Abstract
Owing to its highly biocompatible property as naturally produced nanoscale particle and drug carrying ability, exosome has attracted much interest in the biomedical area. Versatile functions of exosome in biological system play an important role in elucidating mysterious and unknown biological processes and pathological disease progression. For usage of exosome as brain disease therapeutics, even though the ability of exosomes crossing blood brain barrier (BBB) is not well clearly proven, the small size and their own characteristics possessing cell-derived molecular contents may provide great and beneficial tools for brain delivery and brain-associated disease therapy. A variety of trials related to bioapplications using stem cell-derived exosome in regenerative therapy or autologous exosome shuttling inhibitor targeting brain disease-associated protein marker enhance possibility of exosome toward clinical application. The radionuclide PET or SPECT imaging of radiolabeled exosome will be clearly able to provide accurate clues for analyzing their whole body distribution, targeting efficacy, and the degree of non-specific tissue uptake. In this perspective, the practical information on thranostics of exosome for brain delivery and therapy is offered and radionuclide-based exosome applicability will be dealt with.
Keywords: Exosome, Theranostics, Radionuclide imaging, In vivo targeted therapy, Regenerative therapy
Introduction
The concept of exosomes that is known as one of the small nano-sized extracellular vesicles (EV) released from nearly all cells was first recognized in the early 1980s. Exosomes deliver cell’s intrinsic molecular components as a form of RNA and protein to distant or neighboring cells to alter their phenotype and function. Since exosome is known to play an important role in innate and adoptive immunity, diverse biological functions such as stem cell maintenance, inflammation, and cell angiogenesis have been identified. Also, exosome has been linked with triggering pathological process in cancer, neurodegenerative disease, which can be considered the ideal therapeutic target.
Exosome that is naturally produced from our body has been intensively noticed as potential diagnostic and therapeutic agent owing to its high biocompatibility and infinite applicability. Significant interest and bold R&D investment on exosome has greatly increased in recent years even though discovery and history of exosome is just 40 years. Indeed, exosome has long been thought to have a role of merely tiny waste disposal from cells. But, after exosome is recognized to contribute to physiological or pathological function as a significant messenger, many basic scientists and physicians start to apply exosome to their own biological or medicinal area.
From this atmosphere, the National Institutes of Health (NIH) started to realize the significance of extracellular vesicle on a broad range of biomedical applicability. For the effort of accelerating the advancement of the basic research and application on exosome, this government institute started to be seeking grant applications for research projects regarding exosome-based disease detection, diagnosis, and therapy.
As well as government interest, industrial effort has been made for the purpose of its mass production or medicinal application. Many bio venture companies, especially developing cell-based therapeutics, are hoping to harness exosome that is purified from cell culture medium for the commercial purpose. One of the exosome-specialized leading companies, ExoCoBio established in 2017, has received about $46 million through series A and B funding round in total from domestic venture capital within 2 years to establish GMP-manufacturing facility for mass production of exosome therapeutics. This bio-company has pursued improvement of skin tissue regeneration using stem cell exosome in cosmeceutical purpose as well as stem cell exosome-based disease therapy. Also, Codiak BioSciences founded in 2015 is applying the natural properties of exosomes capable of loading chemical or macromolecule drug for disease targeting and therapy.
Potential Role of Exosome in Drug Delivery and In Vitro Diagnostic Purpose
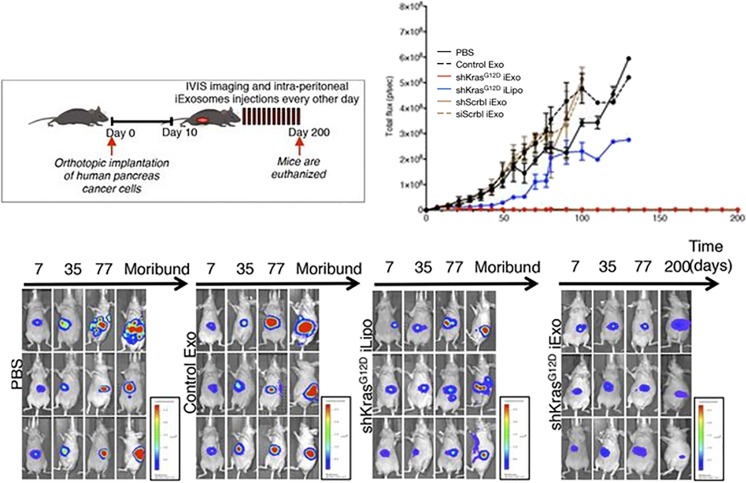
Exosome has contributed to roughly five different research areas (drug delivery system, in vitro diagnosis, stem cell alternatives, cancer vaccine, and cosmetics) for biomedical application [1–10]. Ability of exosome that is capable of simply encapsulating small molecule drug, peptide, and nucleic acid as drug delivery conveyor provides substantial merit for targeted disease therapy [11–14]. Raghu Kalluri’s group clearly demonstrated the superiority of exosome as siRNA delivery therapeutics compared to its counterpart, liposome. CD47-possessing exosome is recognized by circulating monocyte as a self, and circulation time increases, which has greater chance to be accumulated in tumor area [15]. The shRNA against oncogenic KRAS mutant mRNA was transferred into human fibroblast exosome using electroporation, and intraperitoneal administration of engineered exosome (iExosome) in pancreas tumor-bearing mice was conducted at every other day until 200 days. Compared to the same counterpart group using liposome, drug-loaded iExosome significantly inhibits tumor growth, and therapeutic effect of iExosome for pancreas cancer was maintained until 200 days (Fig. 1). However, bolus injection of exosome via intravenous route eventually causes a very high rate of liver accumulation, which results in low targeting efficacy to the tumor area. Liver evading method to make exosome drug send to the disease site more efficiently should be developed.
Fig. 1.
Therapeutic effectiveness of drug-loaded exosome for pancreas cancer. Human foreskin fibroblast (BJ)-derived exosome was loaded with shRNA for KrasG12D using electroporation. Ten days after orthotopic administration of human pancreas cancer cells, approximately 108 iExosomes (0.15–0.20 μg/injection) were intraperitoneally injected into the nude mice every other days until 200 days. Time course semi-quantitative data for growth of luciferase-expressing pancreas tumor in mice revealed that iExosomes but not iLiposomes completely suppress tumor volume in pancreas tumor-bearing mice until 200 days. Reprinted with permission from [15]
For the diagnostic purpose, abundance of exosome, approximately 3 × 109/mL in blood serum, offers good original source to detect disease biomarker. A variety of customized microfluidic chip platform has been rapidly developed for simple, rapid detection of exosome biomarker in blood, urine [16–18]. Urinary exosome isolated from urine sample of idiopathic Parkinson’s disease patient contains the elevated level of Ser(P)-1292 LRRK2, which correlated with poor cognitive performance [19]. Rapid detection of exosome biomarker via simple sample collection will be able to contribute to prediction, classification, and therapeutic effect evaluation of Parkinson’s disease patient. However, we need to consider that exosome is so heterogeneous. Certain exosome contents are vacant inside, or the amount of target protein or RNA content varies as the individual exosome. Thus, exosomal content analysis needs to be performed in single exosome level.
Therapeutic Function of Stem Cell-Derived Exosome
For application of stem cell-derived exosome, MSC-derived exosomes exerted reduction in infarct size by 45% in myocardial ischemia/reperfusion injury [19, 20]. The glycolytic enzyme, anti-apoptotic protein, and anti-inflammatory factors are contained in mesenchymal stem cells (MSC)-derived exosomes, which leads to increase in ATP production and myocardial viability, and decrease in oxidative stress and inflammation with functional improvement [21]. Also, stem cell-derived exosome participates in stimulating skin rejuvenation by promoting the synthesis of collagen and elastin [22].
There are several clear evidences on therapeutic benefit using stem cell-derived exosome in neurological diseases. Human dental pulp stem cell-derived exosome showed reduction in the apoptosis of dopaminergic neuron treated with 6-hydroxydopamine (OHDA) in specialized three-dimensional cell culture condition [23]. Also, exosomes loaded with antioxidant enzyme, catalase exerted sustained release and neuroprotective effect by decreasing ROS levels in PC12 cells. Intranasal administration of catalase-carrying exosome showed high accumulation in the mouse brain and protective effect in Parkinson’s disease mouse [24]. Also, interesting evidence dealing with the beneficial effect of long noncoding RNA in exosome was reported [25]. Indeed, it was surprised at existence of long noncoding RNA that is more than 200 nucleotide in size with three-dimensional structure inside exosome. Bassit et al. documented that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was found in the exosomes derived from human adipose stem cells and increases survival of neurons by mediating PKCδII alternative splicing [25].
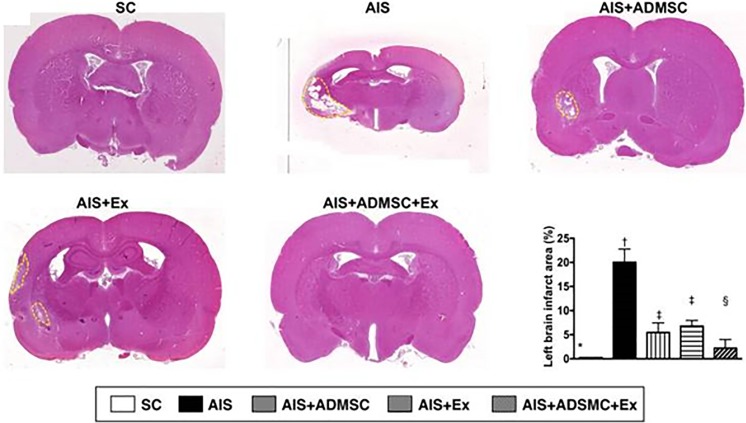
General curiosity on availability of allo-or xeno-graft of exosome for disease therapy can be occurred to facilitate rapid application of exosome therapeutics to clinic. The mini-pig adipose-derived mesenchymal stem cell (ADMSC) and ADMSC-derived exosome therapy were conducted to compare therapeutic effect on acute ischemic stroke (AIS) [26]. The significant improvement in brain infarct volume became apparent in treatment group of ADMSC, exosome, and ADMSC-exosome together, but not in AIS. Intravenous injection of 100 μg of exosome at 3 h after establishment of AIS revealed reduction in brain infarct volume (Fig. 2) and functional improvement accompanied with decreased inflammation and oxidative stress in AIS model. Interestingly, xenograft of pig-originated ADMSC-exosome was immune privileged in rat AIS model. Indeed, ADMSC has immunomodulatory and anti-inflammatory function. And, compared to cell therapy, exosome in much smaller size that contains less surface antigen in exosomal membrane seems to have less immunogenic property, which could be recognized as more safe and effective therapeutics.
Fig. 2.
Reduction of brain infarction volume using ADMSC-derived exosomes in ALS. The brain infarct area was measured on day 60 after acute ischemic stroke (ALS). Xenograft of adipose-derived mesenchymal stem cell exosome via intravenous route showed reduced infarction size, measured by H&E staining (100×) F. Significant improvement in left brain infarct size was found after dual treatment of ADMSC and ADMSC exosome. Reprinted with permission from [26]
Blood Brain Barrier Penetration of Exosome and the Engineered Exosome Therapeutics for Treatment of Brain Disease
The most key challenge in exosome-based brain delivery and therapy is the lack of evidence and uncertainty on exosome to cross blood brain barrier (BBB). Theoretically, major job of exosome is to deliver information to its target cells by transferring donor cell’s inherent molecular signature to change the phenotype and function of target cells. From this aspect, the neuron, astrocyte, and microglia in the brain parenchymal continue to release its own exosome and exosome might take long journey to other peripheral tissues (ex. intestine) along with blood vessel. If so, crossing BBB would be necessary on exosome to perform its own work. However, whether exosome passes through BBB or how endothelial cell uptake mechanism of exosome is involved in remains question mark. In vitro brain microvascular endothelial cell (BMEC) monolayer culture system has been widely used to mimic in vivo BBB environment in microfluidic chip or transwell system [27–29]. One report investigated movement of exosome via BBB using in vitro BBB model [30]. Gaussia luciferase gene fused with lactadherin (hGluc-Lact), membrane protein was used for reporter-based exosome tracing. The hGluc-Lact-293T exosome was shown to cross BBB by interacting with BMEC. Interestingly, exosomes were selectively passing through BMEC monolayer activated by TNF-α to make pro-inflammatory stroke-like condition, while exosomes did not cross BMEC under non-treated normal condition. Approximately 10% of exosome was crossing BMEC monolayer in transwell chamber at 18 h after TNF-α treatment [30]. Blocking study on multiple endocytosis pathways clearly exhibited that internalization of exosome by BMECs is based on clathrin- or caveolae-dependent pathway. However, in this study, real-time fate of exosome in cytoplasmic area and exocytosis route is not well investigated. If we think about the general cellular uptake process of exosome to recipient BMEC, exosomal membrane taken up by BMEC is likely to be fused with endosomal membrane and get molecular contents of exosome released into cytoplasm of target cells. So, maybe, the fate of exogenous exosome secreted from donor cells would be finished after endocytosis, and newly produced de novo exosome would be generated and exit from cells. But, this phenomenon is not well understood, because technique for real-time tracking of exosome from endocytosis to exocytosis is not developed. And, also we could not exclude the feasible phenomenon that the exosome would be directed taken up by cells and the same exosome would be escaped from cells via transcytosis. For the brain-targeted delivery of exosome, despite difficulties in brain delivery of exosome due to BBB, Matthew JA Wood’s group successfully achieved brain delivery of drug-loaded exosome for the purpose of Alzheimer’s disease therapy [31]. DNA plasmid construct containing rabies virus glycoprotein (RVG)-fused Lamp2b was transfected into dendritic cells (DC), and autologous DC exosome loaded with siRNA via electroporation was injected back into mouse. RVG-exosome specifically targeted neuronal cells via its corresponding acetylcholine receptor after crossing BBB and showed dramatic knockdown of BACE1 expression in normal C57BL/6 mice. These findings provide therapeutic potential of exosome for therapeutic siRNA delivery to the brain. However, we need to consider that intrinsic size of exosome ranging from 40 to 100 nm would not be able to avoid being accumulated in the liver tissue when exosomes are systemically injected. High engulfment of the injected exosome by resident Kupffer cells or hepatocytes that cause the non-specific knockdown of gene in the liver could be a major issue to reduce the targeting efficacy of drug-loaded exosome. And, also the accurate amount of exosome uptaken in each organ over time should be quantitatively calculated. Even though many previous reports suggested the excellent therapeutic response of exosome, researches providing in vivo tracking information of exosome such as exact whole body distribution are required [32–39].
In Vivo Radionuclide Imaging of Exosome and Nuclear Thranostics for Brain
To trace organ distribution and excretion of the grafted exosome, adopting the non-invasive imaging techniques is necessary for simple and reliable approach to achieve its goal. Of the different imaging modalities, radionuclide and magnetic resonance imaging techniques can be used for clinical application. MR contrast agent such as such as ultra-small superparamagnetic iron oxide nanoparticles (USPIO) should be labeled with exosome for MR-based tracing of exosome with high spatial resolution [40, 41]. However, based on MR imaging, it may not be easy to distinguish clear location of exosome from non-specific MR signal with high sensitivity in certain organ. Even though radionuclide-based whole body imaging of exosome can offer highly sensitive signal in the certain organ region of interest, the amount of previously reported literatures regarding radioisotope-labeled exosome using PET/CT and SPECT/CT imaging are not enough to better understanding in vivo fate of exosome quantitatively. Currently, only several studies successfully achieved the radioisotope-based in vivo biodistribution of EVs. Morishita et al. adopted the iodine-125, which has long half-lie (60 days), and utilized high affinity of 125I-biotin derivative and streptavidin-lactadherin, exosomal membrane protein for radiolabeling of melanoma exosome [42]. From this study, 125I-melanoma exosome injected via intravenous route in mouse revealed the biphasic radioactivity pattern in the blood, showing very rapid circulation time (1.5 min half-life at first α phase) and much slower reduction (346 min half-life at second β phase) through two compartment model. Also, the injected exosomes were rapidly accumulated in the liver with 17% of injection dose (ID) at 1 min and reached a plateau at 30 min with 39% ID. From this radionuclide-based exosome biodistribution approach, pharmacokinetic information capable of quantitative measurement in the radiolabeled exosome was simply provided.
In our earlier work, we adopted the highly lipophilic radiotracer, 99mTc-HMPAO known as uncharged and lipophilic brain perfusion imaging agent for exosome labeling [43]. Substantial merit in using 99mTc-HMPAO for exosome labeling is to be processed in very simple procedure with mild reaction condition. Glutathione reductase which is highly abundant inside exosome in certain cell type converts 99mTc-HMPAO to hydrophilic form which is trapped inside exosome. The inter-luminal radiolabeling of exosome mimetic nanovesicle (ENV) with 99mTc-HMPAO preserves its integrity on exosomal surface membrane proteins and physiological properties. This study demonstrated that 99mTc-HMPAO-ENVs injected via intravenous route was highly accumulated in the liver at initial time point and seemed to be excreted through intestinal area, compared to mouse group injected with 99mTc-HMPAO itself that showed different distribution pattern with high brain uptake.
Quantitative measurement in radionuclide imaging modality provides useful information to evaluate how much amount of the injected exosomes are localized in the certain area of the brain, and we can also verify how long the localized exosomes are retained and when it is excreted from the brain tissue. However, if very small amount of exosomes injected reach the brain tissue, PET/CT or SPECT/CT could not detect the radionuclide activity of radiolabeled exosome, because it is under detection threshold. Thus, more advanced labeling method to maximize loading efficiency of radioisotope in single exosome to enhance sensitivity of exosome should be developed to be able to observe tissue distribution of individual exosome in microscopic level. Or, more innovative radionuclide imaging device that can detect a very small amount of exosome could help to understand more accurate efficacy of tissue uptaken.
Nuclear theranostics of exosome can be harnessed in the destructive cancer therapy as well as stem cell-based regenerative therapy. Tissue regeneration-induced chemical compound or peptide can be easily loaded inside stem cell-derived exosome via sonication or electroporation method, and the signal of radioisotope labeled in exosome can guide the routes of exosomal movement to the target tissue of interest such as the brain. For the brain cancer-targeted therapy, exosome can be labeled with both theranostic radioisotope such as 177Lu and brain cancer-specific ligand. Also, exosome enables combination therapy by designing theranostic radiolabeled exosome carrying anti-neoplastic drug great for brain tumor-targeted diagnosis and therapy. After exosome therapeutics reach the brain tumor area, margin of brain tumor can be precisely examined via gamma ray-emitting signals, and therapeutic radioisotope and chemodrug work together to enhance killing effect of brain tumor. Likewise, even though there are many challenges on complexity of exosome for clinical application such as examination of exact identification, classification, biogenesis of exosome in basic biology field and the issues for immune response, in vivo delivery, clinical guideline preparation, and the radiotheranostic technique of exosome will be able to make huge contribution, in terms of developing new types of therapeutics and understanding in vivo situation of exosome therapeutics in clinical level.
Funding
This research was supported by a grant of the Research-Driven Hospital Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1277) and National Research Foundation of Korea grant funded by Ministry of Science and ICT (MSIT) (2015M3C7A1028926, 2017M3C7A1048079).
Conflict of Interest
Do Won Hwang declares no conflict of interest.
Ethical Approval
This perspective does not contain any studies with human participants or animals.
Informed Consent
As a review article, obtaining informed consent was waived.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81–94. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinheiro A, Silva AM, Teixeira JH, Gonçalves RM, Almeida MI, Barbosa MA, et al. Extracellular vesicles: intelligent delivery strategies for therapeutic applications. J Control Release. 2018;289:56–69. doi: 10.1016/j.jconrel.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong JPK, Stevens MM. Strategic design of extracellular vesicle drug delivery systems. Adv Drug Deliv Rev. 2018;130:12–16. doi: 10.1016/j.addr.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee K, Fraser K, Ghaddar B, Yang K, Kim E, Balaj L, et al. Multiplexed profiling of single extracellular vesicles. ACS Nano. 2018;12:494–503. doi: 10.1021/acsnano.7b07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–3577. doi: 10.1039/C7LC00592J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodarzi P, Larijani B, Alavi-Moghadam S, Tayanloo-Beik A, Mohamadi-Jahani F, Ranjbaran N, et al. Mesenchymal stem cells-derived exosomes for wound regeneration. Adv Exp Med Biol. 2018;251:1–13. doi: 10.1007/5584_2018_251. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cell. 2018;7:E226.1–E226.10. doi: 10.3390/cells7120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartz M, Darlington A, Afzal MZ, Strande JL. Exosomes exert cardioprotection in dystrophin-deficient cardiomyocytes via ERK1/2-p38/MAPK signaling. Sci Rep. 2018;8:16519–16533. doi: 10.1038/s41598-018-34879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyama Y, Ito T, Hasegawa A, Eriguchi M, Inaba T, Ushigusa T, et al. Exosomes derived from tumor cells genetically modified to express mycobacterium tuberculosis antigen: a novel vaccine for cancer therapy. Biotechnol Lett. 2016;38:1857–1866. doi: 10.1007/s10529-016-2185-1. [DOI] [PubMed] [Google Scholar]
- 11.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2013;65:331–335. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Vashisht M, Rani P, Onteru SK, Singh D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol. 2017;183:993–1007. doi: 10.1007/s12010-017-2478-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Liao C, Zuo P, Liu Z, Ye BC. Magnetic-based microfluidic device for on-chip isolation and detection of tumor-derived exosomes. Anal Chem. 2018;90:13451–13458. doi: 10.1021/acs.analchem.8b03272. [DOI] [PubMed] [Google Scholar]
- 17.Fang S, Tian H, Li X, Jin D, Li X, Kong J, et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS One. 2017;12:e0175050.1–e0175050.13. doi: 10.1371/journal.pone.0175050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao H, Chung J, Lee K, Balaj L, Min C, Carter BS, et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun. 2015;6:6999–7018. doi: 10.1038/ncomms7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N, et al. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov Disord. 2016;31:1543–1550. doi: 10.1002/mds.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Yoo SM, Park HH, Lim HJ, Kim YL, Lee S, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun. 2017;493:1102–1108. doi: 10.1016/j.bbrc.2017.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17:932–939. doi: 10.1016/j.jcyt.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Bassit G, Patel RS, Carter G, Shibu V, Patel AA, Song S, et al. MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology. 2017;158:183–195. doi: 10.1210/en.2016-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–74556. doi: 10.18632/oncotarget.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Wang N, Cai B, Wang GY, Li J, Piao XX. In vitro model of the blood-brain barrier established by co-culture of primary cerebral microvascular endothelial and astrocyte cells. Neural Regen Res. 2015;10:2011–2017. doi: 10.4103/1673-5374.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Y, Yao Y, Tsirka SE, Cao Y. Cell-culture models of the blood-brain barrier. Stroke. 2014;45:2514–2526. doi: 10.1161/STROKEAHA.114.005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YI, Abaci HE, Shuler ML. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017;114:184–194. doi: 10.1002/bit.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 32.Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1–15. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 34.Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399–1410. doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapchak PA, Boitano PD, de Couto G, Marbán E. Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp Neurol. 2018;307:109–117. doi: 10.1016/j.expneurol.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187–192. doi: 10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Cheng Q, Hu G, Deng T, Wang Q, Zhou J, et al. Extracellular vesicles in mesenchymal stromal cells: a novel therapeutic strategy for stroke. Exp Ther Med. 2018;15:4067–4079. doi: 10.3892/etm.2018.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–185. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 40.Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, Benati D, et al. Labeling and magnetic resonance imaging of exosomes isolated from adipose stem cells. Curr Protoc Cell Biol. 2017;75:3.44.1–3.44.15. doi: 10.1002/cpcb.23. [DOI] [PubMed] [Google Scholar]
- 41.Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, Benati D, et al. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int J Nanomedicine. 2016;11:2481–2490. doi: 10.2147/IJN.S104152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morishita M, Takahashi Y, Nishikawa M, Sano K, Kato K, Yamashita T, et al. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J Pharm Sci. 2015;104:705–713. doi: 10.1002/jps.24251. [DOI] [PubMed] [Google Scholar]
- 43.Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci Rep. 2015;5:15636–15646. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]