Abstract
γ-aminobutyric acid type B (GABAB) receptors, G protein-coupled receptors (GPCRs) for GABA, are obligate heterodimers of two homologous subunits, GB1 and GB2. Typical for family C GPCRs, the N termini of both GB1 and GB2 contain a domain with homology to bacterial periplasmic amino acid-binding proteins (PBPs), but only the GB1 PBP-like domain binds GABA. We found that both GB1 and GB2 extracellular N termini are required for normal coupling of GABAB receptors to their physiological effectors, Gi and G protein-activated K+ channels (GIRKs). Receptors with two GB2 N termini did not respond to GABA, whereas receptors with two GB1 N termini showed increased basal activity and responded to GABA with inhibition, rather than activation, of GIRK channels. This GABA-induced GIRK current inhibition depended on GABA binding to the chimeric GB1/2 subunit (the GB1 N-terminal domain attached to the heptahelical domain of GB2), rather than the wild-type GB1 subunit. Interestingly, receptors with reciprocal exchange of N-terminal domains between the subunits were functionally indistinguishable from wild-type receptors. We also found that peptide linkers between GB1 and GB2 PBP-like domains and respective heptahelical domains could be altered without affecting receptor function. This finding suggests that other contacts between the PBP-like and heptahelical domains underlie ligand-induced signal transduction, a finding likely to be relevant for all family C GPCRs.
Gamma aminobutyric acid (GABA), a major inhibitory neurotransmitter in the central nervous system, activates GABAA and GABAC ligand-gated Cl− channels, as well as G protein-coupled GABAB receptors. Activation of GABAB receptors results in generation of slow inhibitory postsynaptic potentials through the stimulation of G protein-activated K+ channels (Kir3 channels or GIRKs), as well as inhibition of neurotransmitter release through the inhibition of voltage-gated Ca2+ channels. In addition, activation of GABAB receptors results in the inhibition of adenylyl cyclase.
GABAB receptors are heterodimers of two homologous subunits, GB1 and GB2 (1–4). Both GABAB receptor subunits belong to the family C (class III) of G protein-coupled receptors (GPCRs), together with metabotropic glutamate receptors (mGluRs), extracellular Ca2+-sensing receptor (CaR), and some pheromone and taste receptors. Only heterodimeric GABAB receptors are functional (1–3), and the surface expression of assembled complexes is regulated through a dimerization-dependent trafficking checkpoint (5, 6). Interestingly, a similar example of obligate heterodimerization recently has been reported for T1R2 and T1R3; upon coassembly, these two family C GPCRs form a sweet taste receptor (7). In contrast, homodimerization, mediated at least in part by formation of N-terminal disulfide bonds, was reported for mGluR1 (8, 9), mGluR5 (10), and CaR (11).
All family C GPCRs have a large extracellular N terminus that contains a domain with homology to bacterial periplasmic amino acid-binding proteins (PBPs, ref. 12). In most cases, but not in the case of GB2, this PBP-like domain was demonstrated to bind ligands (13–15). It is not known, however, how ligand binding to the PBP-like domain is transmitted to the rest of the receptor. Crystallographic structural analysis of the mGluR1 PBP-like domain revealed a dimer of two clamshell-shaped protomers in either open (resting) or closed (active) conformation; both structures were observed in the absence of ligand (16). The active conformation, with a shorter distance between the C termini of the two protomers, is stabilized by glutamate binding. The authors of that study thus suggested that ligand binding to the PBP-like domains draws the heptahelical domains of the two subunits closer, thereby triggering G protein signaling (Fig. 1A). Because this model predicts that receptor signaling depends on movements of the peptide linker between the PBP-like and heptahelical domains, we will refer to it as the peptide-linker model of receptor activation. (In mGluRs and CaR, this peptide linker includes a cysteine-rich domain, which is not present in GB1 and GB2.) Recently, it was suggested that the peptide-linker model of receptor activation might apply to GABAB receptors as well, as illustrated in Fig. 1Ba (17). However, there is an alternative scenario consistent with the currently available data: PBP-like domains might form direct contacts with extracellular loops and/or transmembrane helices of the two heptahelical domains, and the nature of these contacts might change upon ligand binding. This model, referred to as the direct contact model of receptor activation, is illustrated in Fig. 1Bb.
Figure 1.
Models of family C receptor signaling. (A) Model of mGluR1 activation based on the crystal structure of the mGluR1 PBP-like domain and illustrated here as in ref. 16. Glutamate binding stabilizes the closed conformation of the two PBP-like protomers, which results in shortening of the distance between the protomer C termini. It was hypothesized that as a result the two heptahelical domains are drawn closer, thus triggering G protein signaling (16); we refer to this scenario as the peptide-linker model of receptor activation because it relies on the movements of the peptide linker between the PBP-like and heptahelical domains. In mGluRs and CaR, but not GABAB receptors, this linker contains a cysteine-rich (CR) domain. The receptor segments shown in white [the peptide linker, transmembrane segments (TM), and the intracellular C terminus (IC)] are represented schematically. The white spheres represent glutamate. (B) Two models of GABAB receptor activation. The peptide-linker model of receptor activation (a) is based on the mGluR1 model. Note the presence of the C-terminal coiled–coil interaction in assembled GABAB receptors. The direct contact model of receptor activation is shown in b. In this scenario, the ligand-binding associated conformational change of the PBP-like domain is transmitted to the rest of the receptor through direct contacts of the PBP-like and heptahelical domains. The GB1 subunit is shown in black and the GB2 subunit in gray; the light gray spheres represent GABA.
To identify the roles of GB1 and GB2 PBP-like domains in the GABAB receptor heterodimer and gain further insight into the mechanism of activation of family C receptors, we constructed a number of GB1 and GB2 mutant proteins and tested their ability to activate GIRK channels upon coexpression in Xenopus oocytes. We found that heterodimerization is a prerequisite for the normal function of the N-terminal ligand-binding domain of GABAB receptors. In addition, we show that alterations of the peptide linker between the PBP-like domains and heptahelical domains of GB1 and GB2 did not affect receptor function, contrary to the predictions of the peptide-linker model of receptor activation. Thus, it is likely that other contacts between the PBP-like and heptahelical domains underlie receptor signaling — a scenario likely to be conserved among family C GPCRs.
Materials and Methods
Molecular Biology.
Standard molecular biology protocols were adopted from ref. 18. For oocyte expression, constructs were made either in pGemHE (19) or pGemHEm (pGemHE vector with modifications in the linearization linker). GIRK1, GIRK2, wild-type (wt) GB1, and wt GB2 were used in the form described in ref. 5. Because they are functionally identical, we used hemagglutinin-tagged and nontagged constructs interchangeably throughout the study. All mutant receptors were constructed by sequential overlap extension PCR. The breakpoint protein sequence for the GB1/2 chimera reads RFLS-LPLY; for the GB2/1 chimera, it reads RKIS-QKLF. All PCR-amplified stretches of DNA were verified by sequencing. The expression of all nonfunctional chimeric proteins was confirmed by Western blotting of oocyte homogenates (not shown).
Electrophysiology.
Stage V–VI Xenopus oocytes were prepared and maintained as described in ref. 20. cRNAs were prepared by using AmpliScribe T7 kits (Epicentre Technologies, Madison, WI), and oocytes were injected with ≈1–2 ng of GIRK1/GIRK2 cRNAs and ≈5–10 ng of each receptor subunit cRNA, 24–48 h before recording. Recordings were done in modified ND96 solution (100 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/6 mM Hepes, pH 7.4) or in 40K solution (where 40 mM NaCl was replaced with 40 mM KCl). GABA (Research Biochemicals, Natick, MA) or SCH 50911 (Tocris Cookson, Ellisville, MO) were dissolved in 40K solution and applied by bath superfusion. Currents were measured by using standard two-electrode voltage clamp recording (GeneClamp 500B amplifier, pclamp software, Axon Instruments, Foster City, CA). Current-voltage relationships were assayed by square voltage steps from −130 to +30 mV, in 10-mV increments, from the holding potential of −30 mV. To correct for leak and endogenous oocyte currents, traces recorded in modified ND96 solution in the beginning or end of each recording were subtracted off-line from traces recorded in 40K solution. For plots and statistical comparisons, currents recorded between 100 and 116.6 ms after the start of the voltage pulse were averaged to reduce the random and 60-Hz noise. For summary plots and statistical comparisons, only currents recorded at −120 mV were analyzed. The relative activation of GIRK current by GABA depended on the size of the basal current (the larger the basal current, the smaller the relative activation); this was true for all active receptors tested and is a general feature of the oocyte expression system (N. Dascal, personal communication). We tried to minimize this effect by, in each batch, analyzing only the oocytes that showed basal currents comparable to basal currents observed in oocytes injected with wt receptor constructs. Nonetheless, the data points collected were not normally distributed and, where appropriate, were statistically analyzed using Kruskal–Wallis one-way ANOVA on ranks.
Results
GB1 and GB2 N-Terminal Domains.
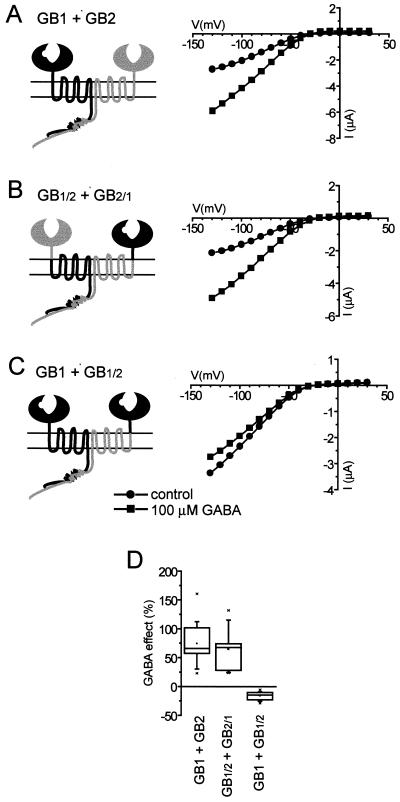
To investigate the roles of the GB1 and GB2 N-terminal extracellular domains in the GABAB heterodimeric complex, we first constructed two chimeric receptor subunits, GB1/2 and GB2/1, with N-terminal domains exchanged; in each case, the chimeric boundary was set two residues before the first transmembrane segment. We then tested the ability of these chimeric receptor subunits to couple to GIRK1/GIRK2 K+ channels in Xenopus oocytes when coexpressed with wt GB1, wt GB2, or each other; results of these experiments are summarized in Fig. 2. Coexpression of GB1/2 with GB2/1, which preserves the normal heterodimeric composition of the GABAB receptor N-terminal domain, resulted in receptors functionally indistinguishable from wt GB1/GB2 heterodimers (Fig. 2 B and D). In contrast, combination of GB2/1 and GB2, which produces receptors with two GB2 N-terminal domains, was not able to activate GIRK channels in response to GABA (not shown). Interestingly, coexpression of GB1 with GB1/2, which gives rise to GABAB receptors with two N-terminal domains of GB1, resulted in the inhibition of GIRK current upon GABA application (Fig. 2 C and D). Although this inhibition was smaller than the activation of GIRK currents induced by wt GABAB receptors, the time courses of the two responses were very similar (not shown). Because of the small magnitude of GB1/GB1/2-mediated responses, we did not attempt to determine the whole dose–response dependence; however, just as in the case of wt GABAB receptors, 50 μM, 100 μM, and 1 mM doses of GABA were equally effective (not shown).
Figure 2.
Chimeras GB1/2 and GB2/1 were functionally assayed by coexpression with GIRK1/GIRK2 channels in Xenopus oocytes. In this assay, binding of GABA to functional GABAB receptors results in activation of inwardly rectifying K+ current; see Materials and Methods for experimental details. The I–V plots from representative cells (A–C) and summary plot for all of the cells tested (D) are shown. In this and subsequent figures, GB1 subunit is schematically represented in black and GB2 subunit in gray; the notch in GB1 subunit represents the GABA-binding site. In all figures, summarized data (which were not normally distributed) are represented as statistical box charts (the horizontal lines in the box denote 25th, 50th, and 75th percentile values, the error bars denote 5th and 95th percentile values, the asterisks denote 1st and 99th percentile values, and the square symbol in the box denotes the mean). For summary plot and statistical analysis, currents were measured at −120 mV; GABA responses are expressed as a percentage of basal GIRK currents recorded in 40K solution just before GABA application. (A) wt GABAB receptors, composed of GB1 and GB2, respond to GABA application by GIRK current activation. (B) In cells coexpressing chimeras GB1/2 and GB2/1, GABA application results in GIRK activation that is no different from the activation mediated by wt receptors. (C) In contrast, chimera GB1/2 coexpressed with GB1 inhibits GIRK current upon GABA application.
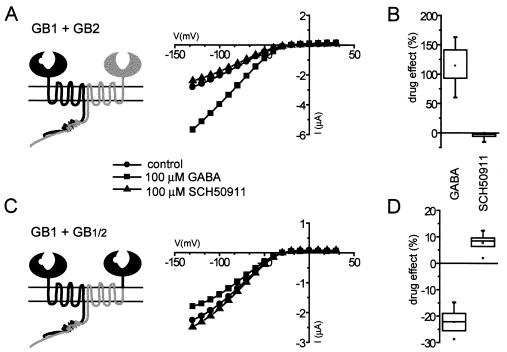
The inhibition of GIRK current upon activation of the GB1/GB1/2 chimera is consistent with a receptor exhibiting elevated basal activity. Because GIRK channels expressed in Xenopus oocytes have high basal activity independent of receptor expression, we were not able to address this possibility directly. To investigate it indirectly, we measured the effect of GABAB receptor competitive antagonist SCH 50911 (21) on either GB1/GB2- or GB1/GB1/2-expressing oocytes (Fig. 3). When oocytes expressing wt GB1/GB2 receptors were superfused with 100 μM SCH 50911, we observed either no effect or a slight inhibition of GIRK current; the largest inhibition (−15.4%) was observed in the cell shown in Fig. 3A. Median SCH 50911 response (−4.0%) was only 3.4% of median GABA response (117.2%) recorded in the same oocytes (Fig. 3B). In contrast, application of SCH 50911 to GB1/GB1/2-expressing oocytes resulted in consistent activation of GIRK current, which, on average, amounted to 33.3% of the GABA-induced inhibition recorded in the same cells (median SCH 50911 response in these oocytes was 7.7%, whereas median GABA response was −23.1%; Fig. 3D). These results suggest that GB1/GB1/2 chimeric receptors possess high basal activity, and that GABA acts as an inverse agonist, stabilizing the inactive receptor conformation. In contrast, wt GB1/GB2 receptors exhibit low basal activity, and GABA behaves as an agonist, stabilizing the active receptor conformation.
Figure 3.
(A and B) Application of the competitive GABAB receptor antagonist SCH 50911 to cells expressing wt GB1/GB2 receptors results in a slight inhibition of basal GIRK current, indicating that wt GABAB receptors have low basal activity. (C and D) In contrast, in oocytes expressing GB1/GB1/2 receptors, the magnitude of the SCH 50911-induced GIRK activation was 33.3% of the magnitude of the GABA-induced inhibition recorded in the same cells. Thus, GB1/GB1/2 receptors have high basal activity, and GABA-mediated inhibition of GIRK current reflects the stabilization of the inactive receptor conformation. The I–V plots from representative cells are shown (A and C); data from cells tested with both drugs are summarized (B and D).
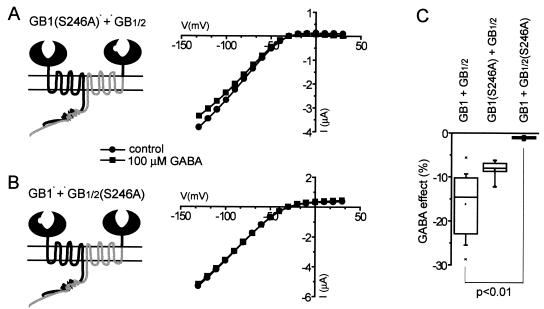
The GB1/GB1/2 chimeric receptor has two GABA-binding sites, in contrast to the wt GB1/GB2 receptor. To determine which of the binding sites is responsible for GABA-induced inhibition of GIRK currents, we mutated serine-246 to alanine in either GB1 or GB1/2 (in GB2, the equivalent residue is proline). GB1(S246A) does not bind GABA (22); not surprisingly, it did not mediate GABA-induced activation of GIRK current when coexpressed with GB2 in oocytes (not shown). Coexpression of GB1(S246A) with GB1/2, however, resulted in GABA-induced GIRK current inhibition similar to that mediated by GB1/GB1/2 (Fig. 4 A and C). In contrast, the same S246A mutation in GB1/2 completely abolished GABA-mediated inhibition of GIRK currents in oocytes expressing GB1 and GB1/2(S246A) (Fig. 4 B and C). These results indicate that GABA binding to the chimeric GB1/2 receptor subunit, but not to wt GB1 subunit, underlies the aberrant GABA-induced activity of GB1/GB1/2 receptor complex.
Figure 4.
Mutating serine-246 to alanine disrupts the GABA-binding site in GB1, illustrated here by removal of the notch representing GABA-binding site. (A) The function of GB1/GB1/2 receptors was not affected by the S246A mutation in the wt GB1 subunit. (B) In contrast, the analogous mutation in the GB1/2 subunit completely abolished the inhibition of GIRK current mediated by GB1/GB1/2 receptor complex. The I–V plots from representative cells (A and B) and summary plot (C) are shown. Data were statistically analyzed by Kruskal–Wallis one-way ANOVA on ranks, followed by the Dunn's posttest.
GB1 and GB2 Peptide Linkers.
The equal effectiveness of GB1/2/GB2/1 and GB1/GB2 receptors in mediating GABA-induced GIRK current activation suggests that the nature of the connection between the PBP-like and heptahelical domains is not critical. To further explore this issue, we modified the linkers that connect the PBP-like domains of GB1 or GB2 with the respective first transmembrane segments. Two types of modifications were introduced (Fig. 5A). In one set of mutants (GB1ad11 and GB2ad11), a glycine-rich peptide GGGASSASGGG, predicted to form a random coil structure, was introduced in the middle of each linker, extending it by 11 aa. In another set of mutants (GB1xg11 and GB2xg11), this peptide was introduced at the same position as in GB1ad11 and GB2ad11, but the preceding 11 aa of the linker were deleted; in effect, 11 aa of each linker were replaced with the GGGASSASGGG peptide, whereas the length of each linker did not change. The ability of these mutant receptor subunits to activate GIRK current in Xenopus oocytes was then examined; these data are summarized in Fig. 5 B and C.
Figure 5.
Amino acid sequences of ≈40-aa-long peptide linkers that connect the PBP-like domains with the transmembrane segments of GB1 and GB2 (A), the I–V plots from representative cells (B), and the summary plot (C) are shown. In the GB1ad11 and GB2ad11 mutant proteins, the 11-aa-long random coil peptide GGGASSASGG (boxed in gray) was inserted into GB1 and GB2 linkers at the positions shown, which resulted in linker extension. In the GB1xg11 and GB2xg11 mutant proteins, the GGGASSASGG peptide was inserted at the same position, but the preceding 11 residues of either GB1 or GB2 (underlined) were simultaneously deleted; the length of linkers was thus kept constant. Extension of GB1 and GB2 linkers had no effect on the GABA-mediated GIRK current activation, and 11 residues in the GB1 linker may be replaced with the random-coil peptide without functional consequences. Replacement of 11 residues in the GB2 linker prevented receptor cell surface expression (not shown).
Coexpression of GB1ad11 with GB2ad11 resulted in receptors functionally indistinguishable from wt receptors. In contrast, the GB1xg11/GB2xg11 receptor complex was not able to activate GIRK currents in Xenopus oocytes, either because receptors were absent from the cell surface or because cell surface-expressed receptors were not functional. Because the overall linker length in GB1xg11 and GB2xg11 mutant receptor subunits was not modified, we also tested each of them separately, coexpressed with appropriate wt subunits. The GB1xg11/GB2 mutant receptor was able to mediate GABA-induced GIRK current activation as effectively as the wt GB1/GB2 receptor, whereas GB1/GB2xg11 receptor combination did not result in GIRK current activation upon application of GABA. Chemiluminescence measurements revealed that the latter receptor complex was not expressed on the cell surface (not shown), indicating that exchanging 11 linker residues of GB2 with a glycine-rich peptide affected receptor folding and/or assembly. Thus, all linker mutant receptors that were appropriately assembled and expressed on the cell surface were able to activate GIRK channels as well as wt receptors. These results are not compatible with the peptide-linker model of receptor activation (Fig. 1Ba), suggesting that direct contacts of PBP-like and heptahelical domains underlie receptor signaling (Fig. 1Bb).
Discussion
Family C GPCRs are constitutive dimers, and this dimerization is a prerequisite for functional activity. However, it is not known whether dimerization is important for ligand binding and associated conformational changes, G protein coupling, or both. In addition, intramolecular signal transduction of family C GPCRs is not well understood. Heterodimerization is a prerequisite for the plasma membrane expression and function of GABAB receptors, and individual GB1 and GB2 subunits are not functional even if expressed on the cell surface. This allowed us to examine the functional role of each subunit in the GABAB receptor complex and to address the functional significance of dimerization in GABAB receptor signaling (23). The function of different mutant GB1 and GB2 proteins was assessed by coexpressing them in Xenopus oocytes with their physiological effectors, GIRK1/GIRK2 channels (24). Here, we report that (i) both GB1 and GB2 N-terminal domains are necessary for appropriate GABAB receptor function, and (ii) the ligand-induced conformational changes of the GB1/GB2 ligand-binding domain are conveyed to the remainder of the receptor through contacts other than the peptide linker between the PBP-like domains and respective heptahelical domains.
A large N terminus that contains a ligand-binding PBP-like domain is characteristic of all family C GPCRs (12–15). The recent structural analysis of the mGluR1 PBP-like domain revealed that, as prokaryotic PBPs, it undergoes large conformational changes akin to the opening and closing of clamshells (16). The structure also revealed that mGluR1 PBP-like domains exist as a dimer in both the ligand-free and the ligand-bound state, further strengthening the observation that dimerization is functionally essential for family C GPCRs. However, although many other family C receptors function as homodimers, GABAB receptors are heterodimers. What are the roles of two homologous, but nonidentical N-terminal domains in the GABAB receptor heterodimer, and what would happen if we constructed GABAB receptors that were homomers of either GB1 or GB2 in the N-terminal region? These questions are particularly intriguing because the N-terminal domain of GB2 was previously shown not to bind GABA or other GABAB receptor ligands, although it does increase the agonist-binding affinity of GB1 (1, 3). In agreement with the recent report by Galvez et al. (17), our studies show that both the GB1 and GB2 N-terminal domains are necessary for the appropriate function of GABAB receptors (Fig. 2). Chimeric receptors containing two GB2 N-terminal domains (GB2/1/GB2) did not respond to GABA application — not a surprising finding, considering the evidence that the GB2 PBP-like domain does not bind GABA. Remarkably, receptors containing two GB1 N-terminal domains (GB1/GB1/2) responded to GABAB receptor ligands in an aberrant way: application of GABA resulted in the inhibition, rather than activation, of GIRK currents, whereas competitive antagonist SCH 50911 had the opposite effect (Fig. 3). The relative magnitude of SCH 50911 effects, compared to GABA effects, was larger for GB1/GB1/2 than for wt GB1/GB2 receptors, suggesting that GB1/GB1/2 chimeric receptors exhibit increased constitutive activity. One possible interpretation of these results is that the GB2 PBP-like domain normally inhibits GABAB receptor signaling, and that GABA binding to the GB1 PBP-like domain results in the relief of this inhibition. In this scenario, GABA-induced inhibition of GIRK currents by GB1/GB1/2 receptors reflects the fact that the PBP-like domain of GB1 can provide this inhibitory effect only in the GABA-bound state, albeit inefficiently. Similarly, the finding that GB2/1/GB2 receptors, which contain two GB2 PBP-like domains, also exhibit high basal activity (17) would imply that the GB2 PBP-like domain to some extent resembles the GABA-bound GB1 PBP-like domain. In an alternative scenario, the high basal activity of GB1/GB1/2 receptors reflects the shift in the normal equilibrium between active and inactive receptor conformations, such that the active conformation is now stable in the ligand-free state and further stabilized by antagonist SCH 50911, whereas the inactive conformation is stabilized by GABA binding. With either scenario, it is clear that the conformational state of heterodimeric ligand-binding domain affects the constitutive activity of GABAB receptors.
The aberrant effect of GABA on GB1/GB1/2 receptors depended on its binding to the GB1/2 subunit. Importantly, GB1/GB1/2(S246A) receptors were devoid of any GABA-dependent activity; whereas S246A mutation in GB1/2 was sufficient to abolish the GABA-induced inhibition of GIRK current, it did not restore the normal receptor function (Fig. 4). Taken together, our findings indicate that the GB2 N-terminal domain plays an important role in GABAB receptor function that cannot be substituted for by the N-terminal domain of GB1; this finding is in agreement with the recent report that a large internal deletion in the GB2 N terminus results in the complete loss of GABAB receptor activity (25).
Next, we wanted to address how the ligand-induced conformational changes of the PBP-like ligand-binding domains might be transmitted to the rest of the receptor, resulting in G protein activation. A priori, there are three possibilities for the transmission of this signal: (i) through the peptide linker between the PBP-like domains and heptahelical domains (the peptide-linker model of receptor activation; Fig. 1Ba); (ii) through direct contacts of the PBP-like domains with extracellular loops and/or transmembrane segments (the direct contact model of receptor activation; Fig. 1Bb), and (iii) a combination of both mechanisms. Our finding that GABAB receptor function was not affected by exchange of the N-terminal domains between GB1 and GB2 (Fig. 2) can be easily reconciled with the direct contact model of receptor activation. On the other hand, because the recent structural analysis of mGluR1 PBP-like domains revealed that a distance between the C termini of two protomers was shorter in the ligand-stabilized closed conformation (16), the authors of that study as well as Galvez et al. (17) favored the peptide-linker model of receptor activation. To test the different models experimentally, we modified the linkers between the PBP-like domains and the first transmembrane segments of GB1 and GB2. The peptide-linker model of receptor activation predicts the structure of these peptide linkers to be important for receptor conformational changes, hence not likely to tolerate amino acid substitutions or insertion of glycine-rich peptides. In contrast, in the direct contact model of receptor activation, these linker regions are predicted to act only as tethers for the PBP-like domains, and changes in structure and/or length of tethers are likely to be tolerated. We found that the insertion of an 11-aa-long random coil peptide in the linker region of both GB1 and GB2 did not affect the receptor function (Fig. 5), strongly supporting the direct contact model of GABAB receptor activation. The GB1 linker also tolerated the substitution of 11 aa by the same random-coil peptide (Fig. 5). This was not true for the GB2 linker; however, GB1/GB2xg11 heterodimeric receptors were not expressed on the cell surface, suggesting that partial replacement of GB2 with a glycine-rich peptide resulted in a folding and/or assembly defect. Taken together, these results strongly support the direct contact model of receptor activation (Fig. 1Bb).
In summary, the N termini of both GB1 and GB2 play important roles in ligand-mediated receptor activation, underscoring the importance of dimerization for the function of PBP-like ligand-binding domains in family C GPCRs. In addition, GABAB receptor signaling appears to be mediated by direct contacts between the PBP-like domains and the heptahelical receptor core. The overall conservation of family C receptor structure suggests that this direct contact model of receptor activation may apply to other members of this receptor family, but additional work will be needed to test this hypothesis.
Acknowledgments
We thank M. Lazdunski for GIRK2 cDNA, the Howard Hughes Medical Institute sequencing facility for their support, S. Yurkovskaya for invaluable technical assistance, and S. Fried for editorial help. We are also grateful to H. R. Bourne and D. L. Minor for helpful advice on experiments and comments on the manuscript. A National Institute of Mental Health grant to Silvio Conte Center of Neuroscience at University of California, San Francisco, supported this work. M.M.-M. was supported in part by a National Institutes of Health Institutional Research Service Award in Molecular and Cellular Basis of Cardiovascular Diseases. L.Y.J. and Y.N.J. are Howard Hughes Investigators.
Abbreviations
- GPCR
G protein-coupled receptor
- GABA
γ-aminobutyric acid
- PBP
periplasmic amino acid-binding protein
- GIRK
G protein-activated K+ channel
- mGluR
metabotropic glutamate receptor
- CaR
extracellular Ca2+-sensing receptor
- wt
wild-type
References
- 1.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. Nature (London) 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 2.Jones K A, Borowsky B, Tamm J A, Craig D A, Durkin M M, Dai M, Yao W J, Johnson M, Gunwaldsen C, Huang L Y, et al. Nature (London) 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 3.White J H, Wise A, Main M J, Green A, Fraser N J, Disney G H, Barnes A A, Emson P, Foord S M, Marshall F H. Nature (London) 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 4.Kuner R, Köhr G, Grünewald S, Eisenhardt G, Bach A, Kornau H C. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 5.Margeta-Mitrovic M, Jan Y N, Jan L Y. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 6.Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, et al. J Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson G, Hoon M A, Chandrashekar J, Zhang Y, Ryba N J, Zuker C S. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 8.Ray K, Hauschild B C. J Biol Chem. 2000;275:34245–34251. doi: 10.1074/jbc.M005581200. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji Y, Shimada Y, Takeshita T, Kajimura N, Nomura S, Sekiyama N, Otomo J, Usukura J, Nakanishi S, Jingami H. J Biol Chem. 2000;275:28144–28151. doi: 10.1074/jbc.M003226200. [DOI] [PubMed] [Google Scholar]
- 10.Romano C, Miller J K, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y, Goldberg M P, O'Malley K L. Mol Pharmacol. 2001;59:46–53. [PubMed] [Google Scholar]
- 11.Zhang Z X, Sun S, Quinn S J, Brown E M, Bai M. J Biol Chem. 2001;276:5316–5322. doi: 10.1074/jbc.M005958200. [DOI] [PubMed] [Google Scholar]
- 12.O'Hara P J, Sheppard P O, Thøgersen H, Venezia D, Haldeman B A, McGrane V, Houamed K M, Thomsen C, Gilbert T L, Mulvihill E R. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- 13.Malitschek B, Schweizer C, Keir M, Heid J, Froestl W, Mosbacher J, Kuhn R, Henley J, Joly C, Pin J P, et al. Mol Pharmacol. 1999;56:448–454. doi: 10.1124/mol.56.2.448. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto T, Sekiyama N, Otsu M, Shimada Y, Sato A, Nakanishi S, Jingami H. J Biol Chem. 1998;273:13089–13096. doi: 10.1074/jbc.273.21.13089. [DOI] [PubMed] [Google Scholar]
- 15.Bräuner-Osborne H, Jensen A A, Sheppard P O, O'Hara P, Krogsgaard-Larsen P. J Biol Chem. 1999;274:18382–18386. doi: 10.1074/jbc.274.26.18382. [DOI] [PubMed] [Google Scholar]
- 16.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Nature (London) 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 17.Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prézeau L, Pin J P. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1997. [Google Scholar]
- 19.Liman E R, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 20.Collins A, Chuang H, Jan Y N, Jan L Y. Proc Natl Acad Sci USA. 1997;94:5456–5460. doi: 10.1073/pnas.94.10.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong J, Marino V, Parker D A, Kerr D I, Blythin D J. Eur J Pharmacol. 1998;362:35–41. doi: 10.1016/s0014-2999(98)00723-7. [DOI] [PubMed] [Google Scholar]
- 22.Galvez T, Prezeau L, Milioti G, Franek M, Joly C, Froestl W, Bettler B, Bertrand H O, Blahos J, Pin J P. J Biol Chem. 2000;275:41166–41174. doi: 10.1074/jbc.M007848200. [DOI] [PubMed] [Google Scholar]
- 23.Margeta-Mitrovic M, Jan Y N, Jan L Y. Proc Natl Acad Sci USA. 2001;98:14649–14654. doi: 10.1073/pnas.251554498. . (First Published November 27, 2001; 10.1073/pnas.251554498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lüscher C, Jan L Y, Stoffel M, Malenka R C, Nicoll R A. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 25.Jones K A, Tamm J A, Craig D A, Yao W, Panico R. Neuropsychopharmacology. 2000;23:S41–S49. doi: 10.1016/S0893-133X(00)00145-7. [DOI] [PubMed] [Google Scholar]