Abstract
Background
Understanding racial differences in outcomes for atrial fibrillation (AF) may guide interventions to diminish health inequities.
Methods and Results
In a retrospective, cross-sectional study of adults hospitalized with a principal diagnosis of AF using the 2001-2012 National Inpatient Sample, we assessed racial differences for in-hospital. We accounted for case-mix and clustering by race within hospitals to estimate odds ratios (OR) for death associated with individual patient race and hospital racial composition. We identified 676,567 hospitalizations (mean age 71.8 years, 53.6% women) with principal diagnosis of AF (84.2% White, 7.1% Black, 5.0% Hispanic). Black (vs. White) race was associated with 1.63-fold (95% CI, 1.50-1.78) risk of death. Other races had similar risk of death as Whites. Risk of death for Blacks (vs. Whites) declined over time [2001: OR 1.78(95% CI 1.31-2.43); 2012: OR 1.23(95% CI 0.92–1.64)]. Racial differences in deaths within hospitals narrowed, while hospitals with larger proportions of Blacks had persistently worse outcomes than hospitals with fewer Blacks (OR 1.08 per 10% increase in Blacks in 2001 and 2012).
Conclusion
Black patients with a principal diagnosis of AF were more likely to suffer in-hospital death than Whites. Our findings suggest racial disparities based upon individual patients’ race improved over time, but outcomes were persistently worse at hospitals with higher proportions of Black patients, regardless of patients’ races.
Keywords: Atrial fibrillation, Disparities, Outcomes, Race
INTRODUCTION
Atrial fibrillation and atrial flutter (simplified here as AF) affects about 1-3% of adults and causes significant morbidity [1–4]. Prior studies have identified multiple racial differences in AF epidemiology, treatment, and outcomes. Among the elderly or in regional populations, Blacks with AF received stroke prevention or AF-related treatment less often than non-Hispanic Whites (hereafter “Whites”) [5–7] and were more likely to have AF-associated strokes and heart failure (HF) [8–10]. A nationally representative 2-year cross-sectional study showed Black patients hospitalized with AF were more likely to die in the hospital than Whites and less likely to receive catheter ablation [11]. Although racial differences for AF are reported in prior studies of limited geographic, temporal, or age distribution, population-based estimates and temporal trends are unclear. In addition, racial inequities may be related to different treatments delivered based upon the race of an individual patient (from conscious or unconscious bias) or may arise from lower quality of care at hospitals that serve a large proportion of racial minorities. How racial differences for AF relate to variation in outcomes within and across hospitals have not been well-characterized [12-14]. We investigated racial differences in AF-associated procedures and adverse outcomes during more than a decade (2001-2012) using a nationally representative sample of US hospitalizations. Further, we determined racial differences in AF care related to individual patient race as well as hospital-level differences in racial composition.
MATERIAL AND METHODS
We used data from the US National Inpatient Sample and the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality to identify hospitalizations with a principal diagnosis of AF or atrial flutter (International Classification of Diseases Ninth Revision, Clinical Modification, ICD-9-CM, 427.3) during years 2001-2012. As ICD-9-CM does not distinguish between AF or atrial flutter, we will simplify in this paper and refer to both as AF. Prior studies of claims have shown up to 95% sensitivity, 99% specificity, and 70-96% positive predictive value for AF ICD-9-CM 427.3×.[15-17] The NIS has been used extensively to characterize US healthcare utilization [18]. In brief, NIS approximates a 20% stratified sample of non-federal acute care hospitals that are representative of US hospitalizations-including both inpatient and observation status [18].
We excluded hospitalizations for patients age <40years, race categorized as “other” (2.3%) or not documented, or missing essential covariates or hospital data (age, sex, race, admission date, discharge date, or date of in-hospital death) (Table A 1 in the supplementary appendix). We also excluded patients with same-day admission and discharge and length of stay >30 days to avoid bias by patients who are either relatively well or overly complex, respectively. We excluded hospitalizations associated with cardiothoracic surgery or coronary interventions as primary procedures. We assessed racial differences in hospitalization rates for AF. We calculated annual age-adjusted AF hospitalization rates by race using NIS hospital weighting and US census data [19].
Our primary objective was to whether there were racial differences in in-hospital AF procedures and coincident clinical outcomes over a 12-year period. Race and ethnicity were provided by the hospital sources to the NIS datasets. If Hispanic ethnicity was reported by the hospital source, the patient’s race/ethnicity category was defined as Hispanic. Associated cardiovascular conditions included HF and ischemic stroke, and the primary outcome was in-hospital death. Procedures included surgical and catheter ablation, and cardio version. We defined outcomes and procedures by ICD-9-CM codes consistent with prior use and detailed in Table A 2of the supplementary appendix [5,11,20]. Patients of White race were the referents. Models were adjusted for covariates including age, sex, calendar year, comorbid conditions[myocardial infarction (MI), hypertension, diabetes mellitus [21], dementia, chronic obstructive pulmonary disease, chronic liver disease, and chronic kidney disease using enhanced Charlson and Elixhauser ICD-9-CM codes, [22] Table A 2, hospital characteristics (region, location as urban vs. rural, and teaching status), and primary payer (Medicare, Medicaid, private, self-pay). Diagnoses analyzed were limited to the principal diagnosis and the first 14 secondary diagnoses to maintain consistency across the available data within the NIS from 2001-2012.Continuous variables were reported as means and tested using the F test and categorical variables as proportions and tested using the chi-square test.
Associations between race and clinical outcomes or AF procedures were estimated using generalized estimated equations with standard errors accounting for hospital clustering. Temporal trends were assessed with interaction terms for race and year (continuous; race *year). We used a previously described decomposition method to separate racial differences into ‘within-hospital’ and ‘across-hospital’ effects [23,24]. Within-hospital effects were estimates of the difference in outcomes associated with an individual patient’s race after adjusting for each hospital’s overall racial composition. Across-hospital effects were estimates of outcome differences associated with each hospital’s racial composition after adjusting for an individual patient’s race. Across-hospital associations were primarily determined for every 10% increase in the proportion of Black patients across patients hospitalized at a given center. We also determined across-hospital associations for a theoretical 100% change in the proportion of Black patients across hospitals for comparison with the adjusted effect of race as a binary (Black vs. White) variable on death (supplement). We compared within- and across-hospital estimates in 2001 and 2012 to study temporal trends among Blacks compared with Whites.
All analyses were conducted using SAS version 9.4 (Cary, NC), and a two-sided alpha of ≤ 0.05 was deemed statistically significant. The Institutional Review Board at Boston University Medical Center deemed the study as not human subject’s research.
RESULTS
A total of 676, 567 hospitalizations (population-based estimate of 3,329,124) with a principal diagnosis of AF were identified from 2001-2012. Table 1 shows patient characteristics by race. The mean age was 71.8 years and women comprised 53.6% of the sample. The patients with AF were 84.2% White, 7.1% Black, and 5.0% Hispanic, and 1.4% Asian/Pacific Islander. Compared with Whites, patients of other racial groups were more likely to have Medicaid or self-pay for care, and thus have lower socioeconomic status.
Table 1:
Characteristics and co-morbidities for admissions with a primary diagnosis of AF from 2001-2012
| CHARACTERISTICS | White | Black | Hispanic | Asian/PI | All races | P value |
|---|---|---|---|---|---|---|
| N (unweighted) | 569669 | 48036 | 33828 | 9472 | 676567 | |
| N (weighted) | 2803629 | 235762 | 166609 | 45157 | 3329124 | |
| % of weighted participants | 84.20% | 7.10% | 5.00% | 1.40% | 100% | |
| Age, mean years | 72.4 | 66.5 | 69 | 70.7 | 71.8 | <0.001 |
| Women, % | 53.5 | 55 | 53.9 | 52.8 | 53.6 | <0.001 |
| Hospital location: urban, % | 83.9 | 90.9 | 93.4 | 93.7 | 85 | <0.001 |
| Teaching hospital, % | 36.9 | 56.2 | 43.9 | 46.2 | 38.9 | <0.001 |
| Payer | <0.001 | |||||
| Medicare, % | 71.1 | 59.4 | 56.8 | 56.9 | 69.1 | |
| Medicaid, % | 2.3 | 10.2 | 12.7 | 12.5 | 3.7 | |
| Private, % | 23 | 22 | 20.2 | 24.2 | 22.8 | |
| Self-pay, % | 1.9 | 5.2 | 6.2 | 3.7 | 2.5 | |
| No charge, % | 0.2 | 0.7 | 1.2 | 0.3 | 0.3 | |
| Other, % | 1.5 | 2.5 | 2.8 | 2.4 | 1.7 | |
| CO-MORBIDITIES | ||||||
| Diabetes, % | 21.2 | 32.7 | 32.9 | 29.1 | 22.8 | <0.001 |
| Hypertension, % | 65.2 | 78.6 | 71 | 70.5 | 66.6 | <0.001 |
| ACS, % | 31.5 | 27.1 | 29.5 | 27.2 | 31 | <0.001 |
| History of MI, % | 30.9 | 26.8 | 28.6 | 26.3 | 30.4 | <0.001 |
| Dyslipidemia, % | 37 | 30.1 | 33.2 | 37 | 36.3 | <0.001 |
| Dementia, % | 0.8 | 1 | 0.8 | 1 | 0.8 | <0.001 |
| COPD, % | 4 | 2.8 | 2.5 | 2.2 | 3.8 | <0.001 |
| CKD, % | 7.4 | 16.4 | 9.3 | 11 | 8.2 | <0.001 |
| CLD, % | 0.9 | 1.2 | 2 | 1 | 1 | <0.001 |
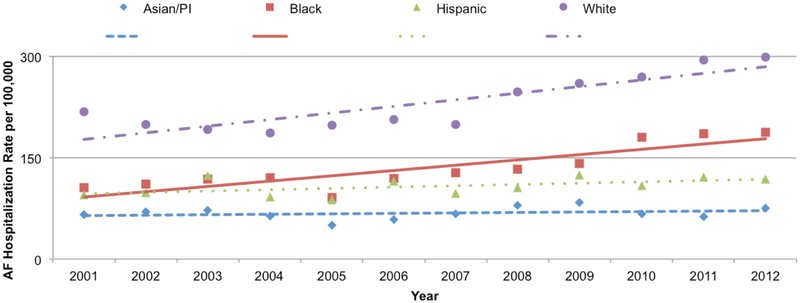
Estimated hospitalization rates increased for all races over the study period. Whites had the highest population-based AF hospitalization rate ranging from 186.1-298.5 per 100,000 US residents during the years 2001-2012 (Figure 1 and Table A 4). AF hospitalization rates were lower for Blacks (range 91.3-187.3 per 100,000) and Hispanics (range 87.3-124.2 per 100,000). Asians/PI (range 49.6-83.7 per 100,000) had the lowest hospitalization rates.
Figure 1:
Rate of Primary Hospitalization for AF per 100,000 US adults (>40 years old) by year and race, NIS 2001-2012.
In unadjusted analysis (Table 2), HF was the most common outcome – occurring in 26.9% of all participants. HF was more prevalent among Blacks (35.1%) than Whites (26.2%). Death occurred in 1.0% of all participants and was also more common among Blacks (1.4%) than Whites (1.0%). Cardio version was the most common procedure for AF and occurred in 14.3% of all hospitalizations. Cardio version was less frequent among Blacks (10.5%) than Whites (15.0%).
Table 2:
Outcomes and procedures by race for admissions with a primary diagnosis of AF from 2001-2012
| OUTCOMES: Unadjusted | White | Black | Hispanic | Asian/PI | All races |
|---|---|---|---|---|---|
| HF, % | 26.2 | 35.1 | 27.6 | 23.7 | 26.9 |
| Stroke, % | 1.8 | 1.2 | 1.4 | 1.3 | 1.7 |
| Died, % | 1.0 | 1.4 | 1.0 | 0.9 | 1.0 |
| PROCEDURES: Unadjusted | |||||
| Catheter ablation, % | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 |
| Surgical ablation, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cardioversion, % | 15.0 | 10.5 | 9.4 | 9.2 | 14.3 |
| Any AF procedure¶, % | 15.3 | 10.8 | 9.6 | 9.4 | 14.6 |
Catheter ablation, surgical ablation, or cardioversion
Blacks [OR 1.67 (95% CI 1.63-1.71)] and Hispanics [OR 1.11 (95% CI 1.09-1.15)] hospitalized for AF had greater odds of comorbid HF compared with Whites across the 2001-2012 year span after adjustment (Table 3, Figure A 1). In contrast, Asians/Pacific Islanders had lower comorbid HF [OR 0.87(95% CI, 0.82-0.91)] than Whites. Stroke was less common in all studied racial groups compared with Whites after adjustment: Blacks [OR0.79 (95% CI, 0.72-0.86)], Hispanics [OR0.83 (95% CI 0.75-0.91)], and Asian/Pacific Islander [OR 0.72(95% CI 0.59-0.86)].
Table 3:
Adjusted association between race and outcomes or procedures for admissions with a primary diagnosis of AF from 2001-2012
| White | Black | Hispanic | Asian/PI | |
|---|---|---|---|---|
| OUTCOMES: Adjusted OR (95% CI) | ||||
| HF# | ref | 1.67*† (1.63, 1.71) |
1.11*† (1.09, 1.15) |
0.87*† (0.82, 0.91) |
| Stroke# | ref | 0.79* (0.72, 0.86) |
0.83* (0.75, 0.91) |
0.72* (059, 0.86) |
| Death | ref | 1.63*† (1.50, 1.78) |
1.09 (0.97, 1.23) |
1.07 (0.86, 1.32) |
| PROCEDURES: Adjusted OR (95% CI) | ||||
| Catheter ablation | ref | 0.78* (0.66, 0.92) |
0.66* (0.53, 0.81) |
0.46*† (0.29, 0.72) |
| Cardioversion | ref | 0.54*† (0.52, 0.56) |
0.52*† (0.50, 0.54) |
0.51* (0.48, 0.55) |
| Any AF procedure¶ | ref | 0.54*† (0.53, 0.56) |
0.52*† (0.50, 0.54) |
0.51* (0.47, 0.55) |
HF outcome adjusted for stroke. Stroke outcome adjusted for HF. Death outcome adjusted for both HF and stroke.
P<.05,
Interaction between race and year
Catheter ablation, surgical ablation, or cardioversion
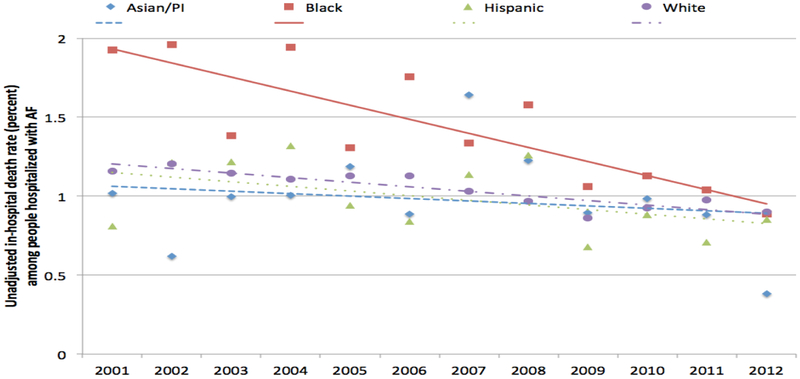
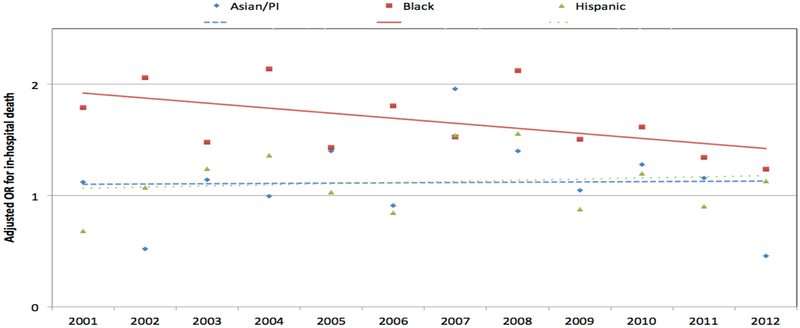
Blacks with AF had 1.63-fold (95% CI 1.50-1.78; unadjusted mortality rate 1.4% vs. 1.0 %) higher risk of in-hospital mortality than Whites after multivariable adjustment (Table 3). Compared with Whites, mortality was similar for Hispanics [OR 1.09(95% CI 0.97-1.23)], and Asians/PI [OR1.07 (95% CI 0.86-1.31)]. The risk of death for Blacks compared to White patients decreased over time (p for interaction<.0001); by year 2012, significant outcome differences based upon race were no longer identified (Figures 2[unadjusted] and 3 [adjusted], Table A 5).
Figure 2:
Unadjusted death, by race and year, NIS 2001-2012. Unadjusted percent of deaths among people hospitalized principally for AF. Linear trend lines by race are shown.
Figure 3:
Adjusted odds ratios for death by race and year among people hospitalized principally for AF. Adjusted for age, sex, comorbidities (diabetes, hypertension, history of myocardial infarction, Dyslipidemia, chronic obstructive pulmonary disease, chronic kidney disease, chronic liver disease, heart failure, and stroke), hospital location, hospital teaching status, payer and region, White = referent. Linear trend lines by race are shown. There are interactions between race and year for Blacks.
Ablation and cardio version were less common among people of all studied ethnic/racial groups compared with Whites. Blacks [OR 0.54 (95% CI 0.53-0.56)], Hispanics [OR 0.52 (95% CI 0.50-0.54]; and Asian/Pacific Islanders [OR 0.51 (95% CI 0.47-0.55)] were less likely to receive AF procedures (Table 3).
Our decomposition analysis primarily evaluated the within- and across-center association of a 10% increase in the proportion of patients at a hospital who were Black (Table 4). Both within-center [OR1.06 (95% CI 1.02-1.09)] and across-center [OR1.08 (95% CI 1.00-1.16)] differences contributed to higher deaths for Blacks compared with Whites in 2001. In 2012 the within-center difference was no longer significant [OR 1.01 (95% CI 0.97-1.04)]; however, the across-center difference persisted [OR1.08 (95% CI 1.01-1.14)]. We also modeled the predicted death between theoretical hospitals with 0% vs. 100% proportion of patients of Black race (Table A 6): within-center association between Black vs. White race and death was reduced [2001: OR 1.71 (95% CI 1.21, 2.41); 2012 OR 1.05 (95% CI 0.74, 1.49)], while the across-center difference persisted [2001: OR 2.06 (95% CI 0.98, 4.32); 2012 OR 2.06 (95% CI 1.16, 3.70)].
Table 4:
Associations between black race, and in-hospital death and procedures adjusting for center confounding for patients hospitalized primarily for AF in NIS 2001 and 2012. OR (95% CI) Referent = White
| Procedures | Death | |||
|---|---|---|---|---|
| 2001 | 2012 | 2001 | 2012 | |
| Adjusted for covariates (presence or absence of Black race vs. White) | ||||
| Combined across- and within-center | 0.55 (0.48, 0.62) |
0.54 (0.50, 0.59) |
1.78 (1.31, 2.43) |
1.23 (0.92, 1.64) |
| Adjusted for covariates and confounding by center (10% increase in the proportion of patients hospitalized who are black) | ||||
| Within-center (similar racial mix) | 0.95 (0.93, 0.96) |
0.94 (0.93, 0.95) |
1.06 (1.02, 1.09) |
1.01 (0.97, 1.04) |
| Across-center (differing racial mix) | 0.93 (0.87, 0.99) |
0.94 (0.90, 0.99) |
1.08 (1.00, 1.16) |
1.08 (1.01, 1.14) |
There were persistent contributions to fewer AF-associated procedures for Blacks compared with Whites for both within-center [2001: OR 0.95(95% CI 0.93, 0.96); 2012: OR 0.94 (95% CI 0.93, 0.95)] and across-center differences [2001: OR 0.93(95% CI 0.87, 0.99); 2012: OR 0.94 (95% CI, 0.90, 0.99)] (Table 4). Models for the predicted procedures between theoretical hospitals with 0% vs. 100% proportion of patients of Black race are shown in Table A 6.
DISCUSSION
We investigated racial differences in outcomes and procedures associated with hospitalization for AF from 2001-2012 in the US. The persistently lower rates of AF hospitalization for Blacks is consistent with studies of community-based cohorts and Medicare beneficiaries [17,25]. Blacks with AF were approximately60% more likely to experience in-hospital mortality and 50% less likely to undergo AF-associated procedures compared with Whites. Reassuringly, racial differences for in-hospital mortality declined over time, and no longer significantly varied in 2012. Importantly, differences in AF treatment based upon an individual patient’s race were more equitable over time within centers. However, hospitals caring for more Black patients continued to show worse mortality among patients hospitalized for AF regardless of the individual patient’s race.
In the United States, people of racial and ethnic minority groups have different patterns of AF care and management than Whites. Our findings are consistent with studies identifying racial differences in Blacks with AF and other cardiovascular diseases. Blacks and Hispanics on average have strokes at younger ages and have greater disability and mortality than Whites [26,27]. In older adults, Medicare data indicate that compared with Whites, Blacks and Hispanics with AF have increased risk of stroke (1.2- to 1.7-fold) and death (1.1- to 1.5-fold) [28]. The finding in our study of fewer strokes among patients who are members of racial minority groups compared with Whites may be related to our focus on patients with a primary hospitalization diagnosis of AF, our study’s inclusion of both prevalent and incident AF, and survival bias within readmitted patients [29].
Optimistically, the racial difference in death between Blacks and Whites appears to be narrowing over a contemporary decade. The within-center component of death narrowed whereas the across-center component persisted. The changing pattern suggests that care within individual hospitals is becoming more equitable. However, Blacks may have continued to have higher death rates because they were more likely to be admitted to poorer performing hospitals, rather than differences in care provided to individuals based upon their race. Prior analyses similarly showed that Blacks, in general, present to poorer performing hospitals after acute MI [12,30]. Further, compared with Whites, Black patients tend to live closer to higher-quality hospitals but are more likely to receive cardiac surgery at lower-quality hospitals [31]. Black patients may feel unwelcome at hospitals which treat predominantly White patients [31]. In making health decisions, racial concordance between physician and patient has been found to be more important for Blacks than Whites [32,33]. Consistent with prior studies, we found that the likelihood of AF procedures was persistently about half that among racial minorities compared with Whites - both across and within hospitals. 7,11Implicit racial stereotypes by providers, patient socioeconomic status, or variation in clinic severity may affect decision-making either subconsciously or overtly [34].
Broad efforts focusing on quality improvement and elimination of disparities, such as those promoted through the Patient Protection and Affordable Care Act of 2010 may provide the impulse to affect meaningful change [35]. Thus, more comprehensive, longitudinal, and contemporary data are needed to assess the current disparities in the context of healthcare systems rapidly adapting at the community, primary care, and hospital levels.
STRENGTHS AND LIMITATIONS
The analysis using NIS datasets is large, nationally representative, multi-ethnic, contemporary, and allows the assessment of time-trends over a 12-year span. Given the nature of the dataset, there are important limitations.
Principally, the NIS data are limited to hospitalization diagnoses and only prevalent comorbid conditions can be assessed. Clinically relevant comorbidities that developed prior to the hospitalization, including those known to be associated with high risk of stroke and mortality such as those in the CHA2DS2-VASC risk prediction rule [36], are not available in the NIS. Second, the data are not longitudinal; we cannot differentiate prevalent from incident AF. Thus, we cannot account for repeated observations, within-patient correlated data, or survival bias. Third, assessment of post-discharge outcomes and causal relationships is limited. Fourth, the data are biased towards sick individuals with multiple hospitalizations. Fifth, the type of AF, prevalence of unrecognized AF, and the decision to hospitalize for AF may vary by race. Sixth, the administrative data available were collected for billing purposes and not clinical care or research [37]. The ICD-9-CM codes used in our study relied on healthcare provider identification and documentation. The assignment of principal diagnosis may reflect reimbursement policies and not clinical importance. Over time, principal diagnosis coding has shifted towards higher reimbursing diagnosis related groups (such as stroke) thus introducing bias.29 In some cases when a patient presents with several acute conditions simultaneously, the diagnosis that has greater reimbursement may be listed in the principal position. Seventh, there is likely residual confounding as administrative data were used for risk adjustment. Particularly, measures of socioeconomic status (including occupation and education), health literacy, medications - including anticoagulation therapy – or clinical prognostic measures, such as vital signs and diagnostic test results, were not available. We used coded secondary diagnoses as surrogates for clinical complexity; however ICD-9 codes do not communicate the severity of comorbidities [38]. Eighth, the NIS samples different hospitals each year and we are not able to adjust for individual hospitals in the analysis. However, the decomposition method allows adjustment for clustering based on the proportion of black patients at a hospital.
Supplementary Material
IMPLICATIONS.
Blacks hospitalized principally for AF were more likely to die and less likely to receive an AF-associated procedure during hospitalization compared with Whites across a dozen years in a contemporary nationally representative sample of adult hospitalizations. Over time, racial differences for in-hospital mortality declined. Within-center differences based on an individual patient’s race have narrowed. However, our findings suggest that Blacks are more likely to be admitted to poorer-performing hospitals. Further public health investigation is warranted to examine the causes for differences in outcomes among Blacks with AF and identify modifiable factors to inform outcome-improving strategies for Blacks with AF.
ABBREVIATIONS:
- AF
Atrial Fibrillation
- HF
Heart Failure
- ICD-9-CM
International Classification of Diseases Ninth Revision, Clinical Modification
- NIS
Nationwide Inpatient Sample
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011; 124:1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc. 2015; 4: e001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013; 44: 3103–3108. [DOI] [PubMed] [Google Scholar]
- 5.Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/Ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke. 2008; 39: 2736–2743. [DOI] [PubMed] [Google Scholar]
- 6.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010; 41: 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015; 12:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson JR, Zahuranec DB, Lisabeth LD, Sánchez BN, Skolarus LE, et al. Mexican Americans with atrial fibrillation have more recurrent strokes than do non-Hispanic whites. Stroke. 2010; 41: 2132–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentile NT, Seftchick MW. Poor outcomes in Hispanic and African American patients after acute ischemic stroke: influence of diabetes and hyperglycemia. Ethn Dis. 2008; 18: 330–335 [PubMed] [Google Scholar]
- 10.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, et al. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality. JAMA Cardiol. 2016; 1: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naderi S, Rodriguez F, Wang Y, Foody JM. Racial disparities in hospitalizations, procedural treatments and mortality of patients hospitalized with atrial fibrillation. Ethn Dis. 2014; 24:144–149. [PubMed] [Google Scholar]
- 12.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005; 112: 2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who you are and where you live: how race and geography affect the treatment of medicare beneficiaries. Health Aff (Millwood). 2004; 33–44. [DOI] [PubMed] [Google Scholar]
- 14.Epstein AM. Health care in America--still too separate, not yet equal. N Engl J Med. 2004; 351:603–605. [DOI] [PubMed] [Google Scholar]
- 15.Glazer NL, Dublin S, Smith NL, French B, Jackson LA, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007; 167: 246–252. [DOI] [PubMed] [Google Scholar]
- 16.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012; 21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. Rockville MD: HCUP Databases. Healthcare Cost and Utilization Project (HCUP). [PubMed] [Google Scholar]
- 19.US Census Bureau. Population Estimates: Historical data 2000s. [Google Scholar]
- 20.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013; 61:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Z, Liu T, Tse G, Gong M, Gladding PA, et al. A Machine Learning Aided Systematic Review and Meta-Analysis of the Relative Risk of Atrial Fibrillation in Patients With Diabetes Mellitus. Front Physiol. 2018; 9: 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 23.Berlin JA, Kimmel SE, Ten Have TR, Sammel MD. An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999; 55: 470–476. [DOI] [PubMed] [Google Scholar]
- 24.Localio AR, Berlin JA, Have TR Ten, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001; 135:112–23. [DOI] [PubMed] [Google Scholar]
- 25.Freeman J V, Wang Y, Akar J, Desai N, Krumholz H. National Trends in Atrial Fibrillation Hospitalization, Readmission, and Mortality for Medicare Beneficiaries, 1999-2013. Circulation. 2017:135; 1227–1239. [DOI] [PubMed] [Google Scholar]
- 26.Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, et al. Racial variation in initial stroke severity. Stroke. 2000; 31: 563–567. [DOI] [PubMed] [Google Scholar]
- 27.Mathur R, Pollara E, Hull S, Schofield P, Ashworth M, et al. Ethnicity and stroke risk in patients with atrial fibrillation. Heart. 2013; 99:1087–1092. [DOI] [PubMed] [Google Scholar]
- 28.Kabra R, Cram P, Girotra S, Vaughan Sarrazin M. Effect of race on outcomes (stroke and death) in patients >65 years with atrial fibrillation. Am J Cardiol. 2015; 116: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindenauer PK, Lagu T, Shieh M-S, Pekow PS, Rothberg MB. Association of Diagnostic Coding with Trends in Hospitalizations and Mortality of Patients with Pneumonia, 2003-2009. JAMA. 2012; 307:1405–1413. [DOI] [PubMed] [Google Scholar]
- 30.Sarrazin MV, Campbell M, Rosenthal GE. Racial differences in hospital use after acute myocardial infarction: does residential segregation play a role? Health Aff (Millwood). 2009; 28:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimick J, Ruhter J, Sarrazin MV, Birkmeyer JD. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood). 2013; 32:1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komaromy M, Grumbach K, Drake M, Vranizan K, Lurie N, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996; 334: 1305–1310. [DOI] [PubMed] [Google Scholar]
- 33.Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000; 19: 76–83. [DOI] [PubMed] [Google Scholar]
- 34.Moskowitz GB, Stone J, Childs A. Implicit stereotyping and medical decisions: Unconscious stereotype activation in practitioners’ thoughts about African Americans. Am J Public Health. 2012; 102: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh HK, Graham G, Glied SA. Reducing racial and ethnic disparities: The action plan from the department of health and human services. Health Aff (Millwood). 2011; 30:1822–1829. [DOI] [PubMed] [Google Scholar]
- 36.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 37.Sarrazin MS, Rosenthal GE. Finding Pure and Simple Truths with Administrative Data. JAMA. 2012; 307: 1433–1435. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan-Sarrazin MS, Lin X, Cram P. The impact of paradoxical comorbidities on risk-adjusted mortality of Medicare beneficiaries with cardiovascular disease. Medicare Medicaid Res Rev. 2011; 1:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.