Abstract
Previously, we reported that neurotensin (NT), which is expressed in the uterus and oviduct, enhanced bovine sperm capacitation and acrosome reactions. As NT mRNA expression in bovine oviducts increases dramatically in the follicular phase, we hypothesized that NT modulates fertilization and subsequent conception in cattle. The objective of this study was to evaluate the effect of NT on embryo development and blastocyst quality. The rate of embryo cleavage was significantly increased by the addition of NT to the fertilization medium. Furthermore, the total number of cells and numbers of cells in the inner cell mass of blastocysts were significantly increased by NT during in vitro fertilization (IVF). These results suggested that NT enhanced the efficiency of early bovine embryo development and blastocyst quality. The expression of NT receptors (NTRs) in sperm, testes, oocytes, and cumulus cells was evaluated to determine whether NT acted via NTRs in sperm alone or in both male and female reproductive cells during IVF. Immunocytochemistry and reverse transcription polymerase chain reaction revealed that NTR1 and NTR2 were expressed in sperm and testes, but not in oocytes and cumulus cells. We propose that NT selectively acts upon sperm via NTR1 and NTR2 during IVF to improve the cleavage rate and quality of blastocysts, which are important determinants of sperm quality for successful conception. This research supports our hypothesis that NT acts as a key modulator of fertilization and conception in cattle. Further studies are necessary to apply our findings to the industrial framework of bovine reproduction.
Keywords: Blastocyst, Bovine sperm, Cumulus-oocyte complex, In vitro fertilization, Neurotensin
Artificial insemination using frozen-thawed semen provides several benefits in the industrial framework of bovine reproduction, such as the efficient use of bulls, promotion of selective breeding, reduction of cost and space, and long-term preservation and transportation of semen. However, in Japan and other countries, conception rates after artificial insemination have continuously declined [1,2,3]. The causes of this problem remain obscure, but one possibility is the poor quality of sperm used for fertilization via artificial insemination [4]. An improvement in sperm quality after freezing and thawing may improve the efficiency of bovine production through artificial insemination. However, there is little information on the effects of improved sperm quality on fertilization and bovine embryo development.
Mammalian spermatozoa are non-functional immediately after ejaculation and need to acquire fertilizing ability (i.e. capacitation) during migration in the female reproductive tract. Only capacitated sperm can undergo the acrosome reaction and fertilize an oocyte. It is well known that various factors in the female reproductive tract are involved in both sperm capacitation and the acrosome reaction. Neurotransmitters such as gamma aminobutyric acid, dopamine, and serotonin also participate in this process [5,6,7].
We previously revealed that neurotensin (NT), which is expressed in the oviduct and uterus, enhanced sperm capacitation and the acrosome reaction in bulls and mice [8, 9]. NT consists of 13 amino acids and has multiple functions in several organs [10,11,12,13]. Three types of NT receptors (NTRs), NTR1, NTR2, and NTR3, have been isolated from mice and cattle. NTR1 and NTR2 belong to a family of G protein-coupled receptors with seven transmembrane spanning domains, whereas NTR3 belongs to a family of sorting receptors [14]. Changes in biological processes induced by NT [15,16,17], including gastrointestinal motility [18] or glutamate signaling in the brain [19], mainly occur via NTR1 or NTR2. We reported that NTR1 was expressed in the neck region of bovine sperm, but the expression and localization of NTR2 in sperm are not well understood [9]. Additionally, the expression of NTR1 and NTR2 in bovine oocytes and cumulus cells remains unknown.
Based on previous results showing that NT regulates sperm function, we hypothesized that NT acts as a modulator of bovine conception. To evaluate this hypothesis, it is essential to investigate the effect of NT on fertilization, embryo development, and blastocyst quality, which are all significant steps involved in successful conception. Interestingly, the mRNA expression of NT in the bovine oviduct was reported to increase by over 20 folds in the follicular phase compared with that in the luteal phase [20]. This supports our hypothesis that NT levels in female reproductive tissues affect successful fertilization and subsequent conception in bovine artificial insemination. Furthermore, NT administration will be potentially useful in bovine artificial insemination because it is easy to deliver NT as an extracellular ligand to the female reproductive tract and/or semen.
In this study, to understand the potential contribution of NT to the improvement in conception rates after bovine artificial insemination, we administered NT in the medium used for in vitro fertilization and examined its effect on embryo development. The expression of NTR1 and NTR2 in sperm, testes, oocytes, and cumulus cells was evaluated to determine whether NT acted via NTRs in sperm alone or in both male and female reproductive cells during in vitro fertilization.
Materials and Methods
Oocyte collection and maturation
Ovaries were collected from Japanese Black cattle or heifers at a local slaughterhouse, transported to the laboratory in a container within 4 h of removal, and placed in saline water at 38.5°C. The follicular fluid and bovine oocytes were aspirated from antral follicles (2–8 mm in diameter) with a 10-ml syringe attached to a 21-gauge needle. After the follicular contents including oocytes had settled, the supernatant was discarded and the sediment was resuspended in Medium 199 (Thermo Fisher Scientific, Waltham, MA, USA). After 10 min, the supernatant was discarded and the sediment was resuspended in Medium 199 again. Cumulus-oocyte complexes (COCs) with a uniform ooplasm were selected in Medium 199.
The isolated COCs were washed three times, resuspended, and matured in TCM199 (Thermo Fisher Scientific) supplemented with 5% fetal bovine serum (Gibco BRL), 0.1 IU/ml follicle-stimulating hormone (Antrin; Kyoritsu Seiyaku, Tokyo, Japan), 50 ng/mL epidermal growth factor (Upstate Biotechnology, Lake Placid, NY, USA), and 0.2 mM pyruvic acid (Nacalai Tesque, Kyoto, Japan) in mineral oil (Nacalai Tesque) at 38.5°C in an atmosphere containing 5% CO2 for 22–24 h.
Sperm preparation
Semen straws from different mature Japanese Black bulls were used for sperm preparation. Frozen semen samples were thawed in a water bath at 37°C for 30 sec, washed with bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA)-free modified Tyrode’s albumin lactate pyruvate (mTALP) medium supplemented with 10 mM caffeine (Wako Pure Chemical Industries, Osaka, Japan) and NT (ab141173, abcam) at 0 (control) or 1 μM, and centrifuged twice at 500 × g for 5 min. After the supernatant was discarded, the final sperm pellet was resuspended in the same medium at a final concentration of 2.0 × 107 sperm/ml.
In vitro fertilization
In vitro fertilization (IVF) was performed using one straw of frozen semen. An equal volume of mTALP medium supplemented with 3 mg/ml BSA and 10 IU/ml heparin (NOVO Heparin, Mochida Seiyaku, Tokyo, Japan) was added to the sperm suspension. The final concentration of NT in the IVF medium was 0.5 μM. The heparin-treated sperm suspension was placed under paraffin oil and incubated at 38.5°C in an atmosphere containing 5% CO2. Groups of COCs (≤ 50) matured in vitro were introduced into 100-μl microdrops of sperm suspension in a 35-mm culture dish. Six hours after the initiation of fertilization, the cumulus cells were removed with a pipette.
Embryo culture
The culture medium was composed of modified synthetic oviduct fluid (mSOF) [21, 22] supplemented with 20 μl/ml essential amino acid solution (50 ×, Gibco BRL), 10 μl/ml nonessential amino acid solution (100 ×, Gibco BRL), 1 mM glycine, 2 mM taurine, insulin-transferrin-selenium supplement (final concentrations of 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium; Sigma-Aldrich), and 6 mg/ml fatty acid-free BSA (Sigma-Aldrich). IVF oocytes were cultured in groups of 10 to 15 in 50-μl drops of mSOF at 38.5°C in an atmosphere containing 5% CO2, 5% O2, and 90% N2. Rates of early cleavage, cleavage, blastocyst formation on day 7, and blastocyst formation on day 8 were assessed at 27 h, 72 h, 168 h, and 192 h, post-IVF. We evaluated not only the cleavage rate but also the early cleavage rate to discuss the relationship between NT and fertility, as fast-cleaving embryos (≤ 27 h) showed higher viability and pregnancy rates than slow-cleaving embryos (> 27 h) in cattle [23, 24]. Cleavage and blastocyst formation rates were calculated based on the percentages of the number of original oocytes and cleaved oocytes, respectively.
Differential staining of inner cell mass (ICM) and trophectoderm (TE) cells
The cell allocation of blastocysts (192 h post-fertilization) was assessed by differential staining of ICM and TE cells, as described previously [25, 26]. Briefly, blastocyst TE cells were stained for 40 sec with 100 μg/ml propidium iodide (Sigma-Aldrich) in a permeabilizing solution of 0.2% (v/v) Triton X-100 (Sigma-Aldrich). Blastocysts were then counterstained and simultaneously fixed for 5 min with 25 μg/ml Hoechst 33342 (Calbiochem, La Jolla, CA, USA) in 99.5% ethanol. Fixed and stained whole blastocysts were mounted, and the number of ICM and TE cells was assessed by fluorescence microscopy. The ICM and TE nuclei were identified by blue and pink to red staining, respectively. We examined approximately half of the developed blastocysts in each treatment group.
Western blot and immunocytochemistry
Western blot was performed to determine the expression of NTR2 in bovine sperm. Sperm pellets were homogenized in ice-cold radioimmunoprecipitation assay buffer containing 50 mM Tris-HCl at pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1% protease inhibitor (Nacalai Tesque) and sonicated on ice to extract total proteins. The sperm suspension was centrifuged and the pellet was collected. After the supernatant was discarded, the sperm pellet was washed with phosphate-buffered saline (PBS) and centrifuged again at 1000 rpm. After the supernatant was discarded, the pellet was resuspended in 2 × sample buffer (Nacalai Tesque), and the extracted solution was boiled for 5 min. Proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with Blocking One (Nacalai Tesque) for 60 min at room temperature. After three washes with PBS containing 0.1% Tween 20, the membrane was incubated with goat polyclonal anti-NTR2 (sc-25050, 1:1000; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C and washed again three times with PBS containing 0.1% Tween 20. Then, the membrane was incubated with horseradish peroxidase-conjugated anti-goat immunoglobulin G (1:2000; Promega, Madison, WI, USA) for 2 h at room temperature. After three washes, the membrane was reacted with Chemilumi-One Super (Nacalai Tesque), and images were obtained using a LAS-3000-mini Lumino Image Analyzer (Fujifilm, Tokyo, Japan).
Immunocytochemical analysis was performed to investigate the localization of NTR2 in bovine sperm. Sperm samples were collected by centrifugation at 1000 rpm for 5 min and fixed with 2% paraformaldehyde and 1% Triton-X in PBS for 15 min at 4°C. They were then washed three times with PBS and blocked with Blocking One (Nacalai Tesque) for 60 min. Next, the sperm suspension was incubated with anti-NTR2 (1:100) overnight at 4°C. After three washes with PBS, the suspension was incubated for 2 h at room temperature with Alexa Fluor 488-conjugated anti-goat immunoglobulin G (1:500; Thermo Fisher Scientific) and Hoechst 33342 (1:5000; Thermo Fisher Scientific). The treated samples were then washed, suspended in PBS, mounted on glass slides, and covered with glass. Images were obtained using an LSM-710 confocal laser microscope (Carl Zeiss, Jena, Germany).
Reverse transcription polymerase chain reaction (RT-PCR)
Testes from three adult Japanese Black bulls at 61, 63, and 66 months of age were provided by Dr Masanori Koyago and Dr Keishi Mizutani (Morioka AI Center, Livestock Improvement Association of Japan, Morioka, Japan). The testes were cut into small pieces, frozen immediately in liquid nitrogen, and stored at −80°C until RNA extraction. Testicular RNA extraction was performed using ISOGEN (Nippon Gene, Tokyo, Japan). Meanwhile, oocytes and cumulus cells were separated by pipetting in PBS supplemented with 0.1% polyvinyl alcohol (PVA; Sigma-Aldrich, USA) and 0.1% hyaluronidase after in vitro maturation for 24 h. After washing with PBS-PVA, the samples were frozen immediately in liquid nitrogen and stored at −80°C until RNA extraction. Total RNAs from oocytes and cumulus cells were extracted using RNeasy micro kits (Qiagen, Venlo, Netherlands). The extracted samples were reverse-transcribed and cDNA was synthesized using ReverTra Ace (TOYOBO, Osaka, Japan). Specific primers were designed using the sequences of NTR1 (forward: TCGGAGTCCGTACCAGCG; reverse: AGTTGTACAGCTCCACAGGC), NTR2 (forward: ATTGTGGCCGTGTATGTCGT; reverse: GCTAGGCACTCAGGGTTGTT), and β-actin (forward: CATCGGCAATGAGCGGTTC; reverse: ACAGCACCGTGTTGGCGTAG) as an internal control. The PCR program consisted of 40 cycles of denaturation at 95°C for 5 sec, annealing at 62°C for 10 sec, and extension at 72°C for 20 sec. The amplified PCR products were electrophoresed onto 2% agarose gels and stained with ethidium bromide. After gel electrophoresis, the gels were visualized by a ChemiDoc Imaging System (Bio-Rad Laboratories, CA, USA).
Statistical analysis
All experiments were performed at least three times. Data are presented as mean ± standard error (SE). Cleavage rates and developmental rates were analyzed by the chi-square test, and cell number data were analyzed by Student’s t-test. P < 0.05 was considered to indicate a significant difference.
Results
Effect of NT on embryo cleavage
We investigated the embryo cleavage rates after IVF to evaluate the effect of adding NT to the fertilization medium. Early cleavage rates and cleavage rates were assessed at 27 h and 72 h, respectively, after IVF. The early cleavage rate was significantly increased by the addition of NT to the IVF medium (Table 1). The cleavage rate of the NT treatment group was also significantly higher than that of the control group. These results suggested that NT enhanced the embryo cleavage rates.
Table 1. Effect of NT on cleavage rate and blastocyst formation rate in vitro.
| Treatment | No. of oocytes | No. (%) of early cleavages | No. (%) of cleavages | No. (%) of blastocysts at day 7 | No. (%) of blastocysts at day 8 |
|---|---|---|---|---|---|
| control | 104 | 38 a (36.5) | 51 a (49.0) | 16 (31.4) | 22 (43.1) |
| NT | 118 | 59 b (50.0) | 76 b (64.4) | 20 (26.3) | 30 (39.5) |
Values with different superscript letters are significantly different (a–b: P < 0.05), n = 3. Early cleavage rate, cleavage rate, blastocyst formation rate on day 7, and blastocyst formation rate on day 8 were assessed at 27 h, 72 h, 168 h, and 192 h, respectively, after IVF. Cleavage rates and blastocyst formation rates were based on the percentages of original oocyte number and cleaved oocyte number, respectively.
Effect of NT on blastocyst development
We evaluated the effect of NT treatment during IVF on blastocyst development by assessing blastocyst formation rates at 168 h and 192 h after IVF. The blastocyst formation rates on both day 7 and day 8 in the NT treatment group were not significantly different from those of the control group (Table 1).
Effect of NT on embryo quality
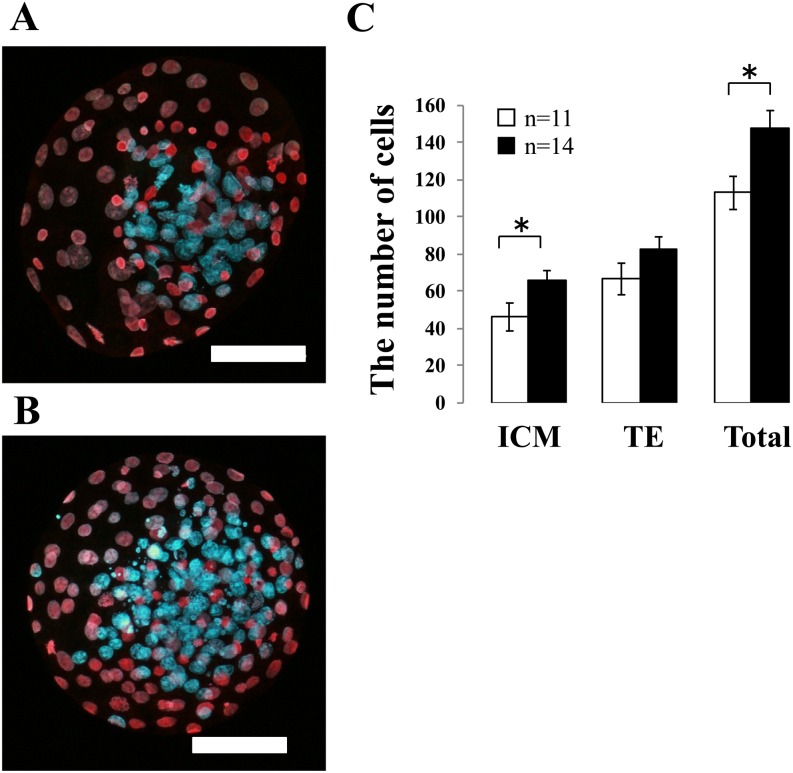
Embryo quality was evaluated by the total number of cells and the number of ICM and TE cells in each blastocyst. At 192 h post-IVF, blastocysts were stained with propidium iodide and Hoechst 33342, and the nuclei of ICM (blue) and TE (pink) cells were counted (Fig. 1A and 1B). The numbers of each cell type in the blastocysts are shown in Fig. 1C. The total number of cells and the number of ICM cells in the NT treatment group were significantly greater than those in the control group.
Fig. 1.
Effect of neurotensin (NT) on embryo quality. Blastocysts at 192 h after fertilization were assessed by differential staining of inner cell mass (ICM) and trophectoderm (TE) cells. Images show a representative blastocyst treated with (A) 0 μM (control) and (B) 1 μM NT. The ICM and TE nuclei were identified by blue and pink to red staining, respectively. Original magnification was × 200, and the scale bar = 50 μm. (C) The number of ICM and TE cells and total cell number were counted. White bars represent the control group and black bars represent the NT group. Data are shown as the mean ± SE (*: P < 0.05). n = total number of blastocysts examined.
Expression of NTR2 in spermatozoa
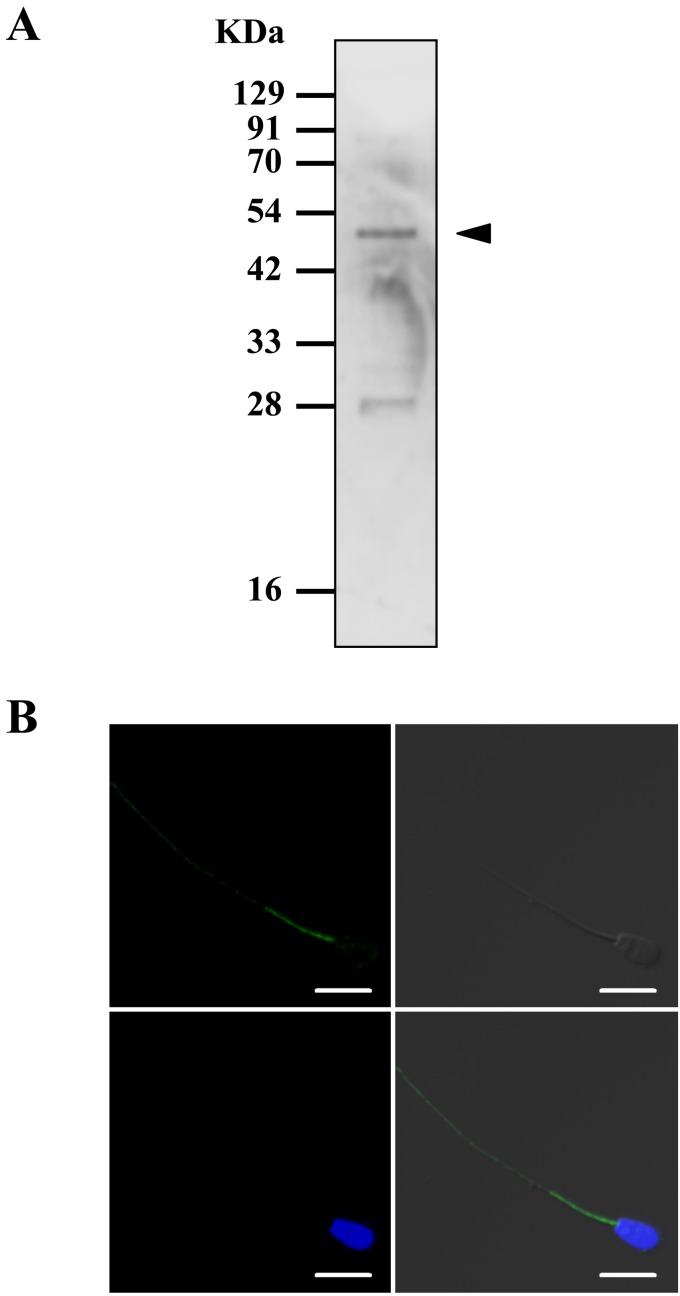
We performed western blot and immunocytochemical analyses to determine the expression and localization of NTR2 in bovine sperm. Western blotting revealed a specific band at approximately 45 kDa, which coincided with the molecular size of NTR2 (Fig. 2A). In addition, immunocytochemical analysis demonstrated the immunoreactivity of NTR2 in bovine sperm tail (Fig. 2B), which was absent in cells that were not incubated with NTR2 antibody (data not shown).
Fig. 2.
Detection of neurotensin receptor 2 (NTR2) expression in bovine sperm. (A) NTR2 expression in bovine sperm was evaluated by western blot. (B) Representative immunocytochemical image showing NTR2 localization in bovine sperm. Note the spot-like immunoreactivity detected at the tail of the sperm. Blue: nuclei (Hoechst 33342). Green: NTR2. Bars = 10 μm.
Expression of NTR1 and NTR2 mRNA in testes, mature oocytes, and cumulus cells
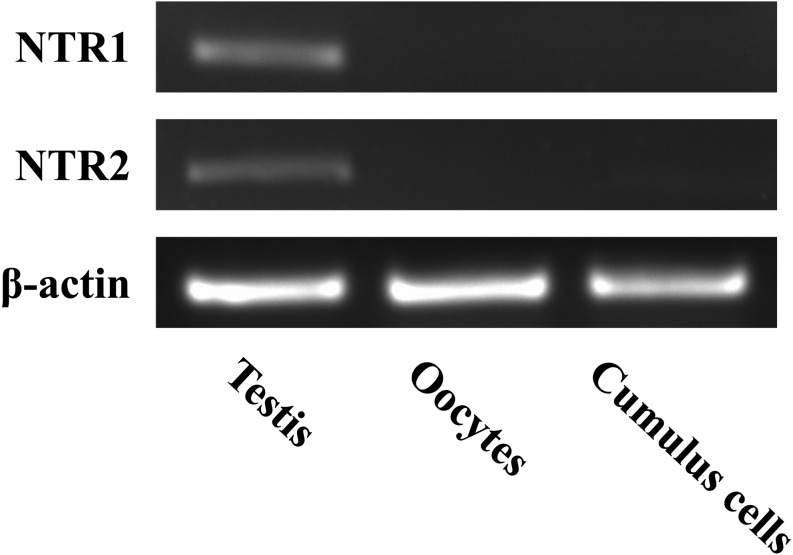
We examined the mRNA expression of NTR1 and NTR2 in testes, mature oocytes, and cumulus cells by RT-PCR with β-tubulin as an internal control. As shown in Fig. 3, NTR1 and NTR2 mRNAs were detected in testes, but not in oocytes and cumulus cells. Combined with the protein expression of NTR1 and NTR2 in bovine sperm (Fig. 2) [9], these findings indicate that NTRs are present only in sperm and testes, but not in female reproductive tissues and cells.
Fig. 3.
Expression of NTR1 and NTR2 mRNA detected by reverse transcription polymerase chain reaction. Oocytes and cumulus cells were matured in vitro for 24 h. Total RNA from testes, oocytes, and cumulus cells were extracted and cDNA was synthesized. Amplified products were obtained by PCR using specific primers, electrophoresed onto 2% agarose gels, and stained with ethidium bromide. The upper, middle, and lower panels represent NTR1, NTR2, and β-actin, respectively.
Discussion
Previously, we reported that NT enhanced sperm capacitation and the acrosome reaction in bulls [9]. It is also known that NT mRNA levels in the bovine oviduct increased by over 20 folds in the follicular phase compared with those in the luteal phase [20]. Based on these results, we hypothesized that NT is a new modulator of fertilization and subsequent conception in cattle. In this study, we investigated whether NT-NTR signaling contributed to the fertilization, embryonic development, and embryo quality required for successful conception.
In this study, the rates of both early cleavage and cleavage were significantly increased by the addition of NT to the IVF medium, suggesting that NT-NTR signaling improved the fertilization rate and/or early embryo development rate. Although the detection of two pronuclei in zygotes is a general method of evaluating fertilization, the high concentration of lipid droplets in bovine but not human and mouse zygotes makes the observation of bovine pronuclei very difficult. Thus, the direct effect of NT on fertilization was unclear in the current study because we were unable to observe and evaluate the number of pronuclei. However, NT likely has a positive effect on fertilization rather than embryo development because NT has been shown to improve sperm functions that are essential for fertilization [9]. Here, NT only improved embryo cleavage rates but not blastocyst formation rates (Table 1). Blastocyst formation rates on both day 7 and day 8 showed no significant differences with or without NT treatment. However, the total number of cells and number of ICM cells in blastocysts were significantly increased by NT during IVF. Since the total cell number and ICM cell number in blastocysts were positively correlated with the fetal development rate in mice [27], and blastocysts with a greater number of cells have an advantage in subsequent development and length of conceptus in cattle [28], these results indicated that NT improved the quality of blastocysts. Overall, we predict that NT enhances the efficiency of fertilization and/or early embryo development and the quality of blastocysts in cattle.
It was predicted that NT improved the cleavage rates and the quality of blastocysts via its receptor on sperm as we previously reported the expression of NTR1 in bovine sperm [9]. However, we could not exclude the possibility that NT in the IVF medium directly affected oocytes and/or cumulus cells via NTRs because the expression of NTRs in oocytes and cumulus cells was not well understood. Therefore, we examined the expression of NTR1 and NTR2 in sperm, testes, oocytes, and cumulus cells to determine whether NT acted via NTRs in sperm alone or in both sperm and female reproductive cells during IVF. The expression of the G protein-coupled receptor NTR2 was detected in bovine sperm by western blot and immunocytochemistry. Interestingly, the localization of NTR2 was different from that previously observed for NTR1. Although the reasons for the different expression remain unknown and we did not evaluate the effect of inhibitors of NTR1 or NTR2, both NTR1 and NTR2 may be functional in sperm because many biological events induced by NT were triggered via both NTR1 and NTR2 [15,16,17]. We also revealed that NTR1 and NTR2 mRNAs were selectively expressed in testes, but not in oocytes and cumulus cells. Moreover, we can exclude the possibility that NT directly affected zygotes or embryos because NT was added to the IVF medium, but not to the in vitro culture medium. Taken together, these findings indicate that NT selectively affected sperm via NTR1 and NTR2 during IVF, and sperm activity improved the cleavage rates and the quality of blastocysts.
Several studies have shown that the characteristics of sperm quality, such as motility, capacitation, and morphology, affect conception in cattle [4, 29,30,31,32]. However, to our knowledge, there are few studies showing that only improving bovine sperm quality could lead to beneficial effects on fertilization or embryo development. This study highlighted the importance of sperm quality, especially sperm capacitation, for successful bovine fertilization and conception.
Next, we discuss how to improve embryo cleavage rates and the quality of blastocysts via NT-NTR signaling in bovine sperm alone during IVF. Most fertilizing sperm undergo the acrosome reaction before reaching the zona pellucida [33]. In addition, the acrosome reaction causes the translocation of IZUMO1, which is an essential protein for sperm-egg fusion to allow subsequent fertilization [34]. These reports indicated that it is necessary to induce the acrosome reaction before the sperm reaches the oocyte. Considering that NT was found to be expressed in the uterus and oviduct, it is possible that NT modulate sperms capacitation and induces the acrosome reaction at the appropriate time and place before the sperm reaches the oocyte. However, it remains controversial where and when it is the best to induce capacitation and the acrosome reaction for successful fertilization. Although precise details about NT-NTR signaling remain undetermined in sperm, the fact that NT appears to prepare bovine sperm for fertilization could result in positive effects upon fertilization and subsequent early embryo development.
Another possibility is that sperm are not represented by a homogeneous population. Some studies have shown that the capacitation state, such as intracellular calcium ion concentration and sperm plasma membrane potential, is not uniform under the same experimental condition or treatment [35, 36], suggesting that only a subpopulation of sperm can undergo capacitation. Similarly, it is well known that only a subpopulation of capacitated sperm can induce the acrosome reaction or hyperactivation [37,38,39]. NT also affected a small population of sperm and enhanced the acrosome reaction in our previous study [9]. Although the reason for this heterogeneity is not understood, one hypothesis is that having sperm at different stages of capacitation could extend the time during which sperm as a whole are able to fertilize, improving the efficiency of fertilization. This hypothesis seems to be reasonable for in vivo fertilization, but it is unknown whether it can be applied to IVF because the oocytes are surrounded by abundant sperm during IVF. In addition, there are many reports that several sperm selection methods can improve the fertilization rate or embryo development rate [40,41,42,43,44]. Therefore, it can be assumed that NT affected only a partial population of optimal sperm and contributed to the improvement in early embryo development and blastocyst quality.
Currently, the continuous decline of conception rates after artificial insemination is an important issue in bovine reproduction. In this study, we successfully improved the quality of blastocysts in vitro via the actions NT through its receptors on sperm. Poor sperm quality may be a factor in the declining conception rates in cattle, and we have reconfirmed the importance of sperm quality for successful fertilization and conception. Additionally, our results support the hypothesis that NT acts as a modulator of bovine conception. Therefore, artificial administration of NT to the female reproductive tract and/or semen may be useful for artificial insemination in bovine. Early embryo cleavage within 27 h after IVF is positively related to successful pregnancy [23, 24], and NT improved the cleavage rates at 27 h post-IVF in this study. Thus, this result also supported the potential use of NT in tackling the problem of bovine reproduction. However, it is necessary to confirm that NT will have a similar effect upon in vivo fertilization and embryo development because all experiments in this study were conducted in vitro. Furthermore, embryo transfer is conducted worldwide in the bovine industry. The production of embryos in vivo or in vitro is essential for this technique, and in vitro embryo production for embryo transfer has increased internationally [45]. In many cases, blastocysts produced in vitro at day 7 to 8 post-fertilization are transferred into recipients, and the quality of blastocysts is a key for successful production of calves. Considering that we succeeded in producing blastocysts with better quality by the addition of NT to the IVF medium, it is possible that NT treatment enhances not only artificial insemination, but also embryo transfer in bovine reproduction.
In conclusion, we propose that NT acts via NTR1 and NTR2 in bovine sperm to subsequently enhance the cleavage rate and cell number of blastocysts. This result suggests that NT may be a new modulator for bovine conception, although further studies are necessary to apply this discovery to the industrial scale of commercial bovine reproduction.
Acknowledgments
The authors thank the staff at the Meat Inspection Office, Sendai, Japan. This work was supported by a Grant-in-Aid for JSPS Fellows (KAKENHI, grant No. JP17J02431). We thank Dr Masanori Koyago and Dr Keishi Mizutani (Livestock Improvement Association of Japan, Inc.) for providing the bovine testes; Dr Yousuke Naniwa (Livestock Improvement Association of Japan, Inc.) for critical reading of the manuscript, and Charles Allan, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Barbat A, Le Mézec P, Ducrocq V, Mattalia S, Fritz S, Boichard D, Ponsart C, Humblot P. Female fertility in French dairy breeds: current situation and strategies for improvement. J Reprod Dev 2010; 56(Suppl): S15–S21. [DOI] [PubMed] [Google Scholar]
- 2.Dochi O, Kabeya S, Koyama H. Factors affecting reproductive performance in high milk-producing Holstein cows. J Reprod Dev 2010; 56(Suppl): S61–S65. [DOI] [PubMed] [Google Scholar]
- 3.Lucy MC. Reproductive loss in high-producing dairy cattle: where will it end? J Dairy Sci 2001; 84: 1277–1293. [DOI] [PubMed] [Google Scholar]
- 4.Kishida K, Sakase M, Minami K, Arai MM, Syoji R, Kohama N, Akiyama T, Oka A, Harayama H, Fukushima M. Effects of acrosomal conditions of frozen-thawed spermatozoa on the results of artificial insemination in Japanese Black cattle. J Reprod Dev 2015; 61: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puente MA, Tartaglione CM, Ritta MN. Bull sperm acrosome reaction induced by gamma-aminobutyric acid (GABA) is mediated by GABAergic receptors type A. Anim Reprod Sci 2011; 127: 31–37. [DOI] [PubMed] [Google Scholar]
- 6.Ramírez AR, Castro MA, Angulo C, Ramió L, Rivera MM, Torres M, Rigau T, Rodríguez-Gil JE, Concha II. The presence and function of dopamine type 2 receptors in boar sperm: a possible role for dopamine in viability, capacitation, and modulation of sperm motility. Biol Reprod 2009; 80: 753–761. [DOI] [PubMed] [Google Scholar]
- 7.Meizel S, Turner KO. Serotonin or its agonist 5-methoxytryptamine can stimulate hamster sperm acrosome reactions in a more direct manner than catecholamines. J Exp Zool 1983; 226: 171–174. [DOI] [PubMed] [Google Scholar]
- 8.Hiradate Y, Inoue H, Kobayashi N, Shirakata Y, Suzuki Y, Gotoh A, Roh SG, Uchida T, Katoh K, Yoshida M, Sato E, Tanemura K. Neurotensin enhances sperm capacitation and acrosome reaction in mice. Biol Reprod 2014; 91: 53. [DOI] [PubMed] [Google Scholar]
- 9.Umezu K, Hiradate Y, Oikawa T, Ishiguro H, Numabe T, Hara K, Tanemura K. Exogenous neurotensin modulates sperm function in Japanese Black cattle. J Reprod Dev 2016; 62: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carraway R, Leeman SE. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem 1976; 251: 7045–7052. [PubMed] [Google Scholar]
- 11.Zhao D, Pothoulakis C. Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides 2006; 27: 2434–2444. [DOI] [PubMed] [Google Scholar]
- 12.Ramez M, Bagot M, Nikolova M, Boumsell L, Vita N, Chalon P, Caput D, Ferrara P, Bensussan A. Functional characterization of neurotensin receptors in human cutaneous T cell lymphoma malignant lymphocytes. J Invest Dermatol 2001; 117: 687–693. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci 1999; 20: 302–309. [DOI] [PubMed] [Google Scholar]
- 14.Pelaprat D. Interactions between neurotensin receptors and G proteins. Peptides 2006; 27: 2476–2487. [DOI] [PubMed] [Google Scholar]
- 15.Dupouy S, Mourra N, Doan VK, Gompel A, Alifano M, Forgez P. The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie 2011; 93: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 16.Miller LA, Cochrane DE, Carraway RE, Feldberg RS. Blockade of mast cell histamine secretion in response to neurotensin by SR 48692, a nonpeptide antagonist of the neurotensin brain receptor. Br J Pharmacol 1995; 114: 1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi R, Wada E, Yamada D, Yoshikawa M, Wada K. Effect of beta-lactotensin on acute stress and fear memory. Peptides 2006; 27: 3176–3182. [DOI] [PubMed] [Google Scholar]
- 18.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates expression of early growth response gene-1 and EGF receptor through MAP kinase activation in human colonic epithelial cells. Int J Cancer 2007; 120: 1652–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonelli T, Fuxe K, Tomasini MC, Mazzoni E, Agnati LF, Tanganelli S, Ferraro L. Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment. Prog Neurobiol 2007; 83: 92–109. [DOI] [PubMed] [Google Scholar]
- 20.Cerny KL, Garrett E, Walton AJ, Anderson LH, Bridges PJ. A transcriptomal analysis of bovine oviductal epithelial cells collected during the follicular phase versus the luteal phase of the estrous cycle. Reprod Biol Endocrinol 2015; 13: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999; 52: 683–700. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992; 37: 963–978. [DOI] [PubMed] [Google Scholar]
- 23.Sugimura S, Akai T, Hashiyada Y, Somfai T, Inaba Y, Hirayama M, Yamanouchi T, Matsuda H, Kobayashi S, Aikawa Y, Ohtake M, Kobayashi E, Konishi K, Imai K. Promising system for selecting healthy in vitro-fertilized embryos in cattle. PLoS One 2012; 7: e36627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimura S, Akai T, Imai K. Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes. J Reprod Dev 2017; 63: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikawa T, Itahashi T, Numabe T. Improved embryo development in Japanese black cattle by in vitro fertilization using ovum pick-up plus intracytoplasmic sperm injection with dithiothreitol. J Reprod Dev 2016; 62: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online 2001; 3: 25–29. [DOI] [PubMed] [Google Scholar]
- 27.Lane M, Gardner DK. Differential regulation of mouse embryo development and viability by amino acids. J Reprod Fertil 1997; 109: 153–164. [DOI] [PubMed] [Google Scholar]
- 28.O’Hara L, Forde N, Kelly AK, Lonergan P. Effect of bovine blastocyst size at embryo transfer on day 7 on conceptus length on day 14: can supplementary progesterone rescue small embryos? Theriogenology 2014; 81: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda K, Fukushima M, Harayama H. Premature capacitation of frozen-thawed spermatozoa from subfertile Japanese black cattle. J Reprod Dev 2007; 53: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira LZ, de Arruda RP, de Andrade AF, Celeghini EC, Reeb PD, Martins JP, dos Santos RM, Beletti ME, Peres RF, Monteiro FM, Hossepian de Lima VF. Assessment of in vitro sperm characteristics and their importance in the prediction of conception rate in a bovine timed-AI program. Anim Reprod Sci 2013; 137: 145–155. [DOI] [PubMed] [Google Scholar]
- 31.Takeda K, Uchiyama K, Kinukawa M, Tagami T, Kaneda M, Watanabe S. Evaluation of sperm DNA damage in bulls by TUNEL assay as a parameter of semen quality. J Reprod Dev 2015; 61: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harayama H, Minami K, Kishida K, Noda T. Protein biomarkers for male artificial insemination subfertility in bovine spermatozoa. Reprod Med Biol 2017; 16: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA 2011; 108: 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci 2012; 125: 4985–4990. [DOI] [PubMed] [Google Scholar]
- 35.Escoffier J, Navarrete F, Haddad D, Santi CM, Darszon A, Visconti PE. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol Reprod 2015; 92: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luque GM, Dalotto-Moreno T, Martín-Hidalgo D, Ritagliati C, Puga Molina LC, Romarowski A, Balestrini PA, Schiavi-Ehrenhaus LJ, Gilio N, Krapf D, Visconti PE, Buffone MG. Only a subpopulation of mouse sperm displays a rapid increase in intracellular calcium during capacitation. J Cell Physiol 2018; 233: 9685–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNutt TL, Killian GJ. Influence of bovine follicular and oviduct fluids on sperm capacitation in vitro. J Androl 1991; 12: 244–252. [PubMed] [Google Scholar]
- 38.Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod 2001; 65: 1606–1615. [DOI] [PubMed] [Google Scholar]
- 39.Lukoseviciute K, Zilinskas H, Januskauskas A. The effect of oestradiol, progesterone and heparin on bovine spermatozoa function after thawing. Reprod Domest Anim 2005; 40: 100–107. [DOI] [PubMed] [Google Scholar]
- 40.Dirican EK, Ozgün OD, Akarsu S, Akin KO, Ercan O, Uğurlu M, Camsari C, Kanyilmaz O, Kaya A, Unsal A. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J Assist Reprod Genet 2008; 25: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worrilow KC, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, Ivani K, Khoury C, Ball GD, Elliot T, Lieberman J. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes--multicenter, double-blinded and randomized controlled trial. Hum Reprod 2013; 28: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Zhu S, He X, Sun R, He Q, Gan Y, Liu S, Funahashi H, Li Y. Application of a microfluidic sperm sorter to in vitro production of dairy cattle sex-sorted embryos. Theriogenology 2016; 85: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Cerezales S, Laguna-Barraza R, de Castro AC, Sánchez-Calabuig MJ, Cano-Oliva E, de Castro-Pita FJ, Montoro-Buils L, Pericuesta E, Fernández-González R, Gutiérrez-Adán A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci Rep 2018; 8: 2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagata MPB, Endo K, Ogata K, Yamanaka K, Egashira J, Katafuchi N, Yamanouchi T, Matsuda H, Goto Y, Sakatani M, Hojo T, Nishizono H, Yotsushima K, Takenouchi N, Hashiyada Y, Yamashita K. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc Natl Acad Sci USA 2018; 115: E3087–E3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore SG, Hasler JF. A 100-Year Review: Reproductive technologies in dairy science. J Dairy Sci 2017; 100: 10314–10331. [DOI] [PubMed] [Google Scholar]