Abstract
This study aimed to investigate the effect of resveratrol supplementation in maturation medium on the developmental ability and bioenergetic\oxidative status of prepubertal goat oocytes selected by brilliant cresyl blue (BCB). Oocytes collected from slaughterhouse-derived ovaries were selected by 13 µM BCB staining and classified as grown BCB+ and growing BCB- oocytes. All oocytes were matured in vitro in our conventional maturation medium and supplemented with 1 µM (BCB+R and BCB-R) and without (Control groups: BCB+C and BCB-C) resveratrol. After 24 h, IVM-oocytes were fertilized with fresh semen and presumptive zygotes were in vitro cultured for 8 days. Oocytes were assessed for blastocyst development and quality, mitochondrial activity and distribution, and levels of GSH, ROS, and ATP. BCB+R (28.3%) oocytes matured with resveratrol presented significantly higher blastocyst development than BCB+C (13.0%) and BCB- groups (BCB-R: 8.3% and BCB-C: 4.7%). Resveratrol improved blastocyst development of BCB-R oocytes at the same rate as BCB+C oocytes. No differences were observed in blastocyst quality among groups. GSH levels were significantly higher in resveratrol groups (BCB+R: 36554.6; BCB-R: 34946.7 pixels/oocyte) than in control groups (BCB+C: 27624.0; BCB-C: 27655.4 pixels/oocyte). No differences were found in mitochondrial activity, ROS level, and ATP content among the groups. Resveratrol-treated oocytes had a higher proportion of clustered active mitochondria in both BCB groups (BCB+R: 73.07%; BCB-R: 79.16%) than control groups (BCB+C: 19.35%; BCB-C: 40%). In conclusion, resveratrol increased blastocyst production from oocytes of prepubertal goats, particularly in better quality oocytes (BCB+).
Keywords: Antioxidant, Blastocyst, In vitro fertilization (IVF), Juvenile ruminants
Goat production in Mediterranean countries is economically and socially important. For these species, artificial insemination (AI) is the most used reproductive technology. However, in vitro embryo production (IVEP) using oocytes derived from prepubertal animals in conjunction with in vitro embryo transfer, termed as juvenile in vitro embryo transfer (JIVET), can accelerate genetic gain by shortening generation intervals. The addition of JIVET to artificial insemination yielded an extra 25 to 60% genetic gain in sheep programs [1]. The first births using JIVET were reported in the late 1970s [2] but the efficiency of this technology remains low, mainly due to the low competence of these oocytes compared to those from adult females. The reduced in vitro embryo development of these oocytes has been related to oocyte structural and molecular abnormalities [3,4,5,6,7], which are signs of their poor quality.
In order to improve in vitro embryo production, the selection of high-quality oocytes is crucial. Brilliant cresyl blue (BCB) staining is a non-invasive method used for the selection of immature oocytes [8]. BCB is a glucose-6-phosphate dehydrogenase (G6PD) substrate, from which it is reduced from blue to a colorless compound. G6PD activity gradually decreases as oocytes reach their maximum growth phase [9]. Thus, grown oocytes present a low G6PD activity and cannot reduce BCB, so they show a blue cytoplasm (BCB+), while growing oocytes with high G6PD activity reduce BCB and present an unstained cytoplasm (BCB-) [10]. Several studies in cattle [11, 12], sheep [13, 14], horses [15], goats [16], buffalos [17] and mice [18] showed that BCB+ oocytes presented higher embryo development competence compared to BCB- oocytes. However, in adult goats, 3.6% of morphologically good oocytes were found to show signs of degeneration following BCB staining [8].
An important factor contributing to the poor quality of in vitro matured oocytes could be their sensitivity to oxidative stress [19]. These oocytes are less able to maintain an appropriate redox homeostasis in response to oxidative stress generated by the in vitro condition compared to those from adult females [20]. This could be caused by an altered synthesis of endogenous antioxidants [20, 21]. The addition of antioxidants to the maturation medium has been proposed as a good strategy to overcome the effect of oxidative stress allowing an increase in oocyte embryo development [19, 22].
Resveratrol (3,4,5-trihydroxy-trans-stilbene) is a small polyphenol synthesized by several plants, such as nuts, mulberry and grapes [23]. This phytoalexin is a potent antioxidant that, by activation of SIRT1, a NAD+ dependent deacetylase belonging to the sirtuin family, induces the upregulation of the endogen antioxidant system [24]. Recent studies highlighted that, SIRT1 acts as sensor of the redox state in oocytes and granulosa cells [24]. Furthermore, resveratrol is involved in the regulation of energy homeostasis [25], metabolism [26], estrogen levels [27], and genomic stability [28]. It has also been observed that resveratrol supplementation during in vitro maturation (IVM) positively affected oocyte quality, fertilization and embryo development outcomes in goats, cattle, and pigs [29].
This study investigated the effect of resveratrol supplementation of IVM media on the developmental ability of prepubertal goat oocytes selected by BCB. To this end the bioenergetic/oxidative status of in vitro matured oocytes, oocyte cleavage, and blastocyst formation and quality, following in vitro fertilization, were analyzed.
Materials and Methods
Materials
Unless stated otherwise, all chemicals were obtained from Sigma-Aldrich Chemical Co (St. Louis, MO, USA).
Oocytes collection and BCB staining
Oocytes were collected from ovaries of slaughtered juvenile (30 to 45-day-old) goats (Capra hircus). Oocytes with two or more complete layers of compact cumulus cells and with a uniform cytoplasm were selected for IVM. In the experiments two and three, morphologically selected oocytes were incubated with 13 µM BCB for 45 min under 5% CO2 in air at 38.5°C. After BCB exposure, oocytes were classified based on cytoplasm coloration, BCB+ (blue) or BCB- (colorless), and were in vitro matured [13].
In vitro maturation, in vitro fertilization (IVF) and embryo culture (IVEC)
Groups of 25–30 oocytes were matured in TCM-199, supplemented with 5 µg/ml follicle-stimulating hormone, 5 µg/ml luteinizing hormone, 1 µg/ml 17 β estradiol, 10 ng/ml EGF, 10% fetal bovine serum, 5 µg/ml gentamycin, 1 mM L-glutamine, and 0.2 mM sodium pyruvate, for 24 h under 5% CO2 in air at 38.5°C. After IVM, the oocytes were inseminated with fresh semen, obtained from three Murciano-Granadino bucks of proven fertility. Highly motile spermatozoa were selected using Bovipure density gradient kit (Nidacon EVB S.L., Barcelona, Spain). Oocytes were transferred into BO-IVF medium (IVF Bioscience; UK) for fertilization with 1 × 106 spermatozoa/mL for 20 h, under 5% CO2, 5% O2 and 90% N2 atmosphere, at 38.5°C. After in vitro fertilization, presumptive zygotes were cultured in BO-IVC (IVF Bioscience; UK) for 8 days, under 5% CO2, 5% O2 and 90% N2 atmosphere, at 38.5°C. The cleavage rate and blastocyst rate/blastocyst cell number were recorded at 48 h and on day 8.
Assessment of nuclear status
After IVM, denuded oocytes were fixed in ethanol and stained with 1 µM Hoechst 33342 solution (Invitrogen) for 1 h. The nuclear configurations were classified, using an epifluorescent microscope (Olympus BX50), as germinal vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), or metaphase II (MII).
Blastocyst differential staining
Analysis of blastocyst cell numbers was performed by differential staining of the inner cell mass (ICM) and trophectoderm (TE) cell compartments [30]. Blastocysts were first incubated, briefly, for 15 sec in TCM199 with 1% Triton X-100 and 100 µg/ml propidium iodide, then transferred into an ethanol solution with Hoechst 33342 for 3 h. A digital image of each blastocyst was taken by epifluorescence microscopy and the numbers of TE (red) and ICM (blue) nuclei were counted using ImageJ software (ImageJ 1.5Oi).
Measurement of glutathione (GSH) and reactive oxygen species (ROS) levels
Denuded oocytes at the MII stage (presence of the first polar body) were incubated in the dark for 30 min with 10 µM 2’7’-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes, Eugene, OR, USA) or 10 µM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (Cell Tracker Blue; CMF2HC; Molecular Probes, Eugene, OR, USA) for reactive oxygen species or glutathione detection, respectively. An epifluorescent microscope with a UV filter (460 nm for ROS and 370 nm for GSH) was used to take digital images, and the fluorescence intensities of the oocytes were analyzed using ImageJ software [31].
Quantification of adenosine 5’-triphosphate (ATP) intracellular content
Groups of 6 MII oocytes were denuded by gentle pipetting and placed into Eppendorf tubes with 200 µl ultrapure water and stored at –80°C until their analysis. The ATP content of oocytes was measured using the adenosine 5-triphosphate bioluminescent somatic cell assay kit (FLASC) as described previously [32]. The bioluminescence generated by ATP-dependent luciferin-luciferase reaction was immediately measured using a luminometer.
Evaluation of mitochondrial distribution and activity
After IVM, MII oocytes were denuded and incubated in the dark for 30 min with 200 nM MitoTracker Orange CMTMRos (Molecular Probes, Eugene, OR, USA), under 5% CO2 in air, at 38.5°C. After incubation, oocytes were fixed in 3% paraformaldehyde for 60 min at 38°C and stained with 1 µM Hoechst 33342 solution. Oocytes were stored at 4°C in the dark until their analysis [13].
Mitochondria analysis was performed using Leica TCS SP5 CLSM with LAS lite 170 Image software equipped with a 405 nm diode laser and a multiphoton laser. In each individual oocyte MitoTracker fluorescence intensities were measured at the equatorial plane as described previously [33]. Leica LAS AF Lite image analysis software package (Leica Microsystems GmbH, Wetzlar, Germany) was used for the quantitative analysis of fluorescence intensity. Mitochondrial distribution patterns were classified in two groups, as previously reported [34], with some modifications: 1) Pattern A: homogeneous fine, with small granulations spread throughout the cytoplasm; 2) Pattern B: heterogeneous clustered, with large granulations spread throughout the cytoplasm or located in specific cytoplasmic domains.
Experimental design
Experiment 1: Effect of resveratrol supplementation at different concentrations on oocyte developmental competence.
The dose-responsive effects of resveratrol on oocyte developmental competence were evaluated. Resveratrol was added to the IVM medium at concentrations of 0.5 µM and 1 µM. A group of oocytes were cultured, in the absence of resveratrol, as control (C). After in vitro fertilization (IVF) cleavage rate, blastocyst formation and cell number were evaluated.
Experiment 2: Effect of 1 µM resveratrol supplementation on the developmental competence of oocytes selected by BCB staining.
Based on the results of experiment 1, we evaluated the effect of 1 µM resveratrol on the developmental competence of oocytes selected by BCB staining. Oocytes were matured in vitro with (BCB+R; BCB-R) or without (BCB+C; BCB-C) 1 µM resveratrol. After IVF and in vitro embryo culture, cleavage rate, blastocyst formation and cell number were assessed.
Experiment 3: Effect of 1 µM resveratrol supplementation on the bioenergetic/oxidative status of oocytes selected by BCB staining.
Oocytes at the metaphase II stage from the different groups were analyzed for: intracellular ROS and GSH levels (Experiment 3a), intracellular ATP content, mitochondrial activity, and organization (Experiment 3b).
Statistical analysis
For each experiment, at least three replicates were carried out. The oocytes used in each replicate were from the same group of abattoir-derived ovaries collected on the same day. After BCB selection, BCB+ and BCB- oocytes were randomly distributed across resveratrol groups.
Statistical analysis was performed using STATA\IC 11.0 software package. Data were first checked for normal distribution and were analyzed using the Shapiro-Wilk test. Data for maturation, cleavage, blastocyst rates and blastocyst cell number, expressed as mean values ± standard errors of mean (SE), were normally distributed and analyzed using one-way ANOVA, followed by Bonferroni’s post-hoc test. Data for intracellular ROS and GSH levels, ATP content and mitochondrial activity, expressed as mean values ± standard errors of mean (SE), were not normally distributed and were analyzed with a non-parametric Kruskal-Wallis test. Active mitochondrial distribution was analyzed by Chi-square and Fischer’s exact tests where appropriate. The overall chi-square was calculated and found to be significant before performing the Fischer’s exact test to detect differences among experiment groups. Differences of 0.05 or less in the probability values were considered significant.
Results
Experiment 1: Effect of resveratrol supplementation at different concentrations on oocyte developmental competence and blastocyst cell number
Results on the effect of resveratrol supplementation, at different concentrations, on oocytes developmental competence after IVF are reported in Table 1. Cleavage and blastocyst rates were lower (P < 0.05) when 0 µM and 0.5 µM resveratrol was added to the IVM medium compared with the addition of 1 µM resveratrol. Total blastocyst cell numbers did not differ among groups (Table 1).
Table 1. Effect of different resveratrol concentrations on embryo development and blastocyst cell numbers of prepubertal goat oocytes (Experiment 1).
| Resveratrol concentration (µM) |
No. oocytes | No. cleaved |
No. blastocysts/cleaved |
No. blastocysts/total | Blastocyst cell number |
|---|---|---|---|---|---|
| (% ± SE) | (% ± SE) | (% ± SE) | (mean ± SE) | ||
| 0 | 164 | 100 (61.2 ± 2.0) a | 11 (10.9 ± 2.5) a | 11 (6.8 ± 1.6) a | 127 ± 10.8 |
| 0.5 | 152 | 89 (56.7 ± 5.3) a | 11 (14.5 ± 3.4) a | 11 (7.9 ± 1.7) a | 167.5 ± 23.8 |
| 1 | 163 | 137 (83.5 ± 2.8) b | 32 (24.5 ± 2.0) b | 32 (20.1 ± 1.3) b | 156.5 ± 13.9 |
a, b Values with different superscript letters within a column differ significantly (P < 0.05). Three replicate trials were performed.
Experiment 2: Effect of 1 µM resveratrol supplementation on the developmental competence of oocytes selected by BCB staining
Supplementation with 1 µM resveratrol during IVM did not affect meiotic progression and the ratio of nuclear maturation within BCB+ or BCB- groups (Table 2). No differences were found in the cleavage rate among groups (Table 3). The blastocyst rate (blastocyst / total oocytes) was higher (P < 0.05) in the BCB+C group compared to that of BCB-C group. Resveratrol supplementation increased the blastocyst rate of BCB- oocytes at the same rate as BCB+C. BCB+R presented a higher (P < 0.05) blastocyst rate than the BCB+C, BCB-C and BCB-R groups (Table 3). The total, ICM and TE blastocyst cell numbers did not differ among groups (Table 3).
Table 2. Effect of 1 µM resveratrol, added to the IVM medium, on meiotic progression of prepubertal goat oocytes selected by brilliant cresyl blue (BCB) staining (Experiment 2).
| Groups | No. oocytes | GV | GVBD | MI | MII |
|---|---|---|---|---|---|
| (% ± SE) | (% ± SE) | (% ± SE) | (% ± SE) | ||
| BCB+C | 53 | 0 | 1 (2.2 ± 1.3) | 4 (7.3 ± 2.3) | 48 (90.4 ± 1.1) |
| BCB+R | 46 | 0 | 0 | 3 (6.5 ± 0.1) | 43 (93.5 ± 0.1) |
| BCB-C | 47 | 0 | 1 (1.9 ± 1.1) | 6 (12.9 ± 0.5) | 40 (85.2 ± 0.6) |
| BCB-R | 50 | 1 (1.9 ± 1.1) | 1 (1.9 ± 1.1) | 8 (16.4 ± 3.1) | 40 (79.9 ± 1.0) |
GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; MII, metaphase II. Three replicate trials were performed.
Table 3. Effect of 1 µM resveratrol, added to the IVM medium, on embryo development and blastocyst cell number of prepubertal goat oocytes selected by brilliant cresyl blue (BCB) staining (Experiment 2).
| Groups | No. oocytes | No. cleaved | No. blastocysts/cleaved | No. blastocysts/total | Blastocyst cell number (mean ± SE) |
||
|---|---|---|---|---|---|---|---|
|
(% ± SE) |
(% ± SE) |
(% ± SE) |
Total | ICM | TE | ||
| BCB+C | 110 | 87 (78.4 ± 3.6) | 14 (16.0 ± 0.5) a | 14 (13.0 ± 0.7) a | 134.6 ± 7.4 | 30.2 ± 3.5 | 106.8 ± 21.8 |
| BCB+R | 116 | 103 (88.3 ± 2.6) | 32 (32.1 ± 1.3) b | 32 (28.3 ± 0.9) b | 167 ± 12.6 | 43.4 ± 4 | 133.4 ± 12 |
| BCB-C | 95 | 67 (71.1 ± 6.9) | 4 (8.3 ± 1.5) a | 4 (4.7 ± 0.4) c | 136 ± 4.9 | 32.5 ± 0.5 | 122.5 ± 28.5 |
| BCB-R | 88 | 67 (78.0 ± 8.9) | 8 (11.1 ± 1.4) a | 8 (8.3 ± 0.8) ac | 120.5 ± 12.4 | 31.3 ± 5.5 | 89.2 ± 8.2 |
a, b, c Values with different superscript letters within a column differ significantly (P < 0.05). Four replicate trials were performed.
Experiment 3: Effect of 1 µM resveratrol supplementation on the bioenergetic/oxidative status of oocytes selected by BCB staining
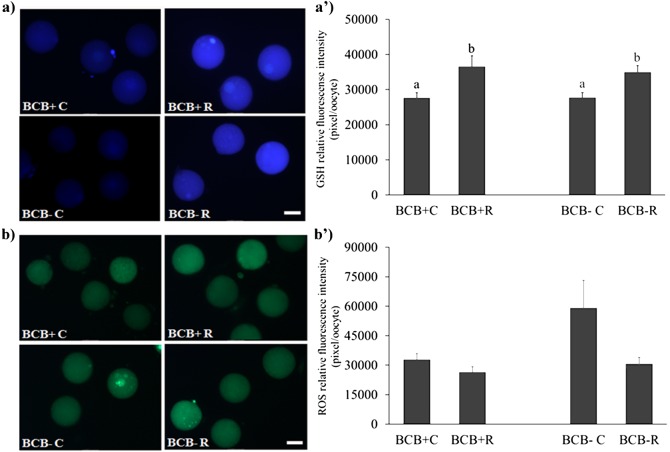
Experiment 3a: Intracellular GSH levels were higher (P < 0.05) in both BCB+ (36554.6 ± 3049.25 pixels/oocyte) and BCB- (34946.8 ± 1877.8 pixels/oocyte) groups treated with resveratrol during IVM compared to their respective controls, BCB+C (27624.0 ± 1513.67 pixels/oocyte) and BCB-C (27655.42 ± 1489.8 pixels/oocyte) groups (Fig. 1). We did not find any significant differences in ROS levels among experimental groups (BCB+C: 32740.3 ± 3165.0; BCB+R: 26314.1 ± 2857.0; BCB-C: 59071.3 ± 14079.0; BCB- R: 30587.3 ± 3337.0 pixels/oocyte) (Fig. 1).
Fig. 1.
Effect of 1 µM resveratrol, added to the in vitro maturation medium, on GSH and ROS intracellular levels of prepubertal goat oocytes selected by brilliant cresyl blue (BCB) staining (Experiment 3a): intracellular GSH (a) and ROS levels (b) of in vitro matured prepubertal goat oocytes. Epifluorescence photomicrographs of MII oocytes stained with CellTracker Blue to determine GSH levels (a’) and with 2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA) to detect ROS (b’). Values with different superscript letters (a vs. b) are significantly different (P < 0.05). Scale bar = 100 µm.
Experiment 3b: The intracellular ATP contents of the oocytes (Fig. 2) were not significantly different among groups (BCB+C: 3586.4 ± 203.6; BCB+R: 3219.0 ± 171.9; BCB-C: 3769.2 ± 267.6; 4083.1 ± 291.6 Fmol/oocyte).
Fig. 2.
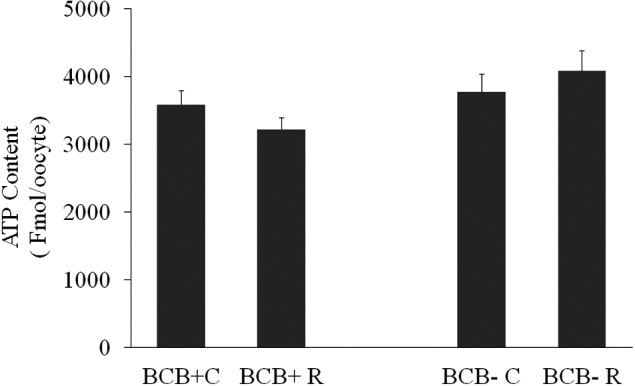
ATP content (mean ± SE) of brilliant cresyl blue (BCB)-selected prepubertal goat oocytes matured with or without 1 µM resveratrol (Experiment 3b).
We did not find any significant differences in the fluorescent image intensities of active mitochondria (BCB+C: 17.2 ± 2.6; BCB+R: 13.5 ± 1.4; BCB-C: 16.5 ± 2.5; BCB-R: 16.0 ± 2.4 AU) (Fig. 3).
Fig. 3.
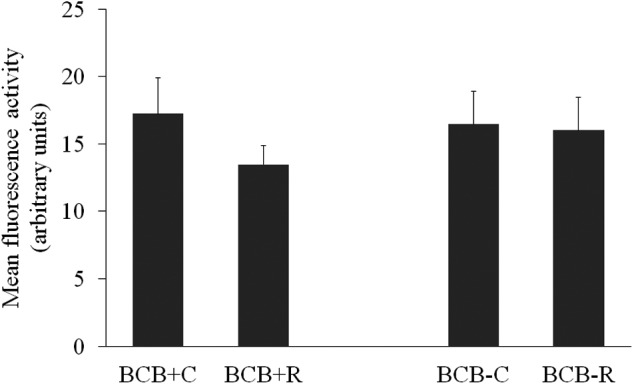
Mitochondrial activity of brilliant cresyl blue (BCB)-selected prepubertal goat oocytes in vitro matured with or without 1 µM resveratrol (Experiment 3b). Fluorescence intensity was measured at the equatorial plane. Values are expressed as arbitrary units (Means ± SE).
Mitochondrial distribution patterns were different (P < 0.05) between control and resveratrol-treated oocytes in both BCB groups. Resveratrol-treated oocytes had a higher (P < 0.05) rate of pattern B mitochondria distribution (BCB+R: 73.07%; BCB-R: 79.16%) compared to controls (BCB+C, 19.35% and BCB-C, 40%; Fig. 4).
Fig. 4.
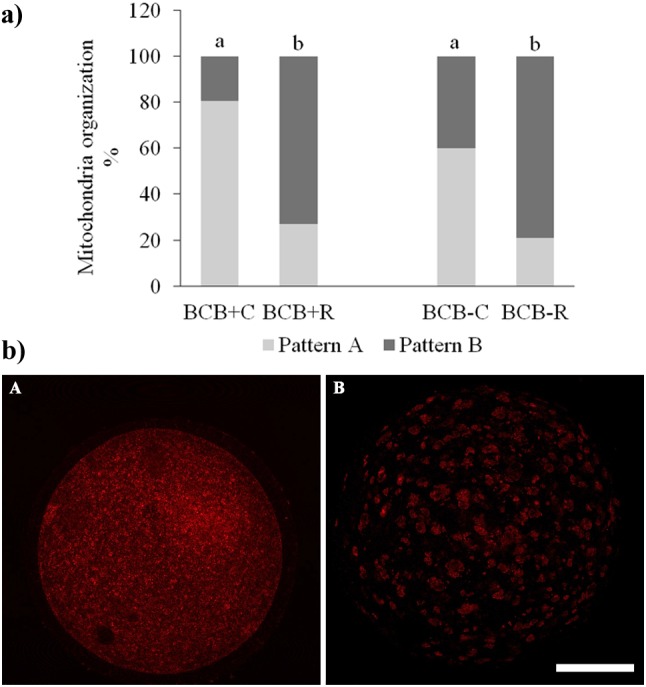
Mitochondrial organization of brilliant cresyl blue (BCB)-selected prepubertal goat oocytes in vitro matured with or without 1 µM resveratrol (Experiment 3b). a) Distribution of mitochondrial aggregation patterns in metaphase II prepubertal goat oocytes, different superscript letters (a vs. b) are significantly different (P < 0.05). b) Representative CLSM images of mitochondrial aggregation patterns in prepubertal goat oocytes after staining with MitoTracker orange CMTM Ros: A) Homogeneous small granulations spread throughout the cytoplasm (pattern A); B) Heterogeneous large granulations spread throughout the cytoplasm or are located in specific cytoplasmic domains (Pattern B). Scale bar = 50 µm.
Discussion
In the present study, we investigated the potential beneficial effect of resveratrol supplementation of the maturation medium on the embryo developmental competence of prepubertal goat oocytes selected by BCB staining.
Increasing evidence proves that the addition of resveratrol during in vitro maturation has positive effects on in vitro embryo production in different species [29]. In adult goats, resveratrol improved the developmental potential of parthenogenetic-derived blastocysts and hand-made cloned blastocysts [35]. Resveratrol acts in a dose-dependent manner and the optimal concentration is species-specific [29]. We have demonstrated that, resveratrol at a concentration of 1 µM, significant increased blastocyst development of prepubertal goat oocytes compared to 0.5 µM and control groups. After selection with BCB, BCB+ oocytes, matured in the presence of resveratrol (BCB+R), developed to the blastocyst stage at significantly higher rates than those of the control group (BCB+C). Moreover, resveratrol positively affected BCB- oocytes, improving their competence to blastocyst development up to the rates of BCB+ oocytes matured in conventional medium.
In our study oocyte nuclear maturation (MII stage) was not affected by resveratrol treatment, which is in line with results from other studies on bovines [36], goats [35] and pigs [37]. On the contrary, Wang et al. (2014) found that resveratrol promoted oocyte nuclear maturation due to its antioxidant properties and the induction of progesterone secretion [38]. We observed that oocytes with low G6PDH activity (BCB+) had higher developmental competence than those with high enzyme activity (BCB-). These findings have been previously shown in our laboratory in goats [16, 39], cattle [12] and sheep [13], and by other authors, in different species [11, 14, 15, 17, 18]. A recent study observed that in spite of similar mitochondrial distribution between both BCB groups, mtDNA content experienced a 1.9-fold increase in BCB+ cattle oocytes which confirmed their higher competence compared to BCB- oocytes [40].
On the other hand, some findings contradicted the utility of this test for selecting competent oocytes in bovines and pigs [8]. Moreover, the existence of a high caspase-3 activity in bovine blastocysts developed from BCB+ oocytes and a higher BCL-2 associated X protein (BAX) protein level in the BCB+ oocytes could imply a harmful effect of this staining [8]. In order to understand the reasons of the positive effect of resveratrol on embryo development of prepubertal goat oocytes, we evaluated the oxidative and bioenergetic status of oocytes. Our findings showed that resveratrol significantly increased intracellular GSH levels of in vitro matured oocytes in both BCB groups. In pigs [37], cattle [38] and goats [35], the beneficial effect of resveratrol on oocyte developmental competence has been associated with its antioxidant activity which increases intracellular GSH levels and decreases ROS levels. In our study, besides the increase in GSH content, resveratrol treatment did not affect ROS levels in both BCB+ and BCB- groups. Antioxidants do not always act in a univocal manner; indeed, increased GSH levels are not always associated with reduced ROS levels, as observed by other authors [22, 31]. Several reports suggest that IVM media supplementation with other antioxidants alleviated oxidative stress during in vitro maturation of poor-quality oocytes and improved early embryo development through a mechanism, including increase in GSH content [41,42,43]. GSH is the major non-protein sulfhydryl compound in mammalian cells and protects cells from oxidative damage [44]. The GSH level in oocytes increased as the oocyte resumed meiosis, and higher concentrations were found in mature oocytes than in immature [45]. The intra-oocyte GSH level can be considered as a marker of cytoplasmic maturity due to the close correlation with embryonic development [46]. In the adult goat, more competent oocytes (BCB+) presented higher intracellular GSH levels and the capacity to develop to the blastocyst stage after parthenogenetic activation [47]. Moreover, a reduction in GSH levels has been correlated with low developmental competence of oocytes derived from prepubertal mice and pigs [20, 21]. Several studies prove that GSH promotes decondensation of the sperm head and male pronucleus formation during fertilization [16,48], but also plays an important role in the development of parthenogenetic embryos [35, 37, 47].
Furthermore, GSH is involved in several biological processes, including DNA and protein synthesis, cell proliferation and protection of mitotic spindle from oxidizing agents [49, 50]. Another finding from our study was the effect of resveratrol on mitochondria organization. In fact, supplementation of the maturation medium with resveratrol induced a modification of active mitochondrial distribution in the cytoplasm of BCB+ and BCB- oocytes from a fine homogeneous pattern to a clustered distribution.
It has been shown that the activation of SIRT1 by resveratrol enhanced mitochondrial biosynthesis and degradation, thus, improving mitochondrial function and the developmental ability of oocytes [51]. In addition, resveratrol treatment could efficiently correct the defective phenotypes of mitochondrial organization in in vitro aged or methylglyoxal-treated mouse oocytes [52, 53]. Mitochondrial distribution and activity are considered good markers of oocyte quality. During in vitro maturation, changes in mitochondrial distribution and activity occur supporting oocytes maturation in cattle [54], dogs [55], goats [6], sheep [13], horses [56], pigs [57] and humans [58].
In a comparative study, Leoni et al. (2015) documented different active mitochondrial organizations in sheep MII-oocyte with high (adult) and low (prepubertal) developmental competence. A fine homogeneous dispersion of active mitochondria was observed at the GV stage in both oocyte types. This organization persisted in prepubertal MII-oocytes while adult MII-oocytes acquired a clustered distribution [34]. A clustered active mitochondrial organization was associated with maturation and high developmental competence in horse [56], dog [59], pig [60] and human [58] oocytes.
In our study, the presence of large clustered granules in resveratrol-treated MII-oocytes, which showed the highest GSH levels and developmental competence, suggests that the clustered mitochondrial phenotype may reflect the correct cytoplasmic maturity.
Quantitative analysis revealed that resveratrol neither affected ATP content nor the mitochondrial activity of prepubertal goat oocytes; indeed no significant difference was found among groups. In contrast, resveratrol treatment increased ATP content and the mitochondrial membrane potential in bovine in vitro matured oocytes [61].
In summary, the results of the present study show that supplementation of resveratrol during in vitro maturation improved embryo development to blastocyst stage, particularly in better quality oocytes (BCB+). Increased GSH levels and mitochondrial cluster distribution, could be some of the mechanisms underlying the positive effect of resveratrol supplementation on oocyte quality.
Acknowledgments
The authors would like to thank the financial support by the Spanish Minister of Science, Innovation and Universities (Project: AGL2017-85837-R).
References
- 1.Granleese T, Clark SA, Swan AA, van der Werf JHJ. Increased genetic gains in sheep, beef and dairy breeding programs from using female reproductive technologies combined with optimal contribution selection and genomic breeding values. Genet Sel Evol 2015; 47: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trounson AO, Willadsen SM, Moor RM. Reproductive function in prepubertal lambs: ovulation, embryo development and ovarian steroidogenesis. J Reprod Fertil 1977; 49: 69–75. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien JK, Dwarte D, Ryan JP, Maxwell WMC, Evans G. Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep. Reprod Fertil Dev 1996; 8: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 4.Gandolfi F, Milanesi E, Pocar P, Luciano AM, Brevini TA, Acocella F, Lauria A, Armstrong DT. Comparative analysis of calf and cow oocytes during in vitro maturation. Mol Reprod Dev 1998; 49: 168–175. [DOI] [PubMed] [Google Scholar]
- 5.Ledda S, Bogliolo L, Leoni G, Naitana S. Cell coupling and maturation-promoting factor activity in in vitro-matured prepubertal and adult sheep oocytes. Biol Reprod 2001; 65: 247–252. [DOI] [PubMed] [Google Scholar]
- 6.Velilla E, Rodríguez-Gonzalez E, Vidal F, Izquierdo D, Paramio MT. Mitochondrial organization in prepubertal goat oocytes during in vitro maturation and fertilization. Mol Reprod Dev 2006; 73: 617–626. [DOI] [PubMed] [Google Scholar]
- 7.Velilla E, Izquierdo D, Rodríguez-González E, López-Béjar M, Vidal F, Paramio MT. Distribution of prepubertal and adult goat oocyte cortical granules during meiotic maturation and fertilisation: ultrastructural and cytochemical study. Mol Reprod Dev 2004; 68: 507–514. [DOI] [PubMed] [Google Scholar]
- 8.Opiela J, Kątska-Książkiewicz L. The utility of Brilliant Cresyl Blue (BCB) staining of mammalian oocytes used for in vitro embryo production (IVP). Reprod Biol 2013; 13: 177–183. [DOI] [PubMed] [Google Scholar]
- 9.Mangia F, Epstein CJ. Biochemical studies of growing mouse oocytes: preparation of oocytes and analysis of glucose-6-phosphate dehydrogenase and lactate dehydrogenase activities. Dev Biol 1975; 45: 211–220. [DOI] [PubMed] [Google Scholar]
- 10.Ericsson S, Boice ML, Funahashi H, Day BN. Assessment of porcine oocytes using brilliant cresyl blue. Theriogenology 1993; 39: 214 Abstract. [Google Scholar]
- 11.Alm H, Torner H, Löhrke B, Viergutz T, Ghoneim IM, Kanitz W. Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology 2005; 63: 2194–2205. [DOI] [PubMed] [Google Scholar]
- 12.Pujol M, López-Béjar M, Paramio MT. Developmental competence of heifer oocytes selected using the brilliant cresyl blue (BCB) test. Theriogenology 2004; 61: 735–744. [DOI] [PubMed] [Google Scholar]
- 13.Catalá MG, Izquierdo D, Uzbekova S, Morató R, Roura M, Romaguera R, Papillier P, Paramio MT. Brilliant Cresyl Blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction 2011; 142: 517–527. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Lin J, Huang J, Wang J, Zhao Y, Chen T. Selection of ovine oocytes by brilliant cresyl blue staining. J Biomed Biotechnol 2012; 2012: 161372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadi-Sangcheshmeh A, Held E, Ghanem N, Rings F, Salilew-Wondim D, Tesfaye D, Sieme H, Schellander K, Hoelker M. G6PDH-activity in equine oocytes correlates with morphology, expression of candidate genes for viability, and preimplantative in vitro development. Theriogenology 2011; 76: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-González E, López-Bejar M, Izquierdo D, Paramio MT. Developmental competence of prepubertal goat oocytes selected with brilliant cresyl blue and matured with cysteamine supplementation. Reprod Nutr Dev 2003; 43: 179–187. [DOI] [PubMed] [Google Scholar]
- 17.Manjunatha BM, Gupta PSP, Devaraj M, Ravindra JP, Nandi S. Selection of developmentally competent buffalo oocytes by brilliant cresyl blue staining before IVM. Theriogenology 2007; 68: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y-G, Liu Y, Zhou P, Lan GC, Han D, Miao DQ, Tan JH. Selection of oocytes for in vitro maturation by brilliant cresyl blue staining: a study using the mouse model. Cell Res 2007; 17: 722–731. [DOI] [PubMed] [Google Scholar]
- 19.Khazaei M, Aghaz F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Int J Fertil Steril 2017; 11: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Y, Wheeler MB, Krisher RL. Disrupted redox homeostasis and aberrant redox gene expression in porcine oocytes contribute to decreased developmental competence. Biol Reprod 2012; 87: 78. [DOI] [PubMed] [Google Scholar]
- 21.Jiao GZ, Cao XY, Cui W, Lian HY, Miao YL, Wu XF, Han D, Tan JH. Developmental potential of prepubertal mouse oocytes is compromised due mainly to their impaired synthesis of glutathione. PLoS One 2013; 8: e58018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sovernigo TC, Adona PR, Monzani PS, Guemra S, Barros F, Lopes FG, Leal C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod Domest Anim 2017; 52: 561–569. [DOI] [PubMed] [Google Scholar]
- 23.Jeandet P, Delaunois B, Aziz A, Donnez D, Vasserot Y, Cordelier S, Courot E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J Biomed Biotechnol 2012; 2012: 579089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S, Jr, D’Alessandro AM, Falone S, Amicarelli F. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev 2015; 2015: 659687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 2012; 15: 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 2007; 26: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat KPL, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res 2001; 61: 7456–7463. [PubMed] [Google Scholar]
- 28.Ortega I, Duleba AJ. Ovarian actions of resveratrol. Ann N Y Acad Sci 2015; 1348: 86–96. [DOI] [PubMed] [Google Scholar]
- 29.Galeati G, Spinaci M. Resveratrol from red grapes: an useful agent for oocyte maturation and subsequent embryonic development. Austin J Invit Fertil 2015; 2: 1–3. [Google Scholar]
- 30.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online 2001; 3: 25–29. [DOI] [PubMed] [Google Scholar]
- 31.Soto-Heras S, Roura M, Catalá MG, Menéndez-Blanco I, Izquierdo D, Fouladi-Nashta AA, Paramio MT. Beneficial effects of melatonin on in vitro embryo production from juvenile goat oocytes. Reprod Fertil Dev 2018; 30: 253–261. [DOI] [PubMed] [Google Scholar]
- 32.Català MG, Roura M, Izquierdo D, Morato R, Hammami S, Paramio MT. Blastocyst development, MPF activity and ATP content of lamb oocytes supplemented with insulin-transferrin-selenium (ITS) and ascorbic acid at IVM. Small Rumin Res 2013; 112: 103–107. [Google Scholar]
- 33.Martino NA, Ariu F, Bebbere D, Uranio MF, Chirico A, Marzano G, Sardanelli AM, Cardinali A, Minervini F, Bogliolo L, Dell’Aquila ME. Supplementation with nanomolar concentrations of verbascoside during in vitro maturation improves embryo development by protecting the oocyte against oxidative stress: a large animal model study. Reprod Toxicol 2016; 65: 204–211. [DOI] [PubMed] [Google Scholar]
- 34.Leoni GG, Palmerini MG, Satta V, Succu S, Pasciu V, Zinellu A, Carru C, Macchiarelli G, Nottola SA, Naitana S, Berlinguer F. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS One 2015; 10: e0124911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee A, Malik H, Saha AP, Dubey A, Singhal DK, Boateng S, Saugandhika S, Kumar S, De S, Guha SK, Malakar D. Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression. J Assist Reprod Genet 2014; 31: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprícigo JF, Morató R, Arcarons N, Yeste M, Dode MA, López-Bejar M, Mogas T. Assessment of the effect of adding L-carnitine and/or resveratrol to maturation medium before vitrification on in vitro-matured calf oocytes. Theriogenology 2017; 89: 47–57. [DOI] [PubMed] [Google Scholar]
- 37.Kwak SS, Cheong SA, Jeon Y, Lee E, Choi KC, Jeung EB, Hyun SH. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012; 78: 86–101. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Tian X, Zhang L, He C, Ji P, Li Y, Tan D, Liu G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril 2014; 101: 577–586. [DOI] [PubMed] [Google Scholar]
- 39.Urdaneta A, Jiménez-Macedo A-R, Izquierdo D, Paramio MT. Supplementation with cysteamine during maturation and embryo culture on embryo development of prepubertal goat oocytes selected by the brilliant cresyl blue test. Zygote 2003; 11: 347–354. [DOI] [PubMed] [Google Scholar]
- 40.Lamas-Toranzo I, Pericuesta E, Bermejo-Álvarez P. Mitochondrial and metabolic adjustments during the final phase of follicular development prior to IVM of bovine oocytes. Theriogenology 2018; 119: 156–162. [DOI] [PubMed] [Google Scholar]
- 41.Park YG, Lee SE, Son YJ, Jeong SG, Shin MY, Kim WJ, Kim EY, Park SP. Antioxidant β-cryptoxanthin enhances porcine oocyte maturation and subsequent embryo development in vitro. Reprod Fertil Dev 2018; 30: 1204–1213. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zhang Z, He C, Zhu K, Xu Z, Ma T, Tao J, Liu G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J Pineal Res 2015; 59: 365–375. [DOI] [PubMed] [Google Scholar]
- 43.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 2012; 20: 249–259. [DOI] [PubMed] [Google Scholar]
- 44.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol 2005; 5: 5–17. [PubMed] [Google Scholar]
- 45.Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev 2003; 64: 106–112. [DOI] [PubMed] [Google Scholar]
- 46.de Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology 2000; 53: 761–771. [DOI] [PubMed] [Google Scholar]
- 47.Abazari-Kia AH, Mohammadi-Sangcheshmeh A, Dehghani-Mohammadabadi M, Jamshidi-Adegani F, Veshkini A, Zhandi M, Cinar MU, Salehi M. Intracellular glutathione content, developmental competence and expression of apoptosis-related genes associated with G6PDH-activity in goat oocyte. J Assist Reprod Genet 2014; 31: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutovsky P, Schatten G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol Reprod 1997; 56: 1503–1512. [DOI] [PubMed] [Google Scholar]
- 49.Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod 1997; 57: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 50.Lafleur MVM, Hoorweg JJ, Joenje H, Westmijze EJ, Retèl J. The ambivalent role of glutathione in the protection of DNA against singlet oxygen. Free Radic Res 1994; 21: 9–17. [DOI] [PubMed] [Google Scholar]
- 51.Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One 2014; 9: e94488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma R, Zhang Y, Zhang L, Han J, Rui R. Sirt1 protects pig oocyte against in vitro aging. Anim Sci J 2015; 86: 826–832. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, He XQ, Huang X, Ding L, Xu L, Shen YT, Zhang F, Zhu MB, Xu BH, Qi ZQ, Wang HL. Resveratrol protects mouse oocytes from methylglyoxal-induced oxidative damage. PLoS One 2013; 8: e77960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Gonçalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod 2001; 64: 904–909. [DOI] [PubMed] [Google Scholar]
- 55.Valentini L, Iorga AI, De Santis T, Ambruosi B, Reynaud K, Chastant-Maillard S, Guaricci AC, Caira M, Dell’Aquila ME. Mitochondrial distribution patterns in canine oocytes as related to the reproductive cycle stage. Anim Reprod Sci 2010; 117: 166–177. [DOI] [PubMed] [Google Scholar]
- 56.Torner H, Alm H, Kanitz W, Goellnitz K, Becker F, Poehland R, Bruessow KP, Tuchscherer A. Effect of initial cumulus morphology on meiotic dynamic and status of mitochondria in horse oocytes during IVM. Reprod Domest Anim 2007; 42: 176–183. [DOI] [PubMed] [Google Scholar]
- 57.Brevini TAL, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod 2005; 72: 1218–1223. [DOI] [PubMed] [Google Scholar]
- 58.Dell’Aquila ME, Ambruosi B, De Santis T, Cho YS. Mitochondrial distribution and activity in human mature oocytes: gonadotropin-releasing hormone agonist versus antagonist for pituitary down-regulation. Fertil Steril 2009; 91: 249–255. [DOI] [PubMed] [Google Scholar]
- 59.De los Reyes M, Palomino J, Parraguez VH, Hidalgo M, Saffie P. Mitochondrial distribution and meiotic progression in canine oocytes during in vivo and in vitro maturation. Theriogenology 2011; 75: 346–353. [DOI] [PubMed] [Google Scholar]
- 60.Torner H, Brüssow K-P, Alm H, Ratky J, Pöhland R, Tuchscherer A, Kanitz W. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre-ovulatory maturation. Theriogenology 2004; 61: 1675–1689. [DOI] [PubMed] [Google Scholar]
- 61.Takeo S, Sato D, Kimura K, Monji Y, Kuwayama T, Kawahara-Miki R, Iwata H. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J Reprod Dev 2014; 60: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]