Supplemental Digital Content is available in the text
Keywords: Burn injury, coagulopathy, manual of operations, -omics, prospective study
ABSTRACT
Introduction:
Provisioning care for traumatically injured patients makes conducting research very proximal to injury difficult. These studies also inherently have regulatory barriers to overcome. Here we outline a protocol for acute-phase enrollment of traumatically injured patients into a prospective observational clinical trial with precise and comprehensive sample acquisition in support of a systems biology approach to a research study.
Methods:
Experts in trauma, burn, blood coagulation, computational biology, and integrative systems biology developed a prospective study that would capture the natural history of coagulation pathology after traumatic injury. Blood was sampled at admission and serial time points throughout hospitalization. Concurrently, demographic and outcomes data were recorded and on-site point-of-care testing was implemented. Protocols were harmonized across sites and sampling protocols validated through demonstration of feasibility and sample quality assurance testing. A novel data integration platform was developed to store, visualize, and enable large-scale analysis of empirical and clinical data. Regulatory considerations were also addressed in protocol development.
Results:
A comprehensive Manual of Operations (MOO) was developed and implemented at 3 clinical sites. After regulatory approval, the MOO was followed to collect 5,348 longitudinal samples from 1,547 patients. All samples were collected, processed, and stored per the MOO. Assay results and clinical data were entered into the novel data management platform for analyses.
Conclusion:
We used an iterative, interdisciplinary process to develop a systematic and robust protocol for comprehensive assessment of coagulation in traumatically injured patients. This MOO can be a template for future studies in the acute setting.
INTRODUCTION
Trauma encompasses a wide spectrum of injuries, ranging from penetrating (i.e., gun shots and stabbings), blunt injury (i.e., motor vehicle collisions) to thermal injury. Patients suffering from these injuries frequently develop shock, which is most often related to hemorrhage and sterile inflammatory processes. Hemorrhage is the leading cause of potentially preventable death in combat casualties and is the second leading cause of death in civilian trauma (1, 2). Coagulopathy is a significant contributor to mortality in these patients (3–7). Patients with thermal injury experience significant tissue injury, fluid shifts, and inflammatory responses that are also associated with derangements in coagulation function (8). The underlying mechanisms of coagulopathy following traumatic hemorrhage or thermal injury are not well characterized, and there are few specific diagnostic tools available. To address this issue, a better mechanistic understanding of this dynamic process, including characterization of distinct coagulopathic states, early identification of predictors of poor outcome, and finally targets for therapeutic intervention are needed to enable the development of improved diagnostics and targeted therapeutics for trauma-induced coagulopathy (TIC). Current therapy is primarily transfusion based, guided by standard coagulation tests or viscoelastic methods when possible. While some targeted therapies (e.g., tranexamic acid, fibrinogen concentrate) have been employed, none are widely accepted and the mechanisms of action are not well understood (9).
The coagulopathy associated with traumatic injuries is the result of multiple factors, which while independent, are also highly interactive. The following factors are believed to be the initiators of the coagulopathy of traumatic shock: tissue injury, shock, dilution, acidosis, hypothermia, and the inflammatory response (10–12). Moreover, multiple factors appear to be involved in the transition from a hypo to a hypercoagulable state. The highly complex etiology and biology of TIC has made it very difficult to study and understand, and the treatment of any one single factor does not routinely improve outcome for the patient. In this regard, traditional clinical research reductionist approaches have not been effective in identifying the causative factors for TIC and, furthermore, various animal models have suggested differing underlying mechanisms (13, 14).
The multifaceted problem of TIC is ideal for utilizing a systems biology approach to understand the specific molecular and biochemical pathways involved
In this report, we present a comprehensive systems biology approach with a large-scale longitudinal enrollment of severely injured trauma patients, to identify mechanisms causing the coagulopathy. It is hoped that identification of mechanisms will ultimately lead to the development of advanced diagnostics and therapeutic interventions to prevent or manage coagulopathy and improve survival after traumatic injury. We report systematic collection of blood samples for a series of assays as well as a large array of clinical data, with subsequent large-scale analysis and input for modeling (Fig. 1). It is our hypothesis that by identifying the dynamics of relevant network biology in coagulopathy (which is distinct from normal coagulation), the unique network nodes that manifest in TIC early after injury can be identified. These network nodes will lead to the identification of candidate biological markers for testing as diagnostic markers and therapeutic targets to prevent, reverse, or minimize TIC.
Fig. 1.
A model for systems biology studies.
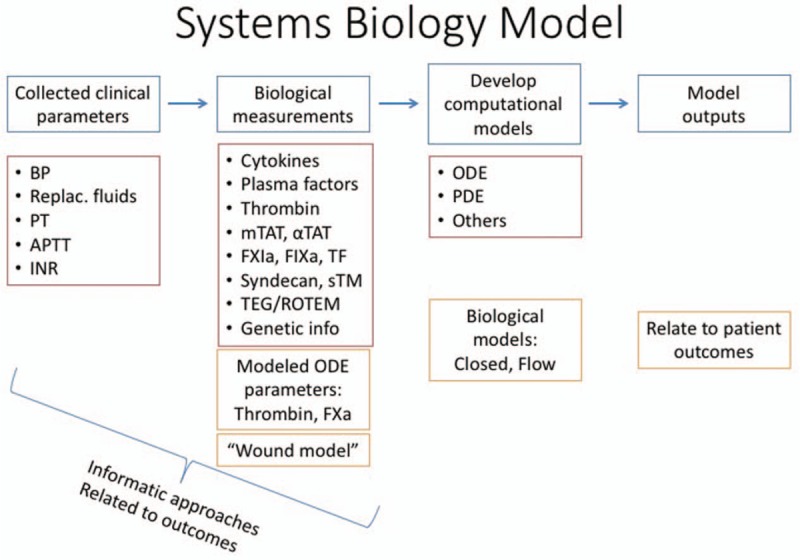
This schematic outlines the model for the protocol we planned to apply in the present work in order to execute a successful systems biology-based research study in a traumatically injured patient population. INR indicates international normalized ratio; ODE, ordinary differential equations; PDE, partial differential equations; PT, prothrombin time; TEG, thromboelastography; Tf, tissue factor.
Need for a prospective longitudinal clinical trial and barriers to completion
Trauma management is challenged by limited diagnostic tests that can readily discriminate between heterogeneous augmentations to coagulation homeostasis, which confounds therapeutic decisions including early administration of plasma or the use of tranexamic acid to block fibrinolysis (15–19). To provide a comprehensive clinical assessment of coagulopathy in trauma, standard laboratory tests of coagulation including the plasma-based prothrombin time (PT; extrinsic pathway) and partial thromboplastin time (intrinsic pathway), platelet count, and fibrinogen level must all be measured. In addition, viscoelastic assessments of whole blood coagulation with thromboelastography (TEG, Haemonetics, Niles, IL) or thromboelastometry (rotational thromboelastometry (ROTEM), TEM International, Munich, Germany) are used to provide a more comprehensive assessment of clot formation and fibrinolysis now using standard protocols (19, 20). Standard clinical laboratory tests (e.g., PT, activated partial thromboplastin time) also only provide a snapshot in time and therefore new biomarkers and more global assessments of hemostasis are warranted in order to begin to tackle the issue of identifying the potential underlying mechanisms in TIC. It is thus our purpose to include additional biomarker measurements that represent a more integrative assessment of thrombin generation, platelet activation, circulating procoagulant activity, contact pathway assessment, endothelial dysfunction, and the pro and anticoagulant and pro and antifibrinolytic balance. We believe that this is essential to understand the pathologies present in TIC that potentially go undetected by routine measurements.
The objective of our study was to codify the acquisition of longitudinal and well-phenotyped clinical samples from trauma and burn patients, particularly in the minutes immediately following injury, to advance mechanistic and translational research progress in TIC and explore the applicability to burn populations. We sought to produce a standard set of harmonized protocols for sample acquisition and handling, similar to what has been done in previous multicenter studies of similar populations (21–23) with novel adaptations to the present study. We developed a Manual of Operations (MOO), for blood sampling that could be used by other trauma studies and under a variety of conditions. An overarching goal of the MOO was to standardize collection, processing, and preservation of blood samples in such a manner as to enable subsequent analysis to determine procoagulant, anticoagulant, fibrinolytic, antifibrinolytic, inflammatory, endothelial, genomic, and other parameters, including short-lived enzyme intermediates. These analyses will be essential for elucidating the underlying mechanisms of TIC.
METHODS
Enrollment
We planned to enroll patients at 3 different academic medical centers, each of which houses a Level 1 trauma center, but with different trauma populations (urban trauma center, rural trauma center, and burn center). Inclusion criteria and proximity to initial trauma were also vetted and maintained with consistency across all sites. Similarly, variability in exclusion criteria was minimized (Table 1). Screening and consent protocols were specific to each site in order to accommodate staffing and existing processes already in place where applicable; however, the time frames for sample collection after screening and enrollment were adhered to per the desired acquisition of acute sampling proximal to injury, and at prescribed subsequent times. Extensive collaboration with each institution's Institutional Review Board (IRB) and Office of Research Integrity, as well as the Department of Defense Human Subjects Research Protection Office was established and maintained in order to develop the most ethical and appropriate consent process including the utilization of legally authorized representatives (LAR).
Table 1.
Inclusion and exclusion criteria used to screen patients
| Inclusion | Exclusion | |
| Burn | Patients at least 18 years of ageArrival to Trauma Unit within 4 h of injuryBurn injuries resulting from flash, flame, contact with hot object or liquid or electrical burn injury | Patients with chemical injuriesPatients who, in the opinion of the Principal Investigator, are not appropriate for inclusion in this study due to preexisting conditionsPatients under 18 years of agePatients currently taking anticoagulantsPatients not fluent in either English or Spanish |
| Trauma | Patients at least 18 years of ageAny acute traumaArrival to Emergency Department as a result of a trauma | Patients under 18 years of agePatients currently taking anticoagulantsPatients with an existing bleeding diathesisPregnant womenIncarcerated individuals |
Data sharing and integrative analytics platform
SysBioCube is the US Army Medical Research and Materiel Command's (USAMRMC) integrated biomedical research data access and analysis platform for health optimization, trauma-related conditions, and diseases of military relevance. It is developed by the Advanced Biomedical and Computational Sciences at the Frederick National Laboratory for Cancer Research, sponsored by the National Cancer Institute. The Systems Biology Collaboration Center, an office hosted at the US Army Center for Environmental Health Research, oversees and coordinates the activities of the SysBioCube.
The goal of the SysBioCube is to serve as a central portal for biomedical research data collection, integration, analysis, mining, and knowledge sharing by the US Army's medical research community, including scientific and technical collaborators from academic and private institutions. Clinical, pathophysiological, psychological, molecular, and biochemical data from human study participants and animal models generated by USAMRMC systems biology study collaborators are uploaded into SysBioCube, and the datasets are made available to approved users through fine grained, role-based access rules. The approved users can access data through different SysBioCube features for browsing, querying, automated integration, and custom visualization for better understanding of the systems biology of diseases.
In the present study, the SysBioCube was selected to be the data repository for all clinical, assay, and sample data and information for all enrolled patients in support of the systems biology protocol.
Clinical data
Clinical data were collected prospectively on all enrolled patients, which captured both injury characteristics and timing, as well as patient demographics. Data were subsequently collected throughout the patient's hospital course as abstracted from charts and records, with a focus on the time frames surrounding blood sample collection (see below) in order to have chronologically associated data. Data of interest included fluid administration, transfusions, operating room procedures, medications, comorbidities, pathologic events (including clot-related such as thromboembolism and disseminated intravascular coagulation), and patient outcomes including mortality or discharge disposition. All physiologic and clinical variables were recorded in REDCap databases at the individual sites, and then later were integrated into the SysBioCube.
Blood collection timeline and tubes
Blood samples were acquired from subjects to include “pretreatment” samples, before blood transfusion or surgical procedures, beginning at presentation to the hospital. For trauma patients, additional samples were obtained at 2, 4, 6, 8, 12, 24, 48, 72, 96, and 120 h. For thermally injured patients, additional time points that were collected after 24 h were collected every 12 h for 7 days, and then at day 14 and day 21 after admission, or until discharge or death.
Given the emergent nature of the clinical setting, we utilized carefully designed systems and protocols to ensure reliable sample collection and integrity for downstream assays. Blood sample collection and handling kits were typically prepared and stored in advance in anticipation of enrollment to include all of the necessary collection tubes, aliquot vials, and labels for tubes, etc. These were prepared in enough quantity to account for at least the first 24 h of enrollment for multiple patients at a time.
Samples were collected in 3.2% citrate, SCAT-144–4.5/5, and PAXgene RNA tubes (Preanalytix, GmBH). The citrate collection inhibits the assembly and function of the primary coagulation complexes by chelating Ca++, while allowing reactivation of the system and estimations of ex-vivo substrate–product flux, through recalcification. The SCAT-144–4.5/5 tube (45 mM EDTA, 100 μM Gly–Gly–Arg Chloromethylketone, 3,000 KIU/mL aprotinin, 5 mM 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride, 200 μM Elastinal, 50 μM E-64, and 10 μM Pepstatin A; Haematologic Technologies, Inc., Essex Junction, VT) is designed to definitively inhibit a wide range of serine, cysteine, aspartyl and metalloproteases allowing us to measure even very low abundance biomarkers indicative of in-situ coagulant and fibrinolytic activity, cellular damage, and cellular activation. The detailed processing of the blood collection tubes and allocation for on-site testing versus central site testing are described in detail in the MOO (Supplement).
Point of care testing
Measurements of the viscoelastic properties of clot formation and clot lysis in whole blood samples are obtained using either rotational thromboelastometry (ROTEM, TEM/IL) or thromboelastography (TEG, Haemonetics). In this protocol, we assessed the extrinsic pathway by using 2 concentrations and sources of tissue factor reagent (Tf); a low-Tf stimulus (5 pM) reagent that is produced in-house and a high-Tf stimulus found in the commercial ROTEM reagent. The concentration of the commercial Tf reagent stimulates a clot time on the order of 8 times faster than the 5 pM in-house Tf. The second pathway involved in the coagulation cascade is the intrinsic or contact pathway, which is activated by either ellagic acid or kaolin, in vitro using ROTEM or TEG. The initiators supply a negatively charged surface on which fXII activation can occur, and are supplied in the commercial ROTEM (ellagic acid) or TEG (kaolin) reagents. Because Tf is not present in the blood normally (note that the first 3 mL of the draw is usually discarded to avoid any phlebotomy-derived Tf entering the blood designated for analysis), it must be added to the reaction in the TEG or ROTEM assay to study the extrinsic pathway. Other initiators and available assays TEG include native (no initiator) or RapidTEG reagent (Haemonetics Inc, Braintree, Mass). TEG and ROTEM assays were completed for a subset of enrolled patient's blood collection time points at all sites as functional point of care testing, to be integrated with all metadata and analyzed for concordance.
Multiomics: mRNA, microRNA, protein, and DNA methylation profiling
Our efforts are aimed to bridge the knowledge gap between gene expression and blood coagulation by characterizing the dynamics of the changes using global multiomic approaches such as dynamic changes in messenger (m)RNA, microRNA, protein, and DNA methylation profiling. For DNA methylation, we used commercially available microarray technology (∼1 million probes).
The expression of these molecules change in response to an insult of some sort (e.g., injury), or an agonist/antagonist. Therefore, blood was collected in a tube to instantly preserve these molecules (e.g., PAXgene/RNAgard tubes [Biomatrica, San Diego, Calif]) and it was used to perform the -omic studies. The results from these studies have the potential to provide the information we are seeking to predict the onset of an irreversible coagulopathic state.
Measurements of coagulation
Panels of conventional and novel biomarkers of all aspects of coagulation will be tested. These include functional, as well as activity and factor concentration assays, and analytes particular to inflammation (chemokines and cytokines, etc.) and vascular endothelial health. The comprehensive set of analyses to be performed on samples collected specifically using methods tailored to the subsequent assays are unique in this study. Examples of biomarkers to be analyzed are meizothrombin (meizothrombin antithrombin complex), factor 5/factor 5a degradation products, endogenous procoagulant activity markers (Tf, factor 11a, factor 9a), lytic potential, markers of ongoing hemostatic activation, complement, and markers of endotheliopathy (syndecan-1).
RESULTS
Development of a manual of operations for the evaluation of trauma-induced coagulopathy
Through the presently described project, one outcome of the program was the group generated-MOO (Supplement). The manual was developed and effectively implemented to define and harmonize the precise blood collection and processing scheme, and clinical data acquisition across sites. As a result, the final data set, when analyzed, should capture the dynamic changes to the biological pathways that occur in response to severe injury. As well, longitudinal information should capture the change that continuously occurs in response to external and internal stimuli (e.g., potential sepsis) thereby providing novel insights into the therapeutic arena.
Final prospective clinical enrollment
Overall, 1,547 patients were enrolled at all 3 sites, and blood samples processed from a total of 5,348 time points. Our enrollment included a nonburn cohort of 1,389 and our burn cohort included 158 patients. For the burn cohort, total body surface area (TBSA) was captured among the patients with a range of 0 to 100 (Table 2). Baseline (time 0) sample collection included 1,241 nonburn (89.3% of total collected) and 153 burn (96.8% of total). Longitudinal collections were heterogenous across sites. A blood and clinical collection protocol was new to 2 of the 3 clinical trauma collection sites. We summarized some of the difficulties in capturing all the longitudinal time points in the Discussion. All samples are stored in the repository for analyses. Clinical collection included documented outcomes such as thromboembolic complications, clotting impairments, mortality as well as ICU days, and ventilator days.
Table 2.
Enrolled burn patients, broken into injury severity (TBSA) cohorts
| TBSA | <10% | 11%–30% | 31%–50% | 51%–70% | 71%–100% |
| Number of Patients | 60 | 58 | 23 | 5 | 12 |
TBSA indicates total body surface area.
For computational systems biology approaches to identifying alterations in hemostasis that might lead to TIC in the trauma nonburn and burn patients, priority samples were selected to include in initial assays (Fig. 2). The first requirement was that patients had a PAXgene tube collected in order to ensure that the critical -omics assays could be completed which are vital to a systems biology approach. The secondary requirement was that the patients had to have an injury severity score established and documented, or a TBSA of injury for the burn patients. This was necessary for subsequent grouping of patients for analysis. Based off of this priority list, we will begin our biomarker evaluation of the patient's hemostatic longitudinal profile in 435 nonburn and 151 burn patients. This will then be integrated with developed computational approaches to deduce any potential alterations in hemostatic pathways that might lead to TIC.
Fig. 2.
Enrollment report.
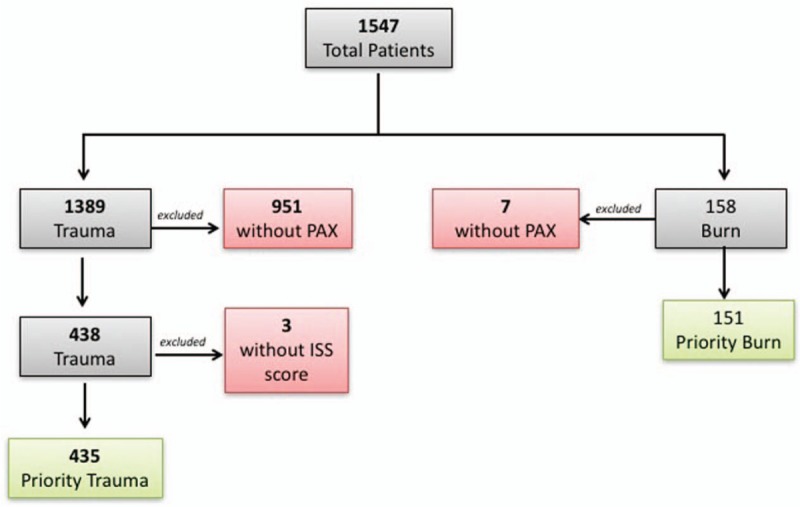
This diagram summarizes the patients that were enrolled at the 3 study sites and how their samples were prioritized for initial assay start up. ISS indicates injury severity score.
SysBioCube
The coagulopathy of trauma (CoT) SysBioCube module was successfully populated with data resulting from the described study. During the initial phase of the CoT project, SysBioCube was intended to be used only as a data sharing portal enabling a secure and common access point for all the collaborators to upload and download all data generated through the consortium. The system allowed for the flexible data sharing required by the project and the consortium was able to share clinical data, biospecimen collection data, assay status, and assay results through the web interface. As the project continued and samples were being accrued at the 3 clinical sites, newer features such as the ‘Sample Tracker’ and ‘Assay Tracker’ were developed and integrated into SysBioCube. ‘Sample Tracker’ was developed for tracking sample aliquot collections at multiple clinical sites. The tool allows clinicians and researchers to track sample availability for conducting planned assays by patients, time points, prioritizations, and biospecimen types (Fig. 3). In addition, the complex collaborations across multiple clinical, assay, and analysis sites necessitated the need to communicate and translate the information on samples and progress of the assays under the study. A dynamic and interactive ‘Assay Tracker’ feature was developed to allow those communications to happen in a seamless intuitive fashion so that the assays can be prioritized based on the types of samples and volumes collected and time lines established for different components of the collaboration to progress smoothly.
Fig. 3.
SysBioCube, Sample Tracker.
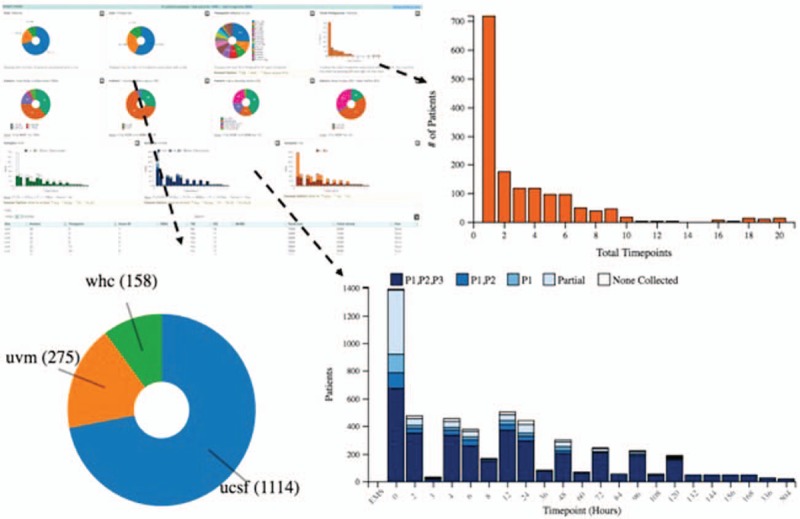
Screen shot of the sample tracker tool and examples of forms of data representation for interactive tracking of the samples collected using this tool. Bar chart displaying the number of time points and patients with available samples, pie chart to display the number of patients from different clinical sites, and stacked bar charts displaying the number of patient samples available at each time point based on the volumes collected.
Repository
Samples were collected and stored at the 3 individual clinical sites, University of California San Francisco, Washington Hospital Center, and University of Vermont during the duration of the study. At the end of the prospective clinical study collection, the samples were transferred to a common repository at the Institute of Surgical Research Fort Sam Houston, San Antonio, Texas. The repository will function as a distribution center from which samples will be shipped to assay sites for analyses. A Steering Committee will oversee and coordinate this process to maximize the impact of appropriate sample utilization. Following a standard embargo period, it is expected that these samples will be made available to researchers in a manner similar to existing NIH sample repositories.
DISCUSSION
With the development and implementation of our Systems Biology Coagulopathy of Trauma and Burn program and the resultant MOO, we initiated the first of its kind a multicenter genomic, proteomic, and clinical assessment of longitudinal samples from civilian trauma subjects. With the resulting knowledge in hand, we expect to address our three primary goals: identify therapeutic targets for drug development; propose laboratory/on-site methods that preemptively identify patients at risk for TIC; and develop recommendations for appropriate prophylactic action to minimize complications.
Subjects were enrolled at 3 different academic medical centers, each of which houses a Level 1 trauma center, but with different trauma populations (urban trauma center, rural trauma center, and burn center). We received approval from IRBs at all 3 institutions and based on previous experiences at each in working with traumatically injured patients, were able to share best practices and successfully justify the utilization of LAR and waivers of informed consent which allowed for consistent sampling at time points proximal to injury in these very ill patients.
Sample consistency and quality, as well as the data recorded regarding sample acquisition, handling, processing, and transfer, were all optimized by developing a detailed protocol regarding the appropriate order and processes for methods of phlebotomy, weighing the evacuated tubes pre and postfilling to ensure consistent anticoagulant concentrations, sample centrifugation, freezing, and storage.
While methods for previous multicenter studies of similar populations have been described (21–23), there are some important points regarding the currently described study and associated MOO that are novel. These include the detailed standardized methods for blood collection, processing, and documentation, which were designed to be specific and accommodating to the planned downstream assays and measurements, even incorporating a specialized tube containing protease inhibitors to allow for a subset of specific functional assays on the isolated plasma. Additionally, a multiassay approach is utilized to include multiomic analyses, protein markers, functional assays, and integration of data (including comprehensive clinical data related to the patients’ hospital course and physiologic status) to allow for computational modeling.
The integration of clinical data regarding not only lab values and physiologic measurements but also timing of surgical procedures, transfusions, and other events with the assay data generated from collected samples is critical in order to truly approach the assessment of a complex process such as coagulation from a systems biology standpoint. Hence, this is the reason for collection of these data in a manner that they can be overlaid with the information gained from assays at the prescribed time points. For example, the interplay between hemostasis, immune response, and endothelial function requires both a high-level view that takes into account the physiologic status of the patient and what their treatment course includes (e.g., did they have a surgical burn excision that morning prior to blood draw? Or did a culture come back positive?) as well as a granular examination of clotting factors and processes potentially being initiated at a molecular level that may serve as early indicators of a subsequent change. The various types of data cannot be analyzed in isolation.
The above described coordinated sample collection is essential for basic science investigation in order to conduct fundamental research into the pathophysiology of TIC. Previous experience with the Thrombolysis in Myocardial Infarction (TIMI) trial (24) showed that samples obtained with ordinary anticoagulants (citrate, EDTA), which do not block the fibrinolytic system, were compromised by postphlebotomy modification of the protein composition. In the trauma environment, the potential for ex-vivo artifact is a particular concern, because pro/anticoagulant, pro/antifibrinolytic, inflammatory cell and tissue proteases are upregulated, with the degree of this upregulation unpredictably influenced by type, severity, and site of injury. Therefore, we incorporated the previously mentioned specialized evacuated tube containing inhibitors to serine, metallo, aspartyl, cysteine, and collagen proteases to prevent postphlebotomy protein alterations that otherwise might occur.
While our analyses were very comprehensive, a limitation of the study was a lack of direct platelet function measurement via aggregometry, noticeably absent in the MOO. However, the inclusion of indirect assessments through onsite viscoelastic analyses in conjunction with platelet counts and fibrinogen concentrations are included.
Lessons Learned:
The fast pace at which events happen in the trauma bay and the urgency of interventions deprioritizes obtaining full sample sets for research; therefore, meticulous preparation and detailed protocols and organization of prelabeled and prepared tubes and documents is critical.
Trauma is occurring in real time and iterative communication is essential with individuals involved at the various stages of sample collection and processing.
Quality control of tubes and reagents must be minded (e.g., bench life of various blood collection tubes vs. how many patients come through the trauma bay).
For specialty tubes, coordinating with the commercial vendor in terms of supply versus demand and functional life time of the tube is necessary.
Development of a system of performance controls for technicians running on-site assays (e.g., the start of every shift a technician runs an appropriate ROTEM/TEG control and saves the data) is likewise necessary.
Several IRB issues were identified, including challenges with waiver of informed consent at 1 site, and the acquisition of genetic material at a different site.
Sample quality was improved by codifying methods of phlebotomy and weighing the evacuated tubes to ensure consistent anticoagulant concentrations.
In summary, we identified a methodology to comprehensively investigate the fundamental, highly networked, molecular, and physiological processes underlying normal coagulation and the perturbations of those processes that result in trauma-induced coagulopathy. We were able to integrate multiple data types [deletion] collected from the onset of the trauma through therapeutic interventions into the SysBioCube which facilitates the use of robust data analytical approaches. These approaches have set the stage to begin to identify biomarkers of disease progression and associate these with appropriate therapeutic strategies as the illness proceeds. Understanding the biology of interacting clotting processes with a high degree of detail will permit the identification of network-based diagnostics and therapeutics through the discovery of clinically relevant and targetable candidate molecules within those networks. The ultimate objective of such findings will lead to reducing mortality and morbidity following trauma and burn injury in both the military and civilian sectors.
Supplementary Material
Footnotes
K.G.M. is the COB, Haematologic Technologies Inc.
Disclaimers: The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as official Department of the Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
Funding: This work was funded by the Department of Defense under the Systems Biology of the Coagulopathy of Trauma portfolio.
The authors report no conflicts of interest.
REFERENCES
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 73 6 Suppl 5:S431–S437, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 60 6 Suppl:S3–S11, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma 54:1127–1130, 2003. [DOI] [PubMed] [Google Scholar]
- 4.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma 55:39–44, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Scott R, Jr, Crosby WH. Changes in the coagulation mechanism following wounding and resuscitation with stored blood; a study of battle casualties in Korea. Blood 9:609–621, 1954. [PubMed] [Google Scholar]
- 6.Simmons RL, Collins JA, Heisterkamp CA, Mills DE, Andren R, Phillips LL. Coagulation disorders in combat casualties. I. Acute changes after wounding. II. Effects of massive transfusion. 3. Post-resuscitative changes. Ann Surg 169:455–482, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maegele M. Acute traumatic coagulopathy: Incidence, risk stratification and therapeutic options. World J Emerg Med 1:12–21, 2010. [PMC free article] [PubMed] [Google Scholar]
- 8.Tejiram S, Brummel-Ziedins KE, Orfeo T, Mete M, Desale S, Hamilton BN, Moffatt LT, Mann KG, Tracy RP, Shupp JW. In-depth analysis of clotting dynamics in burn patients. J Surg Res 202:341–351, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Pusateri AE, Weiskopf RB, Bebarta V, Butler F, Cestero RF, Chaudry IH, Deal V, Dorlac WC, Gerhardt RT, Given MB, et al. Tranexamic acid and trauma: current status and knowledge gaps with recommended research priorities. Shock 39:121–126, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg 245:812–818, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn EL, Moore EE, Breslich DJ, Galloway WB. Acidosis-induced coagulopathy. Surg Forum 30:471–473, 1979. [PubMed] [Google Scholar]
- 12.White NJ. Mechanisms of trauma-induced coagulopathy. Hematology Am Soc Hematol Educ Program 2013:660–663, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet JF, Cohen MJ. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock 32:659–665, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darlington DN, Craig T, Gonzales MD, Schwacha MG, Cap AP, Dubick MA. Acute coagulopathy of trauma in the rat. Shock 39:440–446, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Bouillon B, Brohi K, Hess JR, Holcomb JB, Parr MJ, Hoyt DB. Educational initiative on critical bleeding in trauma: Chicago, July 11–13, 2008. J Trauma 68:225–230, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 13:680–685, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am 92:877–891, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 65:748–754, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Brohi K. Prediction of acute traumatic coagulopathy and massive transfusion: is this the best we can do? Resuscitation 82:1128–1129, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Moore HB, Moore EE, Chin TL, Gonzalez E, Chapman MP, Walker CB, Sauaia A, Banerjee A. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery 156:564–569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg 78:671–686, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 148:127–136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baraniuk S, Tilley BC, del Junco DJ, Fox EE, van Belle G, Wade CE, Podbielski JM, Beeler AM, Hess JR, Bulger EM, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation. Injury 45:1287–1295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med 320:618–627, 1989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


