Abstract
Parkinson's disease is a chronic neurodegenerative disorder characterized by the loss of dopamine neurons in the substantia nigra, decreased striatal dopamine levels, and consequent extrapyramidal motor dysfunction. We now report that minocycline, a semisynthetic tetracycline, recently shown to have neuroprotective effects in animal models of stroke/ischemic injury and Huntington's disease, prevents nigrostriatal dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Minocycline treatment also blocked dopamine depletion in the striatum as well as in the nucleus accumbens after MPTP administration. The neuroprotective effect of minocycline is associated with marked reductions in inducible NO synthase (iNOS) and caspase 1 expression. In vitro studies using primary cultures of mesencephalic and cerebellar granule neurons (CGN) and/or glia demonstrate that minocycline inhibits both 1-methyl-4-phenylpyridinium (MPP+)-mediated iNOS expression and NO-induced neurotoxicity, but MPP+-induced neurotoxicity is inhibited only in the presence of glia. Further, minocycline also inhibits NO-induced phosphorylation of p38 mitogen-activated protein kinase (MAPK) in CGN and the p38 MAPK inhibitor, SB203580, blocks NO toxicity of CGN. Our results suggest that minocycline blocks MPTP neurotoxicity in vivo by indirectly inhibiting MPTP/MPP+-induced glial iNOS expression and/or directly inhibiting NO-induced neurotoxicity, most likely by inhibiting the phosphorylation of p38 MAPK. Thus, NO appears to play an important role in MPTP neurotoxicity. Neuroprotective tetracyclines may be effective in preventing or slowing the progression of Parkinson's and other neurodegenerative diseases.
Parkinson's disease is a common neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra. The loss of dopaminergic afferents from the substantia nigra to the striatum and putamen results in extrapyramidal motor dysfunction, including tremor, rigidity, and bradykinesia (1). The signs and symptoms of Parkinson's disease can be treated with drugs that increase or enhance dopamine function, but these drugs fail to alter disease progression and most produce undesirable side effects, like motor fluctuations and dyskinesias (2). Several neurotoxins induce Parkinson's-like neuropathology in animals, including the neurotoxins 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (3). MPTP selectively destroys dopamine neurons in the substantia nigra, resulting in a Parkinson's-like syndrome in many species, including humans, monkeys, and mice (4, 5). After parenteral administration, MPTP readily enters the brain and is metabolized by astroglia to 1-methyl-4-phenylpyridinium (MPP+) (6). MPP+ is a substrate of the dopamine transporter and is concentrated in nigral dopamine neurons where it inhibits complex I of the mitochondrial electron transport chain, resulting in ATP depletion and subsequent cell death (6). This proposed mechanism of MPTP toxicity implies that dopamine neurons per se are the direct cellular targets of MPTP's neurotoxic action.
Recently, however, an important role for glial activation in MPTP neurotoxicity has been suggested by two observations. First, MPTP administration to mice results in a robust gliosis in the substantia nigra pars compacta (SNpc), which is accompanied by up-regulation of inducible NO synthase (iNOS) (7). Second, mice lacking the iNOS or neuronal NO synthase (nNOS) genes are relatively resistant to MPTP toxicity of dopamine neurons compared with wild-type littermates (7–11). Importantly, however, iNOS-deficient mice still manifest a marked reduction in striatal monoamine levels comparable to wild-type controls after MPTP administration (7, 11). These data, coupled with the demonstration that iNOS expression is up-regulated in the substantia nigra of Parkinson's patients, but not from age-matched controls (12), suggest that glial activation and the resulting release of NO (and perhaps other glial-derived neurotoxic substances) may contribute to the chronic neurodegenerative state that characterizes Parkinson's disease.
Minocycline is a semisynthetic second-generation tetracycline that exerts anti-inflammatory effects that are completely separate and distinct from its antimicrobial action (13). Clinical studies have shown that minocycline, and related tetracyclines, have beneficial anti-inflammatory activity and appear to be useful for treating both rheumatoid arthritis and osteoarthritis (14). Tetracyclines, like minocycline, have been reported to have a number of biological and pharmacological actions including an ability to inhibit matrix metalloproteases, superoxide production from neutrophils, and most recently, iNOS expression in human cartilage and murine macrophages (15–17). Minocycline, one of the more brain penetrable of the tetracyclines, has recently been shown to have neuroprotective effects in models of global and focal ischemia (18, 19). The minocycline-induced reduction in infarct size and increased survival of hippocampal neurons after focal or global ischemia, respectively, were accompanied by a reduced expression of IL-1β-converting enzyme (caspase 1), cyclooxygenase-2, and iNOS mRNA in affected brain regions. Furthermore, a recent report by Chen et al. (20) demonstrated that minocycline treatment delays mortality in the R6/2 mouse model of Huntington's disease, presumably by inhibiting caspase 1 and caspase 3 expression, as well as iNOS activity. We now report that oral administration of minocycline to mice effectively blocks MPTP-induced degeneration of dopamine neurons in the SNpc, almost completely preventing the loss of striatal dopamine and its metabilites. Minocycline treatment also inhibits MPP+-mediated iNOS expression in vivo and potently blocks NO-induced neurotoxicity in vitro. Thus, indirect and/or direct inhibition of NO-mediated neurotoxicity may underlie minocycline's neuroprotective properties. Minocycline and chemically related neuroprotective tetracyclines may be effective in preventing and/or treating Parkinson's disease.
Materials and Methods
Animals and Treatment.
Eight-week-old male C57BL/6 mice (Taconic Farms) were used in all experiments. Mice (5–7 per group) were administered minocycline (60, 90, or 120 mg/kg per day in 5% sucrose; Sigma) by oral gavage before, during, and after MPTP administration. An untreated control group and MPTP-only group were included. The MPTP-treated groups received four injections of MPTP-HCl (20 mg/kg, i.p.) in saline at 2-h intervals in a single day (four injections total) as described (7) and killed at 7 days after the last injection.
Tyrosine Hydroxylase (TH) Immunohistochemistry and Stereological Quantitation of TH-Positive Neurons.
After postfixation and cryoprotection in 30% sucrose/phosphate buffer, the brains were frozen in liquid nitrogen and sectioned serially (40 μm) through the entire midbrain. Tissue sections were incubated successively with rabbit polyclonal anti-TH antibody (1:2,500, Calbiochem), goat biotinylated-conjugated polyclonal anti-rabbit antibody (1:250; Vector Laboratories), and horseradish-peroxidase-conjugated avidin/biotin complex (Vector Laboratories). Sections were then exposed to diaminobenzidine for detection. To adequately quantify TH-positive neurons, we used the nuclear counterstain methyl green (Vector Laboratories) and the stereological method for counting TH-positive neurons as described by Triarhou et al. (21).
Measurement of Dopamine, 3,4-Dihydroxyphenylacetic Acid (DOPAC), and Homovanilic Acid (HVA) Levels in the Striatum and Nucleus Accumbens.
After treatment, the striatum and nucleus accumbens were dissected, frozen on dry ice, and stored at −70°C. HPLC with electrochemical detection was used to simultaneously measure the concentration of dopamine, DOPAC, and HVA in each sample (7, 11, 25).
Measurement of Minocycline and MPP+ Levels in the Midbrain.
Minocycline and MPP+ were determined in the brain samples by using liquid chromatography with mass spectral detection, which consisted of a Hewlett–Packard model 1100 liquid chromatograph with a Hewlett–Packard model 1946 mass selective detector. A gradient of increasing acetonitrile concentrations in water containing 0.05% trifluoroacetic acid was used to elute the samples from a Zorbax SB-C18, 4.6 × 75-mm column (Hewlett–Packard). The mass spectrometer was run in positive ion mode, fitted with an electrospray ion source, and tuned to select the molecular weights of 171.1 for MPP+ and 459.9 for minocycline.
Neuronal Cell Cultures and Assessment of Neuronal Viability.
Cerebellar granule neurons (CGN) were prepared from 8-day-old Sprague–Dawley rat pups (Harlan Breeders, Indianapolis) as described (22). Primary cultures of rostral mesencephalic neurons (RMN) dissected from embryonic day 15 rat embryos (Harlan Breeders) were prepared as described (23). Cultures were used 2 days after preparation. Neuron/glia cocultures were prepared by modification of a method described by McNaught and Jenner (24). Dopamine neurons in primary cultures were visualized by TH immunohistochemistry (23) and quantified by using a Leitz inverted microscope (×200).
Primary Culture of Astrocyte and Microglia Cells.
Briefly, rostral mesencephalic tissue was dissected from embryonic day 15 rat embryos (Harlan Breeders), minced, and incubated in 0.25% trypsin and 0.01% DNase I in PBS for 5 min at 37°C. Cells were resuspended in growth medium then plated in 75-cm2 flasks coated with poly-d-lysine at a density of 2.0 × 107 cells/flask. Mixed glial cultures were maintained in bicarbonate-buffered DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin and passaged twice before use.
Western Blot Analysis.
Western blot analysis was performed on brain extracts from selected regions and cell cytoplasmic extracts. Extracts were size-fractionated on a 4–12% polyacrylamide gradient gel (SDS/NuPAGE) and transferred onto nitrocellulose (Hybond N, Amersham Pharmacia). Blots were then probed with polyclonal or monoclonal antibodies, followed by a secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) and visualized by using enhanced chemiluminescence.
Results
MPTP-Induced Neurotoxicity of Midbrain Dopamine Neurons Is Blocked by Minocycline.
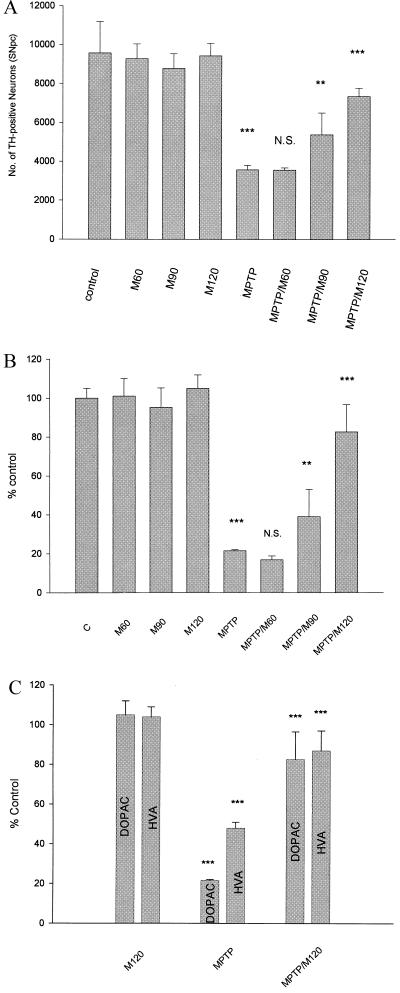
To investigate the neuroprotective effects of minocycline on MPTP-induced dopamine neuronal death in vivo, we treated C57BL/6 mice with minocycline (60, 90, and 120 mg/kg orally) daily for 9 days. On day 3, mice were administered MPTP (4 × 20 mg/kg, i.p.). Seven days after the last dose of MPTP, the brains were analyzed by immunohistochemistry to quantify TH-positive neurons in the SNpc. MPTP treatment reduced the number of TH-positive neurons by ≈63% compared with saline-treated controls (P < 0.001) (Figs. 1 and 2). Mice that received daily treatments of minocycline at either 90 or 120 mg/kg, and MPTP showed increased viable TH-positive neurons in the SNpc, ranging from 37% of control (no minocycline treatment) to 56% (90 mg/kg) and 77% (120 mg/kg) of control after minocycline treatment (P < 0.01 and P < 0.001, respectively) (Fig. 2). The neuroprotective effect of minocycline was dose-dependent as the 60 mg/kg dose of minocycline failed to protect dopamine neurons from MPTP toxicity (Figs. 1 and 2). Minocycline alone did not alters the number of TH-positive neurons significantly.
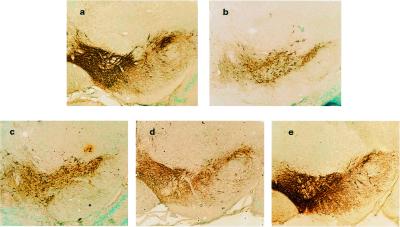
Figure 1.
Minocycline prevents loss of dopamine neurons after MPTP administration. Dopamine neurons and processes were identified by TH immunostaining of representative midbrain sections 7 days after MPTP treatment with or without treatment with minocycline (60, 90, and 120 mg/kg daily, see Materials and Methods for details). (a) dH2O. (b) MPTP treated. (c) MPTP treated after 2 days pretreatment with minocycline 60 mg/kg. (d) MPTP treated after 2 days pretreatment with minocycline 90 mg/kg. (e) MPTP treated after 2 days pretreatment with minocycline 120 mg/kg. Note the marked reduction in TH-positive cell bodies and processes after MPTP administration (compare a and b) and the protection by minocycline (b vs. e). Photomicrographs are from a representative experiment repeated three times with similar results.
Figure 2.
Minocycline prevents loss of TH-positive neurons, striatal dopamine, and dopamine metabolites after MPTP administration. (A) Quantification of TH-positive neurons in the SNpc was carried out as described in the text (21). Minocycline at 90 and 120 mg/kg significantly protects TH-positive neurons from death induced by MPTP exposure (one-way ANOVA; **, P < 0.01; ***, P < 0.001; N.S., not significant) (see text for details). (B and C) Dopamine, HVA, and DOPAC were measured by HPLC (see text and ref. 25 for details). Mice administered MPTP showed significant reductions in striatal dopamine, HVA, and DOPAC compared with controls. Minocycline treatment significantly protected animals from MPTP-induced reductions in dopamine, HVA, and DOPAC (one-way ANOVA; **, P < 0.01; ***, P < 0.001; N.S., not significant). See text for details. Each group consisted of 5–7 animals, and the data are from a representative experiment repeated at least twice with similar results.
Minocycline Blocks MPTP-Induced Loss of Striatal Dopamine and Its Metabolites.
We next measured striatal levels of dopamine and its metabolites, DOPAC, and HVA by HPLC with electrochemical detection. MPTP treatment reduced striatal dopamine, DOPAC, and HVA levels by 78%, 79%, and 52%, respectively. Minocycline treatment dose-dependently blocked the MPTP-induced decrease in striatal dopamine and dopamine metabolites. Mice that received 90 and 120 mg/kg of minocycline had striatal dopamine levels that were 39% and 83% of untreated controls, respectively, compared with only 22% in the MPTP (alone)-treated group (P < 0.01 and P < 0.001, respectively) (Fig. 2B). Minocycline pretreatment had a similar “protective” effect on striatal DOPAC and HVA levels after MPTP administration (Fig. 2C). Consistent with the quantitative data on SNpc dopamine neurons measured by TH immunoreactivity, treatment with 60 mg/kg of minocycline had no significant effect on striatal dopamine or dopamine metabolite levels after MPTP administration. Minocycline pretreatment also blocked the MPTP-induced decrease of dopamine, HVA, and DOPAC in the nucleus accumbens (data not shown).
Minocycline Protects Dopamine Neurons when Administered after MPTP.
We next treated animals with minocycline (120 mg/kg, orally) 4 h and 24 h after MPTP administration. Interestingly, minocycline treatment significantly protects against MPTP-induced dopamine neurotoxicity even 4 h after the last (or 12 h after the first) dose of MPTP. Mice that received minocycline beginning 4 h after MPTP treatment showed increased viable TH-positive neurons in the SNpc, ranging from 36% of control (no minocycline treatment) to 66% of control after minocycline (120 mg/kg) treatment (P < 0.05). Consistent with the quantitative data on SNpc dopamine neurons measured by TH immunoreactivity, minocycline treatment blocked the MPTP-induced decrease in striatal dopamine. Mice that received minocycline (120 mg/kg) had striatal dopamine levels that were 56% of untreated controls, compared with only 19% in the MPTP (alone)-treated group (P < 0.01). Minocycline posttreatment also blocked the MPTP-induced decrease of striatal dopamine metabolites (data not shown). Minocycline treatment, however, failed to protect dopamine neurons when administered 24 h after MPTP administration (data not shown).
Minocycline Does Not Alter Monoamine Oxidase (MAO) Activity Nor Brain MPP+ Levels.
Inhibitors of MAO-B have been found to prevent MPTP-induced neurotoxicity by blocking MPP+ formation in mouse brain (26). To confirm that the neuroprotective effects of minocycline we observed were not caused by decreased metabolism of MPTP to MPP+, we evaluated minocycline as an inhibitor of soluble rat brain MAO-A and MAO-B in vitro (26). We measured MAO-A and MAO-B activity in the presence and absence of minocycline and found that minocycline did not inhibit MAO-A at concentrations as high as 317 μM and MAO-B at concentrations up to 1 mM. By comparison, the mixed MAO-A and MAO-B inhibitor pargyline inhibited soluble rat brain MAO-A and MAO-B with pI50 values of 6.27 μM and 8.19 μM, respectively. Moreover, minocycline treatment had no effect on the concentration of MPP+ in the midbrain of MPTP-treated mice quantified by liquid chromatography with mass spectral detection. MPP+ levels in midbrain were 4.2 ± 0.8 μg/g in untreated or 4.8 ± 1 μg/g in minocycline (120 mg/kg)-treated 3 h after MPTP treatment (P = not significant). Furthermore, minocycline did not inhibit [3H]mazindol binding to membranes expressing human dopamine transporters (data not shown). These data suggest that the neuroprotective effect of minocycline is not caused by reduced metabolism of MPTP to MPP+ or reduced uptake of MPP+ into dopamine neurons.
Minocycline Blocks MPTP-Induced Expression of Midbrain iNOS and Caspase 1.
Because NO synthases and caspase 1 have recently been proposed to mediate (at least in part) MPP+-induced dopamine neuronal death (7–11, 27) and because minocycline has been shown to inhibit ischemia-induced iNOS and caspase 1 expression in brain (18, 19), we measured both iNOS and caspase 1 in midbrain homogeneates of mice treated with MPTP (Fig. 3). Three to 24 h after MPTP administration, both iNOS and caspase 1 were up-regulated in midbrain homogenates as determined by Western blots. Moreover, the latter was blocked by treatment with minocycline (Fig. 3). By contrast, neither MPTP or minocycline had any effect on nNOS expression in these same samples (Fig. 3A).
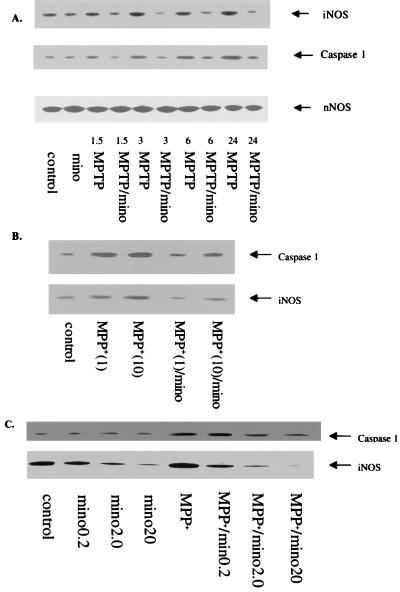
Figure 3.
(A) Minocycline blocks MPTP-induced expression of iNOS and caspase 1 in vivo and in vitro. Immunoblot analyses were performed with polyclonal antibodies against iNOS, nNOS, and caspase 1 (Santa Cruz Biotechnology). Minocycline doses and concentrations as well as the time course after MPTP or MPP+ administration exposure are indicated. MPTP treatment increases iNOS and caspase 1 expression by 3 h posttreatment. Minocycline treatment blocks the increase in both iNOS and caspase 1. Numbers (i.e., 1.5–24) represent the hours of treatment. Note that MPTP treatment fails to alter nNOS expression in these same samples. (B) Minocycline (20 μM) inhibits caspase 1 and iNOS expression induced by MPP+ (1 and 10 μM, 18 h) in primary cultures of mouse astrocytes. Astrocytes from neonatal mouse cerebral cortex were prepared as described (35). Lane 1 (left to right) = control; lane 2 = MPP+ (1 μM); lane 3 = MPP+ (10 μM); lane 4 = MPP+ (1 μM) minocycline (20 μM); and lane 5 = MPP+ (10 μM)/minocycline (20 μM). (C) Minocycline inhibits caspase 1 and iNOS expression induced by MPP+ in a mouse microglial cell line (BV2). BV2 cells (36) were cultured to near confluency and then treated with various concentrations of minocycline (0.2–20 μM) with and without MPP+ (10 μM, 18 h). Note that minocycline reduces basal iNOS expression in BV2 cells and completely blocks iNOS and caspase 1 expression induced by MPP+.
Minocycline Blocks MPP+-Induced Glial Expression of iNOS and Caspase 1 in Vitro.
To extend these in vivo data, we treated primary cultures of mouse astrocytes and BV2 cells (a mouse microglial cell line) with MPP+, with and without minocycline. Exposure of astrocytes or BV2 cells to MPP+ up-regulates both iNOS and caspase 1 expression as revealed by Western blots (Fig. 3 B and C). Pretreatment of cultures with minocycline 2 h before MPP+ treatment dose-dependently reduced MPP+-induced iNOS and caspase 1 expression in both astrocytes and microglia (Fig. 3 B and C).
Minocycline Blocks NO, but Not MPP+-Induced Neurotoxicity in Both CGN and RMN.
We next examined whether minocycline could directly block MPP+-induced toxicity of CGN. CGN represent a relatively homogenous population of neurons that contain ≤5% glia and have been previously shown to be killed by MPP+ exposure (28). CGN were exposed to MPP+ (70 μM) in the absence and presence of minocycline (10 and 50 μM), and cell viability was quantified 24 h later. Minocycline treatment had no effect on MPP+ toxicity of CGN (Fig. 4). Because it has been previously reported that both iNOS and nNOS knockout mice are resistant to MPTP neurotoxicity and that NO is able to potentiate MPP+-induced dopamine neuronal death in vitro (24), we examined whether minocycline could directly block NO-induced neurotoxicity of cultured neurons. Treatment of CGN or RMN with the NO donor sodium nitroprusside (SNP) results in a concentration-dependent cell death (Fig. 4 A and B). Remarkably, NO-induced neurotoxicity of CGN, was almost completely blocked by minocycline in a concentration-dependent manner (IC50 ≈1 μM, Fig. 4C).
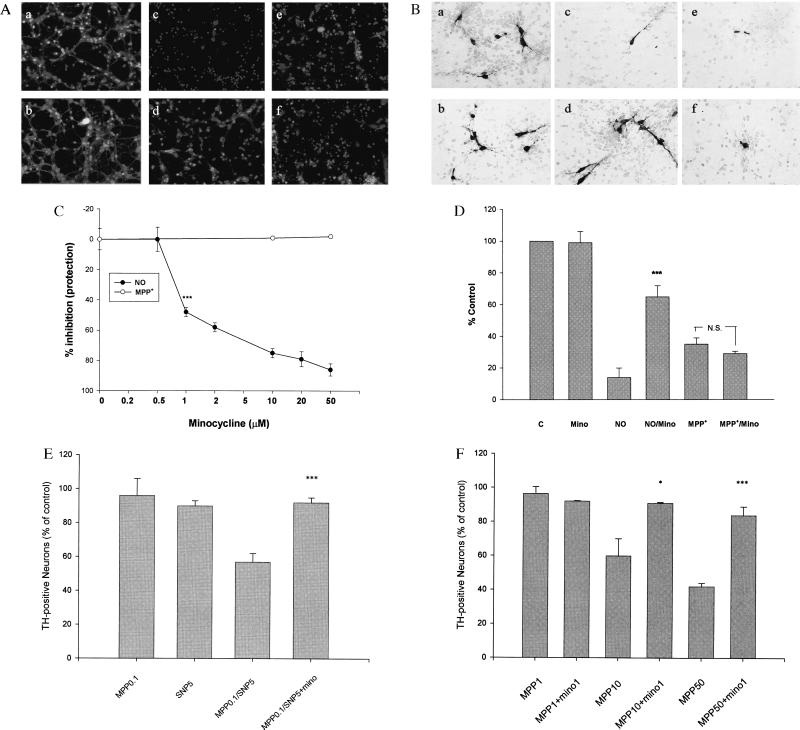
Figure 4.
Effects of minocycline on NO and MPP+ toxicity of cultured CGN and RMN. (A) Minocycline blocks NO-induced neuronal death of CGN, but not MPP+-induced neurotoxicity. CGN were exposed to increasing concentrations of minocycline (0.5–50 μM) for 24 h in the presence of SNP (50 μM, 24 h) or MPP+ (70 μM, 72 h). Viable and dead CGN were quantified by using fluorescein diacetate (yellow-green) and propidium iodide (red) staining as described (22). (a–f) Representative fields of CGN were photographed (×100) after double staining in the absence (a, c, and e) or presence (b, d, and f) of minocycline (20 μM). (a and b) No treatment. (c and d) SNP treatment. (e and f) MPP+ treatment. (C) Quantification of the effects of minocycline on MPP+-treated CGN. Values are expressed as a % of control (untreated) cultures for each concentration of minocycline. Data represent the mean ± SE (bars) values of triplicate determinations from a single but representative experiment repeated three times with similar results (***, P < 0.001 by one-way ANOVA; N.S., not significant). (B) Minocycline blocks NO-induced neuronal death of cultured RMN but not MPP+-induced neurotoxicity. (a–f) Representative fields of fetal RMN (20) were photographed (×200) after TH staining (see text for details). Compare untreated control and minocycline-treated cultures (a and b) with those exposed to 10 μM SNP (NO) (c) or 10 μM MPP+ (e) plus minocycline (10 μM) (d and f). Note that minocycline markedly attenuates NO neurotoxicity (d), but not MPP+ neurotoxicity (f). (D) Quantification of the effects of minocycline on SNP (10 μM) and MPP+ (10 μM)-treated fetal rat RMN. TH-positive cells were counted from photomicrographs like those shown in B above. Data are from a representative experiment repeated twice with similar results (***, P < 0.001 compared with NO alone). (E) Minocycline blocks combined NO/MPP+ toxicity of cultured CGN. Quantification of the effects of minocycline on both SNP (5 μM) and MPP+ (0.1 μM)-treated CGN. Data are from a representative experiment repeated three times with similar results [***, P < 0.001 compared with SNP (5 μM) and MPP+ (0.1 μM) alone]. (F) Minocycline blocks MPP+ neurotoxicity in neuron/glia cocultures. Quantification of the effects of minocycline on MPP+ (1–50 μM)-treated fetal rat RMN/glia cocultures (24). TH-positive cells were quantified from photomicrographs like those shown in B above. Note that in the presence of glia higher concentrations of MPP+ are required to kill dopamine neurons. Nonetheless, in the presence of glia the neurotoxic effects of MPP+ are completely blocked by minocycline. Data are from a representative experiment repeated twice with similar results. (*, P < 0.05; ***, P < 0.001 compared with MPP+ alone).
We next examined the NO-induced loss of dopamine (TH-positive) neurons in primary mesencephalic cultures (Fig. 4 B and D). Again, SNP treatment (10 μM) induced a ≥80% loss of dopamine neurons and the latter was blocked (≥75%) by minocycline (10 μM) (P < 0.01 compared with SNP-treated controls) (Fig. 4D). In separate experiments we showed that minocycline (at concentrations ≤10 μM) had no effect on the generation of NO from SNP under our culture conditions (data not shown). As in CGN, MPP+ toxicity of RMN was unaffected by minocycline treatment (Fig. 4 B and D).
Minocycline Blocks MPP+-Induced Neurotoxicity when Assessed in the Presence of Glia.
To further test our hypothesis that NO is involved in minocycline's protective effect against MPP+-induced neurotoxicity, we treated RMN with both subtoxic concentrations of SNP (5 μM) and MPP+ (0.1 μM) in the absence or presence of minocycline (10 μM). At these concentrations, neither toxin alone resulted in dopamine neuron death, whereas together ≈40% of dopamine neurons were killed (P < 0.01), and the latter was completely blocked by minocycline (Fig. 4E). Finally, to confirm the postulated role of glia in both MPP+ neurotoxicity and minocycline-induced neuroprotection, we treated cultures containing both glia and neurons with MPP+ and minocycline. As predicted, and in contrast to relatively pure neuronal cultures (Fig. 4B), minocycline blocks MPP+-induced dopamine neuronal death in mixed neuron/glia cocultures (Fig. 4F).
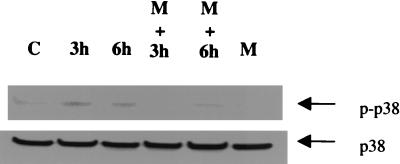
Minocycline Blocks NO-Induced Phosphorylation of p38 Mitogen-Activated Protein Kinase (MAPK) and an Inhibitor of p38 MAPK Blocks NO Toxicity of CGN.
Because Ghatan and colleagues (29) have recently shown that NO-induced apoptosis of neurons is associated with activation of p38 MAPK and that SB203580 (a p38 MAPK inhibitor) blocks NO neurotoxicity, we examined whether minocycline inhibits NO-induced phosphorylation of p38 MAPK in CGN. Pretreatment of CGN with minocycline completely blocks NO-induced p38 MAPK phosphorylation (Fig. 5) without affecting p38 MAPK protein concentration per se. Moreover, as previously reported for cultured cortical neurons (29), SB203580 blocks NO toxicity of CGN (data not shown).
Figure 5.
The effect of NO and minocycline on p38 MAPK phosphorylation in CGN. CGN were exposed to SNP (50 μM) in the absence or presence of minocycline (20 μM) for the indicated times (see text for details). Cell lysates were immunoblotted with anti-phospho-p38 and anti-p38 antibody (New England Biolabs). Note that the increase in phospho-p38 MAPK observed after NO (SNP) treatment is blocked by minocycline (Upper). No changes in p38 MAPK itself was observed (Lower). Similar results were obtained in three independent experiments. C = control, M = minocycline, p-p38 = phosphorylated p38 MAPK; 3 h and 6 h represent the treatment times of SNP.
Discussion
Our data demonstrate that minocycline can effectively protect midbrain dopamine neurons from the toxic effects of MPTP in vivo. Moreover, in contrast to data from iNOS knockout mice (7, 11) minocycline treatment results in a marked “protective effect” on the depletion of dopamine and its metabolites in the striatum and nucleus accumbens after MPTP administration. The neuroprotective effect of minocycline is observed after oral administration even though the oral bioavailability and penetration of minocycline into brain is relatively low in the mouse. However, the oral bioavailability of minocycline and other tetracyclines is considerably higher in humans (30). Both in vivo and in vitro data demonstrate that minocycline treatment inhibits MPTP/MPP+-induced iNOS and caspase 1 expression in astroglia and microglia. Because we demonstrate a more robust neuroprotective effect on striatal dopamine levels in minocycline-pretreated mice administered MPTP than that observed in iNOS knockout mice administered MPTP (7, 11), and because minocycline has no effect on nNOS expression (Fig. 3), we examined whether minocycline could directly inhibit NO-mediated neuronal death in vitro. We demonstrate that NO-induced neuronal death can be directly blocked by minocycline, and at relatively low concentrations (IC50 ≈ 1 μM). Moreover, the latter correlate with the brain levels of minocycline achieved after oral administration (midbrain minocycline levels were 0.32 ± 0.13 μg/g 8 h after treatment with 120 mg/kg). This finding, coupled with recent reports on reduced MPTP toxicity in both iNOS and nNOS knockout mice (7–11), or after treatment with NOS inhibitors (8–10), support an important role for NO in mediating MPTP toxicity. Because minocycline does not directly inhibit MPP+ neurotoxicity in vitro in the absence of glia (Fig. 4 A and B), but does so quite effectively in the presence of glia (Fig. 4 C and D), we argue that the neurotoxicity of MPTP/MPP+ observed in vivo is mediated (at least in part), indirectly, by NO generated from glial iNOS. We also show that subtoxic concentrations of NO and MPP+ can kill CGN when combined and that the latter is blocked by minocycline (Fig. 4C). It seems quite likely, however, that the dopamine transporter-mediated uptake and concentration of MPP+ into dopamine neurons, as well as subsequent inhibition of mitochondrial ATP biosynthesis, renders these neurons particularly vulnerable to NO toxicity (24). Synergistic toxic effects of NO (SNP) and MPP+ were observed in cultured RMN. Thus, we postulate that MPTP neurotoxicity is mediated by both a “direct” (ATP depletion) and “indirect” (NO-mediated) toxic effect on dopamine neurons. Indeed, our data confirm that minocycline is able to block MPP+-induced dopamine neuronal death in cultures containing both glia and neurons.
Recently, Koistinaho and colleagues (18, 19) have demonstrated neuroprotective effects of minocycline in rodent models of both focal and global ischemia. Infarct size, as well as markers of microglial activation, and the induction of iNOS, cyclooxygenase-2, prostaglandin E2 production, and IL-1β expression were significantly reduced even when minocycline treatment was administered 4 h postinsult. In addition, Chen et al. (20) have recently demonstrated a neuroprotective effect of minocycline in the R6/2 transgenic mouse model of Huntington's disease that was associated with inhibition of caspase 1 and 3. Taken together, these reports further suggest that minocycline exerts its neuroprotective effects by “indirectly” inhibiting glial activation and the subsequent release of NO and perhaps cytokines, such as IL-1β (18–20). Although it is likely that such “anti-inflammatory” actions of minocycline undoubtedly contribute to the neuroprotective properties we observe in the MPTP mouse model of Parkinson's disease, our data strongly suggest that minocycline also has a “direct” neuroprotective action as well. Very low concentrations of minocycline are effective in blocking NO toxicity in both CGN and RMN in vitro (Fig. 4).
Although the exact cellular mechanism(s) underlying minocycline's direct neuroprotective activity are unknown, we have also found that minocycline inhibits p38 MAPK phosphorylation/activity in CGN (Fig. 5) as well as microglia (data not shown). p38 MAPK, which is activated by a number of cellular “stresses,” has recently been implicated in neuronal cell death induced by axotomy (31) and excitotoxicity (32). Moreover, Ghatan and colleagues (29) have shown that p38 MAPK mediates neuronal apoptosis induced by NO, and that p38 MAPK inhibitors block NO toxicity of mouse cortical neurons in vitro. Our data, that minocycline treatment of CGN inhibits p38 MAPK activity and that the p38 MAPK inhibitor SB203580 protects CGN from NO toxicity, suggest that inhibition of p38 MAPK may mediate minocycline's direct neuroprotective effects against MPTP/MPP+ toxicity. Indeed, a very recent report has implicated glial p38 MAPK in the neuroprotective actions of minocycline observed against NMDA toxicity in vitro (33). However, our data suggest that minocycline does not directly inhibit p38 MAPK activity but rather inhibits enzyme activation indirectly through reducing phosphorylation, presumably by inhibiting an “upstream” kinase. Additional work will be required to delineate minocycline's exact cellular target(s).
Our findings support an important role for glial activation and NO production in the MPTP model of Parkinson's disease (8–10). Because iNOS expression is up-regulated in the SNpc of Parkinson's patients (12) suggests that a similar mechanism may contribute to the pathogenesis of Parkinson's disease. We caution, however, that MPTP administration, although reliably toxic to dopamine neurons in a variety of species, including humans, may not truly mimic either the etiology or pathophysiology of Parkinson's disease (34). Nevertheless, we also demonstrate that minocycline has robust neuroprotective activity in the MPTP mouse model of Parkinson's disease and provide evidence that this activity is caused by both indirect and direct actions in blocking NO-mediated neurotoxicity. Chemically modified tetracyclines, like minocycline, may prove effective in preventing and/or altering the progression of Parkinson's disease.
Acknowledgments
We thank Drs. Phil Skolnick, Wolfgang Oertel, James Clemens, H. Christian Fibiger, and Marcelle Bergeron for their critical reading of our manuscript.
Abbreviations
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- SNpc
substantia nigra pars compacta
- CGN
cerebellar granule neurons
- RMN
rostral mesencephalic neurons
- iNOS
inducible NO synthase
- nNOS
neuronal NO synthase
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanilic acid
- MAPK
mitogenactivated protein kinase
- TH
tyrosine hydroxylase
- MAO
monoamine oxidase
- SNP
sodium nitroprusside
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Oertel W H, Quinn N P. In: Neurological Disorders: Course and Treatment. Brandt T, Diener H C, Caplan L R, Kennard C, Dichgans J, editors. San Diego: Academic; 1996. pp. 715–772. [Google Scholar]
- 2.Quinn N P. Neurology. 1998;51:S25–S29. doi: 10.1212/wnl.51.2_suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 3.Tolwani R J, Jakowec M W, Petzinger G M, Green S, Waggie K. Lab Anim Sci. 1999;49:363–371. [PubMed] [Google Scholar]
- 4.Langston J W, Ballard J W, Tetrud J W, Irwin I. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 5.Uhl G R, Javitch J A, Snyder S H. Lancet. 1985;1:956–957. doi: 10.1016/s0140-6736(85)91729-5. [DOI] [PubMed] [Google Scholar]
- 6.Tipton K F, Singer T P. J Neurochem. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- 7.Liberatore G T, Jackson-Lewis V, Vukosavic S, Mandir A S, Vila M, McAuliffe W G, Dawson V L, Dawson T M, Przedborski S. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 8.Snyder S H. Nat Med. 1996;2:965–966. doi: 10.1038/nm0996-965. [DOI] [PubMed] [Google Scholar]
- 9.Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews R T, Beal M F. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 10.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson V L, Dawson T M. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz J B. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- 12.Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch E C. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 13.Ryan M E, Ashley R A. Adv Dent Res. 1998;12:149–151. doi: 10.1177/08959374980120011101. [DOI] [PubMed] [Google Scholar]
- 14.Ryan M E, Greenwald R A, Golub L M. Curr Opin Rheumatol. 1996;8:238–247. doi: 10.1097/00002281-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Golub L M, Lee H M, Ryan M E, Giannobile W V, Payne J, Sorsa T. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 16.Gabler W L, Smith J, Tsukuda N. Res Commun Chem Pathol Pharmacol. 1992;78:151–160. [PubMed] [Google Scholar]
- 17.Golub L M, Ramamurthy N S, Llavaneras A, Ryan M E, Lee H M, Liu Y, Bain S, Sorsa T. Ann NY Acad Sci. 1999;878:290–310. doi: 10.1111/j.1749-6632.1999.tb07691.x. [DOI] [PubMed] [Google Scholar]
- 18.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan P H, Koistinaho J. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Proc Natl Acad Sci USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Ona V O, Li M, Ferrante R J, Fink K B, Zhu S, Bian J, Guo L, Farrell L A, Hersch S M, et al. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 21.Triarhou L C, Norton J, Ghetti B. J Neurocytol. 1988;17:221–232. doi: 10.1007/BF01674209. [DOI] [PubMed] [Google Scholar]
- 22.Du Y, Dodel R C, Bales K R, Jemmerson R, Hamilton-Byrd E, Paul S M. J Neurochem. 1997;69:1382–1388. doi: 10.1046/j.1471-4159.1997.69041382.x. [DOI] [PubMed] [Google Scholar]
- 23.Dodel R C, Du Y, Bales K R, Ling Z D, Carvey P M, Paul S M. Neuroscience. 1998;86:701–707. doi: 10.1016/s0306-4522(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 24.McNaught K S, Jenner P. J Neurochem. 1999;73:2469–2476. doi: 10.1046/j.1471-4159.1999.0732469.x. [DOI] [PubMed] [Google Scholar]
- 25.Fuller R W, Perry K W. J Pharmacol Exp Ther. 1989;248:50–56. [PubMed] [Google Scholar]
- 26.Fuller R W, Warren B J, Molloy B M. Biochem Pharmacol. 1970;19:2934–2936. doi: 10.1016/0006-2952(70)90036-5. [DOI] [PubMed] [Google Scholar]
- 27.Klevenyi P, Andreassen O, Ferrante R J, Schleicher J R, Jr, Friedlander R M, Beal M F. NeuroReport. 1999;10:635–638. doi: 10.1097/00001756-199902250-00035. [DOI] [PubMed] [Google Scholar]
- 28.Marini A M, Paul S M. Proc Natl Acad Sci USA. 1992;89:6555–6559. doi: 10.1073/pnas.89.14.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, Youle R J, Morrison R S. J Cell Biol. 2000;150:335–347. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly R G, Kanegis L A. Toxicol Appl Pharmacol. 1967;11:171–183. doi: 10.1016/0041-008x(67)90036-1. [DOI] [PubMed] [Google Scholar]
- 31.Glicksman M A, Chiu A Y, Dionne C A, Harty M, Kaneko M, Murakata C, Oppenheim R W, Prevette D, Sengelaub D R, Vaught J L, Neff N T. J Neurobiol. 1998;35:361–370. [PubMed] [Google Scholar]
- 32.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 33.Tikka T M, Koistinaho J E. J Immunol. 2001;166:7527–7233. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- 34.Jenner P, Schapira A H, Marsden C D. Neurology. 1992;42:2241–2250. doi: 10.1212/wnl.42.12.2241. [DOI] [PubMed] [Google Scholar]
- 35.Dodel R C, Du Y, Bales K R, Gao F, Paul S M. J Neurochem. 1999;73:1453–1460. doi: 10.1046/j.1471-4159.1999.0731453.x. [DOI] [PubMed] [Google Scholar]
- 36.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]