Abstract
Background:
The high rate of non-muscle invasive bladder cancer recurrence is a major challenge in patient management. microRNAs (miRNAs) functionally regulate tumor cell proliferation and invasion, and have strong potential as biomarkers, since they are robust to degradation. The objective of this project was to identify reproducible prognostic miRNAs in resected non-muscle invasive bladder tumor tissue that are predictive of the recurrent tumor phenotype.
Methods:
We utilized patients diagnosed with primary non-muscle invasive bladder cancer in three independent cohorts for a biomarker discovery / validation approach. Baseline tumor tissue from patients with the clinically challenging, non-muscle invasive primary low stage, Ta high-grade and T1 tumors comprised the discovery cohort (n=38). We isolated the tumor tissue RNA and assessed a panel of ~800 microRNAs (miRNAs).
Results:
miR-26b-5p was the top-ranking prognostic tumor tissue miRNA, with a time-to-recurrence Hazard Ratio (HR) 0.043 for levels above vs. below median, (adjusted p-value=0.0003). miR-26b-5p was related to a dose-response reduction in tumor recurrence, and levels above the median were also associated with reduced time-to-progression (adjusted p-value=0.02). We used two independent longitudinal cohorts that included both low-grade and high-grade Ta and T1 tumors for validation and found a consistent relationship between miR-26b-5p and recurrence and progression.
Conclusions:
Our results suggest that miR-26b-5p levels may be prognostic for non-muscle invasive bladder cancer recurrence, and can feasibly be assessed in baseline tumor tissue from a wide variety of clinical settings.
Impact:
Early identification of those non-muscle invasive bladder tumor patients with refractory phenotypes would enable individualized treatment and surveillance.
Keywords: miR, miRNA, bladder cancer, urothelial carcinoma, recurrence
Introduction
Non-muscle invasive bladder tumors are prevalent in the population. An estimated 500,000 patients with a history of urothelial carcinoma currently reside in the U.S.(1). Bladder cancer recurrence rates vary considerably (2) and tumor behavior within a single histopathologic group is highly heterogeneous (3). Of patients diagnosed with non-muscle invasive bladder cancer, 50% to 75% experience recurrences within 6 to 12 years of diagnosis and 10% to 30% of tumors progress to muscle-invasive disease (4). The high rate of disease recurrence and progression is a major challenge in patient management (5). Because we lack reliable predictive markers to distinguish those patients who will experience recurrence, the need to screen all patients for these events frequently (every 3-6 months by the invasive cystoscopy procedure) makes bladder cancer one of the most expensive malignancies (6, 7).
Primary tumor clinicopathologic characteristics used to predict recurrence include multiplicity, tumor size, T category (depth of invasion), presence of carcinoma in situ, tumor grade, and patient gender (8). Patients with low stage (Ta), low grade (LG) tumors can remain disease free for many years, but poorly differentiated tumors with a high grade (HG) often recur within one year and frequently progress to muscle invasive disease (5). Management of these patients with TaHG or tumors extending into the lamina propria (T1) is clinically challenging. A subset of patients experience recurrent or progressing tumors that are refractory to treatment, and bladder removal by cystectomy may need to be performed (9). Early identification of those non-muscle invasive bladder tumor patients with recurrent, progressing, and refractory phenotypes would enable individualized treatment and surveillance recommendations, reducing patient burden and disease mortality.
Non-coding RNAs, particularly the microRNAs (miRNAs) have emerged as useful prognostic biomarkers in cancer in part because their small size makes them stable to degradation and thus robust to variations in sample handling (10). The miRNAs regulate their target genes by binding to specific sites, usually in the 3’ untranslated region (UTR) of the target gene. The miRNA then modifies the target gene via translational repression, cleavage, degradation, or sequestration (11). The objective of this project was to identify reproducible prognostic miRNAs in resected non-muscle invasive bladder tumor tissue that are predictive of the recurrent tumor phenotype as potential biomarkers and molecular therapeutic targets.
Methods
All study procedures were approved by the Committee for the Protection of Human Subjects at Dartmouth College and the Veteran’s Institutional Review Board of Northern New England. We utilized patients diagnosed with primary non-muscle invasive bladder cancer in three independent cohorts for a biomarker discovery / validation approach.
For the Dartmouth-Hitchcock Medical Center (DHMC) cohort, we retrospectively selected a sequential set of bladder cancer patients identified through the hospital tumor registry diagnosed in the years 2008 - 2014. We identified a subset of those patients with the clinically challenging non-muscle invasive histologic types: primary Ta High-Grade and T1 tumors (TaHG / T1) who had archived formalin-fixed, paraffin-embedded (FFPE) tissue blocks available for this miRNA expression project. We followed patients via retrospective review of their electronic medical record to identify recurrence and progression events. We ensured that all tissue samples utilized represented the tissue that was removed prior to the administration of any intravesical immunotherapy or chemotherapy. We reviewed the patient medical records carefully to ascertain clinical information related to recurrence and progression events. The study pathologists reviewed the hematoxylin and eosin (H&E) stained slide and circled the non-cauterized portion, containing tumor. For each tumor, we performed macrodissection on several of the matching 10 micron unstained tissue sections to select only the circled portions to maximize the tumor content of the sample. RNA was isolated from this portion using Qiagen Deparaffinization Reagent followed by the Qiagen AllPrep FFPE tissue kit (Qiagen Inc, Germantown, MD).
For additional corroboration of our miRNA markers, we collected an independent cohort of bladder cancer patients from the White River Junction Department of Veterans Affairs (VA) Medical Center (WRJ VAMC). Using administrative data from the VA National Corporate Data Warehouse, we searched for patients undergoing transurethral resection of a bladder tumor or bladder biopsy at WRJ VAMC (CPT codes 52204, 52214, 52224, 52234, 52235, 52240; ICD9 procedure codes 57.33, 57.49) between 2005 - 2011. This timeframe was chosen because of the availability of high-quality, consistently reported claims data and sufficient retrospective follow-up time to ascertain recurrence and progression outcomes. We obtained clinical information related to recurrence and progression events from the patient medical records. We utilized tissue that was removed without the administration of any intravesical immunotherapy or chemotherapy within the past 3 years. We used one or two 10-micron sections of tumor for deparaffinization and RNA isolation using the Qiagen AllPrep FFPE tissue kit. We did not macrodissect the VA cohort specimens, nor select on the basis of tumor content.
The New Hampshire population-based cohort was comprised of bladder cancer patients diagnosed in the state of New Hampshire (NH) between 2002 – 2004. Eligible cases were residents of the state of New Hampshire at the time of diagnosis identified using the State Cancer Registry, hospital pathology departments, and hospital cancer registries, as previously described (12). Information on bladder tumor clinicopathological features and recurrences was obtained from medical records, or provided by the treating hospital(s) (both inpatient and outpatient records, including any pathology reports) covering the follow-up period. The study pathologist used matching H&E stained slides to ensure that the selected specimens contained a minimum of 75% tumor. We used an entire 10-micron section for deparaffinization and RNA isolation using the Qiagen AllPrep FFPE tissue kit. Notably, this NH population cohort is unselected and included entire tissue sections and a large proportion of Ta low-grade tumors, (in contrast to the DHMC cohort, which we restricted to TaHG/T1 tumors and macrodissected).
Tissue-matched bio-fluid samples
On a subset of DHMC bladder cancer patients with tumor tissue samples, we also collected blood and urine samples. Subjects recruited into the study during their diagnostic appointment, but prior to tumor resection. Urine and peripheral blood samples were collected during that visit or at a subsequent visit, prior to tumor resection. Whole blood was collected in a Vacutainer EDTA(K2) plastic tube (Becton Dickinson, CA) and fractionated by centrifugation at 1,500 × g for 20 minutes at 20°C. Plasma, enriched-white blood cells (WBCs), and red blood cells were aliquoted into cryogenic vials (Corning Incorporated, Corning, NY) and stored at −80°C. Urine was collected mid-stream in Clikseal containers (Therapak Corp, Buford, GA), and subsequently transferred to 15 ml conical tubes (VWR, Radnor, PA) for centrifugation. Urine was centrifuged twice, first at 1,200 × rpm for 20 minutes at 20°C. Supernatant was transferred to fresh conical tube and centrifuged at 2,500 × rpm for 20 minutes at 20°C. Final supernatant was aliquoted into cryogenic vials and stored at −80°C.For comparison with the biofluids, total RNA was extracted from 3× 20 μM slices of FFPE-tumor tissue using Norgen FFPE RNA/DNA Purification Plus Kit (Norgen Biotek Corp., Thorold, ON, Canada). Circulating and exosomal plasma microRNA was isolated from a 200 ul plasma volume using Norgen Plasma/Serum Circulating and Exosomal RNA Isolation Kit. Cell-free microRNA from urine was obtained using Norgen Urine Exosome RNA Isolation Kit from a 4 ml volume of cell-free urine. Enriched-WBC RNA was isolated using Norgen Total RNA Purification Kit. All protocols were performed according to manufacturer’s instructions. Plasma and urine microRNA was purified and concentrated using Amicon Ultra 0.5 columns (Millipore, Billerica, MA), as described (13).
MicroRNA expression levels
Dartmouth Genomics and Microarray Core facility simultaneously assessed approximately 800 miRNA probes using the Nanostring human v3 microRNA expression assay (NanoString Technologies, Seattle, WA). We loaded 200ng of total RNA into the assay. Specific tags were ligated to the 3’ end of each miRNA molecule. MiRNA molecules were then hybridized to a panel of miRNA:tagspecific nCounter capture and barcoded reporter probes. The nCounter Digital Analyzer counted individual fluorescent barcodes and quantified the target RNA molecules present in each sample. We used Nanostring Nsolver 3.0/4.0 software to normalize the count data to the positive controls and to average geometric mean of the top 100 detected miRNAs. We estimated the background level using the counts in the negative controls (mean +2SD), and restricted our analyses to the miRNAs expressed at counts above background in at least 1/3 of the tumors (169 miRNAs in the DHMC cohort). The miRNA levels of samples duplicated across batches were highly correlated (r2=0.99) and the coefficient of variation (%CV) for miR-26b-5p was 5.6 among these samples.
Statistical analysis
We defined first recurrent tumor as any tumor identified following a disease-free remission period, more than 90 days after the date of initial primary bladder tumor diagnosis. These recurrent tumors include subsequent tumors of the same level of invasiveness, as well as those progressing to higher stage/grade. Persistent primary tumors that did not have a remission period were excluded from the analysis of recurrence. Time to recurrence was calculated as the time between the initial diagnosis date and the date of the first recurrence event. We report on overall progression, including tumors with a greater stage or grade than the initial primary bladder tumor; and report the proportion progressing to muscle-invasion or metastasis (14). If no events were reported, the date the patient was last seen documented in the medical record was used for censoring.
Median times to first recurrence, or progression were calculated using the Kaplan-Meier method. Multivariate analysis of time to the first bladder tumor recurrence and progression analyses were performed using Cox-proportional hazards regression analysis with miR-26b-5p levels modeled as a continuous variable, and using median or quintile cut-points. The standard base prognostic model included adjustment for age at diagnosis of first bladder tumor, gender, tumor size (<3, 3+cm), multiplicity (single, multiple), stage (Ta, T1) / grade (low-grade, high grade). miRNA levels were modeled as counts, or using the median as a cutpoint. We constructed time-dependent receiver operating characteristic (ROC) curves and area under the receiver operating characteristic curves (AUCs) using Akitas’s nearest neighbor estimation of the bivariate distribution implemented in the ‘survivalROC’ package (15). We assessed the accuracy of the multivariate models for discriminating patients at high-risk of recurrence using the concordance-index, which has values ranging from 0.5 to 1.0 (perfect discrimination). P-values represent two-sided statistical tests. Analyses were performed using R 3.4.1.
Results
As shown in Table 1, the non-muscle invasive bladder cancer patient cohorts assessed for miRNAs included a majority of male patients (72-100%) diagnosed with bladder cancer at mean ages between 62-72. Multiple tumors were present in a subset (26-38%), and approximately half had large (3+ cm) tumors.
Table 1.
Non-muscle invasive bladder cancer patient characteristics by cohort.
| DHMC TaHG/T1 cohort | VA cohort | NH population cohort | |||||
|---|---|---|---|---|---|---|---|
| n=38 | % | n=24 | % | n=169 | % | ||
| Age | mean±SD | 69.84±11.96 | 72.38±10.32 | 62.67±10.36 | |||
| Gender | Female | 9 | 24% | 0 | 0% | 47 | 28% |
| Male | 29 | 76% | 24 | 100% | 122 | 72% | |
| Multiplicity | Multi | 10 | 26% | 9 | 38% | 50 | 30% |
| Single | 28 | 74% | 14 | 58% | 119 | 70% | |
| Size | Large | 17 | 45% | NA | 69 | 58% | |
| Small | 21 | 55% | NA | 50 | 42% | ||
| Stage/Grade | TaLG | 0 | 0% | 14 | 58% | 131 | 78% |
| TaHG | 10 | 26% | 8 | 33% | 12 | 7% | |
| T1 | 28 | 74% | 1 | 4% | 26 | 16% | |
Within the DHMC TaHG/T1 cohort, we used Cox-regression analysis to assess the association between each of the miRNAs detected in tumor tissue and time-to-first recurrence. Our multivariable model was adjusted for sex, age, multiplicity, tumor size, stage, and grade. Table 2 shows the top P-value ranked tumor tissue miRNAs associated with recurrence. miR-424-5p, miR-125a-5p, and miR-193b-3p showed association trends in the continuous model of miRNA counts, however when analyzed categorically (e.g. using the median as a threshold), the relationships for miR-125a-5p, and miR-193b-3p were not statistically significant, suggesting a non-linear relationship. Although miR-424-5p levels above the median were significantly associated with recurrence (P=0.033), this marker was not statistically significantly associated with progression (P=0.30).
Table 2.
Top-ranking miRNAs associated with non-muscle invasive bladder cancer recurrence.
| miRNA count | miRNA levels ≥ vs < median | miRNA levels ≥ vs < median | ||||||
|---|---|---|---|---|---|---|---|---|
| p-value* | coefficient* | HR* | (95%CI) | p-value* | HR* | (95%CI) | p-value* | |
| Recurrence | Progression | |||||||
| DHMC TaHG/T1 cohort | events=20 of 38 | events=9 of 38 | ||||||
| hsa-miR-26b-5p | 0.00084 | −0.0021 | 0.043 | (0.0079-0.24) | 0.00031 | 0.061 | (0.0059-0.64) | 0.020 |
| hsa-miR-424-5p | 0.006 | 0.0047 | 2.94 | (1.09-7.93) | 0.033 | 2.1 | (0.51-8.68) | 0.3 |
| hsa-miR-125a-5p | 0.0064 | −0.0028 | 0.41 | (0.13-1.33) | 0.14 | 1.2 | (0.25-5.86) | 0.82 |
| hsa-miR-193b-3p | 0.0089 | 0.006 | 1.38 | (0.50-3.85) | 0.54 | 1.47 | (0.30-7.23) | 0.63 |
| Validation: | ||||||||
| VA cohort | events=11 of 23 | events=1 of 23 | ||||||
| hsa-miR-26b-5p | 0.66 | (0.15-2.97) | 0.59 | NA | ||||
| NH population cohort | events=104 of 178 | events=8 of 176 | ||||||
| hsa-miR-26b-5p | 0.71 | (0.47-1.05) | 0.086 | 0.32 | (0.062-1.64) | 0.17 | ||
Multivariable model adjusted for sex, age, multiplicity, tumor size, stage, grade.
Thus, miR-26b-5p was the miRNA most strongly associated with recurrence (continuous miR-26b-5p levels, P=0.00084 adjusted for sex, age, multiplicity, tumor size, stage, grade), and with progression (adjusted P=0.02). miR-26b-5p levels did not differ significantly by sex (P=0.90), multiplicity (P=0.95), tumor size (P=0.62), stage/grade (P=0.06), treatment with chemotherapy (P=0.1), or BCG (P=0.71), and were not correlated with age at diagnosis (P=0.27). The tumor miR-26b-5p counts were lower than the patient-matched histologically normal adjacent tissue in 2/3 of the resected specimens assessed (Supplemental Figure 4). Breaking the tumor tissue miRNA level at the median, the patients with miR-26b-5p above the median had longer time-to-recurrence (Figure 1a). The recurrence Hazard Ratio (HR) for tumor tissue miR-26b-5p was 0.043, ≥ vs. <median (P-value=0.0003, adjusted for sex, age, multiplicity, tumor size, stage, grade) (Table 2), meeting the Bonferroni corrected p-value threshold (alpha 0.05 / 169 miRNAs). The addition of chemotherapy and BCG treatment to the model did not materially affect the statistical significance (recurrence P-value=0.00025, progression P-value=0.012). Dividing the miR-26b-5p levels into quintiles, we observe a dose-response relationship with time-to-recurrence (Figure 1c). Using the concordance-index, we assessed the accuracy of the multivariable models for discriminating patients at high-risk of recurrence. The base-model (sex, age, multiplicity, size, stage, grade) had a concordance-index of 0.63. The concordance-index improved with the addition of miR-26b-5p to 0.81 (model includes sex, age, multiplicity, size, stage, grade, and miR-26b-5p).
Figure 1. Prognosis of DHMC discovery cohort by baseline tumor tissue miR-26b-5p levels.
Kaplan-Meier plots depict recurrence (a, c) and progression (b, d) probability in patient subgroups based on the miR-26b-5p levels in their primary TaHG/T1 tumors. Patients with miR-26b-5p levels ≥median (black line) vs. <median (grey line) had lower recurrence (adjusted P=0.00031, panel a) and progression probabilities (adjusted P=0.02, panel b). In panels c, d, the shades depict the quintile, with dark lines depicting higher miR-26b-5p levels.
We also evaluated tumor tissue miR-26b-5p in relation to bladder cancer progression to a higher stage or grade. Stage progression to muscle invasive disease or metastasis occurred in 33% of these events. Patients with miR-26b-5p ≥ vs. <median (adjusted p-value=0.02), or in higher quintiles consistently had a lower risk of progression (Table 2, Figure 1b, 1d). Based on the consistency of the association with both recurrence and progression risk, we focused on validating miR-26b-5p as a prognostic marker across other patient cohorts.
We assessed miR-26b-5p levels in an independent cohort of patient from the VA Medical Center in White River Junction (WRJ VAMC) (n=20 patients with 11 recurrence events and a single progression event). The Kaplan-Meier plot shows the probability of recurrence is lower among VA patients with miR-26b-5p levels above the median (HR 0.66), although the curves begin to intersect as time moves to 17 months (Figure 2a).
Figure 2. Prognosis of the VA replication cohort by baseline tumor tissue miR-26b-5p levels.
Kaplan-Meier plots depict recurrence (11 events of 23) and progression probability (1 event of 23). Patients with miR-26b-5p levels ≥median (black line) vs. <median (grey line) had lower probability of recurrence out to 10 months of follow-up (panel a).
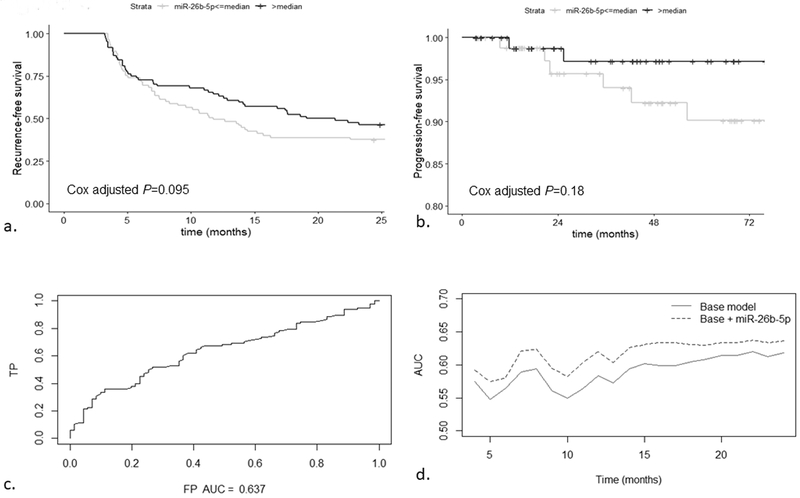
We then assessed the value of miR-26b-5p in a broader community setting using the NH population cohort. Kaplan-Meier plots show a consistent, lower risk of recurrence and of progression with miR-26b-5p levels above the median (Figure 3a, 3b). Analyses stratified into Ta low-grade and Ta high-grade / T1 (Supplemental Figure 1), and by World Health Organization / International Society of Urological Pathology (WHO/ISUP) classification subsets show similar effects (Supplemental Figure 2). We constructed ROC curves to evaluate the ability of our models to discriminate patients who have recurrence from those who do not. The area under the ROC curve (AUC) at 24 months was 0.64 for the model containing miR-26b-5p, plus the base factors (sex, age, multiplicity, tumor size, stage, grade) (Figure 3c). The model containing miR-26b-5p has a consistently higher AUC (mean 0.62) compared to the base model (mean 0.59) throughout the two-years of follow-up (P=0.00027). We also assessed the accuracy of the multivariable models for discriminating patients at high-risk of recurrence. The concordance-index for the model containing base factors (sex, age, multiplicity, tumor size, stage, grade) remained at 0.60 with the addition of miR-26b-5p for the Ta patients, however for T1 patients, the discrimination improved from 0.66 for the base-model to 0.75 with the addition of miR-26b-5p.
Figure 3. Prognosis of the NH population-based replication cohort by baseline tumor tissue miR-26b-5p levels.
Kaplan-Meier plots depict recurrence (98 events of 169) and progression probability (8 events of 167). Patients with miR-26b-5p levels ≥median (black line) vs. <median (grey line) had lower recurrence (adjusted P=0.095, panel a) and progression probabilities (adjusted P=0.18, panel b). Panel c shows the area under the ROC curve (AUC) is 0.64 for discrimination of patients with recurrence at 24-months in the model containing age, gender, grade, stage, multiplicity, and miR-26b-5p level in the baseline tumor. Panel d shows that the addition of miR-26b-5p level significantly increases the AUC compared to the base model throughout the follow-up period (P=0.00027).
Blood and urine can also be convenient for assessment of prognostic miRNA levels. We used a set of blood and urine samples that were patient matched to tumor tissue to assess the levels of the tumor tissue miR-26b-5p in the corresponding bio-fluid samples. Tumor tissue miR-26b-5p levels were not significantly correlated with blood plasma (P=0.19), white blood cell (WBC) (P=0.36), or urine levels (P=0.45) (Supplemental Figure 3).
Discussion
The objective of this project was to identify prognostic miRNAs in resected non-muscle invasive bladder tumor tissues. We utilized non-muscle invasive bladder cancer patient cohorts spanning three different settings: a referral hospital cohort, a Veterans Administration hospital, and a population-based study including community hospitals. We identified miR-26b-5p as consistently showing prognostic value for bladder tumor recurrence and progression. The linear nature of miR-26b’s relationship with prognosis make it a better biomarker prospect than the other miRNAs identified. The utility of this miRNA was validated in unselected tissue sections from community hospital settings.
Gottardo et al. identified suppressed expression of miR-25b among a group of 10 miRNAs dysregulated in a cross-sectional screen of n=25 urothelial carcinoma vs. n=2 normal bladder tissue samples (16) a finding replicated by Miyamoto et al. comparing a Japanese cohort of n=69 bladder tumors, n=23 normal epithelia (P=0.0006). Our finding that miR-26b-5p is a predictor of recurrence and progression across several longitudinally followed cohorts of non-muscle invasive bladder cancer patients is unique, and strongly supports the in vitro work demonstrating a tumor suppressive role for miR-26b-5p (17). While focused on the role of procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) as a potential miR-26a-5p/miR-26b-5p target, these cell culture transfection studies also demonstrated a functional role for miR-26b. MiR-26b-5p transfection inhibited proliferation, migration, and matrigel invasion of bladder tumor cell lines, compared to miR-control (17).
miR-26b is contained within an intron of the phosphatase gene carboxy-terminal domain RNA polymerase II polypeptide (CTDSP). miR-26b is expressed as part of the CTDSP2 transcript, and together they block the G1/S-phase cell cycle transition by cooperatively activating the checkpoint protein pRb (18). c-Myc is overexpressed in approximately half of non-muscle invasive bladder tumors, with levels that are not correlated with tumor stage or grade (19). c-Myc is capable of transcriptionally repressing both CTDSP and miR-26b (18), possibly explaining the low miR-26b levels observed in some tumors. The levels of the mature form of miR-26b can also be controlled by inhibiting the processing of the precursor pre-miR-26b (20). Because Myc has been challenging and intractable as a direct therapeutic target, targeting miR-26b is a potential alternative intervention strategy to reduce risk of recurrence.
miR-26b levels were significantly lower in serum samples of prostate cancer patients relative to non-cancer controls P<0.001 (21). However, our study within bladder cancer patients showed that tumor tissue miR-26b levels did not correlate with the patient-matched biofluid, suggesting that the baseline resected tumor will likely be the more useful specimen. The tissue miR-26b levels were associated with recurrence risk even without macrodissecting the tissue specimens, increasing the logistical feasibility of this potential biomarker.
Our study provides unique longitudinal assessment of the relationship between miRNAs in non-muscle invasive bladder cancer patients’ baseline tumor tissue and their future recurrence and progression outcomes. The addition of miR-26b-5p to our multivariable models improved the accuracy for discrimination of patients at elevated risk for recurrence. The validation of this model in an external population with community hospital patients suggests potential clinical utility. Limitations of this study include a small number of progression events in the replication datasets, hampering our ability to draw definitive conclusions regarding the reproducibility of this endpoint. We deliberately restricted the Dartmouth Hitchcock Medical Center (DHMC) cohort to patients with the more clinically challenging non-muscle invasive tumors (26% Ta high-grade, 74% T1), to ensure we identified markers addressing the needs of this subgroup. In contrast, the majority of the patients in the replication phase from the White River Junction Veteran’s Affairs Medical Center (WRJ VAMC) and New Hampshire (NH) population cohorts had low stage (Ta) low-grade tumors (58 and 78%, respectively). The VA patients included some index tumors that were actually recurrences, in contrast, the DHMC and NH cohorts only assayed miRNAs in a patient’s incident (first) tumor. miR-26b levels were related to recurrence risk across all three cohorts, increasing the validity of our finding.
We identified miR-26b levels in baseline tumor tissue as a potential biomarker for non-muscle invasive bladder cancer recurrence and progression. Our results demonstrate that prognostic miR-26b levels can feasibly be assessed in baseline tumor tissue from a wide variety of clinical settings. Further validation of the prognostic value of this marker in the baseline tumor tissue of additional populations is warranted.
Supplementary Material
Acknowledgements
This publication was funded in part by grant numbers R21CA182659, K07CA102327, and P42ES07373 from the National Cancer Institute, NIH, from the National Institute of Environmental Health Sciences, NIH. The authors acknowledge the following important contributions: the New Hampshire State Cancer Registry, which is supported by the Centers for Disease Control and Prevention’s National Program of Cancer Registries through the New Hampshire Department of Health and Human Services, Division of Public Health Services, Bureau of Public Health Statistics & Informatics, Health Statistics and Data Management Section; The Genomics and Molecular Biology Shared Resources (GMBSR) facilities at the Norris Cotton Cancer Center at Dartmouth with NCI Cancer Center Support Grant 5P30 CA023108-37. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: Disclaimer: Opinions expressed in this manuscript are those of the authors and do not constitute official positions of the U.S. Federal Government or the Department of Veterans Affairs.
Conflicts of interest: The authors declare no potential conflicts of interest.
References
- 1.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013. February;63(2):234–41. [DOI] [PubMed] [Google Scholar]
- 2.Carroll P, Raghavan D, Stein J, Zietman A, editors. The Treatment of Bladder Cancer- Stage by Stage. 2001 AUA Annual Meeting; 2001 June 2, 2001. [Google Scholar]
- 3.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007. June;25(3):285–95. [DOI] [PubMed] [Google Scholar]
- 4.Petrovich Z, Baert L, Boyd SD, et al. Management of carcinoma of the bladder. Am J Clin Oncol 1998. June;21(3):217–22. [DOI] [PubMed] [Google Scholar]
- 5.Honma I, Masumori N, Sato E, et al. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology. 2004. October;64(4):744–8. [DOI] [PubMed] [Google Scholar]
- 6.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–30. [DOI] [PubMed] [Google Scholar]
- 7.Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008. July;180(1):31–7; discussion 7. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Drager BJ. Identifying risk factors in patients with non-muscle-invasive bladder cancer: clinical implications. Eur Urol. 2011. October;60(4):721–3. [DOI] [PubMed] [Google Scholar]
- 9.Liakou CI, Narayanan S, Ng Tang D, Logothetis CJ, Sharma P. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun. 2007;7:10. [PMC free article] [PubMed] [Google Scholar]
- 10.Schubert M, Junker K, Heinzelmann J. Prognostic and predictive miRNA biomarkers in bladder, kidney and prostate cancer: Where do we stand in biomarker development? J Cancer Res Clin Oncol. 2016. August;142(8):1673–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sempere LF, Kauppinen S. Translational Implications of MicroRNAs in Clinical Diagnostics and Therapeutics In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling: Oxford: Academic Press; 2009. p. 2965–81. [Google Scholar]
- 12.Marsit CJ, Houseman EA, Christensen BC, et al. Identification of methylated genes associated with aggressive bladder cancer. PLoS One. 2010;5(8):e12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong DA, Green BB, Seigne JD, Schned AR, Marsit CJ. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer. 2015. November 14;14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Kikuchi E, Mikami S, et al. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urol. 2014. January 8;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000. June;56(2):337–44. [DOI] [PubMed] [Google Scholar]
- 16.Gottardo F, Liu CG, Ferracin M, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol. 2007. Sep-Oct;25(5):387–92. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto K, Seki N, Matsushita R, et al. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br J Cancer. 2016. July 26;115(3):354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Lu Y, Zhang Q, et al. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res. 2012. May;40(10):4615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christoph F, Schmidt B, Schmitz-Drager BJ, Schulz WA. Over-expression and amplification of the c-myc gene in human urothelial carcinoma. Int J Cancer. 1999. April 20;84(2):169–73. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Zhang L, Sun T. Cohesive Regulation of Neural Progenitor Development by microRNA miR-26, Its Host Gene Ctdsp and Target Gene Emx2 in the Mouse Embryonic Cerebral Cortex. Front Mol Neurosci. 2018;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moltzahn F, Olshen AB, Baehner L, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011. January 15;71(2):550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.