Introduction
Loss of skeletal muscle mass and strength is apparent as early as the 4th decade of life and progresses linearly with increasing age.1,2 The term sarcopenia, first introduced by Irwin Rosenberg, was created to describe the loss of muscle mass that accompanies aging. As Rosenberg himself commented, “there is probably no decline in structure and function more dramatic than the decline in lean body mass or muscle mass over the decades of life.”3 Over the last decades, the age-related decline of muscle mass and strength has been a focus of gerontologic research as a means for exploring the aging process and its consequences and has been recognized as a major public health issue.2,4 The development of sarcopenia has been associated with the development of functional impairment, disability, increased risk of falls and fractures, reduced health-related quality of life (HRQOL) loss of independence, and increased risk of death.2,5–7 As sarcopenia is common and related to disability, it has been estimated to cost the US health system ~$18.5 billion per year.8 Although originally described as a strictly age-related phenomenon, the loss of muscle mass and strength with aging is more complex and multifaceted. The purpose of our perspective is to highlight the importance of sarcopenia in older adults with cancer and address existing challenges and future directions of the field.
Relevance in Oncology
Sarcopenia in the oncology setting is an area of growing research interest due its high prevalence in adults with cancer, with a prevalence between 11% and 74% in all adults and often even higher in older adults with cancer, and given its association with adverse outcomes.9–13 Sarcopenia is fundamentally the result of an imbalance in muscle protein turnover.2,14 As cancer is predominantly a disease of older adults, older adults with cancer face the dual threat of age-related sarcopenia and the pro-inflammatory response of cancer-related cachexia (see figure 1). This dual risk makes older adults particularly vulnerable to the adverse outcomes of both of these serious conditions. Furthermore, cancer treatments, including systemic chemotherapy, radiation therapy, and surgery, can all additionally worsen muscle loss through a variety of different mechanisms.
Figure 1:
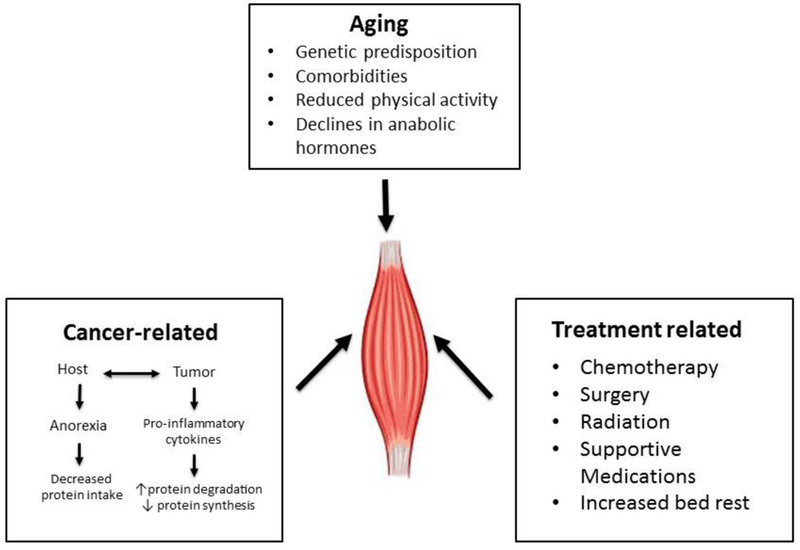
Causes of muscle loss in older adults with cancer.
In general, sarcopenia results in less treatment tolerability, more postoperative complications, and shorter overall survival in adults with cancer, independent of age, gender, cancer stage, and tumor type.9–11 The cause of the shorter overall survival is possibly a combination of vulnerability to adverse outcomes of cancer treatment due to limited physical reserve, and in some cases, the impossibility of further cancer-directed treatment due to limited physical reserve. Furthermore, a higher non-cancer related mortality during and after therapy is described in older adults with cancer.15 Sarcopenia is also highly correlated with the incidence of severe chemotherapeutic toxicity.12,13,16 The association of chemotherapy toxicity with lower muscle mass or lean body mass is most apparent in therapeutics dosed based on body surface area (BSA).16 Moreover, body composition measures have also been associated with decreased HRQOL, physical function impairments, and frailty in cross-sectional analyses.17–19 One recent study found sarcopenia was associated with a decline in physical function independence in older adults undergoing chemotherapy.20 Further understanding the association of sarcopenia with other important outcomes that are of particular importance to older adults with cancer, such as long-term functional independence, disability, and HRQOL, requires further evaluation.
Current Challenges
Although the number of publications on sarcopenia within oncology has grown immensely over the last decade, several major barriers exist in the field of body composition research in oncology that have curtailed progress.
Standardizing terminology
No broadly accepted consensus definition of sarcopenia exists, in oncology or elsewhere. The definition of sarcopenia is probably one of the least agreed upon and most debated topics in body composition research.21 Since the term sarcopenia was first introduced, numerous definitions have been proposed. Moreover, many different modalities have been employed to assess body composition, resulting in further difficulty in developing a unified definition. Computed tomography (CT)-derived body composition analysis is the primary assessment method within oncology given the common use of CT imaging for cancer staging and disease monitoring, but dual-energy X-ray absorptiometry (DEXA) scans are more routinely incorporated outside of oncology.21 Sarcopenia was first defined similar by Baumgartner et al as two standard deviations below the mean muscle mass of healthy younger adults.22 More recently the definition of sarcopenia has broadened to not only involve low muscle mass, but also reduced strength and/or physical performance.23 Within oncology, the most commonly employed definitions of sarcopenia to date have been solely focused on low muscle mass with a variety of cut-points used.13 A recent meta-analysis examining the predictive value of sarcopenia in adults found 11 different definitions of sarcopenia used in studies of patients with solid tumors.9 In this review, we will use the term sarcopenia more broadly to encompass all these definitions given the evolving nature of the term over time. Part of the discrepancy in the definition of sarcopenia between the gerontologic and oncologic literature exists due to the different viewpoints and outcomes under consideration. Geriatrics views sarcopenia in the light of developing disability, whereas oncology is more concerned about low muscle mass and its association with increased mortality and complications from cancer treatments. The lack of a consensus definition has limited the progress in the field due to limited generalizability of study findings. In all publications related to sarcopenia and body composition examinations, it is important to be clear on the definition employed and rationale. Developing cohesion across the field of body composition research in oncology was recently identified as a priority area at the recent National Institutes of Health workshop on understanding the role of muscle and body composition in cancer.
Lack of prospective evidence
Most studies to date on sarcopenia in oncology have been retrospective in nature with a severely limited ability to characterize the patient population, thus limiting our understanding of the underlying mechanisms associated with increased adverse outcomes. How sarcopenia and low muscle mass is related to other risk factors such as frailty and functional impairments as well as other predictors of chemotherapy toxicity, such as the Cancer and Aging Research Group (CARG) calculator, remains unknown as few studies to date have included such assessments. Potentially incorporating sarcopenia into such calculators could further refine chemotherapy prediction in older adults. Prospective evaluation of sarcopenia in older adults with cancer is critically needed to better understand the association of sarcopenia with adverse outcomes and to develop precise and personalized interventions to improve outcomes in the at-risk population.
Future Directions
In order to facilitate the use of body composition variables in to routine oncology care, we must refine our ability to accurately assess muscle measures in real-time without undue burden on providers and patients. Sophisticated auto segmentation software are available to support large-scale implementation of CT-based muscle mass assessment with high fidelity, yet have not been incorporated into routine radiology use.24 With the aid of such tools, lean mass quantification could feasibly be integrated into the routine care of patients undergoing staging or surveillance imaging and thus potentially allow incorporation of lean mass quantification within the electronic medical record to support the use of calculators to aid treatment decision-making regarding the risk of adverse outcomes and targeting of high-risk populations for intervention.
As we gain deeper understanding of the implications and underlying pathophysiology of sarcopenia among older patients with cancer we will develop new strategies to improve outcomes in this population. Interventions to improve muscle mass, density, and strength are particularly attractive since they have the potential to simultaneously improve both quantity and quality of life. Integrative programs with nutritional, exercise, and pharmacologic components can be tailored to the specific needs of older patients. Researchers should also be open-minded to complementary and holistic approaches.
Although body composition in oncology analyzed at the time of diagnosis has gained traction as a predictor of adverse outcomes, more recently there has also been a focus on the impact of cancer treatments on body composition. Several studies have assessed changes overtime during chemotherapy and several common themes have been observed.16 Most systemic chemotherapy agents result in lean muscle mass loses overtime; however, certain agents targeted agents (such as MEK inhibitors) may result in increases in muscle mass compared to cytotoxic chemotherapy.16,25 Deciphering whether these loses are strictly treatment related versus a consequence of the ongoing catabolic effect of cancer is not easily possible. These body composition changes are particularly worrisome in the adjuvant treatment setting with implications that extend into cancer survivorship. As low muscle mass and other body composition variables have been associated with increased risk of falls and fractures, functional dependence, and disability in non-cancer populations,2,6,7 this is a potentially critical area warranting further exploration as a survivorship issue.
Lastly, several studies have demonstrated the inverse relationship between chemotherapy toxicity and muscle mass with higher toxicities rates routinely associated with lower muscle mass.12,13 Sarcopenia may impact chemotherapy pharmacokinetics via alterations in distribution, metabolism, and clearance.26,27 Conventional chemotherapy dosing by BSA does not account for the wide variation in body composition in adults with cancer.28 A wide distribution of lean body mass is seen for any given body weight and this may affect pharmacokinetics of chemotherapy, yet is unaccounted for by standard chemotherapy dosing that only incorporates height and weight.27 Some early studies have examined chemotherapy pharmacokinetics and sarcopenia.16 Mir et al demonstrated that the median sorafenib AUC was significantly higher in sarcopenic patients and also had higher toxicity rates.26 Other small studies have had mixed results.29,30 The cause of this effect likely lies within pharmacokinetic drug effects of sarcopenia; meaning that patients with a low lean body mass experience a lower volume of distribution of chemotherapeutic drugs. Consequently, higher systemic drug levels are observed, resulting in more chemotherapeutic toxicities. More sophisticated chemotherapy drug dosing that incorporates body composition variables may help reduce inter-individual variability in drug levels that could reduce toxicities while maintaining or improving efficacy.21,30
Conclusion:
The use of utilizing of routine CT-based to assess body composition in oncology represents a nascent opportunity to better inform prediction of adverse outcomes, target interventions, and further our understanding of cancer and its treatments on muscle. Older adults with cancer are particularly at risk given the combined insult from agerelated and cancer-related causes of sarcopenia. There is an overall lack of consensus behind the use of terminology across the oncology field and given the retrospective nature of the vast majority of the field to date, many knowledge gaps persist that limit our understanding of many of these associations. Further comprehensive and prospective work is necessary to delineate the role of sarcopenia in older adults with cancer, and to develop targeted intervention strategies to improve outcomes in this high risk population.
Acknowledgments
Funding: Supported in part by the Walter B. Frommeyer Fellowship in Investigative Medicine at the University of Alabama at Birmingham, the American Cancer Society Institutional Research Grant (IRG-60–001-53), and the National Cancer Institute of the National Institutes of Health (K08CA234225). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
References
- 1.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. The journals of gerontology. Series A, Biological sciences and medical sciences 1997;52(5):B267–276. [DOI] [PubMed] [Google Scholar]
- 2.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. The journal of nutrition, health & aging 2008;12(7):433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg IH. Sarcopenia: origins and clinical relevance. The Journal of nutrition 1997;127(5 Suppl):990S–991S. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Current opinion in clinical nutrition and metabolic care 2010;13(1):1–7. [DOI] [PubMed] [Google Scholar]
- 5.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 2006;35(4):409–415. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The journals of gerontology. Series A, Biological sciences and medical sciences 2005;60(3):324–333. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society 2002;50(5):889–896. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. Journal of the American Geriatrics Society 2004;52(1):80–85. [DOI] [PubMed] [Google Scholar]
- 9.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 10.Dunne RF, Roussel B, Culakova E, et al. Characterizing cancer cachexia in the geriatric oncology population. Journal of geriatric oncology 2018. [DOI] [PMC free article] [PubMed]
- 11.Broughman JR, Williams GR, Deal AM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. Journal of geriatric oncology 2015;6(6):442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Seminars in cell & developmental biology 2015. [DOI] [PubMed]
- 13.Rier HN, Jager A, Sleijfer S, Maier AB, Levin MD. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. The oncologist 2016. [DOI] [PMC free article] [PubMed]
- 14.Walrand S, Guillet C, Salles J, Cano N, Boirie Y. Physiopathological mechanism of sarcopenia. Clinics in geriatric medicine 2011;27(3):365–385. [DOI] [PubMed] [Google Scholar]
- 15.McDonald AM, Swain TA, Mayhew DL, et al. CT Measures of Bone Mineral Density and Muscle Mass Can Be Used to Predict Noncancer Death in Men with Prostate Cancer. Radiology 2017;282(2):475–483. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins JJ, Sawyer MB. Interactions of lean soft-tissue and chemotherapy toxicities in patients receiving anti-cancer treatments. Cancer Chemother Pharmacol 2018. [DOI] [PubMed]
- 17.Williams GR, Deal AM, Muss HB, et al. Frailty and skeletal muscle in older adults with cancer. Journal of geriatric oncology 2018;9(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams GR, Deal AM, Muss HB, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget 2017;8(20):33658–33665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nipp RD, Fuchs G, El-Jawahri A, et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. The oncologist 2018;23(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rier HN, Jager A, Meinardi MC, et al. Severe sarcopenia might be associated with a decline of physical independence in older patients undergoing chemotherapeutic treatment. Support Care Cancer 2018;26(6):1781–1789. [DOI] [PubMed] [Google Scholar]
- 21.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 2014;38(8):940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 23.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137(4):231–243. [DOI] [PubMed] [Google Scholar]
- 24.Popuri K, Cobzas D, Esfandiari N, Baracos V, Jagersand M. Body Composition Assessment in Axial CT Images Using FEM-Based Automatic Segmentation of Skeletal Muscle. IEEE transactions on medical imaging 2016;35(2):512–520. [DOI] [PubMed] [Google Scholar]
- 25.Prado CM, Bekaii-Saab T, Doyle LA, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. British journal of cancer 2012;106(10):1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. Plos One 2012;7(5):e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prado CM, Maia YL, Ormsbee M, Sawyer MB, Baracos VE. Assessment of nutritional status in cancer--the relationship between body composition and pharmacokinetics. Anti-cancer agents in medicinal chemistry 2013;13(8):1197–1203. [DOI] [PubMed] [Google Scholar]
- 28.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013;31(12):1539–1547. [DOI] [PubMed] [Google Scholar]
- 29.Massicotte MH, Borget I, Broutin S, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. The Journal of clinical endocrinology and metabolism 2013;98(6):2401–2408. [DOI] [PubMed] [Google Scholar]
- 30.Williams GR, Deal AM, Shachar SS, et al. The impact of skeletal muscle on the pharmacokinetics and toxicity of 5-fluorouracil in colorectal cancer. Cancer Chemother Pharmacol 2018;81(2):413417. [DOI] [PMC free article] [PubMed] [Google Scholar]


