Abstract
The ultimate goal of any scientific development is to increase well-being and human health. Novel strategies are required for the achievement of safe and effective therapeutic treatments beyond the conventional ones, and society needs new requirements for new technologies, moving towards clean and green technology development. Green nanotechnology is a branch of green technology that utilizes the concepts of green chemistry and green engineering. It reduces the use of energy and fuel by using less material and renewable inputs wherever possible. Green nanotechnology, in phytoformulations, significantly contributes to environmental sustainability through the production of nanomaterials and nanoproducts, without causing harm to human health or the environment. The rationale behind the utilization of plants in nanoparticle formulations is that they are easily available and possess a broad variability of metabolites, such as vitamins, antioxidants, and nucleotides. For instance, gold (Au) nanoparticles have attracted substantial attention for their controllable size, shape, and surface properties. A variety of copper (Cu) and copper oxide (CuO) nanoparticles have also been synthesized from plant extracts. Titanium dioxide and zinc oxide nanoparticles are also important metal oxide nanomaterials that have been synthesized from a number of plant extracts. International and domestic laws, government and private-party programs, regulations and policies are being carefully reviewed and revised to increase their utility and nurture these nanoscale materials for commercialization. Inspiring debates and government initiatives are required to promote the sustainable use of nanoscale products. In this review, we will discuss the potential of the utilization of plant extracts in the advancement of nanotechnology.
Keywords: green nanotechnology, phytoformulations, green engineering, gold (Au) nanoparticles, nanoproducts
1. Introduction
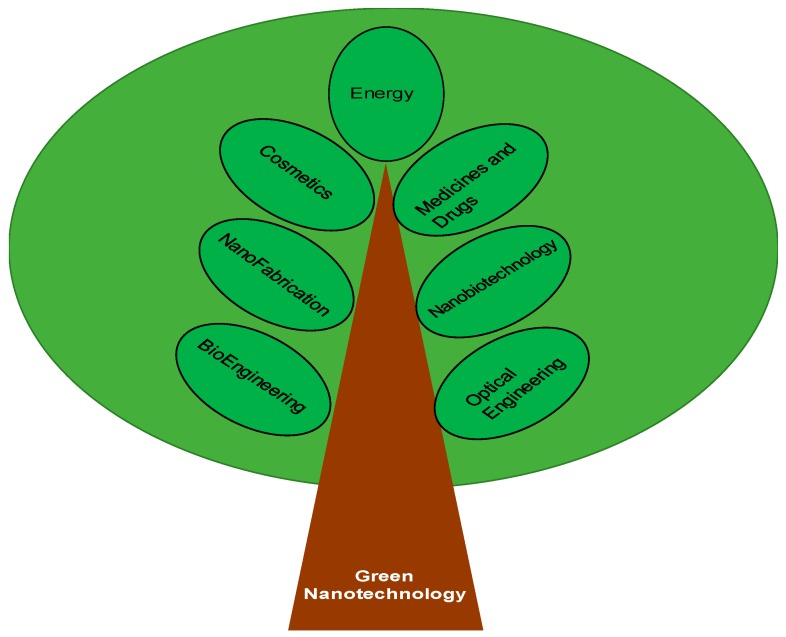
Nanotechnology is cited as a key technology of the 21st century and has generated a great deal of excitement world-wide, but it has been slowed down because of the poor understanding of hazards associated with nanotechnology and fewer policies to manage new risks. Researchers, however, continue to move ahead, engaging themselves to conquer the challenges ranging from managing, producing, funding, regulatory, and technical aspects. Green nanotechnology is a branch of green technology that utilizes the concepts of green chemistry and green engineering, where the word “green” refers to the use of plant products (Figure 1). It reduces the use of energy and fuel by using less material and renewable inputs wherever possible. Furthermore, nanotechnological products, processes, and applications are expected to contribute significantly to environmental and climate protection by saving raw materials, energy, and water, as well as by reducing greenhouse gases and hazardous waste. Increased energy efficiency, reduced waste and greenhouse gas emission, and decreased consumption of non-renewable raw materials are the main advantages of green nanotechnology. Green nanotechnology offers a great opportunity to stop the adverse effects before they occur [1,2].
Figure 1.
Branches of green nanotechnology.
Green nanotechnology does not ascend de novo; rather, it forms on the principles of green chemistry and engineering. Apart from such obvious areas as the development of solar cells, biofuels, and fuel cells, green nanotechnology applications might involve the use of nanomaterials in clean production processes that synthesize nanoparticles, using sunlight or by recycling industrial waste products into nanomaterials. There is some “truly” green nanotechnology, i.e., fully growing nanomaterials in plants—however, they will never reach the scale required for the industrial production of nanomaterials. In order to make a conclusive observation, green nanotechnology needs a full process assessment like other industrially manufactured products [3,4].
2. Herbal Approach for Developing Nanoparticles
The activity of herbal medicines depends on the overall function of active components, as all the constituents provide synergistic action and, thus, improve the therapeutic value. Each active constituent is related to each other and they all play significant roles (Table 1). On the other hand, the insoluble character of most of the drugs of herbal origin leads to lower bioavailability and, because of this, systemic clearance is increased and frequent administration or a higher dose is required—all of which renders the drug a low-class drug for therapeutic use.
Table 1.
Herbal drug-loaded nanoparticles.
| Formulation | Active Ingredients | Biological Activity | Method of Preparation | References |
|---|---|---|---|---|
| Curcuminoids solid lipid nanoparticles | Curcuminoids | Anticancer and antioxidant | Micro-emulsion technique | [8] |
| Glycyrrhizic acid loaded nanoparticles | Glycyrrhizin acid | Antihypertensive and anti-inflammatory | Rotary-evaporated film ultrasonication method | [9] |
| Nanoparticles of cuscuta chinensis | Flavonoids and lignans | Hepatoprotective and antioxidant effects | Nanosuspension method | [10] |
| Artemisinin nanocapsules | Artemisinin | Anticancer | Self-assembly procedure | [11] |
| Berberine-loaded nanoparticles | Berberine | Anticancer | Ionic gelation method | [12] |
| CPTencapsulated nanoparticles | Camptothecin | Anticancer | Dialysis method | [13] |
| Taxel-loaded nanoparticles | Taxel | Anticancer | Emulsion solvent evaporation | [14] |
In phytoformulation research, developing nanotechnology-based dosage forms, e.g., solid lipid nanoparticles (SLNs), polymeric nanoparticles (nanospheres and nanocapsules), proliposomes, liposomes, nanoemulsions, etc., has a great number of advantages for herbal drugs. These include enhancement of solubility and bioavailability, improvement of stability, suppression of toxicity, improvement of pharmacological activity, sustained delivery, improving tissue macrophage circulation, and defense against physical and chemical degradation. Therefore, problems associated with plant medicines can be overcome with nano-sized drug delivery systems (NDDS) of herbal drugs, having a potential future for enhancing their activity. Hence, including nanocarriers as an NDDS in conventional medicine systems would be necessary to combat more chronic diseases like diabetes, cancer, asthma, and others, with the aid of herbal drugs [5,6,7].
3. Nanoparticles Synthesized from Plant Extracts
3.1. Gold and Silver Nanoparticles
Au nanoparticles have gained substantial attention due to their controllable size, shape, and surface properties [15]. Because of these unique properties, gold nanoparticles have been studied for potential applications in areas such as biosensors, hyperthermia therapy [16], antibacterial drugs, genetic engineering, and delivery platforms for therapeutics. Environmentally friendly sources of Au nanoparticles are achieved by employing plants, as they are biological factories via green chemistry-based techniques. The study of nanoparticle syntheses also discovered that a variety of shapes, including rod-shaped, irregular, decahedral, icosahedral, and hexagonal, could be produced, depending on the pH of the reaction medium. Furthermore, a leaf extract of eucalyptus macrocarpa could be used to synthesize gold nanoparticles (Table 2). The results from this study show that spherical particles with a size ranging from 20 to 80 nm were obtained as the main product [17].
Table 2.
A selection of nanoparticles synthesized by various plants.
| Plant | Nanoparticle | Size (nm) | Shape | Reference |
|---|---|---|---|---|
| Aloe vera | Au & Ag | 50 to 350 | Spherical, triangular | [18] |
| Aloe vera | In2O3 | 5 to 50 | Spherical | [19] |
| Citrullus colocynthis | Ag | 31 | Spherical | [20] |
| Curcuma longa | Pd | 10 to 15 | Spherical | [21] |
| Diopyros kaki | Pt | 15 to 19 | Crystalline | [22] |
| Eucalyptus macrocarpa | Au | 20 to 100 | Spherical, triangular, hexagonal | [23] |
| Mangifera indica | Ag | 20 | Spherical, triangular, hexagonal | [24] |
| Rhododendron dauricum | Ag | 25 to 40 | Spherical | [25] |
| Psidium guajava | Au | 25 to 30 | Spherical | [26] |
| Pyrus sp. (Pear fruit extract) | Au | 200 to 500 | Triangular, hexagonal | [27] |
| Terminalia catappa | Au | 10 to 35 | Spherical | [28] |
In addition to synthesizing pure metal nanoparticles by plants in this way, several authors have also reported alloying Au and Ag to investigate the properties of the resulting bimetallic nanoparticles. This bimetallic nanoparticle synthesis comprises a competitive reduction process between two aqueous solutions, each of which contain a different metallic ion precursor that is reacted with a plant extract. In the case of bimetallic nanoparticles, gold has a larger reduction potential than silver, so gold will form first and create the core of the resulting core–shell structure. Subsequently, the reduction of Ag ions, in the same way, results in Ag coalescing on the core to form the shell. There are some plants that have been effectively used to synthesize bimetallic (Au-Ag) nanoparticles, including cashew nut, neem, and West Indies mahogany [29,30,31].
3.2. Copper and Copper Oxide Nanoparticles
A variety of copper (Cu) and copper oxide (CuO) nanoparticles have been synthesized from plant extracts. Cu nanoparticles of magnolia leaf extract have been biologically synthesized to develop stable nanoparticles sized 40 to 100 nm. Furthermore, the activity of Cu nanoparticles has shown potential antibacterial activity against cells of Escherichia coli [32]. Syzygium aromaticum (clove) extracts have been used in the synthesis of Cu nanoparticles with a spherical to granular morphology and a mean particle size of 40 nm [33]. Cu nanoparticles have been synthesized by using the stem latex of Euphorbia nivulia, that is, common milk hedge. These nanoparticles are stabilized by peptides and terpenoids that are present in latex. Furthermore, these nanoparticles are reported to be toxic to human adenocarcinomic alveolar basal epithelial cells [34].
3.3. Palladium and Platinium Nanoparticles
Satishkumar et al. [35] developed palladium nanoparticles from C. zeylanicum (cinnamon) bark extract. During synthesis, the temperature, concentration, and reaction pH of the bark extract changed, but morphology and particle size (15 to 20 nm) were not influenced. Using Annona squamosa (custard apple) peel extract, palladium nanoparticles were also synthesized, ranging in size from 75 to 85 nm [36]. Camellia sinensis (tea) and Coffea arabica (coffee) extracts have been used to synthesize palladium nanoparticles with sizes ranging from 20 to 60 nm and a cubic crystal symmetry in the center [37]. Song et al. [38] reported the first platinum nanoparticles of Diospyros kaki (persimmon) leaf extract, having sizes of 2 to 12 nm. Lately, particle size- and shape-controlled biological synthesis of platinum nanoparticles has also been reported. Plant wood for the nanometer scale has been used for this purpose [39]. For instance, Coccia et al. [40] reported isolated lignin from red pine (Pinus resinosa) for producing palladium and platinum nanoparticles.
3.4. Titanium Dioxide and Zinc Oxide Nanoparticles
These important metal oxide nanomaterials have been synthesized from a number of plant extracts. For instance, Roopan et al. [41] established that TiO2 nanoparticles could be effectively synthesized from Annona squamosa peel; meanwhile, from Nyctanthes arbor-tristis leaf extracts, round particles were found, ranging in size from 100 to 150 nm [42]. Eclipta prostrata leaf extracts could also produce particles, with a size range of 36 to 68 nm [43,44]. Velayutham et al. synthesized TiO2 nanoparticles using Catharanthus roseus leaf extract, ranging in size from 25 to 110 nm and irregularly shaped. The suspension of TiO2 revealed that they were both larvicidal and adulticidal against Bovicola ovis (sheep louse) and Hippobosca maculate (hematophagous fly) [45]. The antioxidant and antibacterial properties of TiO2 nanoparticles, synthesized using an extract from Psidium guajava, were evaluated against Pseudomonas aeruginosa, Staphylococcus aureus, Proteus mirabilis, Aeromonas hydrophila, E. coli, and other pathogens. In addition, the antioxidant and antibacterial properties of TiO2 nanoparticles were evaluated in nanometer scale-up and in bulk [46].
3.5. Indium Oxide (In2O3), Iron Oxide, Lead, and Selenium Nanoparticles
Using a variety of plants, a number of new types of metal oxide and metal nanoparticles were biologically synthesized. Indium oxide nanoparticles were synthesized from aloe vera leaf extract (aloe barbadensis Miller). After primary synthesis, for the production of nanoparticles, the precipitates were thermally treated at 400–600 °C. The size of the synthesized spherical nanoparticles ranged from 5 to 50 nm and the size was dependent on the reaction temperature [47]. In a number of environmental remediation technologies, iron nanoparticles are very important. Thus, a number of studies have focused on green chemistry to synthesize these iron (Fe) nanoparticles. For instance, to synthesize Fe nanoparticles, sorghum bran aqueous extracts have been used. Recently, Pattanayak et al. [48] synthesized spherical iron nanoparticles that had particle sizes of 100 nm from Azadirachta indica (neem) leaf extract. A while ago, Shah et al. synthesized iron nanoparticles from different plant extracts, such as Cymbopogon citratus (lemon grass tea), Datura innoxia, Tridax procumbens, Calotropis procera, Tinospora cordifolia, and Euphorbia milii. Stem extract was used to synthesize the smallest spherical nanoparticles, from 13 nm, the widest size range (43–342 nm) of nanoparticles synthesized from Cymbopogon citratus leaf extract [49]. Lead (Pb) and selenium (Se) are two other significant nanoparticles that have been synthesized biologically. Joglekar et al. [50] were able to synthesize spherical Pb nanoparticles form Jatropha curcas latex, having sizes ranging from 10 to 12.5 nm. Lately, Sasidharan et al. [51] have been able to synthesize spherical selenium (Se) nanoparticles from citrus reticulata peel extract, with a size of 70 nm.
4. Green Synthesis of Metal Nanoparticles
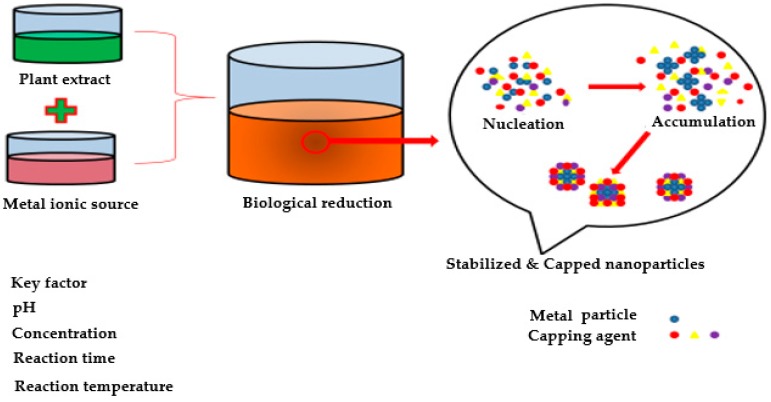
For a long time, it has been known that plants have the potential for biological reduction of metallic ions and hyper-accumulation [52,53]. Because of such remarkable properties, plants have been considered a more environmentally friendly biological method for synthesis of metallic nanoparticles, and also useful for detoxification applications [54]. Plant extracts contain various bioactives, such as alkaloids, proteins, phenolic acids, sugars, terpenoids, and polyphenols, which have been found to have an important role in first reducing and then stabilizing the metallic ions, as shown in Figure 2.
Figure 2.
Biological synthesis of nanoparticles by using plant extracts.
The shape and size of nanoparticles mainly depend on the variation in composition and concentration of active biomolecules of different plants, and their interaction with the aqueous metal ions. Especially in chemical and biological synthesis of nanoparticles, the aqueous metal ion precursors from metal salts are reduced, which results in colour change of the reaction mixture and provides a quantitative indication of nanoparticle formation. More importantly, the nanoparticles synthesized from reducing agents may show general toxicity, engendering serious concern for developing environmentally friendly processes. The process of the formation of nanoparticles begins by mixing a metal–salt solution with a sample of plant extract. During the synthesis of nanoparticles, biochemical reduction of the salt solution starts immediately and the change in colour of the reaction mixture indicates the formation of nanoparticles. During synthesis, initially there is an activation period process in which metal ions are converted to zero-valent state from their mono or divalent oxidation states, so that the nucleation of such reduced metal atoms takes place [55]. Furthermore, the process of nanoparticle synthesis is followed by the integration of smaller neighbouring particles to form larger nanoparticles, which are thermodynamically stable, and, subsequently, the metal ions are reduced biologically. In this way, growth progresses and nanoparticles aggregate to form a variety of shapes such as spheres, cubes, triangles, rods, wires, hexagons, and pentagons. In the final stage of the process, the ability of plant extract to stabilize the nanoparticle finally determines its stable morphology. Significantly, the quality, size, and morphology of the nanoparticles are influenced by properties of the plant extracts; mainly its concentration, reaction time, metal salt concentration, reaction solution pH, and temperature [56,57].
5. Green Nanotechnology: Risk Aspects
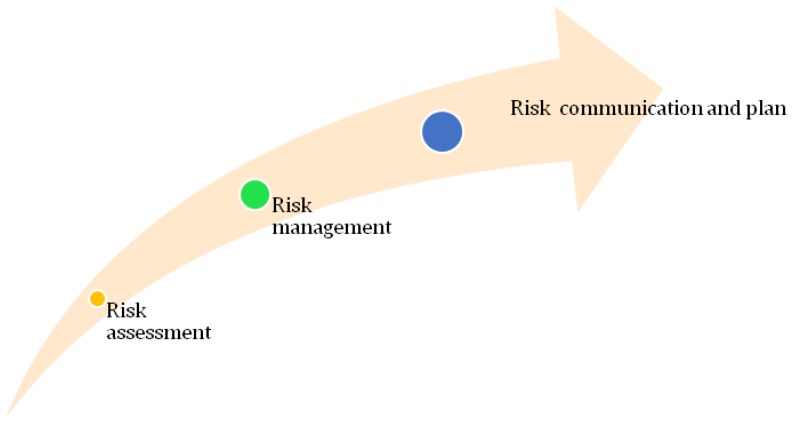
The invention of nanotechnology application in the field of green technology has raised concerns regarding the nanomaterial impact on health and safety of workers. This urgently requires scientific, technological, and governmental efforts to manage such kinds of risks for the workforce. This means detecting genuine risks derived from nanomaterial exposure in the workplace, that is, “risk assessment”: To plan the control measures of “risk management” and, finally, to communicate the plan. Overall, all these steps of risk aspects are critical and will be discussed in the following section, which aims to protect the worker from harm and provide all the benefits of green nanotechnology for society (Figure 3).
Figure 3.
Risk aspects of green nanotechnology.
6. Risk Assessment
Risk assessment of nanomaterials includes the same processing steps that are used in the risk assessment of other types of materials/chemicals [58]. These include hazard identification, hazard characterization, dose-response relationships, and assessment of exposure for the different scenarios. Unfortunately, the risk assessment process of nanomaterials still suffers from a deficiency of toxicological data for a variety of nanomaterials. Furthermore, the definition of vital health effects, such as genotoxicity, pulmonary toxicity, or carcinogenicity in conditions of long-term and low-dose exposure, are approaching realistic scenarios that require attention [59]. For the characterization of occupational nanomaterial risks, the exposure assessment remains a fundamental condition. Efforts should be made to overcome practical barriers that are related to the novelty of green-nanomaterial exposure scenarios, the variations in how to detect and classify nanomaterials, and the queries about the metrics for health-related sampling. That aside, it is also important to carry out biological monitoring studies, which are important to define possible biomarkers of nanomaterials exposure, and effects that are prospectively tested, validated and used in occupational risk evaluation [60].
7. Risk Management
The aim of risk assessment is to provide computable predictions of risks assisting their evidence-based management [61]. To effectively manage the potential risks related to green nanotechnology, a plan of risk management including a hierarchy of controls should be emphasized [62]. The first step of planning is to determine potential exposure to workers, measuring and identifying how this exposure may differ depending on the job task. After identification, potential worker exposure should be managed by using the hierarchy of controls that starts with hazard elimination, adopting a green chemistry by substitution with a non-hazard, and an introduction of engineering controls, including enclosed systems. Administrative control should follow these steps, including training programs by which companies communicate with workers to find out if they have the information to sufficiently understand the routes and nature of potential nanomaterial exposure in the workplace, adequate job procedures, possible risks, preventive and protective measures, and the policies adopted. In this context, it should be important to improve insufficient or inadequate information for workers present on safety data sheets. Risk management, including the use of personal protective equipment (PPE), including respiratory and eye protection, gloves, and lab coats, is the final step for exposure control.
8. Risk Communication
Risk communication is a crucial part of green nanotechnology, relating to the healthy origination and sustainable development of general public transparency. In terms of making available complex technical and health information, risk communication should be made effective, in language that should be accessible and understandable to the general population. Importantly, regulatory scientists, researchers, workforce representatives, industry, and governmental authorities should be engaged in a dialogical progressive communication of the potential green nanotechnology risks, with the objective to form adequate perceptions and attitudes. This is tremendously important to ensure that the spread as well as promotion by mass media involves appropriate information regarding the benefits and challenges of green nanotechnology, protecting public opinion from both unrealistic prospects and excessive consciousness in this regard.
9. Conclusions
As green nanotechnology becomes more commercialized, it will have the potential to become an industry with very strong green credentials. As a general conclusion, it can be said that green nanotechnology involves challenging work in the pharmaceutical industry. Ultimately, however, it improves the quality of life, promotes environmentally friendly commitments, as well as ethical values in the field of nanotechnology.
Acknowledgments
We would like to thank Pharmaceutical Sciences Laboratory, Åbo Akademi University for providing the necessary support.
Author Contributions
Conceptualization, A.V. and S.P.G.; Validation, A.V., and S.P.G.; Formal analysis, A.V., K.K.B. and N.P.; Resources, A.V. and J.M.R.; Writing—original draft preparation, A.V.; Supervision, J.M.R.
Funding
Academy of Finland, grant numbers 309374 (J.M.R.) and 309794 (K.K.B., J.M.R.); Sigrid Jusélius Foundation (N.P.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hullmann A., Meyer M. Publications and patents in nanotechnology. Scientometrics. 2003;58:507–527. doi: 10.1023/B:SCIE.0000006877.45467.a7. [DOI] [Google Scholar]
- 2.Zou H., Wu S., Shen J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008;108:3893–3957. doi: 10.1021/cr068035q. [DOI] [PubMed] [Google Scholar]
- 3.Balbus J.M., Florini K., Denison R.A., Walsh S.A. Protecting workers and the environment: An environmental NGO’s perspective on nanotechnology. J. Nanopart. Res. 2007;9:11–22. doi: 10.1007/s11051-006-9173-7. [DOI] [Google Scholar]
- 4.Benn T.M., Westerhoff P. Nanoparticle Silver Released into Water from Com mercially Available Sock Fabrics. Environ. Sci. Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 5.Besley J.C., Kramer V.L., Priest S.H. Expert opinion on nanotechnology: Risks, benfits, and regulation. J. Nanopart. Res. 2008;10:549–558. doi: 10.1007/s11051-007-9323-6. [DOI] [Google Scholar]
- 6.Guo L., Lui X., Sanchez V., Vaslet C., Kane A.B., Hurt R.H. Window of Opportunity: Designing Carbon Nanomaterials for Environmental Safety and Health. Mater. Sci. Forum. 2007;511–516:544–545. [Google Scholar]
- 7.Hristozov D., Ertel J. Nanotechnology and Sustainability: Benefits and Risk of Nanotechnology for Environmental Sustainability. Forum der Forschung. 2009;22:161–168. [Google Scholar]
- 8.Jayapalan A.R., Lee B.Y., Kurtis K.E. Can nanotechnology be green? Comparing efficacy of nano and microparticles in cementitious materials. Cem. Concr. Compos. 2013;36:16–24. doi: 10.1016/j.cemconcomp.2012.11.002. [DOI] [Google Scholar]
- 9.Som C., Wick P., Krug H., Nowack B. Environmental and health effects of nanomaterials in nanotextiles and façade coatings. Environ. Int. 2011;37:1131–1142. doi: 10.1016/j.envint.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Rickerby D.G., Morrison M. Nanotechnology and the environment: A European perspective. Sci. Technol. Adv. Mater. 2006;8:19–24. doi: 10.1016/j.stam.2006.10.002. [DOI] [Google Scholar]
- 11.Hutchison J.E. Greener nanoscience: A proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano. 2008;2:395–402. doi: 10.1021/nn800131j. [DOI] [PubMed] [Google Scholar]
- 12.Lam C.W., James J.T., McCluskey R., Arepalli S., Hunter R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 13.Schulte P.A., McKernan L.T., Heidel D.S., Okun A.H. Occupational safety and health, green chemistry, and sustainability: A review of areas of convergence. Environ. Health. 2013;12:1186–1476. doi: 10.1186/1476-069X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastlake A., Hodson L., Geraci C.L., Crawford C.A. Critical evaluation of material safety data sheets (MSDS) for engineered nanomaterials. J. Chem. Health Saf. 2012;53:S108–S112. doi: 10.1016/j.jchas.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnovale C., Bryant G., Shukla R., Bansal V. Size, shape and surface chemistry of nano-gold dictate its cellular intraction, uptake and toxicity. Prog. Mater. Sci. 2016;83:152–190. doi: 10.1016/j.pmatsci.2016.04.003. [DOI] [Google Scholar]
- 16.Khan A.K., Rashid R., Murtaza G., Zahra A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm. Res. 2014;13:1169–1177. doi: 10.4314/tjpr.v13i7.23. [DOI] [Google Scholar]
- 17.Manuel C., Toni U., Allan P., Katrin L. Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste. Separations. 2018;5:56. [Google Scholar]
- 18.Hua M., Zhang S., Pan B., Zhang W. Heavy metal removal from water/wastewater by nano-sized metal oxides: A review. J. Hazard. Mater. 2012;211:317–331. doi: 10.1016/j.jhazmat.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Centi M., Perathoner S. Carbon nanotubes for sustainable energy applications. ChemSusChem. 2011;4:913–925. doi: 10.1002/cssc.201100084. [DOI] [PubMed] [Google Scholar]
- 20.Qiao Y., Li C.M. Nanostructured catalysts in fuel cells. J. Mater. Chem. 2011;21:4027–4036. doi: 10.1039/C0JM02871A. [DOI] [Google Scholar]
- 21.Cardellina J.H. Challenges and opportunities confronting the botanical dietary supplement industry. J. Nat. Prod. 2000;65:1073–1084. doi: 10.1021/np0200515. [DOI] [PubMed] [Google Scholar]
- 22.Williamson E.M. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- 23.Samaligy M.S., Afif N.N., Mahmoud E.A. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006;308:140–148. doi: 10.1016/j.ijpharm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Dong L., Jia A., Chang X., Xue H. Preparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicine. Int. J. Biol. Macromol. 2006;38:296–299. doi: 10.1016/j.ijbiomac.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Chen H., Chang X., Du D., Liu W., Yang X. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J. Control. Release. 2006;110:296–306. doi: 10.1016/j.jconrel.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 26.Lawrencea M.J., Reesb G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H., Cui Y., Zhu S., Feng F., Zheng X. Characterization and antimicrobial activity of a pharmaceutical microemulsion. Int. J. Pharm. 2010;395:154–160. doi: 10.1016/j.ijpharm.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as Curecumin: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y.H., Lin J.H., Chou S.C., Chang S., Chung C.C. Berberine-loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: In vitro and in vivo study. Nanomedicine. 2015;10:157–171. doi: 10.2217/nnm.14.76. [DOI] [PubMed] [Google Scholar]
- 30.Bhatt R.L., Vries P., Tulinsky J., Bellamy G., Baker B., Singer J.W. Synthesis and in vivo antitumor activity of poly(L-glutamic acid) conjugates of 20(S)-camptothecin. J. Med. Chem. 2003;46:190–193. doi: 10.1021/jm020022r. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca C., Simões S., Gaspar R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release. 2002;4:273–286. doi: 10.1016/S0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 32.Harikumar P.S., Aravind A. Antibacterial Activity of Copper Nanoparticles and Copper Nanocomposites against Escherichia Coli Bacteria. Int. J. Sci. 2016;2:84–90. doi: 10.18483/ijSci.957. [DOI] [Google Scholar]
- 33.Tran Q.H., Van Quy N., Le A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. 2013;4:1–21. doi: 10.1088/2043-6262/4/3/033001. [DOI] [Google Scholar]
- 34.Vijay Kumar P.P.N., Pammi S.V.N., Kollu P., Satyanarayana K.V., Shameem V.U. Green synthesis and characterization of silver nanoparticles using Boerhaaviadiffusa plant extract and their anti-bacterial activity. Ind. Crops Prod. 2014;52:562–566. doi: 10.1016/j.indcrop.2013.10.050. [DOI] [Google Scholar]
- 35.Dhuper S., Panda D., Nayak P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangiferaindica. Nano Trends J. Nanotechnol. Appl. 2012;13:16–22. [Google Scholar]
- 36.Kalishwaralal K., Deepak V., Pandian S.R.K., Kottaisamy M., BarathManiKanth S., Kartikeyan B., Gurunathan S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B Biointerfaces. 2010;77:257–262. doi: 10.1016/j.colsurfb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Narayanan K.B., Park H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014;140:185–192. doi: 10.1007/s10658-014-0399-4. [DOI] [Google Scholar]
- 38.Mondal S.N., Roy R.A., Laskar I., Sk S., Basu D. Mandal Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swieteniamahogani JACQ) leaves. Colloids Surf. B Biointerfaces. 2011;82:497–504. doi: 10.1016/j.colsurfb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Sharma R., Rana V. Effect of carboxymethylation on rheological and drug release characteristics of Terminaliacatappa gum. Carbohydr. Polym. 2017;175:728–738. doi: 10.1016/j.carbpol.2017.08.047. [DOI] [PubMed] [Google Scholar]
- 40.Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 41.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 42.Brewer E., Coleman J., Lowman A. Emerging technologies of polymeric nanoparticles in cancer drug delivery. J. Nanomater. 2011;2011:1–10. doi: 10.1155/2011/408675. [DOI] [Google Scholar]
- 43.Lim Z.Z.J., Li J.E.J., Yung L.Y.L., Bay B.H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011;32:983–990. doi: 10.1038/aps.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boisselier E., Astruc D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 45.Kundu S., Ghosh S.K., Mandal M., Pal T. Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in micellar medium. Mater. Sci. 2002;25:577–579. doi: 10.1007/BF02710555. [DOI] [Google Scholar]
- 46.Justin T., Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Y.W., Jin R., Mirkin C.A. DNA-modified core–shell Ag/Au nanoparticles. J. Am. Chem. Soc. 2001;123:7961–7962. doi: 10.1021/ja011342n. [DOI] [PubMed] [Google Scholar]
- 48.Shah M., Fawcett D., Sharma S., Tripathy S.K., Poinern G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials. 2015;8:7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Alanazi F.K., Radwan A.A., Alsarra I.A. Biopharmaceutical applications of nanogold. Saudi Pharm. J. 2010;18:179–193. doi: 10.1016/j.jsps.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tedesco S., Doyle H., Blasco J., Redmond G., Sheehan D. Oxidative stress and toxicity of gold nanoparticles in Mytilusedulis. Aquat. Toxicol. 2010;100:178–186. doi: 10.1016/j.aquatox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Nalawade P., Mukherjee T., Kapoor S. High-yield synthesis of multispiked gold nanoparticles: Characterization and catalytic reactions. Colloid Surf. A Physicochem. Eng. Asp. 2012;396:336–340. doi: 10.1016/j.colsurfa.2012.01.018. [DOI] [Google Scholar]
- 53.Kareem A.M., Ismail S., Kundan K. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Plant Sci. 2016;7:303. doi: 10.3389/fpls.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemen S. Metal Hyperaccumulation in Plants: A Review Focusing on Phytoremediation Technology. J. Environ. Sci. Technol. 2011;4:118–138. [Google Scholar]
- 55.Yu J., Dabing Z., Deok-Chun Y. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016;34:7–18. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Niederberger M., Garnweitner G. Reduction of Metal Ions in Polymer Matrices as a Condensation Method of Nanocomposite Synthesis. Eur. J. 2006;12:7282. doi: 10.1002/chem.200600313. [DOI] [PubMed] [Google Scholar]
- 57.Khwaja S., Azamal H., Rifaqat A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018;16:14. doi: 10.1186/s12951-018-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palaniselvam K., Mashitah M., Yusoff G., Natanamurugaraj G. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016;24:473–484. doi: 10.1016/j.jsps.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbara D., Phil S., Günter S., Rothen R. In vitro approaches to assess the hazard of nanomaterials. Nano Impact. 2017;8:99–116. [Google Scholar]
- 60.Peter L., Jutta T., Christian R., Albert B. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018;92:121–141. doi: 10.1007/s00204-017-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker H., Herzberg F., Schulte A., Kolossa-Gehring M. The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int. J. Hyg. Environ. Health. 2011;214:231–238. doi: 10.1016/j.ijheh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Ivo I., Veruscka A., Walter R., Laura L.H., Mark D. Opportunities and challenges of nanotechnology in the green economy. Environ. Health. 2014;13:78. doi: 10.1186/1476-069X-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]