Abstract
Lewy body diseases involve neurogenic orthostatic hypotension (nOH), cardiac noradrenergic deficiency, and deposition of the protein alpha-synuclein (AS) in sympathetic ganglion tissue. Mechanisms linking these abnormalities are poorly understood. One link may be AS deposition within sympathetic neurons. We validated methodology to quantify AS colocalization with tyrosine hydroxylase (TH), a marker of sympathetic noradrenergic innervation, and assessed associations of AS/TH colocalization with myocardial norepinephrine content and cardiac sympathetic neuroimaging data in nOH. Post-mortem sympathetic ganglionic AS/TH colocalization indices and myocardial norepinephrine contents were measured in 4 Lewy body and 3 rare non-Lewy body nOH patients. Sixteen Lewy body and 11 non-Lewy body nOH patients underwent in vivo skin biopsies and thoracic 18F-dopamine positron emission tomographic scanning, with cutaneous colocalization indices expressed vs. cardiac 18F-dopamine-derived radioactivity. Ganglionic AS/TH colocalization indices were higher and myocardial norepinephrine lower in Lewy body than non-Lewy body nOH (p=0.0020, p=0.014). The Lewy body nOH group had higher AS/TH colocalization indices in skin biopsies and lower myocardial 18F-dopamine-derived radioactivity than did the non-Lewy body nOH group (p<0.0001 each). All Lewy body nOH patients had colocalization indices > 1.5 in skin biopsies and 18F-dopamine-derived radioactivity < 6,000 nCi-kg/cc-mCi, a combination not seen in non-Lewy body nOH patients (p<0.0001). In Lewy body nOH AS deposition in sympathetic noradrenergic nerves is related to post-mortem neurochemical and in vivo neuroimaging evidence of myocardial noradrenergic deficiency. These associations raise the possibility that intra-neuronal AS deposition plays a pathophysiological role in the myocardial sympathetic neurodegeneration attending Lewy body nOH.
Keywords: orthostatic hypotension, cardiac neuroimaging, sympathetic nervous system, alpha-synuclein, tyrosine hydroxylase, skin biopsy, Lewy body diseases
Orthostatic hypotension (OH) occurs commonly in the elderly,1 is associated with decreased quality of life and increased morbidity and mortality,2 and is treatable.3 Neurogenic orthostatic hypotension (nOH) is a form of orthostatic hypotension that results from inadequate reflexive release of the sympathetic neurotransmitter norepinephrine (NE) in response to upright posture.4 Symptoms of nOH can be improved by NE precursor therapy,5 especially in the setting of sympathetic noradrenergic deficiency.6
Prominent among causes of nOH are Lewy body diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), and pure autonomic failure (PAF). These diseases involve severe myocardial NE depletion7 and post-mortem evidence for deposition of the protein alpha-synuclein (AS) in catecholaminergic neurons both in the brain and in the sympathetic nervous system.8, 9 In contrast with Lewy body diseases, multiple system atrophy (MSA), which usually also involves nOH, is characterized by AS deposits in glial cells in the central nervous system.10 Lewy body diseases and MSA are now considered to be members of a family of autonomic synucleinopathies.11
Bases for the relationship between alpha-synucleinopathy and myocardial noradrenergic deficiency are poorly understood. In rare patients with autosomal dominantly transmitted familial PD, mutation or replication of the AS gene causes not only the characteristic movement disorder but also nOH, accompanied by neuroimaging evidence of myocardial sympathetic denervation.12, 13 Although post-mortem observations have documented that sporadic Lewy body diseases are associated with cardiac sympathetic denervation,14, 15 the relevance to these findings of AS deposition within sympathetic nerves is unclear.
Denervation may not be the only determinant of myocardial noradrenergic deficiency. Under resting conditions most of tissue catecholamine turnover reflects net leakage from the vesicles into the cytoplasm.16 Lewy body diseases are associated with high catecholamine turnover due to decreased vesicular sequestration and a shift from vesicular sequestration to oxidative deamination of cytoplasmic catecholamines in myocardial sympathetic nerves.17–19 These functional abnormalities would be expected to contribute to myocardial noradrenergic deficiency and could augment formation of toxic products of oxidation of cytoplasmic catecholamines, such as 3,4-dihydroxyphenylacetaldehyde.20 In the present study we used the mono-exponential slope of decline of 18F-dopamine-derived radioactivity between the frames with midpoints about 8 minutes and 25 minutes after initiation of tracer injection (k8’−25’) as an index of myocardial catecholamine turnover. Studies to date have not assessed the relationship between myocardial catecholamine turnover and intra-neuronal AS deposition.
A key deficiency in this area has been the lack of a method to quantify AS deposition in sympathetic noradrenergic nerves. Immunofluorescence microscopic assays have been used to visualize AS in skin biopsies from patients with autonomic synucleinopathies,11, 21–23 but differences in methodologies and inconsistencies in results remain to be resolved,21, 24–26 and the reports have usually been observational or semi-quantitative.11 Under the presumption that sympathetic denervation may decrease tissue AS content because of the deposition of AS within sympathetic nerve fibers that are lost, an “alpha-synuclein ratio” has been proposed, in which AS signal in regions of interest is adjusted for local innervation by the ratio of AS to protein gene product 9.5, a pan-axonal marker.27 No previous study has validated and applied methodology to measure AS deposition within sympathetic noradrenergic nerves.
We devised and validated methodology to quantify colocalization of AS with tyrosine hydroxylase (TH), a marker of sympathetic noradrenergic innervation; and we examined whether AS/TH colocalization in sympathetic ganglion tissue post-mortem is related to myocardial NE depletion and whether AS/TH colocalization in skin biopsies in vivo is related to neuroimaging evidence of cardiac sympathetic denervation and dysfunction in Lewy body nOH.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
The subjects in this study were evaluated at the NIH Clinical Center after having given written informed consent to participate in one or more research protocols approved by the Intramural Research Board of the National Institute of Neurological Disorders and Stroke. Patients had been referred for evaluation of a known or suspected autonomic synucleinopathy. Five healthy control subjects were also studied. Autonomic function testing to identify nOH included continuous blood pressure recording associated with performance of the Valsalva maneuver28 and orthostatic plasma catechols.29
Lewy body nOH
PAF (N=9) was diagnosed based on nOH in patients more than 30 years old without signs of central neurodegeneration, low plasma levels of NE or its neuronal metabolite 3,4-dihydroxyphenylglycol,30 and low myocardial 18F-dopamine-derived radioactivity.31 Such patients also often have olfactory dysfunction.32
Patients with nOH in the setting of DLB (N=2) carried an initial diagnosis of PAF but developed dementia and parkinsonism over years. The patients had a putamen/occipital cortex ratio of 18F-DOPA-derived radioactivity ≤ 2.433 and low myocardial 18F-dopamine-derived radioactivity.
PD with orthostatic hypotension (PD+OH, N=5) was diagnosed from nOH, bradykinesia and cogwheel rigidity, low myocardial 18F-dopamine-derived radioactivity, and a putamen/occipital cortex ratio of 18F-DOPA-derived radioactivity ≤ 2.4.33
Non-Lewy body nOH
MSA (N=6) was diagnosed by progressive parkinsonism or cerebellar ataxia and other supportive clinical features such as poor airway control manifested by dysarthria, stridor, or aspiration and urinary retention requiring bladder catheterization. The patients had decreased cerebrospinal fluid (CSF) levels of 3,4-dihydroxyphenylacetic acid34 and normal interventricular septal myocardial 18F-dopamine-derived radioactivity.17, 31
Among the group of patients with rare non-Lewy body forms of nOH there were 2 with autoimmune autonomic ganglionopathy (AAG), diagnosed by symptoms of pandysautonomia, neuroimaging evidence of intact post-ganglionic sympathetic innervation, and high titers of anti-neuronal nicotinic receptor antibodies.35, 36 Other patients with non-Lewy body nOH had autoimmunity-associated autonomic failure with sympathetic denervation (AAD, N=1),37 baroreflex failure after neck and chest irradiation for lymphoma in the remote past (N=1),38 or PAF with autopsy-proven absence of Lewy bodies in the brain and sympathetic ganglion tissue (N=1).39
Assay validation
In order to validate AS deposition in sympathetic noradrenergically innervated skin constituents and the AS/TH colocalization index as biomarkers of Lewy body forms of nOH, we comprehensively studied patients diagnosed with PD+OH, PAF, or DLB+OH as described above. Seven patients (4 with Lewy body nOH, 3 with non-Lewy body nOH) had post-mortem confirmation of their diagnoses. The AS/TH colocalization index was validated further in sympathetic ganglion tissue obtained post-mortem from Banner Sun Health Research Institute—4 patients with autopsy-proven Lewy body disease and 4 controls without a Lewy body disease.
To exclude non-specific binding of AS antibody, AS was analyzed in samples from rare patients with nOH who would not be expected to have AS deposition in sympathetic noradrenergically innervated skin constituents. The negative controls included 2 patients with AAG, where nOH reflects circulating antibodies to the neuronal nicotinic receptor;36 a patient with AAD,37 a patient who had autopsy-proven PAF without synucleinopathy in the brain or sympathetic ganglion tissue,39 and 5 healthy volunteers.
To exclude non-specific binding of TH antibody, immunoreactive TH was measured in the patient with AAD, who had extremely low plasma and urine levels of catechols and no measurable TH in skin biopsy tissues assayed in our laboratory and independently in another laboratory.37
To adjust for possible concurrent sympathetic noradrenergic denervation or atrophic changes in the sizes of sympathetic noradrenergically innervated skin constituents, AS signal intensity was expressed both in absolute terms and relative to TH or smooth muscle actin (SMA) in the same images.
Skin biopsies
There are obvious individual and ethnic differences in hair distribution, cutaneous vascularity, and sweating along the lateral aspect of the leg—the usual site of skin biopsies—whereas the nape of the neck has relatively abundant hair, blood vessels, and sweat glands. In this study skin biopsies were obtained from the lateral aspect of the leg in 2 patients before the biopsy location was switched to the neck.
Although eccrine sweat glands are well known to receive sympathetic cholinergic innervation, they also receive sympathetic noradrenergic innervation.40–43 The central hypothesis driving our study, that AS deposition within sympathetic noradrenergic nerves is associated with cardiac noradrenergic deficiency in patients with nOH, explains the emphasis on colocalization of AS with immunoreactive TH in nerve fibers surrounding sweat glands.
Skin biopsy samples were placed in Zamboni fixative solution and kept at 4 °C for about 18–20 hours, washed with Sorenson’s phosphate buffer (133 mM, pH 7.6), and placed in 20% glycerol for cryoprotection. Samples were embedded in optimum cutting temperature compound, frozen, and sliced into 8–10 μm thick sections (Histoserv, Germantown, MD).
SMA-containing and sympathetic noradrenergic fibers are arranged in parallel in arrector pili muscles. In eccrine sweat glands SMA surrounds the secretory epithelial cells, and sympathetic noradrenergic nerves surround the SMA. In blood vessels sympathetic noradrenergic nerves surround the SMA. These characteristic features enabled identification of even small pieces of arrector pili, blood vessels, and sweat glands in the relatively thin sections.
Immunolabeling protocols were applied to identify TH using rabbit anti-TH antibody (1:1000, Pel-Freez Biologicals, Rogers, AR), AS using mouse IgG1 monoclonal anti-AS (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), and alpha SMA using mouse IgG2a monoclonal anti-SMA (1:400, Santa Cruz Biotechnology, Santa Cruz, CA) for 24 h at 4 °C. The primary immunoreactions were visualized using appropriate secondary antibodies—Cy3-conjugated anti-rabbit antibody (Jackson Immune Research Labs), Alexa 488-conjugated anti-mouse IgG1 antibody and Alexa 647-conjugated anti-mouse IgG2a antibody (Thermo Scientific, Inc., Rockford, IL) for 1 h at room temperature. Coverslips were mounted on slides with ProLong™ Gold anti-fade reagent (Thermo).
To minimize non-specific binding, the immunostained tissues were treated with TrueBlack™ Lipofuscin Autofluorescence Quencher (Biotium, Inc., Fremont, CA). The tissues mounted on slides were subjected to confocal immunofluorescence microscopy using a Zeiss LSM 880 laser scanning microscope (Carl Zeiss, Germany). The images were captured using proprietary ZEN 2 image acquisition, and the image datasets were processed for image stitching and illumination correction (Zeiss).
Fluorescence intensities were in relative units. We included a negative control on each slide and used the same laser gain and sensitivity settings for all the samples. All the images were analyzed by personnel who were blinded as to the individual clinical diagnosis. For skin biopsies image sizes were 1,024 X 1,024 pixels. Image analysis procedures are described below.
18F-Dopamine PET scanning
18F-Dopamine positron emission tomographic (PET) scanning was done as described previously.44 Briefly, interventricular septal and free wall concentrations of 18F-DA-derived radioactivity were recorded for the 5-minute frame with a mid-point about 8 minutes after initiation of the 3-minute infusion of 1 mCi of the tracer (8’ Radioactivity). Radioactivity concentrations in nCi/cc were adjusted for the radioactivity dose in mCi and the body mass in kg and expressed in units of nCi-kg/cc-mCi. The radioactivity normally peaks at about 8 minutes. To calculate the rate of loss of radioactivity between the frame with the mid-point at 8 minutes and the 10-minute frame with mid-point at 25 minutes after initiation of tracer administration, we obtained the mono-exponential slope of decline in septal radioactivity (k8’−25’). This slope indicates pharmacokinetic turnover, probably as an inverse measure of vesicular storage.18
Other clinical laboratory tests
18F-DOPA scanning without carbidopa pretreatment was conducted as previously published.33 Lumbar puncture was performed to obtain CSF for assays of contents of catechols in our laboratory.34
Clinical 3.0 Tesla magnetic resonance imaging (MRI) was done without administration of contrast. The matrix was 240×240, slice oversampling 1.4, 181 slices at 1.0 mm thickness, acquired resolution 1 mm3.
The University of Pennsylvania Smell Identification Test (UPSIT, Sensonics International, Haddon Heights, NJ), Montreal Cognitive Assessment (MoCA), and Unified Parkinson’s Disease Rating Scale (off levodopa) were administered in most patients.
Post-mortem observations
Post-mortem neuropathologic diagnostic confirmation was obtained for a total of 15 individuals. For 4 patients with Lewy body disease and 4 controls without a Lewy body disease post-mortem neuropathologic descriptions were provided by the Banner Sun Health Research Institute. The remaining 7 patients (4 Lewy body nOH, 3 non-Lewy body nOH) had post-mortem diagnostic confirmation through the University of Miami Brain Endowment Bank or the Laboratory of Pathology at the NIH Clinical Center. Post-mortem intervals were less than 24 hours in each case. AS/TH colocalization indices in sympathetic ganglion tissue were calculated and assays of myocardial NE19 conducted.
Data analysis and statistics
Regions of interest were drawn (white dotted outlines) around arrector pili muscles (Figure 1A and 1D), blood vessels (Figure 1B and 1E), and sweat glands (Figure 1C and 1F). Average fluorescence intensity of AS (green), SMA (blue), and TH (red) within the regions of interest was measured using Fiji software after background subtraction and tabulated for each visualized constituent.
Figure 1: Immunofluorescence microscopic images of sympathetic noradrenergically innervated constituents in skin biopsies.
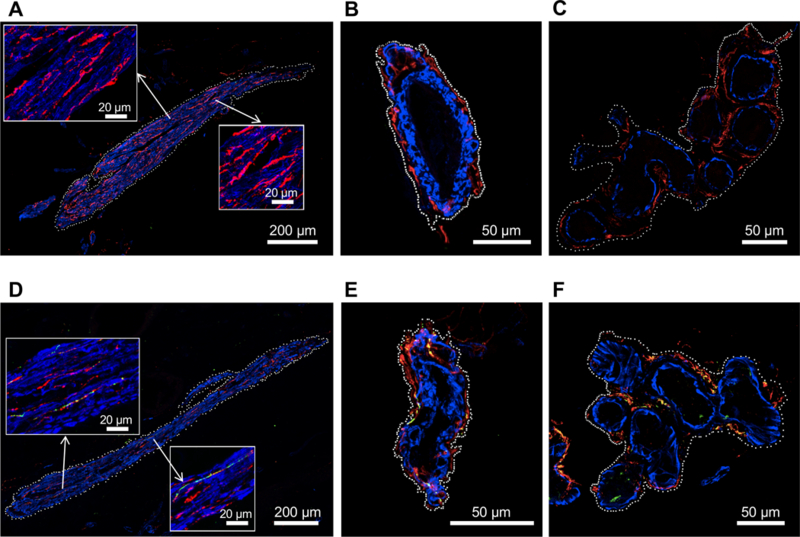
Smooth muscle actin (blue) and tyrosine hydroxylase (TH, red) are present in arrector pili muscles (A, D), blood vessels (B, E), and sweat glands (C, F). White dots indicate placement of regions of interest for quantifying alpha-synuclein (AS, green) and colocalized AS with TH (yellow). Upper panels (A-C) show images from patients with non-Lewy body forms of neurogenic orthostatic hypotension (nOH) and bottom panels (D-F) images from patients with Lewy body (D-F) nOH. Note AS deposition in TH-positive fibers in the Lewy body patients.
For colocalization analyses the method of Jaskolski et al.45 was used, with calculation of the AS/TH colocalization index in the entire image as follows: (1) normalized mean deviation product (nMDP) values for colocalization were tabulated from −1.0 to +1.0 (Figure 2A); (2) the counts corresponding to nMDP values from 0.3 to 1.0 were summed (red or blue boxes in Figure 2A); (3) 0.1 was added, so that the sum of the counts was greater than zero; (4) the log of the number from step (3) was calculated. The formula for the colocalization index therefore was the following.
Figure 2: Alpha-synuclein (AS)/tyrosine hydroxylase (TH) colocalization indices in post-mortem sympathetic ganglion tissue.
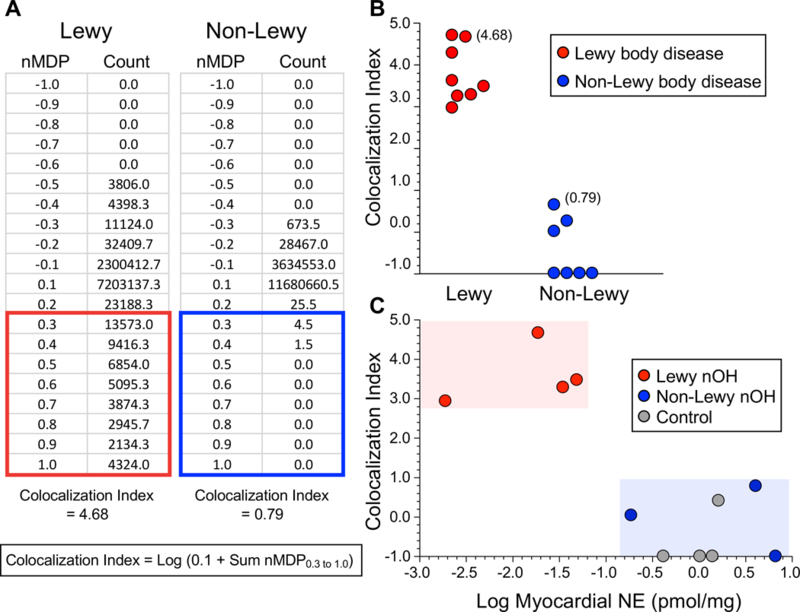
Panel A shows representative normalized mean deviation product (nMDP) values and corresponding colocalization counts from sympathetic ganglion tissue of a patient with a Lewy body disease (red outline) and a control subject without Lewy body disease (blue outline). nMDP is defined as the correlation between individual pairs of pixels. Values for nMDP range from −1.0 to +1.0, where +1.0 means perfect correlation and −1.0 means perfect exclusivity. At the bottom of Panel A is the formula used to calculate the colocalization index. Counts for nMDP values above 0.3 were summed, and 0.1 was added to the sum to avoid taking the log of zero when the sum of counts was zero. If there were no AS/TH colocalization, the index would be −1.0 (the log of 0.1). Panel B shows individual AS/TH colocalization indices from 8 patients with a Lewy body disease (red) and 7 control subjects without a Lewy body disease (blue). Panel C shows a scatterplot relating individual values for the AS/TH colocalization index as a function of the log of the apical myocardial concentration of norepinephrine (NE), expressed in units of pmol/mg wet weight, in Lewy body nOH patients (red), non-Lewy body nOH patients (blue), and control subjects without either nOH or a Lewy body disease (gray). Note complete separation of the Lewy body nOH patients from the non-Lewy body nOH patients and control subjects in terms of both AS/TH colocalization indices and myocardial NE contents.
Mean values (± 1 standard error of the mean) for AS, AS/TH ratios, and AS/SMA ratios for each of the 3 skin constituents and for myocardial NE contents were compared between the Lewy body nOH and non-Lewy body nOH groups by independent-means t-tests conducted on log-transformed data. T-tests were also used to compare mean values for 18F-dopamine-derived radioactivity and clinical and other laboratory parameters between the Lewy body nOH and non-Lewy body nOH groups. Frequencies of values in compared groups were analyzed by Fisher’s exact test. A p value less than 0.05 defined statistical significance.
RESULTS
AS/TH colocalization index in post-mortem tissues
In all 8 patients with Lewy body diseases who had post-mortem sympathetic ganglion tissue assayed the AS/TH colocalization index exceeded 2.9, whereas in all 7 patients with non-Lewy body diseases the AS/TH colocalization index was below 1.0 (p=0.00016; Figure 2B). The mean AS/TH colocalization index in the 4 patients with Lewy body nOH was greater than the mean index in the 7 controls without a Lewy body disease (3.79 ± 0.68 vs. −0.39 ± 0.79, p<0.0001) and than in the 3 patients with non-Lewy body nOH (−0.06 ± 0.52, p=0.04; Table 1).
Table 1:
Post-mortem data from patients with neurogenic orthostatic hypotension (nOH)
| Patient No. | Age at Death | Sex | AS/TH Coloc. Index | Myo. NE |
|---|---|---|---|---|
| Years | SNS Ganglion | pmol/mg | ||
| Lewy body nOH | ||||
| 1 | 83.4 | F | 3.49 | 0.048 |
| 2 | 79.1 | M | 4.68 | 0.019 |
| 3 | 68.9 | M | 3.31 | 0.034 |
| 4 | 77.8 | F | 2.95 | 0.002 |
| Non-Lewy body nOH | ||||
| 5 | 72.8 | M | 0.79 | 4.02 |
| 6 | 54.3 | M | −1.00 | 6.57 |
| 7 | 61.0 | M | 0.04 | 0.18 |
Abbreviations: AS=alpha-synuclein; Coloc.=colocalization; Myo=apical myocardial; NE=norepinephrine; TH=tyrosine hydroxylase.
The 4 patients with Lewy body nOH had lower mean myocardial NE content than did the 7 controls without a Lewy body disease (0.026 ± 0.010 vs. 2.17 ± 0.87 pmol/mg wet weight, p=0.00047) and than the 3 patients with non-Lewy body nOH (3.59 ± 1.86 pmol/mg; p=0.014; Figure 2C).
AS deposition in skin biopsies
All patients with a Lewy body form of nOH had AS deposition in arrector pili muscles, blood vessels, and sweat glands (examples are in Figure 1D-F). Individual values for intensities of AS signal in the Lewy body nOH group were above the ranges in the non-Lewy body nOH group (Figure 3A-C). (One MSA patient had blood vessel AS intensity within the range of values of the Lewy body nOH group, Figure 3B.) Intensity values for AS in arrector pili muscles, blood vessels, and sweat glands of Lewy body nOH patients averaged 37, 13, and 30 times those of non-Lewy body nOH patients (p<0.0001 each, Table 2).
Figure 3: Individual values for alpha-synuclein in regions of interest in arrector pili muscles (A), blood vessels (B), and sweat glands (C), and for the alpha-synuclein/tyrosine hydroxylase colocalization index (D) in skin biopsies from patients with Lewy body neurogenic orthostatic hypotension (nOH, red), non-Lewy body nOH (blue), and healthy volunteers (HV) without either nOH or a Lewy body disease (gray).
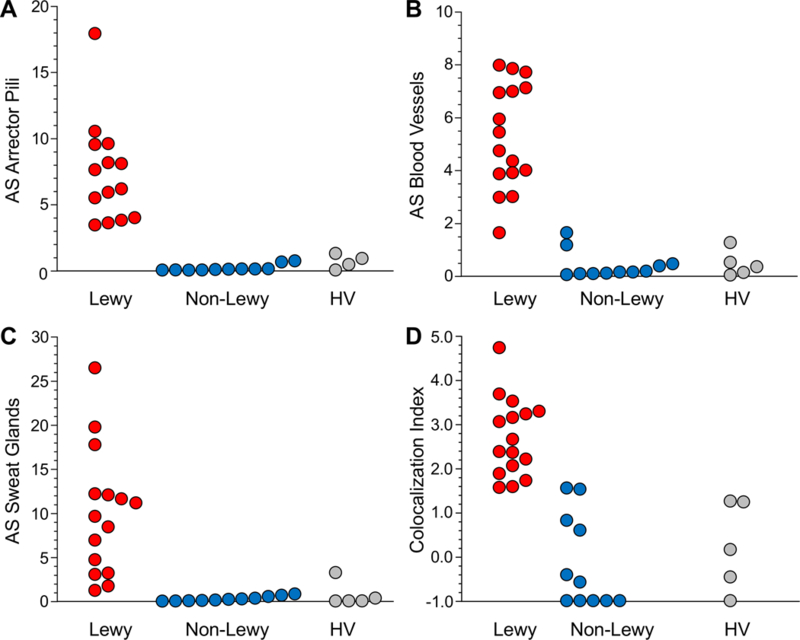
Note separations of the Lewy body nOH from the other groups.
Table 2: Demographic and laboratory comparisons between Lewy body and non-Lewy body groups with neurogenic orthostatic hypotension.
Tabulated are mean values (± SEM), with the numbers of patients in parentheses. Also shown are percentages of patients among those with available data.
| Parameter | Lewy | Non-Lewy | P |
|---|---|---|---|
| Age (years) | 71.3 ± 2.1 (16) | 58.1 ± 4.7 (11) | 0.00862 |
| Sex (M/F) | 9/7 | 6/5 | n.s. |
| Co-localization index | 2.70 ± 0.22 (16) | -0.12 ± 0.32 (11) | <0.0001 |
| AS (Arrector Pili) | 7.43 ± 1.03 (14) | 0.20 ± 0.08 (11) | <0.0001 |
| AS (Blood vessel)) | 5.27 ± 0.50 (16) | 0.41 ± 0.16 (11) | <0.0001 |
| AS (Sweat gland) | 10.07 ± 1.86 (15) | 0.34 ± 0.09 (11) | <0.0001 |
| AS/SMA (Arrector Pili) | 0.27 ± 0.05 (15) | 0.02 ± 0.01 (11) | <0.0001 |
| AS/SMA (Blood vessel) | 0.32 ± 0.10 (16) | 0.04 ± 0.01 (11) | <0.0001 |
| AS/SMA (Sweat gland) | 0.71 ± 0.12 (15) | 0.06 ± 0.03 (11) | <0.0001 |
| AS/TH (Arrector Pili) | 0.73 ± 0.08 (15) | 0.04 ± 0.01 (11) | <0.0001 |
| AS/TH (Blood vessel) | 0.80 ± 0.15 (16) | 0.11 ± 0.03 (11) | <0.0001 |
| AS/TH (Sweat gland) | 1.09 ± 0.12 (15) | 0.11 ± 0.03 (11) | <0.0001 |
| UPSIT (out of 40) | 18.8 ± 1.6 (16) | 32.0 ± 1.0 (11) | <0.0001 |
| Cortical atrophy on MRI | 86% (14) | 0% (9) | <0.0001 |
| Septum 18F-DA (nCi-kg/cc-mCi) | 3,501 ± 378 (16) | 10,814 ± 1,168 (11) | <0.0001 |
| Free Wall 18F-DA (nCi-kg/cc-mCi) | 3,080 ± 247 (16) | 9,476 ± 1,298 (11) | <0.0001 |
| CSF DHPG (pmol/mL) | 9.92 ± 0.87 (15) | 6.32 ± 0.96 (9) | 0.01403 |
| CSF NE (pmol/mL) | 0.78 ± 0.12 (15) | 0.35 ± 0.13 (9) | 0.03347 |
| 18F-DOPA P/A PUT | 0.80 ± 0.03 (16) | 0.89 ± 0.04 (10) | n.s. |
| UPDRS (off levodopa, max. 171) | 31.1 ± 5.7 (15) | 30.9 ± 7.8 (10) | n.s. |
| MoCA (max. 30) | 25.9 ± 1.0 (14) | 26.7 ± 0.6 (11) | n.s. |
| 18F-DOPA PUT/OCC | 2.83 ± 0.25 (16) | 2.87 ± 0.29 (10) | n.s. |
| 18F-DOPA PUT Washout % | 22 ± 32% (16) | 20 ± 4% (10) | n.s. |
| CSF DOPA (pmol/mL) | 3.37 ± 0.24 (15) | 2.85 ± 0.51 (9) | n.s. |
| CSF DOPAC (pmol/mL) | 1.28 ± 0.11 (15) | 1.10 ± 0.191 (9) | n.s. |
| Plasma NE (pmol/mL) | 1.04 ± 0.11 (16) | 1.35 ± 0.36 (11) | n.s. |
| Plasma DHPG (pmol/mL) | 3.95 ± 0.34 (16) | 5.43 ± 1.12 (11) | n.s. |
| Plasma DOPA (pmol/mL) | 8.81 ± 1.13 (16) | 8.62 ± 1.23 (11) | n.s. |
| Plasma DOPAC (pmol/mL) | 8.33 ± 0.92 (16) | 8.38 ± 1.35 (11) | n.s. |
| Skin NE (pmol/mg) | 0.082 ± 0.019 (14) | 0.047 ± 0.020 (10) | n.s. |
| Skin DHPG (pmol/mg) | 0.002 ± 0.001 (12) | 0.003 ± 0.001 (8) | n.s. |
| Skin DOPA (pmol/mg) | 0.062 ± 0.031 (14) | 0.042 ± 0.017 (10) | n.s. |
Abbreviations: 18F-DA=18F-dopamine; AS=alpha-synuclein; CSF=cerebrospinal fluid; DHPG=3,4-dihydroxyphenylglycol; DOPAC=3,4-dihydroxyphenylacetic acid; MoCA=Montreal Cognitive Assessment; MRI=magnetic resonance imaging; NE=norepinephrine; P/A=posterior/anterior ratio; SMA=smooth muscle actin; TH=tyrosine hydroxylase; PUT/OCC=putamen/occipital cortex ratio; UPDRS=Unified Parkinson’s Disease Rating Scale; UPSIT=University of Pennsylvania Smell Identification Test
AS/TH colocalization in skin biopsies
The mean AS/TH colocalization index was greater in the Lewy body nOH than in the non-Lewy body nOH group (p<0.0001; Figure 3D and Table 2). All the Lewy body patients had indices >1.5. Across all subjects, values for the AS/TH colocalization index correlated positively with AS intensities in arrector pili muscles, blood vessels, and sweat glands, with or without adjustment for SMA or TH (Supplementary Table).
Compared to healthy controls, Lewy body nOH patients had AS intensities and AS/TH colocalization indices above the ranges of values in the healthy controls. (An exception was a healthy control with AS intensity within the range of the Lewy body nOH group in sweat glands, Figure 3C).
18F-dopamine-derived radioactivity
All the patients with Lewy body nOH had 18F-dopamine-derived radioactivity less than 6,000 nCi-kg/cc-mCi in the left ventricular free wall (Figure 4A-D). The distribution of septal 18F-dopamine-derived radioactivity in the Lewy body nOH group was broader than the distribution of free wall radioactivity (Figure 4E). The distribution of k8’−25’ values in the Lewy body group overlapped with the distribution in the non-Lewy body group (Figure 4F). Among the patients with non-Lewy body nOH, 1 had low free wall 18F-dopamine-derived radioactivity (Figure 4D), and 3 patients with non-Lewy body nOH had septal radioactivity within the range of values in the Lewy body group (Figure 4E).
Figure 4: Individual values for alpha-synuclein (AS) signal intensity or the AS/tyrosine hydroxylase (TH) colocalization index as a function of myocardial 18F-dopamine-derived radioactivity.
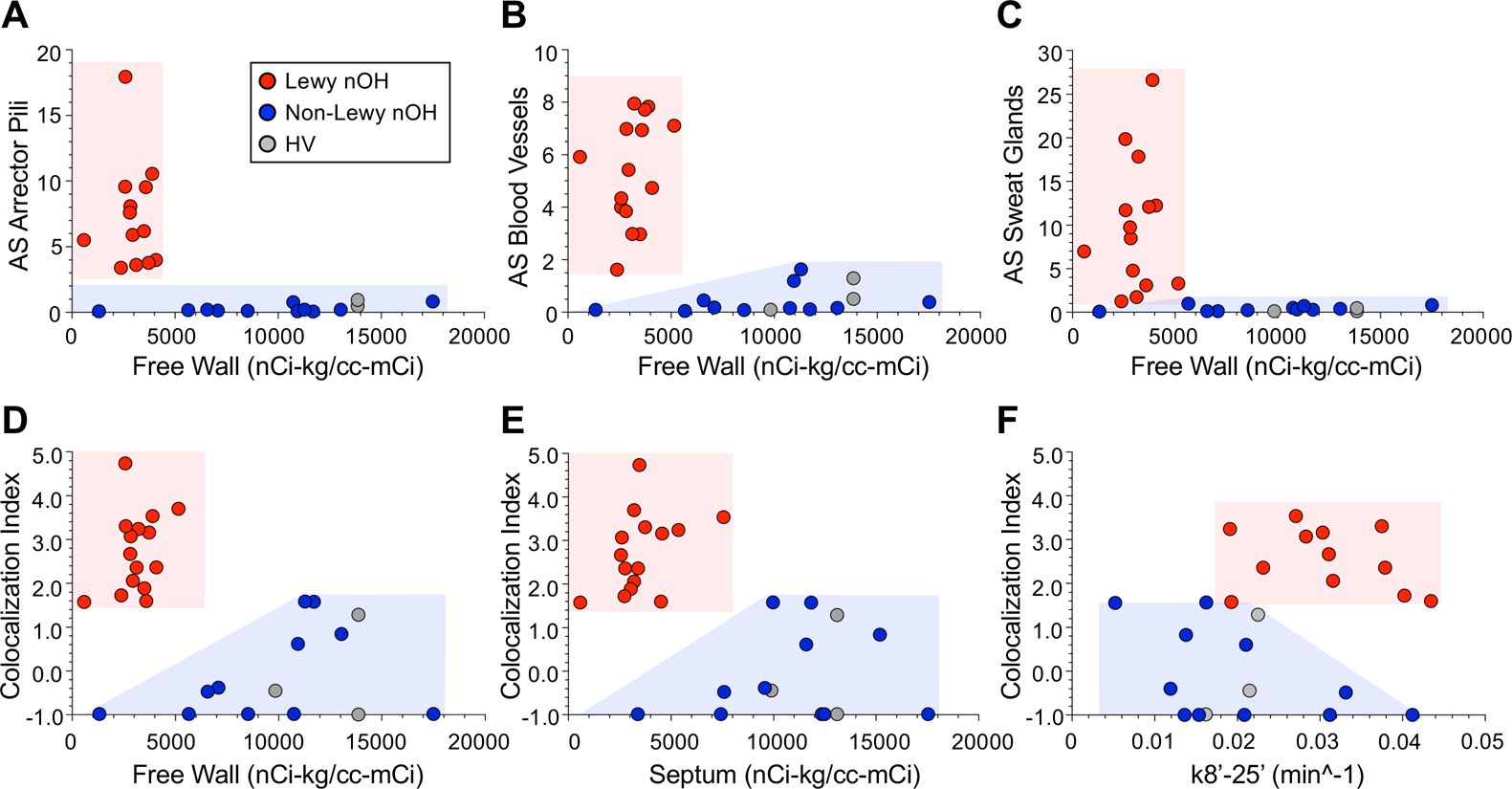
AS signal intensities are shown for regions of interest in arrector pili muscles (A), blood vessels (B), and sweat glands (C) and AS/TH colocalization indices in panels D-F. Panels A-D show data for 18F-dopamine-derived radioactivity in the left ventricular free wall and panel E radioactivity in the interventricular septum. Panel F shows data for the mono-exponential slope of decline of radioactivity between 8 and 25 minutes after initiation of tracer administration (k8’−25’). Red circles indicate patients with Lewy body forms of neurogenic orthostatic hypotension (nOH), blue circles non-Lewy body nOH, and gray circles healthy volunteers without either nOH or a Lewy body disease. Note increased AS signal intensities and AS/TH colocalization indices and low 18F-dopamine-derived radioactivity in all patients with Lewy body nOH.
When individual values for AS in the 3 skin constituents were expressed as a function of 18F-dopamine-derived radioactivity in the left ventricular free wall, the patients with Lewy body nOH were completely separated from the patients with non-Lewy body nOH (Figure 4A-C). All the Lewy body nOH patients had high AS/TH colocalization indices and low free wall and septal 18F-dopamine-derived radioactivity, a combination not seen in any of the non-Lewy body nOH patients (Figure 4D-E, p<0.0001).
Other clinical laboratory data
Scores on the UPSIT distinguished the Lewy body nOH from the non-Lewy body nOH groups (Table 2). Of the 16 patients in the former group, 10 (63%) were anosmic, a finding not observed in the latter group.
Of 14 patients in the Lewy body nOH group who had an MRI scan, cortical atrophy was reported in 12, whereas cortical atrophy was not reported in the 9 non-Lewy body nOH patients who had an MRI scan (Table 2). The Lewy body nOH group did not differ from the non-Lewy body nOH group in mean values for the putamen/occipital cortex ratio of 18F-DOPA-derived radioactivity, washout of putamen 18F-DOPA-derived radioactivity, or the posterior/anterior ratio of putamen 18F-DOPA-derived radioactivity (Table 2).
CSF levels of DHPG and NE were lower in the Lewy body nOH group than in the non-Lewy body nOH group (Table 2). The groups did not differ in mean CSF levels of DOPA or DOPAC. Plasma levels of catechols did not distinguish the two groups; however, mean plasma DHPG and NE levels were lower in the Lewy body nOH group than in the healthy controls (3.95 ± 0.34 vs. 8.50 ± 0.76 pmol/mL and 1.04 ± 0.11 vs. 3.56 ± 0.59 pmol/mL, p<0.0001 each).
Post-mortem data in nOH patients
A total of 7 patients with nOH (4 Lewy body, 3 non-Lewy body) had post-mortem neuropathologic confirmation of their diagnoses. Patient 1 in Table 1 carried a mutation of the gene encoding glucocerebrosidase. In this patient PAF had evolved into DLB+OH and PD+OH. Patient 2 also had PAF evolving into DLB+OH, with PD coming on late in the disease course. Although there was a strong family history of PD, as of this writing no genotypic abnormality has been identified. The patient had severe loss of immunoreactive TH and abundant AS fibrils in sympathetic ganglion tissue. Patient 3 had PAF clinically and by the clinical laboratory tests done at the NIH Clinical Center as described in the Methods section; post-mortem neuropathology showed AS-containing Lewy bodies in the substantia nigra. Patient 4, who was not studied at the NIH, had a clinical history of autonomic failure, cognitive changes, and progressive Parkinsonism. Scattered AS-positive neurites were noted in the substantia nigra, without AS-positive glial cytoplasmic inclusions.
Among the non-Lewy body nOH patients, Patient 5 in Table 1, who was not studied at the NIH, had a clinical and post-mortem neuropathologic diagnosis of MSA. The AS/TH colocalization index was normal post-mortem both in sympathetic ganglion tissue and in skin (data not shown). Patient 6 had a clinical and post-mortem neuropathologic diagnosis of MSA. At the NIH Clinical Center he had normal myocardial 18F-dopamine derived radioactivity (data not shown). Patient 7 had PAF clinically but without Lewy bodies in the brain or sympathetic ganglion tissue.39 The patient had normal AS/TH colocalization indices in both sympathetic ganglion tissue obtained post-mortem and in skin biopsies obtained during life.
DISCUSSION
Mechanisms linking Lewy body diseases with myocardial noradrenergic deficiency have been poorly understood. Here we report evidence for one such mechanism—AS deposition within sympathetic noradrenergic neurons. We found that AS/TH colocalization indices post-mortem in sympathetic ganglion tissue were associated with myocardial NE depletion and that AS/TH colocalization indices in vivo in skin biopsies were associated with neuroimaging evidence of cardiac sympathetic denervation and dysfunction in Lewy body nOH.
We validated and applied two approaches for quantitative analyses of sympathoneural AS deposition. The first was based on the fact that all three sympathetic noradrenergically innervated constituents in skin biopsies—arrector pili muscles, blood vessels, and sweat glands—can be delineated by SMA. Regions of interest were drawn based on the location of sympathetic noradrenergic fibers coursing parallel to SMA in arrector pili muscles, in the adventitia of blood vessels, and around secretory epithelial cells in sweat glands.
The second novel quantitative approach was calculation of the AS/TH colocalization index. We validated this index by analyzing post-mortem images of sympathetic ganglion tissue. Compared to control subjects and patients with non-Lewy body forms of nOH, patients with autopsy proven Lewy body diseases had high AS/TH colocalization indices in sympathetic ganglion tissue. The AS/TH colocalization index in skin biopsies was validated by comparing patients diagnosed with Lewy body vs. patients with rare non-Lewy body forms of nOH. All patients with Lewy body nOH had elevated AS/TH colocalization indices, in contrast with normal indices in most patients with non-Lewy body nOH and healthy controls without a Lewy body disease or nOH.
By both the regions of interest and AS/TH colocalization index methods, Lewy body forms of nOH were characterized by AS deposition in sympathetic noradrenergically innervated skin constituents. For all three constituents the patients with Lewy body nOH had many-fold higher AS signal intensities than did patients with non-Lewy body nOH and healthy controls. Since the AS/TH colocalization index data agreed with the regions of interest data for all three skin constituents (Supplementary Table), a substantial proportion of AS signal seems to reflect AS deposition within sympathetic noradrenergic nerves.
Previous studies described elevated AS levels in skin biopsies from patients with PAF,21, 46 PD,21, 23, 47 and DLB11 and normal levels in MSA,47 but without quantification of AS within sympathetic noradrenergic neurons. Our results agree generally with those in a recent report by Donadio et al. that Lewy body forms of synucleinopathy are associated with intra-neuronal AS deposition in in skin biopsies.11 That study used PGP 9.5, a non-specific axonal marker, whereas ours used TH, a specific marker of sympathetic noradrenergic neurons. A key advantage of the current study was the quantitative methodology used to measure intra-neuronal AS deposition.
Analyses of 18F-dopamine-derived radioactivity over time enabled assessments of cardiac sympathetic innervation and intra-neuronal catecholamine turnover in the same patients from whom skin biopsies were obtained. Peak radioactivity at about 8’ after initiation of the 3-minute infusion of the tracer is a measure of neuronal uptake and therefore an indirect measure of innervation; and the mono-exponential slope of subsequent decline in radioactivity (k8’−25’) is a measure of catecholamine turnover.18 In confirmation of previous reports17, 18 we found that patients with Lewy body forms of nOH had evidence for both denervation and increased turnover. We now report that these abnormalities are associated with increased AS deposition in cutaneous sympathetic noradrenergic nerves.
Limitations
The main limitations of this study relate to generalizability of the results and to the restricted focus on nOH. All the patients evaluated at the NIH Clinical Center were referred for possible autonomic synucleinopathy, were selected in terms of inclusion and exclusion criteria for one or more intramural NINDS research protocols, and agreed to undergo comprehensive inpatient research testing. The results from this study therefore might not apply to the broader population of patients with nOH. Because the study was designed to assess a specific mechanism—intra-neuronal AS deposition—that might link AS with sympathetic noradrenergic deficiency in nOH, whether increased AS/TH colocalization occurs generally in Lewy body diseases independently of nOH cannot be determined from the present data.
Perspectives
We report strong associations of AS deposition in sympathetic noradrenergic nerves in sympathetic ganglion tissue with myocardial NE depletion and of intra-neuronal AS deposition in skin biopsies with neuroimaging evidence of myocardial noradrenergic denervation and dysfunction in Lewy body forms of nOH. These associations raise the possibility that intra-neuronal AS deposition may play a pathophysiological role in sympathetic noradrenergic neurodegeneration.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1. What is New?
Cardiac noradrenergic deficiency and alpha-synucleinopathy are associated with Lewy body diseases. One factor linking these abnormalities may be alpha-synuclein deposition within sympathetic noradrenergic neurons. We validated and applied novel methodology to assess alpha-synuclein colocalization with tyrosine hydroxylase, a marker of sympathetic noradrenergic nerves, in neurogenic orthostatic hypotension.
2. What is Relevant?
In Lewy body diseases neurogenic orthostatic hypotension, supine hypertension, and myocardial noradrenergic deficiency frequently occur together, for reasons that have been obscure.
3. Summary:
We report strong associations between alpha-synuclein/tyrosine hydroxylase colocalization and myocardial noradrenergic denervation and dysfunction in Lewy body forms of neurogenic orthostatic hypotension. These associations raise the possibility that intra-neuronal alpha-synuclein deposition may play a pathophysiological role in sympathetic noradrenergic neurodegeneration.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
SOURCE OF FUNDING: The research reported here was supported by the Division of Intramural Research, National Institute of Neurological Disorders and Stroke.
Abbreviations:
- 18F-DA
18F-dopamine
- AAG
autoimmune autonomic ganglionopathy
- AAD
autoimmunity-associated autonomic failure and sympathetic denervation
- AS
alpha-synuclein
- CSF
cerebrospinal fluid
- DLB
dementia with Lewy bodies
- MRI
magnetic resonance imaging
- MoCA
Montreal Cognitive Assessment
- MSA
multiple system atrophy
- nMDP
normalized mean deviation product
- nOH
neurogenic orthostatic hypotension
- NE
norepinephrine
- OH
orthostatic hypotension
- PAF
pure autonomic failure
- PD
Parkinson disease
- PET
positron emission tomographic
- SMA
smooth muscle actin
- TH
tyrosine hydroxylase
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S): None
REFERENCES
- 1.Saedon NI, Tan MP and Frith J. The prevalence of orthostatic hypotension: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2018; doi: 10.1093/gerona/gly188. [DOI] [PMC free article] [PubMed]
- 2.Angelousi A, Girerd N, Benetos A, Frimat L, Gautier S, Weryha G and Boivin JM. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens 2014;32:1562–71; discussion 1571. [DOI] [PubMed] [Google Scholar]
- 3.Cheshire WP. Chemical pharmacotherapy for the treatment of orthostatic hypotension. Expert Opin Pharmacother 2018;1–13. [DOI] [PubMed]
- 4.Ziegler MG, Lake CR and Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med 1977;296:293–297. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, Nardin R and Freeman R. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation 2003;108:724–8. [DOI] [PubMed] [Google Scholar]
- 6.Palma JA, Norcliffe-Kaufmann L, Martinez J and Kaufmann H. Supine plasma NE predicts the pressor response to droxidopa in neurogenic orthostatic hypotension. Neurology 2018;91:e1539–e1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein DS and Sharabi Y. The heart of PD: Lewy body diseases as neurocardiologic disorders. Brain Res 2019;1702:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R and Goedert M. Alpha-synuclein in Lewy bodies. Nature 1997;388:839–40. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann H, Hague K and Perl D. Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology 2001;56:980–1. [DOI] [PubMed] [Google Scholar]
- 10.Papp MI, Kahn JE and Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 1989;94:79–100. [DOI] [PubMed] [Google Scholar]
- 11.Donadio V, Incensi A, El-Agnaf O, Rizzo G, Vaikath N, Del Sorbo F, Scaglione C, Capellari S, Elia A, Stanzani Maserati M, Pantieri R and Liguori R. Skin alpha-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study. Sci Rep 2018;8:14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein DS, Li ST and Kopin IJ. Sympathetic neurocirculatory failure in Parkinson disease: Evidence for an etiologic role of alpha-synuclein. Ann Intern Med 2001;135:1010–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton A, Gwinn-Hardy K, Sharabi Y, Li ST, Holmes C, Dendi R, Hardy J, Crawley A and Goldstein DS. Association between cardiac denervation and parkinsonism caused by alpha-synuclein gene triplication. Brain 2004;127:768–772. [DOI] [PubMed] [Google Scholar]
- 14.Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K and Takahashi H. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 2008;131:642–50. [DOI] [PubMed] [Google Scholar]
- 15.Isonaka R, Sullivan P, Jinsmaa Y, Corrales A and Goldstein DS. Spectrum of abnormalities of sympathetic tyrosine hydroxylase and alpha-synuclein in chronic autonomic failure. Clin Auton Res 2018;28:223–230. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Esler MD, Meredith IT, Dart A, Cannon RO 3rd, Quyyumi AA, Lambert G, Chin J, Jennings GL and Goldstein DS Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation 1992;85:1775–1785. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein DS, Holmes C, Kopin IJ and Sharabi Y. Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest 2011;121:3320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DS, Holmes C, Sullivan P, Mash DC, Sidransky E, Stefani A, Kopin IJ and Sharabi Y. Deficient vesicular storage: A common theme in catecholaminergic neurodegeneration. Parkinsonism Relat Disord 2015;21:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein DS, Sullivan P, Holmes C, Miller GW, Sharabi Y and Kopin IJ. A vesicular sequestration to oxidative deamination shift in myocardial sympathetic nerves in Parkinson disease. J Neurochem 2014;131:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ and Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem 2013;126:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donadio V, Incensi A, Piccinini C, Cortelli P, Giannoccaro MP, Baruzzi A and Liguori R. Skin nerve misfolded alpha-synuclein in pure autonomic failure and Parkinson disease. Ann Neurol 2016;79:306–16. [DOI] [PubMed] [Google Scholar]
- 22.Donadio V, Cortelli P, Elam M, Di Stasi V, Montagna P, Holmberg B, Giannoccaro MP, Bugiardini E, Avoni P, Baruzzi A and Liguori R. Autonomic innervation in multiple system atrophy and pure autonomic failure. Journal of neurology, neurosurgery, and psychiatry 2010;81:1327–35. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons CH, Garcia J, Wang N, Shih LC and Freeman R. The diagnostic discrimination of cutaneous alpha-synuclein deposition in Parkinson disease. Neurology 2016;87:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delic V, Chandra S, Abdelmotilib H, Maltbie T, Wang S, Kem D, Scott HJ, Underwood RN, Liu Z, Volpicelli-Daley LA and West AB. Sensitivity and specificity of phospho-Ser129 alpha-synuclein monoclonal antibodies. J Comp Neurol 2018;526:1978–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Otano J, Casanova-Molla J, Morales M, Valls-Sole J and Tolosa E. Cutaneous autonomic denervation in Parkinson’s disease. J Neural Transm 2015;122:1149–55. [DOI] [PubMed] [Google Scholar]
- 26.Donadio V, Incensi A, Cortelli P, Giannoccaro MP, Jaber MA, Baruzzi A and Liguori R. Skin sympathetic fiber alpha-synuclein deposits: a potential biomarker for pure autonomic failure. Neurology 2013;80:725–32. [DOI] [PubMed] [Google Scholar]
- 27.Wang N, Gibbons CH, Lafo J and Freeman R. alpha-Synuclein in cutaneous autonomic nerves. Neurology 2013;81:1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein DS and Cheshire WP Jr., Beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver. Clin Auton Res 2017;27:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein DS and Cheshire WP. Roles of catechol neurochemistry in autonomic function testing. Clin Auton Res 2018;3:273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein DS, Holmes C, Sharabi Y, Brentzel S and Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology 2003;60:1327–32. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein DS, Holmes C, Lopez GJ, Wu T and Sharabi Y. Cardiac sympathetic denervation predicts PD in at-risk individuals. Parkinsonism Relat Disord 2018;52:90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein DS and Sewell L. Olfactory dysfunction in pure autonomic failure: Implications for the pathogenesis of Lewy body diseases. Parkinsonism Relat Disord 2009;15:516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S and Eldadah BA. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord 2008;14:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein DS, Holmes C and Sharabi Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 2012;135:1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S and Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin Auton Res 2002;12:281–5. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein DS, Holmes C and Imrich R. Clinical laboratory evaluation of autoimmune autonomic ganglionopathy: Preliminary observations. Auton Neurosci 2009;146:18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DS, Holmes C, Sullivan P, Donadio V, Isonaka R, Zhong E, Pourier B, Vernino S, Kopin IJ and Sharabi Y. Autoimmunity-associated autonomic failure with sympathetic denervation. Clin Auton Res 2017;27:57–62. [DOI] [PubMed] [Google Scholar]
- 38.Sharabi Y, Dendi R, Holmes C and Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension 2003;42:110–6. [DOI] [PubMed] [Google Scholar]
- 39.Isonaka R, Holmes C, Cook GA, Sullivan P, Sharabi Y and Goldstein DS. Pure autonomic failure without synucleinopathy. Clin Auton Res 2017;27:97–101. [DOI] [PubMed] [Google Scholar]
- 40.Uno H Sympathetic innervation of the sweat glands and piloarrector muscles of macaques and human beings. J Invest Dermatol 1977;69:112–20. [DOI] [PubMed] [Google Scholar]
- 41.Schafer MK, Schutz B, Weihe E and Eiden LE. Target-independent cholinergic differentiation in the rat sympathetic nervous system. Proc Natl Acad Sci U S A 1997;94:4149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saga K Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem 2002;37:323–86. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Li H, Chen L, Fang S, Xie S and Lin C. Three-dimensional reconstructed eccrine sweat glands with vascularization and cholinergic and adrenergic innervation. J Mol Histol 2018;49:339–345. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein DS, Holmes C, Cannon RO 3rd, Eisenhofer G and Kopin IJ Sympathetic cardioneuropathy in dysautonomias. N Engl J Med 1997;336:696–702. [DOI] [PubMed] [Google Scholar]
- 45.Jaskolski F, Mulle C and Manzoni OJ. An automated method to quantify and visualize colocalized fluorescent signals. J Neurosci Methods 2005;146:42–9. [DOI] [PubMed] [Google Scholar]
- 46.Shishido T, Ikemura M, Obi T, Yamazaki K, Terada T, Sugiura A, Saito Y, Murayama S and Mizoguchi K. alpha-synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology 2010;74:608–10. [DOI] [PubMed] [Google Scholar]
- 47.Zange L, Noack C, Hahn K, Stenzel W and Lipp A. Phosphorylated alpha-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain 2015;138:2310–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


