Abstract
Background:
Preclinical evidence from male subjects indicates that exposure to psychotropic medications, during early development, results in long-lasting altered responses to reward-related stimuli. However, it is not known if exposure to the antidepressant fluoxetine, in female subjects specifically, changes sensitivity to natural and drug rewards, later in life.
Aims:
The aim of this work was to investigate if exposure to fluoxetine mediates enduring changes in sensitivity to the rewarding properties of cocaine and sucrose, using female mice as a model system.
Methods:
We exposed C57BL/6 female mice to fluoxetine (250 mg/L in their drinking water) for 15 consecutive days, either during adolescence (postnatal day 35–49) or adulthood (postnatal day 70–84). Twenty-one days later, mice were examined on their behavioral reactivity to cocaine (0, 2.5, 5, 7.5 mg/kg) using the conditioned place preference paradigm, or assessed on the two-bottle sucrose (1%) test.
Results:
We found that regardless of age of antidepressant exposure, female mice pre-exposed to fluoxetine displayed reliable conditioning to the cocaine-paired compartment. However, when compared to respective age-matched controls, antidepressant pre-exposure decreased the magnitude of conditioning at the 5 and 7.5 mg/kg cocaine doses. Furthermore, fluoxetine pre-exposure reduced sucrose preference without altering total liquid intake.
Conclusions:
The data suggest that pre-exposure to fluoxetine, during adolescence or adulthood, results in a prolonged decrease in sensitivity to the rewarding properties of both natural and drug rewards in female C57BL/6 mice.
Keywords: Anhedonia, conditioned place preference, C57BL/6, juvenile, reward, selective serotonin reuptake inhibitor
Introduction
Major depressive disorder (MDD) is a severe and debilitating illness that affects millions of people across the globe (Ferrari et al., 2013). The prevalence of this disorder is particularly high within the juvenile population, given that up to 11% of adolescents are diagnosed with MDD (Costello et al., 2002). This is problematic because, if untreated, depressed adolescents display higher suicide attempts (Miranda and Shaffer, 2013), engage in illicit drug use (Copeland et al., 2009), and become involved in risky behavior (Duell et al., 2017). Currently, the most prescribed pharmacotherapeutic agent for the management of juvenile MDD is the selective serotonin reuptake inhibitor (SSRI), fluoxetine (FLX) – as other antidepressants do not consistently ameliorate depressive symptomology within this population (Emslie and Judge, 2000). As a result, there has been a significant increase in the prescription rates of FLX in individuals within their teenage years (Schroder et al., 2017). This is unfortunate, given that pharmacodynamic differences, across numerous psychotropic agents, are commonly reported between developing and adult organisms (Iñiguez et al., 2008; Scalzo and Spear, 1985). Further aggravating this issue, there is a dearth of preclinical studies that have assessed for potential long-lasting side effects as a function of juvenile antidepressant pre-exposure (Izquierdo et al., 2016; Olivier et al., 2011).
Exposure to antidepressants during adolescence, specifically, is concerning because preclinical studies suggest that there are indeed long-lasting neurobiologic alterations as a function of SSRI history. For example, adolescent FLX exposure has been shown to mediate a prolonged increase in sensitivity to anxiogenic stimuli (Homberg et al., 2011; Karpova et al., 2009), induce impairments in spatial memory performance (Sass and Wortwein, 2012), and increase the incentive valence of both natural (Iñiguez et al., 2010a) and drug rewards (Iñiguez et al., 2015). Collectively, this complex behavioral profile may suggest that juvenile FLX exposure mediates a phenotype indicative of altered drug-seeking behavior in adulthood. Nevertheless, it should be noted that the majority of these preclinical studies have been conducted using male subjects as a model system. This is an unexpected experimental approach, given that clinical data suggest that females are more likely than males to be diagnosed with a mood disorder across their lifetime (Kessler, 2003), and thus, are more likely to be prescribed with FLX (Hoffmann et al., 2014). To address this gap in the literature, the purpose of this investigation is to assess the prolonged effects of adolescent FLX exposure on the incentive motivation for cocaine and sucrose, in female C57BL/6 mice.
Materials and methods
Animals
Female C57BL/6 mice were utilized in the current investigation (Charles River, Hollister, California, USA). Mice were housed in polypropylene cages (3–4 per cage), which were bedded with wood shavings, and had access to food and water ad libitum. The colony room was maintained at a temperature between 21–23 C°, under a 12-hour light/dark cycle (lights on at 07:00). All studies were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), and with approval of the Institutional Animal Care and Use Committee at the University of Texas at El Paso, USA.
Drugs
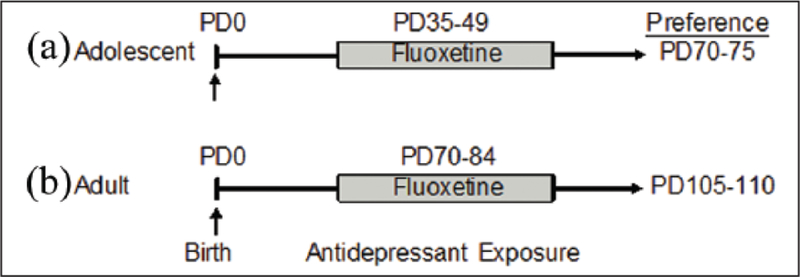
Fluoxetine hydrochloride was purchased from Spectrum Chemicals (Gardena, California) and was dissolved (250 mg/L) in water (vehicle (VEH)). FLX was delivered ad libitum in the drinking water (changed weekly) in light protected bottles (Model PC9RH8.5RD; Ancare, Bellmore, New York, USA). This approach was taken in order to reduce animal attrition due to tissue necrosis post chronic injections (Perrone et al., 2004), or SSRI-induced constipation (Baek et al., 2015). The dose of FLX was selected because it yields a dosage close to 25 mg/kg (Dulawa et al., 2004) – taking into account that females and adolescents metabolize FLX faster than males and/or adults (Anderson, 2005; Hodes et al., 2010; Wegerer et al., 1999). Cocaine hydrochloride was purchased from Sigma-Aldrich (St Louis, Missouri, USA) and was diluted with sterile 9% saline and administered in a volume of 2 mL/kg via intraperitoneal (IP) injection at 0, 2.5, 5, or 7.5 mg/kg. Antidepressant treatment and timeline of experimental design is depicted in Figure 1.
Figure 1.
Experimental design. Separate groups of adolescent (postnatal day (PD)-35) and adult (PD70) female C57BL/6 mice received vehicle (VEH) or fluoxetine (FLX; 250 mg/mL, in their drinking water) for 15 consecutive days. Twenty-one days later, mice were tested on their preference for cocaine (0, 2.5, 5, or 7.5 mg/kg) using the place conditioning test, or sucrose (1%) preference using the two-bottle choice test. Specifically, (a) adolescent FLX pretreated animals initiated behavioral testing at PD70, while (b) adult FLX pre-exposed mice initiated behavioral testing at PD105.
Experimental design
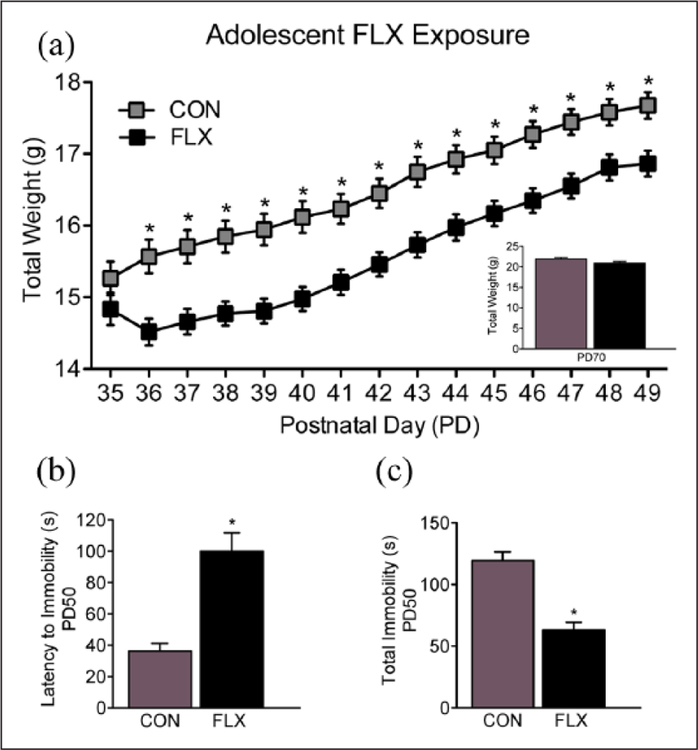
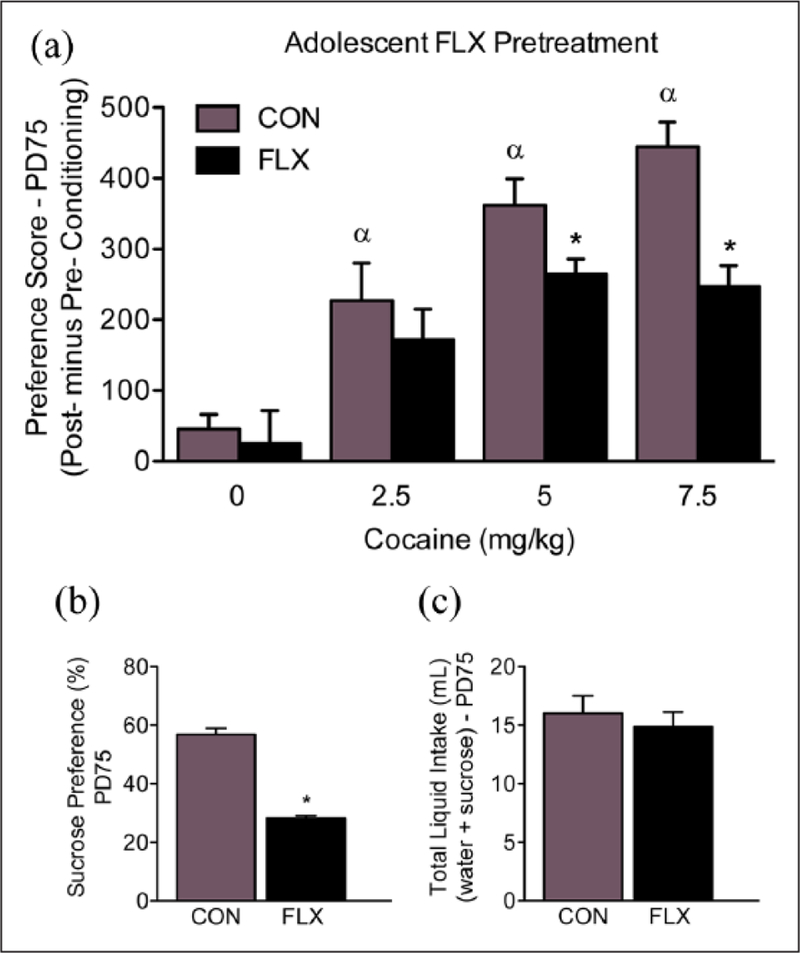
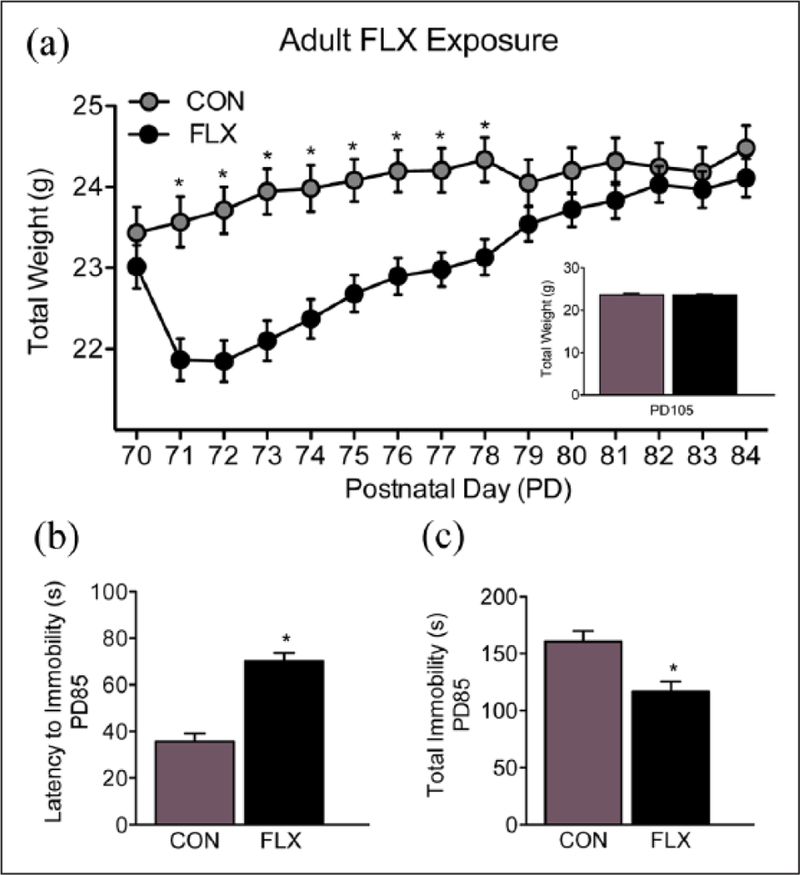
An initial experiment was conducted to examine whether the FLX dose/regimen selected would induce an antidepressant-like behavioral effect in adolescent female mice. To do this, PD35 female mice were exposed to FLX in their drinking water for 15 consecutive days (PD35–49). Twenty-four hour post-FLX exposure (PD50), the adolescent mice were tested on the tail suspension test. Based on the results of this experiment (i.e. decreased immobility; Figure 2(c)), separate groups of PD35 female mice were randomly selected to receive FLX for the same number of days (PD35–49). However, in this case, the female mice were left undisturbed in their home-cage, for 21 days, post antidepressant exposure (Figure 1(a)). At PD70 (i.e. adulthood) the mice were tested on behavioral responses to cocaine using the conditioned place preference (CPP) paradigm, or their sensitivity to a natural reward (sucrose preference test). Next, to examine whether the altered responses to both cocaine and sucrose observed in adulthood (Figure 3) were the results of the age of FLX exposure (adolescence vs adulthood), we conducted a separate set of similar experiments using adult (PD70) female mice (matched for FLX treatment and behavioral testing time; Figure 1(b)). Briefly, as with the adolescent mice, we first examined if the same FLX regimen (250 mg/L; PD70–84) would also mediate an antidepressant-like effect (decrease total immobility; Figure 4(c)) in the tail suspension test, 24 hour post antidepressant exposure (i.e. PD85). Also, we evaluated whether such treatment altered preference for cocaine or sucrose, 21 days post antidepressant exposure (see Figure 5). Separate groups of animals were used across all experiments in order to avoid potential testing carryover effects (see Table 1 for list of experimental groups). A video tracking system (EthovisionXT; Noldus, Leesburg, Virginia, USA) was used to record the behavioral data, except the tail suspension test, which was scored by observers unaware of antidepressant treatment conditions.
Figure 2.
Effects of adolescent fluoxetine (FLX) exposure on body weight gain and the tail suspension test. (a) Body weight increased across days of treatment (postnatal day (PD) 35–49), regardless of FLX or water-control (CON) conditions. However, when compared to CON mice, FLX-exposed mice displayed lower weight gain as of the second day (PD36) of antidepressant exposure (p<0.05). Twenty-one days later (PD70), no differences in body weight were noted between the groups (p>0.05; inset). Data are presented as average weight across days and antidepressant exposure (mean ± standard error of the mean (SEM), in g). (b) Twenty-four hours post antidepressant exposure (PD50), a separate group of adolescent mice was tested in the tail suspension test. FLX-exposed mice displayed higher time (s) to adopt a posture of immobility, (c) as well as a lower time spent immobile, when compared to CON mice (p<0.05). Data are presented as total time in seconds (mean ± SEM). *Significantly different when compared to CON (p<0.05).
Figure 3.
Effects of adolescent fluoxetine (FLX) exposure on reward-related behavior in adulthood. (a) Three-weeks after adolescent antidepressant exposure (postnatal day (PD)-70+), FLX-pretreated mice displayed decreased sensitivity to 5 and 7.5 mg/kg cocaine, when compared to water-pretreated control (CON) mice receiving the same doses of cocaine (n=9–11 per experimental group; p<0.05). *Within cocaine-dose group comparison (p<0.05). αSignificantly different when compared to age-matched controls conditioned to saline (p<0.05). (b) Adolescent FLX pretreatment reduced preference for a 1% sucrose solution three weeks after antidepressant exposure (n=12 per group; p<0.05). (c) No differences in total liquid intake were observed between the experimental groups (p>0.05).
Figure 4.
Effects of adult fluoxetine (FLX) exposure on body weight gain and the tail suspension test. (a) Body weight increased across days of treatment (postnatal day (PD) 70–84), regardless of FLX or water-control (CON) conditions. However, when compared to CON mice, FLX-exposed mice displayed lower weight gain as of the second day of antidepressant exposure (PD71), remaining lower until PD78 (p<0.05). Twenty-one days post FLX exposure (PD105) no differences in body weight were noted between the groups (p>0.05; inset). Data are presented as average weight across days and antidepressant exposure (mean ± standard error of the mean (SEM), in g). (b) Twenty-four hours post antidepressant exposure (PD85), a separate group of adult mice was tested in the tail suspension test. FLX-exposed mice displayed higher time (s) to adopt a posture of immobility, (c) as well as a lower time spent immobile, when compared to CON mice (p<0.05). Data are presented as total time in seconds (mean ± SEM). *Significantly different when compared to CON (p<0.05).
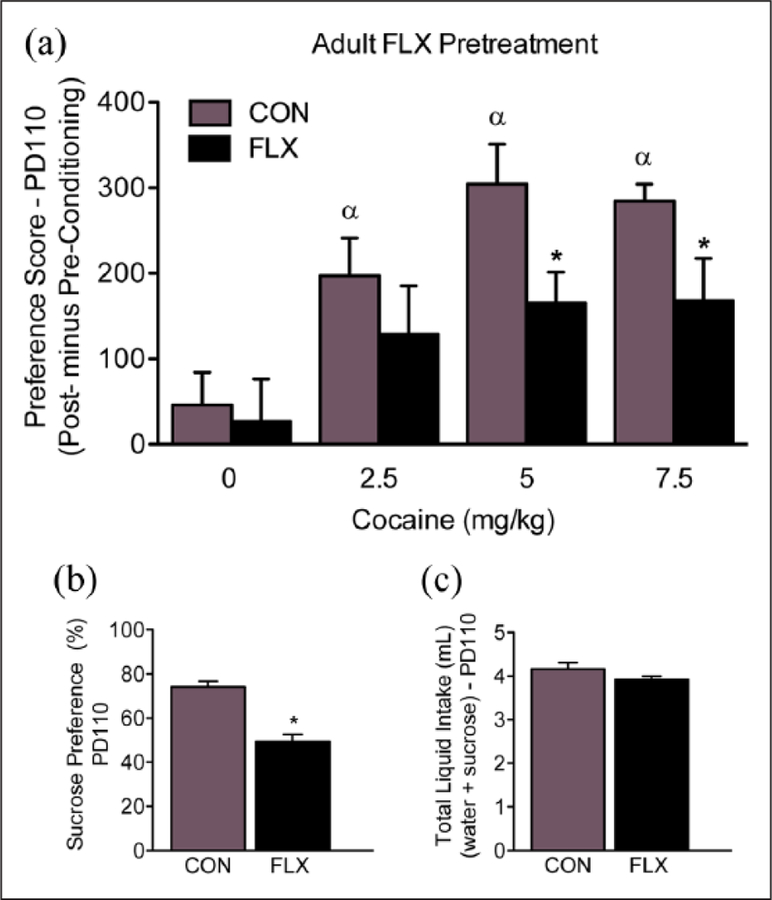
Figure 5.
Enduring effects of adult fluoxetine (FLX) exposure on reward-related behavior. (a) Three-weeks after adult antidepressant exposure, FLX-pretreated mice displayed decreased sensitivity to 5 and 7.5 mg/kg cocaine, when compared to water-pretreated control (CON) mice exposed to the same doses of cocaine (n=10 per experimental group; p<0.05). *Within cocaine group comparison (p<0.05). αSignificantly different when compared to age-matched controls conditioned to saline (p<0.05). (b) Adult FLX pretreatment reduced preference for a 1% sucrose solution three weeks after antidepressant exposure (n=12 per group; p<0.05). (c) No differences in total liquid intake were observed between the experimental groups (p>0.05).
Table 1.
Experimental groups.
| Group | Drug | n | Age | Time | Procedure | Data |
|---|---|---|---|---|---|---|
| 1 | CON | 10 | PD35–49 | 24 h | Tail suspension test (PD50) | Figure 2(b) and (c) |
| FLX | 10 | PD35–49 | ||||
| 2 | CON | 41 | PD35–49 | 21 d | Cocaine place preference (PD70–75) | Figure 3(a) |
| FLX | 40 | PD35–49 | ||||
| 3 | CON | 12 | PD35–49 | 21 d | Sucrose preference (PD70–75) PD35–49 | Figure 3(b) and (c) |
| FLX | 12 | |||||
| 4 | CON | 10 | PD70–84 | 24 h | Tail suspension test (PD85) | Figure 4(b) and (c) |
| FLX | 10 | PD70–84 | ||||
| 5 | CON | 40 | PD70–84 | 21 d | Cocaine place preference (PD105–110) | Figure 5(a) |
| FLX | 40 | PD70–84 | ||||
| 6 | CON | 12 | PD70–84 | 21 d | Sucrose preference (PD105–110) | Figure 5(b) and (c) |
| FLX | 12 | PD70–84 |
CON: control; d, day; FLX: fluoxetine; h, hour; PD: postnatal day.
Tail suspension test
The tail suspension test is a measure of behavioral despair in which rodents are suspended by their tail during a single trial of six minutes. Initially, mice engage in escape-directed activity, but eventually adopt a posture of immobility. Increases in immobility have been characterized as indicative of depressive-like behavior (Iñiguez et al., 2016; Porsolt, 2000), which is reversed by pharmacological antidepressant treatment – a response correlated to human antidepressant efficacy (Cryan et al., 2005). The total time (s) spent immobile during the last five minutes of the test was the dependent variable. If mice climbed up their tail, they were gently returned to the hanging position; mice that climbed up their tail more than two times were excluded from the experiment.
Conditioned Place Preference
CPP was carried out as previously described (Iñiguez et al., 2010b), using a three-compartment apparatus (Alcantara et al., 2014). The compartments differed in floor texture, as well as wall coloring and pattern. On the preconditioning day (Day 1), mice had free access to explore the entire apparatus for 25 min in order to obtain baseline preference to any of the three compartments (side compartments: 23×16×36 cm; middle compartment: 9×16×36 cm, L×W×H). Conditioning trials (25 min, two per day) were given on four consecutive days (Days 2–5). During the conditioning trials, mice received a saline injection (1 mL/kg, IP) and were confined to the preferred compartment of the apparatus (biased procedure; Bardo and Bevins, 2000). After three hours, mice received cocaine (0, 2.5, 5, or 7.5 mg/kg, IP) and were confined to the opposite (non-preferred) side compartment. Doses of cocaine were selected based on prior work (Hilderbrand and Lasek, 2014). On test day (preference, Day 6), mice were again allowed to freely explore the entire apparatus for 25 min (i.e. PD75 for mice that received FLX-pretreatment during adolescence, and PD110 for mice that received FLX-pretreatment as adults). Data were calculated as a preference score by subtracting the time (s) spent in the cocaine-paired side during test day (Day 6) from the time spent on the same compartment during the preconditioning day (Day 1). Thus, a positive number indicates higher preference for the cocaine-paired side, whereas a negative number would indicate avoidance of the cocaine-paired side.
Sucrose preference
The sucrose preference test consisted of a two-bottle procedure in which mice were given the choice between consuming water or a 1% sucrose solution (Wallace et al., 2008). Mice were habituated to drink water from two separate bottles, 21-days post FLX exposure (PD70–74 for the adolescent pretreated group, and PD105–109 for the adult pretreated group). Twenty-four hours later, one of the bottles was replaced with a 1% sucrose solution, while the other bottle contained water (PD75 for the adolescent antidepressant pretreated group, and PD110 for the adult antidepressant pretreated group). The position of the sucrose bottle was counter-balanced (left vs right) across the different cages to control for potential side-preference bias. Preference for sucrose over water (sucrose/(sucrose + water)) was used as a measure for sensitivity to reward (Warren et al., 2011).
Statistics
Data were analyzed using analysis of variance (ANOVA) techniques, with FLX pre-treatment (between measure), days of FLX exposure (repeated measure), and cocaine post-treatment (between measure) as sources of variance. Separate analyses were performed between adolescent and adult groups to avoid age-specific influences on locomotor activity. Tukey post-hoc tests were used to examine all pairwise comparisons. Planned comparisons were also conducted to examine the hypothesis that FLX pretreatment will alter cocaine-induced reward. Two-tailed Student’s t-tests were used for analyses implicating two-group comparisons. Statistical significance was defined as p<0.05. Data are presented as mean ± standard error of the mean (SEM).
Results
FLX decreases body weight in adolescent female mice
Figure 2(a) shows the effects of adolescent antidepressant exposure (PD35–49) on body weight (g). A mixed-design repeated measures ANOVA showed that weight was influenced by a main effect of FLX treatment (between measure: F1,123=10.39, p<0.05), a main effect of day of antidepressant exposure (repeated measure: F14,1722=370.88, p<0.5), as well as their interaction (FLX by day of exposure; F14,1722=5.34, p<0.05). Post-hoc analyses revealed that when compared to controls (n=63), FLX-exposed mice (n=62) displayed lower body-weight as of the second day of treatment, remaining lower throughout FLX exposure (p<0.05, respectively). No enduring differences in body weight, as a function of FLX pre-exposure, were observed at PD70 (i.e. prior to behavioral testing in adulthood, p>0.05; see Figure 2(a) inset).
Adolescent FLX exposure decreases immobility in the tail suspension test
Figure 2(b) and (c) displays the effects of FLX (PD35–49) on the adolescent tail suspension test. Twenty-four hours after antidepressant exposure (PD50), adolescent female mice exposed to FLX displayed an increased latency (s) to become immobile when compared to the VEH group (t18=5.0, p<0.05; Figure 2(b)). The FLX-treated mice also displayed a lower time immobile (t18=6.1, p<0.05), when compared to VEH controls (Figure 2(c)), during the last five minutes of the test (n=10 per group). Together, this indicates that the FLX dose/regimen selected, mediates a traditional antidepressant-like effect in adolescent female C57BL/6 mice.
Adolescent FLX exposure decreases cocaine preference in adulthood
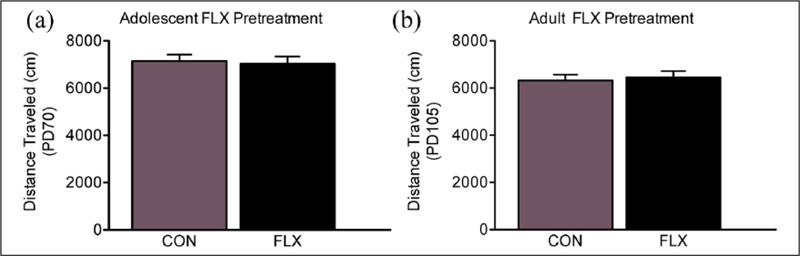
Figure 3(a) shows the lasting effects of adolescent FLX exposure (PD35–49) on cocaine (0, 2.5, 5, or 7.5 mg/kg) CPP in adulthood (PD70+; n=81). Time spent in the cocaine-paired side varied as a function of adolescent FLX exposure (pretreatment main effect: F1,73=11.48, p<0.05), as well as cocaine exposure in adulthood (post-treatment main effect: F3,73=25.69, p<0.05). Neither VEH-nor FLX-pretreatment (n=10 per group) resulted in preference for any of the compartments when mice were conditioned to saline (p>0.05). In contrast, we found that VEH-pretreated mice conditioned to 2.5 (n=11), 5 (n=10), or 7.5 mg/kg (n=10) cocaine, displayed reliable conditioning, when compared to VEH-pretreated/saline-conditioned mice (αp<0.05). Planned comparisons indicated that FLX pretreatment also mediated reliable conditioning to the compartment paired with 2.5 (n=11), 5 (n=9), and 7.5 (n=10) mg/kg cocaine, when compared to FLX-pretreated/saline-conditioned mice (p<0.05, respectively). Interestingly, the FLX-pretreated female mice conditioned to 5 and 7.5 mg/kg cocaine spent significantly less time in the drug-paired compartment when compared to VEH-pretreated mice receiving the same doses of cocaine in adulthood (p<0.05, respectively). No differences in distance traveled (cm), as a function of adolescent FLX pretreatment, were observed during the preconditioning phase (PD70, p>0.05; Figure 6(a)) – indicating no differences in general locomotor activity between the groups.
Figure 6.
Enduring effects of fluoxetine (FLX) exposure on general locomotor activity. Three-weeks after (a) adolescent (postnatal day (PD)-70; or (b) adult (PD105) antidepressant treatment, no differences in distance traveled (cm) were noted between the groups (p>0.05). Data shown as mean ± standard error of the mean (SEM).
Adolescent FLX exposure decreases sucrose preference in adulthood
Figure 3(b) and (c) shows the lasting effects of adolescent FLX exposure (PD35–49) on sucrose preference in adulthood (PD70+). A student’s t test indicated that adult female mice exposed to FLX during adolescence (n=12) displayed a decrease in preference for a 1% sucrose solution when compared to VEH-pretreated (n=12) controls (t22=12.89, p<0.05; Figure 3(b)). No differences in total liquid intake (water + sucrose) were observed between the groups (p>0.05; Figure 3(c)).
FLX decreases body weight in adult female mice
Figure 4(a) shows the effects of adult antidepressant exposure (PD70–84) on body weight (g). A mixed-design repeated measures ANOVA showed that weight was influenced by a main effect of FLX (between measure: F1,122=10.17, p<0.05), a day main effect of antidepressant exposure (repeated measure: F14,1708=68.28, p<0.05), as well as their interaction (FLX by day of exposure; F14,1708=28.74, p<0.05). Post-hoc analyses revealed that when compared to controls (n=62), adult mice exposed to FLX (n=62) displayed lower body weight as of the second day of treatment (PD71), remaining lower till PD78 (p<0.05, respectively). No differences in body weight were observed the last six days of FLX exposure (PD79–84; p>0.05). Similarly, no enduring differences in body weight, as a function of FLX pre-exposure, were observed later in life (PD105), prior to behavioral testing (see Figure 4(a) inset).
Adult FLX exposure reduces immobility in the tail suspension test
Figure 4(b) and (c) displays the effects of FLX (PD70–84) on the adult tail suspension test. Twenty-four hours after antidepressant exposure (PD85), adult female mice exposed to FLX (n=10) displayed an increased latency (s) to become immobile (t18=6.84, p<0.05), when compared to their VEH-treated counterparts (n=10; Figure 4(b)). The FLX-treated mice also displayed a lower time immobile (t18=3.37, p<0.05), when compared to VEH-controls (Figure 4(c)), during the last five minutes of the test. Together, this indicates that the FLX dose/regimen selected, mediates a traditional antidepressant-like effect in adult female C57BL/6 mice.
Adult FLX exposure reduces cocaine preference later in life
Figure 5(a) shows the enduring effects of adult FLX exposure (PD70–84) on cocaine CPP (n=80). Here, the time spent in the cocaine-paired side varied as a function of adult FLX exposure (pretreatment main effect: F1,72 =7.63, p<0.05), as well as cocaine exposure later in life (post-treatment main effect: F3,72=8.72, p<0.05). Neither the VEH-pretreated (n=10) nor the FLX-pretreated (n=10) animals displayed a preference for any of the compartments when they were conditioned to saline (p>0.05). In contrast, preplanned comparisons indicated that female mice pre-exposed with VEH (PD70–84) conditioned to 2.5 (n=10), 5 (n=10), and 7.5 mg/kg (n=10) cocaine showed reliable conditioning when compared to VEH-pretreated/saline-conditioned mice (αp<0.05, respectively). Similarly, the FLX-pretreated mice conditioned to 5 (n=10) and 7.5 (n=10), but not 2.5 (n=10), mg/kg cocaine showed reliable conditioning when compared to FLX-pretreated/saline-conditioned mice (p<0.05). Interestingly, FLX-pretreated mice conditioned to 5 and 7.5 mg/kg cocaine spent significantly less time in the drug-paired compartment when compared to VEH-pretreated mice receiving the same doses of cocaine (p<0.05, respectively). No differences in total distance traveled (cm) were noted between the FLX-pretreated animals and their respective VEH-pretreated controls during the preconditioning phase (PD105; Figure 6(b)).
Adult FLX exposure decreases sucrose preference later in life
Figure 5(b) and (c) shows the effects of adult FLX exposure (PD70–84) on sucrose preference, 21 days post antidepressant exposure (n=12 per group). A student’s t test indicated that FLX pre-exposed mice displayed a decrease in preference for a 1% sucrose solution when compared to VEH-pretreated controls (t22=12.89, p<0.05; Figure 5(b)). No differences in total liquid intake (water + sucrose) were observed between the groups (p>0.05; Figure 5(c)).
Discussion
SSRIs, like FLX, are often prescribed to the adolescent female population for the treatment of numerous illnesses, although, primarily for the management of MDD (John et al., 2016; Schroder et al., 2017; Steiner et al., 1995). This is unexpected, given that pharmacodynamic differences between developing and adult organisms are commonly reported as a function of psychotropic drug exposure (Correll et al., 2011; Iñiguez et al., 2008), and that such treatments mediate long-lasting neurobehavioral alterations (Brooks et al., 2016; Olivier et al., 2011). Another problem is that animal studies examining for potential enduring side effects, as a result of early-life psychotropic exposure, have largely excluded females as subjects. Recent data, in male rodents, indicate that juvenile exposure to FLX increases sensitivity to the rewarding properties of cocaine in adulthood (Iñiguez et al., 2015), potentially altering drug-abuse vulnerability. However, whether adolescent FLX exposure results in prolonged alterations in sensitivity to reward-related stimuli, in females specifically, has not been evaluated. As such, the purpose of the present investigation was to examine if changes in responses to cocaine and sucrose would be observed in adult female mice (PD70+) pre-treated with FLX during adolescence (PD35–49).
To do this, we first assessed whether the FLX dose/regimen selected (250 mg/kg in drinking water for 15 days) would mediate an antidepressant-like effect in juvenile (PD35–49) and adult (PD70–84) female mice. Specifically, we tested whether or not FLX would decrease immobility in the tail suspension test, a behavioral measure of despair that is widely used to evaluate antidepressant-like efficacy across the literature (Cryan et al., 2005). Not surprisingly, independent of age of antidepressant exposure, we found that SSRI treatment increased escape-directed behaviors (i.e. increase in the time to become immobile, along with decreased total immobility; Figures 2(b) and (c) and 4(b) and (c)) – a traditional antidepressant-like effect. Furthermore, adolescent and adult FLX exposure resulted in decreases in body weight-gain across days of treatment (Figures 2(a) and 4(a)), given that FLX increases energy expenditure (Bross and Hoffer, 1995; Scabia et al., 2018), similar to results that others have previously reported (Amodeo et al., 2015). However, the decreases in body weight were not observed 21-days post treatment in either age group, thus, suggesting that FLX history does not result in enduring decreases of body weight in female C57BL/6 mice.
Adolescent FLX exposure decreased sensitivity to both drug- and natural-reward related stimuli in adulthood. Specifically, in the cocaine place conditioning experiments (Figure 3(a)), FLX-pretreated mice displayed lower preference scores for cocaine at the 5 and 7.5 mg/kg doses, when compared to respective saline-pretreated controls exposed to the same cocaine regimen. Importantly, no differences in general locomotor activity were noted between the groups on the preconditioning day (Figure 6(a)), thus, uncovering a decrease in the reward incentive of cocaine (Bardo and Bevins, 2000). To further explore whether this decrease in preference for cocaine would generalize to a natural reward, we examined the effects of adolescent antidepressant exposure on the two-bottle choice sucrose test in adulthood (Figure 3(b) and (c)). Here, we found that FLX-pretreated animals displayed a decrease in preference for a 1% sucrose solution, without altering total liquid intake – a response that is commonly described as an anhedonia-like phenotype (Willner et al., 1987). Interestingly, this behavioral profile is in direct contrast with previous work conducted in male rodents, which shows that the male animals display an enduring increase in preference to both cocaine (Iñiguez et al., 2015) and sucrose (Iñiguez et al., 2010a), as a function of adolescent FLX treatment (20 mg/kg). This is an important finding, as it demonstrates how juvenile SSRI exposure mediates differential effects on reward sensitivity as a function of sex in adulthood.
To evaluate whether the decreased sensitivity to reward-related stimuli was dependent on the age of FLX exposure (i.e. adolescence), we followed with a series of similar experiments using adult (PD70) female mice (see Figure 1(b)), as a positive control group for age of SSRI exposure. Unexpectedly, adult antidepressant pretreatment (PD70–84) resulted in a similar behavioral response when these animals were tested for cocaine preference, 21-days after FLX pre-treatment (Figure 5(a)). Specifically, the adult FLX-pretreated mice conditioned to 5 and 7.5 mg/kg cocaine, displayed lower preference scores when compared to the VEH-pretreated animals exposed to the same doses of the stimulant. Analogously, when assessing sensitivity to sucrose, we found also that FLX pre-exposure decreased preference for the 1% sucrose solution later in life (Figure 5(b) and (c)). Collectively, our data are consistent with previous work conducted in female rodents indicating that neonatal (PD5–18) exposure to SSRIs causes enduring depression-related behaviors, as per reductions in the rewarding properties of sucrose (Popa et al., 2008). Yet, here, we extend these findings to the adolescent (PD35–49) and adult (PD70–84) stages of development.
The clinical implications of our findings are challenging to interpret. For example, reductions in cocaine and sucrose preference may help explain why clinical data suggest that SSRIs may reduce the risk of substance abuse, particularly for cocaine (Moeller et al., 2007; Oliveto et al., 2012; Walsh et al., 1994), implying that if cocaine and sucrose are less rewarding, this phenotype may be indicative of reduced drug abuse potential. However, a different interpretation of this reward devaluation may be indicative of a prolonged anhedonia-like profile after antidepressant exposure (Popa et al., 2008). Supporting this notion, recent clinical findings suggest that SSRI discontinuation leads to enduring decreases in pleasurable stimuli, such as sexual performance (Reisman, 2017). Therefore, our preclinical work demonstrates that FLX pre-exposure could potentially model an endophenotype of depressive-like behavior (Neill et al., 1990) that mimics depressed female patients with SSRI history (Khazaie et al., 2015; Reisman, 2017).
The neurobiological mechanisms underlining this lowered reward behavioral phenotype are currently not known. Since behavioral assessment was conducted 21 days after FLX exposure, we argue that the decreases in sensitivity to cocaine and sucrose are the result of enduring neuroplastic adaptations. For example, in male rodents, adolescent FLX exposure results in long-term molecular signaling alterations within discreet mood-related brain regions, such as the ventral tegmental area (Iñiguez et al., 2014), the amygdala (Homberg et al., 2011), the frontal cortex (Wegerer et al., 1999), and the hippocampal formation (Klomp et al., 2014). Interestingly, in adult female rats, chronic exposure to FLX (20 mg/kg) alters hippocampal neurogenesis one month after antidepressant exposure (Airan et al., 2007), potentially mediating, at least in part, the lowered sensitivity to reward-related behavior observed in the present investigation. Of course, future work will be needed to directly examine how enduring FLX-induced changes in hippocampal neuroplasticity may influence sensitivity to reward-related stimuli, given that this brain region is implicated in modulating preference for cocaine in the place-conditioning paradigm (Meyers et al., 2003, 2006; Nygard et al., 2013).
A limitation of the present investigation is that we did not control for estrous cyclicity in our experimental animals. Estradiol has been proposed to be a modulator of reinforcing stimuli, including drugs of abuse like cocaine (Kerstetter et al., 2012; Kerstetter and Kippin, 2011). As such, future studies will be necessary to delineate the potential role that sex steroids may play in the behavioral responses observed, as a function of FLX pre-exposure. Another limitation of our work is that we are evaluating alterations in reward three-weeks post-FLX exposure in normal animals. However, given that FLX is prescribed to the female population for numerous illnesses in addition to MDD, such as anxiety, premenstrual dysphoric disorder, and pain (Mika et al., 2013; Steiner et al., 1995), we believe that this is an appropriate approach to initially assess for enduring side effects as a function of antidepressant exposure. Lastly, because of metabolic differences between adolescents and adults, it is possible that blood concentrations of FLX and its metabolites differed between the age groups during treatment (since FLX was available ad lib in drinking water). Thus, future studies will be required to determine the exact FLX plasma concentrations that mediate the enduring anhedonia-like behavior, as a function of age.
The high prescription rate of SSRIs for the management of mood-related illnesses in the female population is undeniable (Schroder et al., 2017). Yet, most preclinical studies examining for potential long-lasting antidepressant-induced consequences have mostly included male animals, so the possibility of sex differences in the outcome of drug-induced side effects, later in life, has been largely ignored. Here, we report that exposure to FLX in adolescent and adult female C57BL/6 mice decreases cocaine and sucrose preference, 21-days post antidepressant exposure – a profile indicative of an anhedonia-like phenotype. In light of this, future work will be necessary to delineate the precise neurobiological mechanisms by which FLX history decreases sensitivity to cocaine and sucrose, in females specifically, in an age independent manner.
Acknowledgements
The authors would like to thank Adolfo Andazola-Carmona and Jose Chavez for excellent technical assistance. Student co-authors were supported by the Research Initiative for Scientific Enhancement (RISE- GM100829, GM069621; Israel Garcia-Carachure, David O Sanchez, Samuel A Castillo), Summer Mentoring And Research Training -Methods in Neuroscience of Drug-abuse (SMART MiND-DA033613; David O Sanchez, Miguel Arenivar) Achieve Career Success in Science through Exellence (ACSScellence DUE-1565063; Omar Lira), and Building Infrastructure Leading to Diversity (BUILD-GM118971; Joshua Preciado-Piña) research programs at California State University San Bernardino and/or The University of Texas at El Paso.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Institute of General Medical Sciences (NIH-NIGMS; Grant no. SC2GM109811 to SDI).
Footnotes
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Airan RD, Meltzer LA, Roy M, et al. (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317: 819–823. [DOI] [PubMed] [Google Scholar]
- Alcantara LF, Warren BL, Parise EM, et al. (2014) Effects of psychotropic drugs on second messenger signaling and preference for nicotine in juvenile male mice. Psychopharmacology (Berl) 231: 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo LR, Greenfield VY, Humphrey DE, et al. (2015) Effects of acute or repeated paroxetine and fluoxetine treatment on affective behavior in male and female adolescent rats. Psychopharmacology (Berl) 232: 3515–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GD (2005) Sex and racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 14: 19–29. [DOI] [PubMed] [Google Scholar]
- Baek IS, Park JY and Han PL (2015) Chronic antidepressant treatment in normal mice induces anxiety and impairs stress-coping ability. Exp Neurobiol 24: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT and Bevins RA (2000) Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153: 31–43. [DOI] [PubMed] [Google Scholar]
- Brooks JM, O’Donnell P and Frost DO (2016) Olanzapine treatment of adolescent rats alters adult D2 modulation of cortical inputs to the ventral striatum. Int J Neuropsychopharmacol Epub ahead of print 12 May 2016. DOI: 10.1093/ijnp/pyw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross R and Hoffer LJ (1995) Fluoxetine increases resting energy expenditure and basal body temperature in humans. Am J Clin Nutr 61: 1020–1025. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Costello EJ, et al. (2009) Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch Gen Psychiatry 66: 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ and March JS (2011) Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry 72: 655–670. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, et al. (2002) Development and natural history of mood disorders. Biol Psychiatry 52: 529–542. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C and Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29: 571–625. [DOI] [PubMed] [Google Scholar]
- Duell N, Steinberg L, Icenogle G, et al. (2017) Age patterns in risk taking across the world. J Youth Adolesc Epub ahead of print 19 October 2017. DOI: 10.1007/s10964-017-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, et al. (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29: 1321–1330. [DOI] [PubMed] [Google Scholar]
- Emslie G and Judge R (2000) Tricyclic antidepressants and selective serotonin reuptake inhibitors: Use during pregnancy, in children/adolescents and in the elderly. Acta Psychiatr Scand Suppl 403: 26–34. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, et al. (2013) The epidemiological modelling of major depressive disorder: Application for the Global Burden of Disease Study 2010. PLoS One 8: e69637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand ER and Lasek AW (2014) Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport 25: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Hill-Smith TE, Suckow RF, et al. (2010) Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J Pharmacol Exp Ther 332: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Glaeske G and Bachmann CJ (2014) Trends in antidepressant prescriptions for children and adolescents in Germany from 2005 to 2012. Pharmacoepidemiol Drug Saf 23: 1268–1272. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Blom T, et al. (2011) Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One 6: e16646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Alcantara LF, Warren BL, et al. (2014) Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci 34: 1007–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Aubry A, Riggs LM, et al. (2016) Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress 5: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Cortez AM, Crawford CA, et al. (2008) Effects of aripiprazole and terguride on dopamine synthesis in the dorsal striatum and medial prefrontal cortex of preweanling rats. J Neural Transm 115: 97–106. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, et al. (2015) Fluoxetine exposure during adolescence increases preference for cocaine in adulthood. Sci Rep 5: 15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL and Bolaños-Guzmán CA (2010a) Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry 67: 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Neve RL, et al. (2010b) Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res 214: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Pozos H, Torre Ade L, et al. (2016) Sex differences, learning flexibility, and striatal dopamine D1 and D2 following adolescent drug exposure in rats. Behav Brain Res 308: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John A, Marchant AL, Fone DL, et al. (2016) Recent trends in primary-care antidepressant prescribing to children and young people: An e-cohort study. Psychol Med 46: 3315–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Lindholm J, Pruunsild P, et al. (2009) Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol 19: 97–108. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, et al. (2012) Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology 37: 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA and Kippin TE (2011) Impact of sex and gonadal hormones on cocaine and food reinforcement paradigms. J Addict Res Ther S4: 2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2003) Epidemiology of women and depression. J Affect Disord 74: 5–13. [DOI] [PubMed] [Google Scholar]
- Khazaie H, Rezaie L, Rezaei Payam N, et al. (2015) Antidepressant-induced sexual dysfunction during treatment with fluoxetine, sertraline and trazodone; a randomized controlled trial. Gen Hosp Psychiatry 37: 40–45. [DOI] [PubMed] [Google Scholar]
- Klomp A, Vaclavu L, Meerhoff GF, et al. (2014) Effects of chronic fluoxetine treatment on neurogenesis and tryptophan hydroxylase expression in adolescent and adult rats. PLoS One 9: e97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR and Neisewander JL (2003) Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14: 2127–2131. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, et al. (2006) Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120: 401–412. [DOI] [PubMed] [Google Scholar]
- Mika J, Zychowska M, Makuch W, et al. (2013) Neuronal and immunological basis of action of antidepressants in chronic pain - clinical and experimental studies. Pharmacol Rep 65: 1611–1621. [DOI] [PubMed] [Google Scholar]
- Miranda R and Shaffer D (2013) Understanding the suicidal moment in adolescence. Ann N Y Acad Sci 1304: 14–21. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, et al. (2007) Citalopram combined with behavioral therapy reduces cocaine use: A double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse 33: 367–378. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the care and use of laboratory animals Washington, DC: National Academies Press. [Google Scholar]
- Neill D, Vogel G, Hagler M, et al. (1990) Diminished sexual activity in a new animal model of endogenous depression. Neurosci Biobehav Rev 14: 73–76. [DOI] [PubMed] [Google Scholar]
- Nygard SK, Klambatsen A, Hazim R, et al. (2013) Sexually dimorphic intracellular responses after cocaine-induced conditioned place preference expression. Brain Res 1520: 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, et al. (2012) Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction 107: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, et al. (2011) The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: A review. Prog Neuropsychopharmacol Biol Psychiatry 35: 1400–1408. [DOI] [PubMed] [Google Scholar]
- Perrone JA, Chabla JM, Hallas BH, et al. (2004) Weight loss dynamics during combined fluoxetine and olanzapine treatment. BMC Pharmacol 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, et al. (2008) Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: Evidence from sleep, stress, and behavior. J Neurosci 28: 3546–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD (2000) Animal models of depression: Utility for transgenic research. Rev Neurosci 11: 53–58. [DOI] [PubMed] [Google Scholar]
- Reisman Y (2017) Sexual consequences of post-SSRI syndrome. Sex Med Rev 5: 429–433. [DOI] [PubMed] [Google Scholar]
- Sass A and Wortwein G (2012) The effect of subchronic fluoxetine treatment on learning and memory in adolescent rats. Behav Brain Res 228: 169–175. [DOI] [PubMed] [Google Scholar]
- Scabia G, Barone I, Mainardi M, et al. (2018) The antidepressant fluoxetine acts on energy balance and leptin sensitivity via BDNF. Sci Rep 8: 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo FM and Spear LP (1985) Chronic haloperidol during development attenuates dopamine autoreceptor function in striatal and mesolimbic brain regions of young and older adult rats. Psychopharmacology (Berl) 85: 271–276. [DOI] [PubMed] [Google Scholar]
- Schroder C, Dorks M, Kollhorst B, et al. (2017) Outpatient antidepressant drug use in children and adolescents in Germany between 2004 and 2011. Pharmacoepidemiol Drug Saf 26: 170–179. [DOI] [PubMed] [Google Scholar]
- Steiner M, Steinberg S, Stewart D, et al. (1995) Fluoxetine in the treatment of premenstrual dysphoria. Canadian fluoxetine/premenstrual dysphoria collaborative study group. N Engl J Med 332: 1529–1534. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, et al. (2008) The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci 28: 10272–10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, et al. (1994) Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol 14: 396–407. [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, et al. (2011) Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci 31: 10347–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegerer V, Moll GH, Bagli M, et al. (1999) Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. J Child Adolesc Psychopharmacol 9: 13–24; discussion 25–16. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, et al. (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364. [DOI] [PubMed] [Google Scholar]