Abstract
Lycium barbarum polysaccharide (LBP) exhibits multiple pharmacological and biological effects, including displaying antioxidant and cytoprotective properties. The current study investigated the effects of LBP-supplemented culture medium on mitochondrial distribution, mitochondrial membrane potential (MMP), adenosine triphosphate (ATP) production, mitochondrial deoxyribonucleic acid (mtDNA) copy number, reactive oxygen species (ROS) accumulation, and development of previously-cryopreserved murine two-cell embryos. Results indicate that LBP enhances development of such embryos, and that potential mechanisms include: (1) mitochondrial function enhancement via altering mitochondrial distribution and increasing MMP, ATP production, mtDNA copy number, and expression of genes involved in mitochondrial biogenesis and energy metabolism (NAD-dependent deacetyltransferase sirtuin-1 (SIRT1) and phosphorylated adenosine monophosphate-activated protein kinase (pAMPK)); (2) down-regulation of ROS generation and enhanced expression of the antioxidant genes glutathione peroxidase 4 (GPX4) and superoxide dismutase 1 (SOD1), thereby increasing embryo oxidative stress tolerance; and (3) increased expression of B-cell lymphoma-2 (BCL2), a critical gene for cell survival and embryo development. These results demonstrate that LBP improves development of previously-cryopreserved murine two-cell embryos via restoration of mitochondrial function and down-regulated generation of ROS.
Keywords: Cryopreservation, Lycium barbarum polysaccharide, Mitochondria, Reactive oxygen species, Two-cell embryos
Cryopreservation is an important technique for preservation of endangered species and in assisted reproductive medicine, and has been applied successfully in both humans and livestock species. However, cryopreserved (relative to fresh) embryos exhibit an impaired rate of early embryonic development [1]. This is attributed to the process of cryopreservation, which may induce mitochondrial dysfunction [2] and promote accumulation of reactive oxygen species (ROS) [1].
Mitochondria are membrane-enclosed organelles that play important roles in a variety of cellular activities, including - among many others - maintenance of cellular homeostasis, adenosine triphosphate (ATP) synthesis, and regulation of apoptosis. Previous studies have shown that vitrification during cryopreservation induces altered mitochondrial distribution and decreased mitochondrial potential (MMP) in oocytes [3] and embryos [4], thereby impairing fertilization and embryo development [3]. Mitochondria are closely associated with the generation and scavenging of ROS [4]. Alteration of normal mitochondrial function leads to an increase in ROS production, inducing oxidative stress in the embryo [4]. Consequently, abnormal mitochondrial distribution and mitochondrial damage may significantly impair embryo development.
Lycium barbarum – a well-known traditional Chinese herbal medicine that has been in use for thousands of years – exhibits several biological effects, including antioxidant and cytoprotective activities [5, 6]. Lycium barbarum polysaccharide (LBP) is the major component of Lycium barbarum responsible for such biological activities [7]. A previous study has shown that LBP prevents generation and accumulation of ROS, and has a protective effect against injury in various cells and tissues via mitochondrion-associated pathways [8].
As mentioned above, LBP may have specific effects on previously-cryopreserved embryo development, either as an antioxidant or via regulation of mitochondrial distribution, membrane potential, and functions. The current study investigated the effects of LBP on development of previously-cryopreserved murine two-cell embryos, and examined potential mechanisms involved in producing these effects.
Materials and Methods
Reagents and animals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Immature 8- to 11-week-old Kunming (KM) mice were obtained from the Laboratory Animal Central of Jiujiang University. All mice were fed a standard lab chow diet (Xietong, China) and housed in a single room under conditions of constant temperature (~25–28 ± 2°C), humidity (55 ± 5%), and lighting (alternating 12 h light/dark cycle). The study protocol was reviewed and approved by the Committee for Ethics on Animal Care and Experiments of Jiujiang University (approval No. SYXK(GAN)2017-0001).
LBP preparation
Preparation of LBP proceeded as previously described [7]. In brief, Lycium barbarum fruit was dried at 60°C and ground to a fine powder. Two rounds of lipid removal were carried out using a 2:1 chloroform:methanol solvent mixture, and a single round of oligosaccharide removal was carried out using 80% ethanol at 80°C. After filtering, residues were extracted and concentrated using a rotary evaporator at 60°C, followed by sequential precipitation using 95% ethanol, 100% ethanol, and acetone. After filtering and centrifugation, the precipitate was collected and vacuum-dried.
Embryo collection and culture
Female mice were injected intraperitoneally with 8 IU pregnant mare serum gonadotrophin (PMSG, Ningbo Sansheng, China) to stimulate follicular development for 46 h. Mice were then injected with 8 IU human chorionic gonadotropin (hCG, Ningbo Sansheng, China) to trigger ovulation. After hCG injection, females were exposed to males overnight, and examined for mating (presence of a vaginal plug) the following morning. Two-cell embryos were collected from mated females 46–48 h after hCG injection, at room temperature (25 ± 2°C), and subjected to either initial cryopreservation (including vitrification) or immediate culture (in Chatot-Ziomek-Bavister (CZB) medium [9] containing 75 mg/ml potassium penicillin and 50 mg/ml streptomycin sulfate). Approximately 25 embryos per 50 μl microdrop of CZB (covered with mineral oil) were incubated for 72 h at 37°C in a 5% CO2 atmosphere (100% humidity).
Embryo cryopreservation and thawing
Embryos were cryopreserved using a nylon-mesh holder as described in a previous report [10]. All liquid reagents used in embryo cryopreservation and thawing were pre-warmed to 25 ± 2°C for 2 h. Basal medium consisted of modified Dulbecco’s phosphate-buffered solution (mDPBS) supplemented with 0.3% bovine serum albumin (BSA). Two-cell embryos were initially incubated in basal medium containing 0.5 M sucrose and 25% Ficoll for 3 min. Thereafter, embryos were transferred to vitrification solution I (containing 20% ethylene glycol (EG), 0.5 M sucrose, and 25% Ficoll) and incubated for 20 sec, followed by incubation in vitrification solution II (containing 40% EG, 0.5 M sucrose, and 25% Ficoll) for 20–30 sec. Groups of approximately 30 embryos were loaded onto a nylon-mesh tip and snap-frozen in liquid nitrogen (during which the process of vitrification protects cells from ice crystal damage). For subsequent thawing at 37°C, cryopreserved embryos were transferred from liquid nitrogen into warming solution (0.5 M sucrose basal medium) for 5 min, prior to stepwise transfer through basal medium solutions containing 0.25 M and then 0.125 M sucrose. Thawed embryos were incubated for 5 min in basal medium prior to culture in CZB. Only viable embryos (as identified based on morphologic appearance) were taken forward for culture and the following experiments.
Experimental design
Each experiment employed LBP dissolved in CZB, as well as embryos randomly allocated to different experimental conditions (approximately 25 embryos per condition). To evaluate potential dose-response effects of LBP on in vitro development of previously-cryopreserved two-cell embryos, they were incubated in culture medium supplemented with different LBP concentrations (50, 100, 200, 400, 800, and 1600 μg/ml) for 72 h. This experiment was performed at least in sextuplicate.
Cryopreserved-thawed and fresh two-cell embryos were also cultured with or without 200 μg/ml LBP for 2 h and collected for mitochondrial distribution analysis, MMP analysis, ATP production detection, mitochondrial deoxyribonucleic acid (mtDNA) copy number detection, ROS accumulation detection, quantitative real-time polymerase chain reaction (qRT-PCR), and western blot analysis. This experiment was performed at least in triplicate (three biological replicates).
Blastocyst number
The embryo were cultured in different groups: (1) fresh embryos (no LBP); (2) fresh embryos + LBP (differing concentrations); (3) previously-cryopreserved embryos (no LBP); (4) previously-cryopreserved embryos + LBP (differing concentrations). After the 72 h in vitro culture period, per-group blastocyst number (at least 90 per group) was assessed. After 5 min Hoechst 33342 staining, blastocysts were rinsed with PBS containing 0.1% paraformaldehyde, mounted on a clean glass slide, covered with a coverslip, and examined under an inverted epifluorescence-capable microscope (Nikon, Tokyo, Japan).
RNA isolation and qRT-PCR
Following the 2 h in vitro culture period, approximately 100 embryos per group were subjected to total RNA extraction using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was reverse-transcribed using a PrimeScript® RT reagent Kit (Invitrogen), both according to the manufacturer’s instructions. The qRT-PCR procedure was carried out using Bio-Rad IQ5 and Bio-Rad IQ5 Optical System Software (Bio-Rad, Hercules, CA, USA). PCR cycling conditions were as follows: one cycle for 30 sec at 95°C, followed by 40 cycles for 5 sec at 95°C, and one cycle for 30 sec at 60°C. All primers used are listed in Supplementary Table 1 (online only), and glyceraldehyde-phosphate dehydrogenase (GAPDH) was used as a reference gene. Relative gene expression was quantified using the 2–∆∆CT method. Experiments included at least three biological replicates per sample.
Western blot analysis
Following the 2 h in vitro culture period, 25 embryos per treatment were lysed using 60 μl of 1 × sodium dodecyl sulfonate (SDS) – supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF) and 1 mM sodium orthovanadate – for 20 min on ice, after which lysates were stored at –20°C. Prior to electrophoresis, lysates were heated to 98°C for 5 min, immediately cooled on ice, and centrifuged at 12,000 g for 5 min. Supernatant proteins were separated on a 12% polyacrylamide gel before transfer to polyvinylidene fluoride (PVDF) membranes (Millipore, CA, USA). Membranes were blocked using Tris-buffered saline containing tween (TBST) and supplemented with 5% skim milk, at 25°C for 1 h. Membranes were then incubated overnight at 4°C with primary monoclonal antibodies targeting the phosphorylated and total forms of adenosine monophosphate activated protein kinase (pAMPK and total AMPK; Catalog #s 2535S and MAB3197, Cell Signaling Technology, Beverly, MA, USA), B-cell lymphoma-2 (BCL-2; Catalog # sc-23960, Santa Cruz Biotechnology, CA, USA), BCL-2 associated X protein (BAX; Catalog # sc-4239, Santa Cruz Biotechnology), and GAPDH (Catalog # sc-32233, Santa Cruz Biotechnology), at estimated ratios of 1:1000 (apart from GAPDH, for which the ratio was 1:2000). After washing, membranes were incubated with secondary antibody (1:10 000) conjugated to horseradish peroxidase (HRP; Zhong Shan Golden Bridge Biotechnology, Nanjing, China) at 37°C for 30 min. Finally, immunoreactive bands were visualized using a SuperSignalWest Pico Kit (Thermo, Rockford, Ill., USA) and protein band densitometric analysis was carried out using ImageJ (Version 1.49).
Intracellular ROS detection and quantitation
After the 2 h in vitro culture period, approximately 75 embryos per treatment were transferred into CZB containing 10 mm dichlorofluorescein (DCFH) diacetate (DCHFDA; Beyotime Institute of Biotechnology, China) for ROS detection and quantitation, as described previously [11]. Briefly, embryos were incubated at 37 °C for 30 min in CZB containing DCHFDA, washed three times using CZB, mounted on glass slides in CZB, covered with coverslips, and subjected to laser-scanning confocal microscopy (Nikon, Melville, NY, USA) to visualize fluorescence. Fluorescence intensity was determined by densitometric analysis using ImageJ (Version 1.49).
Mitochondrial distribution and MMP detection
After the 2 h in vitro culture period, approximately 75 embryos per treatment were used to detect mitochondrial distribution and MMP using Rhodamine 123 and JC-1, as previously reported [4]. At an inner MMP < 100 mv (low mitochondrial polarization) JC-1 remains a monomer emitting green fluorescence in the fluorescein isothiocyanate (FITC) channel, whereas at an inner MMP > 140 mv (high mitochondrial polarization) JC-1 forms J-aggregates and emits red fluorescence in the rhodamine isothiocyanate channel. Embryos were incubated in CZB containing 0.1 mg/ml Rhodamine 123 or 1 mg/ml JC-1 at 37°C for 20 min, washed three times using CZB, slide-mounted, and immediately observed via laser-scanning confocal microscopy (Nikon). Excitation and emission wavelengths to detect green fluorescence were 488 nm and 530 nm, respectively. Excitation and emission wavelengths to detect red fluorescence were 525 nm and 590 nm, respectively. Finally, fluorescence intensity was determined by densitometric analysis using ImageJ (Version 1.49).
Determination of intracellular ATP content
After the 2 h in vitro culture period, embryos were collected and their ATP content was determined using a kit assay (Sigma-Aldrich), according to the manufacturer’s instructions. Briefly, 25 embryos per group were transferred to ATP assay buffer before adding 0.2 µl ATP probe, 2 µl ATP converter, and 2 µl Developer Mix. Determination of ATP content proceeded using luminometric analysis (FLUO star OPTIMA, BMG Labtech, Germany), with reference to a standard curve.Estimated ATP content per individual embryo was then determined, as previously described [12,13,14].
Determination of mtDNA copy number
After the 2 h in vitro culture period, cryopreserved-thawed embryos were collected and mtDNA copy number was determined by qRT-PCR, as previously described [12]. Briefly, 25 embryos per group were lysed in 20 μl lysis buffer (20 mM Tris, 0.4 mg/ml proteinase K, 0.9% Nonidet-40, and 0.9% Tween 20) at 55°C for 30 min, followed by 95°C for 5 min. Mitochondrially-encoded reduced nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit 5 gene (MT-ND5) was selected as an index to determine mtDNA copy number [14]. Per-lysate mtDNA copy number was calculated using threshold cycle number (Ct), with reference to a standard curve. Primer sequences for MT-ND5 are listed in Supplmentary Table 1.
Statistical analyses
All experiments were repeated at least in triplicate per group. Data were analyzed using one-way analysis of variance (ANOVA), followed by Fisher’s least significant different test (Fisher LSD) and an independent-samples t-test, within the Statistical Package for the Social Sciences (SPSS) software environment (Version 13.0; SPSS, Chicago, IL, USA). Results were considered statistically significant at P < 0.05.
Results
Effects of LBP on development of previously-cryopreserved two-cell embryos
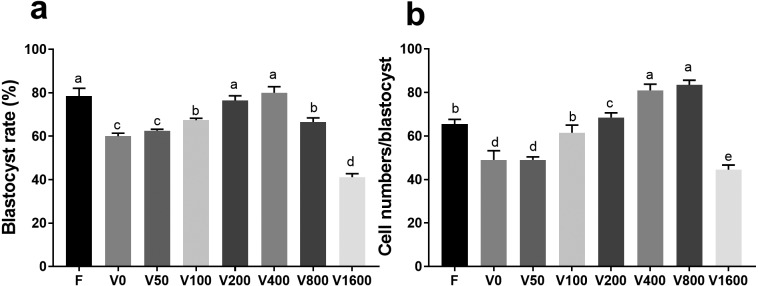
Post-thaw two-cell embryo viability was approximately 90%. When surviving embryos were exposed to increasing LBP concentrations (50,100, 200, 400, 800, and 1600 μg/ml), the rate of blastocyst attainment exhibited a corresponding increase, peaking at 400 μg/ml LBP (Fig. 1a). At 200 μg/ml LBP, no difference in blastocyst attainment rate was observed between LBP-treated previously-cryopreserved and fresh two-cell embryos (relative to LBP-untreated embryos) (Fig. 1a). At LBP concentrations between 200 and 800 μg/ml, blastocyst attainment rate was higher in both previously-cryopreserved and fresh two-cell embryos (relative to LBP-untreated embryos) (Fig. 1b). However, 1600 μg/ml LBP retarded blastocyst attainment rate in both previously-cryopreserved and fresh two-cell embryos (relative to LBP-untreated embryos) (Fig. 1a).
Fig. 1.
Effects of LBP on development of previously-cryopreserved murine two-cell embryos. (a) Effects of LBP on blastocyst attainment rate. (b) Effects of LBP on total per-blastocyst cell numbers. F: fresh two-cell embryos; V: previously-cryopreserved (vitrified) two-cell embryos; V+Number (LBP dose, μg/ml): previously-cryopreserved (vitrified) two-cell embryos cultured in the presence of different LBP doses. Results represent means ± SEM of three independent experiments. Error bar letters denote significant differences (P < 0.05).
Effects of LBP on mitochondrial distribution and MMP in previously-cryopreserved two-cell embryos
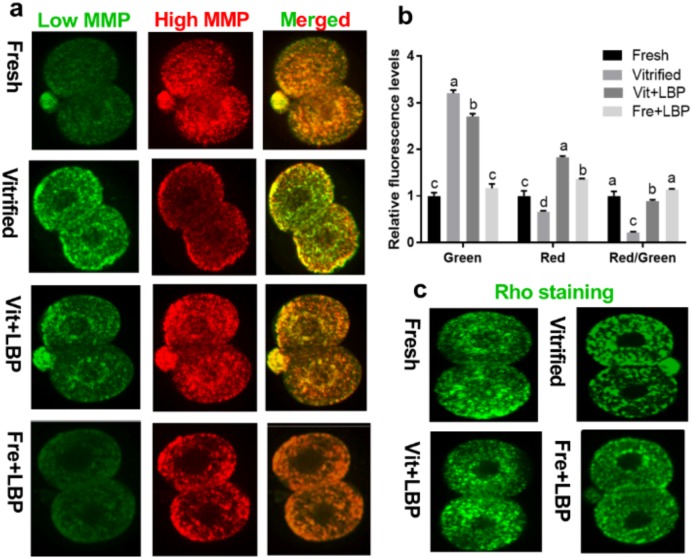
Compared to fresh two-cell embryos, previously-cryopreserved two-cell embryos exhibited less intense red JC-1 fluorescence and more intense green JC-1 fluorescence, resulting in a significantly lower red/green fluorescence ratio (i.e., lower levels of mitochondrial polarization). However, treatment with 200 μg/ml LBP significantly attenuated this prior cryopreservation-associated effect, yielding a red/green fluorescence ratio comparable to that of fresh two-cell embryos (Fig. 2a and c).
Fig. 2.
Effects of LBP on mitochondrial distribution and MMP in previously-cryopreserved murine two-cell embryos after 2 h culture. (a and b) Effects of LBP on MMP. (c) Effects of LBP on mitochondrial distribution. Fresh: fresh two-cell embryos; vitrified: previously-cryopreserved (vitrified) two-cell embryos; Vit + LBP: previously-cryopreserved (vitrified) two-cell embryos cultured in the presence of 200 μg/ml LBP; Fre + LBP: fresh two-cell embryos cultured in the presence of 200 μg/ml LBP. Results represent means ± SEM of three independent experiments. Error bar letters denote significant differences (P < 0.05).
Rhodamine 123 fluorescence showed that mitochondrial distribution in fresh two-cell embryos was homogeneous, while mitochondria in previously-cryopreserved two-cell embryos exhibited significant clustering. Moreover, fluorescence intensity in fresh two-cell embryos exceeded that in previously-cryopreserved two-cell embryos. Again, treatment with 200 μg/ml LBP treatment partially attenuated prior cryopreservation-associated mitochondrial clustering, and enhanced mitochondrial fluorescence intensity in previously-cryopreserved two-cell embryos (Fig. 2c).
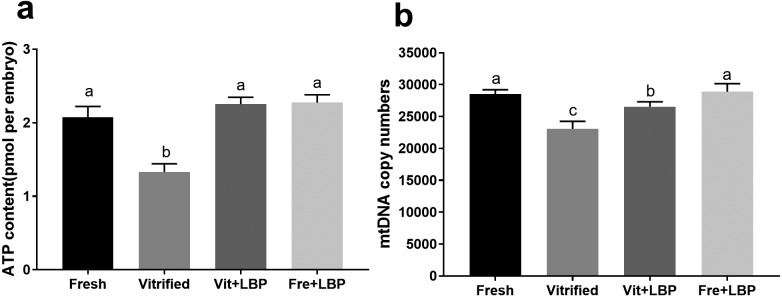
Effects of LBP on ATP content and mtDNA copy number of previously-cryopreserved two-cell embryos
Relative to fresh two-cell embryos, cryopreservation lowered ATP content and mtDNA copy number (Fig. 3). Treatment with LBP increased ATP content of previously-cryopreserved two-cell embryos to the degree that no significant differences could be observed between fresh and LBP-treated previously-cryopreserved embryos (Fig. 3a). While LBP treatment increased mtDNA copy number in previously-cryopreserved embryos, copy number remained lower than that of fresh embryos (Fig. 3b).
Fig. 3.
Effects of LBP on ATP content and mtDNA copy number of previously-cryopreserved murine two-cell embryos after 2 h culture. (a) Effects of LBP on ATP content. (b) Effects of LBP on mtDNA copy number. Fresh: fresh two-cell embryos; vitrified: previously-cryopreserved (vitrified) two-cell embryos; Vit + LBP: previously-cryopreserved (vitrified) two-cell embryos cultured in the presence of 200 μg/ml LBP; Fre + LBP: fresh two-cell embryos cultured in the presence of 200 μg/ml LBP. Results represent means ± SEM of three independent experiments. Error bar letters denote significant differences (P < 0.05).
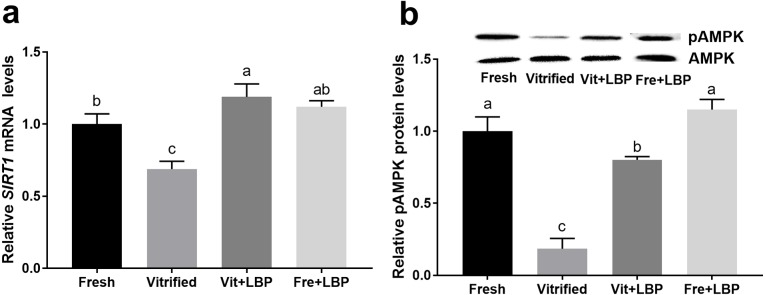
Effects of LBP on SIRT1 mRNA and pAMPK expression by previously-cryopreserved two-cell embryos
Both ATP generation and early embryonic development require NAD-dependent deacetyltransferase sirtuin-1 (SIRT1) and AMPK [12]. Fresh embryos exhibited higher expression levels of SIRT1 mRNA and pAMPK protein than did previously-cryopreserved two-cell embryos, and LBP-treatment of the latter was able to partially attenuate this effect (Fig. 4).
Fig. 4.
Effects of LBP on SIRT1 mRNA and pAMPK protein expression by previously-cryopreserved murine two-cell embryos after 2 h culture in the presence of LBP. (a) Effects of LBP on SIRT1 mRNA expression. (b) Effects of LBP on pAMPK protein expression. Fresh: fresh two-cell embryos; vitrified: previously-cryopreserved (vitrified) two-cell embryos; Vit + LBP: previously-cryopreserved (vitrified) two-cell embryos cultured in the presence of 200 μg/ml LBP; Fre + LBP: fresh two-cell embryos cultured in the presence of 200 μg/ml LBP. Results represent means ± SEM of three independent experiments (pAMPK was normalized to total AMPK). Error bar letters denote significant differences (P < 0.05).
Effects of LBP on ROS generation and antioxidant gene expression by previously-cryopreserved two-cell embryos
Compared to fresh two-cell embryos, previously-cryopreserved two-cell embryos exhibit significantly higher ROS levels, and LBP-treatment of the latter was able to partially attenuate this effect (Fig. 5a and b). Finally, the effects of LBP on antioxidant gene (GPX4, SOD1) expression were examined. Both GPX4 and SOD1 were expressed at lower levels by previously-cryopreserved (relative to fresh) two-cell embryos, and LBP-treatment of the former was able to partially attenuate this effect (Fig. 5c).
Fig. 5.
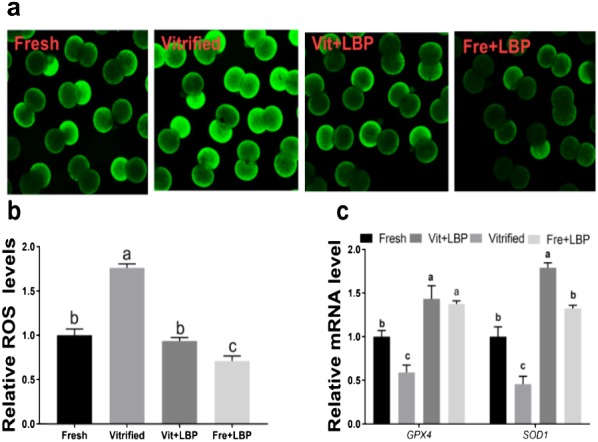
Effects of LBP on ROS generation and antioxidant gene expression by previously-cryopreserved murine two-cell embryos after 2 h culture in the presence of LBP. (a) Effects of LBP on ROS levels. (b) Effects of LBP on GPX4 mRNA expression. (c) Effects of LBP on SOD1 mRNA expression. Fresh: fresh two-cell embryos; vitrified: previously-cryopreserved (vitrified) two-cell embryos; Vit + LBP: previously-cryopreserved (vitrified) two-cell embryos cultured in the presence of 200 μg/ml LBP; Fre + LBP: fresh two-cell embryos cultured in the presence of 200 μg/ml LBP. Results represent means ± SEM of three independent experiments. Error bar letters denote significant differences (P < 0.05).
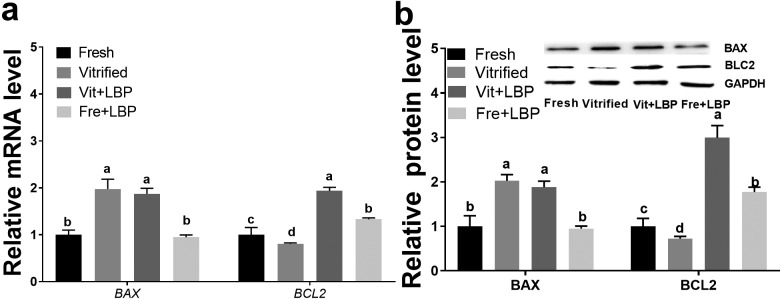
Effects of LBP on BAX and BLC2 expression by previously-cryopreserved two-cell embryos
At both the mRNA and protein levels, expression of BAX was higher in previously-cryopreserved (relative to fresh) two-cell embryos, with LBP treatment of the former having no obvious effect on BAX expression (Fig. 6a and b). In contrast, prior cryopreservation decreased BLC2 expression at both the mRNA and protein levels, and LBP treatment was able to partially attenuate this effect, at both the mRNA and protein levels (Fig. 6a and b).
Fig. 6.
Effects of LBP on BAX and BLC2 expression by previously-cryopreserved murine two-cell embryos after 2 h culture in the presence of LBP. (a) Effects of LBP on BAX and BCL2 mRNA expression. (b) Effects of LBP on BAX and BCL2 protein expression. Fresh: fresh two-cell embryos; vitrified: previously-cryopreserved (vitrified) two-cell embryos; Vit + LBP: previously-cryopreserved (vitrified) two-cell embryos cultured in the presence of 200 μg/ml LBP; Fre + LBP: fresh two-cell embryos cultured in the presence of 200 μg/ml LBP. Results represent means ± SEM of three independent experiments (protein expression was normalized to GAPDH). Error bar letters denote significant differences (P < 0.05).
Discussion
The current study systematically investigated the hypothesized enhancing effects of LBP on development of previously-cryopreserved murine two-cell embryos. Previous studies reported that LBP exerted cytoprotective effects through ROS scavenging [15], up-regulation of SIRT1 expression [16], and inhibition of programmed cell death [15]. However, to date no report has been published regarding the effects of LBP on previously-cryopreserved cells. The present study determined that post-thaw LBP treatment improved the rate of embryonic development of previously-cryopreserved murine two-cell embryos, via mechanisms involving restoration of mitochondrial function and down-regulated ROS production.
We initially evaluated the effect of increasing LBP doses on development of previously-cryopreserved murine two-cell embryos in vitro, and found that LBP enhanced blastocyst attainment rate and total cell number of developing embryos. Although previous studies have reported LBP’s protective effects against many injuries mediated by intracellular and extracellular environments – both in vivo and in vitro [17, 18] – our study is the first to report that LBP effectively promotes post-cryopreservation development efficiency of murine two-cell embryos.
The mitochondrion is an important cellular organelle involved in metabolism and apoptosis, both of which are required for oocyte maturation and embryo development [19, 20]. Cryopreservation-induced alteration of mitochondrial distribution and compromised MMP is a major cause of retarded post-thaw embryo development [20]. The present study demonstrated that LBP mitigates both cryopreservation-induced alteration of mitochondrial distribution and compromised MMP. In further exploring the effects of LBP on mitochondrial function, we demonstrated that LBP supplementation significantly increased ATP content and partially enhanced mtDNA copy number in previously-cryopreserved murine two-cell embryos. Embryonic mitochondrial content and ATP level are positively associated with embryo developmental competence [14] (i.e., play a pivotal role in maintaining cellular metabolism and cell division [21]).
In addition, the present study demonstrated that LBP may assist in recovering mitochondrial function in previously-cryopreserved embryos via increasing expression of SIRT1 and pAMPK, both of which have key roles in mitochondrial biogenesis [3, 20]. Such results are consistent with those of previous animal model studies that demonstrated an LBP-induced increase in SIRT1 [21] and pAMPK expression [16]. However, the mechanism of LBP-induced mitochondrial functional recovery should be the focus of future experiments.
It is well established that a low rate of early embryonic development may be attributed to elevated ROS levels [22, 23], and many studies have indicated that LBP is an excellent antioxidant [5, 6]. The present study confirmed a previous report that cryopreservation induces ROS accumulation in murine two-cell embryos [1] and demonstrated that LBP treatment of thawed embryos diminishes ROS levels, concomitant with accelerated embryo development. This suggests that ROS are at least partially responsible for embryo developmental arrest associated with the cryopreservation. Fresh embryos exhibit antioxidant capacity attributable to enzymes such as GPX4 and SOD1. However, cryopreservation decreases antioxidant enzyme expression in both tissues [24] and murine two-cell embryos [1]. Cryopreservation-induced down-regulated GPX4 and SOD1 expression suggests that cryopreservation may alter gene expression of oocytes [25] and embryos [26].
Mitochondria are the major cellular source of ROS and increased ROS production in mammalian embryos is implicated in developmental arrest and cell death [27]. Additionally, expression of BAX and BCL2 gene family members is associated with embryo development (e.g., BCL2 can protect cells against extracellular insults) [28]. Building on these prior data, we measured expression of key apoptosis-associated molecules via qRT-PCR and western blotting, demonstrating that previously-cryopreserved embryos exhibit up-regulated expression of BAX but mild down-regulation of BCL2 expression. Treatment of previously-cryopreserved embryos with LBP increased BCL2 expression but did not affect BAX expression. Based on the above results, we conclude that embryo developmental arrest and cell death are closely correlated with BCL2 and BAX expression: prior cryopreservation may induce cell death through up-regulation of BAX, while LBP can rescue this phenotype to maintain cell survival by up-regulating BCL2.
Supplementing embryo culture medium with LBP enhances development of previously-cryopreserved murine two-cell embryos. Possible mechanisms include normalization of detrimentally-altered mitochondrial distribution, MMP, ATP production, mtDNA copy number, and ROS levels, as well as opposition of cell death. Thus, LBP treatment may be an effective intervention aimed at improving the quality of previously-cryopreserved embryos, and may consequently be of significant biological and commercial value (e.g., for maintenance of endangered species and in assisted reproductive medicine). However, LBP is a mixture consisting of six monosaccharides, including arabinose, glucose 6‑phosphate, galactose, mannose, xylose, and rhamnose [29]. Further studies are necessary to determine which monosaccharide component(s) play(s) key roles in enhancing development of previously-cryopreserved embryos.
Conflict of interests
The authors declare no competing interests.
Supplementary
Acknowledgments
This study was funded by the Project of Science and Technology of Jiangxi Provincial Education Department (GJJ170966) and the Project of Health Commission of Jiangxi Province (20193010; 20197217).
References
- 1.Gao C, Han HB, Tian XZ, Tan DX, Wang L, Zhou GB, Zhu SE, Liu GS. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res 2012; 52: 305–311. [DOI] [PubMed] [Google Scholar]
- 2.Ahn HJ, Sohn IP, Kwon HC, Jo DH, Park YD, Min CK. Characteristics of the cell membrane fluidity, actin fibers, and mitochondrial dysfunctions of frozen-thawed two-cell mouse embryos. Mol Reprod Dev 2002; 61: 466–476. [DOI] [PubMed] [Google Scholar]
- 3.Cao X, Li J, Xue H, Wang S, Zhao W, Du Z, Yang Y, Yue Z. Effect of vitrification on meiotic maturation, mitochondrial distribution and glutathione synthesis in immature silver fox cumulus oocyte complexes. Theriogenology 2017; 91: 104–111. [DOI] [PubMed] [Google Scholar]
- 4.Zhao XM, Fu XW, Hou YP, Yan CL, Suo L, Wang YP, Zhu HB, Dinnyés A, Zhu SE. Effect of vitrification on mitochondrial distribution and membrane potential in mouse two pronuclear (2-PN) embryos. Mol Reprod Dev 2009; 76: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 5.Xiao J, Liong EC, Ching YP, Chang RCC, Fung ML, Xu AM, So KF, Tipoe GL. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr Diabetes 2013; 3: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, Wang G, Zhong J. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. PLoS One 2014; 9: e110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Q, Li J, Yan J, Liu S, Guo Y, Chen D, Luo Q. Lycium barbarum polysaccharides ameliorates renal injury and inflammatory reaction in alloxan-induced diabetic nephropathy rabbits. Life Sci 2016; 157: 82–90. [DOI] [PubMed] [Google Scholar]
- 8.Kou L, Du M, Zhang C, Dai Z, Li X, Zhang B, Hu X. Polysaccharide purified from Lycium barbarum protects differentiated PC12 cells against L‑Glu‑induced toxicity via the mitochondria‑associated pathway. Mol Med Rep 2017; 16: 5533–5540. [DOI] [PubMed] [Google Scholar]
- 9.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto H, Jiang JY, Tanaka T, Sasada H, Sato E. Vitrification of large quantities of immature bovine oocytes using nylon mesh. Cryobiology 2001; 42: 139–144. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Han J, Zhu CC, Tang F, Cui XS, Kim NH, Sun SC. Exposure to HT-2 toxin causes oxidative stress induced apoptosis/autophagy in porcine oocytes. Sci Rep 2016; 6: 33904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe T, Kawahara-Miki R, Hara T, Noguchi T, Hayashi T, Shirasuna K, Kuwayama T, Iwata H. Modification of mitochondrial function, cytoplasmic lipid content and cryosensitivity of bovine embryos by resveratrol. J Reprod Dev 2017; 63: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Noohi S, Shin JS, Tan SL, Taketo T. Bi-directional communication with the cumulus cells is involved in the deficiency of XY oocytes in the components essential for proper second meiotic spindle assembly. Dev Biol 2014; 385: 242–252. [DOI] [PubMed] [Google Scholar]
- 14.He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci 2016; 17: 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao K, Liu M, Cao J, Yao M, Lu Y, Li J, Zhu X, Yang Z, Wen A. Protective effects of Lycium barbarum polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. Molecules 2014; 20: 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia L, Li W, Li J, Li Y, Song H, Luan Y, Qi H, Ma L, Lu X, Yang Y. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci Rep 2016; 6: 36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang F, Peng T, Yang S, Wang W, Zhang Y, Li H. Lycium barbarum polysaccharide attenuates the cytotoxicity of mutant huntingtin and increases the activity of AKT. Int J Dev Neurosci 2016; 52: 66–74. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Pan X, Li T, Zhang C, Shi N. Lycium barbarum polysaccharides protect against trimethyltin chloride-induced apoptosis via sonic hedgehog and PI3K/Akt signaling pathways in mouse Neuro-2a cells. Oxid Med Cell Longev 2016; 2016: 9826726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagai S, Mabuchi T, Hirata S, Shoda T, Kasai T, Yokota S, Shitara H, Yonekawa H, Hoshi K. Oocyte mitochondria: strategies to improve embryogenesis. Hum Cell 2004; 17: 195–201. [DOI] [PubMed] [Google Scholar]
- 20.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell 2006; 125: 1241–1252. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod 2000; 62: 1745–1753. [DOI] [PubMed] [Google Scholar]
- 22.Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 2001; 7: 175–189. [DOI] [PubMed] [Google Scholar]
- 23.Choi W, Zhang X, Sharma RK, Falcone T, Agarwal A. Effect of oxidative stress on mouse oocyte cytoskeleton and embryo development. Fertil Steril 2005; 84: S19. [Google Scholar]
- 24.Warnick CT, Lazarus HM. Adenine nucleotides during organ storage. Transplant Proc 1977; 9: 1575–1577. [PubMed] [Google Scholar]
- 25.Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, Hamamah S. Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Hum Reprod 2012; 27: 2160–2168. [DOI] [PubMed] [Google Scholar]
- 26.Castillo-Martín M, Yeste M, Pericuesta E, Morató R, Gutiérrez-Adán A, Bonet S. Effects of vitrification on the expression of pluripotency, apoptotic and stress genes in in vitro-produced porcine blastocysts. Reprod Fertil Dev 2015; 27: 1072–1081. [DOI] [PubMed] [Google Scholar]
- 27.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod 1998; 13: 998–1002. [DOI] [PubMed] [Google Scholar]
- 28.Yang MY, Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci 2002; 70: 159–169. [DOI] [PubMed] [Google Scholar]
- 29.Jing L, Jia XW. Lycium barbarum polysaccharide arbitrates palmitate-induced apoptosis in MC3T3‑E1 cells through decreasing the activation of ERS‑mediated apoptosis pathway. Mol Med Rep 2018; 17: 2415–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.