Abstract
Hindbrain ependymocytes are postulated to have a glucose-sensing role in regulating gonadal functions. Previous studies have suggested that malnutrition-induced suppression of gonadotropin secretion is mediated by noradrenergic inputs from the A2 region in the solitary tract nucleus to the paraventricular nucleus (PVN), and by corticotropin-releasing hormone (CRH) release in the hypothalamus. However, no morphological evidence to indicate the neural pathway from the hindbrain ependymocytes to hypothalamic kisspeptin neurons, a center for reproductive function in mammals, currently exists. The present study aimed to examine the existence of a neuronal pathway from the hindbrain ependymocytes to kisspeptin neurons in the arcuate nucleus (ARC) and anteroventral periventricular nucleus (AVPV). To determine this, wheat-germ agglutinin (WGA), a trans-synaptic tracer, was injected into the fourth ventricle (4V) in heterozygous Kiss1-tandem dimer Tomato (tdTomato) rats, where kisspeptin neurons were visualized by tdTomato fluorescence. 48 h after the WGA injection, brain sections were taken from the forebrain, midbrain and hindbrain and subjected to double immunohistochemistry for WGA and dopamine β-hydroxylase (DBH) or CRH. WGA immunoreactivities were found in vimentin-immunopositive ependymocytes of the 4V and the central canal (CC), but not in the third ventricle. The WGA immunoreactivities were detected in some tdTomato-expressing cells in the ARC and AVPV, DBH-immunopositive cells in the A1–A7 noradrenergic nuclei, and CRH-immunopositive cells in the PVN. These results suggest that the hindbrain ependymocytes have neuronal connections with the kisspeptin neurons, most probably via hindbrain noradrenergic and CRH neurons to relay low energetic signals for regulation of reproduction.
Keywords: Ependymocyte, Kisspeptin neuron, Wheat-germ agglutinin
Energetic condition is one of the critical factors for regulating reproductive functions in mammals. Chronic food restriction delays the onset of puberty and causes cessation of the reproductive cycles in female mammals [1]. Experimentally, short-term food restriction suppresses pulsatile secretion of luteinizing hormone (LH) [2] and the steroid-induced LH surge in female rats [3, 4]. These studies suggest that low energetic signals suppress gonadal functions via inhibition of gonadotropin-releasing hormone (GnRH)/LH secretion.
The ependymocytes of the hindbrain have been proposed to be able to sense glucose availability for controlling GnRH/LH secretion. Pancreatic glucokinase (GK), a rate-limiting enzyme for glucose metabolism, and phosphorylated AMP-activated protein kinase (AMPK), an active form of AMPK, are evident in the ependymocytes of the wall of the central canal (CC) and the fourth cerebral ventricle (4V) [5, 6]. This pancreatic glucosensing mechanism is suggested to be GK- and AMPK-dependent [7]. Thus, the central glucosensing mechanism could share a similar molecular cascade with pancreatic β cells. The ependymocytes taken from rat CC show an in vitro increase in intracellular Ca2+ concentrations in response to low extracellular glucose [8] and to AMPK activator (AICAR) [6]. Further, an injection of alloxan, a GK inhibitor, or AICAR, into the 4V suppresses in vivo pulsatile secretion of LH in female rats [6, 9]. An injection of 5-thioglucose, a glucose metabolism inhibitor, into the 4V suppressed the steroid-induced LH surge [10]. These studies imply that the ependymocytes of the hindbrain could play a role as one of the energy-sensing cells through the GK/AMPK cascade to control GnRH/LH pulse and surge.
It is likely that noradrenergic neurons and/or corticotropin-releasing hormone (CRH) neurons mediate the mechanisms relaying the low energetic signals to kisspeptin neurons in the hypothalamic arcuate nucleus (ARC) and anteroventral-periventricular nucleus (AVPV) to regulate GnRH/LH pulse and surge. The ARC kisspeptin neurons, co-expressing neurokinin B (NKB) and dynorphin A, are referred to as KNDy neurons, and are postulated to play an essential role in GnRH/LH pulsatile secretion [11,12,13,14]; AVPV kisspeptin neurons are thought to regulate the preovulatory GnRH/LH surge [15,16,17]. Acute fasting suppressed Kiss1 gene (encoding kisspeptin) expression in the ARC [18], AVPV [19], attenuated LH pulses [20], and surge [3] in female rats. These results suggest that low energetic signals are relayed to the ARC and AVPV kisspeptin neurons to suppress the GnRH/LH pulse and surge. A lesion of the brainstem noradrenergic neurons with saporin-conjugated dopamine β-hydroxylase (DBH) antibody (DSAP) prevented the suppression of estrous cyclicity induced by administration of 2-deoxy-D-glucose (2DG), a glucose metabolic inhibitor, in female rats [21]. Destruction of the A6 noradrenergic nucleus attenuated the preovulatory LH surge [22]. Notably, a local administration of α-methyl-p-tyrosine, a catecholamine synthesis inhibitor, into the PVN rescued LH pulses suppressed by fasting [20] or peripheral administration of 2DG [23]. Further, fasting-induced suppression of LH pulses was blocked by a central administration of CRH antagonist [20] and expression of CRH receptor proteins were observed in the ARC and AVPV kisspeptin neurons [24]. Thus, the noradrenergic and/or CRH neuronal pathways seem to contribute in relaying energetic signals from energy sensor cells in the hindbrain to the hypothalamic kisspeptin neurons to control GnRH/LH secretion. However, no morphological analysis to show the neuronal pathway from the hindbrain ependymocytes to the hypothalamic kisspeptin neurons has been provided yet.
The present study, thus, aims to provide morphological evidence for the neuronal pathway originating from the hindbrain ependymocytes to the kisspeptin neurons in the ARC and AVPV via noradrenergic and/or CRH neurons. To determine this, wheat-germ agglutinin (WGA), a trans-synaptic tracer, was injected into the 4V of female heterozygous Kiss1-tandem dimer Tomato (tdTomato) rats, in which kisspeptin neurons are visualized by tdTomato fluorescence. Then rat brain sections including the hypothalamus and brainstem were subjected to double immunohistochemistry to visualize WGA and several peptides, such as vimentin, a marker of ependymocyte, DBH, a noradrenergic neuronal marker, or CRH.
Materials and Methods
Animals
Three adult female heterozygous Kiss1-tdTomato rats (8 weeks old, 197–214 g) were used in which kisspeptin neurons were visualized by tdTomato fluorescence. The colocalization of tdTomato reporter protein and kisspeptin in the ARC and AVPV were previously described in [25]. The normal reproductive function of the female heterozygous Kiss1-tdTomato rat was also confirmed. The animals were housed under a controlled environment (14 h light and 10 h darkness; light on at 0500 h; temperature, 24 ± 2ºC) with free access to food (CE-2, Clear Japan, Tokyo, Japan) and water. Animals having shown at least 2 consecutive 4-day estrous cycles were bilaterally ovariectomized (OVX) and immediately received a subcutaneous Silastic implant (inner diameter, 1.5 mm; outer diameter, 3.0 mm; length 25.0 mm; Dow corning, Midland, MI) containing estradiol-17β (E2; Sigma-Aldrich, St. Louis, MO) dissolved in peanut oil at 20 μg/ml, 7 days before brain sampling. Animals treated with the E2 implant were previously confirmed to display a diestrous level of plasma E2 [2]. The OVX+low E2 model was chosen because fasting- or glucoprivation-induced suppression of LH pluses was evident in the presence of diestrous level E2 in OVX rats and was able to visualize both AVPV and ARC kisspeptin neurons by tdTomato fluorescence. The Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University, approved the present study.
WGA injection into the fourth ventricle
Biotinylated WGA was purchased from vector laboratories, Inc. (B-1025; Burlingame, CA). A guide cannula (23 G, Plastics one, Roanoke, VA) for WGA administration was stereotaxically implanted into the 4V under the anesthesia with intraperitoneal (ip) injection of ketamine (27 mg/kg, Sankyo, Tokyo, Japan) and xylazine (5.3 mg/kg, Bayer Yakuhin, Tokyo, Japan) cocktail. The cannula tip was placed at 12.5 mm posterior and 8.3 mm ventral to the bregma at midline according to a rat brain atlas [26]. 0.5% WGA (2 μl/head) dissolved in ultrapure water according to a previous paper [27] was injected into the 4V (n = 3) at the rate of 0.5 μl/min with a microinjection pump (EICOM, Kyoto, Japan) through the inner cannula attached to the guide cannula.
Immunohistochemistry
48 h after the 4V WGA injection, the animals were perfused with 0.05 M phosphate buffered-saline (PBS, pH 7.5) followed by 4% paraformaldehyde in 0.05 M PB under the deep anesthesia by ip pentobarbital (40 mg/kg) injection. After perfusion, the brain was immediately removed, postfixed in the same fixative overnight at 4ºC and then immersed in a 30% sucrose-0.05 M PB for 3–5 days at 4ºC. The coronal sections at 50 μm thicknesses were collected in four consecutive series with a cryostat, and were stored at –20ºC in cryoprotectant until further use for immunohistochemistry. A series of the sections were subjected to double-immunohistochemistry for WGA and either vimentin, an ependymal marker, DBH, a noradrenergic neuronal marker, or CRH. The antibodies used in the present study were as follows: rabbit polyclonal anti-WGA (1:10000, Sigma-Aldrich T4144, RRID: AB_261669), chicken polyclonal anti-vimentin (1:2000, Millipore AB5733, RRID: AB_11212377), mouse monoclonal anti-DBH (1:1000, Millipore MAB308, RRID: AB_2245740) and guinea pig anti-CRH (1:5000, Peninsula Laboratories T-5007, RRID: AB_518256). Alexa 488-conjugated anti-rabbit IgG (1:800, Thermo Fisher Scientific A-11008, RRID: AB_143165), Alexa 594-conjugated anti-chicken IgG (1:800, Molecular Probes A-11042, RRID: AB_142803), Alexa 594-conjugated anti-mouse IgG (1:800, Molecular Probes A-21203, RRID: AB_141633) or Alexa 594-conjugated anti-guinea pig IgG (1:800, Jackson ImmunoResearch Labs 106-585-003, RRID: AB_2337442) were used for second anti-bodies to visualize the immunoreactivities. Briefly, free-floating brain sections were rinsed 3 times in 0.05 M PBS containing 0.1% NaN3 and 0.3% Triton-X (PBST) at room temperature between each step. After a 90 min incubation in 10% normal goat serum (NGS) in 0.05 M PBST for blocking nonspecific binding, the sections were incubated with primary antibodies for 2 days for detection of combination of WGA and vimentin and for 7 days for detection of combination of WGA and DBH or combination of WGA and CRH at 4ºC. They were further incubated in a mixture of each secondary antibody solution in 10% NGS blocking buffer for 2 h at room temperature. Finally, sections were mounted onto gelatin-coated glass slides with a drop of Prolong Gold (Life Technologies, Tokyo, Japan), an antifade regent. The sections were examined under a fluorescence microscope with the ApoTome optical sectioning (Carl Zeiss, Oberkochen, Germany).
Quantification of WGA-immunopositive noradrenergic, CRH or kisspeptin neurons
The number of DBH-immunopositive, CRH-immunopositive or tdTomato-positive cells with or without co-expression of WGA was quantified in a series of coronal sections at 200 μm intervals in female heterozygous Kiss1-tdTomato rats injected with WGA into the 4V (n = 3). The number of WGA-immunopositive cells was counted in sections at approximately from 2.28 mm anterior to bregma to 15.96 mm posterior to the bregma. The number of DBH-immunopositive neurons were counted in the hindbrain noradrenergic nuclei (A1–A7) and the area postrema in the coronal sections: A7, from 8.16 mm to 8.76 mm; A6, from 9.36 mm to 10.32 mm; A5, from 9.36 mm to 11.04 mm; A4 from 10.68 mm to 10.92 mm; A3, from 11.16 mm to 11.88 mm; A2, from 13.80 mm to 15.96 mm; A1, from 13.92 mm to 15.96 mm; area postrema, from 13.80 mm to 14.28 mm posterior to the bregma. The number of CRH-immunopositive cells were counted in the PVN and supraoptic nucleus (SON) in the hypothalamus on the sections: PVN, from 1.56 mm to 1.92 mm; SON, from 0.60 mm to 1.72 mm posterior to the bregma. The number of tdTomato-positive cells were counted in the ARC and AVPV in the brain sections: ARC, from 1.72 mm to 4.20 mm posterior to the bregma; AVPV, from 0.12 mm anterior to the bregma to 0.48 mm posterior to the bregma.
Results
Distribution of WGA-immunopositive ependymocytes surrounding the cerebroventricles of female rats
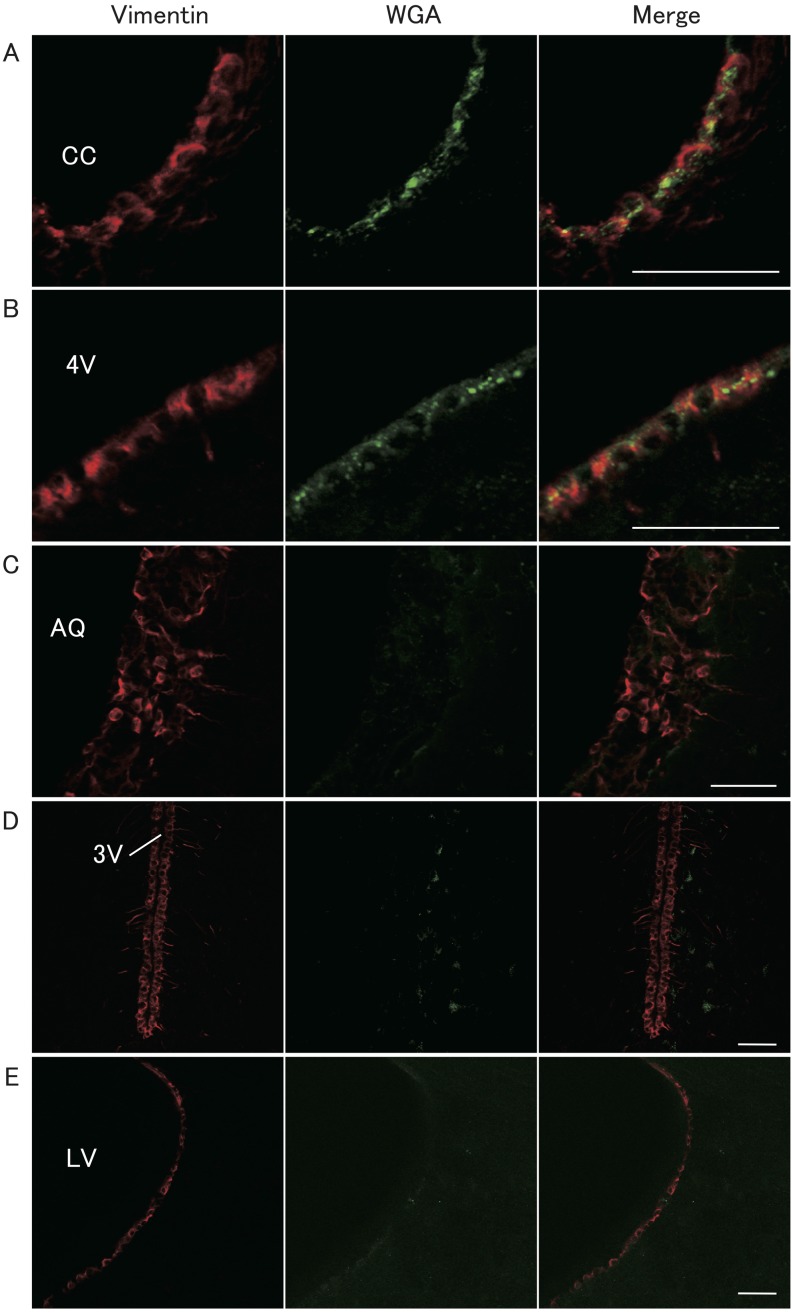
Figure 1 shows vimentin- and WGA-immunopositive cells in representative heterozygous Kiss1-tdTomato female rats injected with WGA into the 4V. Vimentin-immunopositive cells were found in the wall of the cerebral lumen including the lateral ventricle (LV), third ventricle (3V), aqueduct (AQ), 4V and CC (Fig. 1). The granular-like WGA immunoreactivities were found in cell bodies of the vimentin-immunopositive ependymocytes surrounding the CC and 4V (Figs. 1A and 1B) in the all animals. However, few WGA immunoreactivities were found in the vimentin-positive ependymocytes of the wall of the AQ, 3V and LV (Figs. 1C, 1D and 1E).
Fig. 1.
Localization of vimentin and wheat-germ agglutinin (WGA) immunoreactivities in the brain of a representative female rat injected with WGA into the fourth ventricle (4V). Vimentin immunoreactivity was used as a marker of ependymocytes. Vimentin (red, left panels) and WGA (green, middle panels) were visualized by immunohistochemistry and merged cells are shown in the right panels. Scale bars = 50 μm; CC, central canal; AQ, aqueduct; 3V, third ventricle; LV, lateral ventricle.
Distribution of WGA-immunopositive cells in the forebrain, midbrain and hindbrain
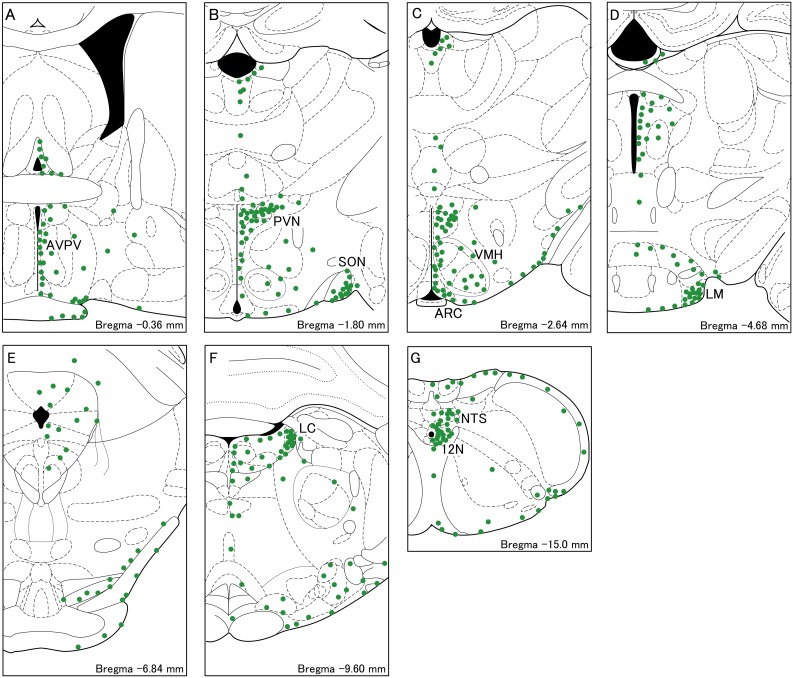
Figure 2 shows schematic illustrations indicating the distribution of WGA-immunopositive cells in the brain of female rats injected with WGA into the 4V. WGA-immunopositive cell bodies were widely distributed in the thalamus, hypothalamus, pons and medulla oblongata (Fig. 2). Among these regions, WGA-immunopositive cell bodies abundantly found in the AVPV (Fig. 2A), PVN (Fig. 2B), SON (Fig. 2B), ARC (Fig. 2C), ventromedial hypothalamus (VMH, Fig. 2C), lateral-mammillary nucleus (LM, Fig. 2D), locus coeruleus (Fig. 2F), hypoglossal nucleus (12N, Fig. 2G), and the solitary tract nucleus (Fig. 2G).
Fig. 2.
Schematic illustrations of WGA-immunopositive cells in the brainstem frontal sections of a representative female rat injected with WGA into the 4V. The distribution of the cell bodies containing WGA (green dots) are shown in A–G. A dot indicates 20 cells. AVPV, the anteroventral-periventricular nucleus; PVN, the paraventricular nucleus; SON, supraoptic nucleus; ARC, the arcuate nucleus; VMH, the ventromedial hypothalamus; LM, the lateral-mammillary nucleus; LC, the locus coeruleus;12N, hypoglossal nucleus; NTS, the solitary tract nucleus.
Colocalization of WGA and tdTomato in the ARC and AVPV of female rats
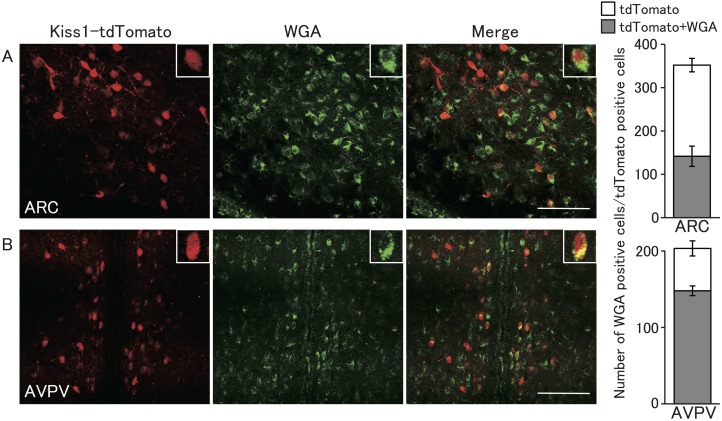
Figure 3 shows tdTomato fluorescence and WGA-immunopositive cells in representative heterozygous Kiss1-tdTomato female rats injected with WGA into the 4V. The tdTomato-positive cells were found in the ARC and AVPV in the hypothalamus, but not in other brain regions. The distribution of the tdTomato-positive cells was consistent with previous studies, in which kisspeptin and tdTomato were colocalized in these nuclei [25]. Co-expression of WGA and tdTomato was found in the both ARC and AVPV of female rats. WGA immunoreactivities were found in 40% of tdTomato-positive cells in the ARC (142 cells out of 352 cells, in mean, n = 3) (Fig. 3A, right graph), and were found in 73% of tdTomato-expressing cells in the AVPV (147 cells out of 203 cells) (Fig. 3B, right graph). In the ARC and AVPV regions, some cells showed only WGA immunoreactivity without tdTomato fluorescence.
Fig. 3.
Localization of WGA immunoreactivity and tandem dimer Tomato (tdTomato)-expressing kisspeptin neurons of the ARC and AVPV in a heterozygous Kiss1-tdTomato female rat injected with WGA into the 4V. Representative photomicrographs of the brain sections including ARC (A) or AVPV (B) visualized by immunohistochemistry for WGA (green) and tdTomato fluorescence (red). Right columns indicate the number of tdTomato-expressing cells with (gray) or without WGA immunoreactivity (open). Values are indicated as means ± SEM. Scale bars = 100 μm.
Localization of WGA immunoreactivity in the DBH-immunopositive cells of the noradrenergic regions and area postrema
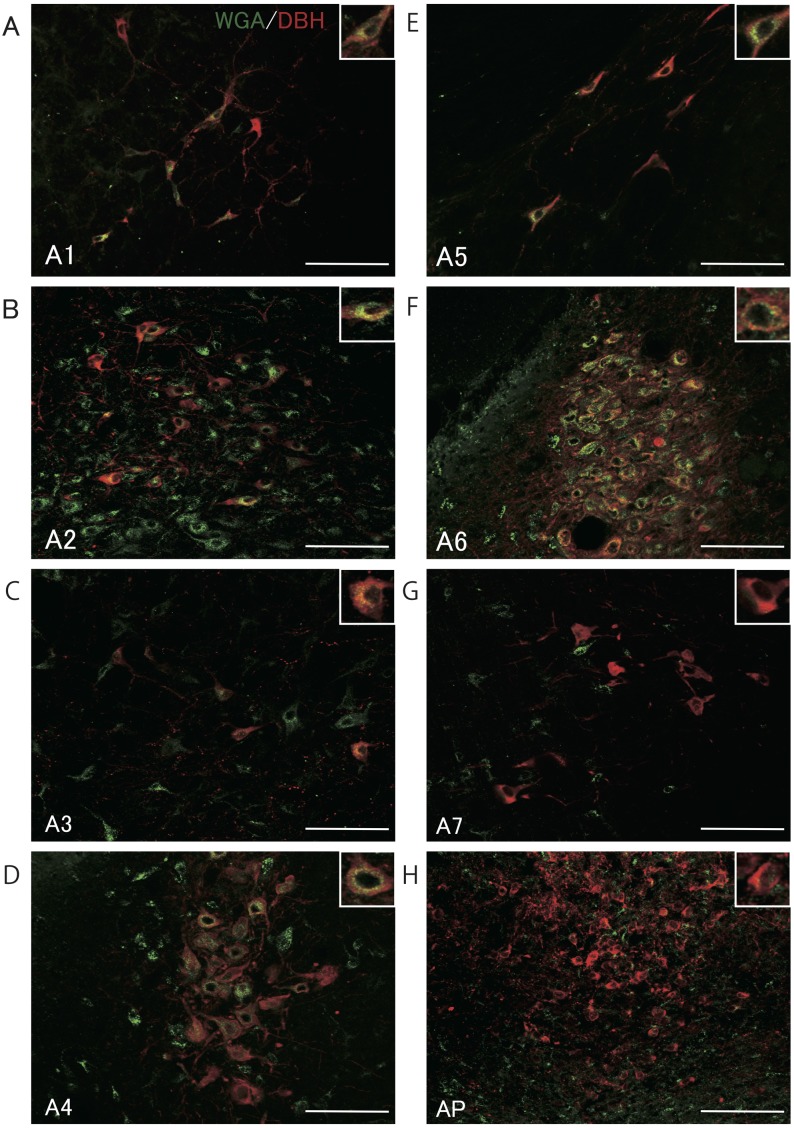
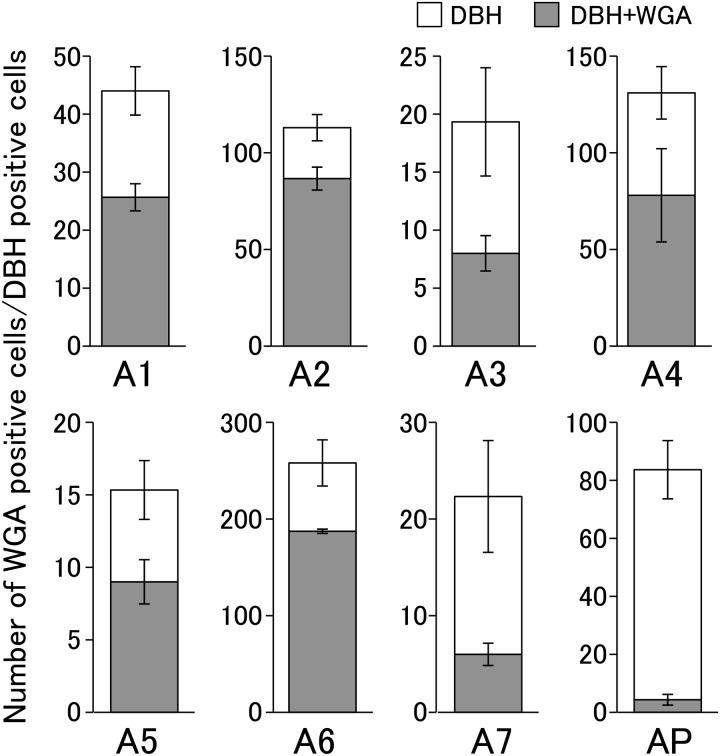
Figure 4 shows WGA- and DBH-immunopositive cells in representative heterozygous Kiss1-tdTomato female rats injected with WGA into the 4V, and Fig. 5 shows the number of DBH-immunopositive cells with or without WGA signals in the A1–A7 regions and area postrema. DBH immunoreactivity was found in cells in the A1–A7 regions (Figs. 4A–4H). Especially, WGA immunoreactivities were detected in most of DBH-positive cells of the A2 region in the solitary tract nucleus (77%; 87 out of 113 cells, in mean, n = 3) and A6 region in the locus coeruleus (73%; 187 out of 258 cells). In the A1, A3–A5 regions, approximately half of DBH-positive cells showed WGA immunoreactivity: A1 (41%; 26 out of 44 cells); A3 (41%; 8 out of 19 cells); A4 (60%; 79 out of 131 cells); A5 (59%; 9 out of 15 cells). The WGA immunoreactivity was also found in the DBH-immunopositive cells in the A7 region (27%; 6 out of 22 cells), however, a few DBH-positive cells showed WGA immunoreactivity in the area postrema (5%; 4 out of 84 cells). Note that the number of DBH-positive cells greatly varied depending on the nuclei.
Fig. 4.
Localization of WGA immunoreactivity and dopamine β-hydroxylase (DBH)-immunopositive cells in A1–A7 regions and the area postrema (AP). Photomicrographs of sections including A1–A7 noradrenergic regions and AP stained for DBH (red) and WGA (green) shown in A–H. Scale bars = 100 μm.
Fig. 5.
Quantitative analysis of co-expression of WGA in the DBH-immunopositive cells of the A1–A7 regions and the area postrema (AP). Columns indicate the number of DBH-immunopositive cells with (gray) or without WGA (open). Values are indicated as means ± SEM.
Localization of WGA immunoreactivity in the CRH-positive cells in the PVN and SON
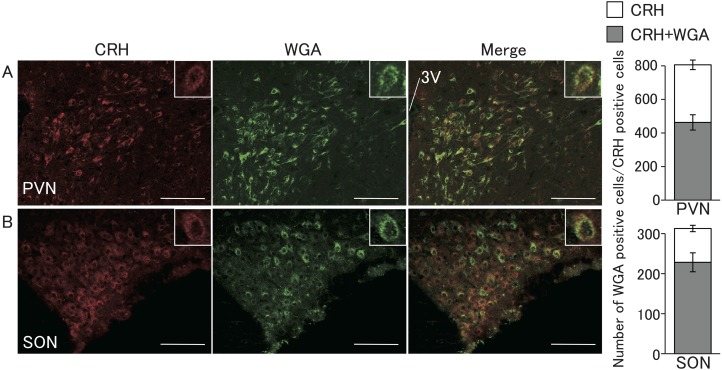
Figure 6 shows WGA- and CRH-immunopositive cells in the PVN and SON in representative female rats injected with WGA into the 4V. CRH-immunopositive cells were found in the parvocellular cellular region of the PVN (Fig. 6A) and throughout the SON (Fig. 6B). WGA-immunopositive cells were also mainly found in the parvocellular region, while few were found in the magnocellular region in the PVN (Fig. 6A) and throughout the SON (Fig. 6B). A large population of the CRH-positive cells showed WGA signals in the PVN (57%; 463 out of 806 cells, in mean, n = 3) and SON (73%; 228 out of 312 cells).
Fig. 6.
Localization of WGA immunoreactivity and corticotropin-releasing hormone (CRH)-immunopositive cells in the PVN and SON. Photomicrographs of sections including the PVN and SON stained by immunohistochemistry for CRH (red) and WGA (green) shown in A and B. Right columns indicate the number of CRH-immunopositive cells with (gray) or without WGA (open). Values are indicated as means ± SEM. Scale bars = 100 μm.
Localization of ependymal fibers and DBH-immunopositive cells in the A2 region
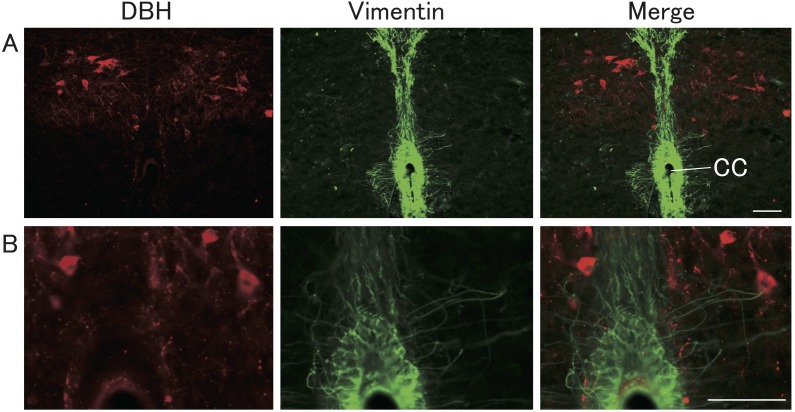
Figure 7 shows vimentin- and DBH-immunopositive cell bodies and fibers in representative E2-treated OVX rats. Some vimentin-immunopositive ependymal fibers were closely located with the DBH-immunopositive cell bodies.
Fig. 7.
Localization of vimentin and DBH immunoreactivities in the brainstem. Representative photomicrographs of sections showing DBH-immunopositive (red) and vimentin-immunopositive (green) cells and fibers in the brainstem including the A2 region of NTS and the central canal (A). High magnification of photomicrographs is shown in B. Scale bars = 50 μm.
Discussion
The present study provides evidence of the neuronal pathway originating from the hindbrain ependymocytes to the kisspeptin neurons located in the ARC and AVPV, because WGA immunoreactivities were found in the tdTomato-expressing kisspeptin neurons in the ARC and AVPV in the female rats injected with WGA into the 4V. To our knowledge, this is the first report to show morphological evidence of the neuronal connection between the hindbrain ependymocytes and hypothalamic kisspeptin neurons. It should be noted that WGA-immunoreactivities were found in the vimentin-immunopositive ependymocytes surrounding the 4V and CC, but not in the 3V and LV in female rats bearing the 4V WGA injection, suggesting that WGA was only taken into the hindbrain ependymocytes not the hypothalamic ones. Previous studies have suggested that the 4V ependymocytes contribute to sensing lower energy availability to control LH release. In vitro intracellular Ca2+ concentration of rat 4V ependymocytes increases in response to low extracellular glucose levels and 4V injection of 2DG suppresses pulsatile LH release in female rats [8, 28]. Taken together, the present results suggest that lowered glucose availability sensed by the hindbrain ependymocytes is conveyed to the hypothalamic kisspeptin neuron, a key player for GnRH secretion in mammals, to suppress LH release.
The present results suggest that hindbrain ependymocytes convey low energy availability signals to kisspeptin neurons via the brainstem noradrenergic neurons and/or the hypothalamic CRH neurons, because WGA-immunoreactivities were found in many of the A1–A6 noradrenergic neurons as well as the PVN/SON CRH neurons. This notion is largely consistent with previous studies showing that the A2 noradrenergic neurons projecting to the PVN and endogenous CRH mediate the fasting-induced suppression of LH pulses in female rats [20, 29, 30]. Further, 4V injection of 2DG or 48-h fasting suppresses LH pulses in rats [2, 20, 28]. The 4V or central canal ependymocytes may have a direct connection with A2 noradrenergic neurons, because the current study showed that ependymal fibers were closely located with the cell bodies and fibers of A2 DBH-immunopositive noradrenergic neurons. Thus, taken together, the low energetic signals sensed by hindbrain ependymocytes may activate A2 noradrenergic neurons and then PVN CRH neurons, consequently suppressing the activities of the ARC kisspeptin neuron, which is suggested to be a key regulator for pulsatile GnRH/LH secretion [11, 12]. Interestingly, WGA signals were found in approximately half of ARC kisspeptin neurons, suggesting that a part of ARC kisspeptin neurons receive energetic signals from the hindbrain ependymocytes. It was suggested that activity of ARC kisspeptin neurons, referred to as KNDy neurons, synchronize with each other by both neuron-glia and neuron-neuron communications via gap junctions and NKB-NKB receptor signaling [31]. Thus, it is speculated that the energetic signals conveyed to a part of ARC kisspeptin neurons from the hindbrain ependymocytes may affect ARC kisspeptin population as a whole to control pulsatile GnRH/LH secretion. The hindbrain ependymocytes-A2 noradrenergic neurons-PVN pathway may be also involved in the induction of feeding: indeed, 4V injection of 2DG increased food intake in male rats [28], and PVN injection of noradrenergic agonists stimulated feeding behavior [32].
In the present study, WGA immunoreactivities were found in the A6 noradrenergic neurons. The A6 noradrenergic neurons are suggested to be involved in the GnRH/LH surge system, because electrolytic or DSAP-induced lesion of the A6 noradrenergic neurons attenuated the preovulatory GnRH/LH surge [22, 33]. AVPV kisspeptin neurons, most of which were WGA-immunopositive in the current study, are recognized to be responsible for the GnRH/LH surge generation: kisspeptin expression in the AVPV is upregulated by a preovulatory increase in circulating estrogen [34,35,36] and electrolytic lesion of the AVPV inhibited the LH surge [37, 38]. Further, acute fasting or peripheral 2DG injection suppresses steroid-induced GnRH/LH surge in female rats [3, 39]. Interestingly, a larger percentage of AVPV tdTomato-positive kisspeptin neurons showed WGA signals than in the ARC tdTomato-positive cells. This may imply that the AVPV kisspeptin neurons could be more sensitive than the ARC kisspeptin neurons. Further, it was reported that AVPV kisspeptin neurons expressed CRH receptor and received CRH immunoreactive fibers [24]: A tracing study suggested that AVPV region receives a projection from PVN neurons [40]. Thus, it is also possible that the PVN CRH neurons may directly project to the AVPV kisspeptin neurons to affect the AVPV kisspeptin neuron activity and consequently GnRH/LH release. Taken together, these results can be interpreted as follows: low energetic signals sensed by the hindbrain ependymocytes would be conveyed to the AVPV kisspeptin neurons via A6 noradrenergic neurons and/or the PVN CRH neurons, consequently resulting in suppression of GnRH/LH surge, and then ovulation. It should be noted that many neuronal fibers from the A6 project to the preoptic area in adult female rats [43], where GnRH neurons were located. This suggests that GnRH neurons may be also projected by A6 noradrenergic neurons directly.
To date, the physiological role of the SON CRH neurons in regulation of hypothalamic kisspeptin neurons and/or gonadotropin release is unclear. Previous studies reported that A1, A2 and A6 noradrenergic neurons project to the SON [41] and kisspeptin immunoreactive fibers were found in the SON [42], suggesting that hindbrain ependymocytes may have a connection with the SON via these noradrenergic and/or kisspeptin neuronal pathways.
The noradrenergic neurons in the A1, A5 and A7 regions, showing WGA immunoreactivities in the present study, may be involved in the control of feeding behavior. These noradrenergic neurons might mediate satiety signals sensed by 4V ependymocytes, because the chemical and electrolytic lesion of the ventral noradrenergic bundle, derived from A1, A5 and A7 cell groups, induced hyperphagia in female rats [44]. In the current study, WGA immunoreactivities were observed in CRH-immunopositive neurons in the PVN and SON, suggesting that the hindbrain ependymocytes have a neuronal connection with the CRH neurons in these nuclei. The CRH neurons could be involved in feeding and metabolism, since it has been reported that 3V injection of CRH reduced food intake and body weight in male rats [45]. Interestingly, few WGA immunoreactivities were found in the area postrema. This suggests that ependymocytes have few connections with the area postrema and that WGA injected into the 4V was unlikely taken into the area postrema cells. It was reported that the area postrema has a role in the regulation of feeding behavior, because that lesion of the area postrema blocked glutamate-induced feeding [46]. The area postrema has been also suggested to mediate the suppressive effect of insulin-induced glucoprivation on pulsatile LH secretion [47]. Thus, the area postrema cells may connect to hypothalamic kisspeptin neurons via an independent pathway from the hindbrain ependymocytes.
In the present study, WGA immunoreactivities were found in the lateral ventral part of the VMH. In the VMH, expression of mRNA of receptors for orexin and leptin, major regulators of food intake, were reported [48, 49]. Energetic signals sensed by the 4V ependymocytes may modulate the role of these receptors: indeed, acute fasting induced an increase in their mRNA expression [50, 51]. We also found WGA immunoreactivities in non-kisspeptin neurons in the ARC. In the ARC, several orexigenic peptides, such as neuropeptide Y, agouti-related protein, and an anorexigenic peptide, such as α-melanocyte-stimulating hormone, are distributed [52]. Future studies are needed to clarify the connection between the hindbrain ependymocytes and these ARC orexigenic and anorexigenic neurons or VMH leptin and orexin receptive neurons. WGA-immunopositive cell bodies were also found in other brain regions, such as A3 and A4 noradrenergic nuclei, LM and 12N. Since few reports are available to show the physiological roles of these nuclei, future studies are required to investigate the role of the interaction between these nuclei and the hindbrain ependymocytes.
In conclusion, the present study suggests that the hindbrain ependymocytes have neuronal connections with the ARC and AVPV kisspeptin neurons through the brainstem adrenergic and/or hypothalamic CRH neurons: most probably, the A2 noradrenergic and then hypothalamic CRH neurons relay the pathway to the ARC kisspeptin neurons to convey the low energetic signals to suppress GnRH/gonadotropin pulses, while A6 noradrenergic neurons may have a role to convey low energetic signals to the AVPV from the ependymocytes to suppress GnRH/gonadotropin surge in mammals.
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Numbers 23380163 and 18H03973 to HT, 24380157 to KM, 16K07987 to NI. We thank Dr Nicola Skoulding for editorial assistance.
Appendix
Table A1. Antibody table.
| Peptide / Protein target | Name of antibody | Manufacturer, Catalog No | Species raised in; monoclonal or polyclonal |
Dilution used | RRID |
|---|---|---|---|---|---|
| WGA | Anti-WGA | Sigma-Aldrich | Rabbit; polyclonal | 1:10000 | AB_261669 |
| Cat# T4144 | |||||
| Vimentin | Anti-Vimentin | Millipore | Chicken; polyclonal | 1:2000 | AB_11212377 |
| Cat# AB5733 | |||||
| DBH | Anti-DBH | Millipore | Mouse; monoclonal | 1:1000 | AB_2245740 |
| Cat# MAB308 | |||||
| CRF | Anti-CRH | Peninsula Laboratories | Guinea pig; unknown | 1:5000 | AB_518256 |
| Cat# T-5007 | |||||
| Rabbit IgG | Alexa 488-conjugated | Thermo Fisher Scientific | Goat; polyclonal | 1:800 | AB_143165 |
| anti-rabbit IgG | Cat# A-11008 | ||||
| Chicken IgG (H+L) | Alexa 594-conjugated | Molecular Probes | Goat; unknown | 1:800 | AB_142803 |
| anti-chicken IgG | Cat# A-11042 | ||||
| Mouse IgG (H+L) | Alexa 594-conjugated | Molecular Probes | Donkey; unknown | 1:800 | AB_141633 |
| anti-mouse IgG | Cat# A-21203 | ||||
| Guinea pig IgG (H+L) | Alexa 594-conjugated | Jackson Immuno Research Labs Cat# 106-585-003 | Goat; polyclonal | 1:800 | AB_2337442 |
| anti-guinea pig IgG |
References
- 1.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev 1992; 16: 235–272. [DOI] [PubMed] [Google Scholar]
- 2.Cagampang FR, Maeda KI, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J Endocrinol 1991; 129: 321–328. [DOI] [PubMed] [Google Scholar]
- 3.Kohsaka A, Watanobe H, Kakizaki Y, Suda T, Schiöth HB. A significant participation of orexin-A, a potent orexigenic peptide, in the preovulatory luteinizing hormone and prolactin surges in the rat. Brain Res 2001; 898: 166–170. [DOI] [PubMed] [Google Scholar]
- 4.McClure TJ, Saunders J. Effects of withholding food for 0−72 h on mating, pregnancy rate and pituitary function in female rats. J Reprod Fertil 1985; 74: 57–64. [DOI] [PubMed] [Google Scholar]
- 5.Maekawa F, Toyoda Y, Torii N, Miwa I, Thompson RC, Foster DL, Tsukahara S, Tsukamura H, Maeda K. Localization of glucokinase-like immunoreactivity in the rat lower brain stem: for possible location of brain glucose-sensing mechanisms. Endocrinology 2000; 141: 375–384. [DOI] [PubMed] [Google Scholar]
- 6.Minabe S, Deura C, Ikegami K, Goto T, Sanbo M, Hirabayashi M, Inoue N, Uenoyama Y, Maeda K, Tsukamura H. Pharmacological and morphological evidence of ampk-mediated energy sensing in the lower brain stem ependymocytes to control reproduction in female rodents. Endocrinology 2015; 156: 2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 2005; 360: 2211–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriyama R, Tsukamura H, Kinoshita M, Okazaki H, Kato Y, Maeda K. In vitro increase in intracellular calcium concentrations induced by low or high extracellular glucose levels in ependymocytes and serotonergic neurons of the rat lower brainstem. Endocrinology 2004; 145: 2507–2515. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M, I’Anson H, Tsukamura H, Maeda K. Fourth ventricular alloxan injection suppresses pulsatile luteinizing hormone release in female rats. J Reprod Dev 2004; 50: 279–285. [DOI] [PubMed] [Google Scholar]
- 10.Singh SR, Briski KP. Septopreoptic mu opioid receptor mediation of hindbrain glucoprivic inhibition of reproductive neuroendocrine function in the female rat. Endocrinology 2004; 145: 5322–5331. [DOI] [PubMed] [Google Scholar]
- 11.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O’Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 2012; 153: 307–315. [DOI] [PubMed] [Google Scholar]
- 13.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151: 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett 2016; 612: 161–166. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008; 28: 8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamura H, Homma T, Tomikawa J, Uenoyama Y, Maeda K. Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann N Y Acad Sci 2010; 1200: 95–103. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki T, Iwasa T, Kinouchi R, Yoshida S, Murakami M, Gereltsetseg G, Yamamoto S, Kuwahara A, Yasui T, Irahara M. Fasting reduces the kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr J 2011; 58: 1003–1012. [DOI] [PubMed] [Google Scholar]
- 19.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol 2008; 20: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 20.Maeda K, Cagampang FR, Coen CW, Tsukamura H. Involvement of the catecholaminergic input to the paraventricular nucleus and of corticotropin-releasing hormone in the fasting-induced suppression of luteinizing hormone release in female rats. Endocrinology 1994; 134: 1718–1722. [DOI] [PubMed] [Google Scholar]
- 21.I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 2003; 144: 4325–4331. [DOI] [PubMed] [Google Scholar]
- 22.Anselmo-Franci JA, Franci CR, Krulich L, Antunes-Rodrigues J, McCann SM. Locus coeruleus lesions decrease norepinephrine input into the medial preoptic area and medial basal hypothalamus and block the LH, FSH and prolactin preovulatory surge. Brain Res 1997; 767: 289–296. [DOI] [PubMed] [Google Scholar]
- 23.Nagatani S, Bucholtz DC, Murahashi K, Estacio MA, Tsukamura H, Foster DL, Maeda KI. Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology 1996; 137: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 24.Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett 2012; 531: 40–45. [DOI] [PubMed] [Google Scholar]
- 25.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam; Boston: Elsevier Academic Press; 2005. [Google Scholar]
- 27.Arvidsson J, Pfaller K. Central projections of C4-C8 dorsal root ganglia in the rat studied by anterograde transport of WGA-HRP. J Comp Neurol 1990; 292: 349–362. [DOI] [PubMed] [Google Scholar]
- 28.Murahashi K, Bucholtz DC, Nagatani S, Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology 1996; 137: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamura H, Nagatani S, Cagampang FR, Kawakami S, Maeda K. Corticotropin-releasing hormone mediates suppression of pulsatile luteinizing hormone secretion induced by activation of alpha-adrenergic receptors in the paraventricular nucleus in female rats. Endocrinology 1994; 134: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 30.Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Effect of corticotropin-releasing hormone antagonist on oestrogen-dependent glucoprivic suppression of luteinizing hormone secretion in female rats. J Neuroendocrinol 1999; 11: 101–105. [DOI] [PubMed] [Google Scholar]
- 31.Ikegami K, Minabe S, Ieda N, Goto T, Sugimoto A, Nakamura S, Inoue N, Oishi S, Maturana AD, Sanbo M, Hirabayashi M, Maeda KI, Tsukamura H, Uenoyama Y. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J Neuroendocrinol 2017; 29: 29. [DOI] [PubMed] [Google Scholar]
- 32.Leibowitz SF. Hypothalamic paraventricular nucleus: interaction between alpha 2-noradrenergic system and circulating hormones and nutrients in relation to energy balance. Neurosci Biobehav Rev 1988; 12: 101–109. [DOI] [PubMed] [Google Scholar]
- 33.Szawka RE, Poletini MO, Leite CM, Bernuci MP, Kalil B, Mendonça LB, Carolino RO, Helena CV, Bertram R, Franci CR, Anselmo-Franci JA. Release of norepinephrine in the preoptic area activates anteroventral periventricular nucleus neurons and stimulates the surge of luteinizing hormone. Endocrinology 2013; 154: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 2006; 26: 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 36.Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology 2010; 58: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 1980; 31: 147–157. [DOI] [PubMed] [Google Scholar]
- 38.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 1982; 34: 395–404. [DOI] [PubMed] [Google Scholar]
- 39.Briski KP, Sylvester PW. Role of endogenous opiates in glucoprivic inhibition of the luteinizing hormone surge and fos expression by preoptic gonadotropin-releasing hormone neurones in ovariectomized steroid-primed female rats. J Neuroendocrinol 1998; 10: 769–776. [DOI] [PubMed] [Google Scholar]
- 40.Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol 2006; 494: 190–214. [DOI] [PubMed] [Google Scholar]
- 41.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 1982; 257: 275–325. [DOI] [PubMed] [Google Scholar]
- 42.Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol 2010; 22: 1101–1112. [DOI] [PubMed] [Google Scholar]
- 43.Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 1977; 127: 25–53. [PubMed] [Google Scholar]
- 44.Ahlskog JE, Hoebel BG. Overeating and obesity from damage to a noradrenergic system in the brain. Science 1973; 182: 166–169. [DOI] [PubMed] [Google Scholar]
- 45.Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol 1988; 255: E255–E259. [DOI] [PubMed] [Google Scholar]
- 46.Ritter S, Stone SL. Area postrema lesions block feeding induced by systemic injections of monosodium glutamate. Physiol Behav 1987; 41: 21–24. [DOI] [PubMed] [Google Scholar]
- 47.Cates PS, O’Byrne KT. The area postrema mediates insulin hypoglycaemia-induced suppression of pulsatile LH secretion in the female rat. Brain Res 2000; 853: 151–155. [DOI] [PubMed] [Google Scholar]
- 48.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 1998; 438: 71–75. [DOI] [PubMed] [Google Scholar]
- 49.Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 1998; 395: 535–547. [PubMed] [Google Scholar]
- 50.Mitchell SE, Nogueiras R, Morris A, Tovar S, Grant C, Cruickshank M, Rayner DV, Dieguez C, Williams LM. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol 2009; 587: 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 2000; 37: 335–344. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000; 404: 661–671. [DOI] [PubMed] [Google Scholar]