 0.664b | 0.001b | ||||||
| Male | 68 | 87.18 | 27 | 60 | 133 | 89.26 | ||
| Female | 10 | 12.82 | 18 | 40 | 16 | 10.74 | ||
| Birth year | 0.248b | 0.019b | ||||||
| 2000 | 22 | 28.21 | 23 | 51.11 | 31 | 20.81 | ||
| 2001 | 56 | 71.79 | 22 | 48.89 | 118 | 79.19 | ||
| Plurality | 0.416b | 1b | ||||||
| Singleton | 75 | 96.15 | 43 | 95.56 | 146 | 97.99 | ||
| Multiple | 3 | 3.85 | 2 | 4.44 | 3 | 2.01 | ||
| Mother’s race | 0.074 | 0.771 | ||||||
| White | 51 | 67.11 | 33 | 73.33 | 118 | 79.19 | ||
| Asian | 19 | 25 | 9 | 20 | 27 | 18.12 | ||
| Other | 6 | 7.89 | 3 | 6.67 | 4 | 2.68 | ||
| Mother’s ethnicity | 0.001 | <0.001 | ||||||
| Non-Hispanic | 60 | 76.92 | 19 | 42.22 | 80 | 53.69 | ||
| Hispanic | 18 | 23.08 | 26 | 57.78 | 69 | 46.31 | ||
| Maternal birth Country | <0.001 | <0.001 | ||||||
| US | 41 | 52.56 | 12 | 26.67 | 63 | 42.28 | ||
| Mexico | 8 | 10.26 | 22 | 48.89 | 57 | 38.26 | ||
| Other | 29 | 37.18 | 11 | 24.44 | 29 | 19.46 | ||
| Maternal age (years) mean (SD) | 30.7 (5.3) | 28.5 (5.1) | 28.3 (5.6) | 0.0016 | 0.0285 | |||
| Maternal age (years) | 0.027 | 0.146 | ||||||
| <20 | 2 | 2.56 | 3 | 6.67 | 8 | 5.37 | ||
| 20–24 | 7 | 8.97 | 6 | 13.33 | 31 | 20.81 | ||
| 25–29 | 18 | 23.08 | 17 | 37.78 | 44 | 29.53 | ||
| 30–34 | 34 | 43.59 | 14 | 31.11 | 48 | 32.21 | ||
| 35+ | 17 | 21.79 | 5 | 11.11 | 18 | 12.08 | ||
| Maternal weight prior to XAFP blood draw (lbs) mean (SD) | 143.6 (26.4) | 147.3 (35.3) | 144.6 (27.2) | 0.7997 | 0.5186 | |||
| Gestational age at XAFP blood draw (days) mean (SD) | 119.9 (8.2) | 118.8 (8.7) | 118.8 (7.4) | 0.3057 | 0.4962 | |||
| Gestational age at birth (days) mean (SD) | 271.0 (14.5) | 265.2 (29.0) | 271.7 (19.0) | 0.753 | 0.1385 | |||
| Gestational age at birth (weeks) | 0.849 | 0.239 | ||||||
| <33 | 2 | 2.56 | 4 | 9.09 | 4 | 2.68 | ||
| 33 to <37 | 7 | 8.97 | 5 | 11.36 | 17 | 11.41 | ||
| ≥37 | 69 | 88.46 | 35 | 79.55 | 128 | 85.91 | ||
t test for means, Chi squared for frequencies (of non-missing/unknown categories), excluding missing data
Fisher’s exact test, excluding missing data
Specimen Collection
Maternal mid-pregnancy and neonatal blood specimens were retrieved from the California Department of Public Health’s prenatal and newborn screening specimen archives. The neonatal specimen archive contains dried bloodspots on nearly every infant, approximately 500,000 per year, born in California since 1980. All newborn blood specimens taken from children were obtained by the heel-stick method usually within 24–48 h of birth. The blood specimens were collected on special S&S filter paper and allowed to dry at room temperature prior to transport to the regional laboratory for routine screening. Blood spots remaining after routine testing were catalogued and stored at −20 °C.
Also included in the archive are approximately 200,000 maternal serum and blood cell pellet specimens collected for routine prenatal Expanded Alphafetoprotein (XAFP) screening at 15–19 weeks gestation. The samples were collected from pregnant women living in three Southern California counties in 2000–2001. Specimens were stored by obstetrical care service providers in serum separator tubes which underwent XAFP testing within 7 days of collection at a central laboratory (median time = 3 days). Leftover specimens were stored at −20 °C after 1–2 days under refrigeration. Consent forms for the XAFP Screening program were distributed at the time of the blood collection which stipulated that specimens and results from prenatal testing could be used for legitimate research purposes given appropriate IRB approval.
Measuring TSH
As part of the state’s Neonatal Screening Program, California Department of Health Services Genetic Diseases Branch routinely tests newborn blood for TSH levels. Blood spots are analyzed with a solid-phase, time-resolved sandwich fluoroimmunoassay dissociation enhanced lanthanide fluoroimmunoassay (DELFIA) (AutoDELFIA; PerkinElmer, Wellesley, MA) using a lanthanide metal europium (Eu) label. Maternal serum samples from this study were measured using an I-labeled immunoradio-metric assay (IRMA).
Statistical Methods
Demographic differences between the ASD, DD, and GP groups were tabulated and t tests (for means) as well as Chi squared tests (for frequencies) were calculated. Correlation of TSH levels in maternal and newborn blood was tested using Pearson correlation coefficients. The distributions of TSH levels in maternal mid-pregnancy serum and newborn blood spots in the ASD group were compared to the distributions in the GP and DD groups using two-sample t-tests and log10-transformed TSH concentrations. Additional comparisons were made for the DD versus the GP group. No samples in this study were below the limit of detection, so no imputations methods were necessary.
To examine the association of TSH levels in maternal mid-pregnancy and newborn blood with child outcome after adjustment for possible confounders, we fit separate conditional and unconditional logistic regression models. Conditional logistic regression models were used to analyze matched data (matched on child sex, birth month, and birth year) for ASD versus GP analyses. Unconditional logistic regression models were used to compare ASD to DD populations as well as DD to GP populations. TSH levels were log10 transformed in order to normalize the skewed values, and case versus control status was regressed on transformed TSH levels with adjustment for several factors either associated with autism in previous epidemiologic studies or related to the maternal and neonatal blood draw. Maternal and neonatal models were fit adjusting for mother’s age, mother’s race (white, Asian, other), Hispanic (yes/no), mother’s place of birth (US, Mexico, Other), and mother’s weight at blood draw. Maternal models were additionally adjusted for gestational age at maternal blood draw, while neonatal models were adjusted for gestational age at birth and baby’s age at blood draw. Maternal weight and gestational age at time of mid-pregnancy blood draw were used because they were related to the maternal blood draw, while maternal age, race/ethnicity, and country of origin have been associated with autism in previous epidemiologic studies (Croen et al. 2002; Magnusson et al. 2012).
An additional set of adjusted logistic regression models were fit comparing the log odds of ASD for individuals with TSH levels in various quartiles. First, quartile cutoff points were based on the distribution of TSH in the control population. These cutoff points were then used to categorize individuals in the ASD group into various quartiles. Logistic regression analyses were then run comparing odds of ASD for individuals with TSH levels in the second, third, or fourth quartile compared to the first quartile representing the lowest levels.
Complete ascertainment of thyroid levels in neonatal blood samples was achieved, while 12.3 % of maternal samples (24 GP mothers, 6 ASD mothers) were missing thyroid values.
Results
The characteristics of the study population are shown in Table 1. Mothers of children with ASD were less likely to be Hispanic or to be born in Mexico compared to GP or DD mothers. Mothers of children with ASD were older than GP and DD mothers (Table 1). The distribution of maternal race was similar across the three groups.
Arithmetic mean concentrations and 95 % confidence intervals for TSH in maternal and neonatal samples are presented in Table 2. The majority of mothers had second trimester TSH levels within the normal range of 0.4 and 2.5 International Micro Units per Milliliter (lIU/mL) (Baloch et al. 2003). As expected, neonatal TSH concentrations were much higher than concentrations in maternal serum samples, and the majority fell within the normal range of 1.3 and 19 lIU/mL (Baskin et al. 2002).
Table 2.
Arithmetic mean TSH concentrations (ng/mL) by diagnostic group
| Maternal serum | N | Untransformed 5–95 % range | Arithmetic mean | 95 % Confidence interval | p value for t test, versus GPa | p value for t test versus DD |
|---|---|---|---|---|---|---|
| GP | 127 | (0.42–3.69) | 1.99 | (1.46–2.52) | - | - |
| DD | 38 | (0.07–2.97) | 1.44 | (1.2–1.68) | 0.09 | - |
| ASD | 72 | (0.32–3.91) | 1.69 | (1.37–2.0) | 0.18 | 0.62 |
| ASD w/regression | 10 | (0.58–2.54) | 1.41 | (0.92–1.90) | 0.48 | 0.81 |
| ASD w/Early Onset | 59 | (0.32–5.63) | 1.76 | (1.39–2.14) | 0.25 | 0.61 |
| Newborn blood spots | N | Untransformed 5–95 % range | Arithmetic mean | 95 % Confidence interval | t test versus GP | t test versus DD |
| GP | 149 | (2.02–14.25) | 6.71 | (5.87–7.55) | - | - |
| DD | 45 | (0.55–11.11) | 4.86 | (3.79–5.93) | 0.00 | - |
| ASD | 78 | (1.27–13.62) | 6.44 | (5.49–7.38) | 0.65 | 0.01 |
| ASD w/regression | 14 | (1.67–16.83) | 6.86 | (4.49–9.22) | 0.76 | 0.07 |
| ASD w/Early Onset | 61 | (1.27–12.99) | 6.40 | (5.30–7.51) | 0.56 | 0.02 |
t tests were done comparing using log10-transformed TSH concentrations to achieve normality
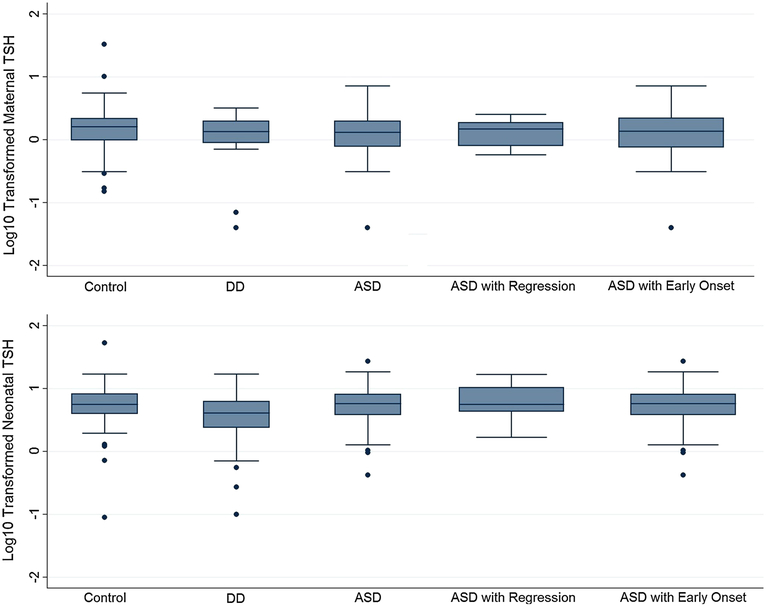
In both maternal and neonatal specimens, TSH levels were lower in the ASD group compared to GP controls, though differences were not significant (Maternal ASD vs. GP means: 1.69 vs. 1.99, p value 0.18, Neonatal ASD vs. GP means: 6.44 vs. 6.71, p value 0.65) (Table 2; Fig. 1). Neonatal TSH concentrations were significantly lower in the DD group compared to the ASD and GP groups.
Fig. 1.
Log10 transformed boxplots of maternal and neonatal TSH levels, by diagnosis category. The median is represented by the center line within each box. The upper and lower boundaries of the solid box are the upper and lower interquartile range. The upper whisker represents the most extreme value within Q3 ? 1.5*(Q3 - Q1) and the lower whisker represents the most extreme value within Q1 – 1.5*(Q3 - Q1)
The correlation of TSH levels between maternal prenatal and newborn specimens was significant, though weak, in the GP population (correlation coefficient 0.21, p value = 0.02), and were non-significant in the ASD and DD populations (Table 3).
Table 3.
Correlation of maternal and neonatal TSH levels
| ASD | DD | GP | Total population | |
|---|---|---|---|---|
| Correlationa | 0.08 | 0.08 | 0.21 | 0.15 |
| p value | 0.50 | 0.61 | 0.02 | 0.02 |
| n | 72 | 38 | 127 | 237 |
Pearson correlation coefficients
After adjustment for covariates, maternal mid-pregnancy TSH levels were inversely associated with ASD risk in the child (ASD vs. GP: AOR = 0.33 [0.12–0.91]), and for Early Onset ASD (Early Onset ASD vs. GP: AOR = 0.31 [0.10–0.98], Table 4). Comparing the DD to the GP population, mothers of children with DD had significantly lower TSH during pregnancy (DD vs. GP: AOR = 0.09 [0.02–0.42]). A similar pattern was seen for neonatal TSH levels, although associations were not statistically significant (Table 4). As a sensitivity analysis, neonatal regression models were run excluding gestational age at birth, and resulting AOR’s were all similar in direction and magnitude (data not shown). Another sensitivity analysis excluding individuals in small strata (other race/ethnicity, Mexico/Other maternal country of birth) yielded similar results (Appendix Table 7). Additional analyses were conducted for quartiles of TSH levels (Table 5). In maternal samples, TSH levels in the second, third, and fourth quartiles were associated with reduced risk for both ASD and Early Onset ASD in children, though only the third quartile demonstrated statistical significance. TSH levels in the fourth quartile were associated with a significantly reduced risk for DD compared to GP. In neonatal samples, no significant associations were seen for quartiles of TSH comparing ASD children to GP children. While neonatal TSH levels in the second, third, and fourth quartiles were associated with AOR’s below 1.0 for DD versus GP, confidence intervals were wide and the reduced risk of DD achieved borderline significance only among children with exposure levels in the third quartile (Table 5). One final analysis fitting an interaction term between gestational age at blood draw and maternal TSH models yielded no significant results (p value for ASD vs. GP model = 0.95), and similar results were seen for the interaction term between gestational age at birth and neonatal TSH models (p value for ASD vs. GP model = 0.68).
Table 4.
Odds ratios and 95 % confidence intervals for ASD associated with log10-transformed maternal and newborn TSH concentrations, the EMA study
| N | ASD versus GPa | N | ASD w/regression versus GPa | N | Early Onset ASD versus GPa | N | ASD versus DDb | N | DD versus GPb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal serum TSH | ||||||||||
| Unadjusted | 198 | 0.53 [0.22–1.29] | 86 | 0.58 [0.07–4.85] | 182 | 0.58 [0.23–1.45] | 110 | 1.31 [0.45–3.86] | 165 | 0.40 [0.13–1.19] |
| Adjusted | 192 | 0.33 [0.12–0.91] | 86 | 0.81 [0.09–6.99] | 176 | 0.31 [0.10–0.98] | 108 | 1.73 [0.43–7.08] | 162 | 0.09 [0.02–0.42] |
| Neonatal bloodspot TSH | ||||||||||
| Unadjusted | 226 | 0.72 [0.29–1.80] | 111 | 1.19 [0.18–7.75] | 205 | 0.67 [0.25–1.82] | 123 | 4.06 [1.33–12.35] | 194 | 0.21 [0.07–0.57] |
| Adjusteda | 216 | 0.61 [0.18–2.14] | 109 | 1.25 [0.07–21.22] | 200 | 0.56 [0.15–2.16] | 120 | 2.00 [0.34–11.66] | 189 | 0.36 [0.08–1.61] |
Conditional logistic regression: adjusted model uses mother’s age, mother’s race (white, Asian, other), Hispanic (yes/no), mother’s place of birth (US, Mexico, Other), and mother’s weight at blood draw. Maternal models also adjust for gestational age at maternal blood draw, while Neonatal models adjust for gestational age at birth and baby’s age at blood draw. Unadjusted models also use conditional logistic regression, accounting for matching by sex, birth month, and birth year
Unconditional logistic regression: adjusted for birth month, birth year, and sex as well as above confounders
Table 5.
Odds ratios and 95 % confidence intervals associated with quartiles of maternal and newborn TSH concentrations, the EMA study
| ASD versus GPa | ASD w/regression versus GPa | Early Onset ASD versus GPa | ASD versus DDb | DD versus GPb | |
|---|---|---|---|---|---|
| Maternal serum TSH | |||||
| Second quartile | 0.41 [0.15–1.12] | 0.29 [0.02–3.73] | 0.53 [0.18–1.54] | 0.78 [0.19–3.27] | 0.57 [0.15–2.08] |
| Third quartile | 0.38 [0.15–0.98] | 1.32 [0.15–11.8] | 0.35 [0.13–0.98] | 0.60 [0.13–2.71] | 0.49 [0.13–1.83] |
| Upper quartile | 0.4 [0.16–1.02] | 0.6 [0.05–6.97] | 0.41 [0.15–1.13] | 1.34 [0.25–7.30] | 0.22 [0.05–0.94] |
| Neonatal bloodspot TSH | |||||
| Second quartile | 0.83 [0.31–2.19] | 0.57 [0.05–5.96] | 0.88 [0.31–2.49] | 1.48 [0.31–7.20] | 0.35 [0.11–1.18] |
| Third quartile | 1.1 [0.43–2.8] | 1.19 [0.18–7.83] | 0.96 [0.33–2.74] | 2.25 [0.42–12.07] | 0.26 [0.07–0.98] |
| Upper quartile | 0.88 [0.33–2.34] | 0.91 [0.12–6.96] | 0.92 [0.32–2.66] | 2.18 [0.47–10.20] | 0.55 [0.16–1.89] |
Conditional logistic regression (maternal models): adjusted model uses mother’s age, mother’s race (white, Asian, other), Hispanic (yes/no), mother’s place of birth (US, Mexico, Other), and mother’s weight at blood draw. Maternal models also adjust for gestational age at maternal blood draw, while Neonatal models adjust for gestational age at birth and baby’s age at blood draw. Unadjusted models also use conditional logistic regression, accounting for matching by sex, birth month, and birth year
Unconditional logistic regression: adjusted for birth month, birth year, and sex as well as above confounders
Discussion
In this population-based, nested case–control study with banked biospecimens from the mother during pregnancy and the child shortly after birth, higher TSH levels in mid-pregnancy maternal serum were associated with decreased odds of a child being diagnosed with an ASD. This association was statistically significant for the overall ASD population, the subpopulation of children diagnosed with Early Onset ASD, and the children diagnosed with DD, after adjustment for confounders. Additionally, odds of a child having DD were significantly decreased when maternal TSH levels were elevated compared to control children. While unadjusted results were not statistically significant, they were similar in magnitude to the adjusted results. While quartile analyses did not yield any significant results, the odds ratios comparing second, third, and fourth quartiles of maternal TSH concentrations to the lowest quartile in ASD versus GP populations were all below the null hypothesis of OR = 1, and thus suggest that particularly low maternal TSH levels during pregnancy may be associated with future diagnosis of ASD in the child. In our population, 4.8 % of mothers and 5.5 % of children were below the recommended normal TSH range (Baloch et al. 2003; Baskin et al. 2002).
A limited number of studies have examined thyroid hormone levels in relation to ASD or DD, but to our knowledge, this is the first study to examine TSH levels during pregnancy and the newborn period. One recent study found a strong relationship between maternal hypothyroxinemia with normal TSH levels during gestational weeks 6–18 and having a child with probable autistic symptoms 6 years later (as defined by the Pervasive Developmental Problems subscale of the Child Behavior Checklist and/or the Social Responsiveness Scale) (OR 3.89, 95 % CI [1.83–8.20]) (Román et al. 2013). Another study found that infants with very low thyroxine (T4) (\3rd percentile) at birth had higher risk of being diagnosed with ASD, although findings were not statistically significant for the entire study population (Hoshiko et al. 2011). One animal study found that some of the developmental delays associated with prenatal alcohol exposure were prevented with thyroid hormone replacement therapy, suggesting that perinatal hypothyroidism could contribute to developmental delays (Gottesfeld and Silverman 1990). This is consistent with human studies which found that women who had hypothyroxinemia during pregnancy had children with delayed mental and motor function when compared to controls (Pop et al. 2003), while another study found that maternal hypothyroxinemia during pregnancy led to higher risk of expressive language delay and non-verbal cognitive delay in the child (Henrichs et al. 2010). A large Danish cohort study reported a significant association between maternal hypothyroidism diagnosed after the birth of the child and increased risk of ASD (Andersen et al. 2014). Combined, these results suggest that low thyroid hormone levels in the perinatal period and/or dysfunctional thyroid hormone regulation could be associated with increased risk of ASD and DD. Because TSH and T3/T4 are linked in a negative feedback loop, thyroid function in an normal individual would typically trigger increased TSH when thyroid hormone levels were low, and vice versa. However, the findings from this study indicate that lower maternal TSH levels are associated with increased risk of ASD in the child. We could not determine if the lower TSH levels were a response to high T3/T4 levels or if TSH levels were chronically low due to a dysfunctional feedback loop.
Results from several studies seem to support dysfunctional thyroid hormone regulation as a risk factor associated with ASD in the child. Familial history of autoimmune thyroid disease has been associated with pervasive developmental disorders including ASD (Sweeten et al. 2003) as well as ASD with regression (Molloy et al. 2006). In our study, TSH levels were not associated with regressive ASD, although the sample size for regressive ASD was very small and effect estimates were imprecise. Two other studies examined TSH levels in children already diagnosed with ASD and found either no difference between children with ASD and controls (Cohen et al. 1980) or that children with ASD have lower levels of TSH (Hashimoto et al. 1991).
The findings of low mid-pregnancy maternal TSH as well as a pattern of lower neonatal TSH being associated with increased risk for ASDs and DD’s in the child is plausible given that thyroid hormones are essential for normal fetal brain development. The fetus is completely dependent on maternal thyroid hormone in early- and midgestation (De Escobar et al. 2004), and late fetal brain development requires normal fetal thyroid production.
Another potential mechanism linking risk of ASD and DD to perinatal TSH levels is through placental abnormalities. Placental abnormalities have been associated with neurological diseases (Scher 2001), including ASDs (Walker et al. 2013). Additionally, placental abnormalities directly influence levels of placental chorionic gonadotropin (Cole 2009; Pillitteri and Pillitteri 2010) which have the capacity to modulate maternal TSH levels (Hershman 2008). Placental chorionic gonadotropin also plays a pivotal role in supporting the fetal adrenal zone (Serón-Ferré et al. 1978) which may play a key role in the normal maturation and development of the fetus (Ishimoto and Jaffe 2011).
Our study has several unique strengths. First, TSH concentrations were assessed during mid-pregnancy in the mother and at birth in the child, two biologically relevant time periods during which neurodevelopment occurs. Second, TSH levels were obtained for mother–child pairs, allowing an examination of maternal versus child effects of TSH levels on ASD and DD risk. Third, analyses were adjusted for a broad set of covariates, thus reducing the likelihood of biased risk estimates due to confounding.
However, several study limitations are worthy of mention. First, we did not directly measure free T4 or T3 thyroid hormone levels, and were thus unable to determine if reduced TSH was a consequence of elevated T4/T3 or related to a dysfunctional TSH-thyroid hormone negative feedback loop. Second, we did not have information on the presence of thyroid conditions during pregnancy, nor on treatment for those conditions. Third, TSH in the mother was measured at only one time point during pregnancy. If the critical effects of TSH on child development occur before or after the second trimester measurement, then a mid-pregnancy measurement may not completely capture the relationship between TSH and ASDs. Fourth, ASD, DD, and control designations were not based on a standardized clinical assessment, but relied on information recorded in service agency records. The use of a standard protocol for expert review of RC records likely minimized misclassification of final ASD and DD status. Some children selected as controls at 3–4 years of age may have been diagnosed at a later age with ASD. This potential misclassification of control status could affect 1–2 children at most, assuming a background ASD prevalence of *1 %.
Any such misclassification of case or control status should be non-differential with respect to TSH levels, and therefore potentially bias our risk estimates toward the null value. Finally, the limited sample size resulted in imprecise estimates of risk for ASD and its subtypes in relation to TSH levels in the mother and the child.
In conclusion, risk of ASD and DD were associated with reduced levels of maternal TSH measured during mid-pregnancy. Given that some causes of thyroid dys-function such as thyroid autoimmunity and thyroid tumors can be regulated during pregnancy, these findings provide additional motivation to treat thyroid disorders (Groot et al. 2012). Future studies including larger sample sizes, direct measurement of TSH and other thyroid hormone levels throughout pregnancy, analysis of ASD phenotypic subgroups, and information on maternal thyroid clinical conditions during pregnancy and their treatment are warranted.
Acknowledgments
Funding was provided by grants from the National Institute of Mental Health (R01-MH72565, L. Croen, PI), the National Alliance for Autism Research (824/LC/01-201-004-00-00, L. Croen, PI), and the California Tobacco-Related Disease Research Program (8RT-0115, M. Kharrazi, PI). We thank Jack Collins, Roxana Odouli and Tiffany Wong for project coordination; Julie Ruedaflores for record review and abstraction; Meredith Anderson and Daniel Najjar for assistance with data management and analysis; and Steve Graham and Debbie Hildebrandt for record linkage and specimen retrieval.
Appendix
Table 6.
Arithmetic mean TSH concentrations (ng/mL) by Demographic and Diagnostic Group
| ASD | DD | GP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Untransformed 5–95 % interval | Arithmetic mean | 95 % Cl | N | Untransformed 5–95 % interval | Arithmetic mean | 95 % Cl | N | Untransformed 5–95 % interval | Arithmetic mean | 95 % Cl | |
| Maternal serum | ||||||||||||
| Total | 72 | (0.32–3.91) | 1.69 | (1.37–2) | 38 | (0.07–2.97) | 1.44 | (1.2–1.68) | 127 | (0.42–3.69) | 1.99 | (1.46–2.52) |
| Country of origin | ||||||||||||
| US | 40 | (0.35–3.79) | 1.62 | (1.2–2.04) | 10 | (0.04–2.97) | 1.68 | (1.04–2.31) | 53 | (0.42–3.73) | 1.72 | (1.47–1.96) |
| Mexico | 6 | (0.32–3.91) | 1.97 | (0.42–3.51) | 17 | (0.07–3.2) | 1.51 | (1.12–1.89) | 50 | (0.42–3.22) | 1.67 | (1.39–1.96) |
| Other | 26 | (0.37–5.63) | 1.73 | (1.18–2.28) | 11 | (0.72–2.09) | 1.12 | (0.83–1.42) | 24 | (0.56–10.14) | 3.26 | (0.47–6.05) |
| Sex of child | ||||||||||||
| Male | 62 | (0.37–3.8) | 1.72 | (1.42–2.03) | 22 | (0.07–2.97) | 1.40 | (1.04–1.77) | 113 | (0.42–3.73) | 2.03 | (1.43–2.62) |
| Female | 10 | (0.32–7.21) | 1.48 | (0–2.95) | 16 | (0.72–2.8) | 1.49 | (1.17–1.81) | 14 | (0.86–3.01) | 1.72 | (1.29–2.14) |
| Race | ||||||||||||
| White | 47 | (0.32–3.8) | 1.70 | (1.3–2.09) | 27 | (0.07–2.97) | 1.54 | (1.22–1.86) | 102 | (0.47–3.47) | 1.81 | (1.57–2.06) |
| Asian | 17 | (0.31–2.66) | 1.44 | (1.11–1.76) | 9 | (0.72–2.09) | 1.18 | (0.83–1.54) | 22 | (0.31–3.39) | 2.82 | (−0.18 to 5.82) |
| Other | 6 | (0.4–5.73) | 2.55 | (−0.02–5.11) | 2 | (1–1.5) | 1.25 | (−1.93 to 4.43) | 3 | (0.88–3.69) | 2.04 | (−1.61 to 5.69) |
| Ethnicity | ||||||||||||
| Hispanic | 16 | (0.32–3.91) | 1.62 | (1.04–2.2) | 21 | (0.07–2.8) | 1.46 | (1.09–1.83) | 60 | (0.39–3.21) | 1.59 | (1.34–1.84) |
| Non-Hispanic | 56 | (0.32–5.63) | 1.71 | (1.33–2.08) | 17 | (0.72–2.97) | 1.41 | (1.09–1.73) | 67 | (0.56–3.81) | 2.36 | (1.37–3.34) |
| Maternal age | ||||||||||||
| <20 | 2 | (0.96–2.7) | 1.83 | (−9.22–12.88) | 2 | (1.63–1.97) | 1.80 | (−0.36 to 3.96) | 7 | (0.29–3.2) | 1.67 | (0.62–2.72) |
| 20–24 | 5 | (0.83–2.72) | 1.49 | (0.58–2.39) | 5 | (0.71–2.16) | 1.63 | (0.9–2.36) | 27 | (0.35–3.69) | 1.80 | (1.36–2.24) |
| 25–29 | 16 | (0.04–5.73) | 1.81 | (0.87–2.75) | 15 | (0.07–2.8) | 1.34 | (0.97–1.71) | 38 | (0.42–3.12) | 1.37 | (1.12–1.62) |
| 30–34 | 33 | (0.32–3.91) | 1.66 | (1.18–2.14) | 12 | (0.04–3.2) | 1.30 | (0.76–1.84) | 41 | (0.76–3.81) | 2.69 | (1.13–4.24) |
| 35+ | 16 | (0.32–3.8) | 1.66 | (1.11–2.22) | 4 | (0.72–2.97) | 1.81 | (0.32–3.3) | 14 | (0.42–10.14) | 2.19 | (0.8–3.57) |
| Newborn blood spots | ||||||||||||
| Total | 78 | (1.27–13.62) | 6.435897 | (5.49–7.38) | 45 | (0.55–11.11) | 4.862 | (3.79–5.93) | 149 | (2.02–14.25) | 6.709195 | (5.87–7.55) |
| Country of origin | ||||||||||||
| US | 41 | (1.27–12.99) | 5.894634 | (4.76–7.02) | 12 | (0.27–17) | 6.480833 | (3.75–9.21) | 63 | (2.52–12.63) | 6.303016 | (5.48–7.12) |
| Mexico | 8 | (1.55–10.57) | 6.55875 | (4.08–9.04) | 22 | (0.71–11.11) | 4.347273 | (2.8–5.9) | 57 | (1.25–15.75) | 7.177368 | (5.27–9.09) |
| Other | 29 | (2.17–16.83) | 7.167241 | (5.21–9.13) | 11 | (0.55–7.81) | 4.125454 | (2.58–5.67) | 29 | (1.29–15.63) | 6.671379 | (5.24–8.11) |
| Sex of child | ||||||||||||
| Male | 68 | (1.44–12.99) | 6.369706 | (5.37–7.37) | 27 | (0.27–9.26) | 4.21963 | (3.05–5.39) | 133 | (1.96–14.32) | 6.860451 | (5.94–7.78) |
| Female | 10 | (1.27–18.42) | 6.886 | (3.42–10.35) | 18 | (0.55–17) | 5.825556 | (3.73–7.92) | 16 | (2.05–14.25) | 5.451875 | (3.93–6.98) |
| Race | ||||||||||||
| White | 51 | (1.44–12.99) | 6.063922 | (5.12–7.01) | 33 | (0.71–12.56) | 5.15 | (3.78–6.52) | 118 | (1.96–14.32) | 6.919576 | (5.9–7.94) |
| Asian | 19 | (0.42–16.83) | 6.635263 | (4.78–8.49) | 9 | (0.55–7.81) | 4.128889 | (2.31–5.95) | 27 | (2.35–10.17) | 6.072222 | (4.84–7.3) |
| Other | 6 | (2.41–27.06) | 9.551667 | (−0.11–19.22) | 3 | (0.27–6.13) | 3.893333 | (−3.97–11.76) | 4 | (3.3–6.32) | 4.8025 | (2.67–6.94) |
| Ethnicity | ||||||||||||
| Hispanic | 18 | (1.04–10.57) | 6.105556 | (4.7–7.51) | 26 | (0.71–12.56) | 4.904231 | (3.22–6.58) | 69 | (1.96–14.32) | 7.221449 | (5.61–8.83) |
| Non-Hispanic | 60 | (1.36–15.23) | 6.535 | (5.37–7.7) | 19 | (0.27–11.11) | 4.804211 | (3.54–6.06) | 80 | (2.36–12.73) | 6.267375 | (5.52–7.02) |
| Maternal age | ||||||||||||
| <20 | 2 | (6.01–6.19) | 6.1 | (4.96–7.24) | 3 | (4.9–11.11) | 9.04 | (0.13–17.95) | 8 | (0.72–16.18) | 8.31875 | (3.51–13.13) |
| 20–24 | 7 | (5.24–9.37) | 6.857143 | (5.49–8.22) | 6 | (0.1–8.19) | 3.698333 | (0.59–6.8) | 31 | (2.77–13.53) | 7.336774 | (4.07–10.61) |
| 25–29 | 18 | (1.67–27.06) | 7.213333 | (4.37–10.06) | 17 | (0.27–17) | 4.921176 | (2.88–6.96) | 44 | (2.02–12.63) | 6.524318 | (5.43–7.62) |
| 30–34 | 34 | (0.96–16.83) | 6.586176 | (5.09–8.08) | 14 | (0.71–12.56) | 4.116429 | (2.42–5.82) | 48 | (2.77–14.32) | 6.578333 | (5.53–7.63) |
| 35+ | 17 | (1.04–10.33) | 5.178235 | (3.7–6.66) | 5 | (1.88–9.26) | 5.638 | (1.27–10.01) | 18 | (0.09–11.98) | 5.713889 | (4.3–7.13) |
Table 7.
Odds ratios and 95 % confidence intervals for ASD associated with log10-transformed maternal and newborn TSH concentrations, the EMA study
| N | ASD versus GPa | N | ASD w/regression versus GPa | N | Early Onset ASD versus GPa | N | ASD versus DDb | N | DD versus GPb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal serum TSH | ||||||||||
| Unadjusted | 130 | 0.41 [0.14–1.21] | 50 | 0.41 [0.04–4.23] | 114 | 0.47 [0.15–1.42] | 79 | 1.35 [0.34–5.46] | 93 | 0.30 [0.07–1.38] |
| Adjusted | 126 | 0.25 [0.07–0.84] | 50 | 0.64 [0.07–6.11] | 110 | 0.27 [0.07–1.01] | 75 | 2.18 [0.39–12.39] | 92 | 0.07 [0.01–0.69] |
| Neonatal bloodspot TSH | ||||||||||
| Unadjusted | 149 | 0.61 [0.18–2.10] | 61 | 0.88 [0.09–8.68] | 129 | 0.53 [0.13–2.06] | 84 | 1.69 [0.34–8.43] | 108 | 0.26 [0.04–1.58] |
| Adjusted3 | 143 | 0.42 [0.10–1.80] | 60 | 1.62 [0.03–85.82] | 127 | 0.35 [0.07–1.74] | 82 | 0.83 [0.07–9.13] | 106 | 0.77 [0.05–13.13] |
Populations excluded from regressions: Mexico/Other as birth country, “Other” race/ethnicity status
Conditional logistic regression: adjusted model uses mother’s age, mother’s race (white, Asian) Hispanic (yes/no), and mother’s weight at blood draw. Maternal models also adjust for gestational age at maternal blood draw, while Neonatal models adjust for gestational age at birth and baby’s age at blood draw. Unadjusted models also use conditional logistic regression, accounting for matching by sex, birth month, and birth year
Unconditional logistic regression: adjusted for birth month, birth year, and sex as well as above confounders
Contributor Information
Vincent M. Yau, Autism Research Program, Division of Research, Kaiser Permanente, 2000 Broadway, Oakland, CA 94612, USA
Marta Lutsky, Autism Research Program, Division of Research, Kaiser Permanente, 2000 Broadway, Oakland, CA 94612, USA.
Cathleen K. Yoshida, Autism Research Program, Division of Research, Kaiser Permanente, 2000 Broadway, Oakland, CA 94612, USA
Bill Lasley, Center for Health and the Environment, University of California Davis, One Shields Ave, Davis, CA 95616, USA.
Martin Kharrazi, Genetic Disease Screening Program, California Department of Public Health, 850 Marina Bay Pkwy, Richmond, CA, USA.
Gayle Windham, Division of Environmental and Occupational Disease Control, California Department of Public Health, 850 Marina Bay Pkwy, Bldg. P, Richmond, CA 94804, USA.
Nancy Gee, Center for Health and the Environment, University of California Davis, One Shields Ave, Davis, CA 95616, USA.
Lisa A. Croen, Autism Research Program, Division of Research, Kaiser Permanente, 2000 Broadway, Oakland, CA 94612, USA
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders DSM-IV-TR Fourth Edition (4th ed.). American Psychiatric Publication. [Google Scholar]
- Andersen S, Laurberg P, Wu C, Olsen J (2014). Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. doi: 10.1111/1471-0528.12681. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al. (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(12), 1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry J-F, et al. (2003). Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid, 13(1), 3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. (2002). American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocrine Practice, 8(6), 457–469. [PubMed] [Google Scholar]
- Bernal J, Guadaño-Ferraz A, & Morte B (2003). perspectives in the study of thyroid hormone action on brain development and function. Thyroid, 13(11), 1005–1012. doi: 10.1089/105072503770867174. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, et al. (2005). Timing of prenatal stressors and autism. Journal of Autism and Developmental Disorders, 35(4), 471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- CDC. (2014). Prevalence of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR 63(No. SS-2):[1–21]. [PubMed]
- Cohen DJ, Young JG, Lowe TL, & Harcherik D (1980). Thyroid hormone in autistic children. Journal of Autism and Developmental Disorders, 10(4), 445–450. [DOI] [PubMed] [Google Scholar]
- Cole LA (2009). New discoveries on the biology and detection of human chorionic gonadotropin. Reproductive Biology and Endocrinology RB&E, 7, 8. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. (2008a). Maternal mid-pregnancy autoantibodies to fetal brain protein: The early markers for autism study. Biological Psychiatry, 64(7), 583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Goines P, Braunschweig D, Yolken R, Yoshida CK, Grether JK, et al. (2008b). Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Research, 1(2), 130–137. doi: 10.1002/aur.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, & Selvin S (2002). Descriptive epidemiology of autism in a California population: Who is at risk? Journal of Autism and Developmental Disorders, 32(3), 217–224. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, & Hendrick V (2011). Antidepressant use during pregnancy and childhood autism spectrum disorders. Archives of General Psychiatry, 68(11), 1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- De Escobar GM, Obregón MJ, & del Rey FE (2004). Maternal thyroid hormones early in pregnancy and fetal brain development. Best Practice and Research Clinical Endocrinology and Metabolism, 18(2), 225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z, & Silverman PB (1990). Developmental delays associated with prenatal alcohol exposure are reversed by thyroid hormone treatment. Neuroscience Letters, 109(1–2), 42–47. [DOI] [PubMed] [Google Scholar]
- Groot LD, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. (2012). Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism, 97(8), 2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New England Journal of Medicine, 341(8), 549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Aihara R, Tayama M, Miyazaki M, Shirakawa Y, & Kuroda Y (1991). Reduced thyroid-stimulating hormone response to thyrotropin-releasing hormone in autistic boys. Developmental Medicine and Child Neurology, 33(4), 313–319. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. (2010). Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The generation R study. The Journal of Clinical Endocrinology and Metabolism, 95(9), 4227–4234. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- Hershman JM (2008). The role of human chorionic gonadotropin as a thyroid stimulator in normal pregnancy. The Journal of Clinical Endocrinology and Metabolism, 93(9), 3305–3306. doi: 10.1210/jc.2008-1461. [DOI] [PubMed] [Google Scholar]
- Hoshiko S, Grether JK, Windham GC, Smith D, & Fessel K (2011). Are thyroid hormone concentrations at birth associated with subsequent autism diagnosis? Autism Research, 4(6), 456–463. doi: 10.1002/aur.219. [DOI] [PubMed] [Google Scholar]
- Ishimoto H, & Jaffe RB (2011). Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocrine Reviews, 32(3), 317–355. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado-Autric R, Ausó E, García-Velasco JV, del Arufe M, Escobar del Rey F, Berbel P, et al. (2003). Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cyto-architecture of the progeny. The Journal of Clinical Investigation, 111(7), 1073–1082. doi: 10.1172/JCI16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler CP, Croen LA, Grether JK, & Van de Water J (2004). Identifying environmental contributions to autism: Provocative clues and false leads. Mental Retardation and Developmental Disabilities Research Reviews, 10(4), 292–302. doi: 10.1002/mrdd.20043. [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, & Amaral DG (2000). Autism spectrum disorders. Neuron, 28(2), 355–363. doi: 10.1016/S0896-6273(00)00115-X. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Rai D, Goodman A, Lundberg M, Idring S, Svensson A, et al. (2012). Migration and autism spectrum disorder: population-based study. British Journal of Psychiatry, 201, 109–115. doi: 10.1192/bjp.bp.111.095125. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Dawson G, Bernier R, Dunn M, et al. (2006). Familial autoimmune thyroid disease as a risk factor for regression in children with Autism Spectrum Disorder: A CPEA Study. Journal of Autism and Developmental Disorders, 36(3), 317–324. doi: 10.1007/s10803-005-0071-0. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, & Escobar del Rey F (2004). Role of thyroid hormone during early brain development. European Journal of Endocrinology/European Federation of Endocrine Societies, 151(Suppl 3), U25–U37. [DOI] [PubMed] [Google Scholar]
- Nordenbæk C, Jørgensen M, Kyvik KO, & Bilenberg N (2013). A Danish population-based twin study on autism spectrum disorders. European Child and Adolescent Psychiatry. doi: 10.1007/s00787-013-0419-5. [DOI] [PubMed] [Google Scholar]
- Pillitteri A, & Pillitteri A (2010). Maternal and child health nursing: Care of the childbearing and childrearing family. Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins. [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, & de Vijlder JJ (2003). Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clinical Endocrinology, 59(3), 282–288. [DOI] [PubMed] [Google Scholar]
- Posthuma D, & Polderman TJC (2013). What have we learned from recent twin studies about the etiology of neurodevelop-mental disorders? Current Opinion in Neurology, 26(2), 111–121. doi: 10.1097/WCO.0b013e32835f19c3. [DOI] [PubMed] [Google Scholar]
- Rodier PM (2004). Environmental causes of central nervous system maldevelopment. Pediatrics, 113(4 Suppl), 1076–1083. [PubMed] [Google Scholar]
- Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VWV, Hofman A, de Rijke YB, Tiemeier H (2013). Association of gestational maternal hypothyroxinemia and increased autism risk. Annals of Neurology. doi: 10.1002/ana.23976. [DOI] [PubMed] [Google Scholar]
- Sadamatsu M, Kanai H, Xu X, Liu Y, & Kato N (2006). Review of animal models for autism: implication of thyroid hormone. Congenital Anomalies, 46(1), 1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Scher MS (2001). Fetal and neonatal neurologic consultations: Identifying brain disorders in the context of fetal-maternal-placental disease. Seminars in Pediatric Neurology, 8(2), 55–73. [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M, Lawrence CC, & Jaffe RB (1978). Role of hCG in regulation of the fetal zone of the human fetal adrenal gland. The Journal of Clinical Endocrinology and Metabolism, 46(5), 834–837. [DOI] [PubMed] [Google Scholar]
- Soldin OP, Lai S, Lamm SH, & Mosee S (2003). Lack of a relation between human neonatal thyroxine and pediatric neurobehavioral disorders. Thyroid, 13(2), 193–198. doi: 10.1089/105072503321319503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. (2013). Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA, 309(6), 570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, & McDougle CJ (2003). Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics, 112(5), e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Jones MB, Zwaigenbaum L, & MacLean JE (1998). Genetics of autism: overview and new directions. Journal of Autism and Developmental Disorders, 28(5), 351–368. [DOI] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, & McConnell R (2013). Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry, 70(1), 71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, et al. (2013). Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biological Psychiatry, 74(3), 204–211. doi: 10.1016/j.biopsych.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR (2008). Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of Neuroendocrinology, 20(6), 784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, & Murphy C (2003). Prevalence of autism in a US metropolitan area. JAMA, 289(1), 49–55. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, & Croen LA (2013). Maternal infection during pregnancy and autism spectrum disorders. J Autism Dev Disord. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]