Abstract
Background
Plasma circulating cell-free (cf) DNA is regarded as a source of tumor DNA. Based on availability of blood tissue for the purposes of early detection of cancer and patients’ follow-up, several studies have evaluated concentration of cf DNA in cancer patients in association with tumor features. In the present study, we assessed concentration of cf DNA in lung cancer patients with two commercial kits (MN and QIAGEN) to find whether it can be used as a prognostic biomarker.
Results
Primary cf DNA concentrations as measured by QIAGEN kit was significantly higher in patients who died in the follow-up period compared with alive patients (P = 0.007). Moreover, the concentrations as measured by both methods were higher in patients who experienced recurrence in the follow-up period compared with patients without recurrence (P = 0.008 and 0.007 for MN and QIAGEN kits respectively). Significant associations were also found between cf DNA concentrations and tumor stage (P = 0.005 and 0.02 for MN and QIAGEN kits respectively). Notably, cf DNA concentration was higher in metastatic tumors compared with non-metastatic tumors in association with number of involved organs. Based on the AUC values, both kits could differentiate metastatic cancers from non-metastatic ones with accuracy of 98%.
Conclusions
The current study highlights the accuracy of cf DNA concentrations for prediction of disease course in lung cancer patients.
Keywords: Circulating free DNA, Lung cancer, Metastasis
Introduction
Circulating cell-free (cf) DNA is thought to be secreted in the blood circulation through necrosis, apoptosis or active release from nucleated cells. Inflammation, trauma and malignancy have been linked with elevated concentrations of cfDNA [1]. Based on the observed release of DNA from tumoral cells during processes such as necrosis and apoptosis, plasma cfDNA is regarded as a source of tumor DNA for the purposes of early detection of cancer and patients’ follow-up [2]. High mortality rate of lung cancer and poor patients’ outcome have prompted researchers to find suitable biomarkers for this kind of cancer. High cf DNA concentrations at baseline have been associated with worse patients’ outcome of cancer patients in some studies [2]. Moreover, the clinical validity of measurement of cfDNA for the estimation of lung cancer survival has been confirmed through meta-analysis of available literature [3]. Besides, quantification of cfDNA has a diagnostic accuracy comparable with conventional blood-based biomarkers for lung cancer screening [4]. Moreover, specific mutations found in cfDNA could act as prognostic and predictive biomarkers for patients with non-small cell lung cancer (NSCLC). For instance, the presence of EGFR activating mutations in cfDNA of these patients has been regarded as a predictive marker for response to EGFR-tyrosine kinase inhibitors [5]. Specifically, the L858R EGFR mutation in cfDNA could predict the overall survival of NSCLC patients [6].
Although several studies have quantified circulating cfDNA in lung cancer patients, no definite evidence indicates the associations between its concentrations and tumor features. The inconsistencies between the results of former studies might be originated from the method of cfDNA quantification. Consequently, in the present study we aimed at quantification of cfDNA in Iranian lung cancer patients with two commercially available kits to find the associations between its concentration and patients’ clinical data in a comparative manner.
Materials and methods
Patients
A total of 44 patients with NSCLC participated in the current study. Patients were hospitalized in Masih Daneshvari Hospital, Tehran during April 2017 to May 2018. The recurrence after initial treatment and patients’ outcome were recorded. All patients had performance status of 1–2 based on the Eastern Cooperative Oncology Group (ECOG) criteria [7]. The study protocol was approved by ethical committee of Tehran University of Medical Sciences. Written informed consent forms were signed by all study participants.
Assessment of free DNA concentrations
Venous blood was gathered in sterile EDTA-coated tubes. Samples were centrifuged at 590×g for 15 min in room temperature. Isolated plasma samples were kept at − 80 °C. cfDNA was extracted from plasma samples using the QIAamp Circulating Nucleic Acid Kit (QIAGEN, Valencia, CA, USA) and NucleoSpin Plasma XS (MN, Germany). cfDNA concentrations were quantified using NanoDrop 2000 (Thermo Scientific). Total concentrations of cf DNA in the plasma were reported. The same volume of plasma was used for cf DNA extraction from all samples (2 ml for extraction with QIAGEN kit and 260 µl for extraction with MN kit based on the guidelines provided by the companies).
Statistical analyses
Statistical analyses were performed in SPSS v.18.0 (IBM Corp., Armonk, NY, USA). Data were reported as mean ± standard deviation (SD). Paired-samples t test was used for comparison of concentrations of cfDNA in each sample as measured by each kit. The significance of association between cfDNA concentrations and tumor features was assessed using independent-samples t test. For all statistical analyses, P < 0.05 was considered as significant. The receiver operating characteristic (ROC) curve was designed to evaluate the suitability of cfDNA concentrations for prediction of recurrence probability and metastatic potential. The Youden index (j) was used to maximize the difference between sensitivity (true-positive rate) and 1—specificity (false-positive rate).
Results
General clinical information of patients
The current study included 44 patients with NSCLC. Patients were followed up until October 2018. During follow-up period 12 of them died. Blood samples were taken from patients before any cancer treatment at initial visit. Table 1 shows General demographic and clinical data of patients.
Table 1.
General demographic and clinical data of patients
| Variables | Values |
|---|---|
| Age (mean ± SD (range)) | 59.79 ± 11.65 (34–84) |
| Cell free DNA concentration—MN Kit (mean ± SD (range)) (ng/ml) | 13.92 ± 4.11 (3.6–22.3) |
| Cell free tumor DNA—QIAGEN Kit (mean ± SD (range)) (ng/ml) | 19.1 ± 6.03 (6.1–32.6) |
| Recurrence after (month) (mean ± SD (range)) | 7.76 ± 5.4 (1–19) |
| Gender | |
| Male | 45.5% |
| Female | 54.5% |
| Smoking | |
| Yes | 22.7% |
| No | 77.3% |
| Stage | |
| II | 2.3% |
| III | 4.5% |
| IV | 93.2% |
| Recurrence | |
| Yes | 56.8% |
| No | 43.2% |
| Current status | |
| Alive | 67.6% |
| Dead | 32.4% |
| Metastasis | |
| Non metastatic | 11.4% |
| Metastasis to one organ | 68.1% |
| Metastasis to two organ | 9.1% |
| Metastasis to three organ | 11.4% |
Comparison of circulating free DNA concentrations as detected by two methods
The mean values (± standard deviation) of circulating DNA concentrations as measured simultaneously by two methods were 13.72 (3.95) (ng/ml) and 19.1 (6.03) (ng/ml) for MN and QIAGEN kits respectively (P < 0.001).
Association between circulating free DNA concentration and patients’ characteristics
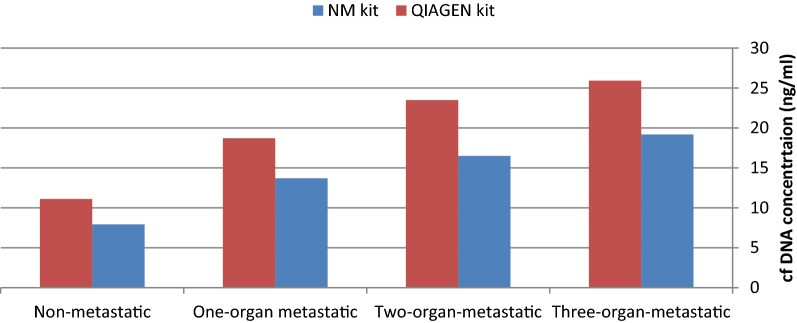
Primary cfDNA concentrations (ng/ml) as measured by QIAGEN kit was significantly higher in patients who died in the follow-up period compared with alive patients (P = 0.007). Moreover, the concentrations as measured by both methods were higher in patients who experienced recurrence in the follow-up period compared with patients without recurrence (P = 0.008 and 0.007 for MN and QIAGEN kits respectively). Significant associations were also found between cfDNA concentrations and tumor stage (P = 0.005 and 0.02 for MN and QIAGEN kits respectively). Notably, cfDNA concentration was higher in metastatic tumors compared with non-metastatic tumors in association with number of involved organs (Fig. 1 and Table 2). Table 2 shows associations between cfDNA concentration and patients’ characteristics.
Fig. 1.
Circulating free DNA concentrations (ng/ml) in association with metastasis potential
Table 2.
Association between cf DNA concentration (ng/mL) and patients’ characteristics
| cfDNA concentration (MN kit) (ng/ml) | P value | cfDNA concentration (QIAGEN kit) (ng/ml) | P value | |
|---|---|---|---|---|
| Gender | ||||
| Male vs. female | 13.96 (4.67) vs. 13.88 (3.69) | 0.94 | 19.21 (6.59) vs. 19.01 (5.67) | 0.91 |
| Smoking | ||||
| Yes vs. no | 13.96 (4.55) vs. 13.9 (4.05) | 0.97 | 19.17 (6.1) vs. 19.08 (6.1) | 0.96 |
| Current status | ||||
| Alive vs. dead | 13.22 (4.56) vs. 16.21 (3.4) | 0.05 | 17.56 (5.78) vs. 23.49 (6.28) | 0.007 |
| Recurrence | ||||
| Yes vs. no | 15.81 (3.45) vs. 12.06 (4.7) | 0.008 | 21.93 (6.09) vs. 16.27 (5.7) | 0.007 |
| Stage | ||||
| II and III vs. IV | 7.66 (2.5) vs. 14.37 (3.84) | 0.005 | 11.43 (2.05) vs. 19.66 (5.84) | 0.02 |
| Metastasis | ||||
| Non metastatic vs. Metastasis to one organ | 7.94 (3.81) vs. 13.69 [3] | 0.02 | 11.1 (3.52) vs. 18.71 (4.73) | 0.01 |
| Non metastatic vs. Metastasis to two organs | 7.94 (3.81) vs. 16.5 (2.29) | 0.001 | 11.1 (3.52) vs. 23.5 (4.98) | 0.002 |
| Non metastatic vs. Metastasis to three organ | 7.94 (3.81) vs. 19.18 (3.45) | < 0.001 | 11.1 (3.52) vs. 25.92 (6.24) | < 0.001 |
| Metastasis to one organ vs. Metastasis to two organs | 13.69 [3] vs. 16.5 (2.29) | 0.33 | 18.71 (4.73) vs. 23.5 (4.98) | 0.26 |
| Metastasis to one organ vs. Metastasis to three organs | 13.69 [3] vs. 19.18 (3.45) | 0.004 | 18.71 (4.73) vs. 25.92 (6.24) | 0.01 |
| Metastasis to two organs vs. Metastasis to three organs | 16.5 (2.29) vs. 19.18 (3.45) | 0.57 | 23.5 (4.98) vs. 25.92 (6.24) | 0.87 |
After partial correction for patients’ gender, circulating DNA concentrations (ng/ml) were not correlated with either age of patients or recurrence time after initial diagnosis (Table 3).
Table 3.
Partial correlation between free DNA concentrations (ng/ml) and age/recurrence time (controlled for gender)
| cfDNA concentration (MN kit) (ng/ml) | cfDNA concentration (QIAGEN kit) (ng/ml) | |||
|---|---|---|---|---|
| R | P value | R | P value | |
| Age | − 0.23 | 0.06 | − 0.18 | 0.12 |
| Recurrence time | − 0.05 | 0.4 | − 0.19 | 0.2 |
ROC curve analysis
We evaluated diagnostic power of cfDNA concentration for prediction of recurrence probability and metastatic potential (Table 4). Both kits had 100% sensitivity for differentiation of recurrence probability (Fig. 2). Based on the AUC values, both kits could differentiate metastatic cancers from non-metastatic ones with accuracy of 98% (Fig. 3).
Table 4.
The results of ROC curve analysis
| Differentiation of recurrence probability | Differentiation of metastatic potential | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate criterion | AUC | Ja | Sensitivity | Specificity | P-valueb | Estimate criterion | AUC | Ja | Sensitivity | Specificity | P-valueb | |
| MN | > 11.3 | 0.71 | 0.43 | 100 | 43.7 | 0.01 | > 10.1 | 0.98 | 0.95 | 95 | 100 | < 0.001 |
| QIAGEN | > 15.1 | 0.74 | 0.5 | 100 | 50 | 0.004 | > 13 | 0.98 | 0.97 | 97.5 | 100 | < 0.001 |
aYouden index, bSignificance level P (Area = 0.5), Estimate criterion: optimal cut-off point for cf DNA concentration
Fig. 2.
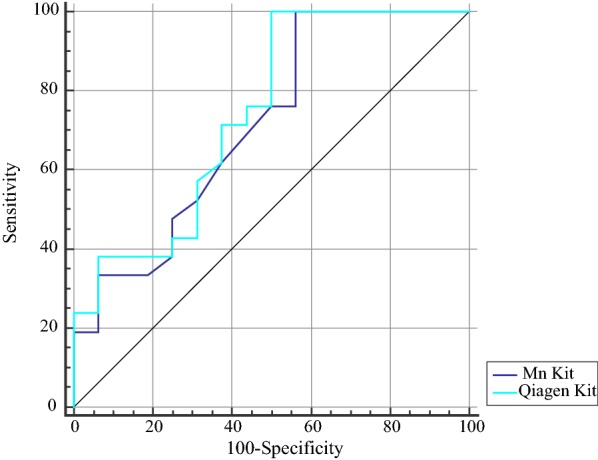
ROC curve analysis for assessment of diagnostic power of cf DNA concentrations for differentiation of recurrence probability
Fig. 3.
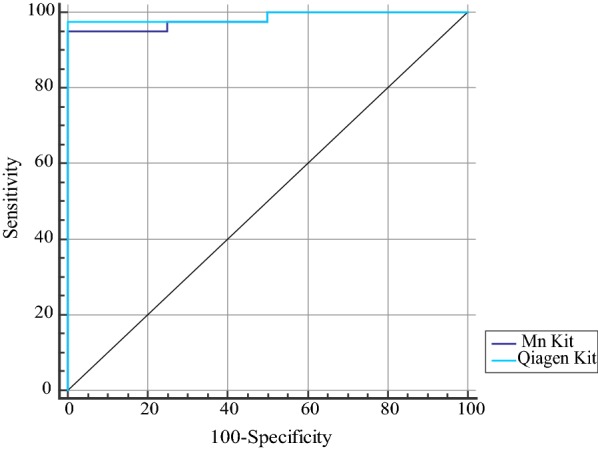
ROC curve analysis for assessment of diagnostic power of cf DNA concentrations (ng/ml) for differentiation of metastatic potential
Discussion
Lung cancer is a human malignancy associated with high mortality rate. Several prognostic factors including tumor stage, performance status, age at diagnosis and response to first-line chemotherapy have been recognized for this kind of cancer [8]. In the present study, we first quantified cfDNA levels in lung cancer patients and then assessed associations between cfDNA concentrations and some of known prognostic factors for lung cancer. Previous studies have measured cfDNA levels using different methods such as spectrophotometry [9], digital polymerase chain reaction (PCR)-based technologies [10] or Alu81-quantitative PCR [11]. We used two commercially available kits for isolation of cfDNA and subsequently quantified cfDNA using NonoDrop equipment. Notably, we found significant associations between cfDNA concentration and some clinical features such as stage, recurrence and metastatic potential. Although we found significant difference in the cfDNA concentration as measured by the mentioned commercially available kits, associations remained significant even when the reported concentrations were low (with MN method). This finding shows the validity of our obtained results. Moreover, cfDNA concentrations were not associated with patients’ age, sex or smoking history which shows the suitability of this source of biomarker for identification of disease status independent of these features. Although the cfDNA levels were associated with recurrence potential, after partial correction for patients’ gender, cfDNA concentrations were not correlated with recurrence time after initial diagnosis which might be explained by the relative low number of samples. Notably, cfDNA concentrations were not only predictive of metastatic potential, but also they could predict the number of involved organs which is potentially indicative of poor outcome. Based on the AUC values, diagnostic power of cfDNA concentration for prediction metastatic potential was higher than its ability to predict recurrence. Such diagnostic power was also independent of isolation method.
In brief, our study highlights the suitability of liquid biopsy as a non-invasive method for prediction of lung cancer prognosis in Iranian patients. As in the present study, we only assessed baseline cfDNA concentrations, future studies are needed to assess cfDNA levels in certain intervals after administration of drugs to explore their relevance with response of patients to each therapeutic regimens. We also showed availability of these methods for clinical practice and could solve the technical problems that were related to the evaluation of the cfDNA concentrations in the blood circulation. The similar results obtained from two mentioned kits indicate the reproducibility of the data.
Conclusion
Based on the results of previous studies indicating that the diagnostic accuracy of quantitative investigation of cfDNA is not inferior to conventional peripheral biomarkers for lung cancer screening [12], such approaches can be used for initial screening of cancer patients as well. However, studies in large sample sizes are needed to explore the differences in cfDNA concentrations between normal individuals, precancerous conditions and cancer patients.
Authors’ contributions
HMM performed experiments. SGF wrote the manuscript. AK, EM, ZEM, SS and BS contributed in patients’ assessments and study design. VKO analysed the data. MG and MHM designed and supervised the study. All authors read and approved the final manuscript.
Acknowledgements
The current study was supported by a Grant from Tehran University of Medical Sciences.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
The consent to publish has been obtained from the participant to report individual patient data.
Ethics approval and consent to participate
The study protocol was approved by ethical committee of Tehran University of Medical Sciences. All study participants provided their written consent for participation in the study.
Funding
The current study was supported by a Grant from Tehran University of Medical Sciences.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- cf
circulating cell-free
- SD
standard deviation
- ROC
receiver operating characteristic
- AUC
area under curve
Contributor Information
Hanifeh Mirtavoos-Mahyari, Email: hanifehmirmah@yahoo.com.
Soudeh Ghafouri-Fard, Email: s.ghafourifard@sbmu.ac.ir.
Adnan Khosravi, Email: Adkhosravi@yahoo.com.
Elahe Motevaseli, Email: e_motevaseli@sina.tums.ac.ir.
Zahra Esfahani-Monfared, Email: yeganenoruzi@yahoo.com.
Sharareh Seifi, Email: sh_seifi@yahoo.com.
Babak Salimi, Email: babak_salmd@yahoo.com.
Vahid Kholghi Oskooei, Email: vahidkholghioskooei@gmail.com.
Mohsen Ghadami, Phone: 00982188953005, Email: mghadami@sina.tums.ac.ir.
Mohammad Hossein Modarressi, Phone: 00982188953005, Email: modaresi@tums.ac.ir.
References
- 1.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 2.Tissot C, Toffart AC, Villar S, Souquet PJ, Merle P, Moro-Sibilot D, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J. 2015;46(6):1773–1780. doi: 10.1183/13993003.00676-2015. [DOI] [PubMed] [Google Scholar]
- 3.Cargnin S, Canonico PL, Genazzani AA, Terrazzino S. Quantitative analysis of circulating cell-free DNA for correlation with lung cancer survival: a systematic review and meta-analysis. J Thorac Oncol. 2017;12(1):43–53. doi: 10.1016/j.jtho.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Shao F, Wu X, Ying K. Value of quantitative analysis of circulating cell free DNA as a screening tool for lung cancer: a meta-analysis. Lung Cancer. 2010;69(2):225–231. doi: 10.1016/j.lungcan.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Ai B, Liu H, Huang Y, Peng P. Circulating cell-free DNA as a prognostic and predictive biomarker in non-small cell lung cancer. Oncotarget. 2016;7(28):44583–44595. doi: 10.18632/oncotarget.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karachaliou N, Mayo-de las Casas C, Queralt C, de Aguirre I, Melloni B, Cardenal F, et al. Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol. 2015;1(2):149–157. doi: 10.1001/jamaoncol.2014.257. [DOI] [PubMed] [Google Scholar]
- 7.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Ost DE, Yeung S-CJ, Tanoue LT, Gould MK. Clinical and organizational factors in the initial evaluation of patients with lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e121S–e141S. doi: 10.1378/chest.12-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva JM, Silva J, Sanchez A, Garcia JM, Dominguez G, Provencio M, et al. Tumor DNA in plasma at diagnosis of breast cancer patients is a valuable predictor of disease-free survival. Clin Cancer Res. 2002;8(12):3761–3766. [PubMed] [Google Scholar]
- 10.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JL, Kim HJ, Choi BY, Lee HC, Jang HR, Song KS, et al. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol Lett. 2012;3(4):921–926. doi: 10.3892/ol.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gedvilaitė V, Schveigert D, Cicėnas S. Cell-free DNA in non-small cell lung cancer. Acta Med Litu. 2017;24(2):138. doi: 10.6001/actamedica.v24i2.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.