Abstract
Background
The male excess in childhood cancer incidence is well established; however, the underlying biologic mechanisms remain unknown. Examining the association between male sex and childhood cancer by single year of age by tumor type may highlight important periods of risk such as variation in growth and hormonal changes, which will inform etiologic hypotheses.
Methods
Using data from the Surveillance, Epidemiology and End Results (SEER) 18 registries (2000–2015), incidence rate ratios (IRR) and 95% confidence intervals (95% CI) were estimated as the measure of association between male sex and childhood cancer by single year of age (0–19).
Results
The IRR for male cancer overall was 1.19 (95% CI: 1.18–1.20) and was similar in magnitude at nearly every year of age. Burkitt lymphoma was strongly associated with male sex (IRRs ≥2 at each year of age). Increased incidence was observed among males for acute lymphoblastic leukemia, Hodgkin and Non-Hodgkin lymphomas for nearly all years of age. Medulloblastoma was the only central nervous system tumor with a significant male predominance at nearly every age. Male sex displayed a consistent inverse association with nephroblastoma and thyroid carcinoma over the ages studied.
Conclusions
Male sex was positively associated with most cancers. The higher incidence rates observed in males remained consistent over the childhood and adolescent periods suggesting that childhood and adolescent hormonal fluctuations may not be the primary driving factor for the sex disparities in childhood cancer. The observed incidence disparities may be due to sex differences in exposures, genetics, or immune responses.
Keywords: childhood cancer incidence, epidemiology, sex differences
INTRODUCTION
The male excess in childhood cancer incidence is well-recognized1, but few studies have focused on sex differences in incidence during the childhood and adolescence periods by single year of age.2 The biologic mechanisms underlying the sex differences in childhood cancer incidence remain largely unexplored. Previously, we estimated the association between male sex and childhood cancer and found it to be independent of established perinatal risk factors3, most notably, birth weight4, an established risk factor for many types of childhood cancer.5 These findings suggest that sex itself may be a biologic risk factor for childhood cancer.
In 1995, Gurney et al.2 characterized the incidence rates of childhood cancer by single year of age (0–14 years) and sex among children diagnosed with cancer using Surveillance, Epidemiology, and End Results (SEER) data (1974–1989). Taking a similar approach but using contemporary tumor classifications and including children aged 0–19 years, we used SEER 18 data (2000–2015) to estimate the incidence rate ratios (IRR) and 95% confidence intervals (95% CI) for sex and childhood cancer type. We estimated the association overall and by single year of age to assess trends in incidence over the childhood and adolescent periods, which may shed light on potential mechanisms such as hormonal variation or periods of rapid growth that may contribute to the increased cancer incidence among males.
METHODS
Using data from the Surveillance, Epidemiology, and End Results (SEER) Program we estimated incidence rate ratios (IRR) for males compared to females for childhood cancer overall and by tumor type for all ages combined and by single year of age at diagnosis. Cases were identified from the SEER 18 Registry6 (2000–2015), which includes cases from Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, the Alaska Native Tumor Registry, Greater California, Greater Georgia, Kentucky, Louisiana, and New Jersey. Individuals with cancer diagnosed between 2000–2015 and aged 0–19 years were included.
The following International Classification of Childhood Cancer (ICCC), 3rd Edition7 categories were included based on sample size for stratification by sex and year of age: I Leukemias ([Ia Acute lymphoid leukemia, Ib Acute myeloid leukemia, Ic Chronic myeloproliferative diseases, Id Myelodysplastic syndrome and other myeloproliferative diseases), II Lymphomas (IIa Hodgkin lymphoma, IIb Non-Hodgkin lymphoma, IIc Burkitt lymphoma), III Central Nervous System (IIIa.1 Ependymomas, IIIb Astrocytomas, IIIc.1 Medulloblastoma, IIIc.2 Primitive Neuroectodermal Tumor [PNET], IIIc.4 Atypical teratoid/rhabdoid tumor), IVa Neuroblastoma, V Retinoblastoma, VIa.1 Nephroblastoma, VIIa Hepatoblastoma, VIII Bone (VIIIa Osteosarcoma, VIIIb Chondrosarcoma, VIIIc Ewing tumor and related sarcomas of bone [Ewing sarcoma]), IX Soft Tissue Sarcomas (IXa Rhabdomyosarcoma and IXb Fibrosarcoma, IXd.1 Ewing tumor and Askin tumor of soft tissue, and IXd.2 peripheral PNET [pPNET] of soft tissue), X Germ cell tumors (GCT) (Xa Intracranial and intraspinal GCT, Xb Extracranial and extragonadal GCT, Xc Malignant gonadal GCT), XI Other malignant epithelial neoplasms and malignant melanomas (XIb Thyroid carcinoma, XId Malignant melanoma, XIf.1 Salivary gland carcinoma).
Statistical analysis
Frequency counts of cancer cases among males and females for each year of age from SEER are presented (Supplemental Table 1). The incidence rate ratio (IRR) denominator was the population counts for each year of age for males and females from SEER 18. Incidence rates, calculated as cases per million per year, were estimated for males and females for all ages combined (age-adjusted) and by single year of age. IRRs and the corresponding 95% confidence intervals (95% CI), estimated using a Normal distribution, were calculated with female serving as the referent population. For each year of age, IRRs for cancers with ≤10 cases were not estimated. Analyses were done using SAS v9.4 and SAS/STAT v14.1. (SAS Institute, Cary, NC). Two-sided hypothesis tests for statistical significance were conducted using an alpha of 0.05.
RESULTS
There were 71,906 cases of childhood cancer identified in SEER 18 (2000–2015). Fifty-three percent were male. The average age at diagnosis for males and females was 9.7 (standard deviation [SD]: 6.7) and 10.0 (SD: 6.4) years, respectively. Overall, male sex was significantly associated with childhood cancer (incidence rate ratio [IRR]: 1.19; 95% CI: 1.18–1.20) (Figure 1, Supplemental Table 2). Male sex was significantly associated with a majority of tumor types, with the exceptions being nephroblastoma, extracranial/extragonadal germ cell tumors (GCT), thyroid carcinoma, malignant melanoma, and salivary gland carcinoma, which were all inversely associated with male sex. The following sections are categorized by ICCC designations. All IRRs for each tumor type by year of age can be found in Supplemental Table 2 and Figure 2.
FIGURE 1.
Male-to-female incidence rate ratios (IRR) and corresponding 95% confidence intervals for childhood cancer by tumor type (ICCC designation) for all ages combined, SEER 18 (2000–2015)
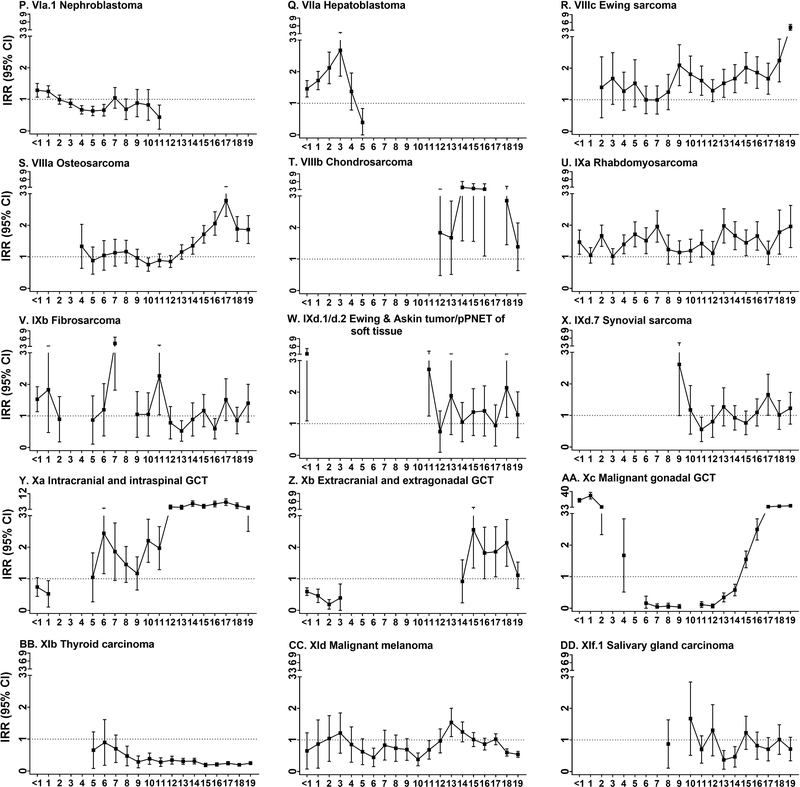
FIGURE 2.
Male-to-female incidence rate ratios (IRR) and 95% confidence intervals (95% CI) for childhood cancer by tumor type (ICCC designation) and single year of age, SEER 18 (2000–2015)
I. Leukemias
The overall IRR for males, compared to females, for acute lymphoblastic leukemia (ALL) was 1.36 (95% CI: 1.33–1.39). Male sex was significantly associated with ALL for every age from 1–19 years, particularly among children >14 years of age where the IRR often exceeded 2. Male sex was associated with AML overall (IRR: 1.18; 95% CI: 1.12–1.24) and by single year of age for 3–5, 7, 9–10, 12, and 14. Male sex was associated with chronic myeloproliferative diseases (CMD) (IRR: 1.23; 95% CI: 1.11–1.35) and myelodysplastic syndrome/other myeloproliferative diseases (MDS) (IRR: 1.36; 95% CI: 1.21–1.51) overall, but no consistent trend was observed when considering single years of age for either disease.
II. Lymphomas
Male sex was strongly associated with each type of lymphoma: Hodgkin (IRR: 1.25; 95% CI: 1.20–1.30), Non-Hodgkin (IRR: 1.80; 95% CI: 1.72–1.87), and Burkitt (IRR: 4.62; 95% CI: 4.29–4.95). In general, estimates for male sex were elevated, excluding the null, for each type at most ages. Burkitt lymphoma displayed the strongest association with male sex. For each year of age, the IRR for male sex and Burkitt lymphoma exceeded 2 and ranged from 2.09 (2 years) to 8.82 (18 years).
III. Central Nervous System (CNS)
There was variation in the association between male sex and CNS tumor types by single year of age. Male sex was strongly associated with medulloblastoma (IRR: 1.76; 95% CI: 1.65–1.88). The IRRs were lower in magnitude for astrocytoma (IRR: 1.13; 95% CI: 1.09–1.17), PNET (IRR: 1.27; 95% CI; 1.13–1.41), ependymoma (IRR: 1.23; 95% CI: 1.13–1.33), and atypical teratoid/rhabdoid tumors (IRR: 1.24; 95% CI: 1.05–1.44). Male sex was consistently associated with medulloblastoma over most ages. There was variation in the association between male sex and ependymoma, PNET, astrocytoma, and atypical teratoid/rhabdoid tumors for single-year age categories, but most estimates included the null.
IVa. Neuroblastoma, V Retinoblastoma, VIa Nephroblastoma, and VIIa Hepatoblastoma
For all ages combined, significant positive associations were observed between male sex and neuroblastoma (IRR: 1.13; 95% CI: 1.07–1.19), retinoblastoma (IRR: 1.17; 95% CI: 1.08–1.26), and hepatoblastoma (IRR: 1.70; 95% CI: 1.53–1.86), but a significant inverse associated was observed between male sex and nephroblastoma (IRR: 0.91; 95% CI: 0.86–0.97). Male sex was significantly associated with neuroblastoma at ages <1, 2–3, and 5 and retinoblastoma at age 2. Male sex was significantly associated with hepatoblastoma from <1–3 years. There was a suggestion of a male excess in hepatoblastoma at age 4, but there was a significant inverse association between male sex and hepatoblastoma at age 5; however, the inverse association observed for male sex and hepatoblastoma at age 5 may be an anomaly as the association between male sex and hepatoblastoma was significant when ages 6–10 were combined (IRR: 1.98; 95% CI: 1.04–2.91; results not shown). Male sex was positively associated with nephroblastoma at ages <1–1, but the associations were inverse for ages 4–6 and age 11 years.
VIII. Bone
Male sex was associated with all bone tumors (osteosarcoma IRR: 1.33; 95% CI: 1.25–1.41; chondrosarcoma IRR: 2.59; 95% CI: 2.08–3.10; Ewing sarcoma IRR: 1.69; 95% CI: 1.56–1.81). While male sex was positively associated with osteosarcoma overall, there was significant inverse association between male sex and osteosarcoma observed at age 10 and significant positive associations between male sex and osteosarcoma for ages 14–19 years. Ewing sarcoma displayed a consistent association with male sex, which was elevated and significant at all ages from 9–19 years, except at age 12 years.
IX. Soft Tissue Sarcomas
Male sex was associated with rhabdomyosarcoma (IRR: 1.42; 95% CI: 1.33–1.51), fibrosarcoma (IRR: 1.14; 95% CI: 1.00–1.28), Ewing tumors, Askin tumors and pPNET of soft tissue combined (IRR: 1.27; 1.06–1.48), but not synovial sarcomas (IRR: 1.09; 95% CI: 0.94–1.23). Nearly all effect estimates were above the null for rhabdomyosarcoma and were significant at ages <1, 2, 4–7, 13–16, and 18–19. Male sex displayed varying associations with the remaining soft tissue sarcomas by year of age.
X. Germ Cell Tumors (GCTs)
Male sex was strongly associated with GCTs particularly intracranial/intraspinal (IRR: 2.73; 95% CI: 2.51–2.95) and malignant gonadal GCTs (IRR: 2.35; 95% CI: 2.24–2.45) for all ages combined. An inverse association was observed for male sex and extracranial/extragonadal GCTs (IRR: 0.83: 95% CI: 0.73–0.92). Male sex was inversely associated with intracranial/intraspinal GCTs at 1 year of age, but displayed a significant positive association for ages 6 and 10–19 years with IRRs above 3 observed for ages 12–19 years. Male sex was inversely associated with extracranial/extragonadal GCTs at ages <1–3 and the association was positive when ages 4–13 were combined (IRR: 1.70; 95% CI: 1.05–2.35; results not shown) and for ages 15–18 individually. Male sex displayed a dynamic range of association with malignant gonadal GCTs. Male sex was significantly associated with gonadal GCTs for <1–2 years of age, the association was inverse from 6–14 (except at age 10), and the association was positive for 15–19 years.
XI. Other malignant epithelial neoplasms and malignant melanomas
Male sex was inversely associated with thyroid carcinoma (IRR: 0.25; 95% CI: 0.23–0.27), malignant melanoma (IRR: 0.78; 95% CI: 0.73–0.84), and salivary gland carcinoma (IRR: 0.79; 95% CI: 0.66–0.93). Male sex displayed a consistent, inverse association with thyroid carcinoma for all years and the associations were significant for ages 8–19. Conversely, the direction of association varied for male sex and malignant melanoma with significant inverse associations observed at ages 6, 10, 11, 18 and 19, and a positive association was observed at age 13. Male sex displayed a significant inverse association with salivary gland carcinoma at ages 13–14.
DISCUSSION
We observed higher childhood cancer incidence among males for most tumor types using SEER data (2000–2015). Importantly, this association persisted when considering individual year of age for numerous tumor types. The positive association between male sex and Burkitt lymphoma was the strongest among all tumor types as reported elsewhere.2,8 The trend of an increased risk among males was also observed for Hodgkin and other Non-Hodgkin lymphomas, similar to a previous report.9 ALL was more frequent among males, as others have reported2,10, and the magnitude of this association increased over 1–19 years of age. Concerning central nervous system (CNS) tumors, all tumor types were more common in males as reported elsewhere, but only medulloblastoma displayed a consistent male predominance over the ages studied.11–13 Male sex was significantly inversely associated with thyroid carcinoma overall and among each year of age, consistent with a previous report.14 Male sex was also inversely associated with nephroblastoma, as previously reported.2
Given the role of perinatal risk factors in childhood cancer etiology3,5,15,16 and the known sex differences in growth-related risk factors17, sex differences in perinatal risk factor profiles are one plausible explanation for the higher incidence of childhood cancer in males. However, our recent work found the male excess in risk for most childhood cancers to be independent of birthweight and other perinatal risk factors.4 The present findings of a consistent, increased incidence among males for all ages combined and by single year of age for cancer overall and most tumor types suggests that sex, and the associated biologic properties, may underlie the male excess in childhood cancer incidence. Some sex disparities in childhood cancer incidence may be due to the male excess in birth defects (overall male:female ratio 1.18; 95% CI: 1.13, 1.24)18 and chromosomal abnormalities such as Down syndrome19, which are associated with an increased risk of childhood cancer20 and leukemia21, respectively. Other biologic mechanisms for the increased risk of childhood cancer among males may depend on sex differences in 1) germline variation and gene expression on the X22,23 and autosomal chromosomes24; 2) immune responses25,26; and 3) pubertal hormone profiles and the corresponding growth rates.27,28
Sex-specific single nucleotide variants (SNVs) associated with childhood cancer may explain some of the observed sex differences in incidence. ALL is the most well- characterized childhood cancer in discovery genome-wide association studies (GWAS).29–32 As the most common childhood malignancy, ALL also has adequate sample size to identify sex-specific SNVs. In our study, the increased ALL incidence among males persisted at nearly every year of age with the exception of <1 year, which might suggest that the MLL-rearrangement, diagnosed in approximately 80% of infant ALL cases33, may operate independently of sex. There are germline SNVs with sex-specific associations for ALL, which may explain some of the observed male excess at younger ages. SNVs that show variation in association with ALL by sex have been reported in ERCC134 (maleOR: 1.94; femaleOR: 1.19) and HLA genes35 (male:female minor allele ORs>14). As such, sex-specific variation for SNVs may account for some of the observed male excess in ALL incidence. Identification of sex-specific SNVs associated with other childhood cancers remains to be completed.
Sex differences in immune function, which is supported by more infectious diseases during childhood among males25,26,36,37 and increased autoimmunity in females26, likely plays a critical role in the observed sex disparities in cancer incidence. The sex differences in immune fuction26 may depend on sex-specific differences in gene expression on the X and autosomal chromosomes, which contains immune-related genes.22 Such differences contribute to sex-variation in immune response and tumor surveillance. There are mechanistic differences between solid and hematologic cancers in evading immune surveillance38, but whether these mechanisms differ by sex is unknown. The immune-related biologic mechanisms underlying sex differences in childhood cancer incidence may be particularly relevant for hematologic malignancies as these arise in cellular components of the immune system. There is evidence that the infectious etiology of ALL may be stronger in boys due to sex differences in germline haplotypes and SNVs in the HLA-DQA1 gene.35,39 The consistent increased incidence we observed among males for all lymphomas may also depend on immune system-related mechanisms as lymphomas are cancers of secondary lymphoid organs.
Early life hormonal variation, especially that of puberty40, may play an important role in the increased cancer incidence we observed among males. While sex differences in the hormonal milieu in early childhood are not as stark as those during adolescence and adulthood25, tumors such as osteosarcoma41, which is driven by rapid bone growth and/or hormonal fluctuations during the pubertal period, may be particularly sensitive to hormonal variation.42,43 In our study, osteosarcoma and Ewing sarcoma displayed male-to-female ratios that fluctuated according to pubertal timing. Among children and adolescents, peak bone growth coincides with the adolescent period27 for both sexes and depends on estrogen, which is produced through aromatization of testosterone in males.44 Variation in pubertal timing and estrogen levels in males and females may regulate the rate of bone growth leading to the observed increased incidence of osteosarcoma among girls aged 10–12 years and the reversal of this trend over the teen years where males had a higher incidence of osteosarcoma and Ewing sarcoma, which showed a marked increase in male excess for ages 18–19 in our study. The extreme male excess of chondrosarcoma relative to other bone tumors was notable and a hormonal or growth-driven pathology may be responsible for the observed trend. Hormones may also indirectly impact the development of ALL and lymphomas. In adults, estrogen may increase the immune response45,46 while increasing testosterone may dampen the immune response47, although these findings warrant validation in pediatric populations.
Other interesting trends were observed for some tumors that should be explored in future mechanistic and etiologic studies. The consistent male excess observed for medulloblastoma but no other CNS tumors may depend on sex differences in neurological development48, which may underlie sex differences in the microenvironment. Similarly, the consistently increased incidence of hepatoblastoma among males, as we and others have reported49, may be the result of sex differences in fetal development and response to environmental exposures during gestation.50
We recognize that the interpretation of the male-to-female IRR in GCTs is not as straightforward as in other tumors. The observed sex differences in incidence our analysis does not account for the sex-specific biologic regulatory mechanisms for GCTs, which originate from the primordial germ cells that give rise to the sperm and the egg in males and females, respectively. The biologic mechanisms underlying the inverse associations for males and extracranial/extragonadal GCT at early ages may result from germ cells that have not reached the gonads and therefore not undergone sex specification, as such, tumor formation may depend on the permissibility of the microenvironment within which the tumor arises.51 The female excess of intracranial/intraspinal and extracranial/extragonadal GCTs among infants (ages <1–1) was striking and reflects established sex differences in tumor location and histology such that females are most commonly diagnosed with extragonadal tumors prior to the age of 5 and these tumors are commonly of yolk sac or teratoma histologies.51 The varying incidence trends observed by sex among malignant gonadal GCTs may be the result of a higher number of germ cells present in males than females.52
In our study, we used a large population-based sample to estimate male-to-female IRRs by single year of age and we included numerous tumor types; however, our findings have some limitations. While we presented unadjusted IRR estimates, these map closely to the ORs observed in our previous work, which were adjusted for known perinatal risk factors4, suggesting that unmeasured confounding is not likely to be a major driver of our observed associations. We were unable to stratify by race/ethnicity in our analyses due to small sample sizes; however, Gurney et al.2 reported similar trends in sex-specific incidence by race. Lastly, we were unable to look at specific trends by tumor subtype, such as ALL cytogenetic subtypes or solid tumor histologic subtypes, which should be explored in more detailed datasets.
In conclusion, we observed that male sex was associated with most childhood cancer types by single year of age. Based on variation in the trends over the childhood and adolescent periods, there is evidence of hormonal variation contributing to the observed incidence patterns, particularly for bone tumors and GCTs. Meanwhile, the trends for hematologic malignancies, suggest that sex itself may play a critical role in the observed male excess in incidence. The mechanisms are likely a combination of genetic and immune-related biologic factors. While sex is not a modifiable risk factor, understanding the biologic mechanisms and conducting risk factor studies by tumor subtype and sex may identify etiologic heterogeneity by sex and may aid in the development of prevention strategies or treatments.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant number T32 CA099936 [LAW]) and the Children’s Cancer Research Fund, Minneapolis, MN.
Abbreviations
- SEER
Surveillance, Epidemiology and End Results
- IRR
incidence rate ratio
- 95% CI
95% confidence interval
- OR
odds ratio
- ALL
acute lymphoblastic leukemia
- ICCC
International Classification of Childhood Cancer
- GCT
germ cell tumor
- PNET
Primitive Neuroectodermal Tumor
- pPNET
peripheral Primitive Neuroectodermal Tumor
- CNS
central nervous system
Footnotes
Conflict of Interest Statement
The authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.American Cancer Society. Cancer in Children & Adolescents. Spec Sect Cancer Child Adolesc. 2014;1(ICCC):25–42. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. [Google Scholar]
- 2.Gurney J, Severson R, Davis S, Robison L. Incidence of Cancer in Children in the United States. Cancer. 1995;75(8):2186–2195. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer: A pooled analysis. Epidemiology. 2009;20(4):475–483. doi: 10.1097/EDE.0b013e3181a5a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams LA, Richardson M, Kehm RD, et al. The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol. 2018;57:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill KA, Murphy MFG, Bunch KJ, et al. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol. 2015;44(1):153–168. doi: 10.1093/ije/dyu265. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2017 Sub (2000–2015). http://www.seer.cancer.gov.
- 7.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 8.Mbulaiteye SM, Biggar RJ, Bhatia K, Linet MS, Devesa SS. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992–2005. Pediatr Blood Cancer. 2009;53(3):366–370. doi: 10.1002/pbc.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartwright RA, Gurney KA, Moorman AV. Sex ratios and the risks of haematological malignancies. Br J Haematol. 2002;118(4):1071–1077. doi: 10.1046/j.1365-2141.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Zhang H, Yang W, et al. Inherited coding variants at the CDKN2A locus influence susceptibility to acute lymphoblastic leukaemia in children. Nat Commun. 2015;6(Jun 24):7553. doi: 10.1038/ncomms8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosemberg S, Fujiwara D. Epidemiology of pediatric tumors of the nervous system according to the WHO 2000 classification: A report of 1,195 cases from a single institution. Child’s Nerv Syst. 2005;21(11):940–944. doi: 10.1007/s00381-005-1181-x. [DOI] [PubMed] [Google Scholar]
- 12.Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72(17):3323–3342. doi: 10.1007/s00018-015-1930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuch JM, Heuch I, Akslen LA, Kvåle G. Risk of primary childhood brain tumors related to birth characteristics: A Norwegian prospective study. Int J Cancer. 1998;77(4):498–503. doi:. [DOI] [PubMed] [Google Scholar]
- 14.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric Thyroid Carcinoma: Incidence and Outcomes in 1753 Patients. J Surg Res. 2009;156(1):167–172. doi: 10.1016/j.jss.2009.03.098. [DOI] [PubMed] [Google Scholar]
- 15.Bjorge T, Sorensen HT, Grotmol T, et al. Fetal Growth and Childhood Cancer: A Population-Based Study. Pediatrics. 2013;132(5):e1265–e1275. doi: 10.1542/peds.2013-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow EJ, Puumala SE, Mueller BA, et al. Childhood cancer in relation to parental race and ethnicity: a 5-state pooled analysis. Cancer. 2010;116(12):3045–3053. doi: 10.1002/cncr.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunde A, Melve KK, Gjessing HK, Skjærven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165(7):734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 18.Michalski AM, Richardson SD, Browne ML, et al. Sex ratios among infants with birth defects, National Birth Defects Prevention Study, 1997–2009. Am J Med Genet Part A. 2015;167(5):1071–1081. doi: 10.1002/ajmg.a.36865. [DOI] [PubMed] [Google Scholar]
- 19.Bishop J, Huether CA, Torfs C, Lorey F, Deddens J. Epidemiologic study of Down syndrome in a racially diverse California population, 1989–1991. Am J Epidemiol. 1997;145(2):134–147. doi: 10.1093/oxfordjournals.aje.a009084. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KJ, Lee JM, Ahsan K, et al. Pediatric cancer risk in association with birth defects: A systematic review. PLoS One. 2017;12(7):1–56. doi: 10.1371/journal.pone.0181246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasle H, Haunstrup Clemmensen I, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355(9199):165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 22.Spatz A, Borg C, Feunteun J. X-chromosome genetics and human cancer. Nat Rev Cancer. 2004;4(8):617–629. doi: 10.1038/nrc1413. [DOI] [PubMed] [Google Scholar]
- 23.Dunford A, Weinstock DM, Savova V, et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet. 2017;49(1):10–16. doi: 10.1038/ng.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kukurba KR, Parsana P, Balliu B, et al. Impact of the X chromosome and sex on regulatory variation. Genome Res. 2016;26(6):768–777. doi: 10.1101/gr.197897.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: When a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 26.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 27.Granados A, Gebremariam A, Lee JM. Relationship Between Timing of Peak Height Velocity and Pubertal Staging in Boys and Girls. J Clin Res Pediatr Endocrinol. 2015;7(3):235–237. doi: 10.4274/jcrpe.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):1–19. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treviño LR, Yang W, French D, et al. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1001–1005. doi: 10.1038/ng.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaemmanuil E, Hosking FJ, Vijayakrishnan J, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1006–1010. doi: 10.1038/ng.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orsi L, Rudant J, Bonaventure A, et al. Genetic polymorphisms and childhood acute lymphoblastic leukemia: GWAS of the ESCALE study (SFCE). Leukemia. 2012;26(12):2561–2564. doi: 10.1038/leu.2012.148. [DOI] [PubMed] [Google Scholar]
- 32.Wiemels JL, Walsh KM, de Smith AJ, et al. GWAS in childhood acute lymphoblastic leukemia reveals novel genetic associations at chromosomes 17q12 and 8q24.21. Nat Commun. 2018;9(1):286. doi: 10.1038/s41467-017-02596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood. 2015;125(26):3977–3988. doi: 10.1182/blood-2015-02-580043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SL, Zhao H, Zhou B, et al. Polymorphisms in ERCC1 and susceptibility to childhood acute lymphoblastic leukemia in a Chinese population. Leuk Res. 2006;30(11):1341–1345. doi: 10.1016/j.leukres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK, Lupo PJ, Scheurer ME, et al. A childhood acute lymphoblastic leukemia genome-wide association study identifies novel sex-specific risk variants. Medicine (Baltimore). 2016;95(46):e5300. doi: 10.1097/MD.0000000000005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washburn T, Medearis D, Child B. Sex differences in susceptibilty to infections. Pediatrics. 1965;35(1):57–64. [PubMed] [Google Scholar]
- 37.Piccini P, Montagnani C, De Martino M. Gender disparity in pediatrics: A review of the current literature. Ital J Pediatr. 2018;44(1):4–9. doi: 10.1186/s13052-017-0437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran EK, Godfrey J, Kline J. Mechanisms of Immune Tolerance in Leukemia and Lymphoma. Trends Immunol. 2017;38(7):513–525. doi: 10.1016/j.it.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor GM, Dearden S, Payne N, et al. Evidence that an HLA-DQAI-DQBI haplotype influences susceptibility to childhood common acute lymphoblastic leukaemia in boys provides further support for an infection-related aetiology. Br J Cancer. 1998;78(5):561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Mirabello L, Pfeiffer R, Murphy G, et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011;22(6):899–908. doi: 10.1007/s10552-011-9763-2.Height. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127(1):100–102. doi: 10.1016/S0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 43.Biro FM, Greenspan LC, Galvez MP. Puberty in girls of the 21st century. J Pediatr Adolesc Gynecol. 2012;25(5):289–294. doi:doi: 10.1016/j.jpag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh D, Sanyal S, Chattopadhyay N. The role of estrogen in bone growth and formation: changes at puberty. Cell Health Cytoskelet. 2011;(3):1–12. doi: 10.2147/CHC.S8916. [DOI] [Google Scholar]
- 45.Khan D, Ahmed SA. The immune System is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6(January):1–8. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan D, Ahmed A. Estrogen and signaling in the cells of the immune system. Adv Neuroimmune Biol. 2012;3(1):73–93. doi: 10.3233/NIB-2012-012039. [DOI] [Google Scholar]
- 47.Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruigrok ANV, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ries L, Smith M, Gurney J, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. NIH Pub No 99–4649. 1999:179 pp. [Google Scholar]
- 50.Spector LG, Birch J. The Epidemiology of Hepatoblastoma. Pediatr Blood & Cancer. 2012;59:776–779. doi: 10.1002/pbc. [DOI] [PubMed] [Google Scholar]
- 51.Poynter JN, Amatruda JF, Ross JA. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer. 2010;116(20):612–625. doi: 10.1002/cncr.25454.Trends. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Møller H, Evans H, Oliver T, et al. Epidemiology of gonadal germ cell cancer in males and females. APMIS. 2003;111(1):43–48. doi: 10.1034/j.1600-0463.2003.11101071.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.