Abstract
Objective.
To investigate roles of the synovial lymphatic system in tissue damage, macrophage subsets, and the therapeutic potential of proteasome inhibitor Bortezomib (Btz) in a mouse model of experimental post-traumatic OA (PTOA).
Methods.
C57BL/6J wild type mice received a meniscal-ligamentous injury to induce PTOA. Lymphangiogenesis was blocked by a vascular endothelial growth factor receptor 3 (VEGFR3) neutralizing antibody. Synovial lymphatic drainage was examined by near-infrared imaging. Joint damage was assessed by histology. RNA-seq and pathway analysis were applied to synovial lymphatic endothelial cells (LECs). Macrophage subsets were identified by flow cytometry and immunofluorescence staining. M1 and M2 macrophages were induced from bone marrow cells and their effects on LECs were examined in co-cultures ± Btz. The effects of Btz on joint damage, LECs and synovial lymphatic drainage were examined.
Results.
VEGFR3 neutralizing antibody reduced synovial lymphatic drainage and accelerated tissue damage in PTOA joints. Synovial LECs from OA joints had dys-regulated inflammatory pathways and expressed high levels of inflammatory genes. M1 macrophages were increased in PTOA joints and promoted expression of inflammatory genes by LECs, which was blocked by Btz. Btz decreased cartilage loss and expression of inflammatory genes by LECs, and improved lymphatic drainage in PTOA joints.
Conclusion.
Experimental PTOA is associated with decreased synovial lymphatic drainage, and increased M1 macrophages and inflammatory gene expression by LECs, which is improved by Btz. Intra-articular administration of Btz may present a new therapy for PTOA treatment, which is associated with improved synovial lymphatic function.
Keywords: macrophages, lymphatic, inflammation, post-traumatic murine osteoarthritis, Bortezomib
INTRODUCTION
Osteoarthritis (OA) is characterized by the degeneration of articular cartilage, subchondral bone sclerosis, angiogenesis and synovitis. Increasing evidence indicates that not only cartilage, but also surrounding soft tissues (referred to as synovium in this study), contribute to OA pathogenesis. Catabolic factors, including pro-inflammatory cytokines, immune cells and proteases, are detected in OA synovium and play a critical role on cartilage degeneration (1, 2). How these factors are removed from the OA synovium has not been well studied.
The lymphatic system plays an important role in maintaining metabolic and tissue fluid homoeostasis. Interstitial fluid, immune cells, proteins and lipids are drained by lymphatic capillaries from interstitial space. They are then transported to lymph nodes, to the thoracic duct and finally to the bloodstream via collecting lymphatic vessels (3). Lymphatic capillaries are comprised of a thin layer of lymphatic endothelial cells (LECs). Collecting lymphatic vessels are covered by one or more layers of lymphatic muscle cells that enable vessel contraction moving the lymph against gravity (4). We reported that the mouse knee contains a synovial lymphatic system that drains intra-articular injected near-infrared dye to iliac lymph nodes. This synovial lymphatic drainage is reduced in joints with meniscal-ligamentous injury (MLI)-induced post-traumatic OA (PTOA), a mouse model of experimental OA (5). However, it is not known if reduced synovial lymphatic drainage is a consequence of PTOA or vice versa.
The function of lymphatic vessels is affected differently by the duration of inflammation (6). Increased lymphangiogenesis, lymphatic vessel contraction and lymph flow occur in areas with acute inflammation. In contrast, reduced lymphatic vessel contraction and lymph flow occur in areas with chronic inflammation. This may be related to high levels of inducible nitric oxide synthase (iNOS) produced by macrophages and LECs. iNOS directly affects lymphatic muscle cells via excess nitric oxide (7, 8). We reported that in mice carrying the tumor necrosis factor transgene (TNF-Tg), a model of rheumatoid arthritis (RA), macrophages are attached to LECs in the collecting lymphatic vessel that drains inflamed joints (9). However, whether macrophages affect LEC function directly remains to be determined. Effective lymphatic drainage promotes inflammation resolution.
Macrophages are plastic cells. Quiescent macrophages respond to different stimuli in vitro and polarize to M1 (pro-inflammatory) and M2 (anti-inflammatory) states at opposite ends of the macrophage phenotype spectrum. M1s and M2s have distinct gene expression profiles (10). M2s promote tumor lymphangiogenesis by producing vascular endothelial growth factor C (VEGF-C), the growth factor for LECs (11). Synovium of OA patients contains both M1s and M2s (12), but the role of these distinct macrophage populations in lymphatics under non-tumor conditions, such as OA, has not been studied.
Bortezomib (Btz) is a proteasome inhibitor that is FDA approved for treating patients with multiple myeloma (13). Btz inhibits pro-inflammatory cytokines in T cells of RA patients (14) and joint inflammation and bone erosion in adjuvant-induced RA mice (15). It suppresses TNF-induced type II collagen degradation and expression of matrix metalloproteinase 13 (MMP13) in human chondrocytes (16). MG132, a proteasome inhibitor that is used for biological research, reduces cartilage loss in surgery-induced OA models in a prevention protocol (17, 18). However, if proteasome inhibition has a therapeutic role in OA, or if it is associated with an improvement of synovial lymphatics is unknown.
We hypothesize that the progression of PTOA is associated with lymphatic vessel inflammation that is caused by M1s. This leads to reduced synovial lymphatic drainage and facilities joint tissue damage, which can be attenuated by intra-articular administration of Btz. We predict that 1) blocking lymphatic function will accelerate OA tissue damage, 2) OA synovial LECs will express high levels of inflammatory genes, 3) OA synovium will have more M1s and M1s will affect LEC function, and 4) Btz will reduce tissue damage, LEC inflammation, and improve synovial lymphatic drainage. In this study, we used MLI-induced PTOA mouse model and demonstrated an association among M1s, LECs and synovial lymphatic drainage in OA joints and therapeutic potential of Btz.
MATERIALS AND METHODS
Experimental PTOA mouse model.
Three month-old C57BL/6J male mice were subjected to meniscal-ligamentous injury (MLI) surgery to induce PTOA according to a standard operation procedure (SOP) established in the Center for Musculoskeletal Research Center (CMSR) (5, 19). In brief, a 5-mm incision was made on the medial aspect of the right joint and the medial collateral ligament was transected to open the joint space. The medial meniscus was detached from its anterior attachment to the tibia, and the anterior half of the detached medial meniscus was removed. In sham surgery, a 5-mm skin incision was made on the medial aspect of the left joint. Mice received Buprenorphine SR to control pain.
Two experiments were conducted. 1) VEGFR3 neutralizing antibody (Ab) (mF4–31C1 by ImClone, New York) was used. MLI mice were treated with VEGFR3 Ab (0.8mg/kg/intraperitoneal (i.p) injection) or IgG, starting at day 3 post-surgery, 3 times/week for 6 weeks, based on a regimen used in TNF-Tg mice (20). 2) MLI mice were subjected to ultrasound imaging at 4 weeks post-surgery to obtain synovial volume (21, 22) and were randomized into 2 groups based on synovial volume: Btz (0.25mg/ml in 5μl/intra-articular (i.a.) injection) or Vehicle (Veh, 1% DMSO) weekly for 7 weeks. All animal use in this study has been approved by the Animal Care and Use Committee at the University of Rochester.
Near-infrared (NIR) indocyanine green (ICG) lymphatic imaging.
NIR-ICG imaging was performed by i.a. injecting 5μl (0.1μg/μl) of ICG (Akorn) into mouse knee joints according to a CMSR SOP (5, 23, 24). The dynamics of ICG fluorescence over the entire leg were visualized using an NIR imaging system. Briefly, the target area was excited with NIR illumination provided by a tungsten halogen nondichroic MR16 light bulb. A fluorescence emission filter was placed behind the lens and in front of a high-sensitivity 1.4-megapixel CCD camera sensor. The camera, NIR excitation and background illumination were controlled using software developed in the LabView programming environment. Synovial lymphatic drainage as indicated by the percentage of ICG clearance was assessed by calculating the percentage difference of ICG signal intensity between the 3- and 6-hour NIR scans from the region of interest (ROIs) in the knee joint after ICG injection (24).
Histology and staining.
Decalcified knee samples were used. For paraffin sectioning, 30 consecutive 4-μm-thick sections were collected and evenly divided to 3 levels. One section from each of the 3 levels was stained with Alcian blue/Hematoxylin/Orange G (AB/OG). For frozen sectioning, 10 consecutive 7-μm-thick sections were collected. Section #4 or #5 was stained with H&E, an adjacent section was subjected to double immunofluorescence staining for lymphatic vessels and macrophage subsets using primary Abs, including podoplanin (PDPN, 1:200 Abcam) for LECs, F4/80 (1:50 BioLegend) for pan macrophages, iNOS (1:50 Santa Cruz) for M1s or CD206 (1:100 R&D) for M2s. Stained sections were scanned with an Olympus VS120 whole slide imager (5). For 3 dimensional (3D) reconstruction, two 30-μm-thick frozen sections were stained with anti-F4/80/iNOS/PDPN or F4/80/CD206/PDPN Abs, respectively, and were scanned with an Olympus FV1000 confocal microscope to collect a series of 20–25 images. Images were imported to Amira6.0 software for 3D reconstruction.
Histomorphometric analysis.
1) Joint tissue damage was assessed on AB/OG-stained sections using an CMSR SOP, including a modified OARSI score, synovitis score and osteophyte numbers (5, 19). Three sagittal joint sections from 3 levels were used for assessments by 4 blinded independent observers. The average score of 3 levels from each observer was calculated. Average scores of all individual observers were used. Inter-rater agreement for each score was evaluated via calculation of Fleiss’ Kappa co-efficient (25). The results indicate that there is no significant inter-observer variation (data not shown). For the OARSI score, measurements of tibial and femoral surfaces were combined as one value. For synovitis score, a subjective scoring system of 0 to 2 was used: 0 = a synovial lining that has 2–3 cell layers or <10μm (normal), 1 = synovial thickening with 5–10 cell layers or 10–20μm thick, 2 = severe thickening of the synovium with >10 cell layers or >20μm thick. For osteophyte, the number of osteophytes protruding from tibial or femoral surface to the joint space was counted. 2) Area of F4/80+ (pan-macrophages), F4/80+/iNOS+ (M1s), F4/80+/CD206+ (M2s) or PDPN+ (lymphatic vessels) was analyzed with ImageProPlus software. The synovial area was segmented manually into ROIs. The positive staining area was calculated as the ratio of positive staining pixels to total pixels: green pixels for pan-macrophage and yellow pixels for M1s or M2s.
LECs.
The mouse LEC line was provided by Dr. S. Ran, which was generated from benign lymphangiomas induced by Freund’s adjuvant (26). Primary LECs were isolated from synovium and surrounding soft tissues as described (Supplemental figure 1)(27). In brief, knee joints with whole tibia and femur were digested with 1 mg/ml collagenase I. Cells were incubated with phycoerythrin (PE)-conjugated anti-mouse PDPN Ab (Biolegend), resuspended in anti-PE microbeads (Miltenyi Biotec), and loaded on an MS Column. PDPN+ cells were eluted with 1 ml MACS buffer. For characterization, cells were stained with PE-conjugated anti-mouse PDPN Ab combined with biotin-conjugated anti-mouse Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (LYVE-1, eBioscience) or goat anti-mouse VEGFR3 Ab (R&D) followed by Texas red-conjugated streptavidin (BD Biosciences) or Alexa flour 488-conjugated anti-goat Ab. Cells were subjected to flow cytometry (BD LSRII 12 color). Results were analyzed by FlowJo7 software. We referred to these PDPN+ cells as synovial LECs.
RNA sequencing, pathway analysis, and quantitative real time PCR (qPCR).
Total RNA was extracted using an RNeasy Mini Kit following the manufacturer’s protocol (Qiagen). RNA sequencing was performed with the Illumina HiSeq2500 high-throughput DNA sequencer (Illimina). Over 1000 differentially expressed genes between OA LECs and sham LECs (fold change ≥1.5 or ≤0.75) were identified. Each data from OA and its counterpart control was independently analyzed by Ingenuity Pathways Analysis (IPA) (Qiagen). IPA analysis focusing on canonical pathways, biological functions and diseases were performed. QPCR was performed by using iQ SYBR Green supermix and an iCycler PCR machine using sequence specific primers (Supplemental table). Fold changes of genes of interest were calculated use control samples as 1.
Cell cultures.
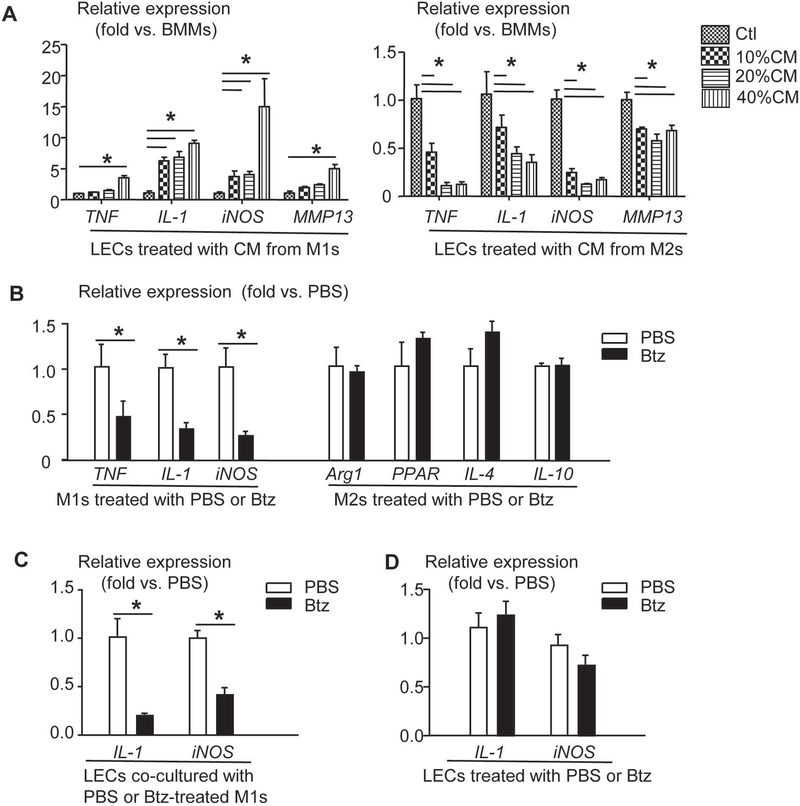
1) Generation of M1s and M2s: bone marrow (BM) cells were isolated from WT mouse tibiae and femora and were cultured with M-CSF for 3 days to generate BM macrophages (BMMs). M1s were induced by 20 ng/mL IL-1 and M2s were induced by 20 ng/mL IL-4. Their phenotypes were confirmed by immunostaining for F4/80+/iNOS+ M1s and F4/80+/CD206+ M2s as described (28)(Supplemental figure 2). 2) The effect of macrophage conditioned medium (CM) on LECs: CM was collected from M1s or M2s. LECs from a murine LEC cell line were treated with 10%, 20% and 40% of CM. Expression of inflammatory genes in LECs were examined by qPCR. 3) The effect of Btz on M1s or M2s. M1s or M2s were treated with Btz, and expression of M1 or M2 effector genes was determined by qPCR, respectively. 4) The effect of Btz on M1-mediated LEC inflammatory gene expression. BMMs on coverslips were induced to M1s and were treated with PBS or Btz. LECs were cultured on plastic dishes separately. Coverslips with PBS or Btz-pre-treated M1s were transferred to LEC dishes, co-cultured and then removed. Expression of inflammatory genes in LECs that left on plastic dishes was examined by qPCR. 5) The effect of Btz on LECs. LECs were treated with PBS or Btz and expression of inflammation genes was assessed by qPCR.
Western blot analysis.
Btz and Veh were i.a. injected into the right or the left joint, respectively. Mice were sacrificed 4 hours later. Synovial tissues and cortical bone of femur were harvested after removal of BM. Proteins were extracted using Ubiquitination Lysis Buffer. Total ubiquitinated proteins were determined by Western blot using an anti-Ubiquitin Ab (Santa Cruz). β-actin was used as a loading control.
Statistical analysis.
All experiments were performed at least twice with similar results. Data are presented as mean ± S.D. An unpaired student t-test was used to compare the 2 groups. One-way ANOVA followed by Tukey post-hoc test was used to compare more than 2 groups. An X-Y linear regression test was used for the determination of any correlation between ICG clearance and OARSI score. P values <0.05 were considered to be statistically significant.
RESULTS
Blockage of lymphangiogenesis worsens experimental OA pathology.
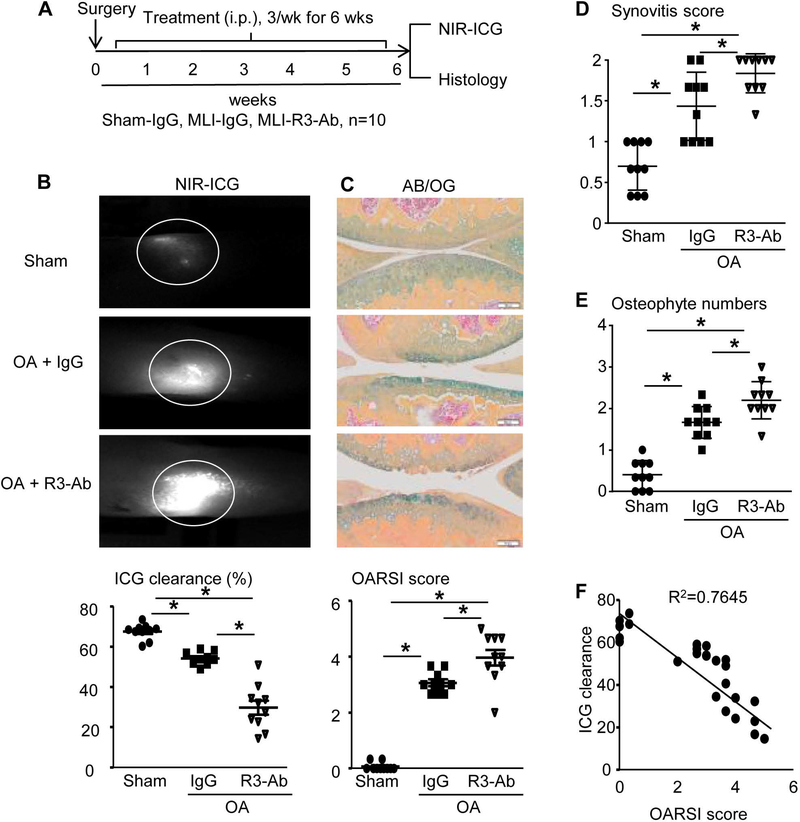
To investigate the cause-effect relationship between lymphatics and OA, we used a VEGFR3 neutralizing Ab to block lymphangiogenesis (20) in MLI-induced PTOA mice. Three groups of mice (n=10 mice/group) were used, including IgG-treated sham or PTOA mice, and VEGFR3 Ab-treated PTOA mice. The treatment (i.p.) was started at day 3 post-surgery, 3 times/week for 6 weeks. Synovial lymphatic drainage and joint tissue damage was assessed (Figure 1A)(5). Knees from IgG-treated MLI mice had a lower percentage of ICG clearance than sham mice, which became significantly worse in VEGFR3 Ab-treated mice (Figure 1B). Histology showed that the MLI procedure caused cartilage loss and fibrillation in joints, which was more severe in VEGFR3 Ab-treated mice. Morphometric analysis revealed higher OARSI score, synovitis and osteophyte formation in IgG-treated MLI joints than in sham joints, which was worse in VEGFR3 Ab-treated MLI joints (Figure 1C–E). There is a strong correlation between ICG clearance and OARSI score (R2=0.7645) (p<0.001) (Figure 1F). These data indicate that blocking lymphangiogenesis reduces lymphatic drainage and accelerates tissue damage in PTOA joints, and that the synovial lymphatic system may play an active role in PTOA progression.
Figure 1. Inhibition of lymphangiogenesis via VEGFR3 blockade exacerbates cartilage destruction in PTOA joints.
(A) Schematic illustration of the experimental design in which WT C57BL/6J mice received MLI or sham surgery, and on day 3 post-surgery were randomized to treatment with anti-VEGFR3 neutralizing antibody (R3-Ab, 0.8 mg/kg, i.p. 3 times/week) or IgG (placebo) for 6 weeks. (B) Mice were subjected to NIR-ICG imaging to quantify synovial lymphatic drainage, and representative images of the ICG remaining in the knee 24hr post-injection (circled region) are shown to illustrate the lack of lymphatic clearance in OA + R3-Ab treated mice. (C) Paraffin sections of knee joints were stained with AB/OG for OARSI scores, and representative micrographs are shown at 10× (bar = 0.1mm). The synovitis score (D) and osteophyte numbers (E) are presented as the mean +/− SD, n=10 mice per group (*p<0.05 via One-way ANOVA followed by Tukey). (F) The relationship between ICG clearance and OARSI score was determined by linear regression analysis (p<0.05 via Pearson’s correlation coefficient; n=30).
Primary LECs from synovium of PTOA joints has an inflammatory phenotype.
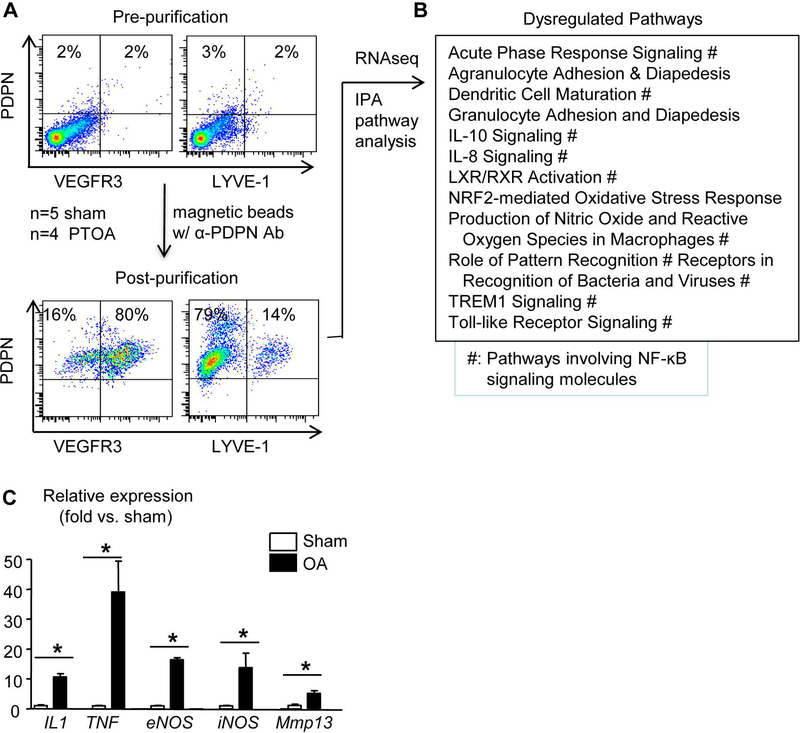
Lymphatic vessels are localized in synovium and soft tissues, mainly around the joint capsule, ligaments, fat pads, and muscles of a normal mouse knee (5). To determine if LECs are changed in the PTOA microenvironment, we purified LECs from these tissues with an anti-PDPN Ab (Supplemental figure 1) (27). Flow cytometric analysis (Figure 2A, n=6 mice) revealed that 80% of PDPN+ cells expressed VEGFR3, a commonly used specific LEC marker (29), and 14% of them expressed LYVE-1, another LEC marker that is known to be expressed by a portion of LECs (30–32). RNA sequencing of synovial LECs from sham (n=5 mice) and OA (n=4 mice) joints revealed approximately 1000 differentially expressed genes. IPA analysis revealed that these genes are involved in 12 dysregulated pathways, 9 of which contain an NF-κB signaling signature (Figure 2B). Since NF-κB regulates expression of a variety of pro-inflammatory genes, we examined the expression levels of IL-1, TNF, endothelial nitric oxide synthase (eNOS), and iNOS in synovial LECs from a separate set of mice that received MLI and sham surgery (n=5 mice/group). We included MMP13 in this panel because it plays a critical role in cartilage degeneration in PTOA. OA synovial LECs had markedly increased expression of inflammatory genes (fold change over sham: IL-1=10.58±1.16, TNF=39.05±10.35, eNOS=16.34±0.78, iNOS=13.78±4.87), while increased MMP13 expression was moderate (fold change over sham: 4.45±0.44) (Figure 2C). These data suggest that LECs in the synovium of experimental PTOA express high levels of inflammatory genes, which we referred to as LEC inflammation.
Figure 2. Lymphatic endothelial cells in PTOA synovium have an inflammatory phenotype.
Mice received MLI or sham surgery as in Fig.1. (A) LECs were isolated from synovium at 6 weeks post-surgery with anti-PDPN antibody, and the enrichment of this PDPN+ population was confirmed by flow cytometry (n=6 mice). (B) RNAseq was performed on the PDPN+ cells, which revealed about 1,000 differentially expressed genes in PTOA vs. sham synovial LECs. Pathway analysis indicated 12 dysregulated pathways (# indicates pathways involving NF-κB signaling; n=4–5 mice/group). (C) Synovial LECs from a different cohort of PTOA mice at 6 weeks post-surgery were subjected to qPCR. Data are mean + SD in which the Sham expression level = 1 (n=5 mice/group; *p<0.05 via unpaired t test).
Experimental PTOA is associated with increased M1 macrophages.
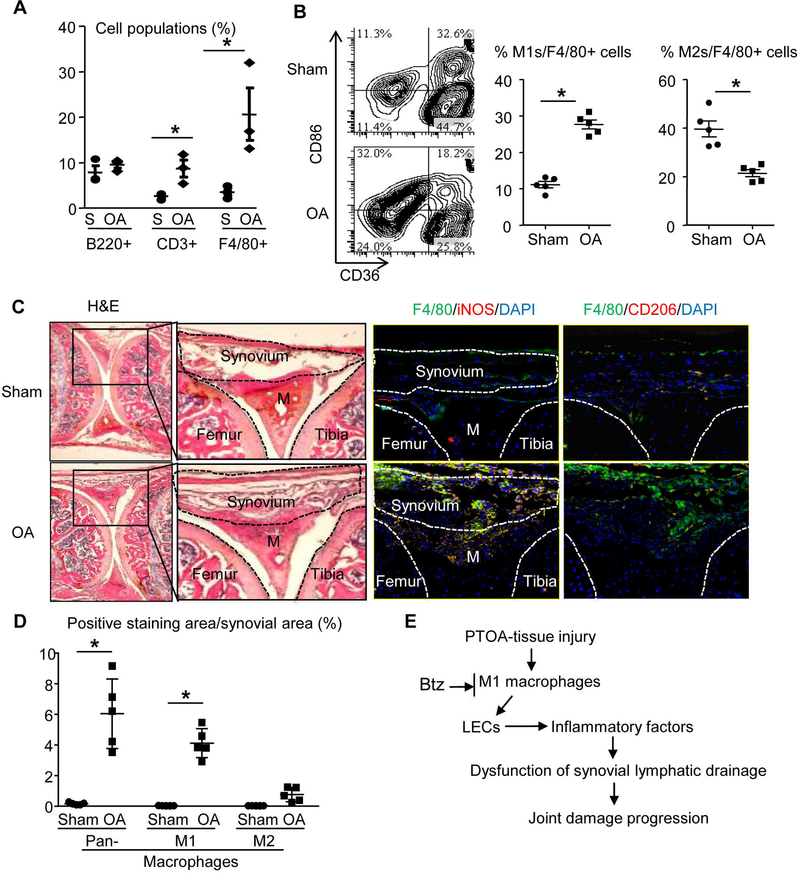
We searched potential cellular mechanisms for LEC inflammation in PTOA joints by examining cell populations in OA vs. sham joints using flow cytometry. Cells from OA synovium had a significant increase in CD3+ T cells and F4/80+ macrophages (Figure 3A). We focused on macrophages due to their high numbers and known role in OA (12) and chronic inflammation. Macrophages are classified into inflammatory M1 or anti-inflammatory M2 subsets (10). Using F4/80+/CD86+/CD36- as M1 and F4/80+/CD86-/CD36+ as M2 cell markers, we found that synovial cells from PTOA mice contained more M1s, but not M2s (Figure 3B). Immunostaining confirmed a markedly increased total of F4/80+ and F4/80+/iNOS+ M1s, but not F4/80+/CD206+ M2s in OA synovium (Figure 3C&D). Based on these data, we hypothesize that in PTOA synovium, M1s cause LEC inflammation, leading to reduced synovial lymphatic drainage and the progression of tissue damage. This process can be attenuated by i.a. administration of Btz (Figure 3E).
Figure 3. Increased M1 macrophages in synovium of PTOA joints. Mice received MLI or sham surgery as in Fig.1, and knee joints were harvested at 6 weeks post-surgery for flow cytometry and histology.
(A) Total synovial cells were subjected to flow cytometry to determine the percentage of B cells (B220+), T cells (CD3+) and macrophages (F4/80+) (*p<0.05; n=3). (B) The % of M1s (CD86+CD36-) or M2s (CD86-CD36+) in total F4/80+macrophages are presented with the mean (*p<0.05; n=5). (C) Frozen sections were stained for H&E, or pan-macrophages (F4/80+, green) plus M1 marker (iNOS+, red) or M2 marker (CD206+, red). Low magnification images (2×) show the region of interests, and inserts of higher magnification images (10×) are shown to illustrate the marked increase in F4/80+/iNOS+ M1s (yellow), but not M2s in PTOA synovium (M = Meniscus). (D) Quantification of F4/80+ macrophages, F4/80+/iNOS+ M1s, and F4/80+/CD206+ M2s (n=5 mice/group; *p<0.05 via Unpaired t test). (E) Proposed model of the association between M1s and LECs in PTOA.
M1 macrophages promote expression of inflammatory factors by LECs, which is blocked by the proteasome inhibitor Bortezomib.
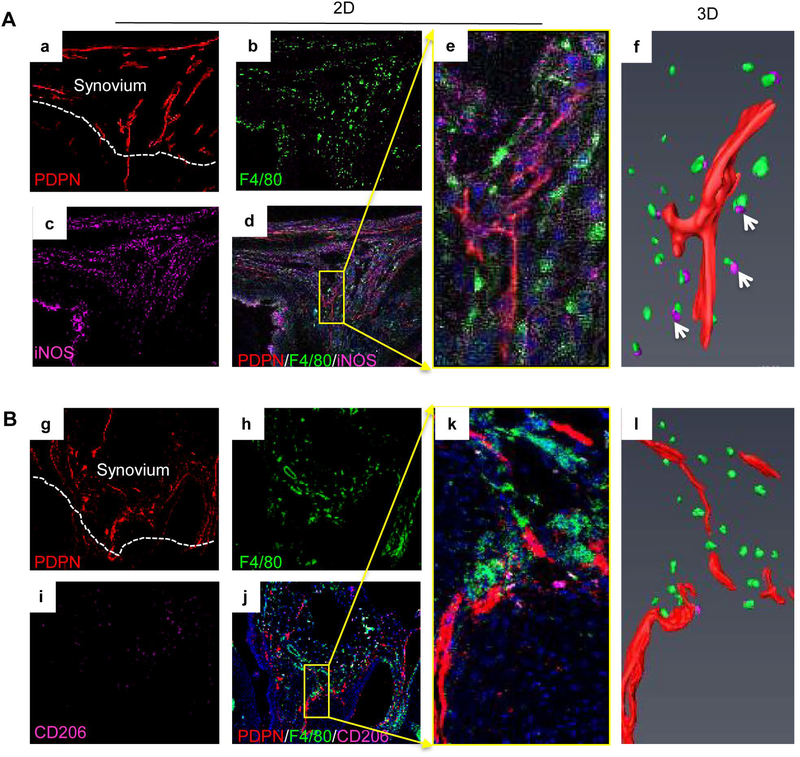
To test if M1s affect LECs in PTOA synovium as proposed in Figure 3E, the potential spatial relationship between lymphatic vessels and macrophage subsets was examined by immunostaining thick sections with Abs for M1s, M2s and LECs. 3D images revealed numerous lymphatic vessels surrounded by M1s, but not M2s (Figure 4A&B, n=4 mice). LECs (a mouse LEC line) were treated with CM from BMMs, M1s or M2s, and expression of inflammatory genes by LECs was examined. Compared to BMM CM, M1 CM increased IL-1 and iNOS levels significantly, but the effect on TNF and MMP13 levels was minimal. In contrast, M2 CM decreased expression of the above genes (Figure 5A). Btz, a clinically used proteasome inhibitor, inhibits inflammation by preventing proteasomal degradation of the negative regulators of NF-κB (14). We found that Btz decreased M1 effector gene expression in M1s, but had no effect on M2 effector gene expression in M2s (Figure 5B). Similar to M1 CM, M1s increased inflammatory gene expression by LECs when they were co-cultured together. This was abolished if M1s were pre-treated with Btz (Figure 5C). Impressively, Btz did not inhibit IL-1-induced inflammatory gene expression if it was added to directly LECs (Figure 5D). These data suggest that Btz inhibits M1-mediated LEC inflammation by mainly affecting M1s, but not LECs.
Figure 4. M1 macrophage accumulation adjacent to lymphatic vessels in PTOA synovium.
Frozen sections (30 μm thick) of knees at 5 weeks post-MLI were immuno-stained with antibodies against PDPN (red) for lymphatic vessels (a), F4/80 (green) for pan macrophages (b), iNOS or CD206 (purple) for macrophage subsets (c). M1s (A) were defined as F4/80/iNOS+ (a-d), and M2s (B) were defined as F4/80/CD206+ cells (g-l). Confocal microscopy was used for z-section imaging to obtain 20–25 consecutive images (e and k) with a step-width of 1μm. PDPN+ lymphatic vessels (red) and M1s or M2s (purple) were detected by Amira to generate 3D images (f and l) in a SurfaceGen module. Arrows in f indicate M1 cells near lymphatic vessels in a 3D image (n=4 mice).
Figure 5. M1 macrophages promote expression of inflammatory genes by LECs, which is inhibited by Bortezomib.
(A) WT BMMs were treated with IL-1 or IL-4 to induce M1s or M2s, respectively. Cells were cultured for 24 hours to generate conditioned media (CM). LECs from a murine LEC cell line were treated with different concentrations of CM for 24 hours. Expression of inflammatory genes in LECs was determined by qPCR (data are mean +/− SD in which Ctl = 1; *p<0.05 via ANOVA; n=3). (B) M1s or M2s were treated with PBS or Btz for 8 hours. Expression of M1 or M2 effector genes was examined by qPCR (data are mean +/− SD in which PBS = 1; *p<0.05 via Unpaired t test; n=3). (C) M1s were pre-treated with Btz for 8 hours, and then co-cultured with LECs for 24 hours. Expression of inflammatory genes in LECs was determined by qPCR (data are mean +/− SD in which PBS = 1; *p<0.05 via Unpaired t test; n=4). (D) LECs were treated with Btz for 8 hours. Expression of inflammatory genes was determined by qPCR (data are mean +/− SD in which PBS = 1; n=3).
Bortezomib reduces expression of inflammatory genes by LECs, joint tissue damage, and improves synovial lymphatic function in experimental PTOA.
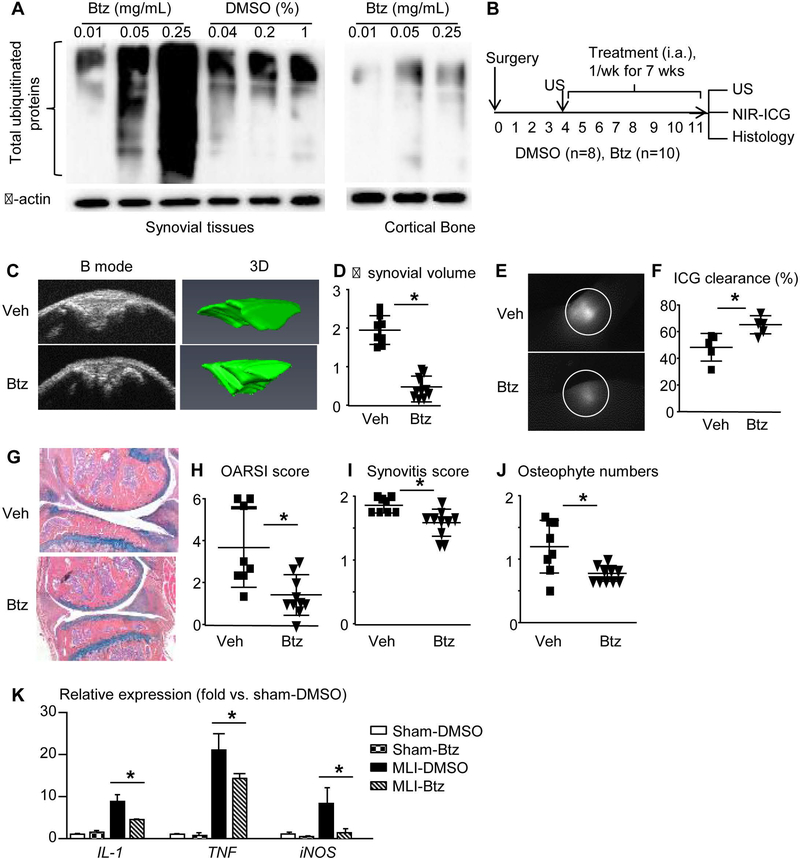
Btz is a drug for treating multiple myeloma. It is given to patients by intravenous or subcutaneous administration. We used i.a. injection of Btz because this route of administration could act mainly within joints at a high local concentration. We demonstrated that Btz, at a dose of 0.25mg/kg, is optimal to inhibit proteasomal degradation of ubiquitinated proteins in synovial tissue (Figure 6A). An intervention protocol was used, with treatment starting at 4 weeks post-MLI. Before the treatment, we performed knee ultrasound to obtain synovial volume (21, 22) and randomized mice into Btz (n=10 mice) and Veh (n=8 mice) groups based on their synovial volume. Mice received a weekly injection for 7 weeks. Mice were subjected to knee ultrasound, and NIR-ICG examination before being sacrificed. Knees were harvested for histology (Figure 6B). Ultrasound imaging showed increased synovial volume by 1.95±0.35 fold in Veh-treated joints. In contrast, increased synovial volume was much less (0.48±0.28 fold) in Btz-treated joints (Figure 6C&D). NIR-ICG lymphatic imaging revealed that Veh-treated joints had significantly reduced ICG clearance (48%±9%), which was restored to 65%±6% by Btz treatment (Figure 6E&F). Histological analyses revealed decreased OARSI score, synovitis score, and osteophyte numbers in Btz-treated joints (Figure 6G–J). To determine if Btz reduces LEC inflammation, we treated sham or MLI mice with i.a. administration of Btz or Veh and compared the expression levels of inflammatory genes by synovial LECs among sham-Veh vs. sham-Btz, and OA-Veh vs. OA-Btz groups. Btz had no effect on sham LECs, but it significantly reduced IL-1, TNF, iNOS, and MMP13 levels in OA LECs (Figure 6K). These data indicate that Btz attenuates joint tissue damage and LEC inflammation, and improves synovial lymphatic drainage in mice with experimental PTOA.
Figure 6. Bortezomib attenuates tissue damage in PTOA joints, improves synovial lymphatic function, and reduces LEC inflammation.
(A) WT mice were used. Different doses of Btz or 1% DMSO (Veh) were i.a. injected to knee joint. Levels of total ubiquitinated proteins in synovial tissues were examined by Western blot at 4 hours post-injection. Cortical bone from femur of the same joint was used as a control. (B) Schematic illustration of the experimental design in which WT C57BL/6J mice received MLI surgery, and 4 weeks later were randomized to DMSO or Btz (1× per week intraarticular injection for 7 weeks). (C) Ultrasound images of B-mode and 3D reconstruction indicate synovial volume at the end of treatment. (D) Changes of synovial volume (mm3) pre- and post-treatment. (E) ICG signal intensity of knee joints 6 hours post-ICG administration. (F) ICG clearance (%). (G) AB/OG stained sections. (H-J) OARSI score, synovitis score, and osteophyte numbers. Data are mean + SD. n=8–10 mice/group. Unpaired t test. *p<0.05. (H) LECs from synovium pooled from 4 joints were subjected to qPCR. Data are mean + SD. n=3 repeats. The fold-changes were calculated using samples from sham-Veh group as 1. Unpaired t test. *p<0.05
DISCUSSION
OA is associated with high levels of catabolic factors and inflammatory cells in the joint space and the soft tissues surrounding the joint. How these factors are cleared has not been well studied. Our previous study reported reduced lymphatic drainage in joints of mice with MLI-induced PTOA following i.a. administration of the NIR dye ICG (5), suggesting an association between the synovial lymphatic drainage and PTOA. However, we do not know why lymphatic drainage is reduced or whether lymphatic vessel dysfunction is a sole consequence of inflammation, or if the lymphatics plays an active role in the PTOA pathogenesis. In this study, we used MLI-induced experimental PTOA mice and demonstrated that blockage of lymphatic function increased joint tissue damage, OA synovial LECs expressed high levels of inflammatory genes, macrophages with M1 phenotype increased in PTOA synovium, and they promoted LECs to express inflammatory genes. In addition, i.a. administration of Btz attenuated cartilage loss, LEC inflammation and improved lymphatic drainage in OA joints. Thus, Btz may provide a new therapy for PTOA, which we suggest could support improved synovial lymphatic function and protection from progressive joint degeneration in this disease.
Lymphangiogenesis requires the transcription factor Prox1 and growth factor VEGF-C/VEGFR3 signal pathway (33, 34). The VEGFR3 neutralizing Ab has been used to investigate the effect of lymphatic blockage on disease process and treatment in numerous pre-clinical studies, including our own (20). Here, we demonstrated that the VEGFR3 blockade exacerbates tissue damage and reduces synovial lymphatic drainage in MLI-induced PTOA (Figure 1). Thus, although our study cannot prove if lymphatic dysfunction leads to OA development, because we treated mice that had already received MLI surgery, we demonstrated, for the first time, a negative association between lymphatic vessel function and joint injury in a mouse model of experimental OA. This makes sense because our preliminary data found that MMP13 protein is removed from PTOA mouse joints to draining lymph nodes (not shown). VEGFR3 neutralizing Ab was given by intraperitoneal injection. It may affect lymphatics systemically. This is unlikely, however, because this Ab mainly blocks newly formed lymphatic vessels, such as in tumor- (25) and inflammation-induced (20) lymphangiogenesis in mice. We did not find swelling or increased body weight in VEGFR3 Ab-treated mice. In the future, we could damage lymphatic vessels locally using an ultrasound-contrast agent as we described (35) and then examine its effect on the pathogenesis of OA and the clearance of catabolic factors.
Inflammation stimulates local lymphangiogenesis, but lymphatic vessels in inflamed areas do not function sufficiently and have slower lymph flow (24). Numerous lymphatic vessels are observed in the synovium of mice with MLI-induced PTOA, but these vessels have an impaired capacity to remove i.a. injected ICG. Synovial LECs from these mice express high levels of iNOS (Figure 2), a phenotype similar to that found in LECs from TNF-Tg RA mice (7). Lymph flow is controlled by active and passive forces placed on collecting lymphatic vessels via lymphatic muscle cells (36). Alternating contraction and relaxation of lymphatic muscle cells propels lymph to draining lymph nodes and eventually to the venous circulation. iNOS relaxes lymphatic muscle cells by producing an excess of nitric oxide. Thus, LECs from PTOA joints may directly affect muscle cells by reducing their contractile function.
Another important finding of our study is that macrophages with the M1 phenotype promote expression of inflammatory factors by LECs (Figure 5). The contribution of macrophages or myeloid cells to lymphangiogenesis has been widely investigated (37–39). Macrophages produce factors, such as VEGF-C, to promote lymphangiogenesis (40). Depletion of macrophages ablates the pro-lymphangiogenesis effect (41). Macrophages may also function as precursors for lymphatic vessels, although this hypothesis has been challenged (42–44). Macrophages are generally classified as pro-inflammatory M1s or anti-inflammatory M2s. M2 macrophages are pro-angiogenic and pro-lymphangiogenic (11) in tumorigenesis. However, in age-related atherosclerosis, M1s, but not M2s, exacerbate atherosclerotic lesions (45), suggesting that M1s mediate tissue damage in the context of chronic inflammation. Zhang et al. reported that skin macrophages have a predominant M1-phenotype in Cox2−/− mice fed with high-salt. This is associated with decreased mature lymphatic duct, dilated lymphatic vessels and decreased lymphatic flow and lymphatic clearance (46, 47). Our findings reveal another function for M1s—involving promotion of LECs to produce inflammatory factors via soluble factors present in conditioned medium. Currently, we do not know the identity of these soluble factors, but IL-1 and TNF, which are highly expressed by M1s, may be involved. Here, we used cells that were induced in vitro to M1 and M2 activation states. However, it has been recognized for many years that these two states inadequately describe the complexity of macrophage responses within in vivo physiological or pathological conditions. Thus, more experiments, such as transcriptomic and systems biology analyses, are needed to further delineate the involvement of macrophage subsets in OA synovium.
OA pathogenesis involves multiple inflammatory pathways and an agent that targets a single pathway may not be very effective, such as anti-IL-1 or anti-TNF drugs (48, 49). Btz affects the ubiquitin-proteasome pathway that controls many inflammatory signaling proteins. Thus, Btz may be more effective at inhibiting inflammation. However, our study has several limitations. First, joints contain many cell types. We do not have evidence to indicate a direct effect of Btz on LECs or other cell types within the joint. We might be able to use a targeted approach by linking Btz with anti-PDPN antibody to deliver Btz to LECs. Second, we only examined one regimen for Btz treatment, e.g. 4 weeks post-MLI and once a week administration. It is possible that other regimens may have better effects, such as giving the drug at an earlier stage or decreasing frequency. Finally, we used a mouse model of experimental PTOA. Whether Btz has an effect on other forms of OA, such as on age-related OA, requires additional study.
In conclusion, we used a combination of imaging, cell biology and morphology in an experimental mouse model of PTOA and found that blockage of lymphangiogenesis accelerates joint tissue damage. Primary LECs isolated from synovium of OA joints have an inflammatory phenotype, which is accompanied by a high number of macrophages that express M1 markers. In vitro, M1s promote production of inflammatory factors by LECs, an effect that is prevented by the proteasome inhibitor Btz. Btz attenuates cartilage loss and expression of inflammatory factors by LECs, and improves lymphatic drainage in OA joints. These findings suggest a possible cellular mechanism for impaired synovial lymphatic function and a therapeutic potential of Btz in joints of PTOA.
Supplementary Material
Supplemental figure 1. Isolation of LECs from synovial and surrounding soft tissues of mouse knee. Knee joints including whole tibia and femur were digested with 1 mg/ml collagenase I for 1 hour. Digestion was stopped by adding 2% fetal bovine serum. Total solution was passed through Falcon 40 μm cell strainer. Cells were collected, washed and incubated with PE-conjugated anti-mouse PDPN Ab at 4°C for 30 minutes. Cells were washed, re-suspended in anti-PE microbeads and rotated at 4°C for another 30 minutes, and then were loaded on the prepared MS Column. PDPN+ cells were pushed out from the column with 1 ml MACS buffer after wash and subjected to RNA analyses or flow cytometic analysis.
Supplemental figure 2. Induction of M1 and M2 macrophages. WT bone marrow macrophages were treated with (20ng/mL) IL-1 or (20ng/mL) IL-4 for 24 hours to induce M1 or M2 phenotype, respectively. Expression of markers for pan-macrophages (F4/80), M1 (iNOS) or M2 (CD206) macrophages was confirmed by immuno-staining with iNOS (1:50 Santa Cruz) and CD206 (1:200 R&D primary antibody, following by Alexa Fluor 568 goat anti rabbit (1:400) and Alexa Fluor 568 rabbit anti goat (1:400) secondary antibody. Images were taken with Olympus 1X71 inverted microscope under 20X magnification.
ACKNOWLEDGMENT
This work was supported by research grants from NIH (AR063650, AR069789, 1S10RR027340–01, AR061307, AR069655, AR056702, TR000042), NYSTEM (C029548), and the Lymphatic Malformation Institute, USA. It also was partially supported by grants from National Natural Science Foundation of China (81330085) and Natural Science Foundation of Henan Province, China (162300410217). We thank Dr. Bronislaw Pytowski, ImClone Systems, New York, for providing anti-mouse VEGFR-3 neutralizing antibody (mF4–31C1).
REFERENCES
- 1.Goldring MB, and Goldring SR. Osteoarthritis. Journal of cellular physiology. 2007;213(3):626–34. [DOI] [PubMed] [Google Scholar]
- 2.Sellam J, and Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature reviews Rheumatology. 2010;6(11):625–35. [DOI] [PubMed] [Google Scholar]
- 3.Dieterich LC, Seidel CD, and Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis. 2014;17(2):359–71. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty S, Davis MJ, and Muthuchamy M. Emerging trends in the pathophysiology of lymphatic contractile function. Semin Cell Dev Biol. 2015;38:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Liang Q, Zuscik M, Shen J, Chen D, Xu H, et al. Distribution and alteration of lymphatic vessels in knee joints of normal and osteoarthritic mice. Arthritis & rheumatology (Hoboken, NJ). 2014;66(3):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von der Weid PY, and Rainey KJ. Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2010;32(6):697–711. [DOI] [PubMed] [Google Scholar]
- 7.Liang Q, Ju Y, Chen Y, Wang W, Li J, Zhang L, et al. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis research & therapy. 2016;18:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, et al. Impaired lymphatic contraction associated with immunosuppression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(46):18784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouta EM, Kuzin I, de Mesy Bentley K, Wood RW, Rahimi H, Ji RC, et al. Treatment of TNF-Tg Mice with Anti-TNF Restores Lymphatic Contraction, Repairs Lymphatic Vessels, and May Increase Monocyte/Macrophage Egress. Arthritis & rheumatology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas SK, and Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11(10):889–96. [DOI] [PubMed] [Google Scholar]
- 11.Quail DF, and Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E, et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22(8):1167–75. [DOI] [PubMed] [Google Scholar]
- 13.Kane RC, Bross PF, Farrell AT, and Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. The oncologist. 2003;8(6):508–13. [DOI] [PubMed] [Google Scholar]
- 14.van der Heijden JW, Oerlemans R, Lems WF, Scheper RJ, Dijkmans BA, and Jansen G. The proteasome inhibitor bortezomib inhibits the release of NFkappaB-inducible cytokines and induces apoptosis of activated T cells from rheumatoid arthritis patients. Clin Exp Rheumatol. 2009;27(1):92–8. [PubMed] [Google Scholar]
- 15.Yannaki E, Papadopoulou A, Athanasiou E, Kaloyannidis P, Paraskeva A, Bougiouklis D, et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis and rheumatism. 2010;62(11):3277–88. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Zhang W, Li F, Guo F, and Chen A. Bortezomib prevents the expression of MMP-13 and the degradation of collagen type 2 in human chondrocytes. Biochemical and biophysical research communications. 2014;452(3):526–30. [DOI] [PubMed] [Google Scholar]
- 17.Radwan M, Wilkinson DJ, Hui W, Destrument AP, Charlton SH, Barter MJ, et al. Protection against murine osteoarthritis by inhibition of the 26S proteasome and lysine-48 linked ubiquitination. Annals of the rheumatic diseases. 2015;74(8):1580–7. [DOI] [PubMed] [Google Scholar]
- 18.Quan R, Huang Z, Yue Z, Xin D, Yang D, Pan J, et al. Effects of a proteasome inhibitor on the NF-kappaB signalling pathway in experimental osteoarthritis. Scandinavian journal of rheumatology. 2013;42(5):400–7. [DOI] [PubMed] [Google Scholar]
- 19.Hamada D, Sampson ER, Maynard RD, and Zuscik MJ. Surgical induction of posttraumatic osteoarthritis in the mouse. Methods in molecular biology (Clifton, NJ). 2014;1130:61–72. [DOI] [PubMed] [Google Scholar]
- 20.Guo R, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT, et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis and rheumatism. 2009;60(9):2666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Bouta EM, Wood RW, Schwarz EM, Wang Y, and Xing L. Utilization of longitudinal ultrasound to quantify joint soft-tissue changes in a mouse model of posttraumatic osteoarthritis. Bone Research. 2017;5:17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouta EM, Banik PD, Wood RW, Rahimi H, Ritchlin CT, Thiele RG, et al. Validation of power Doppler versus contrast-enhanced magnetic resonance imaging quantification of joint inflammation in murine inflammatory arthritis. J Bone Miner Res. 2015;30(4):690–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang YJ, et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis and rheumatism. 2011;63(8):2318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Wood R, Schwarz EM, Wang YJ, and Xing L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum. 2010;62(7):1881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dar QA, Schott EM, Catheline SE, Maynard RD, Liu Z, Kamal F, et al. Daily oral consumption of hydrolyzed type 1 collagen is chondroprotective and anti-inflammatory in murine posttraumatic osteoarthritis. PloS one. 2017;12(4):e0174705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sironi M, Conti A, Bernasconi S, Fra AM, Pasqualini F, Nebuloni M, et al. Generation and characterization of a mouse lymphatic endothelial cell line. Cell Tissue Res. 2006;325(1):91–100. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Wang H, Zhou X, Li X, Sun W, Dellinger M, et al. Lymphatic Endothelial Cells Produce M-CSF, Causing Massive Bone Loss in Mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32(5):939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W, Zhang H, Wang H, Chiu YG, Wang M, Ritchlin CT, et al. Targeting Notch-Activated M1 Macrophages Attenuates Joint Tissue Damage in a Mouse Model of Inflammatory Arthritis. J Bone Miner Res. 2017;32(7):1469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda Y, Amano I, Hata M, Kojima H, and Sawa Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta histochemica et cytochemica. 2010;43(2):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson LA, Prevo R, Clasper S, and Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. The Journal of biological chemistry. 2007;282(46):33671–80. [DOI] [PubMed] [Google Scholar]
- 31.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, and Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. The Journal of experimental medicine. 2006;203(12):2763–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nisato RE, Harrison JA, Buser R, Orci L, Rinsch C, Montesano R, et al. Generation and characterization of telomerase-transfected human lymphatic endothelial cells with an extended life span. The American journal of pathology. 2004;165(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohela M, Bry M, Tammela T, and Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Current opinion in cell biology. 2009;21(2):154–65. [DOI] [PubMed] [Google Scholar]
- 34.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature immunology. 2004;5(1):74–80. [DOI] [PubMed] [Google Scholar]
- 35.Bouta EM, Ju Y, Rahimi H, de Mesy-Bentley KL, Wood RW, Xing L, et al. Power Doppler ultrasound phenotyping of expanding versus collapsed popliteal lymph nodes in murine inflammatory arthritis. PloS one. 2013;8(9):e73766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiological reviews. 1990;70(4):987–1028. [DOI] [PubMed] [Google Scholar]
- 37.Kerjaschki D The crucial role of macrophages in lymphangiogenesis. Journal of Clinical Investigation. 2005;115(9):2316–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zumsteg A, and Christofori G. Myeloid cells and lymphangiogenesis. Cold Spring Harbor perspectives in medicine. 2012;2(6):a006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing L, and Ji RC. Lymphangiogenesis, myeloid cells and inflammation. Expert review of clinical immunology. 2008;4(5):599–613. [DOI] [PubMed] [Google Scholar]
- 40.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113(22):5650–9. [DOI] [PubMed] [Google Scholar]
- 41.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–52. [DOI] [PubMed] [Google Scholar]
- 42.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12(2):230–4. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, et al. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122(14):1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. Journal of Clinical Investigation. 2005;115(9):2363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couchie D, Vaisman B, Abderrazak A, Mahmood DFD, Hamza MM, Canesi F, et al. Human Plasma Thioredoxin-80 Increases with Age and in apoE−/− Mice Induces Inflammation, Angiogenesis and Atherosclerosis. Circulation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang MZ, Yao B, Wang Y, Yang S, Wang S, Fan X, et al. Inhibition of cyclooxygenase-2 in hematopoietic cells results in salt-sensitive hypertension. The Journal of clinical investigation. 2015;125(11):4281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stegbauer J, and Coffman TM. Skin tight: macrophage-specific COX-2 induction links salt handling in kidney and skin. The Journal of clinical investigation. 2015;125(11):4008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis and rheumatism. 2009;61(3):344–52. [DOI] [PubMed] [Google Scholar]
- 49.Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Annals of the rheumatic diseases. 2015;74(9):1697–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Isolation of LECs from synovial and surrounding soft tissues of mouse knee. Knee joints including whole tibia and femur were digested with 1 mg/ml collagenase I for 1 hour. Digestion was stopped by adding 2% fetal bovine serum. Total solution was passed through Falcon 40 μm cell strainer. Cells were collected, washed and incubated with PE-conjugated anti-mouse PDPN Ab at 4°C for 30 minutes. Cells were washed, re-suspended in anti-PE microbeads and rotated at 4°C for another 30 minutes, and then were loaded on the prepared MS Column. PDPN+ cells were pushed out from the column with 1 ml MACS buffer after wash and subjected to RNA analyses or flow cytometic analysis.
Supplemental figure 2. Induction of M1 and M2 macrophages. WT bone marrow macrophages were treated with (20ng/mL) IL-1 or (20ng/mL) IL-4 for 24 hours to induce M1 or M2 phenotype, respectively. Expression of markers for pan-macrophages (F4/80), M1 (iNOS) or M2 (CD206) macrophages was confirmed by immuno-staining with iNOS (1:50 Santa Cruz) and CD206 (1:200 R&D primary antibody, following by Alexa Fluor 568 goat anti rabbit (1:400) and Alexa Fluor 568 rabbit anti goat (1:400) secondary antibody. Images were taken with Olympus 1X71 inverted microscope under 20X magnification.