Abstract
Multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) are T cell-driven autoimmune diseases of the central nervous system (CNS) where interleukin (IL)-17-producing Th17 cells promote damage and are pathogenic. Conversely, tolerogenic dendritic cells (DCs) induce regulatory T (Treg) cells and suppress Th17 cells. Chloroquine (CQ) suppresses EAE through the modulation of DCs by unknown mechanisms. Here we show that signal transducer and activator of transcription (STAT) 1 is necessary for CQ-induced tolerogenic DCs (tolDCs) to efficiently suppress EAE. We observed that CQ induces phosphorylation of STAT1 in DCs in vivo and in vitro. Genetic blockage of STAT1 abrogated the suppressive activity of CQ-treated DCs. Our findings show that STAT1 is a major signaling pathway in CQ-induced tolDCs and may shed light on new therapeutic avenues for the induction of tolDCs in autoimmune diseases, such as MS.
Keywords: dendritic cells, chloroquine, experimental autoimmune encephalomyelitis, tolerogenic dendritic cells, immunomodulation
Introduction
Among cells that are capable of antigen-presentation, DCs arise as professional APCs with the ability to modulate T cell differentiation towards IFN-γ-producing Th1 cells and Th17 cells and other subsets of Th cells that are involved in productive immunity against invading pathogens or deleterious molecules. DCs also stimulate the differentiation and maintenance of Treg cells in the periphery of the immune system which keeps auto-reactive T cells in control. The expression of pathogen-associated molecular pattern receptors (PRRs) prompts DCs to sense the environment and, upon stimulation, respond accordingly through production of cytokines (IL-12, IL-23), chemokines (CXCL3, CCL19/21) and reactive metabolites (NO, Arg) [1]. DCs are also modulated by products from vitamin metabolism, such as retinoic acid (vitamin A) and 1,25-dihydroxyvitamin D (vitamin D), which skews their activity to a tolerogenic state where they induce Treg cell expansion and suppress inflammatory T cells [2, 3]. Conversely, DCs play a pathogenic role in autoimmunity where they express high levels of MHC-II and co-stimulatory molecules CD80 and CD86 leading to an increased ability to activate T cells [4, 5]. Overall, DCs are major players of a crosstalk between the innate and adaptive immune system and their activity greatly influences the outcome of the immune response.
Modulation of dendritic cells with drugs or parasite antigens has yielded promising results in autoimmune disease models. Previously, we showed that chloroquine (CQ), an antimalarial drug, suppressed EAE through modulation of DC function [6]. CQ-treated bone marrow-derived DCs (BMDCs) were able to induce Treg cells in vitro and suppress EAE in a Nitric Oxide-dependent pathway [7, 8]. These observations place DCs as target cells for the development of new therapeutic approaches. In this sense, the characterization of intracellular pathways that lead to generation and function of tolDC is of the utmost need in order to develop approaches that efficiently reduce EAE/MS in an Ag-specific manner. Signaling through STAT proteins has been shown to play controversial roles in DC activation. STAT1 activation in DCs, but not T cells, ultimately leads to the development of protective Th1 immunity to protozoa [9–11]. Type I IFNs, such as MS-first line drug IFN-β, signals through STAT1 suggesting that this pathway may present a tolerogenic role in MS [12]. STAT1 is also necessary for DC maturation and differentiation [13, 14]. However, the role of STAT-1 signaling tolDC function is not completely understood.
In this study, we show that following CQ administration, DCs upregulated the expression of STAT1 in vivo and in vitro. In the absence of STAT1 signaling, CQ-treated BMDCs did not suppress MOG35–55-reactive Th17 cells. CQ-treated STAT1−/− BMDCs failed to suppress EAE development. Together, these observations show that STAT1 signaling is necessary to lDC activity.
Results and Discussion
CQ-induced phosphorylation of STAT1 is necessary to induce tolDCs
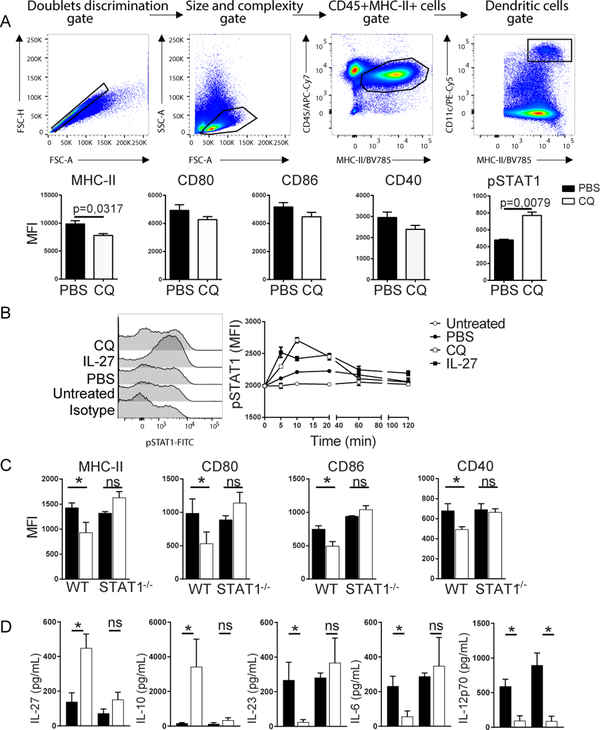
CQ induces tolDCs in EAE mice [6]; however, the effect of CQ on DCs from naïve mice is not known. We treated naïve C57BL/6 mice with CQ for five consecutive days and analyzed the phenotype of spleen DCs 30 minutes after the last dose of CQ. DCs from CQ-treated mice expressed significantly lower levels of MHC class II but not CD80, CD86 and CD40 compared to PBS-treated mice (Figure 1A). Interestingly, we detected higher levels of pSTAT1 in DCs from CQ-treated mice than PBS-treated mice (Figure 1A). To test whether CQ induces STAT1 phosphorylation in vitro, we generated mature BMDCs and exposed them to PBS or CQ. Mature BMDCs exposed to IL-27 served as a positive control as it is known that IL-27 signals through STAT1 [15]. Our results evidenced higher pSTAT1 expression at 5 min, 20 min, 40 and 60 min following CQ exposure compared to PBS-treated mature BMDCs (Figure 1B). pSTAT1 expression was also induced in IL-27-treated mature BMDCs. These results show that CQ induces pSTAT1 in mature BMDCs in vitro. CQ also induced pSTAT expression in immature BMDCs (not shown). STAT proteins serve as mediators of gene expression following cytokine-receptor binding at the cell surface. Upon cytokine receptor activation, cytosolic STAT1 is phosphorylated and form homodimers or heterodimers with STAT2 and migrate to the nucleus where they promote gene expression [16]. The role of STAT1 in DCs remains controversial as it has been shown that STAT1 is necessary for development of a protective immune response while it was also shown that lack of STAT1 renders DCs inflammatory [9, 13, 17].To evaluate whether STAT1 plays a role in the expression of molecules associated with Ag presentation in DCs, mature WT and STAT1−/−BMDCs were subjected to CQ treatment (50 μM) for 18h. Our data show that, opposed to WT BMDCs, CQ is unable to reduce expression of MHC-II, CD80 and CD86 in STAT1−/− BMDCs (Figure 1C). Cytokine dosage in supernatants revealed that CQ induced IL-27 and IL-10 while inhibiting IL-23 and IL-6 in WT, but not in STAT1−/− BMDCs (Figure 1D). IL-12p70 was reduced in CQ-treated BMDCs independently of STAT1 (Figure 1D).
Figure 1 – CQ-induced phosphorylation of STAT1 is necessary to induce tolDCs.
A) Gating strategy and expression pattern of MHC class II and co-stimulatory molecules CD80, CD86, CD40 and pSTAT1 inside gated DCs. Each group consisted of 5 mice. B) Detection of pSTAT1 in BMDCs at different time-points after CQ treatment. Each time-point was cultivated in triplicates. C) Expression of MHC-II and co-stimulatory molecules CD80, CD86 and CD40 in CQ-treated BMDCs generated from WT and STAT1−/− mice. D) Cytokine dosage from culture supernatants described in (C). Cells were cultivated in triplicates. Analysis were carried out with nonparametric Mann-Whitney test where values of p<0.05 (*) were considered significant. ns, not significant. ns, not significant. ND, not detected. Graphs depict mean±SEM from one representative experiment out of three independent experiments with similar results.
STAT1 is necessary for tolDC function
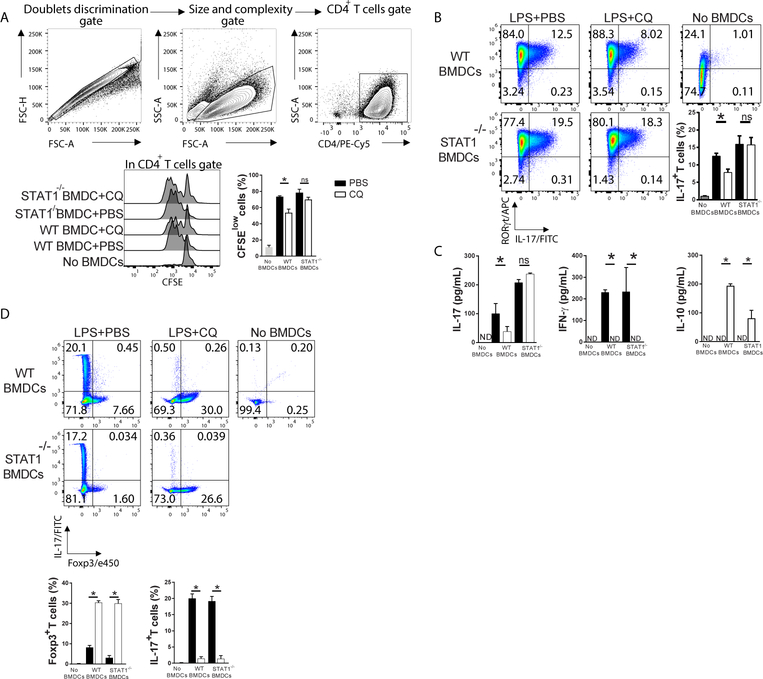
We investigated the ability of CQ-treated STAT1-deficient BMDCs to stimulate T cell proliferation. MOG35–55-pulsed CQ-treated WT and STAT1−/− mature BMDCs were cultivated with CFSE-labelled MOG35–55-specific naïve 2D2 CD4+ T cells. We found that CQ-treated WT BMDCs significantly suppressed T cell proliferation (Figure 2A). CQ-treated STAT1−/− mature BMDCs stimulated T cell proliferation to the same levels as PBS-treated WT and STAT1−/− BMDCs (Figure 2A). In MS patients and EAE mice, high T cell reactivity towards myelin is observed and MS lesions reveal the presence of IL-17-producing T cells [18]. Administration of anti-IL-17 antibodies ameliorates EAE and IL-17−/− mice develop significantly less severe disease compared to WT counterparts [19]. Our data show that WT mature BMDCs induced high expression of RORγt and IL-17 in naïve T cells, whereas CQ-treated WT BMDCs, but not STAT1−/−, significantly suppressed Th17 differentiation (Figure 2B). IL-17 dosage in supernatants followed a similar pattern (Figure 2C). Interestingly, IL-10 was increased while IFN-γ was decreased in CQ-treated BMDCs irrespective of STAT1 deficiency (Figure 2C). Altogether, the phenotype of T cells has a positive correlation with the cytokine pattern of CQ-treated BMDCs (Figure 1D). When cultivated in the presence of TGF-β (2ng/mL), CQ-treated WT and STAT1−/− BMDCs induced Foxp3+ T cells while PBS-treated BMDCs induced IL-17+ T cells (Figure 2D), which shows that the Treg-inducing ability of CQ-treated BMDCs is STAT1-independent, at least in the presence of exogenous TGF-β.
Figure 2 – STAT1 is necessary for tolDC function.
A) Gating strategy and CFSE decay of naïve CD4+ T cells co-cultured with CQ-treated WT and STAT1−/− BMDCs in the presence of MOG35–55 (10 μg/mL) for 72h. B) At the end of the culture period, cells were stimulated with PMA and Ionomycin in the presence of GolgiPlug for 4h and stained with fluorochrome-conjugated antibodies against surface and intracellular markers for FACS analysis. C) Supernatants were collected and assayed for detection of IL-17, IFN-γ and IL-10. D) Co-cultures were conducted in the presence of rhTGF-β1 (2ng/mL) and the frequency of Foxp3+ and IL-17+ T cells was assessed. Cells were cultivated in triplicates. Analysis were carried out with One-Way ANOVA and Bonferroni post-test where values of p<0.05 (*) were considered significant. ns, not significant. ns, not significant. ND, not detected. Graphs depict mean±SEM from one representative experiment out of three independent experiments with similar results.
STAT1−/− BMDCs fail to suppress Th17 cells in the CNS of EAE mice
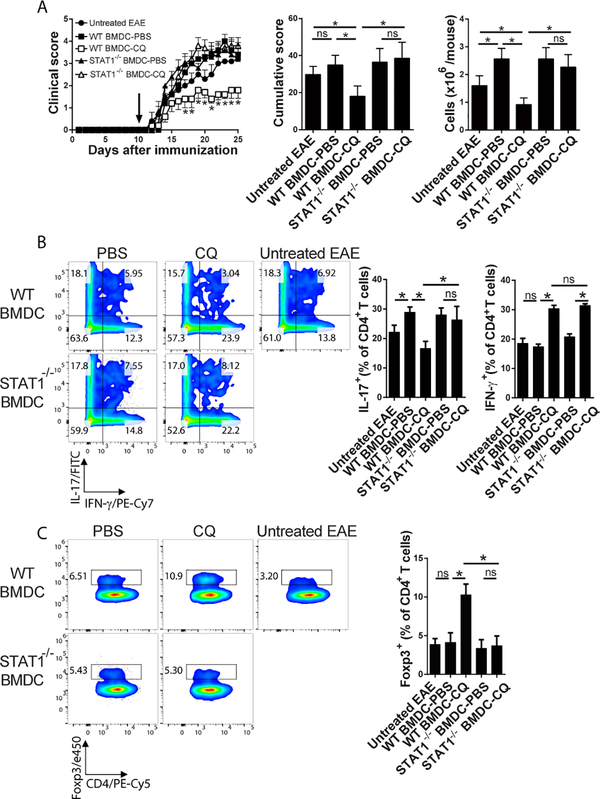
To investigate whether lack of STAT1 would abrogate the therapeutic potential of CQ-treated BMDCs, we adoptively transferred CQ-treated WT and STAT1−/− BMDCs to EAE mice. Mice without BMDC transfer or transferred with PBS-treated BMDCs served as controls. In accordance with our published results [7, 8], CQ-treated WT BMDCs significantly suppressed EAE compared to PBS-treated WT BMDCs and untreated EAE mice (Figure 3A). Conversely, STAT1−/− BMDCs were unable to suppress disease as both CQ and PBS-treated BMDCs induced similar clinical scores (Figure 3A). Analysis of the cells that infiltrated the CNS showed that mice recipient of CQ-treated WT BMDCs presented significantly lower levels of IL-17+ CD4+ T cells compared to PBS-treated counterparts (Figure 3B). STAT1−/− BMDCs failed to suppress infiltration of IL-17+ CD4+ T cells in the CNS of EAE mice (Figure 3B). Opposed to our in vitro data (Figure 2B–D), CQ-treated WT and STAT1−/− BMDCs induced higher IFN-γ+CD4+ T cells in the CNS of EAE mice (Figure 3B). WT BMDCs induced infiltration of Treg cells while STAT1−/− BMDCs failed to do so (Figure 3C).
Figure 3 – STAT1−/− BMDCs fail to suppress Th17 cells in the CNS of EAE mice.
1.5×106 CQ-treated STAT1−/− and WT BMDCs were intravenously transferred to EAE mice ten days after immunization. Each experimental group consisted of 5 mice. A) Clinical and cumulative course of disease was recorded. CNS infiltrating cells were enriched and counted on day 17 pi. Arrow indicates time of adoptive transfer of BMDCs. B) Analysis of IFN-γ+ and IL-17+ CD4+ T cells in the CNS of EAE mice. C) Analysis of Foxp3+ CD4+ T cells in the CNS of EAE mice. Analysis were carried out with Two-Way (clinical score) or One-Way (cumulative scores and CNS infiltration) ANOVA and Bonferroni post-test where values of p<0.05 (*) were considered significant. ns, not significant. Graphs depict mean±SEM from one representative experiment out of three independent experiments with similar results.
STAT1 signaling is negatively controlled by suppressors of cytokine signaling-1 (SOCS1) [20], where SOCS-1 binds to the cytoplasmic domain of IFNGR1 and inhibits phosphorylation of JAK2 [21]. Phosphorylation of JAK2 is a necessary step for docking and phosphorylation of STAT1, leading to successful signaling. Our results show that CQ-treated BMDCs have higher phosphorylation of STAT1, but we did not analyze whether this was a direct or indirect effect of CQ. CQ could potentially inhibit SOCS1 and indirectly favor STAT1 phosphorylation. In summary, our results show that CQ, a weak base used as an antimalarial drug, has a new immunomodulatory facet: the modulation of DCs by favoring STAT1 signaling. We observed that CQ induced STAT1 phosphorylation in vivo and in vitro and that blockade of this signaling pathway resulted in abrogation of the modulatory effect of CQ.
Methods
Mice
WT C57BL/6 mice, from the Multidisciplinary Center for Biological Research (University of Campinas), 2D2 (MOG35–55-specific T cell receptor transgenic), STAT1−/− (B6.129S(Cg)-Stat1tm1Dlv/J) from the Jackson Labs, and were used in this study. Mice were 8-week-old at the start of experiments and were kept in specific-pathogen free condition, in a controlled temperature and photoperiod environment (12 h:12 h, 20°C), with autoclaved food and water ad libitum throughout the experiment. The Institutional Committee from UNICAMP approved the protocols involving laboratory animals. All efforts were made to minimize animal suffering.
CQ treatment and assessment of pSTAT1 expression in DCs in vivo
Treatment with CQ followed a previously described paper [6]. Mice were dissected 30 min after the last dose of CQ and the spleen cells were collected and fixed with PBS+1% Paraformaldehyde for 20 minutes at room temperature. Fixed cells were permeabilized with 95% Methanol for 20 minutes at −20°C. Samples were incubated with fluorochrome-conjugated antibodies for 20 minutes at 4°C. Antibodies used were: anti-mouse CD11c (clone N418), anti-mouse MHC-II (M5/114.15.2), anti-mouse CD80 (16–10A1) and anti-mouse CD40 (1C10) from eBioscience. Anti-mouse p-STAT1 (4a) was from BD Biosciences. 100,000 events were acquired from each sample in a Flow Cytometer (FACSAria, BD Biosciences) and analyzed in FlowJo VX (FlowJo Inc.).
Generation of BMDCs and in vitro modulation
Dendritic cells were generated from bone marrow-derived precursors, as previously described [7]. DCs were cultured with complete RPMI medium containing 50 μM CQ, 100 ng/mL lipopolysaccharide (LPS) from Escherichia coli O111:B4 (Sigma-Aldrich) and 10 μg/mL MOG35–55 peptide (GenScript) for 18h. BMDCs without CQ served as control. BMDCs were used in flow cytometry analysis of surface markers related to DC maturation status, antigen-presenting assays and adoptive transfer experiments. Cytokine detection was carried out by ELISA (R&D Systems).
Kinect analysis of pSTAT1 expression in vitro
For analyses of pSTAT1 expression, 1×106 freshly collected BMDCs were allowed to rest for 1h and later exposed to CQ (50 μM), LPS (100 ng/mL) or IL-27 (20 ng/mL) for 5, 10, 30, 60 and 120 minutes. Unstimulated DCs served as baseline of pSTAT1 expression. At each time-point, cells were immediately fixed and stained as described above.
Analysis of T cell differentiation after co-culture with modulated dendritic cells
For Ag-presentation assays, 2×104 BMDCs were seeded in each well of a U-bottom 96 well plate. 2×105 freshly isolated CFSE-stained naïve CD4+ T cells (Miltenyi Biotec) were seeded into each well of the DC-seeded plates. Naïve CD4+ T cells cultivated without BMDCs served as controls. Cells were cultured in complete IMDM containing 10 μg/mL MOG35–55 for 72h. After incubation, cells were activated with Phorbol Myristate Acetate (PMA, 50 ng/mL, Sigma-Aldrich) and Ionomycin (500 ng/mL, Sigma-Aldrich) in the presence of Brefeldin A (1 μg/mL, Sigma-Aldrich) for 4h. Surface and intracellular staining was performed as described above. In one set of experiments, co-cultures were conducted in the presence of recombinant human TGF-β1 (2ng/mL, R&D Systems). Antibodies used in these set of experiments were anti-mouse CD4 (RM4–5), IL-17A (TC11–18H10), IFN-γ (XMG1.2) and IL-10 (JES5–16E3). All antibodies were from BD Biosciences. Foxp3 (FJk16s) was from eBioscience. 100,000 events were acquired from each sample in a Flow Cytometer (FACSAria, BD Biosciences) and analyzed in FlowJo VX (FlowJo Inc.).
EAE induction and DC transfer
Active EAE was induced and evaluated in mice according to a previously published paper [7]. Clinical signs and weight variation were followed and graded daily according to a score method, were 0: no sign, 1: flaccid tail, 2: hind limbs weakness, 3: hind limbs paralysis, 4: hind paralysis and fore limbs weakness, 5: full paralysis/dead. 1.5 × 106 DCs were intravenously transferred to EAE mice 10 days after immunization.
Isolation of CNS-infiltrating cells and flow cytometry analysis
Isolation of CNS infiltrating cells followed a previously described protocol [22]. Cells were stimulated with PMA+Ionomycin+Brefeldin A, surface and intracellular staining analysis as described above. Antibodies used in these set of experiments were anti-mouse CD4 (RM4–5), IL-17A (TC11–18H10), IFN-γ (XMG1.2), IL-10 (JES5–16E3) and Foxp3 (FJK16s).
Statistical analyses
For clinical score and weight comparisons, collected data were analyzed by two-way ANOVA with Bonferroni post-test. Analyses between two groups were performed by non-parametric Mann-Whitney test. All analyses were performed in GraphPad prism software (version 5.0, Prism).
Acknowledgments
This work was supported by the Sao Paulo Research Foundation (FAPESP, #2014/19492–3 to LV and #2014/02631–0 to RT) and by the National Institutes of Health (NIH Grant AI124386). LV is a Fellow of the National Council of Technological and Scientific Development (CNPq/Brazil).
References
- 1.Takenaka MC and Quintana FJ, Tolerogenic dendritic cells. Semin Immunopathol 2017. 39: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Aar AM, Sibiryak DS, Bakdash G, van Capel TM, van der Kleij HP, Opstelten DJ, Teunissen MB, Kapsenberg ML and de Jong EC, Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J Allergy Clin Immunol 2011. 127: 1532–1540 e1537. [DOI] [PubMed] [Google Scholar]
- 3.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML and de Jong EC, Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol 2015. 8: 265–278. [DOI] [PubMed] [Google Scholar]
- 4.McFarland HF and Martin R, Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 2007. 8: 913–919. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, Yao X, Puck JM, Straus SE and Lenardo MJ, Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 1999. 98: 47–58. [DOI] [PubMed] [Google Scholar]
- 6.Thome R, Moraes AS, Bombeiro AL, Farias Ados S, Francelin C, da Costa TA, Di Gangi R, dos Santos LM, de Oliveira AL and Verinaud L, Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS One 2013. 8: e65913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thome R, Issayama LK, DiGangi R, Bombeiro AL, da Costa TA, Ferreira IT, de Oliveira AL and Verinaud L, Dendritic cells treated with chloroquine modulate experimental autoimmune encephalomyelitis. Immunol Cell Biol 2014. 92: 124–132. [DOI] [PubMed] [Google Scholar]
- 8.Verinaud L, Issayama LK, Zanucoli F, de Carvalho AC, da Costa TA, Di Gangi R, Bonfanti AP, Ferreira IT, de Oliveira AL, Machado DR and Thome R, Nitric oxide plays a key role in the suppressive activity of tolerogenic dendritic cells. Cell Mol Immunol 2015. 12: 384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LM and Scott P, STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J Immunol 2007. 178: 7259–7266. [DOI] [PubMed] [Google Scholar]
- 10.Schneider AG, Abi Abdallah DS, Butcher BA and Denkers EY, Toxoplasma gondii triggers phosphorylation and nuclear translocation of dendritic cell STAT1 while simultaneously blocking IFNgamma-induced STAT1 transcriptional activity. PLoS One 2013. 8: e60215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kernbauer E, Maier V, Stoiber D, Strobl B, Schneckenleithner C, Sexl V, Reichart U, Reizis B, Kalinke U, Jamieson A, Muller M and Decker T, Conditional Stat1 ablation reveals the importance of interferon signaling for immunity to Listeria monocytogenes infection. PLoS Pathog 2012. 8: e1002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platanias LC, Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005. 5: 375–386. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SH, Yu CR, Mahdi RM, Ebong S and Egwuagu CE, Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol 2004. 172: 2307–2315. [DOI] [PubMed] [Google Scholar]
- 14.Vakkila J, Demarco RA and Lotze MT, Coordinate NF-kappaB and STAT1 activation promotes development of myeloid type 1 dendritic cells. Scand J Immunol 2008. 67: 260–269. [DOI] [PubMed] [Google Scholar]
- 15.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC and Quintana FJ, IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 2013. 14: 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuai K and Liu B, Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 2003. 3: 900–911. [DOI] [PubMed] [Google Scholar]
- 17.Pilz A, Kratky W, Stockinger S, Simma O, Kalinke U, Lingnau K, von Gabain A, Stoiber D, Sexl V, Kolbe T, Rulicke T, Muller M and Decker T, Dendritic cells require STAT-1 phosphorylated at its transactivating domain for the induction of peptide-specific CTL. J Immunol 2009. 183: 2286–2293. [DOI] [PubMed] [Google Scholar]
- 18.Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, Mari ER, Safavi F, Leist TP, Zhang GX and Rostami A, Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-beta Therapy. J Immunol 2015. 194: 5085–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K and Iwakura Y, IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006. 177: 566–573. [DOI] [PubMed] [Google Scholar]
- 20.Song MM and Shuai K, The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem 1998. 273: 35056–35062. [DOI] [PubMed] [Google Scholar]
- 21.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S and Yoshimura A, A new protein containing an SH2 domain that inhibits JAK kinases. Nature 1997. 387: 921–924. [DOI] [PubMed] [Google Scholar]
- 22.Thome R, Moore JN, Mari ER, Rasouli J, Hwang D, Yoshimura S, Ciric B, Zhang GX and Rostami AM, Induction of Peripheral Tolerance in Ongoing Autoimmune Inflammation Requires Interleukin 27 Signaling in Dendritic Cells. Front Immunol 2017. 8: 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]