Abstract
Radiometals have been commonly used in medical applications, and utilization of such metals continues to be an attractive research area. In particular, a variety of radiometals have been developed and implemented for molecular imaging. For such applications, 89Zr has been one of the most interesting radiometals currently used for tumor targeting. Several chemical ligands were developed as 89Zr chelators, and new coordinating methods have also been developed more recently. In addition, immuno-positron emission tomography (PET) studies using 89Zr-labeled monoclonal antibodies have been performed by several scientists. In this review, recent advances to the coordination of 89Zr and the utilization of 89Zr in PET studies are described.
Keywords: 89Zr, Positron emission tomography (PET), Coordination, Ligand
Introduction
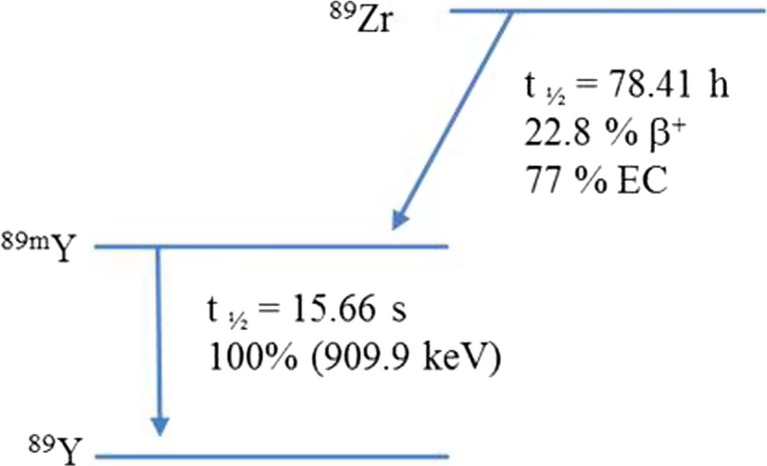
Zirconium-89 (89Zr), with an atomic number of 40, has useful biomedical applications. This is due to its favorable decay characteristics, a half-life of 78.41 h, which make it suitable for labeling biomolecules, such as antibodies, for imaging (Fig. 1, Table 1). Nowadays, 89Zr is considered an important positron-emitting radionuclide used for the development of novel radiopharmaceuticals for positron emission tomography (PET). In particular, 89Zr has been widely used for immuno-PET studies due to ideal physical characteristics.
Fig. 1.
Zirconium-89 decay
Table 1.
Properties of 89Zr
| t½ (h) | Methods of production | Decay mode | Eβ+ (keV) | References |
|---|---|---|---|---|
| 78.41 | 89Y(p,n)89Zr | β+ (22.7%) EC (77%) |
909 | [1] |
Production of 89Zr
There are several reaction pathways that produce 89Zr, such as the 89Y(p,n)89Zr reaction, 89Y(d,2n)89Zr reaction, natZr(p,pxn)89Zr reaction, natSr(α,xn)89Zr reaction, and 90Zr(n,xn)89Zr reactions (Table 2) [5, 6, 12–14]. The first two of these reactions are common pathways to produce 89Zr due to the availability of 89Y from natural sources. The Zweit group utilized natural yttrium pellets to produce 89Zr using the 89Y(d,2n)89Zr reaction: the starting material was irradiated with a 16–7-MeV optimum energy beam of deuterons and then purified in an ion-exchange column to obtain a 66.6-MBq/μAh yield of 89Zr with a minor fraction of long-lived 88Zr (0.008%). Using a similar reaction, high-purity 89Zr production was experimentally reported by Tang and co-workers and theoretically calculated by the Sadeghi group [3, 15]. Despite the higher yield of the 89Y(d,2n)89Zr reaction compared to the 89Y(p,n)89Zr reaction, application of the 89Y(d,2n)89Zr reaction in medical accelerators is still restricted. This is due to the fact that common small medical cyclotrons are not capable of producing the high-energy deuterons required for the 89Y(d,2n)89Zr reaction. Although several medical cyclotrons, such as the GE PETtrace 800 or IBA Cyclone 18/9, have two beam currents, the deuteron energy still is not sufficient to produce a high yield of 89Zr. Hence, the 89Y(p,n)89Zr reaction is the more practical approach to the production of 89Zr in these kinds of machines.
Table 2.
Several reactions for 89Zr production
| No. | Nuclear reaction | Target | Product chemical form | Yield (MBq/μAh) | Time of irradiation | Energy (MeV) | Beam current (μA) | Thickness of target | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 89Y(d,2n)89Zr | Pellet | Chloride | 66.6 ± 5.6 | 12–20 min | 16–7 | 3–5 | 240–340 mg cm−2 | [2] |
| 2 | 89Y(d,2n)89Zr | Magnetron sputtering | Chloride | 58 ± 5 | 1 h | 13 | 10–15 | 25 μm | [3] |
| 3 | 89Y(p,n)89Zr | Magnetron sputtering | Chloride | 44 ± 4 | 1 h | 14 | 10–30 | 25 μm | [3] |
| 4 | 89Y(p,n)89Zr | Foil | Oxalate | 38.9 | 40 min | 13 | 10 | 286 mg cm−2 | [4] |
| 5 | 89Y(p,n)89Zr | Thin foil | Oxalate | 13 | 2 h | 11.4–10 | 10 | 57 mg cm−2 | [5] |
| 6 | 89Y(p,n)89Zr | Foil | Oxalate | 56.2 ± 4.1 | 2–5 h | 15 | 15 | 100 μm | [6] |
| 7 | 89Y(p,n)89Zr | Foil | Oxalate | 12.5 ± 0.5 | 2 h | 18–10 | 12 | 150 μm | [7] |
| 8 | 89Y(p,n)89Zr | Foil | Oxalate | 48.9 ± 4.4 | 1 h | 12.8 | 45 | 640 μm | [8] |
| 9 | 89Y(p,n)89Zr | Sputtered layer | Oxalate | 48.1 | 1 h | 14 | 100 | 25 μm | [9] |
| 10 | 89Y(p,n)89Zr | Sputtered coin | Oxalate | 6.4–18 | 30 min or 2 h | 12.5 or 12.8 | 10–40 | 90–250 μm | [10] |
| 11 | 89Y(p,n)89Zr | Y(NO3)3 solution (2.75 M) | Oxalate | 4.36 ± 0.48 | 2 h | 14 | 40 | Liquid target | [11] |
The first 89Y(p,n)89Zr reaction was carried out by Link and co-workers who employed an 89Y source on Y foil which was irradiated with 13 MeV protons. After irradiation, the Y foil was dissolved in HCl solution, and 89Zr(IV) was extracted via multistep extraction using 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione (TTA) and then HNO3/HF. Purification by anion exchange with 1 M HCl/0.01 M oxalate resulted in an 80% yield of 89Zr (99.99% purification). A similar protocol was reported by the Dejesus group using a thin Y foil [4, 5]. Based on the same starting material of a Y foil target, several studies modified parameters such as foil thickness, time of irradiation, energy, and beam current in the attempt to improve production yields [6–8]. However, the increase of beam energy over 13 MeV inevitably causes the undesirable production of long-lived 88Zr via the 89Y(p,2n)88Zr reaction. Recently, the Queern group worked on the production of 89Zr using sputtered yttrium on niobium coin. They found that a reduction of beam energy from 17.8 to 12.8 MeV or 12.5 MeV using a 0.75-mm-thick aluminum degrader yielded good results with no 88Zr observed [10].
The use of solid targets can be limited by a lack of facilities, so liquid targets have also been utilized to produce 89Zr. For instance, Pandey and co-workers irradiated yttrium (III) nitrate in nitric acid solution. Although their results showed a yield of only 4.4 MBq/μAh for 2 h of irradiation at a 40-μA beam current, which is barely adequate for a solid target, this yield was still better than what has been achieved with conventional liquid targets [11].
Coordination Chemistry and Ligands of 89Zr
Desferrioxamine and Its Derivatives
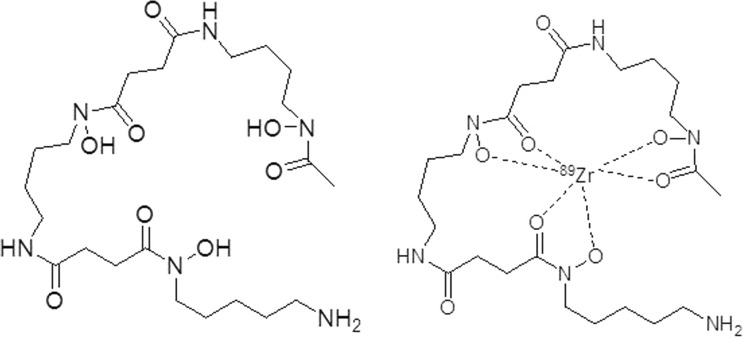
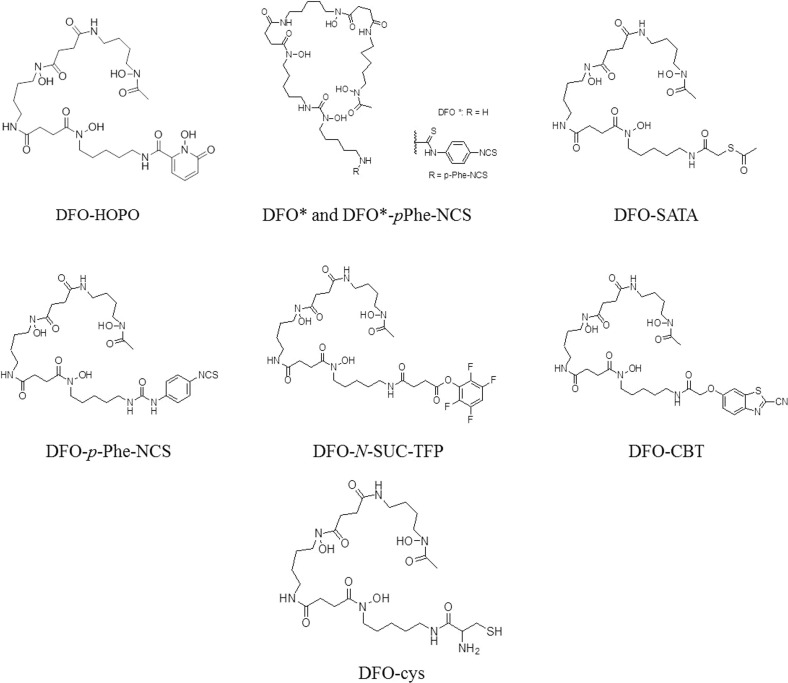
In order to effectively utilize 89Zr, coordination chemistry has been applied to study various chelates. The chelate first utilized for 89Zr is also currently the widely used: desferrioxamine (DFO). As showed in Fig. 2, DFO, which contains three RCO-N(R′)-OH motifs, is a hydroxamate-type siderophore that chelates with 89Zr to form a 89Zr-DFO complex, which is used in 89Zr-immuno-PET studies. Complexes with 89Zr based on the iron-chelator Desferal, DFO (L23), which includes hexadentate coordination of three hydroxamate units, and its derivatives have also been used in 89Zr-PET studies. However, 89Zr-DFO has been known to have some disadvantages, such as poor stability. Since its hexadentate complex is not saturated by a stably octa-coordinated Zr4+ sphere, 89Zr-DFO instability has been observed in several animal model experiments [6, 16]. Due to the importance of developing ligands for zirconium-89-based radiopharmaceuticals, especially for immuno-PET imaging, several DFO derivatives have been reported (Fig. 3), such as N-(S-acetyl) mercaptoacetyldesferal (SATA-DFO) [17] and 2,3,5,6-tetrafluorphenoxy (TFP)-N-succinyldesferal-Fe [18]. These modifications were prepared for bifunctional mAb coupling; however, both protocols showed several drawbacks. For example, an unstable thioether linker exists between maleimide-mAb and SATA-DFO at physiological pH and a complicated six-step reaction is used to prepare mAb-N-succinyldesferal-89Zr, consisting of carboxylation of the amine, protection with Fe(III), activation of the ester, attachment with a mAb, deprotection of Fe(III) from complex, and labeling with 89Zr radionuclide [19].
Fig. 2.
Structure of DFO and its 89Zr-complex
Fig. 3.
DFO derivatives
A simple two-step synthesis to prepare bifunctional 89Zr-labeled mAb via p-isothiocyanatobenzyl-desferrioxamine (DFO-Bz-NCS) was reported by Perk and co-workers more recently. This complex was described to be stable due to the strong and steady thiourea bond between the monoclonal antibodies and the chelator. Although this process proved to be a fast and effective method to acquire 89Zr-labeled mAbs, the restricted water solubility of the DFO-Bz-NCS precursor required experimental skill to prevent aggregation and precipitation of the antibody. Also, despite the stability of thiourea linker, it was reported to be easily cleaved by radiation in some buffers that contain chlorinated compounds [20, 21]. Another rapid and specific conjugation between modified-[89Zr]Zr-DFO and RGP peptides by the click reaction was described by Gao and co-workers. The modification of DFO at the terminal amine with 2-cyanobenzothiazole (CBT) or 1,2-aminothiol (cys) produced [89Zr]Zr-DFO-CBT or [89Zr]Zr-DFO-cys, respectively. Luciferin linkage formation from the click reaction of those with their complementary functionality on RGP peptides showed a high stability with an almost intact complex upon cysteine challenge [22].
Octadentate coordination using DFO-1-hydroxy-2-pyridone (DFO-HOPO) was first described by White and co-workers [23]. This study employed the DFO-HOPO ligand as a plutonium(IV) chelator for treatment of plutonium poison. Low toxicity and a stable octadentate coordination complex with Pu(IV) were observed when the 1,2-HOPO compound was introduced to the DFO molecule. Allott adopted this method and utilized DFO-HOPO to evaluate the stability of octadentate as an 89Zr chelator. Results showed 89Zr-DFO-HOPO to be stable compared to 89Zr-DFO with no demetallation during radio-ITLC analysis, and no bone uptake of 89Zr was observed within 24 h after 89Zr injection. Moreover, 89Zr-DFO-HOPO showed inertness to transchelation by EDTA or serum. DFO*, a modification of DFO by adding one more hydroxamic acid part, was reported as the first octadentate chelator for 89Zr labeling molecules with improved stability [24, 25]. A few years later, the bifunctional chelator DFO*-pPhe-NCS was prepared as an octadentate chelator with 89Zr. 89Zr-DFO*-mAb demonstrated greater stability than the previous hexadentate 89Zr-DFO-mAb with more than twice the intact tracer when stored at room temperature. Yet, solubility is still a challenge for the thiourea structure [26].
Other Hydroxamate-Type Chelators
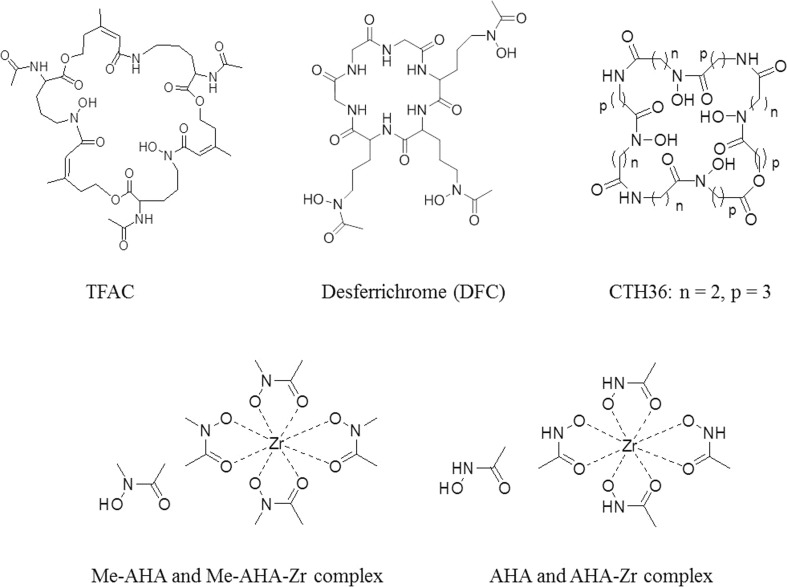
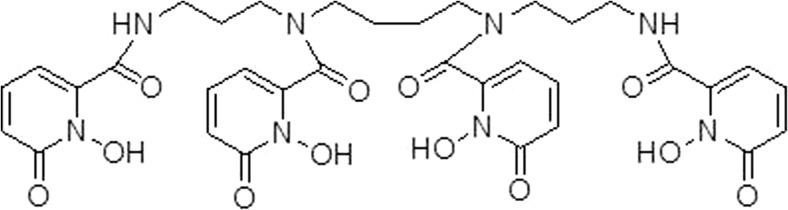
To expand the utilization of hydroxamate-type coordination with 89Zr, many hydroxamate-containing non-DFO structures have been developed (Fig. 4). Guérard and co-workers reported the simplest structures, acetohydroxamic acid (AHA) and its methylated derivative (Me-AHA), as ligands to coordinate with Zr(IV) and 89Zr(IV). Based on X-ray crystallography and potential titration, these studies found a metal to ligand ratio of 1:4 and octadentate coordination with Zr(IV) that was supposedly better than results with DFO. Through an 89Zr labeling complexation study, Me-AHA showed a better activity complex than did AHA. This can be explained by a higher electron density of the oxygen atom (N–O) of Me-AHA which forms a strong bond toward the 89Zr radionuclide [27]. Recently, two bifunctional tetrahydroxamate ligands were synthesized by Rousseau and co-workers based on an iminodipropionamide scaffold. This work was a modification of their previous study that elongated the aliphatic chain on the main ligand and reduced the distance between ligand and isothiocyanate moiety. Despite improved stability, the biodistribution and PET imaging properties of these ligands showed no significant differences compared to those this group studied previously or to DFO [28].
Fig. 4.
Other hydroxamate-type ligands
Macrocyclic structures including hydroxamate moieties have also been developed, such as triacetylfusarinine C (TFAC), desferrichrome (DFC), and tetrahydroxy octaazacyclohexatriacontan-octaone (CTH36). This type of ligand was reported to form steadier coordination than linear ligands. In addition, as a result of the macrocycle effect, a ligand that has a macrocyclic structure could possess an advantage due to the strong stability of the complex [29–32].
Other Types of Chelators
There is a similar structure between hydroxypyridone (HOPO) and hydroxamate; hence, HOPO was also employed as a polydentate hydroxypyridone ligand. The development of a HOPO ligand for chelating 89Zr radionuclides was reported by Deri and co-workers. 3,4,3-(LI-1,2-HOPO), which has four hydroxypyridone moieties (Fig. 5), could make an octadentate 89Zr complex which significantly enhances stability compared to DFO in DFT calculations. The 89Zr-HOPO complex was inert to transchelation in EDTA and serum challenge tests. In serum, the 89Zr complex was an almost intact radiotracer after a 7-day incubation. 89Zr-HOPO also possesses satisfactory biological behavior such as rapid renal excretion and low radioactivity in bone tissue. Conjugation of 89Zr-HOPO with antibodies to make bifunctional ligands is currently an active area of research [33].
Fig. 5.
Structure of 3,4,3-(LI-1,2-HOPO)
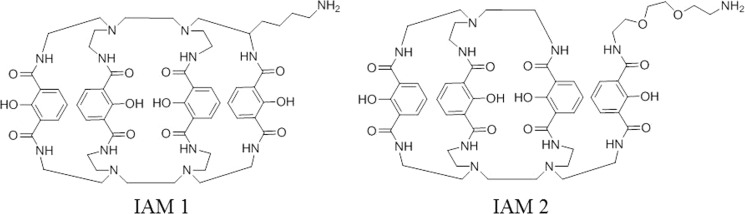
Hydroxyisophthalimide (IAM) ligands, originally used for lanthanides, were also described to produce stable 89Zr complexes. Bhatt group investigated two analogs of IAM, including IAM 1 and IAM 2 which differed by one pensile IAM group (Fig. 6). The result showed that the stability of 89Zr-IMA 1 was greater than that of 89Zr-DFO which was in turn greater than that of 89Zr-IMA 2 (with 72%, 41%, and 26% tracer intact, respectively, after a 7-day incubation with DTPA). However, in the amino model, 89Zr-IAM 1 and 89Zr-IAM 2 accumulated much more in the kidneys, liver, and bone than did 89Zr-DFO. 89Zr-immuno-PET imaging with 89Zr-IAM 1 is currently still under further investigation [34].
Fig. 6.
Structure of hydroxyisophthalimide (IAM) ligands
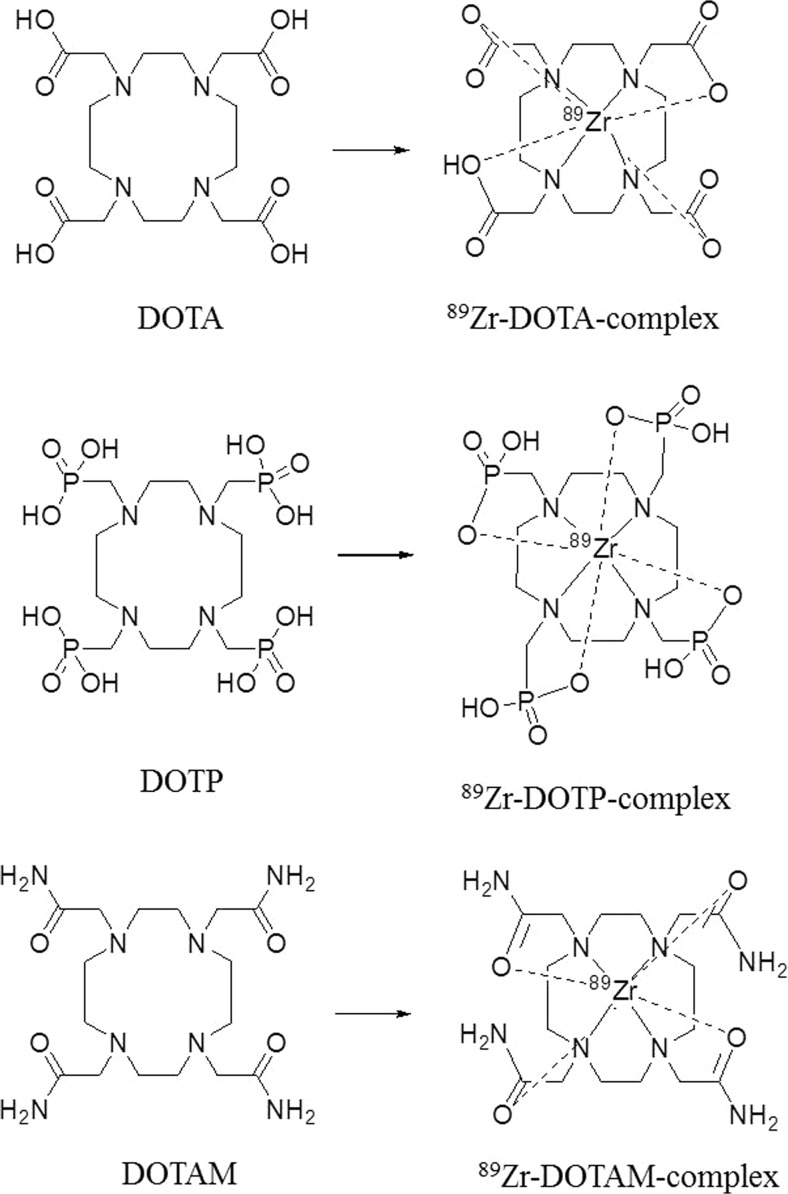
Ligands containing carboxylate and amino donors, such as EDTA and DTPA, have also been reported to complex with 89Zr [35]. Recently, the Wadas group used various kinds of tetraazamacrocycle ligands, namely, DOTA, DOTP, and DOTAM, to react with 89ZrCl4 to form Zr complexes (Fig. 7) [36]. The stability of resulting Zr-complexes (Zr-DOTA, Zr-DOTP, Zr-DOTAM) which were tested with an excess amount of EDTA or a high concentration of metal ions (Fe , Zn , Co , Cu , Mg , Gd , Ga ) was showed as following order: Zr-DOTA >> Zr-DOTP> Zr-DOTAM> Zr-DFO. In additions, they found that Zr-DOTA was stable, showing no change even after 7 days.
Fig. 7.
Structure of tetraazamacrocylic ligands and their 89Zr complexes
In in vivo biodistribution experiments, 89Zr-DOTAM showed a large amount of radioactivity in the liver and spleen, while 89Zr-DOTA showed relatively low radioactivity in the liver, kidneys, and bone. Results from 89Zr-DOTP were generally similar to those from 89Zr-DOTA, except that high amounts of radioactive material were found in the bone with 89Zr-DOTP. Based on these results, dynamic PET imaging studies were conducted using 89Zr-DOTA and 89Zr-DFO. In contrast, 89Zr-DFO accumulates significantly in the kidneys after 4 and until 24 h. However, 89Zr-DOTA accumulates less in the kidneys and bones than does 89Zr-DFO. A small amount of 89Zr-DOTA was observed in the bladder at 4 h, and after 24 h, the radioactivity in the bladder was found to be negligible. Thus, it was found that 89Zr-DOTA was easily cleared from the living body over a short period of time. Therefore, we confirmed that 89Zr-DOTA could be effectively applied to precision medicine without the disadvantages that come with 89Zr-DFO, which is currently used.
Immuno-PET Studies Using 89Zr
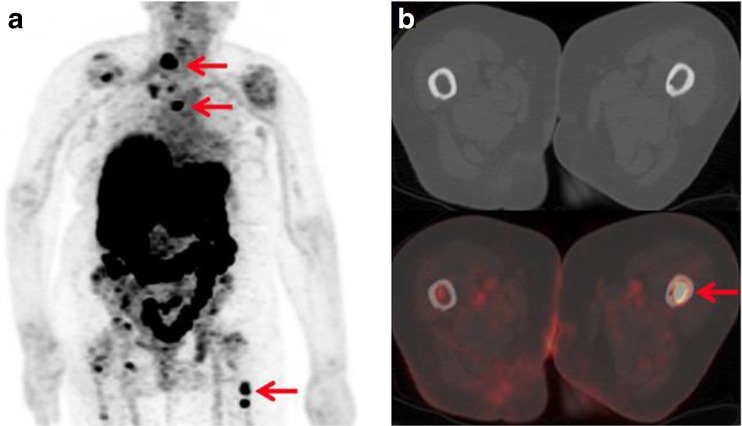
In order to apply 89Zr to precision medicine, immuno-PET studies using 89Zr-labeled monoclonal antibodies (mAbs) have been carried out by various researchers (Table 3). For instance, measurements of metastasis in persons with breast cancer have been carried out using trastuzumab [49]. Trastuzumab is a target for human epidermal growth factor receptor 2 (HER2), which has been used to diagnose HER2-positive breast cancer, and thus, treatment with trastuzumab has shown positive results in patients with HER2-positive breast cancer and gastric cancer [50, 51]. In one case, HER2-negative early breast cancer patients were found to have HER2-positive cancer metastases with PET/CT scans using 89Zr-trastuzumab (Fig. 8) [49]. In addition, 89Zr-trastuzumab PET was used to evaluate the alteration of HER2 expression in patients with HER2-positive breast cancer after they were treated with the anti-angiogenic agent NVP-AUY922, the novel heat shock protein 90 (HSP90) inhibitor. This study suggested that 89Zr-immuno-PET can be useful for determining the alteration of antigen expression and for monitoring the response to treatment with anti-cancer agents [52].
Table 3.
Application of 89Zr-mAb in clinical oncology studies
| Year | mAb | Target | Tumor type | Refs. |
|---|---|---|---|---|
| 2006 | Chimeric mAb U36 | CD44v6 | Head and neck cancer | [37] |
| 2012 | Ibritumomab-tiuxetan | CD20 | B cell lymphoma | [38] |
| 2013 | Bevacizumab | VEGF-A | Breast cancer | [39] |
| 2014 | Bevacizumab | VEGF-A | Neuroendocrine tumors | [40] |
| 2015 | Fresolimumab | TGF-β | Glioma | [41] |
| 2016 | MMOT0530A | MSLN | Pancreatic, ovarian cancer | [42] |
| 2017 | Cetuximab | EGFR | Head and neck, lung cancer | [43] |
| 2017 | Rituximab | CD20 | B cell lymphoma | [44] |
| 2017 | Lumretuzumab | HER3 | Multiple cancer types | [45] |
| 2017 | Bevacizumab | VEGF-A | Metastatic renal cell carcinoma | [46] |
| 2018 | Trastuzumab | HER2 | Breast cancer | [47] |
| 2018 | Atezolizumab | PD-L1 | Bladder cancer, non-small cell lung cancer, triple-negative breast cancer | [48] |
Fig. 8.
Eighty-three-year-old woman with primary ER-positive/HER2-negative invasive ductal breast carcinoma. a89Zr-trastuzumab maximum intensity projection demonstrates several foci of 89Zr-trastuzumab avidity that localize to osseous structures. b Axial CT and 89Zr-trastuzumab PET/CT demonstrate 89Zr-trastuzumab avidity in proximal left femur. Reprinted with permission from ref. [49]. Copyright 2016 Society of Nuclear Medicine
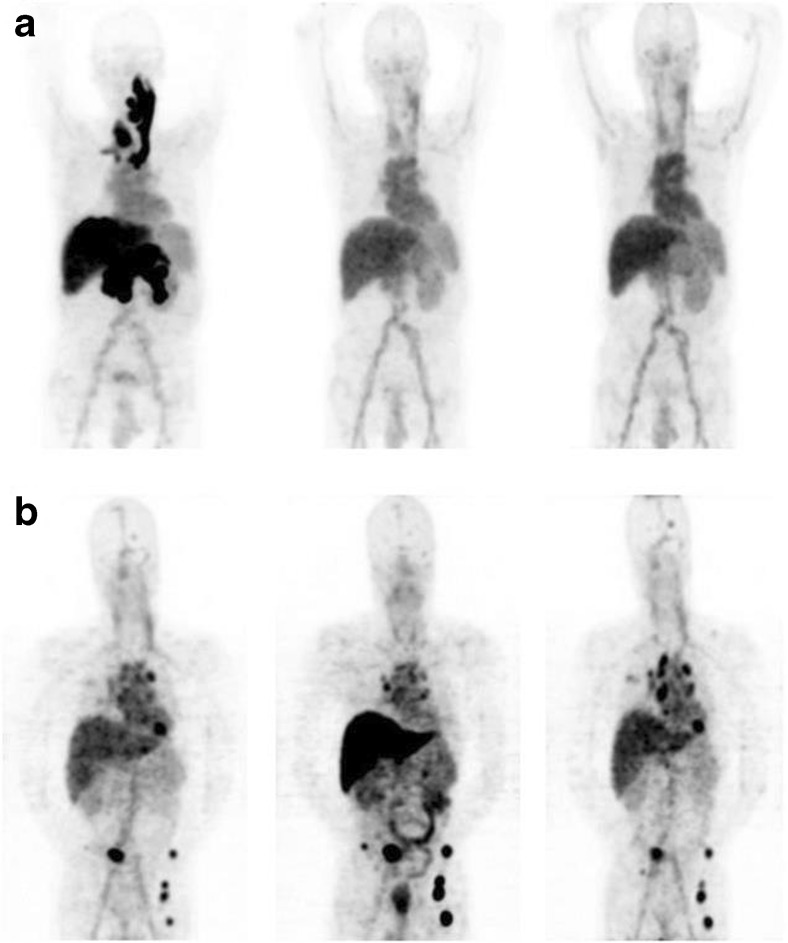
Studies targeting vascular endothelial growth factor A (VEGF-A) have also been conducted using 89Zr-labeled mAbs [39, 40, 52–54]. VEGF-A is overexpressed in malignant breast tumors and ductal carcinoma in situ and is known to be associated with various diseases. Bevacizumab has been reported as a monoclonal antibody that targets VEGF-A, and it has been successfully utilized in several studies. In particular, 89Zr-bevacizumab PET has been used for various ailments such as breast cancer, pelvic cancer, renal cell carcinoma, and neuroendocrine tumors to effectively identify the biological properties of the tumor and confirm the effectiveness of treatment (Fig. 9).
Fig. 9.
PET images of heterogeneous 89Zr-bevacizumab accumulation in tumor lesions of metastatic renal cell carcinoma patients. a Serial 89Zr-bevacizumab PET scans of patient with RCC metastases in the pancreas, liver, and thyroid, with associated jugular and portal vein thrombosis at baseline (left) and 2 (middle) and 6 weeks (right) after the start of bevacizumab/IFNα. b Serial 89Zr-bevacizumab PET scans of patient with RCC metastases in the lungs, mediastinal lymph nodes, bone, and brain at baseline (left) and 2 (middle) and 6 weeks (right) after the start of sunitinib—that is, after 2 sunitinib-free weeks. Reprinted with permission from ref. 54. Copyright 2015 Society of Nuclear Medicine
EGFR is also another interesting target antigen. Cetuximab is a widely known agent to target EGFR. Attachment of cetuximab to EGFR prohibits binding of growth factor to the receptor, and the receptor tyrosine kinase activity is prevented. Thus, biological events such as cell growth, proliferation and differentiation, and cellular invasiveness and apoptosis can be slowed or stopped. 89Zr-cetuximab has been used to evaluate patients with advanced colorectal cancer; tumor uptake was investigated via checking the biodistribution of this labeled antibody [55].
Visualization of metastatic prostate cancer is critical to monitoring the treatment of metastatic prostate cancer. HuJ591 was developed for selectively targeting the extracellular domain of prostate-specific membrane antigen (PSMA), which most prostate cancers express. An immuno-PET imaging study in patients with metastatic prostate cancer using 89Zr-huJ591 was performed [56]. In this case, 5 mCi of 89Zr-huJ591 was injected into 10 patients, and its distribution, elimination, and lesion accumulation were examined. In the PET image, 89Zr-huJ591 was found to accumulate in lesions in the bone and soft tissues more effectively than 99mTc-MDP or FDG. In particular, when analyzing images using 89Zr-huJ591, 11 out of 12 lesions were positive, which proved to be superior to corresponding PET scans using FDG that yielded only 9 positive results.
Another study used 89Zr-labeled cmAb U36 to detect head and neck squamous cell carcinoma (HNSCC) tumors in 20 patients. This study suggested that most of primary tumors were identified by 89Zr-immuno-PET, and performance results of 89Zr-immuno-PET for the detection of lymph node metastasis were no different from those of computed tomography (CT) or magnetic resonance imaging (MRI) [37].
There are no important drug targets for cancers such as pancreatic and ovarian carcinoma. However, it was reported that membrane-bound surface glycoprotein mesothelin (MSLN) is overexpressed in pancreatic and ovarian cancer. Thus, the anti-MSLN antibody MMOT0530A was discovered as a potential imaging biomarker [42, 57]. PET studies using 89Zr-MMOT0530A indicated that its tumor uptake in patients with either pancreatic cancer or ovarian cancer could be clearly visualized. IHC studies suggested that MSLN expression levels, determined with IHC scores, were strongly associated with the intensity of tumor uptake of 89Zr-MMOT0530A.
Conclusion
These results suggest that further study of 89Zr will solve current shortcomings and contribute to molecular imaging research using PET, such as 68Ga. In particular, development of new coordinate chemistry for 89Zr labeling has led to wider application of 89Zr in clinical studies. In oncology, 89Zr-immuno-PET techniques have significantly enhanced tumor detection and the efficiency of treatment. Until now, there has been no standard scale for the use of 89Zr. Thus, some image processing steps, including measurements of tumor uptake and data analysis, should be validated and standardized for wider usage. Overall, based on previous studies, it can be expected that 89Zr will be more successfully applied to the diagnosis and treatment of patients via 89Zr-immuno-PET in the future.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07047572).
Conflicts of Interest
Minh Thanh La, Van Hieu Tran, and Hee-Kwon Kim declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with animals or human participants performed by any of the authors.
Informed Consent
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kasbollah A, Eu P, Cowell S, Deb P. Review on production of 89Zr in a medical cyclotron for PET radiopharmaceuticals. J Nucl Med Technol. 2013;41:35–41. doi: 10.2967/jnmt.112.111377. [DOI] [PubMed] [Google Scholar]
- 2.Zweit J, Downey S, Sharma HL. Production of no-carrier-added zirconium-89 for positron emission tomography. Appl Radiat Isot. 1991;42:199–201. doi: 10.1016/0883-2889(91)90074-B. [DOI] [Google Scholar]
- 3.Tang Y, Li S, Yang Y, Chen W, Wei H, Wang G, et al. A simple and convenient method for production of 89Zr with high purity. Appl Radiat Isot. 2016;118:326–330. doi: 10.1016/j.apradiso.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Link JM, Krohn KA, Eary JF, Kishore R, Lewellen TK, Johnson MW, et al. 89Zr for antibody labeling and positron emission tomography. J Labeled Compd Radiopharm. 1986;23:1297–1298. [Google Scholar]
- 5.Dejesus OT, Nickles RJ. Production and purification of 89Zr, a potential PET antibody label. Int J Rad Appl Instrum A. 1990;41:789–790. doi: 10.1016/0883-2889(90)90030-K. [DOI] [Google Scholar]
- 6.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walther M, Gebhardt P, Grosse-Gehling P, Würbach L, Irmler I, Preusche S, et al. Implementation of 89Zr production and in vivo imaging of B-cells in mice with 89Zr-labeled anti-B-cell antibodies by small animal PET/CT. Appl Radiat Isot. 2011;69:852–857. doi: 10.1016/j.apradiso.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Siikanen J, Tran TA, Olsson TG, Strand SE, Sandell A. A solid target system with remote handling of irradiated targets for PET cyclotrons. Appl Radiat Isot. 2014;94:294–301. doi: 10.1016/j.apradiso.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Meijs WE, Herscheid JDM, Haisma HJ, Wijbrandts R, van Langevelde F, Van Leuffen PJ, et al. Production of highly pure no-carrier added 89Zr for the labelling of antibodies with a positron emitter. Appl Radiat Isot. 1994;45:1143–1147. doi: 10.1016/0969-8043(94)90029-9. [DOI] [Google Scholar]
- 10.Queern SL, Aweda TA, Massicano AVF, Clanton NA, El Sayed R, Sader JA, et al. Production of Zr-89 using sputtered yttrium coin targets. Nucl Med Biol. 2017;50:11–16. doi: 10.1016/j.nucmedbio.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Pandey MK, Engelbrecht HP, Byrne JF, Packard AB, DeGrado TR. Production of 89Zr via the 89Y(p,n)89Zr reaction in aqueous solution: effect of solution composition on in-target chemistry. Nucl Med Biol. 2014;41:309–316. doi: 10.1016/j.nucmedbio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Uddin MS, Khandaker MU, Kim KS, Lee YS, Lee MW, Kim GN. Excitation functions of the proton induced nuclear reactions on natural zirconium. Nucl Inst Meth Phys Res B. 2008;266:13–20. doi: 10.1016/j.nimb.2007.10.010. [DOI] [Google Scholar]
- 13.Kandil SA, Spahn I, Scholten B, Saleh ZA, Saad SMM, Coenen HH, et al. Excitation functions of (α,xn) reactions on natRb and natSr from threshold up to 26MeV: possibility of production of 87Y, 88Y and 89Zr. Appl Radiat Isot. 2007;65:561–568. doi: 10.1016/j.apradiso.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Lewis VE, Zieba KJ. A transfer standard for d + t neutron fluence and energy. Nucl Inst Meth. 1980;174:141–144. doi: 10.1016/0029-554X(80)90422-X. [DOI] [Google Scholar]
- 15.Sadeghi M, Enferadi M, Bakhtiari M. Accelerator production of the positron emitter zirconium-89. Ann Nucl Energy. 2012;41:97–103. doi: 10.1016/j.anucene.2011.11.014. [DOI] [Google Scholar]
- 16.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijs WE, Haisma HJ, Van Der Schors R, Wijbrandts R, Van Den Oever K, Klok RP, et al. A facile method for the labeling of proteins with zirconium isotopes. Nucl Med Biol. 1996;23:439–448. doi: 10.1016/0969-8051(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 18.Verel I, Visser GWM, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GAMS. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- 19.Lewis MR, Shively JE. Maleimidocysteineamido-DOTA derivatives: new reagents for radiometal chelate conjugation to antibody sulfhydryl groups undergo pH-dependent cleavage reactions. Bioconjug Chem. 1998;9:72–86. doi: 10.1021/bc970136v. [DOI] [PubMed] [Google Scholar]
- 20.Perk LR, Vosjan MJWD, Visser GWM, Budde M, Jurek P, Kiefer GE, et al. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur J Nucl Med Mol Imaging. 2010;37:250–259. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vosjan MJWD, Perk LR, Visser GWM, Budde M, Jurek P, Kiefer GE, et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc. 2010;5:739. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- 22.Gao F, Ieritano C, Chen KT, Dias GM, Rousseau J, Bénard F, et al. Two bifunctional desferrioxamine chelators for bioorthogonal labeling of biovectors with zirconium-89. Org Biomol Chem. 2018;16:5102–5106. doi: 10.1039/C8OB01434E. [DOI] [PubMed] [Google Scholar]
- 23.White DL, Durbin PW, Jeung N, Raymond KN. Specific sequestering agents for the actinides. 16. Synthesis and initial biological testing of polydentate oxohydroxypyridinecarboxylate ligands. J Med Chem. 1988;31:11–18. doi: 10.1021/jm00396a005. [DOI] [PubMed] [Google Scholar]
- 24.Allott L, Da Pieve C, Meyers J, Spinks T, Ciobota DM, Kramer-Marek G, et al. Evaluation of DFO-HOPO as an octadentate chelator for zirconium-89. Chem Commun. 2017;53:8529–8532. doi: 10.1039/C7CC03572A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patra M, Bauman A, Mari C, Fischer CA, Blacque O, Häussinger D, et al. An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem Commun. 2014;50:11523–11525. doi: 10.1039/C4CC05558F. [DOI] [PubMed] [Google Scholar]
- 26.Vugts DJ, Klaver C, Sewing C, Poot AJ, Adamzek K, Huegli S, et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET. Eur J Nucl Med Mol Imaging. 2017;44:286–295. doi: 10.1007/s00259-016-3499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guérard F, Lee Y-S, Tripier R, Szajek LP, Deschamps JR, Brechbiel MW. Investigation of Zr(iv) and 89Zr(iv) complexation with hydroxamates: progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem Commun. 2013;49:1002–1004. doi: 10.1039/C2CC37549D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseau J, Zhang Z, Wang X, Zhang C, Lau J, Rousseau E, et al. Synthesis and evaluation of bifunctional tetrahydroxamate chelators for labeling antibodies with 89Zr for imaging with positron emission tomography. Bioorganic Med Chem Lett. 2018;28:899–905. doi: 10.1016/j.bmcl.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Zhai C, Summer D, Rangger C, Franssen GM, Laverman P, Haas H, et al. Novel bifunctional cyclic chelator for 89Zr labeling–radiolabeling and targeting properties of RGD conjugates. Mol Pharm. 2015;12:2142–2150. doi: 10.1021/acs.molpharmaceut.5b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams CJ, Wilson JJ, Boros E. Multifunctional desferrichrome analogues as versatile 89Zr(IV) chelators for immunoPET probe development. Mol Pharm. 2017;14:2831–2842. doi: 10.1021/acs.molpharmaceut.7b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Edwards DS. Bifunctional chelators for therapeutic lanthanide radiopharmaceuticals. Bioconjug Chem. 2001;12:7–34. doi: 10.1021/bc000070v. [DOI] [PubMed] [Google Scholar]
- 32.Seibold U, Wängler B, Wängler C. Rational design, development, and stability assessment of a macrocyclic four-hydroxamate-bearing bifunctional chelating agent for 89Zr. Chem Med Chem. 2017;12:1555–1571. doi: 10.1002/cmdc.201700377. [DOI] [PubMed] [Google Scholar]
- 33.Deri MA, Ponnala S, Zeglis BM, Pohl G, Dannenberg JJ, Lewis JS, et al. Alternative chelator for 89Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO) J Med Chem. 2014;57:4849–4860. doi: 10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatt NB, Pandya DN, Xu J, Tatum D, Magda D, Wadas TJ. Evaluation of macrocyclic hydroxyisophthalamide ligands as chelators for zirconium-89. PLoS One. 2017;12:e0178767–e0178e77. doi: 10.1371/journal.pone.0178767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandya DN, Bhatt N, Yuan H, Day CS, Ehrmann BM, Wright M, et al. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem Sci. 2017;8:2309–2314. doi: 10.1039/C6SC04128K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Börjesson PKE, Jauw YWS, Boellaard R, de Bree R, Comans EFI, Roos JC, et al. Performance of immuno–positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin Cancer Res. 2006;12:2133–2140. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- 38.Rizvi SN, Visser OJ, Vosjan MJ, van Lingen A, Hoekstra OS, Zijlstra JM, et al. Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur J Nucl Med Mol Imaging. 2012;39:512–520. doi: 10.1007/s00259-011-2008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaykema SBM, Brouwers AH, Lub-de Hooge MN, Pleijhuis RG, Timmer-Bosscha H, Pot L, et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J Nucl Med. 2013;54:1014–1018. doi: 10.2967/jnumed.112.117218. [DOI] [PubMed] [Google Scholar]
- 40.van Asselt SJ, Oosting SF, Brouwers AH, Bongaerts AHH, de Jong JR, Lub-de Hooge MN, et al. Everolimus reduces 89Zr-bevacizumab tumor uptake in patients with neuroendocrine tumors. J Nucl Med. 2014;55:1087–1092. doi: 10.2967/jnumed.113.129056. [DOI] [PubMed] [Google Scholar]
- 41.den Hollander MW, Bensch F, Glaudemans AW, Oude Munnink TH, Enting RH, den Dunnen WF, et al. TGF-β antibody uptake in recurrent high-grade glioma imaged with 89Zr-fresolimumab PET. J Nucl Med. 2015;56:1310–1314. doi: 10.2967/jnumed.115.154401. [DOI] [PubMed] [Google Scholar]
- 42.Lamberts LE, Menke-van der Houven van Oordt CW, ter Weele EJ, Bensch F, Smeenk MM, Voortman J, et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody–drug conjugate treatment. Clin Cancer Res. 2016;22:1642–1652. doi: 10.1158/1078-0432.CCR-15-1272. [DOI] [PubMed] [Google Scholar]
- 43.van Loon J, Even AJG, Aerts HJWL, Öllers M, Hoebers F, van Elmpt W, et al. PET imaging of zirconium-89 labelled cetuximab: a phase I trial in patients with head and neck and lung cancer. Radiother Oncol. 2017;122:267–273. doi: 10.1016/j.radonc.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Jauw YWS, Zijlstra JM, de Jong D, Vugts DJ, Zweegman S, Hoekstra OS, et al. Performance of 89Zr-labeled-rituximab-PET as an imaging biomarker to assess CD20 targeting: a pilot study in patients with relapsed/refractory diffuse large B cell lymphoma. PLoS One. 2017;12:e0169828. doi: 10.1371/journal.pone.0169828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensch F, Lamberts LE, Smeenk MM, Jorritsma-Smit A, Lub-de Hooge MN, Terwisscha van Scheltinga AGT, et al. 89Zr lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin Cancer Res. 2017;23:6128–6137. doi: 10.1158/1078-0432.CCR-17-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Es SC, Brouwers AH, Mahesh SVK, Leliveld-Kors AM, de Jong IJ, Lub-de Hooge MN, et al. 89Zr-bevacizumab PET: potential early read out for efficacy of everolimus in metastatic renal cell carcinoma patients. J Nucl Med. 2017;58:905–910. doi: 10.2967/jnumed.116.183475. [DOI] [PubMed] [Google Scholar]
- 47.Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, et al. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45:2300–2306. doi: 10.1007/s00259-018-4099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–1858. doi: 10.1038/s41591-018-0255-8. [DOI] [PubMed] [Google Scholar]
- 49.Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nucl Med. 2016;57:1523–1528. doi: 10.2967/jnumed.115.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database of Syst Rev. 2012. [DOI] [PMC free article] [PubMed]
- 51.Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. doi: 10.1186/s12885-016-2092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaykema SBM, Schröder CP, Vitfell-Rasmussen J, Chua S, Oude Munnink TH, Brouwers AH, et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014;20:3945–3954. doi: 10.1158/1078-0432.CCR-14-0491. [DOI] [PubMed] [Google Scholar]
- 53.Bahce I, Huisman MC, Verwer EE, Ooijevaar R, Boutkourt F, Vugts DJ, et al. Pilot study of 89Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res. 2014;4:35. doi: 10.1186/s13550-014-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oosting SF, Brouwers AH, van Es SC, Nagengast WB, Oude Munnink TH, Lub-de Hooge MN, et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J Nucl Med. 2015;56:63–69. doi: 10.2967/jnumed.114.144840. [DOI] [PubMed] [Google Scholar]
- 55.Oordt CWM-vH, Gootjes EC, Huisman MC, Vugts DJ, Roth C, Luik AM, et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget. 2015;6:30384–30393. doi: 10.18632/oncotarget.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandit-Taskar N, O'Donoghue JA, Beylergil V, Lyashchenko S, Ruan S, Solomon SB, et al. 89Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:2093–2105. doi: 10.1007/s00259-014-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ter Weele EJ, Terwisscha van Scheltinga AGT, Kosterink JGW, Pot L, Vedelaar SR, Lamberts LE, et al. Imaging the distribution of an antibody-drug conjugate constituent targeting mesothelin with 89Zr and IRDye 800CW in mice bearing human pancreatic tumor xenografts. Oncotarget. 2015;6:42081–42090. doi: 10.18632/oncotarget.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]