Abstract
Semi-volatile organic compounds (SVOCs) are used extensively in consumer and personal care products; electronics; furniture; and building materials and are detected in most indoor environments. As a result, human exposure to mixtures of SVOCs is wide-spread. However, very few studies have measured biomarkers of exposure to multiple SVOC classes, and exposure determinants have not been thoroughly explored, particularly for young children. In this study, we investigated biomarkers of exposure to SVOCs among children (age 3–6 years), who may experience higher exposures and be more susceptible to adverse health outcomes than other age groups. We enrolled 203 participants in the Toddlers Exposure to SVOCs in Indoor Environments (TESIE) study (181 provided urine samples and 90 provided serum samples). We quantified 44 biomarkers of exposure to phthalates, organophosphate esters (OPEs), parabens, phenols, antibacterial agents and per- and polyfluoroalkyl substances (PFASs); we detected 29 of the 44 biomarkers in > 95% of samples, and many biomarkers were detected at higher median concentrations than those previously reported in the U.S. general population. Demographic characteristics were associated with differences in concentrations. In general, non-Hispanic white race and higher maternal education were associated with lower concentrations, even after adjusting for other potential confounding variables. Our results suggest that outdoor temperature at the time of biospecimen collection may be a particularly important and under-evaluated predictor of biomarker concentrations; statistically significant relationships were observed between 10 biomarkers and outdoor temperature at the time of collection. A complex correlation structure was also observed among the biomarkers assessed. By and large, statistically significant correlations between biomarkers of exposure to phthalates, parabens, phenols, and OPEs were positive. Conversely, although PFASs were positively correlated with one another, they tended to be negatively correlated with other biomarkers where significant associations were observed. Taken together, our results provide evidence that the assessments of SVOC-associated health impacts should focus on chemical mixtures.
Keywords: Semi-volatile organic compounds (SVOCs, children, exposure
1. Introduction
Semi-volatile organic compounds (SVOCs) are used extensively in consumer and personal care products; electronics; furniture; and building materials. SVOCs are detected in many indoor environments [1], and as a result, exposure to SVOCs is widespread [2–6]. For example, data suggest that phthalates (plasticizers), phenols, and organophosphate ester (plasticizers and flame retardants) are among the most abundant compounds measured in indoor air and dust, and their urinary metabolites, which are used as exposure biomarkers, are detected ubiquitously in samples of the U.S. and global populations [2, 7–12]. Past research demonstrates that young children bear a greater exposure burden for many SVOCs, potentially due to differences in children’s behavior and physiology [13–17]. For example, young children put their hands in their mouths more frequently than adults leading to increased ingestion of house dust and therefore higher exposure to some SVOCs [13].
The primary paradigm for evaluating the impacts of SVOCs on children’s health has been to evaluate exposure to single chemicals or a class of chemicals with similar properties. However, the toxicity of real-world SVOC exposures (i.e., those that occur in mixtures and with other chemical, social, and biological stressors) may not be easy to predict based on single chemical studies [18–20]. Understanding the cumulative impacts of chemical mixtures is broadly recognized as a critically important step in advancing public health, but unfortunately, research in this area remains quite limited. Identifying characteristics associated with chemical exposures, and patterns of co-exposure could provide a method of prioritizing health research on mixtures [21].
The overarching goal of the Toddlers Exposure to SVOCs in Indoor Environments (TESIE) Study is to assess young children’s exposure to mixtures of SVOCs and their potential impacts on health and development. As a part of the TESIE Study, we collected samples from the home environment to examine pathways of exposure, and biological specimens for the assessment of biomarkers of SVOC exposure. We prioritized compounds for assessment based on their widespread use, common detection in indoor environments, and demonstrated potential to impact children’s health [2–6, 9, 22–26]. Thus far, we have measured biomarkers of exposure to organophosphate esters (OPEs), phthalates, parabens, phenols, antibacterial agents and per- and polyfluoroalkyl substances (PFASs) for TESIE children. Here, in this first paper, we present and discuss data on biomarkers measured in children’s serum and urine, and examine associations with demographic and seasonal characteristics potentially related to exposure. Additionally, we investigate patterns of co-exposure to mixtures of SVOCs as the identification of these patterns could be helpful in identifying and prioritizing target mixtures of SVOCs to evaluate with respect to potential health risks. As the TESIE Study provides a wealth of data to assess not only pathways of SVOC exposure that drive children’s internal dose, but also to assess health impacts of exposure mixtures, we additionally outline our planned future research.
1. Methods
1.1. Study population
The Newborn Epigenetic STudy (NEST) was a prospective pregnancy cohort designed to identify early exposures associated with epigenetic changes that contribute to children’s health [27]. NEST invited pregnant women (~11 weeks gestation) receiving prenatal care at Duke’s Division of Maternal and Fetal Medicine between from 2005–2011 to participate. A total of 2,595 women enrolled in NEST and their demographics generally reflect those of the catchment area (i.e., racially, ethnically and socioeconomically diverse).
We enrolled a subset of families participating in NEST to the TESIE Study. Available funding dictated the sample size goal of 200 children. We re-contacted NEST mothers by phone, email, or mail and invited to participate in the TESIE Study. NEST Study newsletters, websites, and social media pages also included information about the TESIE Study and asked families interested in participating to contact our research team. Because our research was focused on young children’s exposures, only children six years of age or younger (n=1292; 48.2% of NEST children) were eligible for TESIE. We enrolled a total of 203 children from 190 families in the TESIE Study between August 2014 and April 2016 (Table 1). Enrolled siblings were mainly multiple births (e.g., twins or triplets); however, one mother had two children enrolled in NEST from two different pregnancies. The Institutional Review Board at the Duke University Medical Center reviewed and approved all study protocols. Mothers provided informed consent prior to participation in NEST and mothers or another primary care-giver and legal guardian (e.g., the father or grandparent) provided informed consent for children’s participation in the TESIE Study. No identifiable information was provided to the Centers for Disease Control and Prevention (CDC). As such, the involvement of the CDC laboratory did not constitute engagement in human subjects research.
Table 1:
Selected characteristics of 203 children participating in the TESIE Study.
| Characteristic | N or mean | % or range |
|---|---|---|
| Child sex | ||
| male | 113 | 55.7% |
| female | 90 | 44.3% |
| Child age | ||
| months (average and range) | 53.9 | 38–73 |
| 38–47 months | 34 | 16.7% |
| 48–59 months | 130 | 64.0% |
| 60–73 months | 39 | 19.2% |
| Maternal race/ethnicity | ||
| non-Hispanic white | 84 | 41.4% |
| non-Hispanic black | 75 | 36.9% |
| Hispanic white | 41 | 20.2% |
| Other | 3 | 1.5% |
| Maternal educational | ||
| less than college graduate | 113 | 55.7% |
| college graduate or more | 90 | 44.3% |
| Average temperature during sample collection (°C mean and range) | 15.5 | −4.4–29.4 |
| Season during sample collection | ||
| winter | 53 | 26.1% |
| spring | 45 | 22.2% |
| summer | 34 | 16.7% |
| fall | 71 | 35.0% |
We conducted home visits for all enrolled TESIE Study families. At these visits study staff collected environmental samples, including dust, product wipes (e.g. vinyl flooring and television wipes), silicone wristband monitors, and passive air samples, using standardized protocols. Study staff conducted detailed environmental assessments that included information about the home (e.g., room dimensions and flooring types) and the products and furnishings in the home (e.g., television and furniture sizes and types). Parents completed questionnaires about their child’s environment, behavior and health. During home visits trained study personnel also measured, children’s height, weight and waist circumference. We also collected wipes of children’s hands and foreheads, and obtained biospecimens, including urine, blood and fecal samples from each child. Details on the collection of the urine and blood samples used to assess exposure biomarkers are provided below. Detailed methods regarding other samples collected will be provided in future publications utilizing these samples.
2.2. Urine collection and analysis
During home visits, we provided families with kits for the collection of children’s urine. Neither 24- nor 48-hour complete urine samples were deemed feasible for young children. Thus children collected three individual spot urine samples at convenient times over a single 48-hour period. Collection kits included three urine collection pans (i.e., toilet hats) and three standard polypropylene specimen collection cups. During the collection period, urine was kept frozen in the home. After three voids were collected, samples were transported to Duke’s research laboratory on ice and were stored in polypropylene collection cups at −20°C. Prior to analysis, urine samples were thawed, thoroughly mixed, and equal volumes from each of the three voids were pooled and aliquoted for various analyses.
One pooled urine aliquot was used for the assessment of six OPE metabolites using published methods [28, 29]. A second pooled aliquot was shipped to the Centers for Disease Control and Prevention (CDC) for the assessment of biomarkers of phthalates and other personal care and consumer product chemicals (see Table 2 for the full list of biomarkers). Previously described methods were used to measure 14 phthalate metabolites [6] and two metabolites of di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), an ortho-phthalate ester replacement used in plastics. Additionally, biomarkers of other personal care and consumer product chemicals (i.e., parabens, phenols, antibacterial agents) were measured in urine using previously described methods [30, 31]. Standard QA/QC practices, including laboratory blanks, were used for all analyses. The method detection limit (MDL) was calculated as 3*S0, where S0 is the standard deviation as the concentration approaches zero [32]. S0 was determined from measurements of low-level standards prepared in urine for samples analyzed at the CDC and for laboratory blanks for samples analyzed at Duke.
Table 2:
Detection frequencies and descriptive statistics for biomarkers in children’s urine samples. OPEs (n=181), phenols (n=180), parabens (n=180), antibacterials (n=180), and phthalates and DINCH (n=180). All concentrations corrected for specific gravity.
| Class | Compound | Abbreviation | MDL ng/mL | % Detect | Median ng/mL | Min ng/mL | 95th Percentile ng/mL |
|---|---|---|---|---|---|---|---|
| OPE Metabolites | bis(2-chloro-isopropyl) phosphate | BCIPP | 0.14 | 80 | 0.46 | ND | 1.9 |
| diphenyl phosphate | DPHP | 0.12 | 99 | 2.3 | ND | 17 | |
| bis(1,3-dichloro-2-propyl) phosphate | BDCIPP | 0.07 | 100 | 6.0 | 0.15 | 36 | |
| 1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate | BCIPHIPP | 0.18 | 97 | 1.3 | ND | 6.8 | |
| isopropyl-phenyl phenyl phosphate | ip-PPP | 0.02 | 100 | 7.1 | 0.42 | 25 | |
| tert-butyl phenyl phenyl phosphate | tb-PPP | 0.08 | 94 | 0.23 | ND | 2.3 | |
| Phenols | 2,4-dichlorophenol | 2,4-DCP | 0.10 | 97 | 1.0 | ND | 32 |
| 2,5-dichlorophenol | 2,5-DCP | 0.10 | 98 | 6.6 | ND | 1325 | |
| benzophenone-3 | BP-3 | 0.40 | 98 | 25 | ND | 1297 | |
| bisphenol A | BPA | 0.20 | 100 | 2.1 | 0.45 | 12 | |
| bisphenol F | BPF | 0.20 | 38 | -- | -- | 11 | |
| bisphenol S | BPS | 0.10 | 99 | 0.94 | ND | 7.2 | |
| Parabens | butyl paraben | B-PB | 0.10 | 34 | -- | -- | 9.2 |
| ethyl paraben | E-PB | 1.0 | 62 | 1.4 | ND | 127 | |
| methyl paraben | M-PB | 1.0 | 99 | 57 | ND | 2052 | |
| propyl paraben | P-PB | 0.10 | 100 | 8.5 | 0.10 | 275 | |
| Antibacterials | triclocarban | TCC | 0.10 | 37 | -- | -- | 8.5 |
| triclosan | TCS | 1.7 | 73 | 5.7 | ND | 71 | |
| Phthalate and DINCH Metabolites | monoethyl phthalate | MEP | 1.2 | 100 | 39 | 3.2 | 254 |
| mono-n-butyl phthalate | MBP | 0.40 | 100 | 20 | 2.5 | 91 | |
| mono-isononyl phthalate | MNP | 0.90 | 53 | 1.2 | ND | 7.6 | |
| mono-hydroxybutyl phthalate | MHBP | 0.40 | 98 | 2.8 | ND | 14 | |
| mono-hydroxyisobutyl phthalate | MHiBP | 0.40 | 100 | 7.1 | 0.90 | 25 | |
| mono-isobutyl phthalate | MiBP | 0.80 | 100 | 19 | 1.8 | 77 | |
| monobenzyl phthalate | MBzP | 0.30 | 100 | 17 | 1.3 | 361 | |
| mono-3-carboxypropyl phthalate | MCPP | 0.40 | 99 | 3.8 | ND | 18 | |
| mono-2-ethylhexyl phthalate | MEHP | 0.80 | 72 | 1.9 | ND | 11 | |
| mono-2-ethyl-5-oxohexyl phthalate | MEOHP | 0.20 | 100 | 13 | 1.9 | 48 | |
| mono-2-ethyl-5-hydroxyhexyl phthalate | MEHHP | 0.40 | 100 | 20 | 1.9 | 80 | |
| mono-2-ethyl-5-carboxypentyl phthalate | MECPP | 0.40 | 100 | 31 | 8.8 | 121 | |
| mono carboxyisooctyl phthalate | MCOP | 0.30 | 100 | 21 | 2.2 | 175 | |
| mono carboxyisononyl phthalate | MCNP | 0.20 | 100 | 4.3 | 0.78 | 24 | |
| cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester | MHINCH | 0.40 | 97 | 2.6 | ND | 14 | |
| cyclohexane-1 2-dicarboxylic acid monocarboxyisooctyl ester | MCOCH | 0.50 | 86 | 1.5 | ND | 6.9 |
Specific gravity (SG) was measured in each sample using a digital handheld refractometer (Atago). Urine biomarkers concentrations were specific gravity corrected prior to statistical analysis to account for urine dilution (as in [33]).
2.3. Serum collection and analysis
Serum samples were obtained from children during home visits by a certified phlebotomist using venipuncture or a finger stick, depending on the preference of the parent and child. All samples were collected in serum separator tubes and were processed and stored at −20°C prior to analysis. The following PFASs were quantified in serum at the CDC using previously described methods [34]: linear and branched perfluorooctane sulfonate (n-PFOS and Sm-PFOS, respectively), linear and branched perfluorooctanoate (n-PFOA and Sb-PFOA, respectively), perfluorononanoate (PFNA), perfluorohexane sulfonate (PFHxS), perfluorodecanoate (PFDA), perfluorooctane sulfonamide (FOSA), N-methyl-perfluorooctane sulfonamido acetate (MeFOSAA), and N-ethyl-perfluorooctane sulfonamido acetate (EtFOSAA). As done for urinary metabolites, standard QA/QC practices were used for the assessment of PFASs and MDLs were calculated as described above [32].
2.4. Outdoor temperature
Because the physiochemical properties of SVOCs indicate that exposure may be related to temperature, we additionally evaluate the impact of outdoor temperatures on biomonitoring data. During home visits, the week of specimen collection was also noted. Data on the average outdoor daily temperature during the week of sample collection was obtained from the National Weather Service. Central heating and air conditioning are exceedingly common in North Carolina; 92% of homes had central heating and cooling and an additional 5% had window air condition units. As a result, indoor temperatures were considered to be relatively constant in our study area and were not evaluated.
2.5. Statistical analysis
Based on a prior literature and preliminary analyses indicating that distributions of biomarker concentrations were heavily right skewed, we used non-parametric statistics or log10-transformed biomarkers concentrations prior to analysis. We conducted statistical analyses for biomarkers when detection frequencies were >50%. For these biomarkers, we accounted for values below the MDL by imputing metabolite values from a truncated normal distribution. We imputed 25 datasets in which the upper bound for each metabolite was set at the MDL and imputed values conditional on covariates. We combined measures of association from statistical analyses (described below) using the MIANALYZE procedure in SAS (version 9.4).
We analyzed relationships between biomarker concentrations (log10-transformed) and predictors using regression analysis. Predictors examined in this paper are limited to maternal demographic characteristics (maternal race and education), child age and sex, and average outdoor temperature during the week urine and serum samples were collected. Because the TESIE Study included multiple children from the same family, we used generalized estimating equations (GEEs) that allowed us to account for residual within-family correlations. Fully adjusted models, which account for all covariates of interest, are presented. Beta coefficients from these models were exponentiated and represent the multiplicative change in biomarker concentrations for each unit of increase for continuous variables (child age and outdoor temperature during the sample collection) or relative to the reference category for categorical variables (maternal race and education and child sex).
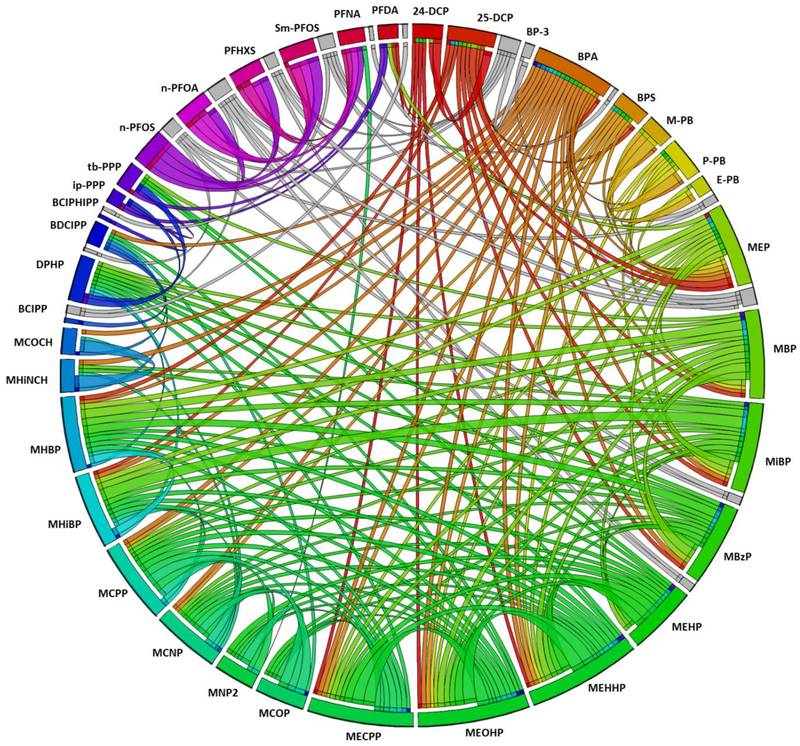
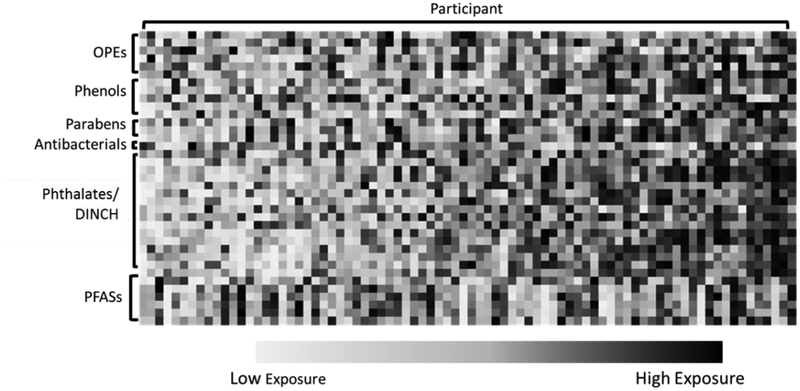
To explore patterns for co-exposure, we assessed correlations between biomarkers using Spearman correlations. Correlations were based on the maximum sample size; all available data were used as opposed to limiting to participants with complete data for all biomarkers. A Circos plot was generated to display Spearman correlations [35]. To provide further insights into patterns of co-exposure, we conducted a secondary assessment among participants with complete data for all biomarkers (n=80). For these participants, we ranked concentrations of each biomarker relative to other TESIE participants (ranks of 1–80) and then generated a heat map displaying the ranks for each participant and biomarker for visual comparison.
3. Results and Discussion
3.1. TESIE cohort
Of the 203 children enrolled in the TESIE Study, slightly more than half were male (55.7%). Children ranged in age from 38 to 73 months at the time of assessment (median 54 months of age; Table 1). Approximately 41% of mothers reported non-Hispanic white race/ethnicity; the remainder reported non-Hispanic black (37%), Hispanic (20%) or other race/ethnicity (2%). Participants reporting other race/ethnicity were excluded from adjusted analyses (n=3). The majority of TESIE mothers had not completed a four-year college degree at the time their child was born (55.7%). Biospecimens were collected from the fall of 2014 to the spring of 2016, with the majority of samples collected in the fall and winter months. A total of 181 TESIE participants (89%) provided urine and 90 (44%) provided serum [80 (39%) participants provided both urine and serum]. One urine sample was lost to processing after the assessment of OPEs and prior to the assessment of other urinary biomarkers. Supplemental Table S1 provides the demographic characteristics for children with each type of biospecimen.
Compared to the parent NEST cohort, TESIE had a slightly greater percentage of non-Hispanic white participants (NEST: 34% non-Hispanic white, 44% non-Hispanic black, 18% Hispanic). TESIE Study mothers also tended to have greater educational attainment than NEST mothers; approximately 33% of NEST mothers were 4-year college graduates at the time of their child’s birth.
3.2. Detection and distribution of biomarkers
Detection frequencies and distributions for all assessed urinary biomarkers are displayed in Table 2. Exposure to SVOCs was ubiquitous among children in the TESIE cohort; 29 of the 44 biomarkers measured were detected in >95% of samples. OPE urinary metabolites were detected frequently (>80% of samples for all metabolites); isopropyl-phenyl phenyl phosphate (ip-PPP) was detected at the highest median concentration, followed by bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) and diphenyl phosphate (DPHP). Phenols, except for bisphenol F, and propyl (P-PB) and methyl paraben (M-PB) were detected in nearly all samples. M-PB concentrations were higher than other parabens with a median concentration of 57 ng/mL. Triclosan was detected in 73% of samples. All of the phthalate and DINCH metabolites assessed were detected in > 53% of samples and the majority were detected in nearly every sample. Monoethyl phthalate (MEP) was detected at the highest median concentration (39 ng/mL) while monoisononyl phthalate (MNP) concentrations were, on average, the lowest of the phthalate and DINCH metabolites assessed (median=1.2 ng/mL). Linear and branched PFOS, branched PFOA, PFHxS and PFNA were detected in nearly every serum sample obtained from TESIE children (Table 3). PFDA was detected in 59% of samples; other PFASs were detected in <50% of samples with the exception of FOSA which was not detected in any samples.
Table 3:
Detection frequencies and descriptive statistics PFASs in children’s serum samples (n=90).
| Biomarkers | Abbreviation | % Detecta | Median ng/mL | Min ng/mL | 95th Percentile ng/mL |
|---|---|---|---|---|---|
| 2-(N-ethyl-perfluorooctane sulfonamido) acetate | EtFOSAA | 1 | -- | -- | -- |
| 2-(N-methyl-perfluorooctane sulfonamido) acetate | MeFOSAA | 42 | -- | -- | 0.80 |
| Perfluorodecanoate | PFDA | 59 | 0.20 | ND | 0.50 |
| perfluorohexane sulfonate | PFHxS | 100 | 0.70 | 0.20 | 2.0 |
| Perfluorononanoate | PFNA | 99 | 0.40 | ND | 1.0 |
| perfluorooctane sulfonamide | FOSA | 0 | -- | -- | -- |
| branched perfluorooctanoates | Sb-PFOA | 49 | -- | -- | 0.44 |
| perfluoromethylheptane sulfonates | Sm-PFOS | 98 | 0.50 | ND | 1.3 |
| n-perfluorooctanoate | n-PFOA | 100 | 1.6 | 0.40 | 3.3 |
| n-perfluorooctane sulfonate | n-PFOS | 99 | 2.0 | ND | 5.0 |
Method detection limit = 0.1 ng/mL for all PFASs.
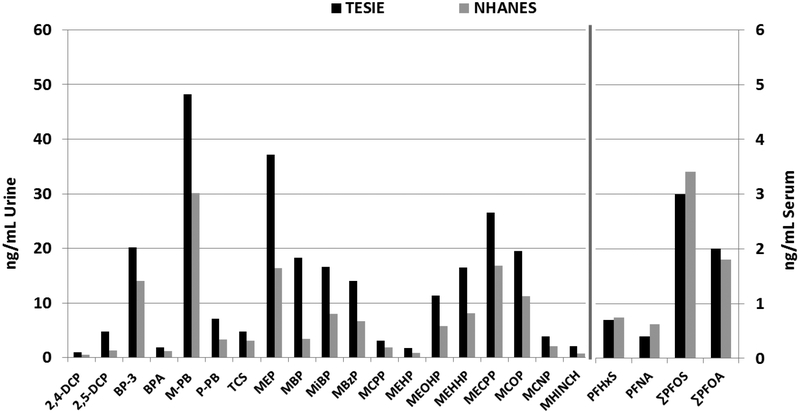
Biomarkers of exposure to phthalates, phenols, parabens and antibacterials were available for a pilot study of 122 children age 3–5 participating the 2013–2014 National Health and Nutrition Examination Survey (NHANES [36]) and were used as a comparison to TESIE (comparing raw concentrations as methods of accounting for dilution—specific gravity and creatinine—differed between studies). In general, children in TESIE had higher median concentrations of urinary biomarkers than children in NHANES (Figure 1; [36]). For example, parabens were 1.4 (methyl paraben [M-PB]) to 2.2 (propyl paraben [P-PB]) times higher among TESIE children. Many of the phthalate metabolites, and in particular monobenzyl phthalate, were also higher in TESIE children. Similarly, concentrations of cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester (MHINCH), an emerging phthalate metabolite were slightly higher in TESIE children than children in NHANES, and are also higher than those reported previously similar aged children in other regions (e.g. [37]).
Figure 1:
Comparison of median biomarker concentrations between TESIE and two subsamples of 2013–2014 NHANES children age 3–5 years (122 children for urinary metabolites [36] and 118 for serum PFASs [34]).
Concentrations of serum PFAS have also been measured in a representative subsample of children participating in 2013–2014 NHANES (age 3–5; n=118; [34]). Median serum PFASs concentrations were very similar to or slightly lower among TESIE children as compared to NHANES (Figure 1; [34]). Differences in TESIE and NHANES biomarker concentrations could reflect the presence of confounding factors. TESIE children were different from NHANES with respect to other demographic characteristics. For example, although children in TESIE were generally racially and ethnically diverse, the percentage of Hispanic children was lower in TESIE, particularly compared to the subset of children 3–5 with urine biomarker measures (65% were Hispanic in the NHANES pilot sample [36]). However, as Hispanic ethnicity was related to higher levels of urinary biomarkers for many compounds in our analyses, we would have expected these differences to be related to lower levels of exposure in TESIE. In addition, children in the NHANES sample were from geographic locations throughout the United States and our study sample was only from North Carolina.
3.3. Race and ethnicity as predictors of children’s biomarker concentrations
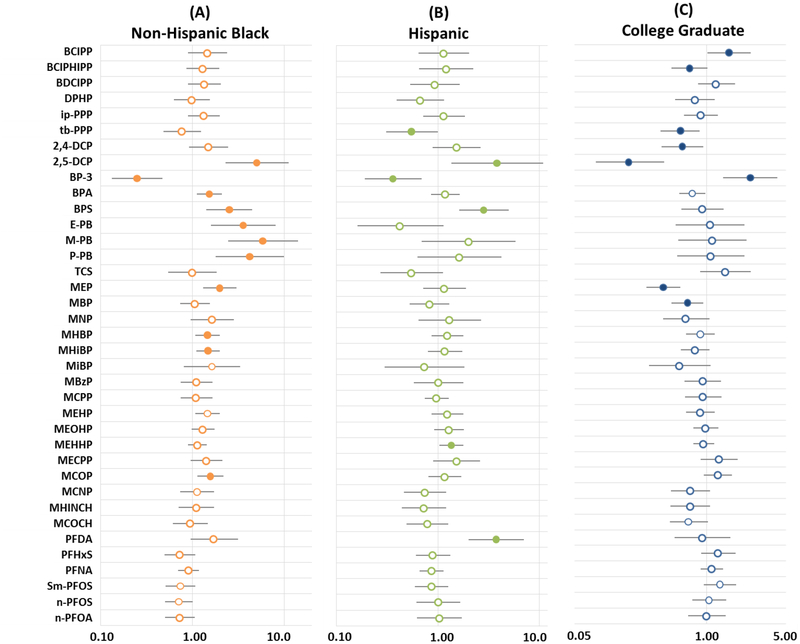
Associations were observed between maternal characteristics (i.e., race/ethnicity and educational attainment) and several of the biomarkers assessed in TESIE children. After adjustment for other covariates (i.e., maternal education, child’s age and sex, and average temperature during sample collection), non-Hispanic black or Hispanic race and ethnicity was generally associated with higher concentrations of urinary biomarkers than non-Hispanic white race (Figure 2; Supplemental Table S2). In particular, among both non-Hispanic black and Hispanic children, 2,5-dichlorophenol was elevated relative to non-Hispanic whites. For example, 2,5-dichlorophenol urinary concentrations were nearly 4 times higher in non-Hispanic blacks compared to whites (10β=4.95, 95% Confidence Interval (CI): 2.25–10.91). Our findings are consistent with previous reports suggesting that 2,5-dichlorophenol exposure may be higher among non-Hispanic blacks and Hispanics [5], though the reasons for this association remain unknown. Benzophenone-3, which is commonly used in sunscreen, was significantly lower among non-Hispanic blacks and Hispanics relative to non-Hispanic white participants, potential reflecting greater sunscreen use by non-Hispanic white participants [38]. Urinary concentrations of parabens also were significantly higher in non-Hispanic blacks than other participants. Parabens are commonly used as antimicrobial preservatives in cosmetics, pharmaceuticals, and food and beverage processing. Previously, paraben urinary concentrations were found to be higher in women, particularly non-Hispanic black women, a finding that was attributed to greater use of personal care products [39]. Although data suggest that adults use more personal care products than children on average, young children may use body/face lotion and sunscreen more frequently than adults [40]. It is also possible that children are incidentally exposed to personal care products used by other members of their families. Data from TESIE children are consistent with past research and suggest that concentrations of several phthalate metabolites are higher among non-Hispanic blacks [e.g. MEP, MHBP, mono-hydroxyisobutyl phthalate, mono carboxyisooctyl phthalate (MCOP), and mono-2-ethylhexyl phthalate]. With the exception of PFDA which was significantly higher in serum samples from Hispanic children (10β=3.76, 95% Confidence Interval (CI): 2.00–7.04), PFASs serum concentrations were generally lower among non-Hispanic black and Hispanic children compared to non-Hispanic white children, though results did not reach statistical significance. Similar PFASs associations were reported previously [41, 42].
Figure 2:
Estimated multiplicative change in biomarker concentration by maternal characteristics. Change for (A) non-Hispanic black and (B) Hispanic children compared to non-Hispanic white children and (C) for the children of college graduates compared to children of mothers with less education. Values >1 indicate elevated concentration relative to the reference group. All analyses adjusted for child age and sex; maternal race/ethnicity and education; and average outdoor temperature at the time of collection. Filled circles are used where p<0.05 and bars indicate 95% confidence intervals.
3.4. Maternal educational attainment as a predictor of biomarker concentrations
Patterns of association were not as clear for maternal education. Higher education (i.e., completing a four year college degree) tended to be associated with decreases in urinary concentrations of phenols (2,5-dichlorophenol, 2,4-dichlorophenol and BPA) and some phthalate and OPE metabolites (ip-PPP, tb-PPP and MEP). There are many possible explanations for this pattern including differences in housing stock [5]. Interestingly, bis(2-chloro-isopropyl) phosphate (BCIPP) concentrations were high among children of college graduates, but concentrations of 1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate (BCIPHIPP) were lower. Both compounds are thought to be metabolites of the same parent (TCIPP), but the two have not been significantly correlated in past assessments [43], potentially suggesting different metabolic pathways.
Benzophenone-3 was significantly higher among children of mothers who completed a college degree (10β=2.69; 95% CI: 1.45, 4.98). Data suggest that this relationship could be related to the use of sunscreen [42]. Higher educational attainment also tended to be associated with PFASs. These data are consistent with NHANES data suggesting positive correlation between the poverty to income ratio and several PFASs [41, 42]. Reasons for this pattern are unknown but may be the result of diet and lifestyle differences [41].
3.5. Sex as a predictor of biomarker concentrations
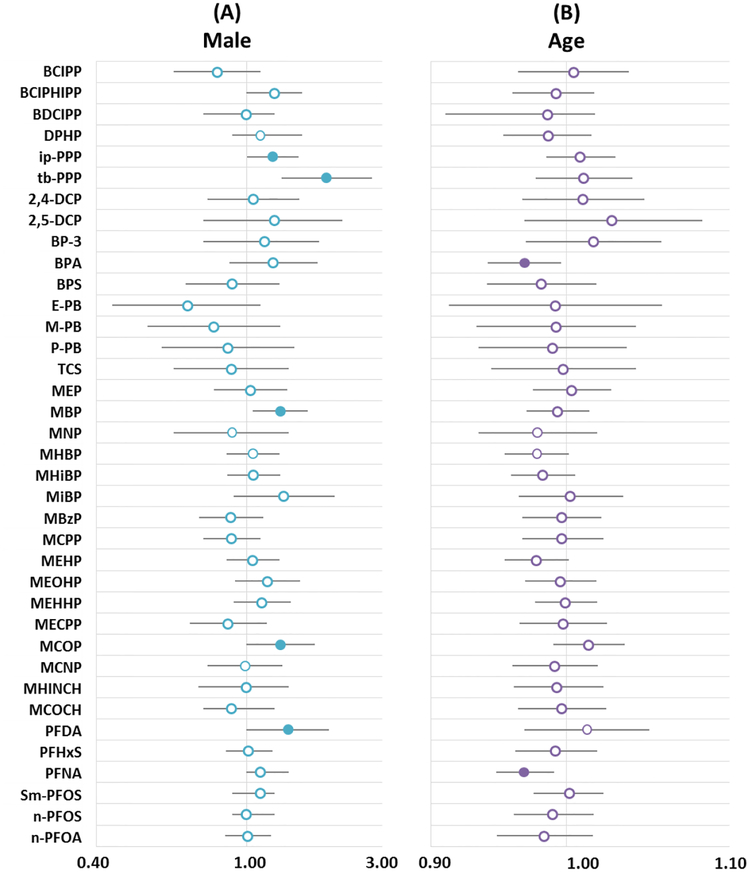
In general, biomarker concentrations were lower among males than females (Figure 3). The use of personal care products (which is more common among women) has been posited as a possible explanation for this pattern for some SVOCs among adults [4, 39]. However, given the ages of the participants in the TESIE Study, we would expect sex differences in the use of personal care products to be less pronounced than those of adults (e.g. on average, young girls generally do not wear make–up with the same frequency as adult women). Interestingly, more statistically significant associations with child sex were observed for BPA and PFNA, compounds for which the primary route of exposures is likely diet [44].
Figure 3:
Estimated multiplicative change in biomarker concentration. (A) Multiplicative change in biomarker concentrations among male children as compared to female children and (B) change for each month increase in age. All analyses adjusted for child age and sex; maternal race/ethnicity and education; and average outdoor temperature at the time of collection. Filled circles are used where p<0.05 and bars indicate 95% confidence intervals.
3.6. Age as a predictor of biomarker concentrations
We originally hypothesized that children’s age could be inversely associated with exposure biomarkers among TESIE children due to expected age differences in exposure-related behaviors (e.g., mouthing behavior). The direction of observed associations, however, was variable. OPEs and phenols tended to increase with each year of age. Associations between child age and three individual phthalate biomarkers were positive and statistically significant (MBP, MNP, and MCOP); however, other associations were mixed. Child age was generally positively associated with PFASs, though associations did not reach statistical significance. Our data suggest parabens may be an exception to this pattern; data suggest that paraben concentrations in urine may decrease with age (though associations were imprecisely estimated and not statistically significant). It is possible that patterns would differ in a younger cohort. Although we had planned to enroll children at younger ages, the median age of children in the TESIE study was 54 months (range 38–73 months), a point at which hand-to-mouth behavior may already have decreased relative to behavior frequencies in toddlers.
3.7. Outdoor temperature as a predictor of biomarker concentrations
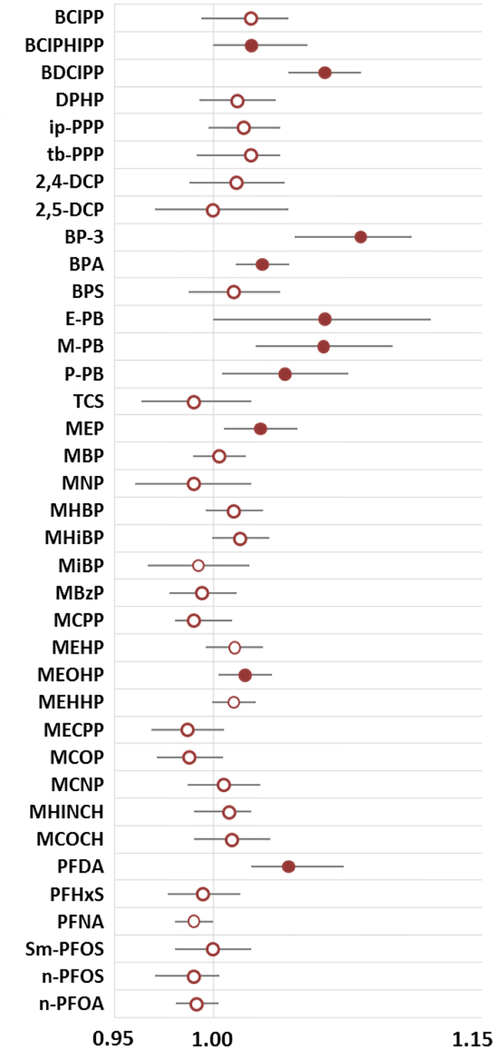
Interestingly, average weekly temperature at the time of sample collection was significantly and positively associated with a number of urinary biomarkers (Figure 4). This finding has been reported previously for metabolites of organophosphate flame retardants [2, 45], a finding which may be attributable to their use in cars. In the summer sun, it is possible that high temperatures increase volatilization and exposure inside the car. Benzophenone-3 was also significantly associated with temperature which could be attributed to increased sunscreen use in warmer months. In addition to benzophenone-3, Ferguson et al. recently reported higher concentrations of MEP, M-PB and P-PB, among sunscreen users [46]; we also observed statically significant increases in these compounds in warmer sample collection periods. Other possible explanations for the patterns we observed include increased volatilization and as a result, higher ambient air concentrations which could increase inhalation or dermal exposures. Indeed, emission rates of SVOCs from products found in home environments are positively associated with indoor temperatures [47]. Although the vast majority of the TESIE participants’ homes had central air conditioning and heating (92%) suggesting that indoor temperatures would be relatively stable throughout the year, we did not measure indoor temperatures. Conversely, outdoor temperature varies considerably in central NC and could be related to exposure kinetics for the assessed compounds (e.g. increased volatilization and exposure in cars in warmer months, or working outside in the summer).
Figure 4:
Estimated multiplicative change in biomarker concentration for a one degree Celsius in average high temperature during the sample collection period. All analyses adjusted for child age and sex and for maternal race/ethnicity and education. Filled circles are used where p<0.05 and bars indicate 95% confidence intervals.
Seasonal changes in behavior could also explain patterns. For example, participants may spend more time indoors or outdoors when it is hotter (or colder depending on the climate of a region). It is also possible that summer clothing exposes more skin, which could, in turn, enhance dermal uptake of compounds found in the home. Similarly, increased humidity and sweating in summer months could impact dermal absorption of SVOCs. For example, recent research suggests that children’s clothing is a possible source of exposure to some SVOCs (e.g. BPA, BPS and BP-3) [48]. It is possible that dermal absorption of these compounds is greater in warmer months. Evaluating the primary routes of exposure for individual SVOCs could provide additional insights into associations with temperature. Regardless of the reasons for temperature associations, this finding is particularly important, at least for some chemicals, in epidemiologic studies which aim to capture long-term exposure with spot urine samples. Seasonal variation in exposure biomarkers could increase exposure misclassification if urinary concentrations are assessed at a single point in time or during a single season.
3.8. Relationships between biomarkers
Figure 5 and Supplemental Table S3 depict Spearman correlations between exposure biomarkers. Statistically significant correlations between biomarkers were common and generally positive. Correlations tended to be strongest between chemicals in the same class and particularly for metabolites of the same parent [e.g., the two DINCH metabolites, MHINCH and cyclohexane-1 2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH) were strongly positively correlated with an rs=0.93 and p<0.0001]. Interestingly, biomarkers of exposure to some compounds with very different use patterns were also correlated. For example, BDCIPP, the metabolite of the flame retardants TDCIPP, was correlated with several phthalate metabolites. Similarly, BPA was correlated with BDCIPP. This could suggest a common source or pathway of exposure; perhaps some children put their hands in their mouths more frequently, leading to higher levels of exposure to chemicals commonly detected in household dust. In contrast, although PFASs were strongly correlated with one another, correlations with other compounds were generally negative. For some PFASs, past research suggests the predominant pathway of exposure may be diet and drinking water while the home environment and consumer products may be more important sources of sources of exposure to many of the other compounds assessed here [49–51]. Different pathways of exposure could explain negative associations between PFASs and other compounds. It is also possible that the differences in the toxicokinetics of PFASs and the other biomarkers could explain inverse associations; the elimination half-life of PFASs is on the order of years whereas the other compounds assessed in the TESIE Study are rapidly metabolized (i.e., t1/2 ~ hours or days).
Figure 5:
Circos plot displaying Spearman correlations between biomarkers. Correlations are represented by ribbons (i.e., lines) between variables with the width of the ribbon corresponding to the strength of the correlation such that a wider band represents a stronger correlation. Rainbow colored bands are used for positive correlations (using a different color for each biomarker) and negative correlations are shown in gray scale. Only Spearman correlations >0.25 or <−0.25 and statistically significant at p<0.05 are displayed.
Figure 6 displays the relative ranking of each biomarker for individual participants’ exposure (organized from the lowest average ranking across all biomarkers to the highest). Interestingly, despite the correlations between biomarkers (Figure 5), exposure profiles of individual participants varied considerably; there were no participants that had below average levels for all biomarkers assessed. The participant with the lowest average biomarker ranking had urinary concentrations of triclosan and several OPEs that were above average. Conversely, the participant with the highest average biomarker ranking had below average levels of several biomarkers (i.e., E-PB and triclosan). Similar to the correlation analysis, overall patterns appeared to differ between PFASs and other biomarkers.
Figure 6:
Biomarker ranking relative to the cohort average for the 80 TESIE participants with both serum and urinary biomarker measurements. Each column depicts rankings for single a participant and each row depicts one biomarker. Blue colors indicate below average concentrations and pink indicates above average concentrations.
Cumulatively, our investigation suggests that different classes of SVOCs in the home environment are mainly positively correlated, although still quite varied among individuals. The primary pathway of exposure may be an important driver of relationships and warrants further investigation. Regardless of the reasons for associations, correlations between SVOCs in the home are likely to complicate toxicological and epidemiologic assessments of single chemicals or classes of chemicals. Although methods of assessing mixtures have come to the forefront in recent years as a research priority, available methods are data intensive.
Our results should be interpreted in the context of several important limitations. Although our assessment of biospecimen was one of the most comprehensive to date in terms of the number of compounds assessed, our study population was relatively small. In addition, there is the potential for selection bias as a result of the strategy we used to recruit NEST families for our follow-up study. Although our study population is similar to the overall NEST cohort, TESIE mothers were more likely to be non-Hispanic white and highly educated (the variables were included in adjusted analysis). Nonetheless, the diversity of our cohort allowed for the evaluation of relationships between race/ethnicity and biomarker concentrations. All data from this study were collected from North Carolina children and as a consequence, results may not be generalizable to other populations. This is particularly true given the associations we observed between many of the assessed biomarkers and outdoor temperature during the sample collection period because patterns of correlation will differ based on geography. In addition, while the biomarkers assessed provide a snap shot of exposure, they may not reflect exposure patterns over time, particularly for rapidly eliminated compounds. In an attempt to address this issue we pooled samples collected over a 48 hour period for urinalysis, nonetheless, a single 48 hour period may not accurately capture longer-term exposure. In addition, although many of the SVOCs we assessed are commonly detected in indoor environments, we are not able to determine the relative contributions of exposure from diet, indoor air and dust or other sources using biomarker information alone. This will be explored in our future research using data available from this project. Thus far we have assessed 44 biomarkers of exposure to SVOCs using targeted analytic approaches. While we consider the assessment of these mixtures to be a valuable contribution, additional SVOCs found in the home environment (e.g., pesticides)—as well as other compounds or agents (VOCs, metals, particulate matter, etc.)—could impact children’s health and should be considered in future assessments. In addition to assessing exposure to known indoor contaminants, non-targeted analytic approaches could be particularly important in identify relevant mixtures, and will be a focus area for the TESIE Study in the future.
3.9. Future Directions
Understanding patterns of co-exposure may help to prioritize common SVOC mixtures for assessments of potential health impacts in toxicological and epidemiologic studies. Work investigating the impacts of SVOCs on children’s health is ongoing in the TESIE Study and will focus on the impacts of a child’s exposome on growth, development and long-term health. Equally important in promoting children’s health is the identification of exposure sources and pathways in the home environment. Extensive information was collected during TESIE home visits pertaining to housing characteristics and potential modifiers of exposure (e.g., cleaning frequency and time-activity patterns). Coupled with samples of numerous products and surfaces in the home, these data will be used to assess the specific exposure contributors and dominant exposure routes for SVOCs. For example, Phillips and Hammel (2018) investigates pathways of exposure to OPEs in TESIE and reports that hand-to-mouth contact and dermal absorption are important pathways of exposure. Investigating pathways of exposure to other compounds may help to explain the correlations we observed between SVOCs and could be useful in identifying populations susceptible to higher than background exposures.
4. Conclusions
Taken together, data from the TESIE Study clearly demonstrate universal, but quite variable exposure to SVOCs (as indicated by biomarker concentrations). Many of the biomarkers quantified correlated suggesting exposure to multiple chemicals, and perhaps that many of the exposure pathways or sources are similar in children (e.g. house dust exposures). Our results additionally suggest that certain demographic characteristics (e.g., race/ethnicity, socioeconomic status) are likely associated with higher exposure to some SVOCs, and that seasonality in biospecimen collections may be an under-evaluated confounder in some epidemiological studies. Lastly, this research highlights the importance of considering exposure to multiple classes of compounds (e.g. mixtures) as researchers continue to focus on exploring the role of environmental chemical exposures in human diseases (e.g. asthma, autism, diabetes, etc).
Supplementary Material
Acknowledgements
Funding for TESIE was provided by grants from the U.S. Environmental Protection Agency (Grant 83564201) and NIEHS (R01 ES016099). We also gratefully acknowledge the NEST study team, particularly Cathrine Hoyo and Susan Murphy (PIs of the NEST cohort) and Rachel McGuire (NEST Study database manager). The authors wish to thank Kayoko Kato, Manori Silva, Prabha Dwivedi, and Tao Jia for the quantification of chemicals biomarkers at the CDC. We also thank Brett Doherty for his assistance in developing statistical code for multiple imputations. We additionally thank TESIE families for their participation in this research.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References:
- 1.Weschler CJ and Nazaroff WW, Semivolatile organic compounds in indoor environments. Atmospheric Environment, 2008. 42(40): p. 9018–9040. [Google Scholar]
- 2.Hoffman K, et al. , Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environmental Science & Technology Letters, 2017. 4(3): p. 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calafat AM, et al. , Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect, 2007. 115(11): p. 1596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva MJ, et al. , Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect, 2004. 112(3): p. 331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye X, et al. , Urinary concentrations of 2,4-dichlorophenol and 2,5-dichlorophenol in the U.S. population (National Health and Nutrition Examination Survey, 2003–2010): trends and predictors. Environ Health Perspect, 2014. 122(4): p. 351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevention)., C.C.f.D.C.a., Fourth National Report on Human Exposure to Environmental Chemicals. Available: http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf [accessed 17 December 2017], 2015.
- 7.Rudel RA, et al. , Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol, 2003. 37(20): p. 4543–53. [DOI] [PubMed] [Google Scholar]
- 8.Abb M, et al. , Phthalates in house dust. Environ Int, 2009. 35(6): p. 965–70. [DOI] [PubMed] [Google Scholar]
- 9.Hauser R and Calafat AM, Phthalates and human health. Occup Environ Med, 2005. 62(11): p. 806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zota AR, Calafat AM, and Woodruff TJ, Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect, 2014. 122(3): p. 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitro SD, et al. , Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ Sci Technol, 2016. 50(19): p. 10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudel RA, et al. , Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ Sci Technol, 2010. 44(17): p. 6583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moya J, Bearer CF, and Etzel RA, Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics, 2004. 113(4 Suppl): p. 996–1006. [PubMed] [Google Scholar]
- 14.Hubal EA, et al. , The challenge of assessing children’s residential exposure to pesticides. J Expo Anal Environ Epidemiol, 2000. 10(6 Pt 2): p. 638–49. [DOI] [PubMed] [Google Scholar]
- 15.Toms LM, et al. , Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environ Health Perspect, 2009. 117(9): p. 1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt CM, et al. , Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol, 2014. 48(17): p. 10432–8. [DOI] [PubMed] [Google Scholar]
- 17.Larsson K, et al. , Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int, 2014. 73: p. 323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rappaport SM, Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol, 2011. 21(1): p. 5–9. [DOI] [PubMed] [Google Scholar]
- 19.Juarez PD, et al. , The public health exposome: a population-based, exposure science approach to health disparities research. Int J Environ Res Public Health, 2014. 11(12): p. 12866–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlin DJ, et al. , Unraveling the health effects of environmental mixtures: an NIEHS priority. Environ Health Perspect, 2013. 121(1): p. A6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapraun DF, et al. , A Method for Identifying Prevalent Chemical Combinations in the U.S. Population. Environ Health Perspect, 2017. 125(8): p. 087017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto M, Hirata-Koizumi M, and Ema M, Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul Toxicol Pharmacol, 2008. 50(1): p. 37–49. [DOI] [PubMed] [Google Scholar]
- 23.Jurewicz J and Hanke W, Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health, 2011. 24(2): p. 115–41. [DOI] [PubMed] [Google Scholar]
- 24.Rappazzo KM, Coffman E, and Hines EP, Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int J Environ Res Public Health, 2017. 14(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carignan CC, et al. , Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environmental Health Perspectives, 2017. 125(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bledzka D, Gromadzinska J, and Wasowicz W, Parabens. From environmental studies to human health. Environment International, 2014. 67: p. 27–42. [DOI] [PubMed] [Google Scholar]
- 27.Hoyo C, et al. , Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). Bmc Public Health, 2011. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt CM, et al. , Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int, 2016. 94: p. 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Eede N, et al. , Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A, 2013. 1303: p. 48–53. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, et al. , Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem, 2005. 77(16): p. 5407–13. [DOI] [PubMed] [Google Scholar]
- 31.Ye X, et al. , Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2006. 844(1): p. 53–9. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JK, Quality Assurance of Chemical Measurements, ed. C. Lewis Publishers, MI: 1987. [Google Scholar]
- 33.Boeniger MF, Lowry LK, and Rosenberg J, Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J, 1993. 54(10): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 34.Ye X, et al. , Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health, 2018. 221(1): p. 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzywinski M, et al. , Circos: an information aesthetic for comparative genomics. Genome Res, 2009. 19(9): p. 1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calafat AM, et al. , Co-exposure to non-persistent organic chemicals among American pre-school aged children: A pilot study. International Journal of Hygiene and Environmental Health, 2017. 220(2): p. 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correia-Sa L, et al. , Exposure of Portuguese children to the novel non-phthalate plasticizer di-(iso-nonyl)-cyclohexane-1,2-dicarboxylate (DINCH). Environ Int, 2017. 102: p. 79–86. [DOI] [PubMed] [Google Scholar]
- 38.Zamoiski RD, et al. , Self-reported sunscreen use and urinary benzophenone-3 concentrations in the United States: NHANES 2003–2006 and 2009–2012. Environmental Research, 2015. 142: p. 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calafat AM, et al. , Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect, 2010. 118(5): p. 679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manova E, et al. , Use Patterns of Leave-on Personal Care Products among Swiss-German Children, Adolescents, and Adults. International Journal of Environmental Research and Public Health, 2013. 10(7): p. 2778–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato K, et al. , Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol, 2011. 45(19): p. 8037–45. [DOI] [PubMed] [Google Scholar]
- 42.Tyrrell J, et al. , Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001–2010. Environment International, 2013. 59: p. 328–335. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman K, et al. , Predictors of urinary flame retardant concentration among pregnant women. Environ Int, 2017. 98: p. 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalowicz J, Bisphenol A - Sources, toxicity and biotransformation. Environmental Toxicology and Pharmacology, 2014. 37(2): p. 738–758. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman K, et al. , Predictors of urinary flame retardant concentration among pregnant women. Environ Int, 2017. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson KK, et al. , Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. Journal of Exposure Science and Environmental Epidemiology, 2017. 27(3): p. 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang Y and Xu Y, Emission of phthalates and phthalate alternatives from vinyl flooring and crib mattress covers: the influence of temperature. Environ Sci Technol, 2014. 48(24): p. 14228–37. [DOI] [PubMed] [Google Scholar]
- 48.Xue J, Liu W, and Kannan K, Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing. Environ Sci Technol, 2017. 51(9): p. 5279–5286. [DOI] [PubMed] [Google Scholar]
- 49.Trudel D, et al. , Estimating consumer exposure to PFOS and PFOA. Risk Anal, 2008. 28(2): p. 251–69. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman K, et al. , Monitoring Indoor Exposure to Organophosphate Flame Retardants: Hand Wipes and House Dust. Environ Health Perspect, 2015. 123(2): p. 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stapleton HM, et al. , Flame retardant associations between children’s handwipes and house dust. Chemosphere, 2014. 116: p. 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.