Abstract
In a previous study on monovular cows, follicles revealed a mean antral (follicular fluid) temperature 1.54°C cooler than rectal temperatures in ovulating cows, whereas no such temperature differences were detected in non-ovulating cows. The present study adds to our previous work, this time considering 24 bi-ovular cows (one follicle per ovary). In order to increase the number of pre-ovulatory follicles failing to ovulate, this study was performed under heat-stress conditions. Follicular temperatures of the ovulating follicles (n = 31) were 0.93°C significantly cooler (P < 0.0001) than rectal temperatures, whereas no significant differences in temperature were found in non-ovulating follicles (n = 17). Eight cows became pregnant. The results of the present study indicate that, similar to those in monovular cows, pre-ovulatory follicles in bi-ovular cows were cooler than deep rectal temperatures and those temperature gradients were not found in follicles showing ovulation failure.
Keywords: Bilateral asymmetry, Dairy cattle, Ovulation failure, Temperature gradients
Pre-ovulatory follicles may be more than 1.0°C cooler than ovarian stroma in rabbits, pigs, cows, and women and both compartments are cooler than jugular and deep rectal temperatures [1, 2]. However, original observations on temperature gradients in reproductive tissues have not achieved prominence or been widely accepted, in part because they are counter-intuitive. They may also have been regarded as artifacts due to the use of anesthetics and open surgery (laparotomy). Nevertheless, set in a functional context, temperature gradients in female reproductive tissues can influence the success of key processes such as the final maturation of oocytes and their corresponding ovarian follicles, shedding of an oocyte at ovulation, gamete transport within the oviducts, and sperm penetration of the zona pellucida at fertilization [3].
More recently, while avoiding complications of anesthesia and surgery, temperatures in pre-ovulatory follicles have been measured directly in monovular cows by means of a thermistor probe. The probe was introduced via the vagina and guided into a pre-ovulatory follicle using ultrasonic monitoring [4]. Fluid temperatures within the antrum were 1.54°C cooler than rectal temperatures in cows that ovulated, but no significant depression of temperature was recorded in cows that failed to ovulate [4]. In a recent study on 622 cows, cows with two co-dominant follicles showed different ovulation patterns to those with one dominant follicle [5]. The objective of the present study was to assess whether follicular cooling in bi-ovular cows occurs in a similar manner to that in monovular cows.
All measurements were performed under heat stress conditions, ranging from 72 to 86 THI values. Of the 24 cows finally included in the study, three showed ovulation failure in both ovaries, 10 ovulated in both ovaries and 11 ovulated in only one ovary: four in the left and seven in the right ovary (Table 1). Three of the 11 single-ovulating cows became pregnant, whereas pregnancy was registered in five of the 10 double-ovulating cows, one of them carrying twins. None of the three non-ovulating cows became pregnant.
Table 1. Summarized findings concerning pre-ovulatory follicular and deep rectal temperatures.
| Right ovary | Left ovary | Both ovaries | ||
|---|---|---|---|---|
| Temperature (°C) | ||||
| All follicles | 38.0 ± 0.76 (24) | 38.1 ± 1.07 (24) | 38.0 ± 0.92 (48) | |
| Ovulating follicles | 37.7 a ± 0.65 (17) | 37.4 a ± 1.00 (14) | 37.6 c ± 0.83 (31) | |
| Non-ovulating follicles | 38.8 b ± 0.46 (7) | 38.9 b ± 0.31 (10) | 38.9 d ± 0.37 (17) | |
| Follicles cooler than rectum (differential) | –0.89 ± 0.33 (17) | –0.89 ± 0.34 (17) | –0.89 ± 0.33 (34) | |
| Follicular diameter (mm) | ||||
| All follicles | 14.8 ± 2.62 (24) | 15.2 ± 3.12 (24) | 15.0 ± 2.85 (48) | |
| Ovulating follicles | 14.9 ± 2.75 (17) | 15.4 ± 3.18 (14) | 15.2 ± 2.91 (31) | |
| Non-ovulating follicles | 14.4 ± 2.44 (7) | 14.8 ± 3.16 (10) | 14.6 ± 2.80 (17) | |
Mean values ± S.D.; n between parentheses. Values with different superscript differ significantly within columns when tested by the Bonferroni test: a–b (P < 0.001); c–d (P < 0.0001).
The mean rectal temperature (± S.D.) was 38.6 ± 0.45°C, ranging from 37.9 to 39.2°C. Follicular fluid temperatures of the ovulating follicles were 0.93 ± 0.57°C significantly cooler (P < 0.0001) than rectal temperatures, ranging from –0.30 to –3.10°C. No significant differences among temperatures were found in non-ovulating follicles. All non-ovulating follicles showed temperatures equal to or higher than their corresponding rectal temperatures, except for three follicles in which temperatures were 0.10, 0.10, and 0.20°C cooler than rectal temperatures. The partners of two of these three cooler follicles ovulated. Mean temperatures of the ovulating follicles were significantly lower than those of the non-ovulating follicles, whereas follicular diameter was similar for ovulating and non-ovulating follicles (Table 1). Significant right-left differences were not found.
In the present study, employing the model of bi-ovular lactating dairy cows, the observations endorse our previous findings on monovular cows, showing that pre-ovulatory follicles are significantly cooler than those that do not subsequently ovulate [4].
Of critical interest is the functional condition of the follicle after puncture and subsequent ovulation. In other words, does damage to the follicular wall compromise or prevent formation of a functional corpus luteum after ovulation? Based now on a large series of observations, we have no evidence that this is possible. The corpus luteum that in due course forms following the puncture of a mature follicle can support a normal pregnancy.
It remains unknown in this experimental approach to ovarian function precisely when a pre-ovulatory follicle commences its divergence in temperature from that of large follicles that do not subsequently ovulate. In fact, nine of the non-ovulating follicles of the 11 single ovulating cows did not experience cooling, in contrast with their ovulating partners. Furthermore, it is not clear exactly when the maximum depression in temperature occurs. Although these points do not alter the overall conclusion drawn from these studies, they are relevant to any proposed explanations for the mechanism(s) underlying the lowering of follicular temperature. They may also be relevant to interpretations of this unusual component of ovarian physiology when attempting to place the phenomenon in an evolutionary context [6].
In our previous study, working under similar conditions but only with monovular cows, follicular temperatures lower than rectal temperatures were not registered in non-ovulating cows [4]. In the current study, three non-ovulating follicles were 0.10 or 0.20°C cooler than rectal temperatures, a decrease lower than the minimum of the range (–0.30°C) for the ovulating follicles. Although three follicles constitute a very small sample, the present results may suggest that a threshold of cooling is necessary to provoke the process of ovulation. A further point to address in a more extensive study would be to determine whether there is any relationship between the extent of cooling and the chances of pregnancy.
The difference between the mean follicular temperature decrease obtained in bi-ovular cows (–0.93°C) and that previously obtained in monovular cows (–1.54°C; 4) suggests an influence of the smaller follicular size of bi-ovular follicles (15.2 mm) compared with that of monovular follicles (18.5 mm). The ratio of antral fluid volume to that of granulosa cell mass is, among other factors, a key component in the process of follicular temperature depression [3]. This ratio could be reduced in bi-ovular follicles, facilitating a less efficient generation of intra-ovarian temperature gradients than in monovular follicles. Physiochemical endothermic reactions and heat removal through blood flow, which may favor the process of ovarian follicular cooling, might be reduced in smaller follicles [7, 8].
As an overall conclusion, results of the present study indicate that in a similar manner to monovular cows, pre-ovulatory follicles in bi-ovular cows were cooler than deep rectal temperatures and such temperature gradients were not found in follicles showing ovulation failure.
Methods
Experimental animals
This study was performed on a commercial dairy herd of Holstein-Friesian lactating dairy cows in north-eastern Spain (41.13 latitude, –2.4 longitude), from July to September 2017. Only healthy cows producing more than 30 kg of milk per day, free of detectable reproductive disorders, and free of clinical diseases during the study period (days –5 to 7 of insemination) were included. Cows were synchronized with a progesterone-based protocol for fixed-time insemination [9] using a controlled intravaginal progesterone-releasing device (CIDR; CIDR, containing 1.38 g of progesterone; Zoetis Spain SL, Alcobendas, Madrid, Spain) plus GnRH (100 μg gonadorelin diacetate tetrahydrate im; Cystoreline, CEVA Salud Animal, Barcelona, Spain) upon CIDR insertion. The CIDR was left in place for 5 days. The animals were given a PGF2α analogue (500 μg cloprostenol im; PGF Veyx Forte, Ecuphar Veterinaria, Barcelona, Spain) on CIDR removal. Twenty-four and 36 h later, the cows received a second PGF2α dose and a second GnRH dose, respectively, and were inseminated 50–56 h after CIDR removal. The cows were selected for temperature measurements at the time of insemination. A combination of ultrasonography and manual rectal palpation was used to confirm cows in estrus and ready for insemination [10]. Only cows with two bilateral co-dominant (ovulatory) follicles (one follicle per ovary) over 10 mm in diameter in the absence of a corpus luteum, with the uterus highly tonic and contractile to the touch, and vaginal discharges of copious and clear fluid were included in the study. The final study population consisted of 24 cows.
Temperature measurements and experimental design
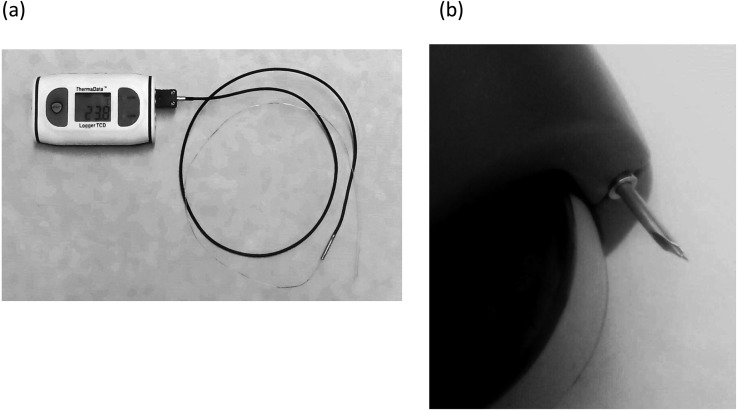
Temperatures within each pre-ovulatory follicle and approximately 20 cm deep within rectum were measured using a fine thermistor probe [4] (Fig. 1a). The probe was programmed for temperature readings every 12 sec (ThermaDataTM Logger TCD; E.T.I. Electronic Temperature Instruments, West Sussex, UK). For temperature measurements, a portable B-mode ultrasound scanner (E.I. Medical IBEX LITE; E.I. Medical Imaging, Loveland CO, USA) equipped with a convex 5–10 MHz (E.I. Medical IBEX MC8.0 10-6 Microconvex; E.I. Medical Imaging, Loveland CO, USA) transducer for transvaginal use was used. The thermistor bead was introduced into a sterile 17G 50-cm long needle with an echogenic tip (COVA needle type-A; Misawa Medical Industry, Tokyo, Japan) for follicle puncture (Fig. 1b) [4].
Fig. 1.
The apparatus with a thermistor probe that was introduced into a 17G ovum-pick-up needle of the ultrasound probe for measuring temperatures in the pre-ovulatory follicular antrum, and 20 cm into the rectum (a). The tip of the thermistor probe within the ovum pick-up needle of the ultrasound probe (b).
Immediately before temperature measurements, the vulva and the perineal region of the cow were washed with a disinfectant solution. The transducer probe, coated with a sterile preservative and with the needle containing the thermistor probe, was positioned and pre-warmed for 60 sec into the dorsal vaginal fornix, to the left or right of the cervix depending on the side of the pre-ovulatory follicle to be measured. The ovary with the pre-ovulatory follicle was then positioned transrectally against the tip of the transducer probe so that the follicle was separated only by the vaginal wall. The vaginal wall was then pierced in a cranial direction through the fornix with the needle, which was introduced into the follicular antrum as previously described [4]. The needle with the thermistor probe was maintained for 36 sec in order to perform three readings. Immediately after the third reading, the needle was carefully removed from the follicular antrum and positioned to perform measurements in the contralateral ovary. Finally, the needle was positioned within the tip of the transducer, just emerging 1‒2 mm, and the transducer was introduced 20 cm deep into the rectum for 36 sec and three readings. The mean of the three readings was used as the temperature for each point. Cows did not show any type of discomfort during the intra-follicular puncture.
Cows received artificial insemination (AI) immediately after temperature measurements. Ovulation was registered as the disappearance of the pre-ovulatory follicle 24 h after insemination, and the presence of a corpus luteum assessed seven days post-AI. Pregnancy diagnosis was performed by ultrasonography 28 days post-AI. All procedures were approved by the Ethics Committee on Animal Experimentation of the University of Lleida (license number CEEA.06-01/12).
A clear negative effect on reproductive performance of lactating dairy cows related to heat stress from May to September has been described extensively in our geographical area [11, 12]. In effect, ovulation failure increases dramatically under heat stress conditions [4, 13]. Therefore, in order to increase the number of pre-ovulatory follicles failing to ovulate, this study was performed under heat-stress conditions. The maximum temperature-humidity index (THI) was registered on the day of the AI and THI values higher than 72 were considered to be heat-stress conditions [14].
Statistical analysis
Differences between temperatures were identified by a one-way ANOVA using the SPSS software package, version 17.0 (SPSS, Chicago, IL, USA). When significant differences were detected, the Bonferroni test was used to examine all possible pairwise comparisons.
References
- 1.Hunter RHF, Einer-Jensen N, Greve T. Presence and significance of temperature gradients among different ovarian tissues. Microsc Res Tech 2006; 69: 501–507. [DOI] [PubMed] [Google Scholar]
- 2.Hunter RHF. Temperature gradients in female reproductive tissues. Reprod Biomed Online 2012; 24: 377–380. [DOI] [PubMed] [Google Scholar]
- 3.Hunter RHF. Physiology of the Graafian follicle and ovulation. Cambridge: Cambridge University Press; 2003, p. 224−294. [Google Scholar]
- 4.López-Gatius F, Hunter R. Clinical relevance of pre-ovulatory follicular temperature in heat-stressed lactating dairy cows. Reprod Domest Anim 2017; 52: 366–370. [DOI] [PubMed] [Google Scholar]
- 5.López-Gatius F, Garcia-Ispierto I, Serrano-Pérez B, Hunter RHF. The presence of two ovulatory follicles at timed artificial insemination influences the ovulatory response to GnRH in high-producing dairy cows. Theriogenology 2018; 120: 91–97. [DOI] [PubMed] [Google Scholar]
- 6.Hunter RHF, López-Gatius F, López-Albors O. Temperature gradients in vivo influence maturing male and female gametes in mammals: evidence from the cow. Reprod Fertil Dev 2017; 29: 2301–2304. [DOI] [PubMed] [Google Scholar]
- 7.Luck MR, Griffiths S, Gregson K, Watson E, Nutley M, Cooper A. Follicular fluid responds endothermically to aqueous dilution. Hum Reprod 2001; 16: 2508–2514. [DOI] [PubMed] [Google Scholar]
- 8.Ng KYB, Mingels R, Morgan H, Macklon N, Cheong Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum Reprod Update 2018; 24: 15–34. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Ispierto I, López-Gatius F. Effects of different five-day progesterone-based fixed-time AI protocols on follicular/luteal dynamics and fertility in dairy cows. J Reprod Dev 2014; 60: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Gatius F. Factors of a noninfectious nature affecting fertility after artificial insemination in lactating dairy cows. A review. Theriogenology 2012; 77: 1029–1041. [DOI] [PubMed] [Google Scholar]
- 11.López-Gatius F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology 2003; 60: 89–99. [DOI] [PubMed] [Google Scholar]
- 12.García-Ispierto I, López-Gatius F, Bech-Sabat G, Santolaria P, Yániz JL, Nogareda C, De Rensis F, López-Béjar M. Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 2007; 67: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 13.López-Gatius F, López-Béjar M, Fenech M, Hunter RHF. Ovulation failure and double ovulation in dairy cattle: risk factors and effects. Theriogenology 2005; 63: 1298–1307. [DOI] [PubMed] [Google Scholar]
- 14.De Rensis F, Garcìa-Ispierto I, López-Gatius F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015; 84: 659–666. [DOI] [PubMed] [Google Scholar]