Abstract
Purpose
Epidermal growth factor receptor (EGFR) exon 20 insertion mutations account for approximately 4% of all EGFR mutations. Given the rarity of this mutation, its clinical outcomes are not fully established.
Materials and Methods
Between 2009 and 2017, non-small cell lung cancer (NSCLC) patients who showed an exon 20 insertion were retrospectively reviewed for clinical characteristics and outcomes, including responses to chemotherapy (CTx) or targeted therapy.
Results
Of 3,539 NSCLC patients who harbored an activating EGFR mutation, 56 (1.6%) had an exon 20 insertion. Of the advanced NSCLC patients, 27 of 1,479 (1.8%) had an exon 20 insertion. The median overall survival was 29.4 months (95% confidence interval 9.3 to 49.6) for 27 advancedNSCLC patients. The 22 patientswho received systemic CTx achieved a 50.0% response rate and a 77.2% disease control rate, with 4.2 months of progressionfree survival. Six patients received EGFR tyrosine kinase inhibitors (TKIs). Three of the four patients that had only an exon 20 insertion showed progressive disease, while one showed stable disease. The othertwo patients had an exon 20 insertion and another EGFR mutation and achieved a partial response.
Conclusion
The incidence of an exon 20 insertion mutation is rare in Korea and occasionally accompanied by other common EGFR mutations. Although the response to systemic CTx. in these patients is comparable to that of patients with other mutations, the response rate to firstor second-generation EGFR TKIs is quite low. Therefore, the development of a more efficient agent is urgently needed.
Keywords: Non-small cell lung cancer, Exon 20 insertion, Epidermal growth factor receptor, Mutation, EGFR tyrosine kinase inhibitor
Introduction
Lung cancer is a leading cause of cancer death worldwide [1,2]. In Korea, 24,000 cases are newly diagnosed, and 17,440 patients die of lung cancer each year [2]. Approximately 80-85% of lung cancers are classified as non-small cell lung cancer (NSCLC) [3]. The discovery of relevant genomic abnormalities in NSCLC has led to the development of novel, targeted chemotherapeutic agents. It has also caused a paradigm shift in the treatment of NSCLC, particularly for those with non-squamous cell carcinoma. Epidermal growth factor receptor (EGFR) mutations represent the most prevalent drug-treatable targets and are detected in approximately 40% of NSCLC cases in Asian patients, and in 10%-20% of cases in Caucasian patients [4,5]. EGFR mutations are commonly found in never-smokers, females, and patients with adenocarcinoma. The most common activating EGFR mutations include an in-frame deletion in exon 19 and the L858R mutation in exon 21. Together, these account for 90% of EGFR mutations [6].
EGFR tyrosine kinase inhibitors (TKIs) are associated with a highly effective and durable response in NSCLC patients with these common EGFR mutations, often yielding 9-13 months of progression-free survival (PFS) and more than 24 months of overall survival (OS) [7-9]. Other, less commonly observed EGFRmutations (such as G719X or L861Q) account for 2%-3% of EGFR mutations and are also considered responsive to EGFR TKIs [10].
EGFR exon 20 insertion mutations are typically located just after the C-helix of the tyrosine kinase domain of EGFR, and their incidence varies between 1% to 9% of all EGFR mutations [11-15]. Given the rarity of these mutations and the fact they are mostly studied in surgically resected patients, the clinical characteristics and outcomes of advanced NSCLC patients with EGFR exon 20 insertion mutations have not been fully established.
Materials and Methods
1. Patients
Between January 2009 and December 2017, histologically confirmed Samsung Medical Center NSCLC patients with activating EGFR mutations were selected from an institutional database. Among them, patients with an EGFR exon 20 insertion mutation were retrospectively analyzed for clinicopathological characteristics, responses to systemic chemotherapy or targeted agents, PFS, and OS.
2. EGFR mutation tests
Mutational analyses of EGFR (exons 18-21) were performed as previously described by directional sequencing, by the peptide nucleic acid clamp method, or by next-generation sequencing [16].
3. Statistical analysis
All available data were retrospectively collected using a standardized case report form. OS and PFS were calculated using the Kaplan-Meier method. The Cox proportional hazards regression model was used to evaluate the impact of collected variables on PFS and OS. Two-sided p-values were set at a 0.05 significance level. All analyses were performed using SPSS ver. 23.0 software (IBM Corp., Armonk, NY).
4. Ethical statement
Institutional Review Board (IRB) approval was obtained from Samsung Medical Center (SMC, Seoul, Korea, SMC 2018-02-019). The IRB approved waiver of informed consent.
Results
1. Prevalence of EGFR mutations
From January 2009 to December 2017, 3,539 patients showed positive results in the EGFR mutation test. Among them, 1,712 (48.3%) had an exon 19 deletion, 1,451 (41.0%) had L858R, 132 (3.7%) had G719X, 92 (2.6%) had L861Q, 56 (1.6%) had an exon 20 insertion, and 34 (1.0%) had S768I (S1 Fig.). Among the 56 patients with an exon 20 insertion, eight had an additional mutation: four had an exon 19 deletion, two had L858R, one had G719S, and one had S768I.
Of the 3,539 total patients, 1,479 had advanced NSCLC. These patients included 752 (50.8%) with an exon 19 deletion, 557 (37.7%) with L858R, 49 (3.3%) with G719X, 49 (3.3%) with L861Q, 27 (1.8%) with an exon 20 insertion, and 17 (1.1%) with S768I (Fig. 1).
Fig. 1.
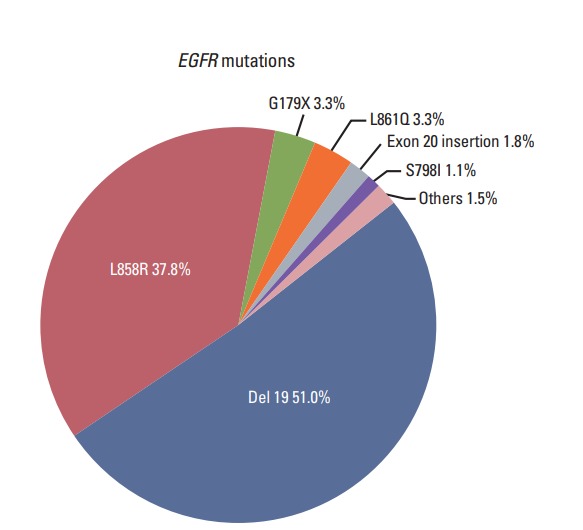
Distribution of epidermal growth factor receptor (EGFR) mutations in advanced non-small cell lung cancer patients (n=1,479).
2. Characteristics of patients with exon 20 insertion mutation
Among total 3,539 patients, 3,163 patients were identified that having common EGFR mutations, meanwhile 376 patients were uncommon EGFR mutations. Demographic characteristics of total 3,539 patients were summarized in Table 1. Women, adenocarcinoma, and early staged NSCLC patients were more common in NSCLC patients with common EGFR mutations.
Table 1.
Clinical characteristics of total 3,539 patients with NSCLC
| Common (n=3,163) | Uncommon (n=376) | p-value | |
|---|---|---|---|
| Age at diagnosis (yr) | 61 (19-92) | 62 (27-87) | NS |
| Sex | |||
| Female | 2,008 (63.5) | 217 (57.7) | 0.032 |
| Male | 1,155 (36.5) | 159 (42.3) | |
| Histology | |||
| ADC | 3,077 (97.3) | 335 (89.1) | < 0.001 |
| SqCC | 47 (1.5) | 20 (5.3) | |
| Others | 39 (1.2) | 21 (5.6) | |
| Stage | |||
| I-IIIA | 1,866 (59.0) | 194 (51.6) | 0.007 |
| IIIB-IV | 1,297 (41.0) | 182 (48.4) |
Values are presented as median (range) or number (%). NSCLC, non-small cell lung cancer; NS, non-significant; ADC, adenocarcinoma; SqCC, squamous cell carcinoma.
Baseline characteristics of 27 advanced NSCLC patients harboring an exon 20 insertion mutation are summarized in Table 2. The median follow-up duration was 12.1 months (range, 0.9 to 62.4 months). The median age at diagnosis was 60 years (range, 43 to 75 years), and 41% of patients were female. A majority of patients (78%) were never-smokers, and most (96%) had adenocarcinoma. The most common metastatic sites were the central nervous system (40.7%), lung (33.3%), bone (33.3%), and liver (18.5%). Of note, 23 patients had only an exon 20 insertion mutation, while four patients had an additional activating mutation: two had L858R, one had S768I, and one had G719S (Table 2).
Table 2.
Baseline characteristics of patients with an exon 20 insertion mutation
| Early-stage (n=29) | Refractory NSCLC (n=27) | Total (n=56) | |
|---|---|---|---|
| Age at diagnosis (yr) | 60 (36-78) | 60 (43-75) | 60 (36-78) |
| Sex | |||
| Female | 14 (48.2) | 11 (40.7) | 25 (44.6) |
| Male | 15 (51.8) | 16 (59.3) | 33 (55.4) |
| Histology | |||
| ADC | 29 (100) | 26 (96.3) | 55 (98.2) |
| SqCC | 0 | 1 (3.7) | 1 (1.8) |
| ECOG | |||
| 0 | 8 (27.6) | 1 (3.8) | 9 (16.1) |
| 1 | 21 (72.4) | 19 (70.3) | 40 (71.4) |
| 2 | 0 | 7 (25.9) | 7 (12.5) |
| Smoking history | |||
| Never | 19 (65.5) | 21 (77.8) | 40 (71.4) |
| Current | 6 (20.7) | 3 (11.1) | 9 (16) |
| Ex | 4 (13.8) | 3 (11.1) | 7 (12.6) |
| Metastasis | |||
| CNS | - | 11 | - |
| Bone | - | 9 | - |
| Liver | - | 5 | - |
| Lung | - | 9 | - |
| Exon 20 mutation | |||
| Insertion only | 25 (8-6.2) | 23 (85.2) | - |
| Double mutation | |||
| Insertion+S768I | - | 1 (3.7) | - |
| Insertion+G719S | - | 1 (3.7) | - |
| Insertion+Deletion 19 | 4 (13.8) | - | - |
| Insertion+L858R | - | 2 (7.4) | - |
Values are presented as median (range) or number (%). NSCLC, non-small cell lung cancer; ADC, adenocarcinoma; SqCC, squamous cell carcinoma; ECOG, Eastern Cooperative Oncology Group; CNS, central nervous system.
3. Types of exon 20 insertion mutations
Among 27 patients with exon 20 insertions, only 17 had available data for amino acid position changes. All exon 20 insertions were clustered between Val769 and Val775. His-773_Val774insHis was the most common insertion mutation (n=7, 41.2%), followed by Val774_Cys775insHisVal (n=3, 17.7%) and His773_Val774insProHis (n=2, 11.7%). Each of the following mutations was found one time: His773_Val-774insAsnProHis, Pro772_His773insThrThrPro, Pro772_ His-773insGlyAsnPro, Val769_Asn771insValGlyVal, and Asp-770_Asn771insGly (Table 3).
Table 3.
Amino acid sequences of 17 NSCLC patients with an exon 20 insertion
| No. | Sex | Age (yr) | Smoking Hx | ECOG | Initial stage | Exon 20 mutation | EGFR TKI | Chemotherapy | Best response | PFS | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 58 | Never | 1 | IIIA | c.2319_2320 ins CAC (p.H773_V774insH) | N/A | Premetrexed+cisplatin | SD | 17.0 | 46.4 |
| 2 | M | 73 | Never | 2 | IA | c.2319_2320 ins CAC (p.H773_V774insH) | N/A | N/A | 44.8 | ||
| 3 | M | 75 | Never | 1 | IV | C.2316_2317 ins GGCAACCCC (p.P772_H773insGNP) | N/A | Premetrexed+cisplatin | SD | 2.7 | 3.8 |
| 4 | M | 63 | Never | 1 | IIIA | c.2319_2320 ins CCCCAC (p.H773_V774insH) | N/A | Paclitaxel+cisplatin | PD | 0.9 | 9.7 |
| 5 | M | 50 | Never | 1 | IB | c.2305_2313 ins GTGGGGGTC (p.V769_N771insVGV) | N/A | N/A | 22.5 | ||
| 6 | F | 55 | Never | 1 | IV | c.2310_2311 ins GGT (p.D770_N771insG) | N/A | Premetrexed+cisplatin | PD | 1.9 | 6.1 |
| 7 | F | 63 | Never | 2 | IV | c.2322_2323 ins CACGTG (p.V774_C775insHV) | N/A | Premetrexed+cisplatin | SD | 2.1 | 18.4 |
| 8 | F | 55 | Never | 1 | IA | c.2322_2323 ins CACGTG (p.V774_C775insHV) | N/A | Premetrexed+cisplatin | PR | 13.0 | 26.2 |
| 9 | F | 56 | Never | 1 | IIIA | c.2319_2320 ins AACCCCCAC (p.H773_V774insNPH) | N/A | Docetaxel+cisplatin | PR | 2.7 | 29.5 |
| 10 | M | 60 | Never | 2 | IB | c.2319_2320 ins CCCCAC (p.H773_V774insPH | N/A | Premetrexed+cisplatin | PR | 2.6 | 46.4 |
| 11 | M | 48 | Never | 2 | IV | c.2315_2316 ins GACAACCCC (p.P772_H773insTTP) | N/A | Premetrexed+cisplatin | PR | 2.4 | 12.4 |
| 12 | F | 62 | Never | 1 | IA | c.2321_2322 ins CCACGT (p.V774_C775insHV) | N/A | N/A | 44.1 | ||
| 13 | F | 52 | Never | 1 | IA | c.2319_2320insCAC (p.H773_V774insH) | N/A | N/A | 35.2 | ||
| 14 | M | 60 | Never | 1 | IIIA | c.2319_2320insCAC (p.H773_V774insH) | N/A | Etoposide+cisplatin | PD | 2.6 | 25.9 |
| 15 | M | 60 | Never | 1 | IIIA | c.2319_2320insCAC (p.H773_V774insH) | N/A | Etoposide+cisplatin | SD | 17.2 | 26.2 |
| 16 | F | 66 | Never | 1 | IIIA | c.2318_2319 ins TAACCCCAG (p.H773_V774insPH) | N/A | Premetrexed+cisplatin | SD | 2.8 | 62.4 |
| 17 | M | 65 | Never | 1 | IA | c.2319_2320insCAC (p.H773_V774insH) | N/A | Premetrexed+cisplatin | PR | 2.9 | 36.9 |
The most frequent amino acid change was His773_Val774insHis (41.1%), followed by Val774_Cys775insHisVal (17.6%) and His773_Val774insProHis (11.7%).
NSCLC, non-small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; ECOG, Eastern Cooperative Oncology Group; EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; PFS, progression-free survival; OS, overall survival; F, female; N/A, not available; M, male; SD, stable disease; PD, progressive disease; PR, partial response.
4. Treatment responses and clinical outcomes
Of the 27 patients with exon 20 insertions, 22 received platinum-based systemic chemotherapy. The overall response rate (ORR) was 50.0%, and the disease control rate was 77.2%. The median PFS was 4.2 months (95% confidence interval [CI], 1.7 to 6.6), and the median OS was 29.4 months (95% CI, 9.3 to 49.6) (Fig. 2). In contrast, all patients with a double mutation were still alive at the time of last follow-up (November 15, 2017; OS range, 2.2 to 15.0 months). A Cox proportional regression analysis revealed that central nervous system metastasis was associated with poor OS (p=0.043, data not shown).
Fig. 2.
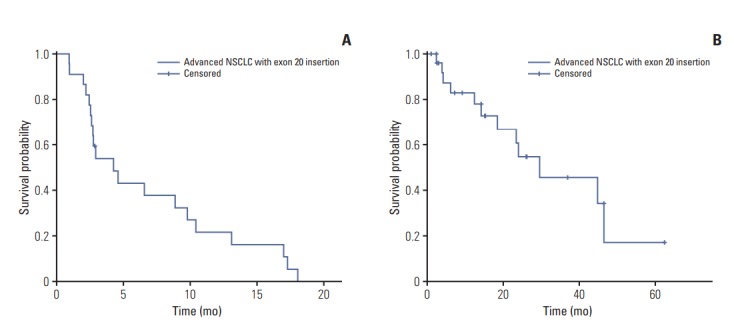
Kaplan-Meier survival curves. (A) Progression-free survival of patients with an exon 20 insertion receiving systemic chemotherapy. (B) Overall survival of patients with an exon 20 insertion. NSCLC, non-small cell lung cancer.
Six patients were treated with an EGFR TKI. Three patients received a reversible EGFR TKI (erlotinib), and the other three received an irreversible EGFR TKI (two received afatinib, and one received osimertinib). Among the four patients with only an exon 20 insertion, three had progressive disease and one had stable disease. In contrast, the two doublemutation patients achieved a partial response to treatment (Table 4).
Table 4.
Clinical information and treatment outcomes of six patients who received EGFR TKIs
| Sex | Age (yr) | Exon 20 type | ECOG | Smoking history | Initial stage | TKI type | EGFR TKI response | PFS |
|---|---|---|---|---|---|---|---|---|
| F | 44 | Insertion | 1 | Never-smoker | IV | Erlotinib | PD | 0.7 |
| M | 65 | Insertion | 2 | Never-smoker | IV | Afatinib | PD | 0.9 |
| M | 60 | Insertion | 1 | Current smoker 30PY | IV | Erlotinib | PD | 2.6 |
| M | 48 | Insertion | 0 | Ex-smoker 20PY | IV | Erlotinib | SD | 11.4 |
| M | 62 | Insertion+L858R | 1 | Ex-smoker 10PY | IIB | Afatinib | PR | 1.9 |
| F | 43 | Insertion+G719S | 1 | Never-smoker | IV | Osimertinib | PR | 2.8 |
EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; ECOG, Eastern Cooperative Oncology Group; PFS, progression-free survival; F, female; M, male; PD, progressive disease; SD, stable disease; PR, partial response.
Discussion
We found that exon 20 insertion mutations represented 1.6% of all EGFR mutations, regardless of the cancer stage. Exon 20 insertions represented 1.8% of the EGFR mutations found in cases of advanced NSCLC, consistent with previous reports [6,11,12,14]. These patients shared similar characteristics with patients harboring other common EGFR mutations in that the majority had adenocarcinoma (96%) and were never-smokers (78%).
Amino acid sequencing data from the exon 20 insertion mutations indicated that the majority (88.2%) showed changes after His773. The most frequent amino acid change was His773_Val774insHis (41.1%), followed by Val774_Cys-775insHisVal (17.6%) and His773_Val774insProHis (11.7%). All amino acid changes occurred between Val769 and Val-775, in a region located just after the C-helix of the EGFR tyrosine kinase domain. These findings are consistent with the results of Yasuda et al. [17]. We observed three novel amino acid sequence variants: Pro772_His773insGlyAsnPro, Val769_Asn771insValGlyVal, and Pro772_His773insThrThr-Pro. This suggests that exon 20 insertion mutations are highly variable and heterogeneous. Although the clinical significance of these novel sequences has not been established, efforts to find new amino acid sequences in exon 20 mutations are warranted.
It has been suggested that exon 20 insertions and other types of EGFR mutations are mutually exclusive [11-14,16]. Among the 56 patients with exon 20 insertions in our study, eight had an additional EGFR mutation to the exon 20 insertion, such as L858R, an exon 19 deletion, S768I, or G719S. Patients with a double mutation comprising an exon 20 insertion and L858R or G719S initially achieved a partial response to EGFR TKIs. However, the PFS was short (1.9-2.9 months), suggesting that exon 20 insertion might be dominant over the other more common mutation. Further validation of these results is needed. As expected, patients with an exon 20 insertion only who received EGFR TKIs had a low response rate and short PFS (~2.6 months; range, 0.7 to 11.4), consistent with previous reports [12,14,15,20].
One patient showed a long duration of stable disease following treatment with an EGFR TKI. Some exon 20 insertions (such as A763_Y764insFQEA) are relatively sensitive to EGFR TKIs, and this patient may have had such a variant [12,21].
In general, exon 20 insertion mutation in NSCLC is associated with lack of sensitivity to first-generation EGFR TKIs, such as erlotinib or gefitinib [12-14,18,19], and so does our study (Table 5). The exact mechanism underlying this lack of sensitivity has not been fully established. It is possible that exon 20 insertions occurring after the C-helix might induce conformational changes that affect the binding affinity to EGFR inhibitors, promoting resistance to EGFR TKIs [17]. Kosaka et al. showed that replacing Asp770 with Gly770 restored sensitivity to EGFR TKIs by allowing Arg776 access. This facilitated a C-helix conformational change and substrate binding in Ba/F3 cells transduced with an EGFR exon 20 insertion mutation [22].
Table 5.
Published studies evaluating clinical response to EGFR TKIs in NSCLC patients with exon 20 insertion
| Study | Type of EGFR Exon 20 mutation | Patients treated with EGFR TKIs | ORR to TKI (%) | PFS to TKI | OS |
|---|---|---|---|---|---|
| Tu et al. [18] | Insertion 20 | 12 | 0 | 3.0 (1.3-4.7) | 12.5 (0-25.5) |
| Lund-Iversen et al. [13] | Insertion 20 | 3 | 0 | - | - |
| Arcila et al. [15] | Insertion 20 | 5 | 40 | 2.5 | > 48 mo |
| Naidoo et al. [12] | Insertion 20 | 11 | 27 | - | - |
| Yasuda et al. [17] | Insertion 20 | 19 | 11 | - | - |
| Kuiper et al. [19] | Insertion 20 | 16 | 0 | 2.9 (2.3-3.6) | 9.7 |
| Current study | Insertion 20 | 4 | 25 | 2.6 (0.7-11.4) | 29.4 (9.3-49.6) |
EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer; ORR, objective response rate; PFS, progression free survival; OS, overall survival.
In one pre-clinical study, patient derived xenografts cells harboring an EGFR exon 20 insertion showed a partial response to second-generation (afatinib) and third-generation (osimertinib and rociletinib) TKIs [23]. Another pre-clinical study showed that a novel, mutant-selective inhibitor of EGFR (nazartinib) showed promising results in vitro in cancer-derived cell lines expressing exon 20 insertions. Nazartinib was effective for the duration of the therapeutic window, at only half the maximal inhibitory concentration (IC50) [24]. But in clinical trials to date, promising results have not been observed with currently available agents designed to target EGFR [25,26]. Therefore, further studies are needed to enhance our understanding of the EGFR structure and to clarify the mechanisms by which exon 20 insertions affect patient responses to EGFR TKIs. As part of these efforts, one phase II study of poziotinib in patients with advanced NSCLC and EGFR exon 20 mutations had been conducted. In this study, seven of 11 patients achieved a partial response to poziotinib, suggesting its promising efficacy [27].
In this study, the advanced NSCLC patients with exon 20 insertion had median OS of 29.4 months (95% CI, 9.3 to 49.6), which is consistent with median OS of advanced NSCLC with common EGFR mutations [8,9]. The ORR and PFS to platinum-based chemotherapy were 50% and 4.2 months, respectively. Considering the poor outcomes with EGFR TKIs, platinum-based systemic chemotherapy is considered standard treatment for advanced NSCLC with an exon 20 insertion mutation [12,20].
The present study has certain limitations. Given the small number of patients and retrospective nature of the analysis, patients analyzed in this cohort might not be representative of all types of advanced NSCLC with EGFR exon 20 insertion mutations. Furthermore, the various techniques used in this study to detect EGFR mutations have inherently different levels of specificity and sensitivity. Nevertheless, we analyzed one of the largest cohorts of its kind, with more than 3,000 patients with activating EGFR mutations.
In conclusion, exon 20 insertion mutations are rare in Korea, and are occasionally accompanied by common EGFR mutations. Although the response to systemic chemotherapy in these patients is comparable to that in patients with more common EGFR mutations, the response rate to first- or second-generation EGFR TKIs is quite low. For this reason, the development of a more efficient chemotherapeutic agent is urgently needed.
Footnotes
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305. doi: 10.4143/crt.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–72.: doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Fukui T, Otani S, Hataishi R, Jiang SX, Nishii Y, Igawa S, et al. Successful rechallenge with erlotinib in a patient with EGFR mutant lung adenocarcinoma who developed gefitinib-related interstitial lung disease. Cancer Chemother Pharmacol. 2010;65:803–6. doi: 10.1007/s00280-009-1212-5. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-smallcell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as firstline treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 9.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–74. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu JY, Wu SG, Yang CH, Gow CH, Chang YL, Yu CJ, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–82. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 12.Naidoo J, Sima CS, Rodriguez K, Busby N, Nafa K, Ladanyi M, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib. Cancer. 2015;121:3212–20. doi: 10.1002/cncr.29493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund-Iversen M, Kleinberg L, Fjellbirkeland L, Helland A, Brustugun OT. Clinicopathological characteristics of 11 NSCLC patients with EGFR-exon 20 mutations. J Thorac Oncol. 2012;7:1471–3. doi: 10.1097/JTO.0b013e3182614a9d. [DOI] [PubMed] [Google Scholar]
- 14.Beau-Faller M, Prim N, Ruppert AM, Nanni-Metellus I, Lacave R, Lacroix L, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25:126–31. doi: 10.1093/annonc/mdt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12:220–9. doi: 10.1158/1535-7163.MCT-12-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Lim SH, Kim M, Kim S, Jung HA, Chang WJ, et al. Is there any predictor for clinical outcome in EGFR mutant NSCLC patients treated with EGFR TKIs? Cancer Chemother Pharmacol. 2014;73:1063–70. doi: 10.1007/s00280-014-2442-8. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177. doi: 10.1126/scitranslmed.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu HY, Ke EE, Yang JJ, Sun YL, Yan HH, Zheng MY, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96–102. doi: 10.1016/j.lungcan.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Kuiper JL, Hashemi SM, Thunnissen E, Snijders PJ, Grunberg K, Bloemena E, et al. Non-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatment. Br J Cancer. 2016;115:1504–12. doi: 10.1038/bjc.2016.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8:179–84. doi: 10.1097/JTO.0b013e3182779d18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin YT, Liu YN, Wu SG, Yang JC, Shih JY. Epidermal growth factor receptor tyrosine kinase inhibitor-sensitive exon 19 insertion and exon 20 insertion in patients with advanced nonsmall-cell lung cancer. Clin Lung Cancer. 2017;18:324–32. doi: 10.1016/j.cllc.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Kosaka T, Tanizaki J, Paranal RM, Endoh H, Lydon C, Capelletti M, et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res. 2017;77:2712–21. doi: 10.1158/0008-5472.CAN-16-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Xu X, Cai J, Ning J, Wery JP, Li QX. NSCLC harboring EGFR exon-20 insertions after the regulatory C-helix of kinase domain responds poorly to known EGFR inhibitors. Int J Cancer. 2016;139:171–6. doi: 10.1002/ijc.30047. [DOI] [PubMed] [Google Scholar]
- 24.Masuzawa K, Yasuda H, Hamamoto J, Nukaga S, Hirano T, Kawada I, et al. Characterization of the efficacies of osimertinib and nazartinib against cells expressing clinically relevant epidermal growth factor receptor mutations. Oncotarget. 2017;8:105479–91. doi: 10.18632/oncotarget.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen YC, Tseng GC, Tu CY, Chen WC, Liao WC, Chen WC, et al. Comparing the effects of afatinib with gefitinib or Erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer. 2017;110:56–62. doi: 10.1016/j.lungcan.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Janne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res. 2011;17:1131–9. doi: 10.1158/1078-0432.CCR-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24:638–46. doi: 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


