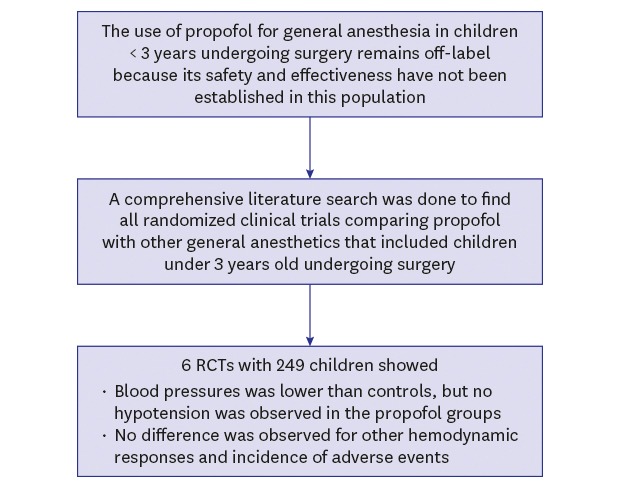
Abstract
Background
Despite well-known advantages, propofol remains off-label in many countries for general anesthesia in children under 3 years of age due to insufficient evidence regarding its use in this population. This study aimed to evaluate the efficacy and safety of propofol compared with other general anesthetics in children under 3 years of age undergoing surgery through a systematic review and meta-analysis of existing randomized clinical trials.
Methods
A comprehensive literature search was conducted of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials to find all randomized clinical trials comparing propofol with another general anesthetic that included children under 3 years of age. The relative risk or arcsine-transformed risk difference for dichotomous outcomes and the weighted or standardized mean difference for continuous outcomes were estimated using a random-effects model.
Results
A total of 249 young children from 6 publications were included. The children who received propofol had statistically significantly lower systolic and diastolic blood pressures, but hypotension was not observed in the propofol groups. The heart rate, stroke volume index, and cardiac index were not significantly different between the propofol and control groups. The propofol groups showed slightly shorter recovery times and a lower incidence of emergence agitation than the control groups, while no difference was observed for the incidence of hypotension, desaturation, and apnea.
Conclusion
This systematic review and meta-analysis indicates that propofol use for general anesthesia in young healthy children undergoing surgery does not increase complications and that propofol could be at least comparable to other anesthetic agents.
Keywords: Propofol, General Anesthesia, Infant, Child, Systematic Review
Graphical Abstract
INTRODUCTION
Pediatric surgery commonly requires general anesthesia, and propofol is one of the most widely used intravenous anesthetics. Propofol is known to have many advantages over other anesthetic agents, including rapid induction of anesthesia, early recovery, and fewer complications such as postoperative nausea and vomiting. A review of propofol use in pediatric anesthesia and sedation described the pharmacokinetics of propofol and comprehensively discussed its benefits and related concerns.1
Propofol is listed in the World Health Organization Model List of Essential Medicines for Children, a list of medicines that satisfy the most important needs in pediatric health care,2 and its regulatory approval status varies somewhat among countries. For example, 1% propofol is licensed for induction and maintenance of anesthesia in all children older than 1 month in the UK3 and European Union member countries including Germany4 and Italy.5 However, the use of propofol for induction of anesthesia is approved only in children ≥ 3 years of age and for maintenance of anesthesia in children ≥ 2 months of age in the United States,1 and the use of propofol for general anesthesia in certain age groups remains off-label in some Asian countries, including Japan and Korea,6,7 because its safety and effectiveness have not been established in this population.
Since conducting clinical trials in young children has several challenges,8 large well-designed studies are lacking, and establishing firm evidence for young patients is difficult. For this reason, many drugs administered to children during anesthesia are not approved for pediatric use, and the off-label use of these drugs is an accepted practice.9
A systematic review on propofol use in neonates was published in 2011,10 and it concluded that no practice recommendation could be made since only 1 study, which assessed propofol for nonemergency neonatal endotracheal intubation, was included. A few small trials, with sample sizes of less than 60, have evaluated the use of propofol for general anesthesia in children younger than 3 years old.11,12 Considering the difficulty of conducting trials in such young children, an overview of propofol use in pediatric anesthesia based on the existing literature will be beneficial. This study aimed to systematically identify trials that compared propofol used for general anesthesia with other general anesthetics in children under 3 years old undergoing surgery and to suggest the current best available evidence based on all existing studies.
METHODS
Search strategy
A comprehensive literature search of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials was conducted from inception to February 12, 2018. A predefined search strategy was used for each database without language restriction. The main key words used for the search were “propofol,” “infant,” “child,” “anesthesia,” and “randomized controlled trial.” The search strategy used for MEDLINE is available in Supplementary Table 1. The reference lists of the included articles and review papers were scanned to identify additional eligible studies.
Inclusion and exclusion criteria
All parallel randomized clinical trials (RCTs) of propofol versus any other anesthetic agent for general anesthesia in pediatric surgery that enrolled children under 3 years old were considered eligible for inclusion. Articles with no relevant outcomes were excluded. The outcomes of interest were achievement of adequate intubation conditions, hemodynamic responses (systolic blood pressure [SBP], diastolic blood pressure [DBP], heart rate [HR], stroke volume index [SVI], cardiac output [CO], and cardiac index) after induction of anesthesia, adverse events (hypotension, desaturation, apnea, postoperative nausea and vomiting, and emergence agitation), and recovery times (time to extubation, eye-opening, and emergence). Publications with only an abstract were excluded.
Study selection and data extraction
The titles and abstracts of the references identified by the search strategy were screened, and clearly irrelevant references were excluded. Full texts of the remaining articles were obtained and assessed for eligibility, based on the defined inclusion criteria. The following information from the original papers was extracted using a standardized data collection form: number; age range; and American Society of Anesthesiologists (ASA) score of participants; type of surgery; premedication; agents used to induce and maintain anesthesia in each group; publication year; country; and the pre-defined outcomes. For hemodynamic responses after induction, the lowest values of the reported means were extracted.
Quality assessment of included studies
The risk of bias of the included studies was assessed for the 5 domains of adequacy of random sequence generation, allocation sequence concealment, blinding of outcome assessment, completeness of data, and possible selective reporting using the Cochrane Collaboration's tool for assessing risk of bias.13 For the first 4 domains, we classified studies as ‘adequate’ if they had a low risk of bias, ‘inadequate’ if they had a high risk of bias, or ‘unclear’ if they had insufficient information to determine the risk. We explored possible selective reporting of study outcomes by comparing the outcomes described in the Methods section with those provided in the Results section in each article.
Statistical analysis and synthesis
All analyses were performed by the usage of propofol in general anesthesia, as an induction agent or a maintenance agent of anesthesia. For dichotomous outcomes, the relative risk (RR) with 95% confidence intervals (CIs) was used to summarize treatment effects. An arcsine-transformed difference (AD) was used if no events were observed in any trials.14 For continuous outcomes, the weighted mean difference (WMD) was used when the outcome measurements in all studies were made using the same definition and scales. When discrepancies in measurement units of the outcome were present, the standardized mean difference (SMD) was used. For studies with 2 control groups, the results of the propofol group were split into 2 groups with smaller sample sizes and included as 2 comparisons.13
A random-effects model was employed to obtain pooled estimates, and the results were illustrated using forest plots. Heterogeneity across individual studies was assessed using the χ2 test for the Cochrane Q statistic and I2.15 An I2 value > 60% or a χ2 P value < 0.1 was considered to indicate substantial statistical heterogeneity between studies. Visual inspection of funnel plots was conducted to assess potential publication bias. Where meta-analysis was not possible, a summary with descriptive statistics is presented. All analyses were done in R version 3.4.0 using the metafor package.16
RESULTS
Description of included studies
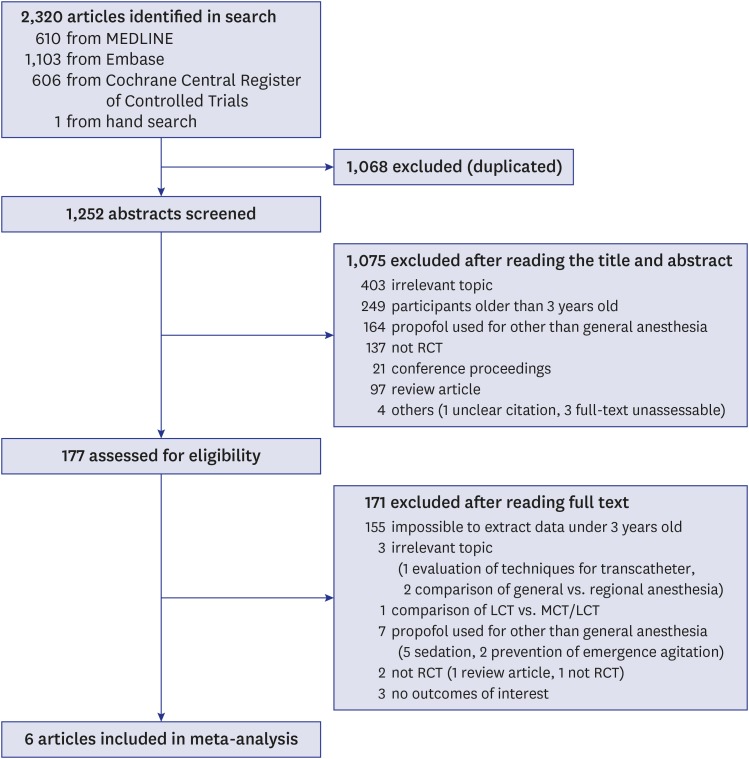
The initial search strategy yielded 1,252 potentially relevant articles. After screening the titles and abstracts, 1,075 records were excluded, and the remaining 177 articles were retrieved for the final determination of eligibility. After we reviewed the full texts of 177 potentially relevant articles and reference lists, 6 articles11,12,17,18,19,20 were included in the analysis (Fig. 1).
Fig. 1. Flow diagram of the literature search.
RCT = randomized clinical trial, LCT = long-chain triglyceride, MCT = medium-chain triglyceride.
The baseline characteristics of the 6 publications are summarized in Table 1. A total of 249 young children who underwent general anesthesia were included, and the participants' average age ranged from 4.3 months to 14.7 months.
Table 1. Characteristics of the included randomized controlled trials comparing propofol with other general anesthesia.
| Source | No. of patients | Anesthesia induction | Anesthesia maintenance | Premedication | ASA class | Type of surgery | Participant's age | Country | |
|---|---|---|---|---|---|---|---|---|---|
| Inclusion criteria | Mean ± standard deviation (range) or Mean [interquartile range] in months | ||||||||
| Aun et al.17 | 9 | Propofol 2.5 mg/kg | Halothane 0.5% + N2O 70% | Oral diazepam syrup 0.4 mg/kg + EMLA cream | I–II | Elective surgery | 8 months to under 2 years | 14.4 (8.4–24) | Hong Kong |
| 9 | Thiopentone 5 mg/kg | 13.2 (8.4–24) | |||||||
| Schrum et al.11 | 20 | Propofol 3 mg/kg | Halothane 1%–3% + N2O 60% | No | Healthy full-term infants | Elective inguinal hernia repair surgery | 1 to 12 months | 7.0 ± 3.8 | USA |
| 20 | Thiopental 5 mg/kg | 7.4 ± 4.1 | |||||||
| 19 | Halothane 2% | 6.8 ± 3.7 | |||||||
| Wodey et al.18 | 10 | Propofol 2 mg/kg | No details provided | Midazolam 0.3 mg/kg rectally | I–II | Elective surgery | 1 to 12 months | 6.2 (1–11) | France |
| 10 | Thiopentone 5 mg/kg | 4.8 (1–8) | |||||||
| Cohen et al.19 | 28 | Sevoflurane 8% + N2O 60% | Propofol-EDTA 12 mg/kg/h + N2O 60% | No | I–II | Elective ambulatory surgery | < 3 years | 14.7 ± 9.2 (2–33) | USA |
| 28 | Sevoflurane 1.5%–2.5% + N2O 60% | 13.2 ± 9.2 (1.5–35) | |||||||
| Steinmetz et al.20 | 17 | Sevoflurane 8% + N2O 70% | Propofol 7 mg/kg/h + remifentanil 0.8 µg/kg/min | No | I–II | Elective cleft lip and palate surgery | 4 to 6 months | 4.3 [4.1–4.5] | Denmark |
| 22 | Sevoflurane 2%–3% | 4.3 [4.1–4.9] | |||||||
| Cheng et al.12 | 28 | Sufentanil 1 µg/kg | Propofol 6–8 mg/kg/h + sufentanil 2 µg/kg/h | Midazolam 0.2 mg/kg for patients > 6 months | II–IV | Elective complex congenital cardiac surgery requiring cardiopulmonary bypass | 1 month to under 3 years | 6.3 ± 1.5 | China |
| 29 | Dexmedetomidine 0.5–0.7 µg/kg/h + sufentanil 2 µg/kg/h | 6.6 ± 2.1 | |||||||
Propofol (2–3 mg/kg) was compared to thiopentone (5 mg/kg) and halothane (2%) as a sole agent for induction of anesthesia in 3 publications.11,17,18 For maintenance of anesthesia, the combined use of propofol with ethylenediaminetetraacetic acid (EDTA) (12 mg/kg/h) and N2O was compared to the combination of sevoflurane (1.5%–2.5%) and N2O,19 the combination of propofol (7 mg/kg/h) and remifentanil (0.8 μg/kg/min) was compared to sevoflurane (2%–3%),20 and the combined use of propofol (6–8 mg/kg/h) and sufentanil (2 μg/kg/h) was compared to the combination of dexmedetomidine (0.5–0.7 μg/kg/h) and sufentanil (2 μg/kg/h).12
The surgery types were elective cardiac procedures requiring cardiopulmonary bypass in 1 study,12 elective cleft lip and palate operations in 1 study,20 elective inguinal hernia repair procedures in 1 study,11 elective ambulatory procedures including repair of bilateral inguinal hernias, orchiopexy, hypospadias, and cleft lip or palate; adenoidectomy; and strabismus surgery in 1 study19 and other elective procedures in 2 studies.17,18
Risk of bias in the included studies
The results of the assessment of risk of bias are provided in Supplementary Table 2.
In 3 of the 6 included articles (50%), patients were randomized by computer-generated numbers, while the others did not give any information on randomization. Two articles (33.3%) maintained allocation concealment in a double-blind manner or by providing no advance knowledge of the allocation sequence. The remaining 4 publications did not provide any description of allocation concealment. The outcome assessors were blind to the treatments in 5 articles (83.3%), and 1 publication did not report whether treatment blinding was used. Completeness of data, which was assessed as adequate if statistical analysis was performed on an intention-to-treat basis or if the percentage of follow-up loss was less than 10%, was achieved in 5 studies (83.3%), and 1 study was classified as ‘unclear’ since the study enrolled children up to 12 years old and did not describe the age of the excluded cases. No instances of possible selective reporting of outcomes were observed.
Hemodynamic responses
Hemodynamic responses were presented in all publications.
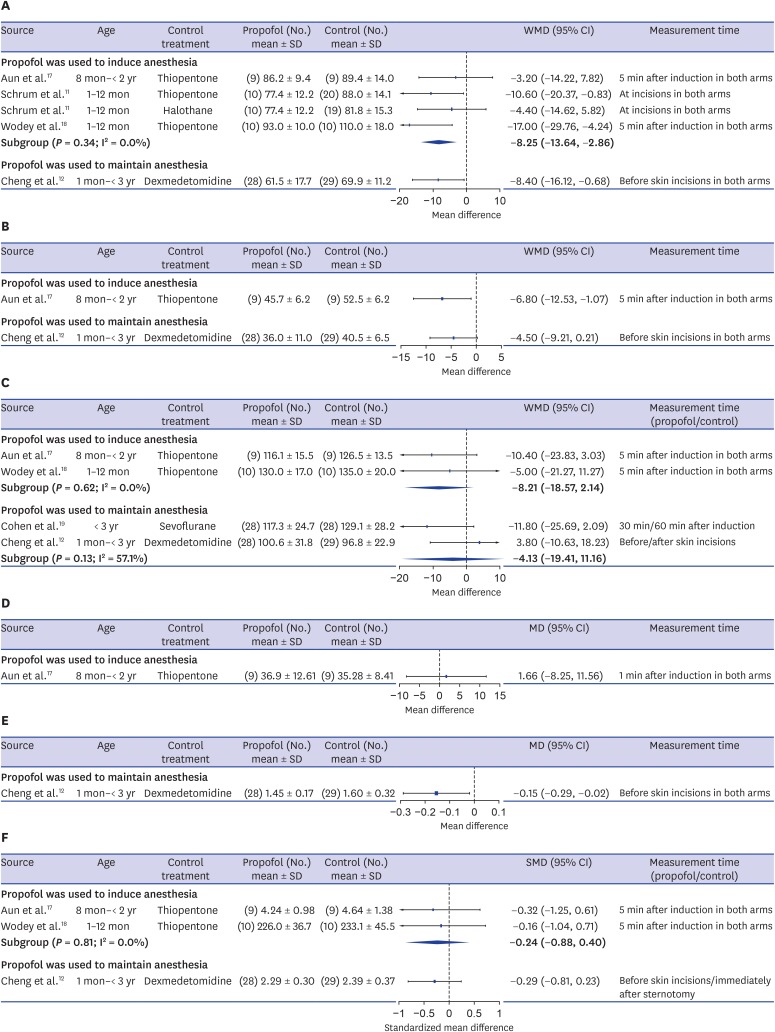
SBP was described in 4 publications.11,12,17,18 In the 3 studies11,17,18 where propofol was administered for anesthesia induction, the participants were ASA I–II infants or toddlers younger than 2 years old who underwent elective inguinal hernia repair or other elective procedures. Propofol (2–3 mg/kg) was compared to thiopentone (5 mg/kg) or halothane (2%) as a sole agent. In the study that used propofol to maintain anesthesia, the patients were ASA II–IV children under 3 years who underwent complex congenital cardiac surgery requiring cardiopulmonary bypass, and the combined use of propofol (6–8 mg/kg/h) and sufentanil (2 μg/kg/h) was compared to the combination of dexmedetomidine (0.5–0.7 μg/kg/h) and sufentanil (2 μg/kg/h).12 A consistent tendency was found for SBP to be lower in the propofol group than in the thiopentone or halothane groups when propofol was used to induce anesthesia (WMD, −8.25 mmHg; 95% CI, −13.64 to −2.86; I2 = 0.0%), and a significantly lower SBP was observed in the propofol-sufentanil group than in the dexmedetomidine-sufentanil group when propofol was used to maintain anesthesia (mean difference [MD], −8.40 mmHg; 95% CI, −16.12 to −0.68) (Fig. 2A).
Fig. 2. Forest plots of hemodynamic responses. (A) Minimum systolic blood pressure. (B) Minimum diastolic blood pressure. (C) Minimum heart rate. (D) Minimum stroke volume index. (E) Minimum cardiac output. (F) Minimum cardiac index.
SD = standard deviation, WMD = weighted mean difference, CI = confidence interval, MD = mean difference, SMD = standardized mean difference.
Two studies reported DBP.12,17 Toddlers who received propofol as an induction agent had significantly lower DBP than those administered thiopentone (MD, −6.80 mmHg; 95% CI, −12.53 to −1.07),17 and toddlers who received propofol-sufentanil as maintenance agents had lower DBP than those who received dexmedetomidine-sufentanil (MD, −4.50 mmHg; 95% CI, −9.07 to 0.21)12 (Fig. 2B).
Information about HR was presented in 5 trials, 2 of which17,18 compared propofol as an induction agents and 3 of which12,19,20 evaluated the combined use of propofol with other agents as maintenance agents. However, 1 article20 was excluded in the pooling of results since it described the median and interquartile range of the lowest HR during surgery. In the study that reported HR but did not report either SBP or DBP,19 ASA I–II children under 3 years old underwent elective ambulatory surgery and the combined use of propofol-EDTA (12 mg/kg/h) and N2O was compared to the combination of sevoflurane (1.5%–2.5%) and N2O. When propofol was used to induce anesthesia, a non-significantly lower HR was shown in the propofol group than in the thiopentone group in both studies (WMD, −8.21 beats/min; 95% CI, −18.57 to 2.14; I2 = 0.0%). When propofol was used with other agents to maintain anesthesia, children who received propofol did not show a significant difference from those who received sevoflurane or dexmedetomidine (WMD, −4.13 beats/min; 95% CI, −19.41 to 11.16; I2 = 57.1%). The propofol-N2O group showed a somewhat lower HR than the sevoflurane-N2O group in elective ambulatory surgery (MD, −11.80 beats/min; 95% CI, −25.69 to 2.09); however, the propofol-sufentanil group showed a slightly higher HR than the dexmedetomidine-sufentanil group in complex congenital cardiac surgery (MD, 3.80 beats/min; 95% CI, −10.63 to 18.23) (Fig. 2C). In the publication that presented the median and interquartile range (IQR) of the HR during surgery,20 the combined use of propofol with remifentanil was compared to sevoflurane for anesthesia maintenance in ASA I to II infants undergoing cleft lip and palate repair surgery. The propofol-remifentanil group showed a lower HR than the sevoflurane group (propofol group: median, 102 beats/min; IQR, 94–105; sevoflurane group: median, 118 beats/min; IQR, 112–125) and the difference between the 2 median values was similar to the treatment effect in the study that compared propofol-N2O to sevoflurane-N2O as maintenance agents.19
The SVI was reported in only 1 study,17 which compared propofol to thiopentone as a sole agent to induce anesthesia in toddlers who underwent elective surgery. There was no significant difference in the SVI between the 2 groups (MD, 1.66 mL/beat/m2; 95% CI, −8.25 to 11.56) (Fig. 2D).
One trial,12 which compared propofol-sufentanil to dexmedetomidine-sufentanil for maintaining anesthesia in children undergoing complex congenital cardiac surgery, presented both CO and cardiac index, and 2 other studies,17,18 which compared propofol to thiopentone as an induction agent in infants and toddlers undergoing elective surgery, described cardiac index. The CO was significantly lower in children who received propofol than in those who received dexmedetomidine (MD, −0.15 L/min; 95% CI, −0.29 to −0.02) (Fig. 2E).
The cardiac index was measured in units of L/min/m2 in 2 studies12,17 and mL/kg/min in 1 trial.18 There was a nonsignificant cardiac index decrease in the propofol group compared to the thiopentone group when propofol was used as an induction agent (SMD, −0.24; 95% CI, −0.88 to 0.40; I2 = 0.0%) and a similar treatment effect to that of dexmedetomidine was shown when propofol was used to maintain anesthesia (SMD, −0.29; 95% CI, −0.81 to 0.23) (Fig. 2F).
Recovery times
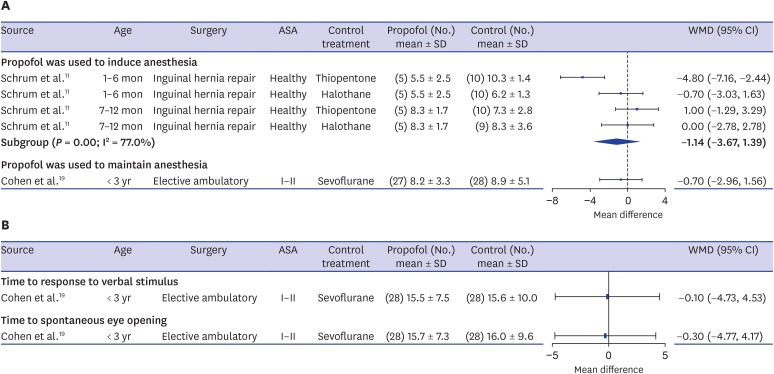
Time to extubation was reported in 3 articles, including 1 study where propofol was used to induce anesthesia11 and 2 studies where propofol was evaluated for maintaining anesthesia.19,20 However, 1 study that presented the median and IQR20 was excluded in the pooling of results. In the remaining 2 studies, propofol was used as a sole induction agent in infants undergoing inguinal hernia repair surgery and the combined use of propofol-EDTA and N2O to maintain anesthesia was evaluated in children under 3 years undergoing elective ambulatory surgery respectively. Although time to extubation was defined slightly differently, both were judged to correspond to the time from discontinuation of anesthetic agents to awake extubation.
When propofol was used to induce anesthesia, the treatment effect varied somewhat according to the patients' age. In infants under 6 months old, the propofol group showed a significantly shorter extubation time than the thiopentone group (MD, −4.80 minutes; 95% CI, −7.16 to −2.44) and a slightly shorter extubation time than the halothane group (MD, −0.70 minutes; 95% CI, −3.03 to 1.63). However, no differences were shown among the 3 groups in infants aged 7 to 12 months (thiopentone: MD, 1.0 minutes; 95% CI, −1.29 to 3.29; halothane: MD, 0.0 minutes; 95% CI, −2.78 to 2.78). When propofol was used to maintain anesthesia, the propofol-N2O group showed a slightly, but not significantly, shorter extubation time than those who received sevoflurane-N2O (MD, −0.70 minutes; 95% CI, −2.96 to 1.56) (Fig. 3A). Meanwhile, the study that summarized median values20 evaluated the combined use of propofol with remifentanil compared to sevoflurane for maintaining anesthesia in 4- to 6-month-old infants undergoing cleft lip and palate repair surgery and showed a non-significantly shorter extubation time in the propofol-remifentanil group (propofol-remifentanil group: median, 10 minutes; IQR, 7–25; sevoflurane group: median, 15 minutes; IQR, 10–18).
Fig. 3. Forest plots of recovery times. (A) Time to extubation (min). (B) Time to emergence/eye opening (min).
SD = standard deviation, WMD = weighted mean difference, CI = confidence interval.
One study19 also reported both the time to response to verbal stimulus and the time to spontaneous eye-opening. Children who received propofol with N2O showed slightly, but not significantly, shorter times to emergence and eye-opening than those who received sevoflurane and N2O (Fig. 3B).
Adverse events
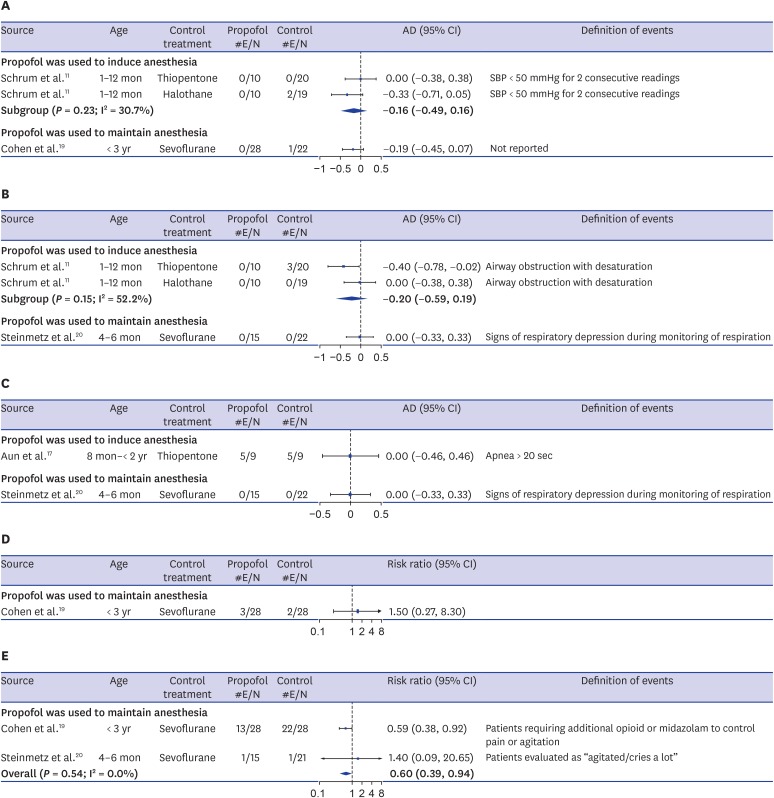
Descriptions regarding hypotension were provided by 2 articles.11,19 Hypotension was defined as SBP less than 50 mm Hg for 2 consecutive readings in 1 study,11 but no specific definition was provided in other study.19 The incidence of hypotension was not significantly different between groups for both usages of propofol (induction and maintenance of anesthesia) (induction: AD, −0.16; 95% CI, −0.49 to 0.16; maintenance: AD, −0.19; 95% CI, −0.45 to 0.07). When propofol was compared to thiopentone and halothane as an induction agent in healthy infants, 2 infants in the halothane group experienced intraoperative hypotension, while none did so in either the propofol or thiopentone group.11 When propofol-N2O was compared to sevoflurane-N2O for anesthesia maintenance in ASA I-II toddlers, 1 child treated with sevoflurane experienced hypotension (Fig. 4A).19
Fig. 4. Forest plots of adverse events. (A) Hypotension. (B) Desaturation. (C) Apnea. (D) Postoperative vomiting. (E) Emergence agitation.
#E/N= number of patients with events/number of total patients, AD = arcsine-transformed difference, CI = confidence interval, SBP = systolic blood pressure.
Desaturation was described by 2 publications11,20 and a similar effect was observed for both usages of propofol (induction: AD, −0.20; 95% CI, −0.59 to 0.19; maintenance: AD, −0.12; 95% CI, −0.38 to 0.13). In the study that used propofol as an induction agent, no incidence of desaturation was observed in either the propofol and halothane groups, while 3 out of 20 (15%) infants who received thiopentone experienced post-extubation airway obstruction with desaturation.11 In the study that used propofol to maintain anesthesia in infants undergoing cleft lip and palate repair surgery, none showed any signs of respiratory depression in either the propofol or sevoflurane groups (Fig. 4B).20 Additionally, the study19 that compared propofol-N2O to sevoflurane-N2O for anesthesia maintenance in children undergoing elective ambulatory surgery stated that oxygen saturation was similar in both groups, but did not present any descriptive statistics.
The incidence of apnea was described in 2 studies,17,20 and no difference was shown when propofol was used for either induction or maintenance of anesthesia (induction: AD, 0.0; 95% CI, −0.46 to 0.46; maintenance: AD, 0.0; 95% CI, −0.33 to 0.33). When propofol was compared to thiopentone as an induction agent in toddlers undergoing elective surgery, 56% of the participants in each group experienced apnea for over 20 seconds at induction. However, no patients in either group showed any signs of respiratory depression when propofol-remifentanil was compared to sevoflurane for anesthesia maintenance in infants undergoing cleft lip and palate repair surgery (Fig. 4C).
Postoperative nausea and vomiting (PONV) was described in only 1 publication.19 When propofol-N2O was compared to sevoflurane-N2O for anesthesia maintenance in ASA I–II toddlers undergoing elective ambulatory surgery, 10.7% of the propofol-N2O group and 7.1% of the sevoflurane-N2O group experienced PONV (RR, 1.50; 95% CI, 0.27 to 8.30) (Fig. 4D).
Two studies in which propofol was used to maintain anesthesia19,20 presented data on emergence agitation during recovery. One study19 compared propofol-N2O to sevoflurane-N2O in toddlers and reported the number of patients requiring additional opioid or midazolam treatment to control pain or agitation during recovery. Another study20 evaluated propofol-remifentanil compared to sevoflurane in 4- to 6-month-old infants. They presented the frequencies of participants classified into 4 levels of agitation (“calm,” “active/restless,” “cries a little,” and “agitated/cries a lot”), and we considered the infants classified as “agitated/cries a lot” as patients who experienced emergence agitation. In a comparison of propofol with sevoflurane in toddlers, 46.4% of the propofol group received additional opioid or midazolam treatment to control pain or agitation, in contrast to 78.6% of the sevoflurane group. However, 6.7% of the propofol group and 4.8% of the sevoflurane group were evaluated as “agitated/cries a lot” in 4- to 6-month-old infants, and the low incidence of emergence agitation in both groups was due to administration of fentanyl before and after extubation. A meta-analysis of 2 studies found that propofol significantly reduced the incidence of emergence agitation compared to sevoflurane (RR, 0.60; 95% CI, 0.39 to 0.94; I2 = 0.0%) (Fig. 4E).
Publication bias
Although assessing funnel plot asymmetry is not appropriate with a small number of included publications, we explored funnel plot asymmetry for minimum SBP and HR after induction (i.e., the outcomes that were reported in the most studies) and the funnel plots were not suggested to be asymmetrical (Supplementary Fig. 1).
DISCUSSION
We conducted a systematic review and meta-analysis to evaluate propofol use for general anesthesia in children under 3 years old undergoing surgery. This meta-analysis included 6 RCTs incorporating 249 children. Most of the included studies were performed with ASA I–II children undergoing elective surgery, except for 1 study that was conducted among ASA II–IV children undergoing complex congenital cardiac surgery. Propofol was compared to thiopentone or halothane as an induction agent, and the combined use of propofol with other agents was compared to sevoflurane or dexmedetomidine for anesthesia maintenance.
Significantly lower SBP and DBP were reported when propofol was used either to induce or to maintain anesthesia. However, no incidence of hypotension was reported in the propofol groups, whereas some children who received halothane to induce anesthesia11 and sevoflurane to maintain anesthesia experienced hypotension19 in the included studies. HR was not significantly lower in the propofol groups when propofol was used either to induce or to maintain anesthesia. The SVI and cardiac index did not differ between the propofol and thiopentone groups when propofol was used as a sole induction agent in healthy toddlers. Meanwhile, when propofol was used as a maintenance agent in cardiac operations, the cardiac index in the propofol group was insignificantly lower than that in dexmedetomidine group and significantly lower CO was observed in the propofol group.
Rapid recovery is a well-known advantage of propofol.1 Our review found that the propofol groups showed slightly shorter times to extubation, eye-opening, and emergence than the thiopentone, halothane, and sevoflurane groups, and this effect was particularly pronounced when propofol was compared with thiopentone for anesthesia induction in healthy infants aged 1–6 months.
The incidence of adverse events, including hypotension, desaturation, apnea, and postoperative vomiting, did not significantly differ between propofol and comparator groups when propofol was used either to induce or to maintain anesthesia, while definitions of those adverse events were slightly different among studies or not clearly specified in some studies. With respect to the incidence of emergence agitation, the propofol groups showed significantly lower incidence compared to sevoflurane groups.
No studies reported intubation conditions, but 3 trials21,22,23 that compared propofol to thiopentone, halothane, or sevoflurane in children ≤ 3 years old presented assessments of the intubation conditions. When we pooled their results, achievement of an adequate intubation condition was significantly less common in the groups that received propofol (RR, 0.62; 95% CI, 0.48–0.81), although those trials did not satisfy our inclusion criteria.
A retrospective study,24 which compared propofol anesthesia by target-controlled infusion to volatile anesthesia in children below 3 years old, found no difference between the 2 groups in all vital signs and incidence of adverse events after surgery. In a RCT, which compared methods for intubating laryngeal airway in children less than 9 years old and used propofol to induce anesthesia in both arms, incidence of adverse events including desaturation and vomiting was comparable to the results of included studies in our systematic review.25 Meanwhile, the only trial26 included in the systematic review on propofol use in neonates undergoing sedation or anaesthesia for procedures10 found that the number of infants who required multiple intubation attempts was lower in propofol group (39% in the propofol group vs. 57% in the morphine-atropinesuxamethonium group) and the incidence of clinically significant side effects was not different between groups. When propofol sedation using target control infusion was compared between children under 3 years and older than 3 years, either the incidence of adverse events or the time-to-recovery was not significantly different between those age groups.27 These results also support our findings that propofol could be at least comparable to other anesthetic agents.
This review has several limitations to be taken into account when interpreting the results. First, there was substantial clinical heterogeneity in the studies' characteristics, including the participants' age, type of surgery, the anesthetic agents that were compared, premedication, and other anesthetic adjuvants. Exploration of the effects of potential factors on outcomes was not possible since only 6 trials were found, and this issue necessitates a cautious interpretation of our findings. Nevertheless, this review provides valuable insight into the overall effects of propofol, as would be expected in a large clinical trial covering diverse settings in clinical practice. Second, the meta-analysis was conducted with aggregated data, which limits a further elaboration of results due to the absence of individual patient data. In particular, hemodynamic responses were extracted from the minimum among the reported mean values for each group. This might yield less representative results, but some directionality of the treatment results can be assessed. Thirdly, the number of all existing relevant studies for this review was only 6 and the sample size in each study was also relatively small, ranging from 18 to 59. This confirms the difficulty of undertaking pediatric clinical trials, particularly for this age group. Although the data may seem insufficient, considering the challenges of conducting pediatric clinical trials, such integrative analyses could serve as a substitute, similar to a moderate-sized clinical trial covering diverse settings.
Based on a quantitative overview of primary studies, we found that propofol use for general anesthesia in healthy children under 3 years old who underwent surgery did not increase complications, indicating that propofol could be at least comparable with other anesthetic agents. Since propofol is used widely in practice, labeling of propofol for this young population should be considered positively in countries where propofol use for pediatric anesthesia remains off-label; furthermore, related reimbursement policies need to be considered. Given the difficulties of conducting pediatric clinical trials, it would be desirable to gather further supporting evidence through future studies based on real-world data collected from clinical practice.
Footnotes
Funding: This study was supported by a grant of the Korean Health Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HC15C1523). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Hahn S, Lee JH, Kim HS.
- Data curation: Hong H, Choi Y, Jang MJ, Kim S.
- Formal analysis: Hong H.
- Investigation: Hong H, Hahn S.
- Methodology: Hong H, Hahn S.
- Validation: Hahn S, Lee JH, Kim HS.
- Writing - original draft: Hong H.
- Writing - review & editing: Hahn S.
SUPPLEMENTARY MATERIALS
Search strategy for MEDLINE
Quality assessment of risk of bias using Cochrane Collaboration's tool
Funnel plots. (A) Systolic blood pressure. (B) Heart rate.
References
- 1.Chidambaran V, Costandi A, D'Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29(7):543–563. doi: 10.1007/s40263-015-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Model List of Essential Medicines for Children. 6th ed. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.British Medical Association; Royal Pharmaceutical Society; Royal College of Paediatrics and Child Health; Neonatal and Paediatric Pharmacists Group. BNF for Children 2011–2012 [Internet] [Accessed 21 Jun, 2017]. www.bnfc.org.
- 4.Karen T, Schlager GW, Bendix I, Sifringer M, Herrmann R, Pantazis C, et al. Effect of propofol in the immature rat brain on short- and long-term neurodevelopmental outcome. PLoS One. 2013;8(5):e64480. doi: 10.1371/journal.pone.0064480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvo I, Landoni G, Mucchetti M, Cabrini L, Pani L. Use and reimbursement of off-label drugs in pediatric anesthesia: the Italian experience. Paediatr Anaesth. 2014;24(6):625–631. doi: 10.1111/pan.12403. [DOI] [PubMed] [Google Scholar]
- 6.Korea Institute of Drug Safety & Risk Management. Drug Saf Inf. 2013;(52):94–105. [Google Scholar]
- 7.Shimazawa R, Ikeda M. Approval status and evidence for WHO essential medicines for children in the United States, United Kingdom, and Japan: a cross-sectional study. J Pharm Policy Pract. 2017;10(1):4. doi: 10.1186/s40545-016-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppini R, Simons SH, Mugelli A, Allegaert K. Clinical research in neonates and infants: challenges and perspectives. Pharmacol Res. 2016;108:80–87. doi: 10.1016/j.phrs.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Smith MC, Williamson J, Yaster M, Boyd GJ, Heitmiller ES. Off-label use of medications in children undergoing sedation and anesthesia. Anesth Analg. 2012;115(5):1148–1154. doi: 10.1213/ANE.0b013e3182501b04. [DOI] [PubMed] [Google Scholar]
- 10.Shah PS, Shah VS. Propofol for procedural sedation/anaesthesia in neonates. Cochrane Database Syst Rev. 2011;(3):CD007248. doi: 10.1002/14651858.CD007248.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Schrum SF, Hannallah RS, Verghese PM, Welborn LG, Norden JM, Ruttiman U. Comparison of propofol and thiopental for rapid anesthesia induction in infants. Anesth Analg. 1994;78(3):482–485. doi: 10.1213/00000539-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X, Zuo Y, Zhao Q, Gu E, Huang Y. Comparison of the effects of dexmedetomidine and propofol on hemodynamics and oxygen balance in children with complex congenital heart disease undergoing cardiac surgery. Congenit Heart Dis. 2015;10(3):E123–30. doi: 10.1111/chd.12228. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2011. [Google Scholar]
- 14.Rücker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28(5):721–738. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 17.Aun CS, Sung RY, O'Meara ME, Short TG, Oh TE. Cardiovascular effects of i.v. induction in children: comparison between propofol and thiopentone. Br J Anaesth. 1993;70(6):647–653. doi: 10.1093/bja/70.6.647. [DOI] [PubMed] [Google Scholar]
- 18.Wodey E, Chonow L, Beneux X, Azzis O, Bansard JY, Ecoffey C. Haemodynamic effects of propofol vs thiopental in infants: an echocardiographic study. Br J Anaesth. 1999;82(4):516–520. doi: 10.1093/bja/82.4.516. [DOI] [PubMed] [Google Scholar]
- 19.Cohen IT, Finkel JC, Hannallah RS, Goodale DB. Clinical and biochemical effects of propofol EDTA vs sevoflurane in healthy infants and young children. Paediatr Anaesth. 2004;14(2):135–142. doi: 10.1111/j.1460-9592.2004.01160.x. [DOI] [PubMed] [Google Scholar]
- 20.Steinmetz J, Holm-Knudsen R, Sørensen MK, Eriksen K, Rasmussen LS. Hemodynamic differences between propofol-remifentanil and sevoflurane anesthesia for repair of cleft lip and palate in infants. Paediatr Anaesth. 2007;17(1):32–37. doi: 10.1111/j.1460-9592.2006.01999.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajan S, Gotluru P, Andews S, Paul J. Evaluation of endotracheal intubating conditions without the use of muscle relaxants following induction with propofol and sevoflurane in pediatric cleft lip and palate surgeries. J Anaesthesiol Clin Pharmacol. 2014;30(3):360–365. doi: 10.4103/0970-9185.137268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annila P, Viitanen H, Reinikainen P, Baer G, Lindgren L. Induction characteristics of thiopentone/suxamethonium, propofol/alfentanil or halothane alone in children aged 1–3 years. Eur J Anaesthesiol. 1999;16(6):359–366. doi: 10.1046/j.1365-2346.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- 23.Viitanen H, Tarkkila P, Mennander S, Viitanen M, Annila P. Sevoflurane-maintained anesthesia induced with propofol or sevoflurane in small children: induction and recovery characteristics. Can J Anaesth. 1999;46(1):21–28. doi: 10.1007/BF03012509. [DOI] [PubMed] [Google Scholar]
- 24.Kang P, Jang YE, Kim EH, Lee JH, Kim JT, Kim HS. Safety and efficacy of propofol anesthesia for pediatric target-controlled infusion in children below 3 years of age: a retrospective observational study. Expert Opin Drug Saf. 2018;17(10):983–989. doi: 10.1080/14740338.2018.1524460. [DOI] [PubMed] [Google Scholar]
- 25.Kim MS, Lee JH, Han SW, Im YJ, Kang HJ, Lee JR. A randomized comparison of the i-gel with the self-pressurized air-Q intubating laryngeal airway in children. Paediatr Anaesth. 2015;25(4):405–412. doi: 10.1111/pan.12609. [DOI] [PubMed] [Google Scholar]
- 26.Ghanta S, Abdel-Latif ME, Lui K, Ravindranathan H, Awad J, Oei J. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics. 2007;119(6):e1248–e1255. doi: 10.1542/peds.2006-2708. [DOI] [PubMed] [Google Scholar]
- 27.Oh TK, Kwon K. Issues in propofol sedation for pediatric patients using target control Infusion (TCI): safety in children 1–3 years old versus children older than 3 years of age. Pediatr Ther. 2016;6(2):291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy for MEDLINE
Quality assessment of risk of bias using Cochrane Collaboration's tool
Funnel plots. (A) Systolic blood pressure. (B) Heart rate.