Abstract
Background:
Epidemiological knowledge and predictors of melanoma among children and adolescents in multi-ethnic populations are limited.
Procedure:
Using data from the Texas Cancer Registry (TCR) and the Surveillance, Epidemiology, and End Results (SEER) 13 database, we identified incident melanoma cases diagnosed at 0-20 years old during 1995-2013 in Texas and the U.S., respectively. Using negative binomial regression, associations between demographic factors and melanoma incidence rates (IR) were evaluated by calculating incidence rate ratios (IRR) and 95% confidence intervals (CI). Annual percent change in IRs was assessed with joinpoint regression.
Results:
Overall, the melanoma IR was 4.16 (TCR, n=634) and 4.84 (SEER, n=1,260) per million. Females, adolescents, non-Hispanic (NH) whites, and Hispanics had higher IRs compared to other groups (p<0.05). In adjusted analyses, Hispanics had a higher incidence of melanoma than NH non-whites (Texas IRR=2.17, 95% CI: 1.30, 3.61; SEER IRR=2.88, 95% CI: 1.97, 4.21). In Texas, NH whites with melanoma were more likely to live in low poverty areas, whereas the opposite trend was observed in Hispanics. Melanoma IRs increased throughout 1995-2004 followed by an average annual decrease of 7.6% (95% CI: −12.6%, −2.2%) in Texas and 6.0% (95% CI: −8.5%, −3.4%) in SEER during 2005-2013 (p<0.05). However, these decreasing trends were not observed among Hispanics or those <10 years old.
Conclusion:
While the overall melanoma IR in children and adolescents appears to be decreasing, this trend is not evident among Hispanics and young children, implicating the need for further research investigating the etiologies and risk factors in these groups.
Keywords: melanoma, epidemiology, pediatric oncology, rare tumors, Hispanic
Introduction
Melanoma is the fifth most common cancer1 and, although rare, is the most common skin malignancy in those <20 years old in the United States (U.S.), with an average annual incidence rate (IR) of 5.5-6.0 cases per million.2,3 Although children and adolescents diagnosed with melanoma have a 5-year survival rate of 85-93%,4–7 those with advanced disease have a significantly poorer prognosis with a 5-year survival rate as low as 68% for those with regional metastatic disease and 11.8% for those with distant metastases.8
After decades of increasing melanoma incidence among those <20 years old, particularly among adolescents, recent reports have observed shifting trends in melanoma incidence in the U.S. since the mid-2000’s.2,3 However, it is unclear whether recently observed decreases in melanoma IRs impact all segments of the pediatric and adolescent population, particularly among younger children and Hispanics due to lack of in-depth characterization of these groups. Our objective was to evaluate demographic and socioeconomic predictors and recent trends of malignant melanoma incidence among children and adolescents in (1) Texas, a state characterized by a large Hispanic population and with a large, population-based cancer registry, and (2) the Surveillance, Epidemiology, and End Results (SEER) database, which includes data from a group of 13 cancer registries in the U.S. and is representative of the general U.S. population.
Methods
Texas study population
We identified cases of cutaneous malignant melanoma diagnosed at <21 years of age during the period of 1995 to 2013 in the Texas Cancer Registry (TCR). The TCR, under the Texas Department of State Health Services, is a large population-based registry with Gold Certification from the North American Association of Central Cancer Registries. Using the International Classification of Diseases for Oncology, third edition (ICD-O-3), we included cases with a histology code of 8720-8780 or 8790, as indicated by the International Classification of Childhood Cancer, third edition (ICCC-3) site group XIe for malignant melanomas.9 In addition, only melanoma diagnoses with a primary site code of C44.0-C44.9 (skin) and a malignant behavior classification were included. We excluded diagnoses that were not the first primary malignancy. Information on the corresponding at-risk population in Texas was obtained from the 2000 and 2010 U.S. Census, including total counts of the corresponding 0-20 year-old population by sex, age, race/ethnicity, and county.10,11
Using the primary site information for each tumor identified in the TCR, the tumor site was categorized as head/neck, trunk, upper extremity, lower extremity, and not otherwise specified (NOS). Tumor stage was defined as localized, regional, distant, or unstaged using the SEER summary stage variable from the TCR. From the TCR, we also obtained information on residential county at the time of diagnosis and demographic characteristics including age at diagnosis (categorized as <5, 5 to <10, 10 to <15, and 15-20 years old), sex, and race/ethnicity (categorized as non-Hispanic [NH] white, Hispanic, and other). Information on individual-level socioeconomic status (SES) is not available from the TCR, therefore county-level poverty was used as a proxy for SES. Using data from the U.S. Census, counties with low poverty were defined as those with ≤15% of households below the poverty level, and counties with high poverty were defined as those with >15% of households below the poverty level.
SEER study population
The U.S. National Cancer Institute’s SEER program collects data from several population-based cancer registries from across the U.S. (Texas is not included) and is demographically representative of the U.S. population as a whole.12 We assessed melanoma incidence using SEER 13, which includes cancer incidence data for the study period of 1995 to 2013 from 13 registries in the U.S. and covers 14% of the total U.S. population.12 Cutaneous melanoma cases were defined using the same ICD-O-3 codes used to define cases from the TCR as described above. Only first primary malignant cases diagnosed at <21 years of age were included. All other variables (age at diagnosis, sex, race/ethnicity, and county-level poverty) were defined in the same manner as used in the TCR cohort.
Cases were identified using the SEER*Stat software13. An individual-level listing of the selected cases along with information on each case’s diagnosis and demographic characteristics was extracted using SEER*Stat’s Case Listing session. Based on the unavailability of the SEER 13 database file within the Case Listing session, melanoma cases were first identified using the SEER 18 database14, which includes data from the SEER 13 registries (diagnoses starting in 1992 to the most current year)15 as well as data from registries that have more recently started to participate in SEER (diagnoses starting in 2000 to the most current year). Once all cases were extracted, we generated the final analytic file to include only cases from SEER 13, which covers the entire study period (1995 to 2013). The 2000 U.S. Census total population was used as the corresponding at-risk population for assessing incidence.
Statistical Analysis
We tabulated descriptive statistics, including frequencies and proportions of melanoma cases by demographic and clinical factors, for Texas and for SEER 13, for the period of 1995 to 2013. We calculated age-adjusted IRs (aIRs) and confidence intervals (CIs) for the overall population over the study period as well as by demographic group, year of diagnosis, and primary tumor site.16 The aIRs were standardized by age category and year of diagnosis and were calculated per million person-years using the underlying at-risk population. To assess predictors of melanoma incidence, incidence rate ratios (IRR) and 95% CIs were estimated using negative binomial regression. Negative binomial regression was used instead of Poisson regression due to overdispersed data (i.e., the variance was greater than the mean). Robust standard errors were used to help control for downward bias of standard errors in the negative binomial regression models, and the natural logarithm of total person-years in the underlying population was used as the population offset. Sex, race/ethnicity, age at diagnosis, county-level poverty, and year of diagnosis were assessed as potential predictors. Variables with a p-value of <0.250 in univariable analyses were included in a final multivariable model to simultaneously evaluate predictors of melanoma incidence and generate adjusted IRRs (aIRR) and 95% CIs. Due to the high proportion of Hispanics in the Texas population, interaction terms for race/ethnicity (NH white and Hispanic) and sex, age at diagnosis, and county-level poverty were tested in the multivariable model to assess whether predictors of melanoma varied across these race/ethnicity groups. Subsequently, the multivariable regression model was conducted separately for NH whites and Hispanics in stratified analyses. Only cases with available data for each of the predictor variables were included in the regression analyses. STATA version 13 (College Station, TX) was used for all data analyses including the estimation of aIRs and all IRRs.
Annual percent change (APC) and 95% CIs in melanoma incidence in Texas and SEER 13 over the period of 1995 to 2013 were assessed using the National Cancer Institute’s Joinpoint Regression Program (version 4.2.0.2).17 We evaluated annual changes in the overall trend in melanoma IRs in addition to changes in IR trends by demographic groups, which included sex, race/ethnicity (NH white and Hispanic), and age at diagnosis (<10 years old and 10-20 years old). The independent variable was defined as the calendar year using 1-year increments. We allowed for up to two joinpoints, wherein a joinpoint indicates a statistically significant change (two-sided p<0.05) in trend or slope, and we relied on the best-fit joinpoint regression model with the least number of joinpoints. Using STATA 13, melanoma IR trends by calendar year were plotted using locally weighted scatterplot smoothing (LOWESS) with a bandwidth of 25%.
Results
Table 1 presents the distributions and aIRs by demographic and clinical characteristics of cutaneous malignant melanoma incident cases identified among children and adolescents during the period of 1995 to 2013 in the TCR and SEER 13. A total of 634 cases were identified from the TCR, and 1,260 cases were identified from SEER 13. The overall aIR in Texas was 4.16 (95% CI: 4.10, 4.21) per 1,000,000, and was slightly higher in SEER 13 with 4.84 (95% CI: 4.78, 4.90) per 1,000,000. In Texas, the majority of cases were non-Hispanic white (85.8%) followed by Hispanic (11.1%), whereas a smaller proportion of the SEER 13 case population was Hispanic (6.6%). Of the 19 (3.0%) cases classified as other race in Texas, 10 (52.6%) were non-Hispanic black. Information on race/ethnicity was missing for 21 cases (3.3%) in Texas and 55 cases (4.4%) in SEER 13. The majority of cases were between 10 and 20 years old at diagnosis (Texas: 87.6%; SEER 13: 91.4%), were female (Texas: 56.0%; SEER 13: 60.2%), and lived in counties with low levels of poverty (i.e., wealthier counties) (Texas: 73.2%; SEER 13: 77.5%). The largest proportion of diagnosed tumors were located in the trunk (Texas: 34.7%; SEER 13: 36.5%) and head/neck regions (Texas: 22.4%; SEER 13: 20.4%). The majority of tumors were localized (Texas: 48.6%; SEER 13: 78.5%) and the smallest proportion of cases presented with distant metastases (Texas: 2.2%; SEER 13: 2.2%).
TABLE 1.
Average annual age-adjusted incidence rates for childhood and adolescent malignant melanoma, 1995-2013
| Texas |
SEER 13 |
|||
|---|---|---|---|---|
| Characteristic | No. (%) | IRa (95% CI) | No. (%) | IRa (95% CI) |
| Overall | 634 (100) | 4.16 (4.10, 4.21) | 1,260 (100) | 4.84 (4.78, 4.90) |
| Sex | ||||
| Male | 279 (44.0) | 3.02 (2.93, 3.11) | 502 (41.4) | 4.03 (3.92, 4.13) |
| Female | 355 (56.0) | 4.76 (4.65, 4.87) | 758 (60.2) | 5.67 (5.53, 5.80) |
| Race/ethnicityb | ||||
| Non-Hispanic White | 526 (85.8) | 10.30 (10.16, 10.45) | 1079 (89.5) | 6.95 (6.83, 7.08) |
| Hispanic | 68 (11.1) | 1.31 (1.26, 1.37) | 79 (6.6) | 1.48 (1.42, 1.55) |
| Otherc | 19 (3.1) | 0.53 (0.49, 0.58) | 47 (3.9) | 0.48 (0.44, 0.52) |
| Age at Diagnosis, years | ||||
| 0-4 | 36 (5.7) | 0.98 (0.93, 1.04) | 38 (3.0) | 0.62 (0.57, 0.67) |
| 5-9 | 43 (6.8) | 1.16 (1.11, 1.22) | 70 (5.6) | 1.07 (1.01, 1.13) |
| 10-14 | 91 (14.4) | 2.52 (2.43, 2.61) | 154 (12.2) | 2.47 (2.38, 2.56) |
| 15-20 | 464 (73.2) | 10.81 (10.65, 10.97) | 998 (79.2) | 14.08 (13.88, 14.27) |
| Year of Diagnosisd | ||||
| 1995-1998 | 91 (14.4) | 2.82 (2.73, 2.92) | 229 (18.2) | 4.17 (4.06, 4.30) |
| 1999-2002 | 132 (20.8) | 4.09 (3.98, 4.21) | 286 (22.7) | 5.22 (5.08, 5.36) |
| 2003-2006 | 181 (28.6) | 5.64 (5.50, 5.77) | 329 (26.1) | 6.00 (5.86, 6.15) |
| 2007-2010 | 148 (23.3) | 4.63 (4.51, 4.75) | 246 (19.5) | 4.49 (4.36, 4.62) |
| 2011-2013 | 82 (12.9) | 3.42 (3.30, 3.54) | 170 (13.5) | 4.14 (4.00, 4.28) |
| County-level Povertye | ||||
| High | 170 (26.8) | 3.32 (3.22, 3.43) | 283 (22.5) | 2.67 (2.57, 2.76) |
| Low | 464 (73.2) | 4.15 (4.05, 4.26) | 977 (77.5) | 6.50 (6.37, 6.64) |
| Primary site | ||||
| Head/Neck | 142 (22.4) | 0.93 (0.91, 0.96) | 257 (20.4) | 0.99 (0.96, 1.01) |
| Trunk | 220 (34.7) | 1.44 (1.41, 1.47) | 460 (36.5) | 1.77 (1.73, 1.80) |
| Upper extremity | 121 (19.1) | 0.79 (0.77, 0.82) | 233 (18.5) | 0.90 (0.87, 0.92) |
| Lower extremity | 131 (20.7) | 0.86 (0.83, 0.88) | 284 (22.5) | 1.09 (1.06, 1.12) |
| NOS | 20 (3.2) | 0.13 (0.12, 0.14) | 26 (2.1) | 0.10 (0.09, 0.11) |
| Stage at diagnosis | ||||
| Localized | 308 (48.6) | 2.02 (1.98, 2.06) | 989 (78.5) | 3.80 (3.75, 3.85) |
| Regional | 97 (15.3) | 0.64 (0.62, 0.66) | 202 (16.0) | 0.78 (0.75, 0.80) |
| Distant | 14 (2.2) | 0.09 (0.08, 0.10) | 27 (2.2) | 0.10 (0.09, 0.11) |
| Unstaged | 215 (33.9) | 1.41 (1.38, 1.44) | 42 (3.3) | 0.16 (0.15, 0.17) |
Abbreviations: CI, confidence interval; IR, age-adjusted incidence rates; NOS, not otherwise specified
Per 1,000,000; IRs are standardized by age category and year with the exception of the IRs for each age category of diagnosis which are standardized by year only.
Information on race/ethnicity is missing for 3.3% (n=21) and 4.4% (n=55) melanoma cases in Texas and SEER 13, respectively.
Includes non-Hispanic non-Whites.
Four-year increments, except for 2011-2013 which is a three-year increment.
Low area-level poverty category includes those living in a county with ≤15% of households below the poverty level; High area-level poverty category includes those living in a county with >15% of households below the poverty level.
The associations between demographic characteristics and the incidence of cutaneous malignant melanoma in Texas and SEER 13 are presented in Table 2, and the number of cases and person-years included in the regression analyses are presented in Supplementary Table 1. After adjusting for other factors, the incidence of melanoma was higher among females compared to males (Texas aIRR=1.33, 95% CI: 1.12, 1.57; SEER 13 aIRR=1.59, 95% CI: 1.42, 1.80); was more than 10-fold higher among those 15-20 years old compared to those <5 years old (Texas aIRR=10.25, 95% CI: 7.23, 14.54; SEER 13 aIRR=19.79 (95% CI: 14.14, 27.72); was >2 times higher among Hispanics compared to NH non-whites (Texas aIRR=2.17, 95% CI: 1.30, 3.61; SEER 13 aIRR=2.88, 95% CI: 1.97, 4.21); and was >16 times (95% CI: 10.61, 26.65) and >18 times (95% CI: 13.33, 24.77) higher among NH whites compared to NH non-whites in Texas and SEER 13, respectively. Melanoma IRs were higher in counties with low poverty compared to counties with high poverty (Texas aIRR=1.22, 95% CI: 1.02, 1.46; SEER 13 aIRR=1.17, 95% CI: 1.00, 1.37). The results from the univariable regression analyses for both the Texas and SEER cohorts were similar to those for the respective multivariable regression analyses in each cohort.
TABLE 2.
Associations between demographic characteristics and malignant melanoma incidence rates among children and adolescents, 1995-2013
| Texas |
SEER 13 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Univariable IRR (95% CI) | p-value | Multivariable Adjusted IRRa (95% CI) | p-value | Univariable IRR (95% CI) | p-value | Multivariable Adjusted IRRa (95% CI) | p-value |
| Sex | ||||||||
| Male | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| Female | 1.26 (1.05, 1.52) | 0.012 | 1.33 (1.12, 1.57) | 0.001 | 1.65 (1.44, 1.90) | <0.001 | 1.59 (1.42, 1.80) | <0.001 |
| Age at diagnosis, years | ||||||||
| 0-4 | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| 5-9 | 1.21 (0.77, 1.91) | 0.411 | 1.18 (0.76, 1.86) | 0.459 | 1.64 (1.08, 2.50) | 0.021 | 1.58 (1.05, 2.37) | 0.029 |
| 10-14 | 2.73 (1.82, 4.08) | <0.001 | 2.49 (1.68, 3.70) | <0.001 | 4.09 (2.79, 6.01) | <0.001 | 3.51 (2.43, 5.08) | <0.001 |
| 15-20 | 11.19 (7.83, 15.98) | <0.001 | 10.25 (7.23, 14.54) | <0.001 | 24.73 (17.49, 34.98) | <0.001 | 19.79 (14.14, 27.72) | <0.001 |
| Race/ethnicity | ||||||||
| Other | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| Hispanic | 2.01 (1.21, 3.34) | 0.007 | 2.17 (1.30, 3.61) | 0.003 | 2.81 (1.90, 4.14) | <0.001 | 2.88 (1.97, 4.21) | <0.001 |
| Non-Hispanic White | 16.62 (10.46, 26.40) | <0.001 | 16.82 (10.61, 26.65) | <0.001 | 19.12 (14.03, 26.06) | <0.001 | 18.10 (13.22, 24.77) | <0.001 |
| County-level povertyb | ||||||||
| High | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| Low | 1.48 (1.22, 1.80) | <0.001 | 1.22 (1.02, 1.46) | 0.030 | 1.80 (1.46, 2.22) | <0.001 | 1.17 (1.00, 1.37) | 0.049 |
| Year of diagnosisc | ||||||||
| 1995-1998 | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| 1999-2002 | 1.41 (1.05, 1.91) | 0.024 | 1.42 (1.07, 1.87) | 0.014 | 1.38 (1.11, 1.71) | 0.004 | 1.32 (1.09, 1.59) | 0.004 |
| 2003-2006 | 1.94 (1.46, 2.58) | <0.001 | 1.92 (1.48, 2.50) | <0.001 | 1.51 (1.22, 1.86) | <0.001 | 1.52 (1.27, 1.81) | <0.001 |
| 2007-2010 | 1.58 (1.16, 2.14) | 0.004 | 1.52 (1.13, 2.04) | 0.005 | 1.22 (0.98, 1.52) | 0.078 | 1.15 (0.95, 1.39) | 0.148 |
| 2011-2013 | 1.12 (0.80, 1.57) | 0.521 | 1.11 (0.81, 1.54) | 0.514 | 1.15 (0.91, 1.46) | 0.245 | 1.08 (0.88, 1.33) | 0.448 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio
Adjusted IRRs are mutually adjusted for all variables listed in the table.
Four-year increments, except for 2011-2013 which is a three-year increment.
Low county-level poverty category includes those living in a county with ≤15% of households below the poverty level; High county-level poverty category includes those living in a county with >15% of households below the poverty level.
Statistically significant interactions were observed between race/ethnicity (NH whites and Hispanics) and (1) age at diagnosis (Texas pinteraction<0.001; SEER 13 pinteraction<0.001) and (2) county-level poverty (Texas pinteraction=0.005; SEER 13 pinteraction=0.031), indicating that age at diagnosis and county-level poverty may predict melanoma incidence differently in NH whites compared to Hispanics. Therefore, multivariable regression analyses were repeated and stratified by NH whites and Hispanics (Table 3; the number of cases and person-years that were included in the stratified regression analyses are presented in Supplementary Table 2). Stratified results showed that Hispanics who were 15-20 years old had a 3.41-times (95% CI: 1.71, 6.80) and 7.15-times (95% CI: 3.00, 17.01) higher incidence compared to those <5 years old in Texas and SEER 13, respectively, while NH whites who were 15-20 years old had a 13.59-times (95% CI: 9.18, 21.80) and 31.36-times (95% CI: 20.29, 48.46) higher incidence compared to those <5 years old in Texas and SEER 13, respectively. In Texas, while incidence rates were 41% (95% CI: 1.14, 1.73) higher among NH whites living in areas with low county-level poverty compared to those with high county-level poverty, the inverse was observed among Hispanics (aIRR: 0.64; 95% CI: 0.40, 1.02). In contrast, in SEER 13, there were no differences in IRs by county-level poverty among NH whites; however, Hispanics living in counties with low poverty had an 85% higher melanoma incidence compared to Hispanics living in counties with high poverty. The interaction between race/ethnicity and sex was also evaluated in the unstratified multivariable regression model and was not statistically significant (Texas pinteraction=0.559; SEER 13 pinteraction=0.218).
TABLE 3.
Associations between demographic characteristics and malignant melanoma incidence rates among children and adolescents, 1995-2013: stratified by race/ethnicity
| Texas |
SEER 13 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic Whites
(n=526) |
Hispanics (n=68) |
Non-Hispanic Whites
(n=1,079) |
Hispanics (n=79) |
|||||
| Characteristic | Adjusted IRRa (95% CI) | p-value | Adjusted IRRa (95% CI) | p-value | Adjusted IRRa (95% CI) | p-value | Adjusted IRRa (95% CI) | p-value |
| Sex | ||||||||
| Male | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| Female | 1.37 (1.14, 1.63) | 0.001 | 1.19 (0.75, 1.90) | 0.464 | 1.57 (1.39, 1.78) | <0.001 | 2.04 (1.20, 3.47) | 0.009 |
| Age at diagnosis, years | ||||||||
| 0-4 | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| 5-9 | 0.99 (0.55, 1.76) | 0.966 | 1.22 (0.54, 2.79) | 0.631 | 1.83 (1.09, 3.07) | 0.022 | 1.99 (0.78, 5.10) | 0.151 |
| 10-14 | 3.20 (1.99, 5.14) | <0.001 | 1.10 (0.46, 2.62) | 0.833 | 4.78 (2.99,7.64) | <0.001 | 3.71 (1.54, 8.90) | 0.003 |
| 15-20 | 13.59 (8.85, 20.87) | <0.001 | 3.41 (1.71, 6.80) | <0.001 | 31.36 (20.29, 48.46) | <0.001 | 7.15 (3.00, 17.01) | <0.001 |
| County-level povertyb | ||||||||
| High | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| Low | 1.41 (1.14, 1.73) | <0.001 | 0.64 (0.40, 1.02) | 0.063 | 1.03 (0.88, 1.20) | 0.728 | 1.85 (1.22, 2.79) | 0.004 |
| Year of diagnosisc | ||||||||
| 1995-1998 | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| 1999-2002 | 1.47 (1.09, 1.97) | 0.011 | 1.00 (0.42, 2.38) | 1.00 | 1.35 (1.12, 1.65) | 0.002 | 1.20 (0.51, 2.80) | 0.677 |
| 2003-2006 | 2.03 (1.54, 2.68) | <0.001 | 1.28 (0.59, 2.80) | 0.532 | 1.53 (1.28, 1.84) | <0.001 | 1.56 (0.74, 3.28) | 0.245 |
| 2007-2010 | 1.51 (1.11, 2.06) | 0.008 | 1.42 (0.67, 3.02) | 0.363 | 1.10 (0.90, 1.34) | 0.370 | 1.96 (0.88, 4.38) | 0.100 |
| 2011-2013 | 1.03 (0.73, 1.47) | 0.852 | 1.39 (0.63, 3.10) | 0.414 | 1.00 (0.81, 1.25) | 0.974 | 2.11 (0.98, 4.53) | 0.057 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio
Adjusted IRRs are mutually adjusted for all variables listed in the table.
Four-year increments, except for 2011-2013 which is a three-year increment.
Low county-level poverty category includes those living in a county with ≤15% of households below the poverty level; High county-level poverty category includes those living in a county with >15% of households below the poverty level.
Table 4 and Figure 1 show the annual changes in IR trends over the study period for both Texas and SEER 13. The number of cases included in the IRs are presented in Supplementary Table 3. Overall, the melanoma IR increased annually between 1995-2004 in both Texas (APC: 12.0%, 95% CI: 5.2%, 19.2%) and SEER 13 (APC: 4.1%, 95% CI: 1.4%, 6.8%), and decreased between 2005-2013, the end of the study period (Texas APC: −7.6%, 95% CI: −12.6%, −2.2%; SEER 13 APC: −6.0%, 95% CI: −8.5%, −3.4%). Similar trends were observed among females, those 10-20 years old, and NH whites, both in Texas and SEER 13 where the IRs increased between 1995 and 2004-2006 and then decreased through the end of the study period. Other demographic groups did not follow this pattern. Annual IR trends among males remained relatively unchanged throughout the study period in Texas and in SEER 13. Among young children (0-9 years old), the IR increased by 7.2% (95% CI: 2.8%, 11.7%) annually over the entire study period in Texas; similarly, IRs in young children increased by 1.5% (95% CI: −2.1%, 5.2%) annually throughout the study period in SEER 13, though this difference was not statistically significant. Among Hispanics, the IR increased by 5.3% (95% CI: −0.6%. 11.6%) annually in Texas and by 2.0% (95% CI: −2.2%, 6.4%) annually in SEER 13, though these differences were not statistically significant.
TABLE 4.
Annual percent change in malignant melanoma incidence rates among children and adolescents, 1995-2013
| Texas |
SEER 13 |
|||
|---|---|---|---|---|
| Characteristic | AAPC (95% CI), % | APC (95% CI), % | AAPC (95% CI), % | APC (95% CI), % |
| Overall | 1.8 (−2.1, 5.7) | 12.0 (5.2, 19.2)a, b | −1.1 (−2.8, 1.3) | 4.1 (1.4, 6.8)a, b |
| −7.6 (−12.6, −2.2)a, c | −6.0 (−8.5, −3.4)a, c | |||
| Sex | ||||
| Male | −0.1 (−3.2, 3.1) | −0.1 (−3.2, 3.1) | −1.2 (−3.3, 0.9) | −1.2 (−3.3, 0.9) |
| Female | 2.6 (−1.9, 7.2) | 13.8 (6.9, 21.1)a, d | −1.0 (−3.3, 1.3) | 4.6 (1.0, 8.4)a, b |
| −9.8 (−16.5, −2.7)a, e | −6.4 (−9.7, −3.9)a, c | |||
| Age at Diagnosis, years | ||||
| 0-9 | 7.2 (2.8, 11.7)a | 7.2 (2.8, 11.7)a | 1.5 (−2.1, 5.2) | 1.5 (−2.1, 5.2) |
| 10-20 | 0.9 (−3.4, 5.4) | 12.3 (4.8, 20.4)a, b | −1.1 (−2.9, 0.7) | 5.1 (1.7, 8.6)a, f |
| −9.4 (−15.1, −3.3)a, c | −5.8 (−8.0, −3.5)a, g | |||
| Race | ||||
| Non-Hispanic White | 0.5 (−4.5, 5.7) | 12.7 (4.1, 22.1)a, b | 0.3 (−1.4, 2.0) | 7.6 (4.3, 11.1)a, f |
| −10.4 (−17.0, −3.3)a, c | −5.2 (−7.3, −3.1)a, g | |||
| Hispanic | 5.3 (−0.6, 11.6) | 5.3 (−0.6, 11.6) | 2.0 (−2.2, 6.4) | 2.0 (−2.2, 6.4) |
Abbreviations: AAPC, average annual percent change throughout the 1995-2013 period; APC, annual percent change estimated with joinpoints at various years
Statistically significant at the 0.05-level
APC for 1995-2004
APC for 2005-2013
APC for 1995-2005
APC for 2006-2013
APC for 1995-2003
APC for 2004-2013
Figure 1.
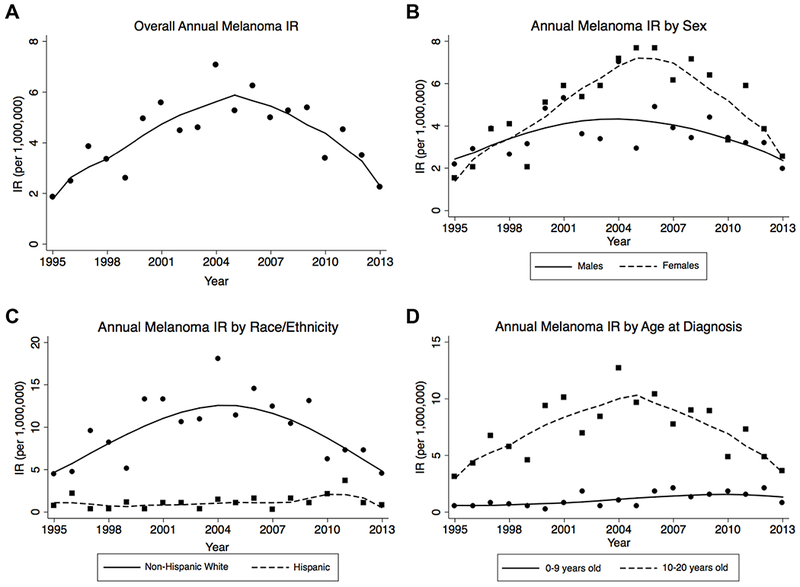
Malignant melanoma incidence rates in Texas, 1995-2013. Points represent incidence rates (IR) for each given year, and the curves generated using LOWESS.
Discussion
Prior studies have examined the incidence of melanoma in children and adolescents, but data specifically describing multi-ethnic pediatric and adolescent populations are limited. In our study we sought to describe and compare melanoma IR trends in children and adolescents in the U.S. using data from SEER 13, a large registry-based database representative of the U.S. population, and from the TCR, which captures information on cancer cases diagnosed throughout Texas, a state with a large Hispanic population.
Similar to previous reports of pediatric melanoma in the U.S.,7,18,19 we observed an overall increase from 1995 to 2013 in melanoma IRs among females, adolescents, and NH whites in both our study populations. Following a steady increasing trend in melanoma IRs since 1995, overall pediatric malignant melanoma IRs have been declining since 2005 by 7.6% and 6.0% annually in Texas and the U.S., respectively, with similar trends seen in the adolescent and NH white subgroups. Similar decreases in the U.S. since the early- to mid-2000s have been previously reported,2,3 and decreases starting as early as the 1990s have been reported in other studies of melanoma incidence from high-income countries with predominantly Caucasian populations in Europe and Australia.20–22
While the overall IR of malignant melanoma has been decreasing since the mid-2000s, this trend was not observed in all demographic groups. We observed that melanoma IRs are statistically significantly higher in Hispanics compared to NH non-whites in both the TCR and SEER 13 cohorts during the study period. In addition, the decreasing IR trend starting in 2005-2006 observed in NH whites and those 10-20 years old was not observed in Hispanics and those <10 years old. Melanoma IRs in these populations appear to be unchanged over the period of 1995 to 2013. Few studies have evaluated melanoma among Hispanic children alone or have specifically sought to understand risk factors in young children. However, some studies have reported that younger children with melanoma are more ethnically diverse18 and are more likely to present with advanced disease.18,23 As melanoma IRs do not appear to be declining among Hispanics and young children, the need to identify risk factors for melanoma in these groups to inform prevention strategies is further underscored.
Several factors may contribute to the lack of declining melanoma IRs in Hispanics. Both the self-perceived and healthcare provider-perceived risk of melanoma among Hispanic children may be falsely low. A study of Hispanic and NH white high school students in Florida, another state with a large Hispanic population, found that Hispanic students, after adjusting for other factors, believed their chance of developing skin cancer was lower than that of their NH white peers. They were also less likely to have received education on performance of skin self-evaluations and were more likely to use tanning beds.24 In addition, compared to other racial/ethnic groups in the U.S., Hispanic children and adolescents are more likely to be uninsured or to have lack of sufficient access to healthcare.25 Lastly, while public health prevention efforts over the last two decades have primarily targeted fair-skinned populations,26 the proportion of Hispanics in the U.S. population has rapidly increased. Hispanics are now the largest ethnic minority in the U.S., comprising 17.8% of the population.27 Though data regarding stage at diagnosis and outcomes by ethnic group in pediatric melanoma are extremely limited, several studies inclusive of adults have reported that minorities including Hispanics with melanoma are more likely to present with regional or distant disease,24,28–30 further underscoring the need for public health strategies directly targeting Hispanics.
Previous studies of melanoma in adults in the U.S. have demonstrated a correlation between increasing melanoma incidence and higher SES.31,32 Among the Texas pediatric and adolescent population, we observed a similar association in NH whites; however, we observed the opposite in Hispanics. Our results suggest that in Texas, the IR of melanoma was higher in Hispanics living in areas with higher poverty compared to Hispanics living in areas with lower poverty, though this finding was not statistically significant. This finding highlights that Hispanic children in this demographic group may need specific targeted public health and/or screening strategies to prevent and detect melanoma. Other studies have found that adults with melanoma with lower SES have an increased thickness of the primary site at diagnosis, with a stronger association in Hispanics,33,34 and Hispanics with melanoma have a 3-fold increased risk of disease-specific mortality compared to NH whites.35 Moreover, Hispanic children with melanoma and children within the lowest SES quartile with melanoma have a significantly higher mortality risk.25 Thus, Hispanic children in the high poverty group may be at risk for these poorer outcomes.
The etiology of melanoma in children and adolescents remains relatively less understood than that of adults. There are several known clinical and environmental risk factors for melanoma. Family history of melanoma accounts for approximately 10% of all melanoma cases, including those diagnosed during childhood and adolescence.36 In addition, there is an increased prevalence of melanoma occurring in: (1) those with large congenital melanocytic nevi; (2) those with the autosomal recessive disorder xeroderma pigmentosum; or (3) those who are immunosuppressed, either due to inherited immunodeficiencies or immunosuppressive therapy.35,37 In addition, the most important environmental risk factor for melanoma, particularly in adolescents, is exposure to ultraviolet radiation (UVR) either through sunlight or tanning bed use.36 While these risk factors are well-established for melanoma diagnosed in adulthood, and, to an extent, adolescence, the etiology of melanoma diagnosed in younger children and adolescents is not well understood and may reflect biological differences in tumor development in these groups.
The strengths of our study include the use of the TCR, a large, population-based registry in a population with large proportion of Hispanics and other race/ethnicity groups. These data allowed for a comprehensive assessment of melanoma IR predictors and trends among the Hispanic population, which has not been extensively assessed in previous reports of pediatric and adolescent melanoma. Through utilizing the TCR and SEER 13 databases, we were able to identify a large number pediatric and adolescent cases of melanoma, a relatively rare disease, which provided power to assess the independent demographic groups.
Even with the relatively large sample size of Hispanics included in our study compared to others, the rarity of pediatric melanoma in combination with the lower IR in Hispanics compared to NH whites still makes drawing definitive conclusions on subsets of the Hispanic population a challenge. For example, the trend of a higher IR of melanoma in Hispanics living in high poverty counties in Texas was not observed in the SEER 13 population. Sample size may be a contributing factor, as only 68 and 79 incident cases from Texas and SEER 13, respectively, were identified in our assessment. Additionally, while area-based measures of SES are frequently used as proxies for individual-level SES, it is possible that there was some misclassification bias introduced by using county-level poverty.
To our knowledge, this is the first report to suggest that melanoma IR trends in children may differ by demographic group. Previous studies evaluating risk factors for pediatric melanoma have primarily focused on NH white populations. While the overwhelming majority of melanoma diagnoses occur among this population, there have been no studies evaluating demographic predictors of pediatric melanoma within independent race/ethnicity groups outside of the NH white population. Our findings suggest that IR trends among Hispanic children and those <10 years old may not follow the general pediatric melanoma trends. Gaining a better understanding of the factors contributing to the development of melanoma in these groups will help inform optimal prevention strategies targeting these populations.
Supplementary Material
Acknowledgements
Cancer incidence data for Texas have been provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, 1100 W 49th Street, Austin, TX 78756, http://www.dshs.state.tx.us/tcr/default.shtm. SN was supported by the National Cancer Institute of the National Institutes of Health under award number 5K12CA090433.
Abbreviations:
- aIR
Age-adjusted incidence rate
- aIRR
Adjusted incidence rate ratio
- APC
Annual percent change
- CI
Confidence interval
- ICCC-3
International Classification of Childhood Cancer, third edition
- ICD-O-3
International Classification of Diseases for Oncology, third edition
- IR
Incidence rate
- LOWESS
Locally weighted scatterplot smoothing
- NH
Non-Hispanic
- NOS
Not otherwise specified
- SEER
Surveillance, Epidemiology, and End Results
- SES
Socioeconomic status
- TCR
Texas Cancer Registry
- UVR
Ultraviolet radiation
- U.S.
United States
Footnotes
Conflict of Interest
The authors have no conflict to declare.
References
- 1.National Cancer Institute. Cancer Stat Facts: Cancer of Any Site. https://seer.cancer.gov/statfacts/html/all.html. Accessed May 18, 2018.
- 2.Campbell LB, Kreicher KL, Gittleman HR, Strodtbeck K, Barnholtz-Sloan J, Bordeaux JS. Melanoma Incidence in Children and Adolescents: Decreasing Trends in the United States. J Pediatr. 2015;166(6):1505–1513. doi: 10.1016/j.jpeds.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134(4):e945–955. doi: 10.1542/peds.2013-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Averbook BJ, Lee SJ, Delman KA, et al. Pediatric melanoma: analysis of an international registry. Cancer. 2013;119(22):4012–4019. doi: 10.1002/cncr.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brecht IB, De Paoli A, Bisogno G, et al. Pediatric patients with cutaneous melanoma: A European study. Pediatr Blood Cancer. 2018;65(6):e26974. doi: 10.1002/pbc.26974. [DOI] [PubMed] [Google Scholar]
- 6.Dean PH, Bucevska M, Strahlendorf C, Verchere C. Pediatric Melanoma: A 35-year Population-based Review. Plast Reconstr Surg Glob Open. 2017;5(3):e1252. doi: 10.1097/gox.0000000000001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23(21):4735–4741. doi: 10.1200/jco.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 8.Lange JR, Palis BE, Chang DC, Soong SJ, Balch CM. Melanoma in children and teenagers: an analysis of patients from the National Cancer Data Base. J Clin Oncol. 2007;25(11):1363–1368. doi: 10.1200/jco.2006.08.8310. [DOI] [PubMed] [Google Scholar]
- 9.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 10.2000 Census of Population and Housing, Summary File 3. US Census Bureau, 2002. [Google Scholar]
- 11.2010 Census Summary File 2, US Summary. US Census Bureau, 2012. [Google Scholar]
- 12.National Cancer Institute. About the SEER Program. https://seer.cancer.gov/about/index.html. Accessed May 18, 2018.
- 13.Surveillance Research Program, National Cancer Institute. SEER*Stat software (www.seer.cancer.gov/seerstat).
- 14.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973-2013 varying) - Linked to County Attributes - Total U.S., 1969-2014 Counties; In: National Cancer Institute, Surveillance Research Program. [Google Scholar]
- 15.Registry Groupings in SEER Data and Statistics. https://seer.cancer.gov/registries/terms.html. Accessed January 14, 2019.
- 16.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Joinpoint Regression Program (Version 4.2.0.2). June 2015. Statistical Methodology and Applications Research Program. [Google Scholar]
- 18.Austin MT, Xing Y, Hayes-Jordan AA, Lally KP, Cormier JN. Melanoma incidence rises for children and adolescents: an epidemiologic review of pediatric melanoma in the United States. J Pediatr Surg. 2013;48(11):2207–2213. doi: 10.1016/j.jpedsurg.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Wong JR, Harris JK, Rodriguez-Galindo C, Johnson KJ. Incidence of childhood and adolescent melanoma in the United States: 1973-2009. Pediatrics. 2013;131(5):846–854. doi: 10.1542/peds.2012-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggen CAM, Durgaram VVL, van Doorn R, et al. Incidence and relative survival of melanoma in children and adolescents in the Netherlands, 1989-2013. J Eur Acad Dermatol Venereol. 2018;32(6):956–961. doi: 10.1111/jdv.14665. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson PM, Fredrikson M. Cutaneous malignant melanoma in children and adolescents in Sweden, 1993-2002: the increasing trend is broken. Int J Cancer. 2007;121(2):323–328. doi: 10.1002/ijc.22692. [DOI] [PubMed] [Google Scholar]
- 22.Wallingford SC, Iannacone MR, Youlden DR, et al. Comparison of melanoma incidence and trends among youth under 25 years in Australia and England, 1990-2010. Int J Cancer. 2015;137(9):2227–2233. doi: 10.1002/ijc.29598. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton EC, Nguyen HT, Chang YC, et al. Health Disparities Influence Childhood Melanoma Stage at Diagnosis and Outcome. J Pediatr. 2016;175:182–187. doi: 10.1016/j.jpeds.2016.04.068. [DOI] [PubMed] [Google Scholar]
- 24.Ma F, Collado-Mesa F, Hu S, Kirsner RS. Skin cancer awareness and sun protection behaviors in white Hispanic and white non-Hispanic high school students in Miami, Florida. Arch Dermatol. 2007;143(8):983–988. doi: 10.1001/archderm.143.8.983. [DOI] [PubMed] [Google Scholar]
- 25.Larson K, Cull WL, Racine AD, Olson LM. Trends in Access to Health Care Services for US Children: 2000-2014. Pediatrics. 2016;138(6). doi: 10.1542/peds.2016-2176. [DOI] [PubMed] [Google Scholar]
- 26.Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15(3):248–253. doi: 10.1177/107327480801500308. [DOI] [PubMed] [Google Scholar]
- 27.Facts for Features: Hispanic Heritage Month 2017. https://www.census.gov/newsroom/facts-for-features/2017/hispanic-heritage.html. Accessed May 18, 2018.
- 28.Black WC, Goldhahn RT, Jr., Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123(10):1331–1334. [PubMed] [Google Scholar]
- 29.Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166(17):1907–1914. doi: 10.1001/archinte.166.17.1907. [DOI] [PubMed] [Google Scholar]
- 30.Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of california cancer registry data, 1988-93. Cancer Causes Control. 1997;8(2):246–252. [DOI] [PubMed] [Google Scholar]
- 31.Harrison RA, Haque AU, Roseman JM, Soong SJ. Socioeconomic characteristics and melanoma incidence. Ann Epidemiol. 1998;8(5):327–333. [DOI] [PubMed] [Google Scholar]
- 32.Lee JA, Strickland D. Malignant melanoma: social status and outdoor work. Br J Cancer. 1980;41(5):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cockburn MG, Zadnick J, Deapen D. Developing epidemic of melanoma in the Hispanic population of California. Cancer. 2006;106(5):1162–1168. doi: 10.1002/cncr.21654. [DOI] [PubMed] [Google Scholar]
- 34.Pollitt RA, Clarke CA, Swetter SM, Peng DH, Zadnick J, Cockburn M. The expanding melanoma burden in California hispanics: Importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117(1):152–161. doi: 10.1002/cncr.25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neier M, Pappo A, Navid F. Management of melanomas in children and young adults. J Pediatr Hematol Oncol. 2012;34 Suppl 2:S51–54. doi: 10.1097/MPH.0b013e31824e3852. [DOI] [PubMed] [Google Scholar]
- 36.Kanavy HE, Gerstenblith MR. Ultraviolet radiation and melanoma. Semin Cutan Med Surg. 2011;30(4):222–228. doi: 10.1016/j.sder.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Pappo AS. Melanoma in children and adolescents. Eur J Cancer. 2003;39(18):2651–2661. doi: 10.1016/j.ejca.2003.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


