Abstract
Purpose:
We described patellofemoral alignment and trochlear morphology at one and five years after anterior cruciate ligament reconstruction (ACLR), and evaluated the associations between alignment and trochlear morphology (at one year) and worsening patellofemoral osteoarthritis (OA) features by five years. We also evaluated the associations between alignment and morphology to self-reported pain and function (Knee injury and Osteoarthritis Outcome Score, KOOS) at five years.
Materials and methods:
In this longitudinal observational study, we followed 73 participants (mean age 29[9] years, 40% women) from one- to five-years after ACLR. Using MRI, we measured alignment and morphology, and scored cartilage and bone marrow lesions at both time points. We used mixed effects and linear regression models to achieve our stated aims.
Results:
Greater lateral patella displacement increased risk of cartilage worsening (Odds Ratio [95% CI]: 1.09 [1.01, 1.16]); while less lateral tilt (0.91 [0.83, 0.99]) and greater trochlear angle (0.88 [0.77, 1.00]) were protective. Greater medial trochlear inclination increased risk of bone marrow lesion worsening (1.12 [1.04, 1.19]); while greater trochlear angle was protective (0.80 [0.67, 0.96]). Greater lateral displacement was associated with worse self-reported KOOS sport and recreation scores (β [95% CI]: −11.0 [−20.9, −1.2]) and quality of life scores (−10.5 [−20.4, −0.7]).
Conclusions:
Lateral displacement, lateral tilt, and morphology at 1 year post-ACLR altered the risk of worsening patellofemoral OA features four years later. Lateral displacement was the only measure associated with worse self-reported symptoms at five years. These findings may lead to novel treatment strategies for secondary prevention after ACLR.
Keywords: Anterior cruciate ligament reconstruction, patellofemoral osteoarthritis, magnetic resonance imaging, patellar alignment, trochlear morphology
1. Introduction
Traumatic knee injury, such as anterior cruciate ligament (ACL) rupture, results in a 2 – 4 fold increased odds of developing knee osteoarthritis (OA) compared to non-injured knees [1,2]. Despite patient perspectives [3], the increased risk of developing knee OA following ACL injury is not mitigated by surgical reconstruction (ACLR) [4-6]. Although typically considered a disease of the tibiofemoral joint, OA following ACLR occurs frequently in the patellofemoral joint [5, 7-9] with a median prevalence of nearly 50% 10-15 years post-ACLR [10]. Patellofemoral OA following ACLR is associated with pain [9]. Therefore, it is clinically important to identify risk factors for patellofemoral OA following ACLR that could serve as targets for physical therapy intervention, particularly in the early stages of disease where the potential for disease modification is most promising [11].
Aberrant biomechanics are believed to be a key causal mechanism for OA onset and progression in the general population and particularly in individuals following ACLR [12, 13]. While ACLR is effective at restoring the tibiofemoral anteroposterior laxity typically present following ACL rupture, altered patellofemoral tracking is observed in ACL deficient knees and may persist despite ACLR [13, 14]. Abnormal patellar alignment and trochlear morphology may lead to decreased joint contact area and increased joint stress [15, 16]. Thus, irrespective of whether patellar malalignment or abnormal trochlear morphology exists prior to, or develops following, ACL injury or reconstruction, it may increase the risk of patellofemoral OA [17, 18]. We recently reported that patellar alignment and trochlear morphology were cross-sectionally associated with prevalent patellofemoral OA features one year post-ACLR [18]. However, we were unable to infer causality. Moreover, healing of both the ligament graft as well as surrounding tissue (such as subchondral bone) continues for at least two years after ACLR towards baseline joint health and function [19]. Therefore, following a cohort beyond this early recovery period is important to better understand the longitudinal associations between alignment and morphology, and symptomatic and structural decline, in the patellofemoral joint.
The aims of our current longitudinal study were to: (i) describe patellofemoral alignment and trochlear morphology at one and five years post-ACLR, including evaluating change over time; (ii) determine whether alignment and morphology measured at one year increase the risk of worsening patellofemoral OA features at five years; and (iii) evaluate the associations between alignment and morphology at one year to self-reported pain and function at five years.
2. Materials and Methods
2.1. Study design
In this longitudinal observational study, we originally included 111 participants who had undergone ACLR approximately one year prior [7, 18]. Participants were aged 18-50 years at the time of surgery. Briefly, exclusion criteria included: i) any injury to, or symptoms in, the ACLR knee prior to the index ACL injury; ii) ACLR > 15 months prior to enrolment; iii) injury or follow-up surgery within the first year following ACLR; and iv) any other condition that influenced daily function. In the present study, all participants evaluated at baseline (i.e. 1 year post-ACLR) were eligible for the five year post-ACLR follow-up. We noted participant-reported secondary injuries/surgeries to the ACLR knee that occurred between baseline (one year post-ACLR) and follow-up (five years post-ACLR).
Single-bundle ACLR with hamstring-tendon autograft was performed arthroscopically by one of two orthopaedic surgeons (TSW, HGM) [7]. Surgery took place a median of three months after injury [7]. All participants received similar physiotherapy treatment after ACLR [7].
2.2. Magnetic resonance imaging
MR images were acquired at baseline and follow-up with a 3.0 T MR scanner (Philips Achieva, NL) using a 16-channel knee coil. Participants were positioned in supine with knees relaxed and slightly flexed. MR sequences included: (i) a proton density-weighted 3D VISTA sequence (repetition time/echo time [TR/TE] 1300/27 ms; field of view [FOV] 150 mm2; 0.35 mm isotropic; echo train length 64 ms; scan time 6 min 11 s); (ii) sagittal short-tau inversion recovery (STIR) sequence (TR/TE 3,850/30 ms; FOV 160 mm2; slice thickness 2.5 mm; slice gap 1.2 mm; inversion time 180 ms; voxel size 0.45 × 0.50 × 2.5 mm); and (iii) axial proton-density turbo spin-echo (TSE) sequence (TR/TE 3,850/34 ms; FOV 140 mm2; slice thickness 2.5 mm; slice gap 2.0 mm; voxel size 0.5 × 0.55 × 2.5 mm).
2.3. Patellofemoral alignment and trochlear morphology
We used the 3D PD VISTA images to evaluate patellar alignment and trochlear morphology. A single reader (EMM) independently evaluated all alignment and morphology measures at baseline and follow-up – images from each time point were measured more than one year apart (i.e., images were not read paired). We assessed four common measures of alignment: patellar height (Insall-Salvati ratio [20]), lateral displacement (bisect offset [21]), and lateral patellar tilt (patellar tilt angle [21] and lateral patellar tilt angle [22]) (see Figure 1). We also assessed five measures of trochlear bony morphology (sulcus angle, lateral trochlear inclination, medial trochlear inclination, trochlear angle, and trochlear depth) [21, 23]. In the sagittal plane, we selected the MRI slice with the widest oblique distance across the patella for evaluating Insall-Salvati ratio. In the axial plane, we selected two slices: the slice with the most prominent posterior condylar line, as well as the slice with the widest patella [24].
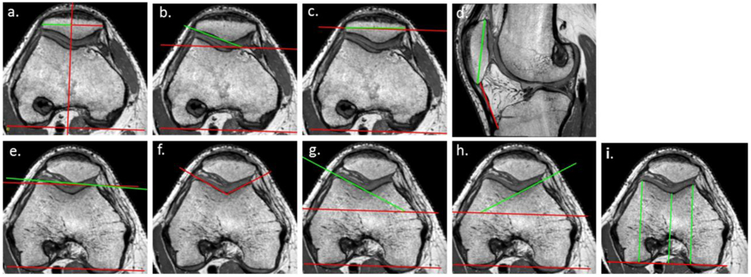
Figure 1.
a. Bisect offset: percentage of the widest patella length that is lateral to the line through deepest part of trochlea running perpendicular to the posterior condylar line (PCL). A higher percentage is more laterally displaced; b. Lateral patellar tilt: angle between PCL and the interior bony margin of the patella lateral facet. A higher angle is less lateral tilt; c. Patellar tilt: angle between PCL and patellar width. A higher angle is more lateral tilt; d. Insall–Salvati Ratio ratio of patellar tendon length to longest patella length. Larger number indicates higher position of patella; e. Trochlear angle: angle between PCL and anterior condylar line. Higher angle indicates deeper sulcus laterally; f. Sulcus angle: angle between the lateral and medial trochlear facet margins. Higher number indicates shallower sulcus; g. Lateral trochlear inclination: angle between PCL and lateral trochlear facet. A larger angle indicates a deeper sulcus laterally; h. Medial trochlear inclination: angle between PCL and medial trochlear facet. A larger angle indicates a deeper sulcus medially; i. Difference in length between: (i) line from posterior condylar line to deepest part of sulcus, and (ii) average length of two lines joining posterior condylar line to anterior femoral condyles.
We used Osirix Lite version 9.5 (Pixmeo Sarl, Switzerland) to calculate all alignment and morphology measures. Intra-rater reliability was previously established with intra-class correlation coefficients (ICC[1,3]) of ≥ 0.94 [18].
2.4. Worsening of patellofemoral OA features
A single experienced musculoskeletal radiologist (AG) read and scored all MR images semi-quantitatively using MRI Osteoarthritis Knee Score (MOAKS) [25]. Reliability has been previously established for these methods (inter-rater weighted k 0.64 – 1.00) [25]. Images at the two time points were read paired. Using MOAKS, the patellofemoral joint was divided into four subregions: the medial and lateral femoral trochlea, and medial and lateral patella (the central median ridge is included in the medial patella for scoring purposes). We analysed changes in cartilage defects, bone marrow lesions (BMLs) and osteophytes from baseline to follow-up. We defined worsening of each of these OA features using existing criteria [26]. Specifically, we defined cartilage worsening as an increase in the percentage of full-thickness cartilage damage or size of any cartilage lesion by at least one grade; BML worsening as any increase in the size or number of BMLs; and osteophyte worsening as any increase in size of osteophytes, providing the worsening is to at least a ‘definite’ osteophyte (i.e. ≥ Grade 2) [25, 26]. Thus, the concept of worsening incorporates both incidence and progression, as recommended previously to overcome methodological challenges in the study of risk factors for OA in both ACL injured and other populations [26, 27].
2.5. Self-reported pain and function
We evaluated five different patient-reported subscales of the Knee injury and Osteoarthritis Outcome Score (KOOS): four of the original subscales (Symptoms, Pain, Sport and Recreation, and Quality of Life) [28], and one additional subscale (Patellofemoral Pain and Osteoarthritis subscale) [29]. Participants completed these questionnaires at baseline and follow-up. We did not include the Activities of Daily Living subscale of KOOS in the present study due to ceiling effects observed in young active populations [5]. For each KOOS subscale, possible scores range from 0 to 100, with 100 representing no problems in each domain [28].
2.6. Statistical analyses
We described alignment and morphology at baseline and follow-up as mean (SD) for the full sample. Then, using previously published non-OA reference values [20, 22, 23, 30], we reported number (percentage) of the sample whose alignment and morphology values exceeded reference values. Finally, we reported the number (percentage) of the sample whose change in alignment and morphology from baseline to follow-up exceeded previously established minimal detectable change values (MDC95) in either direction (see Supplementary Table 1) [18, 30].
We evaluated the longitudinal association between alignment and morphology at baseline and worsening of OA features (cartilage damage, BMLs, osteophytes) in all patellofemoral subregions from baseline to follow-up. To do this, we conducted mixed effects Poisson regression with robust estimates of variance, with participant as a random factor in the model to account for correlation among participant-specific subregions. We included age in each model as indicated by our previous cross-sectional study at one year post-ACLR [18], where older individuals had greater odds of having patellofemoral OA. In addition, we considered sex, BMI, and a square term for alignment/morphology in each model, and included these terms only if they statistically improved the model. We then reported adjusted relative risk per degree (or per percent) difference in each exposure variable (i.e. each alignment or morphology measure). We reported adjusted relative risk for any worsening in the patellofemoral joint, as well as by lateral and medial patellofemoral compartments separately.
Finally, we used linear regression to evaluate whether alignment or morphology at baseline was associated with KOOS scores at five years, adjusting for baseline KOOS scores. In addition to including the baseline KOOS score as a covariate, we also considered age, BMI, sex and the square term for alignment or morphology in each model, including these variables if they statistically improved the model as described above. We reported the adjusted difference in KOOS score per degree (or percentage) difference in alignment or morphology.
For all analyses, in addition to analysing exposures as continuous variables, we created dichotomous variables based on previously established reference values to identify individuals with malalignment or trochlear dysplasia [20, 22, 30]. We then ran additional models with binary exposures in measures where a substantial number of participants (which we defined as ≥ 10% of the sample) exceeded these reference values.
All statistical analyses were completed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
3. Results
Complete demographic and MRI data were available for 73 participants at both one and five years post-ACLR (Tables 1 and 2). Excluded participants did not complete the five-year assessment for the following reasons: pregnant (n=1), lost to follow-up (n=10), unable/declined to attend due to distance or time constraints (n=16); removed from study by participating surgeon due to conflict with participation in another study (n=4); more than five years between original injury and surgery (n=6) [31]; and incorrect knee imaged at follow-up visit (n=1). Those lost to follow-up were not significantly different in baseline age, sex or BMI from those included in the present study (p > 0.05).
Table 1.
Participant characteristics and KOOS scores (n=73)
| Baseline | Follow-up | |
|---|---|---|
| Age at surgery, mean (SD) | 29 (9) | |
| Women, n (% ) | 29 (40%) | |
| BMI kg/m2, mean (SD) | 25.7 (3.6) | 26.2 (3.5) |
| KOOS Symptoms, median (IQR) | 85.7 (75.0, 92.9) | 89.0 (75.0, 96.0) |
| KOOS Pain, median (IQR) | 91.7 (88.9, 97.2) | 97.0 (92.0, 100.0) |
| KOOS Sport and Recreation, median (IQR) | 85.0 (70.0, 95.0) | 90.0 (80.0, 100.0) |
| KOOS Quality of Life, median (IQR) | 68.8 (56.3, 81.3) | 81.0 (69.0, 94.0) |
| KOOS Patellofemoral, median (IQR) | 84.1 (70.5, 90.9) | 90.9(81.8, 97.7) |
BMI = body mass index; KOOS = Knee injury and Osteoarthritis Outcome Score, IQR = interquartile range
Table 2.
Description of alignment and morphology values (n=73)
| Measure* | Baseline | Follow-up |
|---|---|---|
| Insall Salvati ratio, mean (SD) | 1.03 (0.12) | 1.02 (0.12) |
| Low patella (≤0.8) | 2 (3%) | 3 (4%) |
| High patella (≥ 1.2) | 4 (5%) | 5 (7%) |
| Increased from 1 to 5 yrs (≥ 0.06) | 5 (7%) | |
| Decreased from 1 to 5 yrs (≥ 0.06) | 5 (7%) | |
| Bisect offset (%), mean (SD) | 55.14 (6.27) | 55.14 (5.70) |
| High lateral displacement (≥ 61.2%) | 10 (14%) | 12 (17%) |
| Increased from 1 to 5 yrs (≥ 5.63%) | 2 (3%) | |
| Decreased from 1 to 5 yrs (≥ 5.63%) | 3 (4%) | |
| Lateral patellar tilt angle (°), mean (SD) | 11.69 (4.91) | 10.77 (4.60) |
| High lateral tilt (≤12.3°) | 39 (53%) | 43 (61%) |
| Increased lateral tilt from 1 to 5 yrs (≥ 2.44°) | 24 (34%) | |
| Decreased lateral tilt from 1 to 5 yrs (≥ 2.44°) | 6 (9%) | |
| Patellar tilt angle (°), mean (SD) | 10.46 (4.87) | 10.08 (4.95) |
| High lateral tilt (≥ 17.2°) | 6 (8%) | 5 (7%) |
| Increased lateral tilt from 1 to 5 yrs (≥ 2.99°) | 3 (4%) | |
| Decreased lateral tilt from 1 to 5 yrs (≥ 2.99°) | 5 (7%) | |
| Sulcus angle (°), mean (SD) | 122.98 (8.40) | 121.21 (7.85) |
| Wide sulcus angle (≥ 141.5°) | 2 (3%) | 1 (1%) |
| Increased from 1 to 5 yrs (≥ 7.59°) | 0 (0%) | |
| Decreased from 1 to 5 yrs (≥ 7.59°) | 5 (7%) | |
| Lateral trochlear inclination (°), mean (SD) | 28.23 (4.97) | 28.75 (5.65) |
| Low (≤ 18.6°) | 2 (3%) | 2 (3%) |
| Increased from 1 to 5 yrs (≥ 4.02°) | 6 (9%) | |
| Decreased from 1 to 5 yrs (≥ 4.02°) | 3 (4%) | |
| Medial trochlear inclination (°), mean (SD) | 32.90 (5.69) | 34.43 (5.81) |
| Low (≤ 18.6°) | 3 (4%) | 2 (3%) |
| Increased from 1 to 5 yrs (≥ 4.41°) | 11 (16%) | |
| Decreased from 1 to 5 yrs (≥ 4.41°) | 5 (7%) | |
| Trochlear angle (°), mean (SD) | 0.20 (2.66) | −0.09 (2.91) |
| Low (≤−6.0°) | 0 (0%) | 0 (0%) |
| High (≥ 4.7°) | 5 (7%) | 2 (3%) |
| Increased from 1 to 5 yrs (≥ 2.00°) | 3 (4%) | |
| Decreased from 1 to 5 yrs (≥ 2.00°) | 9 (13%) | |
| Trochlear depth (mm), mean (SD) | 7.98 (1.29) | 8.17 (1.31) |
| Low (≤4.47 mm) | 0 (0%) | 1 (1%) |
| Increased from 1 to 5 yrs (≥ 0.8 mm) | 6 (9%) | |
| Decreased from 1 to 5 yrs (≥ 0.8 mm) | 3 (4%) |
Reference values are derived from previous publications for Insall-Salvati ratio [20], lateral patellar tilt angle [22], trochlear depth and remaining measures [30], MDC95 values are derived from previous publications for Insall-Salvati ratio, bisect offset, lateral patellar tilt angle, trochlear angle, sulcus angle [18], and for patellar tilt angle, lateral trochlear inclination and medial trochlear inclination [30] (see Supplementary Table 1).
3.1. Alignment and morphology
The proportion of participants with alignment and morphology values exceeding reference values was generally low at both time points (Table 2). Only two alignment measures had more than 10% of participants exceeding reference values: bisect offset and lateral patellar tilt angle.
Based on MDC95 values, few individuals had a change in alignment or morphology values large enough to suggest true change from one to five years (Table 2). The most notable change was in lateral patellar tilt angle, where 24 (34%) participants exceeded the MDC95 for a decrease in the measure, suggesting an increase in lateral tilt.
3.2. Worsening of patellofemoral OA features
Cartilage worsening and BML worsening is illustrated in two study participants in Figure 2. Three alignment and morphology measures at one year post-ACLR were associated with cartilage worsening in the lateral patellofemoral compartment over time: higher bisect offset; lower lateral patellofemoral tilt angle (i.e., higher lateral tilt); and lower trochlear angle (Table 3). There were no associations with the medial patellofemoral compartment, or with the patellofemoral joint as a whole.
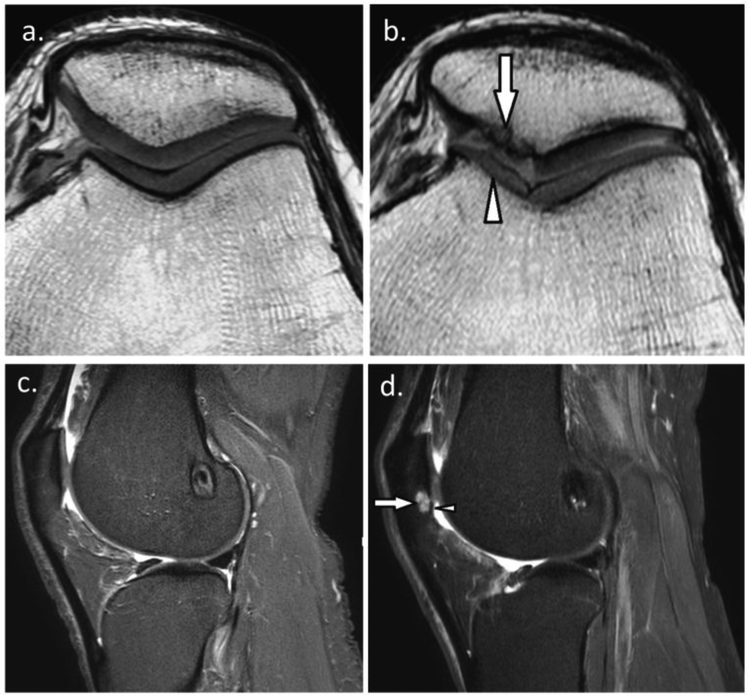
Figure 2.
a. 26-year-old man with ACL reconstruction. Baseline sagittal short-tau inversion recovery (STIR) MRI shows normal cartilage of the patellofemoral joint and absence of bone marrow lesion, and b. Follow-up MRI at the same level shows new small superficial focal cartilage defect at the inferior pole of the patella (arrowhead) with associated subchondral bone marrow lesion (arrow); c. 31-year-old man with ACL reconstruction. Baseline axial proton density-weighted MRI shows normal patellofemoral cartilage, and d. Follow-up MRI at the same level shows diffuse superficial thinning of the medial trochlear cartilage (arrowhead) and large full thickness cartilage loss at the medial patella (arrow) with denuded bone area.
Table 3.
Cartilage worsening: adjusted risk ratio per degree (or per percentage) of exposure variable (alignment or morphology at one year)
| Patellofemoral joint | Lateral | Medial | |
|---|---|---|---|
| Number of subregions with worsening | 70/584 | 22/292 | 48/292 |
| Insall Salvati ratio × 100* (%) | 0.99 (0.96, 1.02) | 0.99 (0.95, 1.02) | 1.00 (0.96, 1.03) |
| Bisect offset (%) | 1.03 (0.98, 1.09) | 1.09 (1.01, 1.16) | 1.01 (0.96, 1.07) |
| ≥ 61.6%^ | 1.26 (0.51, 3.10) | 2.43 (0.91, 6.50) | 0.81 (0.28, 2.34) |
| Lateral patellar tilt angle (°) | 0.95 (0.90, 1.01) | 0.91 (0.83, 0.99) | 0.97 (0.91, 1.04) |
| ≤ 12.3°^ | 1.53 (0.73, 3.20) | 2.87 (0.95, 8.65) | 1.19 (0.54, 2.64) |
| Patellar tilt angle (°) | 1.03 (0.96, 1.09) | 1.06 (0.97, 1.15) | 1.01 (0.94, 1.09) |
| Sulcus angle (°) | 0.98 (0.94, 1.03) | 0.99 (0.93, 1.04) | 0.98 (0.94, 1.03) |
| Lateral trochlear inclination (°) | 1.02 (0.95, 1.08) | 0.98 (0.90, 1.07) | 1.03 1.11) |
| Medial trochlear inclination (°) | 1.02 (0.97, 1.08) | 1.05 (0.97, 1.13) | 1.01 (0.96, 1.07) |
| Trochlear angle (°) | 0.97 (0.86, 1.09) | 0.88 (0.77, 1.00) | 1.02 (0.88, 1.17) |
| Trochlear depth (mm) | 1.14 (0.86, 1.52) | 1.15 (0.79, 1.68) | 1.14 (0.83, 1.55) |
Age was included as a covariate in all models. Note analyses are all subregion-based, thus sample size for each model includes sample size (n=73) times the number of subregions (4 for full patellofemoral joint, 2 each for lateral and medial) times the number of MOAKS scores included (2, cartilage damage size and percentage that is full-thickness). Bold indicates p < 0.05
Insall-Salvati Ratio was transformed to for analyses in order to calculate a risk ratio that could be clinically interpreted per point difference in ratio (a risk ratio in a change in Insall-Salvati Ratio of 1 is not meaningful)
Dichotomized exposure variables: only measures where at least 10% of the sample exceeded reference values were analysed as both continuous and dichotomous exposure variables and are reported here
Two measures were associated with full patellofemoral joint and lateral patellofemoral BML worsening: higher medial trochlear inclination; and lower trochlear angle (Table 4). There were no associations with the medial patellofemoral compartments, and no associations between any patellar alignment measures and BML worsening.
Table 4.
BML worsening: adjusted risk ratio per degree (or per percentage) of exposure variable (alignment or morphology at one year)
| Patellofemoral joint | Lateral | Medial | |
|---|---|---|---|
| Number of subregions with worsening | 27/584 | 7/292 | 20/292 |
| Insall Salvati ratio × 100* (%) | 1.02 (0.98, 1.06) | 1.03 (0.97, 1.09) | 1.02 (0.98, 1.07) |
| Bisect offset | 0.96 (0.86, 1.08) | 1.07 (0.94, 1.21) | 0.92 (0.82, 1.04) |
| ≥61.6%^ | 1.13 (0.15, 8.26) | 2.43 (0.25,23.35) | 0.74 (0.10,5.48) |
| Lateral patellar tilt angle | 0.98 (0.86, 1.12) | 0.92 (0.77, 1.10) | 1.00 (0.86, 1.16) |
| ≤12.3°^ | 0.95 (0.30, 3.03) | 1.15 (0.16, 8.23) | 0.89 (0.25, 3.17) |
| Patellar tilt angle | 1.06 (0.94, 1.19) | 1.11 (0.98, 1.26) | 1.04 (0.90, 1.20) |
| Sulcus angle | 0.99 (0.94, 1.04) | 0.96 (0.86, 1.07) | 1.00 (0.94, 1.06) |
| Lateral trochlear inclination | 1.00 (0.90, 1.11) | 0.99 (0.81, 1.21) | 1.00 (0.89, 1.13) |
| Medial trochlear inclination | 1.07 (1.00, 1.15) | 1.12 (1.04, 1.19) | 1.06 (0.98, 1.14) |
| Trochlear angle | 0.85 (0.73, 0.99) | 0.80 (0.67, 0.96) | 0.87 (0.74, 1.02) |
| Trochlear depth | 0.95 (0.65, 1.39) | 1.21 (0.84, 1.74) | 0.86 (0.52, 1.42) |
Age was included as a covariate in all models. Note subregion-based analyses include sample size (n=73) times the number of subregions (4 for full patellofemoral joint, 2 each for lateral and medial) times the number of MOAKS scores included (2, number of BMLs and size).
Bold indicates p < 0.05
Insall-Salvati Ratio was multiplied by 100 for analyses to calculate a risk ratio that could be clinically interpreted per point difference in ratio (a risk ratio in a change in Insall-Salvati Ratio of 1 is not meaningful)
Dichotomized exposure variables: only measures where at least 10% of the sample exceeded reference values (i.e., were malaligned) were analysed as both continuous and dichotomous variables, and are reported here
Osteophyte worsening occurred in only five participants, thus we did not evaluate the associations between alignment or morphology and osteophyte worsening.
3.3. Self-reported pain and function
Bisect offset was the only measure associated with any KOOS subscale (Table 5). Specifically, when dichotomized, a bisect offset of at least 61.6% had lower KOOS scores for Sport and Recreation (mean 11 points lower), and for Quality of Life (mean 11 points lower).
Table 5.
Association between alignment or morphology (i.e. per degree or per percentage) at one year and KOOS scores at five years
| Symptoms | Pain | Sport Rec | QOL | Patellofemoral | |
|---|---|---|---|---|---|
| Insall Salvati ratio*100 | 0.10 (−0.14, 0.34) | 0.12 (−0.02, 0.26) | 0.14 (−0.14, 0.42) | 0.14 (−0.14, 0.42) | 0.14 (−0.05, 0.32) |
| Bisect offset | −0.23 (−0.71, 0.25) | −0.14 (−0.42, 0.14) | −0.25 (−0.81, 0.31) | −0.40 (−0.96, 0.16) | −0.24 (−0.61, 0.13) |
| ≥61.6% | −1.44 (−10.13, 7.24) | −3.08 (−8.09, 1.92) | −11.02 (−20.88, −1.16) | −10.52 (−20.40, −0.65) | −4.41 (−11.12, 2.30) |
| Lateral patellar tilt angle | −0.00 (−0.63, 0.63) | 0.12 (−0.24, 0.48) | 0.01 (−0.70,0.73) | 0.21 (−0.52, 0.93) | 0.12 (−0.36, 0.59) |
| ≤12.3° | −0.19 (−6.27, 5.89) | −2.70 (−6.15, 0.76) | −4.40 (−11.37, 2.57) | −4.70 (−11.78, 2.37) | −1.41 (−6.07, 3.25) |
| Patellar tilt angle | 0.06 (−0.58, 0.70) | −0.02 (−0.39, 0.34) | 0.01 (−0.72, 0.74) | 0.37 (−0.35, 1.10) | 0.02 (−0.47, 0.50) |
| Sulcus angle | 0.18 (−0.18, 0.53) | −0.01 (−0.22, 0.20) | −0.14 (−0.55, 0.28) | −0.10 (−0.52, 0.32) | −0.07 (−0.35, 0.21) |
| Lateral trochlear inclination | 0.06 (−0.55, 0.67) | 0.24 (−0.12, 0.60) | 0.10 (−0.62, 0.81) | 0.33 (−0.38, 1.05) | 0.24 (−0.23,0.71) |
| Medial trochlear inclination | −0.18 (−0.73, 0.36) | 0.06 (−0.25, 0.37) | 0.53 (−0.08, 1.15) | 0.25 (−0.38, 0.87) | 0.18 (−0.24, 0.60) |
| Trochlear angle | 0.31 (−0.86, 1.47) | 0.03 (−0.65, 0.70) | −0.47 (−1.81, 0.88) | 0.12 (−1.21, 1.46) | −0.11 (−1.00, 0.78) |
| Trochlear depth | −0.74 (−3.12, 1.63) | −0.29 (−1.69, 1.12) | 0.33 (−2.41, 3.07) | −0.33 (−3.13, 2.47) | −0.07 (−1.94, 1.81) |
KOOS score at one year was included as a covariate in all models. Bold indicates p < 0.05
We conducted sensitivity analyses in a subsample with ten of the 73 participants removed who had sustained new injuries between baseline and follow-up. Changes in effect sizes were negligible, though significance changed for trochlear angle and structure, and for bisect offset with KOOS pain and quality of life subscales (see Supplementary Tables 2-4)
4. Discussion
This first longitudinal evaluation of patellofemoral alignment and morphology post-ACLR found abnormal alignment to be prevalent one year following ACLR (bisect offset 14%, lateral patellar tilt angle 53%); while abnormal morphology was less common. Change in alignment and morphology over time was also uncommon, though lateral patellar tilt angle exceeded MDC95 values in 34% of our sample, possibly indicating increased lateral tilt over time. Lateral displacement, lateral tilt, and morphology at one year post-ACLR was associated with increased risk of worsening patellofemoral cartilage or BMLs four years later. Lateral displacement was the only measure associated with worse self-reported symptoms at five years.
Interestingly, more lateral patellar tilt increased the risk of PF cartilage worsening when measured using lateral patellar tilt angle, but not when measured with patellar tilt angle, both of which aim to quantify patellar tilt. This may indicate that lateral patellar tilt angle may be more sensitive for identifying future OA risk. A second unexpected result was that higher medial trochlear inclination at baseline was associated with both lateral and overall patellofemoral BML worsening. It may be that a more protruded medial trochlea encourages lateralization of the patella which would explain the latter findings.
Other measures of patellar alignment (Insall-Salvati ratio, patellar tilt angle) and trochlear morphology (lateral trochlear inclination, sulcus angle) were not associated with structural worsening. This is particularly interesting given that these measures are associated with non-traumatic patellofemoral OA [17, 30]. It may be that different factors drive post-traumatic patellofemoral OA (e.g. synovial inflammation/effusion and acute muscle inhibition following trauma) compared to non-traumatic OA. Alignment and morphology may also influence pathology over longer time periods than captured in the current study (i.e. >5 years). However, malalignment and trochlear dysplasia were uncommon in our sample, and this low prevalence may also explain the negative findings. In our study, both variables with prevalent malalignment (bisect offset, lateral patellar tilt angle) were associated with lateral patellofemoral cartilage worsening. Finally, it may be that measuring alignment with a participant in supine and at rest may not adequately quantify functional malalignment that may be occurring during high level dynamic tasks, which are compromised after ACLR [14, 32, 33].
Significant findings were found predominantly in the lateral patellofemoral compartment, despite medial patellofemoral worsening being more common in the present study. This extends previous findings in the general patellofemoral OA population where alignment and morphology are typically more strongly associated with lateral compartment damage than medial [30]. It is not clear what factors are driving medial patellofemoral worsening in this sample. It may relate to increased tibial rotation excursion which is seen during functional tasks in individuals after ACLR who develop patellofemoral OA compared to those who do not [34]. The increased tibial rotation excursion may contribute to OA by altering patellofemoral contact pressures and joint stress [35].
Only one measure, increased bisect offset, was associated with KOOS scores at five years, specifically greater problems with sports and recreation, and reduced quality of life. Using established minimal important change values for KOOS scores in individuals with patellofemoral pain or OA (Sport and Recreation 15 points; Quality of Life 10 points) [29], only the change in Quality of Life subscale appears to be clinically meaningful. Nonetheless, increased lateral displacement may influence participation and quality of life, constructs that would otherwise be expected to improve from one to five years following ACLR. Further research is needed to explore these associations and possible mechanisms underpinning them. It may be that structural features (i.e. cartilage lesions) mediate associations between lateral displacement and self-reported outcomes. This is supported by our previous three year follow-up study whereby patellofemoral cartilage lesions at baseline were associated with worse symptoms on all KOOS subscales at three years post-ACLR[36], combined with the present study showing lateral displacement increased risk of both cartilage worsening and worse KOOS scores. Confirming such a relationship would require a larger sample size and more complex mediation analyses.
Importantly, patellar alignment may be modifiable [22, 37] and may therefore represent a promising target following ACLR for optimizing both structural and symptom outcomes. Alignment and morphology could be evaluated alongside other known risk factors for post-traumatic patellofemoral OA[8, 31, 34, 38, 39] to inform clinical decision making. The results of our study warrant further research to determine whether mechanistic factors contributing to patellofemoral OA can be effectively addressed in ACLR rehabilitation.
A limitation of the current study was that, because it was an ancillary study of an existing cohort, an a priori sample size calculation was not performed. Based on the proportion of our sample with excessive lateral displacement (bisect offset, 14%), and the proportions of those with and without lateral patellofemoral cartilage worsening, 39 participants in each group would be needed to achieve significance, and to have 39 participants with lateral displacement would require a total sample size of 279. Second, we acknowledge substantial loss to follow-up, though note there were no differences in baseline demographics between those who did and did not participate at follow-up.Third, we did not have access to pre-operative (or pre-injury) images or a control group (non-injured or ACL deficient), and therefore are unable to determine whether alignment or morphology differed as a result of ACL injury or reconstruction, or whether baseline values were pre-existing [14, 40, 41]. The literature suggests that alignment, but not morphology, may change as a result of ACL injury, and is at least partially restored following ACLR [14, 40-42]. It is also unknown whether graft type contributes to changes in alignment. However, regardless of the timeline, we found that having malalignment after ACLR may increase risk of patellofemoral OA. Fourth, multiple testing raises the possibility of Type I error., Fifth, the thresholds to define malalignment and dysplasia were derived from a non-OA cohort of adults over 50 years old [30], a sample with meniscus injury [20], and a young healthy control group [22]. It is not known to what extent these measures differ by age or pathology. Finally, the MDC95 values we used were derived from two readings of individual MR images. These values do not consider error from imaging at two different time points. We believe this would result in misclassifying individuals as having changed when in reality change did not occur. Moreover, the clinical relevance of these changes is unknown. Further studies are required to establish minimal clinically important change values to improve clinical interpretation of changes in alignment and morphology over time.
5. Conclusions
We observed that more lateral displacement (bisect offset), more lateral patellar tilt, and a shallower trochlea one year post-ACLR was associated with lateral patellofemoral cartilage worsening from baseline to follow-up. In addition, a lower trochlear angle and higher medial trochlear inclination was associated with patellofemoral BML worsening, particularly in the lateral compartment. Malalignment in the axial plane was prevalent, while sagittal plane malalignment and abnormal morphology were uncommon in our sample of individuals post-ACLR. Change in alignment or morphology from one to five years was also uncommon, though lateral tilt may have increased over time. Lateral displacement, as measured by bisect offset, may be a risk factor for both structural worsening of the patellofemoral joint as well as reduced function and quality of life following ACLR. Because bisect offset may be modifiable with treatment, it may represent a viable treatment target post-ACLR.
Supplementary Material
Highlights.
Abnormal patellar alignment, but not trochlear morphology, was common one year following ACLR
Change in patellar alignment and trochlear morphology over time was rare, though lateral tilt may increase over time
Increased lateral displacement and lateral tilt, and altered trochlear morphology at 1 year post-ACLR increased risk of patellofemoral cartilage or BML worsening four years later
Lateral displacement was associated with worse self-reported outcomes at five years
Acknowledgments
Funding and conflicts of interest
This work was supported by Arthritis Australia (Grant in Aid), La Trobe University’s Sport, Exercise and Rehabilitation Research Focus Area (Project Grant), the Queensland Orthopaedic Physiotherapy Network (Project Grant), the University of Melbourne (Research Collaboration Grant), and the University of British Columbia’s Centre for Hip Health and Mobility (Society for Mobility and Health). E. Macri received funding support from the Australian Endeavour Award Research Fellowship and Vanier Canada Graduate Scholarship (CIHR). J. Stefanik and E. Macri were supported by NIH/NIGMS U54-GM104941, and J. Stefanik was also supported by NIH/NIAMS K23AR070913. B. Patterson is a recipient of a National Health and Medical Research Council (NHMRC) postgraduate scholarship (GNT 1114296). A. Culvenor’s work was supported by an NHMRC Early Career Fellowship (Neil Hamilton Fairley Clinical Fellowship, GNT 1121173). A Guermazi is the president of Boston Imaging Core Lab (BICL), LLC, and a consultant to Merck Serono, TissueGene, Genzyme, AstraZeneca, OrthoTrophixs, Pfizer and GE Healthcare. T. Whitehead reports personal fees from a Smith and Nephew Clinical Fellowship and personal fees from Smith and Nephew speaking engagement, outside the submitted work. H. Morris reports personal fees from Oceania Orthopaedics Clinical Fellowship, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP, Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis, Osteoarthritis Cartilage 23(4)(2015)507–515. [DOI] [PubMed] [Google Scholar]
- [2].McAlindon T, Zhang Y, Hannan M, Naimark A, Weissman B, Castelli W, Felson D, Are risk factors for patellofemoral and tibiofemoral knee osteoarthritis different?, Journal of Rheumatology 23(2)(1996)33 2–337. [PubMed] [Google Scholar]
- [3].Bennell KL, Van Ginckel A, Kean CO, Nelligan RK, French SD, Stokes M, Pietrosimone B, Blackburn T, Batt M, Hunter DJ, Patient knowledge and beliefs about knee osteoarthritis after anterior cruciate ligament injury and reconstruction, Arthritis Care Res (Hoboken) 68(8)(2016)1180–1185. [DOI] [PubMed] [Google Scholar]
- [4].Luc B, Gribble PA, Pietrosimone BG, Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis, J Athlet Train 49(6)(2014)806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS, Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial, Bmj 346(2013)f232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nordenvall R, Bahmanyar S, Adami J, Mattila VM, Felländer-Tsai L, Cruciate ligament reconstruction and risk of knee osteoarthritis: the association between cruciate ligament injury and post-traumatic osteoarthritis, a population based nationwide study in Sweden, 1987–2009, PLoS One 9(8)(2014)e104681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Khan KM, Beck N, van Leeuwen J, Crossley KM, Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation, Arthritis rheumatol 67(4)(2015)946–955. [DOI] [PubMed] [Google Scholar]
- [8].Lee DW, Yeom CH, Kim DH, Kim TM, Kim JG, Prevalence and Predictors of Patellofemoral Osteoarthritis after Anterior Cruciate Ligament Reconstruction with Hamstring Tendon Autograft, Clin Orthop Surg 10(2)(2018)181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Culvenor AG, Lai CC, Gabbe BJ, Makdissi M, Collins NJ, Vicenzino B, Morris HG, Crossley KM, Patellofemoral osteoarthritis is prevalent and associated with worse symptoms and function after hamstring tendon autograft ACL reconstruction, British Journal of Sports Medicine 48(6)(2014)435–439. [DOI] [PubMed] [Google Scholar]
- [10].Culvenor AG, Cook JL, Collins NJ, Crossley KM, Is patellofemoral joint osteoarthritis an underrecognised outcome of anterior cruciate ligament reconstruction? A narrative literature review, British Journal of Sports Medicine 47(2)(2013)66–70. [DOI] [PubMed] [Google Scholar]
- [11].Pollard T, Gwilym S, Carr A, The assessment of early osteoarthritis, Bone & Joint Journal 90(4)(2008)411–421. [DOI] [PubMed] [Google Scholar]
- [12].Felson DT, Osteoarthritis as a disease of mechanics, Osteoarthritis Cartilage 21(1)(2013)10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amano K, Pedoia V, Su F, Souza RB, Li X, Ma CB, Persistent Biomechanical Alterations After ACL Reconstruction Are Associated With Early Cartilage Matrix Changes Detected by Quantitative MR, Orthop 4(4)(2016)2325967116644421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G, The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint, Am J Sports Med 36(6)(2008)1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Besier TF, Gold GE, Delp SL, Fredericson M, Beaupré GS, The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint, Journal of Orthopaedic Research 26(12)(2008)1627–1635. [DOI] [PubMed] [Google Scholar]
- [16].Liao T-C, Yang N, Ho K-Y, Farrokhi S, Powers CM, Femur Rotation Increases Patella Cartilage Stress in Females with Patellofemoral Pain, Medicine and science in sports and exercise 47(9)(2015)1775–1780. [DOI] [PubMed] [Google Scholar]
- [17].Macri EM, Stefanik JJ, Khan KM, Crossley KM, Is tibiofemoral or patellofemoral alignment or trochlear morphology associated with patellofemoral osteoarthritis? A systematic review, Arthritis Care Res (Hoboken) 68(10)(2016)1453–1470. [DOI] [PubMed] [Google Scholar]
- [18].Macri EM, Culvenor AG, Morris HG, Whitehead TS, Russell TG, Khan KM, Crossley KM, Lateral displacement, sulcus angle and trochlear angle are associated with early patellofemoral osteoarthritis following anterior cruciate ligament reconstruction, Knee Surg Sports Traumatol Arthrosc (2017)1–8. [DOI] [PubMed] [Google Scholar]
- [19].Nagelli CV, Hewett TE, Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations, Sports Medicine 47(2)(2017)221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Insall J, Salvati E, Patella Position in the Normal Knee Joint 1, Radiology 101(1)(1971)101–104. [DOI] [PubMed] [Google Scholar]
- [21].Stefanik JJ, Zumwalt AC, Segal NA, Lynch JA, Powers CM, Association between measures of patella height, morphologic features of the trochlea, and patellofemoral joint alignment: the MOST study, Clin Orthop Relat Res 471(8)(2013)2641–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crossley K, Marino G, Macilquham M, Schache A, Hinman R, Can patellar tape reduce the patellar malalignment and pain associated with patellofemoral osteoarthritis?, Arthritis Rheum 61(12)(2009)1719–1725. [DOI] [PubMed] [Google Scholar]
- [23].Harbaugh CM, Wilson NA, Sheehan FT, Correlating femoral shape with patellar kinematics in patients with patellofemoral pain, Journal of Orthopaedic Research 28(7)(2010)865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stefanik JJ, Roemer FW, Zumwalt AC, Zhu Y, Gross KD, Lynch JA, Frey-Law LA, Lewis CE, Guermazi A, Powers CM, Association between measures of trochlear morphology and structural features of patellofemoral joint osteoarthritis on MRI: the MOST study, Journal of Orthopaedic Research 30(1)(2012)1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW, Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score), Osteoarthritis Cartilage 19(8)(2011)990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Runhaar J, Schiphof D, van Meer B, Reijman M, Bierma-Zeinstra S, Oei E, How to define subregional osteoarthritis progression using semi-quantitative MRI Osteoarthritis Knee Score (MOAKS), Osteoarthritis Cartilage 22(10)(2014)1533–1536. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Y, Niu J, DT Felson, Choi HK, Nevitt M, Neogi T, Methodologic challenges in studying risk factors for progression of knee osteoarthritis, Arthritis Care Res (Hoboken) 62(11)(2010)1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD, Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure, J Orthop Sports Phys Ther 28(2)(1998)88–96. [DOI] [PubMed] [Google Scholar]
- [29].Crossley KM, Macri EM, Cowan SM, Collins NJ, EM Roos, The patellofemoral pain and osteoarthritis subscale of the KOOS (KOOS-PF): development and validation using the COSMIN checklist, Br J Sports Med (2017)bjsports-2016-096776. [DOI] [PubMed] [Google Scholar]
- [30].Macri EM, Felson DT, Zhang Y, Guermazi A, Roemer FW, Crossley KM, Khan KM, Stefanik JJ, Patellofemoral morphology and alignment: reference values and dose-response patterns for the relation to MRI features of patellofemoral osteoarthritis, Osteoarthritis Cartilage (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Patterson BE, Culvenor AG, Barton CJ, Guermazi A, Stefanik JJ, Morris HG, Whitehead TS, Crossley KM, Worsening Knee Osteoarthritis Features on Magnetic Resonance Imaging 1 to 5 Years After Anterior Cruciate Ligament Reconstruction, The American journal of sports medicine (2018)0363546518789685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ristanis S, Giakas G, Papageorgiou CD, Moraiti T, Stergiou N, Georgoulis AD, The effects of anterior cruciate ligament reconstruction on tibial rotation during pivoting after descending stairs, Knee Surg Sports Traumatol Arthrosc ll(6)(2003)360–365. [DOI] [PubMed] [Google Scholar]
- [33].Webster KE, Feller JA, Tibial rotation in anterior cruciate ligament reconstructed knees during single limb hop and drop landings, Clinical Biomechanics 27(5)(2012)475–479. [DOI] [PubMed] [Google Scholar]
- [34].Culvenor A, Perraton L, Guermazi A, Bryant A, Whitehead T, Morris H, Crossley K, Knee kinematics and kinetics are associated with early patellofemoral osteoarthritis following anterior cruciate ligament reconstruction, Osteoarthritis Cartilage 24(9)(2016)1548–1553. [DOI] [PubMed] [Google Scholar]
- [35].Lee TQ, Morris G, Csintalan RP, The influence of tibial and femoral rotation on patellofemoral contact area and pressure, J Orthop Sports Phys Ther 33(11)(2003)686–693. [DOI] [PubMed] [Google Scholar]
- [36].Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Whitehead TS, Morris HG, Crossley KM, Early patellofemoral osteoarthritis features one year after anterior cruciate ligament reconstruction: symptoms and quality of life at three years, Arthritis Care Res (Hoboken) 68(6)(2016)784–792. [DOI] [PubMed] [Google Scholar]
- [37].Callaghan M, Guney H, Reeves N, Bailey D, Doslikova K, Maganaris C, Hodgson R, Felson D, A knee brace alters patella position in patellofemoral osteoarthritis: a study using weight bearing magnetic resonance imaging, Osteoarthritis Cartilage 24(12)(2016)2055–2060. [DOI] [PubMed] [Google Scholar]
- [38].Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K, Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial, The American journal of sports medicine 42(5)(2014)1049–1057. [DOI] [PubMed] [Google Scholar]
- [39].Wang H-J, Ao Y-F, Jiang D, Gong X, Wang Y-J, Wang J, Yu J-K, Relationship between quadriceps strength and patellofemoral joint chondral lesions after anterior cruciate ligament reconstruction, The American journal of sports medicine 43(9)(2015)2286–2292. [DOI] [PubMed] [Google Scholar]
- [40].Muellner T, Kaltenbrunner W, Nikolic A, Mittlboeck M, Schabus R, Vecsei V, Anterior cruciate ligament reconstruction alters the patellar alignment, Arthroscopy 15(2)(1999)165–168. [DOI] [PubMed] [Google Scholar]
- [41].de Vasconcelos DP, de Paula Mozella A, de Sousa Filho PGT, Oliveira GC, Cobra HAdAB, Alteracões radiográficas femoropatelares na insuficiência do ligamento cruzado anterior, Revista brasileira de ortopedia 50(1)(2015)43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shin CS, Carpenter RD, Majumdar S, Ma CB, Three-dimensional in vivo patellofemoral kinematics and contact area of anterior cruciate ligament–deficient and–reconstructed subjects using magnetic resonance imaging, Arthroscopy: The Journal of Arthroscopic & Related Surgery 25(11)(2009)1214–1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.