Abstract
Background
In most western industrialised countries, second generation (atypical) antipsychotics are recommended as first‐line drug treatments for people with schizophrenia. In this review, we specifically examine how the efficacy and tolerability of one such agent ‐ aripiprazole ‐ differs from that of other comparable second generation antipsychotics.
Objectives
To review the effects of aripiprazole compared with other atypical antipsychotics for people with schizophrenia and schizophrenia‐like psychoses.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (November 2012), inspected references of all identified studies for further trials and contacted relevant pharmaceutical companies, drug approval agencies and authors of trials for additional information.
Selection criteria
We included all randomised clinical trials (RCTs) comparing aripiprazole (oral) with oral and parenteral forms of amisulpride, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone or zotepine for people with schizophrenia or schizophrenia‐like psychoses.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated risk ratios (RR) and their 95% confidence intervals (CI) on an intention‐to‐treat basis based on a random‐effects model. Where possible, we calculated illustrative comparative risks for primary outcomes. For continuous data, we calculated mean differences (MD), again based on a random‐effects model. We assessed risk of bias for each included study and used GRADE approach to rate quality of evidence.
Main results
We now have included 174 trials involving 17,244 participants. Aripiprazole was compared with clozapine, quetiapine, risperidone, ziprasidone and olanzapine. The overall number of participants leaving studies early was 30% to 40%, limiting validity (no differences between groups).
When compared with clozapine, there were no significant differences for global state (no clinically significant response, n = 2132, 29 RCTs, low quality evidence); mental state (BPRS, n = 426, 5 RCTs, very low quality evidence); or leaving the study early for any reason (n = 240, 3 RCTs, very low quality evidence). Quality of life score using the WHO‐QOL‐100 scale demonstrated significant difference, favouring aripiprazole (n = 132, 2 RCTs, RR 2.59 CI 1.43 to 3.74, very low quality evidence). General extrapyramidal symptoms (EPS) were no different between groups (n = 520, 8 RCTs,very low quality evidence). No study reported general functioning or service use.
When compared with quetiapine, there were no significant differences for global state (n = 991, 12 RCTs, low quality evidence); mental state (PANSS positive symptoms, n = 583, 7 RCTs, very low quality evidence); leaving the study early for any reason (n = 168, 2 RCTs, very low quality evidence), or general EPS symptoms (n = 348, 4 RCTs, very low quality evidence). Results were significantly in favour of aripiprazole for quality of life (WHO‐QOL‐100 total score, n = 100, 1 RCT, MD 2.60 CI 1.31 to 3.89, very low quality evidence). No study reported general functioning or service use.
When compared with risperidone, there were no significant differences for global state (n = 6381, 80 RCTs, low quality evidence); or leaving the study early for any reason (n = 1239, 12 RCTs, very low quality evidence). Data were significantly in favour of aripiprazole for improvement in mental state using the BPRS (n = 570, 5 RCTs, MD 1.33 CI 2.24 to 0.42, very low quality evidence); with higher adverse effects seen in participants receiving risperidone of general EPS symptoms (n = 2605, 31 RCTs, RR 0.39 CI 0.31 to 0.50, low quality evidence). No study reported general functioning, quality of life or service use.
When compared with ziprasidone, there were no significant differences for global state (n = 442, 6 RCTs, very low quality evidence); mental state using the BPRS (n = 247, 1 RCT, very low quality evidence); or leaving the study early for any reason (n = 316, 2 RCTs, very low quality evidence). Weight gain was significantly greater in people receiving aripiprazole (n = 232, 3 RCTs, RR 4.01 CI 1.10 to 14.60, very low quality evidence). No study reported general functioning, quality of life or service use.
When compared with olanzapine, there were no significant differences for global state (n = 1739, 11 RCTs, very low quality evidence); mental state using PANSS (n = 1500, 11 RCTs, very low quality evidence); or quality of life using the GQOLI‐74 scale (n = 68, 1 RCT, very low quality of evidence). Significantly more people receiving aripiprazole left the study early due to any reason (n = 2331, 9 RCTs, RR 1.15 CI 1.05 to 1.25, low quality evidence) and significantly more people receiving olanzapine gained weight (n = 1538, 9 RCTs, RR 0.25 CI 0.15 to 0.43, very low quality evidence). None of the included studies provided outcome data for the comparisons of 'service use' or 'general functioning'.
Authors' conclusions
Information on all comparisons is of limited quality, is incomplete and problematic to apply clinically. The quality of the evidence is all low or very low. Aripiprazole is an antipsychotic drug with an important adverse effect profile. Long‐term data are sparse and there is considerable scope for another update of this review as new data emerge from ongoing larger, independent pragmatic trials.
Plain language summary
Aripiprazole versus other atypical antipsychotics
In many countries in the industrialised world there has been a huge growth in the prescription of medication for people with mental health problems, taken orally as a tablet or by injection. Atypical and second generation antipsychotic drugs have become ever more popular, because they are thought to help people with mental health problems who do not respond quite so well to initial treatment. These newer drugs hold the promise of both reducing symptoms, such as hearing voices or seeing things, and reducing problematic side effects, such as sleepiness, weight gain, and shaking.
However, there is little research and comparison of the ways in which drugs differ from one another. This review examines the effectiveness of aripiprazole with other new antipsychotics. Originally the review included 12 research trials. After an update search carried out in November 2012, 162 trials were added. Most of these trials were from China and although new data were added to the review, overall conclusions did not change. The review now has five comparisons with aripiprazole being compared with clozapine, olanzapine, quetiapine, risperidone and ziprasidone.
For people with schizophrenia it may be important to know that aripiprazole may not be as good or effective as olanzapine but that it has less side effects. Aripiprazole is similar in effectiveness to risperidone and somewhat better than ziprasidone. Aripiprazole had less side‐ effects than olanzapine and risperidone (such as weight gain, sleepiness, heart problems, shaking and increased cholesterol levels). Aripiprazole was not as good as ziprasidone for dealing with restlessness or people’s inability to sit still. Comparison with other antipsychotic drugs as a group showed that people preferred taking aripiprazole. However, people with schizophrenia as well as mental health professionals and policy makers should know that the evidence is limited and mostly of low or very low quality. More trials and research is required, including on outcomes such as: quality of life; the views of service users and carers; and patient preference.
This plain language summary has been written by a consumer from Rethink Mental Illness, Benjamin Gray. Email: ben.gray@rethink.org
Summary of findings
Summary of findings for the main comparison. COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE for schizophrenia.
| COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: inpatient and outpatient Intervention: COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE | |||||
| Global state: No clinically significant response Follow‐up: up to 12 weeks | Low1 | RR 1.05 (0.87 to 1.27) | 2132 (29 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 100 per 1000 | 105 per 1000 (87 to 127) | |||||
| Moderate1 | ||||||
| 150 per 1000 | 157 per 1000 (131 to 190) | |||||
| High1 | ||||||
| 200 per 1000 | 210 per 1000 (174 to 254) | |||||
| Mental state: as measured by BPRS BPRS (high score = poor) | The mean mental state: as measured by BPRS in the intervention groups was 0.22 lower (1.44 lower to 1 higher) | 426 (5 studies) | ⊕⊝⊝⊝ very low2,3,4,5 | |||
| Leaving the study early ‐ Any reason Follow‐up: up to 12 weeks | Low1 | RR 1.41 (0.46 to 4.29) | 240 (3 studies) | ⊕⊝⊝⊝ very low2,3 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate1 | ||||||
| 50 per 1000 | 70 per 1000 (23 to 214) | |||||
| High1 | ||||||
| 100 per 1000 | 141 per 1000 (46 to 429) | |||||
| Quality of life: as measured by WHO‐QOL‐100 WHO‐QOL‐100 (low score = poor) Follow‐up: up to 12 weeks | The mean quality of life: as measured by WHO‐QOL‐100 in the intervention groups was 2.59 higher (1.43 to 3.74 higher) | 132 (2 studies) | ⊕⊝⊝⊝ very low6 | |||
| Adverse effects: extrapyramidal effects Follow‐up: up to 12 weeks | Low1 | RR 1.91 (0.75 to 4.85) | 520 (8 studies) | ⊕⊝⊝⊝ very low2,3,5 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate1 | ||||||
| 50 per 1000 | 96 per 1000 (38 to 242) | |||||
| High1 | ||||||
| 100 per 1000 | 191 per 1000 (75 to 485) | |||||
| General functioning ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Service use ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk: moderate risk approximately equates to that of the control group risk in the study population. 2 Risk of bias: rated 'serious' ‐ majority of the included studies had inadequate study design ‐ unclear randomisation, allocation concealment and blinding. Some also had selective reporting concerns. 3 Imprecision: rated 'serious' ‐ most of these included studies are of small samples with small effect size and wide confidence interval. The combined effect was not statistically significant. 4 Imprecision: rated 'serious' ‐ we were unable to obtain direct binary measure of mental state, thus used BPRS score as an indicator. 5 Publication bias: rated 'strongly suspected' ‐ only a small number of studies favouring intervention group were identified. 6 Indirectness: rated 'serious' ‐ we were unable to obtain direct binary measure of quality of life, thus employed WHO‐QOL‐100 rating score as an indicator.
Summary of findings 2. COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE for schizophrenia.
| COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: inpatient and outpatient Intervention: COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE | |||||
| Global state: No clinically significant response (as defined by original studies) | Low1 | RR 0.92 (0.64 to 1.32) | 991 (12 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 50 per 1000 | 46 per 1000 (32 to 66) | |||||
| Moderate1 | ||||||
| 100 per 1000 | 92 per 1000 (64 to 132) | |||||
| High1 | ||||||
| 150 per 1000 | 138 per 1000 (96 to 198) | |||||
| Mental state: as assessed by PANSS positive symptom scale score PANSS positive symptom subscale (high score = poor) Follow‐up: up to 12 weeks | The mean mental state: as assessed by PANSS positive symptom scale score in the intervention groups was 0.97 lower (2.34 lower to 0.41 higher) | 583 (7 studies) | ⊕⊝⊝⊝ very low4,5,6,7 | |||
| Leaving the study early Follow‐up: up to 12 weeks | Low1 | RR 0.8 (0.22 to 2.87) | 168 (2 studies) | ⊕⊝⊝⊝ very low2,8 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate1 | ||||||
| 50 per 1000 | 40 per 1000 (11 to 143) | |||||
| High1 | ||||||
| 100 per 1000 | 80 per 1000 (22 to 287) | |||||
| Quality of life: as measured by WHO‐QOL‐100 Follow‐up: up to 12 weeks | The mean quality of life: as measured by WHO‐QOL‐100 in the intervention groups was 2.6 higher (1.31 to 3.89 higher) | 100 (1 study) | ⊕⊝⊝⊝ very low8,9,10,11 | |||
| Adverse effects: extrapyramidal symptoms Follow‐up: up to 12 weeks | Low1 | RR 2.8 (0.64 to 12.31) | 348 (4 studies) | ⊕⊝⊝⊝ very low2,7,12,13 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate1 | ||||||
| 50 per 1000 | 140 per 1000 (32 to 616) | |||||
| High1 | ||||||
| 100 per 1000 | 280 per 1000 (64 to 1000) | |||||
| General functioning ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Service use ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk: moderate risk approximately equates to that of the control group risk in the study population. 2 Risk of bias: rated 'serious' – majority of the included studies had inadequate study design ‐ unclear randomisation, allocation concealment and blinding. A large proportion of them also had selective reporting concerns. 3 Imprecision: rated 'serious' – most of these included studies are of small samples with small effect size and wide confidence interval. The 95% confidence interval of the combined estimated effect was not statistically significant. 4 Imprecision and risk of bias: rated 'serious' ‐ most of the studies included are of small sample size, the overall pooled estimate of effect is not significant. 5 Inconsistency: rated 'serious' ‐ unexplained heterogeneity is high (70%). 6 Indirectness: rated 'serious' ‐ we were unable to obtain direct binary measure of mental state, thus used the best approximate measure available as an indicator. 7 Publication bias: rated 'strongly suspected' ‐ only small number of studies were identified ‐ publication bias likely. 8 Imprecision and publication bias: rated 'serious' ‐ only small number of studies with poor methodological design favouring intervention group were identified ‐ publication bias likely 9 Risk of bias: rated 'serious' – the only included study has unclear study design (randomisation, allocation concealment, blinding were unclear) and concerns of selective reporting. 10 Indirectness: rated 'serious' ‐ we are unable to obtain a direct binary measure of quality of life, thus used the best available proximate measure of WHO‐QOL‐100 as an indicator. 11 Imprecision: rated 'very serious' ‐ only one study is available on this outcome. The sample size is small and the CI of effect estimate is wide. 12 Inconsistency: rated 'serious' ‐ unexplained heterogeneity is around 53%. 13 Imprecision: rated 'serious' ‐ overall event rate is small (<300).
Summary of findings 3. COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE for schizophrenia.
| COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: inpatient and outpatient Intervention: COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE | |||||
| Global state: No clinically significant response (as defined by the original studies) | Low1 | RR 1.08 (0.96 to 1.21) | 6381 (80 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 50 per 1000 | 54 per 1000 (48 to 61) | |||||
| Moderate1 | ||||||
| 100 per 1000 | 108 per 1000 (96 to 121) | |||||
| High1 | ||||||
| 150 per 1000 | 162 per 1000 (144 to 182) | |||||
| Mental state: as measured by BPRS BPRS (high score = poor) Follow‐up: up to 12 weeks | The mean mental state: as measured by BPRS in the intervention groups was 1.33 lower (2.24 to 0.42 lower) | 570 (5 studies) | ⊕⊝⊝⊝ very low4,5,6 | |||
| Leaving the study early Follow‐up: up to 12 weeks | Low1 | RR 1.02 (0.79 to 1.32) | 1239 (12 studies) | ⊕⊝⊝⊝ very low6,7 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate1 | ||||||
| 100 per 1000 | 102 per 1000 (79 to 132) | |||||
| High1 | ||||||
| 150 per 1000 | 153 per 1000 (119 to 198) | |||||
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Adverse effects: extrapyramidal symptoms Follow‐up: up to 12 weeks | Low1 | RR 0.39 (0.31 to 0.5) | 2605 (31 studies) | ⊕⊕⊝⊝ low2,8 | ||
| 100 per 1000 | 39 per 1000 (31 to 50) | |||||
| Moderate1 | ||||||
| 300 per 1000 | 117 per 1000 (93 to 150) | |||||
| High1 | ||||||
| 400 per 1000 | 156 per 1000 (124 to 200) | |||||
| General functioning ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Service use ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk: moderate risk approximately equates to that of the control group risk of the study population. 2 Risk of bias: rated 'serious' ‐ majority of the included studies had inadequate study design ‐ unclear randomisation, allocation concealment and blinding. A large proportion of them also had selective reporting concerns. 3 Imprecision: rated 'serious' ‐ 95% CI around the pool estimate of effect was not statistically significant. 4 Risk of bias: rated 'serious' ‐ only a small number of publications with poor methodological design favouring intervention group were identified ‐ publication bias likely. 5 Indirectness: rated 'serious' ‐ we were unable to find direct binary measure of mental state, thus used the best available data as an indicator. 6 Publication bias: rated 'strongly suspected' ‐ overall event number is small (<300) and the pooled effect estimate is not statistically significant. 7 Imprecision: rated 'serious' ‐ overall event number is small (<300). 8 Publication bias: rated 'strongly suspected' ‐ most of the studies identified were of poor methodological quality favouring intervention group ‐ publication bias likely.
Summary of findings 4. COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE for schizophrenia.
| COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: inpatient and outpatient Intervention: COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE | |||||
| Global state: No clinically significant response (as defined by the original studies) | Low1 | RR 0.97 (0.62 to 1.52) | 442 (6 studies) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 100 per 1000 | 97 per 1000 (62 to 152) | |||||
| Moderate1 | ||||||
| 150 per 1000 | 146 per 1000 (93 to 228) | |||||
| High1 | ||||||
| 200 per 1000 | 194 per 1000 (124 to 304) | |||||
| Mental state: as measured with BPRS BPRS (high score = poor) Follow‐up: up to 12 weeks | The mean mental state: as measured with BPRS in the intervention groups was 2.2 lower (4.97 lower to 0.57 higher) | 247 (1 study) | ⊕⊝⊝⊝ very low5,6,7 | |||
| Leaving the study early Follow‐up: up to 12 weeks | Low1 | RR 0.94 (0.66 to 1.34) | 316 (2 studies) | ⊕⊝⊝⊝ very low2 | ||
| 150 per 1000 | 141 per 1000 (99 to 201) | |||||
| Moderate1 | ||||||
| 250 per 1000 | 235 per 1000 (165 to 335) | |||||
| High1 | ||||||
| 350 per 1000 | 329 per 1000 (231 to 469) | |||||
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Adverse effects: weight gain Follow‐up: up to 12 weeks | Low1 | RR 4.01 (1.1 to 14.6) | 232 (3 studies) | ⊕⊝⊝⊝ very low2,3,8 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate1 | ||||||
| 40 per 1000 | 160 per 1000 (44 to 584) | |||||
| High1 | ||||||
| 100 per 1000 | 401 per 1000 (110 to 1000) | |||||
| General functioning ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Service use ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk: moderate risk approximately equates to that of the control group risk in the study population. 2 Risk of bias: rated 'serious' ‐ majority of the included studies had inadequate study design ‐ unclear randomisation, allocation concealment and blinding. A large proportion of them also had selective reporting concerns. 3 Imprecision: rated 'serious' ‐ the total event number is small (<300) and that the 95% CI of the pooled best estimate of effect is not statistically significant. 4 Publication bias: rated 'strongly suspected' ‐ only a small number of trials with small sample size were identified. It is likely that these trials with low methodological quality would have exaggerated intervention effect. 5 Risk of bias: rated 'serious' ‐ the only included study has serious concern with selective reporting and unclear allocation concealment and incomplete outcome. 6 Indirectness: rated 'serious' ‐ we were unable to find direct binary measure of mental state, thus used BPRS score as an indicator. 7 Imprecision: rated 'serious' ‐ only one study is identified, the estimate of effect is not statistically significant. 8 Publication bias: rated 'strongly suspected' ‐ only small number of trials with poor methodological quality were identified ‐ publication bias likely.
Summary of findings 5. COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE for schizophrenia.
| COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: inpatient and outpatient Intervention: COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE | |||||
| Global state: No clinically significant response Follow‐up: up to 12 weeks | Low1 | RR 1.06 (0.96 to 1.17) | 1739 (11 studies) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 200 per 1000 | 212 per 1000 (192 to 234) | |||||
| Moderate1 | ||||||
| 350 per 1000 | 371 per 1000 (336 to 409) | |||||
| High1 | ||||||
| 500 per 1000 | 530 per 1000 (480 to 585) | |||||
| Mental state: as measured with PANSS PANSS (high score = poor) Follow‐up: up to 12 weeks | The mean mental state: as measured with PANSS in the intervention groups was 0.61 higher (0.23 lower to 1.46 higher) | 1500 (11 studies) | ⊕⊝⊝⊝ very low2,5,6,7 | |||
| Leaving the study early Follow‐up: up to 12 weeks | Low1 | RR 1.15 (1.05 to 1.25) | 2331 (9 studies) | ⊕⊕⊝⊝ low2,7 | ||
| 200 per 1000 | 230 per 1000 (210 to 250) | |||||
| Moderate1 | ||||||
| 350 per 1000 | 402 per 1000 (367 to 438) | |||||
| High1 | ||||||
| 500 per 1000 | 575 per 1000 (525 to 625) | |||||
| Quality of life: as measured with GQOLI‐74 GQOLI‐74 (low score = poor) | The mean quality of life: as measured with gqoli‐74 in the intervention groups was 1.26 lower (6.37 lower to 3.85 higher) | 68 (1 study) | ⊕⊝⊝⊝ very low7,8,9,10 | |||
| Adverse effects: weight gain Follow‐up: up to 12 weeks | Low1 | RR 0.25 (0.15 to 0.43) | 1538 (9 studies) | ⊕⊝⊝⊝ very low2,3,7 | ||
| 100 per 1000 | 25 per 1000 (15 to 43) | |||||
| Moderate1 | ||||||
| 200 per 1000 | 50 per 1000 (30 to 86) | |||||
| High1 | ||||||
| 300 per 1000 | 75 per 1000 (45 to 129) | |||||
| General functioning ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| Service use ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study measured or reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk: moderate risk approximately equates to that of the control group risk of the study population. 2 Risk of bias: rated 'serious' ‐ majority of the included studies had inadequate study design ‐ unclear randomisation, allocation concealment and blinding. Some of them also had selective reporting concerns. 3 Imprecision: rated 'serious' ‐ total number of events is small (<300) and that the 95% CI of pooled estimate of effect is not statistically significant. 4 Publication bias: rated 'strongly suspected' ‐ only small number of trials with small sample size were identified. It is likely that these small studies with poor methodological quality have negatively affected on the estimate of treatment effect. 5 Indirectness: rated 'serious' ‐ we are unable to find direct binary measure of mental state, thus used PANSS score as an indicator. 6 Imprecision: rated 'serious' ‐ the overall pooled estimate of effect was not statistically significant and with wide confidence interval. 7 Publication bias: rated 'strongly suspected' ‐ only a small number of studies with poor methodological deign were identified ‐ publication bias likely. 8 Risk of bias: rated 'serious' ‐ unclear randomisation, allocation concealment, blinding and serious concern with selective reporting. 9 Indirectness: rated 'serious' ‐ we are unable to find direct binary measure of quality of life, thus used GQOLI‐74 score as an indicator. 10 Imprecision: rated 'very serious' ‐ only one study with serious methodological concerns was identified and the estimate of effect is not significant.
Background
Description of the condition
Schizophrenia is usually a chronic and disabling psychiatric disorder, which afflicts approximately one per cent of the population worldwide, affecting male and female patients in similar proportions. The annual incidence of schizophrenia averages 15 per 100,000 population and the risk of developing the illness over one's lifetime averages 0.7% (Tandon 2008). Its typical manifestations include 'positive' symptoms such as fixed, false beliefs (delusions) and perceptions without cause (hallucinations), 'negative' symptoms such as apathy and lack of drive, disorganisation of behaviour and thought, and catatonic symptoms such as mannerisms and bizarre posturing (Carpenter 1994). The degree of suffering and disability is considerable with 80% to 90% of those affected not working (Marvaha 2004) and up to 10% dying prematurely (Tsuang 1978). In the age group of 15 to 44 years, schizophrenia is among the top 10 leading causes of disease‐related disability in the world (WHO 2001). Conventional antipsychotic drugs, such as chlorpromazine and haloperidol, have traditionally been used as first line antipsychotics for people with schizophrenia (Kane 1993). The introduction and subsequent use of clozapine in the United States of America identified that clozapine seemed to be more effective, and was associated with fewer movement disorders than existing agents such as chlorpromazine (Kane 1988). These results boosted the development and marketing of new/second/atypical generation antipsychotics (SGAs).
Description of the intervention
There is no good definition of what constitutes an atypical/second generation antipsychotic, but they were initially said to differ from older generation drugs in that they did not cause movement disorders (catalepsy) in rats at clinically effective doses (Arnt 1998). The terms new or second generation to describe clozapine, a very old drug, are equally poor descriptors. According to treatment guidelines (APA 2004; Gaebel 2006), SGAs include drugs such as amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone and zotepine. It is unclear whether some old and inexpensive compounds such as sulpiride, perazine or even low‐dose chlorpromazine, have similar properties (Möller 2000). High expectations were raised for these SGAs as regards their alleged superiority in a number of areas such as compliance, cognitive functioning, negative symptoms, movement disorders, quality of life and efficacy in treatment‐resistant schizophrenia.
How the intervention might work
Aripiprazole is said to be the prototype of a new and third generation of antipsychotics; the so called dopamine‐serotonin system stabilisers. It is reported to exert its antipsychotic effects by acting as a partial agonist at D2 dopamine and 5‐HT1a serotonin receptors and as an agonist at 5‐HT2 serotonin receptors. It has been postulated that through the above receptor site actions, and hence dopamine and serotonin system stabilisation, a partial D2 agonist would be able to act as an antagonist in pathways where an abundance of dopamine was producing psychosis, yet it would stimulate receptors as an agonist at sites in which low dopaminergic tone would produce adverse effects (e.g. areas mediating motor movement and prolactin release (Rivas‐Vasquez 2003)). Aripiprazole, however, also has an affinity to other receptors including D3, D4, 5‐HT2c, 5HT7, alpha‐1 adrenergic and histamine receptors. This may explain adverse effects associated with this compound such as somnolence, headache, gastrointestinal upset and light headedness (FDA 2002). The recommended target dose for aripiprazole is 10‐15 mg per day (dose range 10‐30 mg/day). Phase III trials were initially conducted in Japan in 1995 and the drug was granted Approved Status by the FDA (USA) on the 15 November 2002 for the treatment of schizophrenia. Aripiprazole has since been licensed in most countries worldwide.
Why it is important to do this review
The debate as to how far the second generation antipsychotic drugs improve these outcomes compared to conventional antipsychotics continues (Duggan 2005) and results from recent studies were sobering (Jones 2006; Lieberman 2005). Nevertheless, in some parts of the world, particularly in western industrialised countries, SGAs have become the mainstay of treatment. Second generation antipsychotics also differ in terms of their costs. Amisulpride and risperidone, for example, are already generic in many countries. Therefore, the question as to whether they differ from each other in their clinical efficacy becomes increasingly important. In this review we aim to summarise evidence from randomised controlled trials comparing aripiprazole with other SGAs. This acts as a continuum to the comparisons previously published by EL‐Sayeh 2006, Leucht 2008 and Komossa 2009.
This review was published in early 2013 with a vast number of Chinese studies in awaiting classification, thus we have updated it again in June 2013.
Objectives
To review the effects of aripiprazole compared with other second generation/atypical antipsychotics for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We included both open and double‐blinded, randomised controlled trials. We included open trials as we felt that important data that could potentially have an impact on the results might otherwise be overlooked. Where a trial was described as "double‐blind" but it was only implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these 'implied randomisation' studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
We included people with schizophrenia and other types of schizophrenia‐like psychosis (e.g. schizophreniform and schizoaffective disorders), irrespective of the diagnostic criteria used. There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
1. Aripiprazole
Any oral form of application, any dose.
2. Other new/atypical antipsychotic drugs
These include amisulpride, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, zotepine: any oral or parenteral form of application, any dose.
Types of outcome measures
We grouped outcomes into the short term (up to 12 weeks), medium term (13‐26 weeks) and long term (over 26 weeks).
Primary outcomes
1. Global state
No clinically important response ‐ as defined by the individual studies (e.g. global impression less than much improved or less than 50% reduction on a rating scale) ‐ medium term
2. General functioning
No clinically important change in general functioning ‐ medium term
3. Adverse effects
Clinically important specific adverse effects ‐ medium term
Secondary outcomes
1. Global state
1.1 No clinically important change in global state (as defined by individual studies) 1.2 Relapse (as defined by the individual studies)
2. Mental state
2.1 No clinically important change in general mental state score 2.2 Average endpoint general mental score 2.3 Average change in general mental state score 2.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia) 2.5 Average endpoint specific symptom score 2.6 Average change in specific symptom score
3. Leaving the studies early
3.1 Any reason, adverse events, inefficacy of treatment
4. Quality of life/satisfaction with treatment
4.1 No clinically important change in general quality of life 4.2 Average endpoint general quality of life score 4.3 Average change in general quality of life score
5. General functioning
5.1 No clinically important change in general functioning,‐ short and long term 5.2 Average endpoint general functioning score 5.3 Average change in general functioning score
6. Cognitive functioning
6.1 No clinically important change in overall cognitive functioning 6.2 Average endpoint of overall cognitive functioning score 6.3 Average change of overall cognitive functioning score
7. Service use
7.1 Number of patients hospitalised
8. Adverse effects
8.1 Number of participants with at least one adverse effect 8.2 Clinically important specific adverse effects (cardiac effects, death, movement disorders, prolactin increase and associated effects, weight gain, effects on white blood cell count), short and long term 8.3 Average endpoint in specific adverse effects 8.4 Average change in specific adverse effects
9. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used the GRADE profiler to import data from Review Manager (RevMan) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' tables.
Global state
Mental state
Leaving the study early
Quality of Life
Adverse effects
General functioning
Service use
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
1. Update search
We searched the Cochrane Schizophrenia Group Trials Register (5 November 2012) using the phrase:
[ ( (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in title, abstract or index terms of REFERENCE) or ( (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in interventions of STUDY)]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
2. Previous electronic search
Please see Appendix 1.
Searching other resources
1. Reference searching
We inspected the reference lists of all studies identified in the search for more trials.
2. Personal contact
Where possible, we contacted the first author of each included study for missing information.
3. Drug companies
We contacted the manufacturers of all atypical antipsychotics included for additional data in the 2011 update.
Data collection and analysis
For previous data collection and analysis methods please see Appendix 2.
Selection of studies
Review author TS inspected the reports for this update. TS resolved any doubts by discussion with other review authors, and where there was still doubt, TS acquired the full article for further inspection. Once the full articles were obtained, TS decided whether the studies met the review criteria. Twenty per cent of the references were randomly checked by HXM for reliability. Any disagreements were resolved by discussion. For any persistent disagreement, TS sought further information from authors of studies and added these trials to the list of those awaiting assessment. (See also Figure 1, Figure 2 and Figure 3 for detailed flow chart of the selection process).
1.
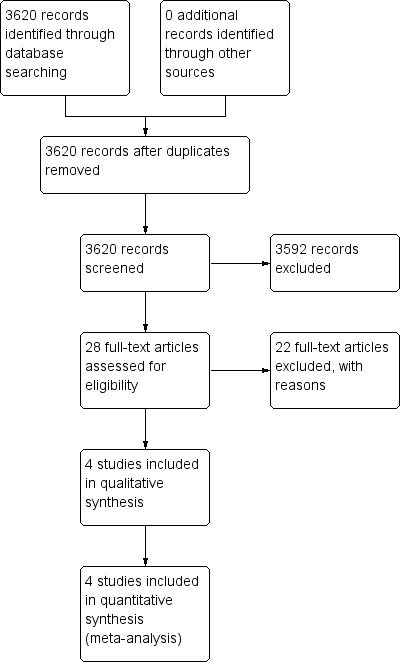
Study flow diagram: original search
2.
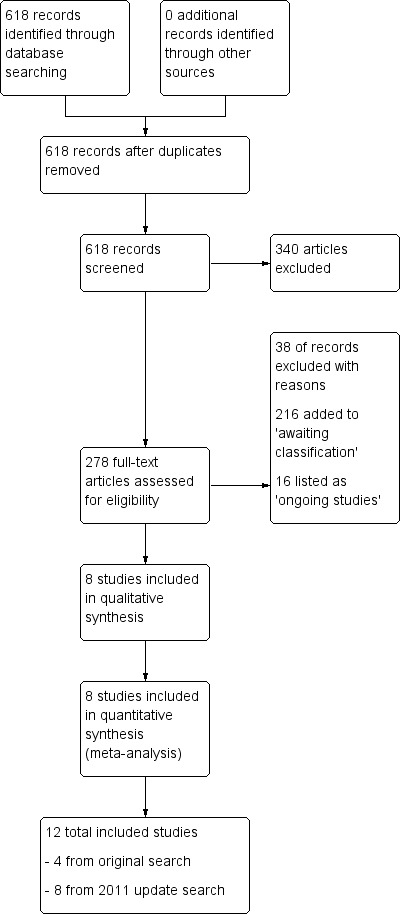
Study flow diagram: update 2011
3.
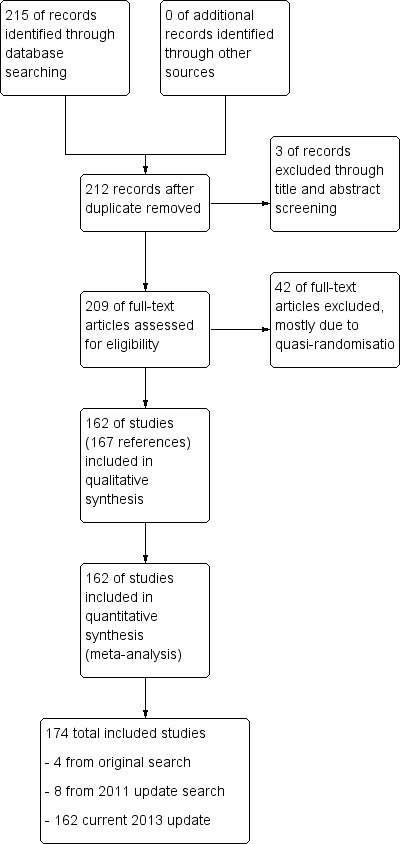
Study flow diagram: update 2012
Data extraction and management
1. Extraction
For this update, TS and HXM extracted data from included studies. If data were presented only in graphs and figures, TS and HXM extracted data whenever possible. When further information was necessary, TS contacted authors of studies in order to obtain missing data or for clarification. If studies were multicentre, where possible, TS extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a. the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we have noted whether or not this is the case in Description of studies.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011, Chapter 9.4.5.2).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion:
a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors;
b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996));
c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS, (Kay 1986)), which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score.
Endpoint scores on scales often have a finite start and end point and these rules can be applied. We entered skewed endpoint data from studies of fewer than 200 participants in as other data within the data and analyses tables rather than into an analysis. Skewed data pose less of a problem when looking at mean if the sample size is large; we entered such endpoint data into syntheses.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not; we entered skewed change data into analyses regardless of the size of study.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for aripiprazole.
Assessment of risk of bias in included studies
For this update, review author TS worked independently by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This new set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
Where inadequate details of randomisation and other characteristics of trials were provided we contacted the authors of the studies in order to obtain additional information.
We have noted the level of risk of bias in both the text of the review and in the Table 1; Table 2; Table 3; Table 4; Table 5.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The Number Needed to Treat/Harm (NNT/H) statistic with its CI is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' tables, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated the mean difference (MD) between groups. We would prefer not to calculate effect size measures (standardised mean difference (SMD). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
No cluster‐randomised trials were identified in our search; however, if reviews in the future include such trials, where clustering is not accounted for in primary studies, we will present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, we will seek to contact first authors of such studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering is been incorporated into the analysis of primary studies, we will present these data as if from a non cluster‐randomised study, but adjust for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If, in future updates of this review cluster trials are identified, cluster studies will be appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, if we encountered such trials, we planned only to use data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added these and combined them within the two‐by‐two table. If data were continuous, we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). Although high rates of premature discontinuation are a major problem in this field, we felt that it was unclear which degree of attrition leads to a high degree of bias. We, therefore, did not exclude outcomes on the basis of the percentage of participants completing them. However, we addressed the attrition problem in all parts of the review, including the abstract. For this purpose, we calculated, presented and commented on frequency statistics (overall rates of leaving the studies early in all studies and comparators pooled and their ranges). We assumed that the people who discontinued the studies for any reason did not show any response to the treatment.
2. Binary
We presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). We undertook a sensitivity analysis to test how prone the primary outcomes are to change when data only from people who complete the study to that point are compared with the ITTanalysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case of continuous outcomes, we preferred to use ITT results, but if not available we used completer data.
3.2 Standard deviations
If SDs were not reported, we first tried to obtain the missing values from the authors. If not available, where there are missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group means, and either a P value or T value available for differences in mean, we can calculate them according to the rules described in the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE* square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, T or F values, CIs, ranges or other statistics. If these formulae do not apply, we would have calculated the SDs according to a validated imputation method, which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless would have examined the validity of the imputations in a sensitivity analysis excluding imputed values, had we imputed any values.
3.3 Last observation carried forward
We anticipated that in many studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). We nevertheless used LOCF data being aware that many results are the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we discussed these fully.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we discussed these fully.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the ChI2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from ChI2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant ChI2 statistic was interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Handbook (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. Where possible, for both dichotomous and continuous data we used the random‐effects model for data synthesis.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses ‐ only primary outcomes
We did not anticipate any subgroup analyses.
2. Investigation of heterogeneity
If inconsistency was high, we reported this. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, then we did not pool data and discussed relevant issues. We know of no supporting research for this 10% cut‐off, but we used prediction intervals as an alternative to this unsatisfactory state. When unanticipated clinical or methodological heterogeneity was obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way so as to imply randomisation. For the primary outcomes, we included these studies and, if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we entered all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumptions and when we used data only from people who completed the study to that point. If there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumptions and when we used data only from people who completed the study to that point. A sensitivity analysis was undertaken to test how prone results were to change when completer‐only data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available): allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
We planned to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials. However, no cluster‐randomised trials were identified for this update.
In future updates, if cluster randomised trials are included we will undertake sensitivity analyses. If we note substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we will not pool data from the excluded trials with the other trials contributing to the outcome, but present them separately.
5. Skewed data
We planned sensitivity analyses a priori for examining the change in the robustness of the sensitivity to including studies with potentially skewed data.
6. Comparator dose
A recent report showed that some of the comparisons of atypical antipsychotics may have been biased by using inappropriate comparator doses (Heres 2006). We, therefore, also analysed whether the exclusion of studies with inappropriate comparator doses changed the results of the primary outcome and the general mental state.
Comparator doses were considered inappropriate where they exceeded BNF and Martindale recommended maximum doses (BNF 2013; Martindale 2013).
Aripiprazole: usual maintenance dose of 15 mg daily; range 10 to 15 mg once daily; maximum dose 30 mg daily.
Clozapine: usual dose 200 to 450 mg daily; maximum dose 900 mg daily.
Quetiapine: usual range 300 to 450 mg daily in two divided doses; maximum dose 750 mg daily.
Risperidone: usual dose 4 mg daily; maximum dose 50 mg every two weeks.
Ziprasidone: (oral) 20 mg twice daily, increased if necessary up to maximum 80 mg twice daily; usual maintenance dose 20 mg twice daily; (intramuscular (IM) for acute agitation) 10 to 20 mg as required; maximum dose 40 mg daily for three consecutive days (to switch to oral therapy as soon as possible).
Olanzapine: usual range 5 to 20 mg daily; maximum dose 20 mg daily.
Results
Description of studies
For a substantive description of studies please see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
1. Original search (2005/2007)
The first search yielded 3620 reports of which 28 were closely inspected. After excluding 22 studies, six publications on four trials and two comparisons could be included: aripiprazole versus olanzapine (two) and aripiprazole versus risperidone (two) (Komossa 2009) (Figure 1).
2. Updated search (November 2011)
The search we undertook for the first update of the review (Khanna 2013) identified 618 further results and, after close inspection, we included eight more studies; making a total of 12 studies altogether. We excluded 38 trials, added 216 trials to 'Studies awaiting classification' (due to the need for translation or data extraction) and 16 trials to 'Ongoing studies' category (Khanna 2013) (Figure 2).
3. Updated search (November 2012)
The 2012 updated search for this current version of the review yielded 215 new citations; 209 full‐text articles were assessed for eligibility, and ultimately 162 studies (from 167 references) were included. The total amount of included studies in this current review is 174 (Figure 3).
Included studies
The 174 included studies randomised 17,244 participants with the diagnosis of schizophrenia or schizoaffective disorder. All studies were described as randomised. We included both double blind and open label studies. Many were sponsored by pharmaceutical companies with some pecuniary interest in the result.
1. Length of studies
One trial was only five days long. The vast majority were short term with a duration of three to eight weeks. Seven medium‐term studies ranged from 20 to 26 weeks, and long‐term studies from 28 weeks to two years.
2. Setting
Studies reported inpatient and outpatient settings; the vast majority of studies were undertaken in China, where, it seems, aripiprazole is being heavily marketed.
3. Participants
Participants were diagnosed with varying diagnostic criteria; Diagnostic Statistical Manual version 4 (DSM‐IV); clinical diagnosis (or no mention). The vast majority of participants in included studies were diagnosed using the Chinese Classification of Mental Disorders (CCMD‐3). Participants were usually relatively chronically ill with mean ages in the late thirties.
4. Study size
The sample size varied from n = 40 (Zhang 2008b) to n = 1599 (Tandon 2006) people.
5. Interventions
5.1 Aripiprazole
Doses ranged between 2.5 to 30 mg/day.
5.2 Control drugs
Other atypical drugs, namely olanzapine, risperidone, ziprasidone and quetiapine were used as controls. As some studies did not elucidate doses it can only be presumed that therapeutic doses were employed.
6. Outcomes
6.1 Leaving the study early
Thirty‐five studies reported on participants leaving the study early due to any reason.
6.2 Rating scales
Details of scales that provided usable data are shown below. Reasons for exclusion of data from other instruments are given under 'Outcomes' in the Characteristics of included studies.
6.2.1 Global state scales
6.2.1.1 Clinical Global Impression Scale ‐ CGI Scale (Guy 1976) This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven‐point scoring system is used with low scores showing decreased severity and/or overall improvement.
6.2.1.2 Investigator's Assessment Questionnaire ‐ IAQ (Tandon 2005) The IAQ is a quantifiable clinical tool that can provide detailed information regarding common safety, efficacy and tolerability concerns that patients experience while taking antipsychotics. It has been shown to highly correlate with time to study discontinuation which is a common measure of effectiveness.
6.2.1.3 Arizona Sexual Experience Scale ‐ ASEX (McGahuey 2000) This is a brief scale for self‐rating of sexual function. It has five items and is rated in five steps. Possible scores range from five to 30 with higher scores indicating more sexual dysfunction.
6.2.2 Mental state scales
6.2.2.1 Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986) This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from one ‐ absent to seven ‐ extreme. It can be divided into three sub scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity.
6.2.2.2 Positive and Negative Syndrome Scale‐Excited Component ‐ PANSS‐EC (Chaichan 2008) The PANSS‐EC scale is derived as a sub scale of PANSS and is a simple scale used to measure the degree of agitation. The scale consists of five items (poor impulse control, tension, hostility, uncooperativeness, excitement), each being ranked from one to seven giving a potential maximum score of 35 points.
6.2.2.3 Brief Psychiatric Rating Scale – BPRS (Overall 1962) This consists of 18 to 24 items (depending on the version) each rated on a scale from one (absent) to seven (extreme).
6.2.3 Adverse effects scales
6.2.3.1 Abnormal Involuntary Movement Scale ‐ AIMS (Guy 1976) This scale has been used to assess tardive dyskinesia, a long‐term, drug‐induced movement disorder and short‐term movement disorders such as tremor.
6.2.3.2 Simpson Angus Scale ‐ SAS (Simpson 1970) This 10‐item scale (with a scoring system of zero to four on each item) measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of parkinsonism.
6.2.3.3 Barnes Akathisia Scale ‐ BAS (Barnes 1989) The scale comprises items rating the observable, restless movements that characterise akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from zero (normal) to three (severe). In addition, there is an item for rating global severity (from zero (absent) to five (severe)). A low score indicates low levels of akathisia.
6.2.4 Quality of Life
6.2.4.1 Euro‐QoL‐5D ‐ EQ‐5D (Jelsma 2001) The EuroQoL‐5D is a generic preference based measure of health‐related quality of life. It has five domains which are mobility, self‐care, usual activities, pain/ discomfort and anxiety/ depression, each having three short questions. The EQ‐5D Utility score assesses all five items on a scale of zero to one, where zero represents worst possible health and one represents perfect health. The EQ‐5D Health Dimension Scale assesses each item on three possible scores where one is the best and three the worst score.
6.2.4.2 Quality of Life Scale – QLS (Heinrichs 1984) Quality of Life Scale (QLS), a 21‐item scale divided into four domains including Interpersonal relations, Instrumental role, Intrapsychic foundations and common objects/activities scored on a seven‐point scale with lowest score indicating severe dysfunction.
6.2.4.3 Impact of Weight on Quality of Life ‐ IwQOL‐Lite (Kolotkin 2002) This is a survey instrument that is used to quantitatively assess an individual's perception of how their weight affects their day‐to‐day life. This instrument is especially valuable to obesity researchers, clinicians, psychologists, medical device and/or pharmaceutical companies seeking to validate the effectiveness of their treatments for obesity using metrics that go beyond the physical measurements of weight loss.
6.2.4.4 World Health Organisation Quality of Life Scale (WHOQoL‐Bref, O'Carroll 2000) The WHOQoL‐Bref is a 26‐item self‐report comprising satisfaction with health, psychological functioning, social relationships and environmental opportunities. Each item is scored on a five‐point scale from one (poor) to five (worse). The Chinese version of the WHOQoL scale, the generic quality of life inventory (GQOLI‐74), was also used in included Chinese studies.
6.2.1 Global state scales
6.2.1.1 Clinical Global Impression Scale ‐ CGI Scale (Guy 1976) This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven‐point scoring system is used with low scores showing decreased severity and/or overall improvement.
6.2.1.2 Investigator's Assessment Questionnaire ‐ IAQ (Tandon 2005) The IAQ is a quantifiable clinical tool that can provide detailed information regarding common safety, efficacy and tolerability concerns that patients experience while taking antipsychotics. It has been shown to highly correlate with time to study discontinuation which is a common measure of effectiveness.
6.2.1.3 Arizona Sexual Experience Scale ‐ ASEX (McGahuey 2000) This is a brief scale for self‐rating of sexual function. It has five items and is rated in five steps. Possible scores range from five to 30 with higher scores indicating more sexual dysfunction.
6.2.2 Mental state scales
6.2.2.1 Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986) This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from one ‐ absent to seven ‐ extreme. It can be divided into three sub scale for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity.
6.2.2.2 Positive and Negative Syndrome Scale‐Excited Component ‐ PANSS‐EC (Chaichan 2008) The PANSS‐EC scale is derived as a sub scale of PANSS and is a simple scale used to measure the degree of agitation. The scale consists of five items (poor impulse control, tension, hostility, uncooperativeness, excitement) each being ranked from one to seven giving a potential maximum score of 35 points.
6.2.2.3 Brief Psychiatric Rating Scale – BPRS (Overall 1962) This consists of 18 to 24 items (depending on the version) each rated on a scale from one (absent) to seven (extreme).
6.2.3 Adverse effects scales
6.2.3.1 Abnormal Involuntary Movement Scale ‐ AIMS (Guy 1976) This scale has been used to assess tardive dyskinesia, a long‐term, drug‐induced movement disorder and short‐term movement disorders such as tremor.
6.2.3.2 Simpson Angus Scale ‐ SAS (Simpson 1970) This 10‐item scale (with a scoring system of zero to four on each item) measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of parkinsonism.
6.2.3.3 Barnes Akathisia Scale ‐ BAS (Barnes 1989) The scale comprises items rating the observable, restless movements that characterise akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from zero (normal) to three (severe). In addition, there is an item for rating global severity (from zero (absent) to five (severe)). A low score indicates low levels of akathisia.
6.2.4 Quality of Life
6.2.4.1 Euro‐QoL‐5D ‐ EQ‐5D (Jelsma 2001) The EuroQoL‐5D is a generic preference based measure of health‐related quality of life. It has five domains which are mobility, self‐care, usual activities, pain/ discomfort and anxiety/ depression, each having three short questions. The EQ‐5D Utility score assesses all five items on a scale of zero to one, where zero represents worst possible health and one represents perfect health. The EQ‐5D Health Dimension Scale assesses each item on three possible scores where one is the best and three the worst score.
6.2.4.2 Quality of Life Scale – QLS (Heinrichs 1984) Quality of Life Scale (QLS), a 21‐item scale divided into four domains including Interpersonal relations, Instrumental role, Intrapsychic foundations and common objects/activities scored on a seven‐point scale with lowest score indicating severe dysfunction.
6.2.4.3 Impact of Wieght on Quality of Life ‐ IwQOL‐Lite (Kolotkin 2002) This is a survey instrument that is used to quantitatively assess an individual's perception of how their weight affects their day‐to‐day life. This instrument is especially valuable to obesity researchers, clinicians, psychologists, medical device and/or pharmaceutical companies seeking to validate the effectiveness of their treatments for obesity using metrics that go beyond the physical measurements of weight loss.
6.2.4.4 World Health Organization Quality of Life Scale (WHOQoL‐Bref, O'Carroll 2000)
The WHOQoL‐Bref is a 26‐item self‐report comprising satisfaction with health, psychological functioning, social relationships and environmental opportunities. Each item is scored on a five‐point scale from one (poor) to five (worse). The Chinese version of the WHOQoL scale, the generic quality of life inventory (GQOLI‐74), was also used in included Chinese studies.
6.3 Adverse effects
Adverse effects were mainly recorded in open interviews. In addition, continuous data were provided for weight, QTc time and cholesterol levels.
6.4 Missing outcomes
No information was provided on the number of people hospitalised or global functioning. This can be an important and useful measure of the efficacy of medications being used. Also, not one study reported on functional outcomes, such as living skills, ability to live independently or employment. These trials clearly are more explanatory than pragmatic, focusing on whether in ideal circumstances aripiprazole has an effect rather than whether it would be useful in everyday routine care (Thorpe 2009).
Excluded studies
There are a total of 79 excluded studies in this 2012 update. The most common reason for exclusion was because of lack of randomisation (see Characteristics of excluded studies).
Awaiting classification
Four studies are awaiting assessment (Wang 2006f; Zhao 2006a; Zheng XR 2008; 陶建青, 2007). Three of these were because of inconsistent reporting of denominators. Since we were unable to get clarification from the authors, we decided to leave these trials in Studies awaiting classification until further information becomes available. Please refer to Characteristics of studies awaiting classification for further details.
Ongoing studies
There are 16 studies in this category (see Characteristics of ongoing studies). There appeared to be much ongoing research activity in 2005 but completed studies have not yet been identified. We have contacted authors for current information but have received no updates.
Risk of bias in included studies
For details please refer to the 'Risk of bias' table for each study and Figure 4 and Figure 5 for the graphic overview.
4.
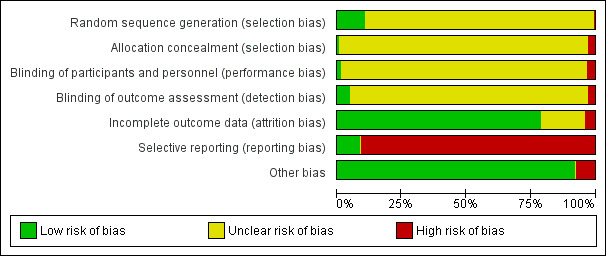
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The vast majority of included studies were rated as an 'unclear' risk of bias. Eighteen trials were rated as a 'low' risk of bias and described randomisation methods in some detail (Figure 5). Three of these used computer‐generated voice recognition systems. It remained unclear whether there was a risk of bias. Only one study described the method of allocation concealment ‐ by means of a sealed envelope (Wei 2006).
Blinding
Only 11 included studies were described as double blind (Chen 2007a; Du 2006; Feng 2006; Fleischhacker 2008; Guo 2006; Kane 2009; Kinon 2004; McQuade 2004; Potkin 2003; Xu 2010; Zimbroff 2007). Chan 2007 and Potkin 2003 described using identical capsules for blinding. Zimbroff 2007 used double dummy administration of medication. The other studies did not provide any information on the blinding procedure. No study examined whether blinding was effective. Six studies were open label (Chen 2009; Kern 2006; Kerwin 2007; Tandon 2006; Wlodzmierz 2006; Wolf 2007), and two described single‐blinding (Zhang 2009a; Zhu 2005). We found that the adverse effect profiles of examined compounds are quite different which may have made blinding difficult. We therefore conclude that the risk of bias for objective outcomes (e.g. death or laboratory values) was low but there was considerable risk of bias for subjective outcomes.
Incomplete outcome data
In two studies the rates of participants leaving the study early were higher than 30% (McQuade 2004; Potkin 2003); in McQuade 2004 it was as high as 72%. Overall attrition was about 35% to 40%. Last observation carried forward (LOCF) method to account for participants leaving the study early was used in studies that made clear attrition numbers, which we now know to be a far from ideal assumption (Leucht 2007). Only McQuade 2004, however, analysed the study completers in a secondary analysis assuming that a participant who left the study prematurely would not have had a change of condition if he/she had stayed in the study. This assumption can also be misleading. The majority of new data from Chinese studies states no incomplete data ‐ therefore, this domain is largely rated across studies as a 'low' risk of bias. Twenty‐eight studies did not mention or make explicit any participants lost through attrition, and so were rated as an 'unclear' risk of bias.
Selective reporting
In terms of selective reporting there was a high risk of bias in the majority of included studies. Overall, only 13 studies rated as a 'low' risk of bias, and only one rated as 'unclear', meaning that the remainder of the 174 included studies (160) were rated at a 'high' risk of bias with not all recorded outcomes reported. Six studies reported only adverse events that occurred in at least 5% to 10% of participants (Chan 2007; Fleischhacker 2008; Kane 2009; Potkin 2003; Zimbroff 2007). By this procedure rare, but important adverse events, can be missed. Kinon 2004 reported adverse effects that occurred in at least 1% of patients. McQuade 2004 did not report on the PANSS positive sub score despite having recorded it. See funnel plots for the primary outcome of no clinically significant response (Figure 6; Figure 7; Figure 8; Figure 9), where distrubution of studies is relatively symmetrical, except for comparison 5.1 (Figure 9), perhaps owing to less included studies.
6.
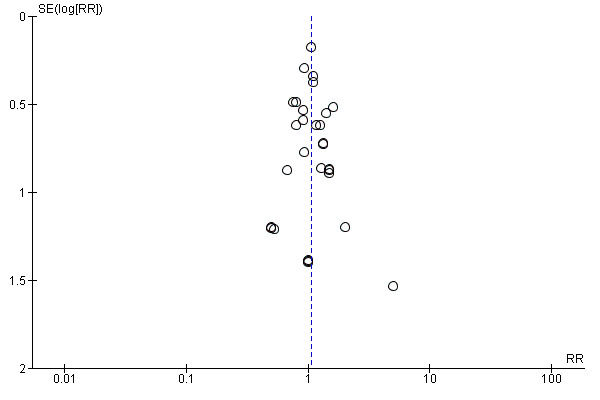
Funnel plot of comparison: 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, outcome: 1.1 Global state: 1. No clinically significant response (as defined by the original studies).
7.
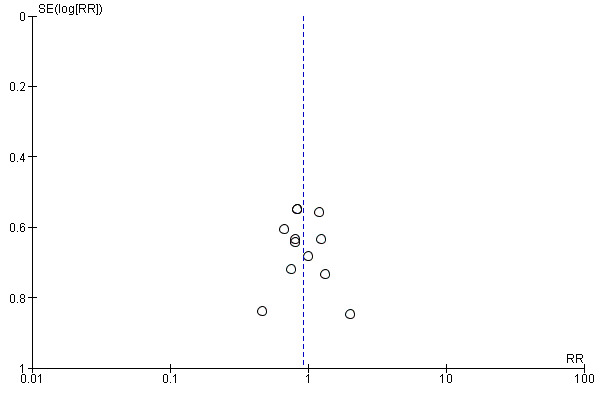
Funnel plot of comparison: 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, outcome: 2.1 Global state: 1.No clinically significant response (as defined by original studies).
8.
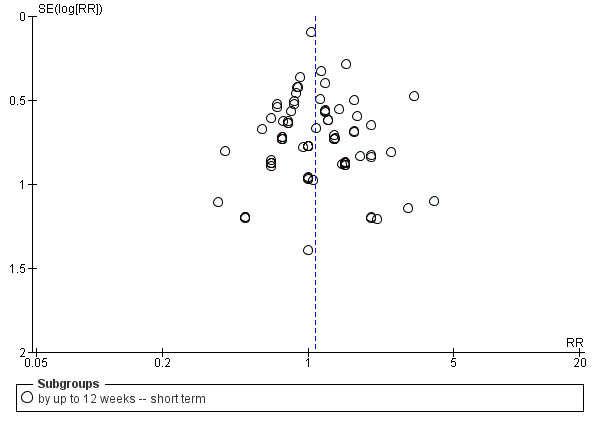
Funnel plot of comparison: 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, outcome: 3.1 Global state: 1. No clinically significant response (as defined by the original studies).
9.
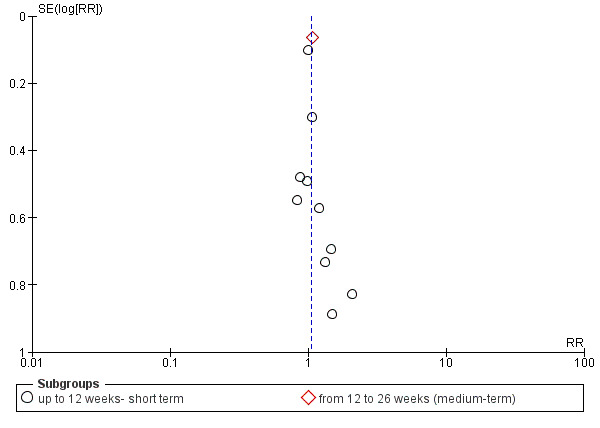
Funnel plot of comparison: 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, outcome: 5.1 Global state: 1.No clinically significant response (as defined by the original studies).
Other potential sources of bias
Many included studies were sponsored by the manufacturer of aripiprazole or the comparator drug. There is evidence that pharmaceutical companies may be selective in reporting the benefits and disadvantages of their own compounds (Heres 2006).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
We used risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data, with their respective 95% confidence intervals (CIs) throughout.
COMPARISON 1: ARIPIPRAZOLE versus CLOZAPINE
1.1 Global state: 1. No clinically significant response (as defined by the original studies)
Data demonstrate there was no difference between groups (29 RCTs, n = 2132, RR 1.05 CI 0.87 to 1.27, Analysis 1.1).
1.1. Analysis.
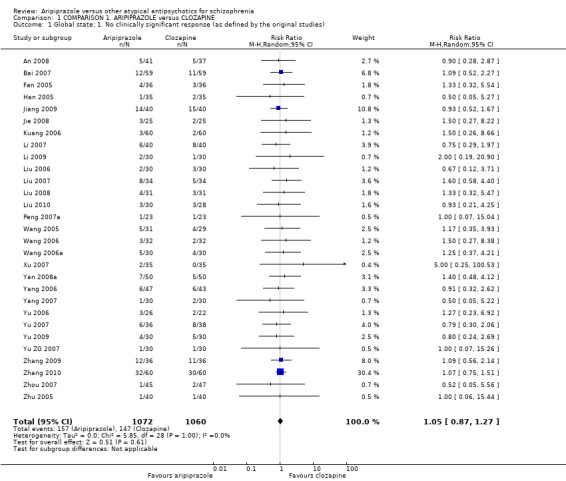
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 1 Global state: 1. No clinically significant response (as defined by the original studies).
1.2 Mental state: 1. Specific ‐ binary outcomes
1.2.1 Anxiety and agitation
In the short term, there was significant favour (P = 0.01) for clozapine with higher levels of anxiety seen in participants receiving aripiprazole (11 RCTs, n = 732, RR 2.62 CI 1.21 to 5.70), however, with a slight degree of heterogeneity (ChI2 = 13.34; df = 10; P = 0.21; I2 = 25%). Data demonstrate no difference between groups at medium term (1 RCT, n = 90). Data for levels of agitation at short term demonstrated no difference between groups (1 RCT, n = 60, Analysis 1.2).
1.2. Analysis.
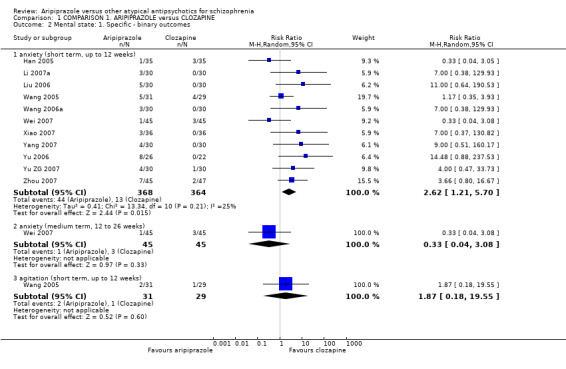
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 2 Mental state: 1. Specific ‐ binary outcomes.
1.3 Mental state: 2. Average endpoint scores of various scales (short term, up to 12 weeks, high = poor)
Data for all scales demonstrated no significant difference, including the BPRS (5 RCTs, n = 426); PANSS (23 RCTs, n = 1638), however with considerable heterogeneity present (ChI2 = 61.84; df = 22; P = 0.0; I2 = 64%); and SANS (1 RCT, n = 50, Analysis 1.3).
1.3. Analysis.
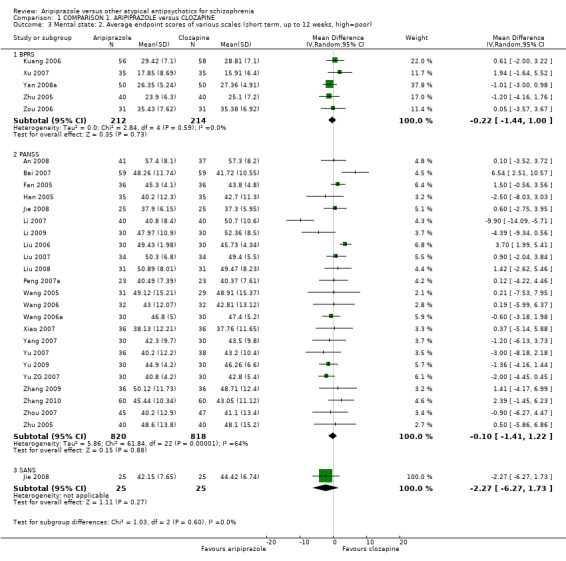
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 3 Mental state: 2. Average endpoint scores of various scales (short term, up to 12 weeks, high=poor).
1.4 Mental state: 3. Average endpoint scores of various scales (medium term, 12 to 26 weeks, high = poor)
When using PANSS, data from 3 RCTs significantly favoured (P = 0.0004) aripiprazole (n = 236, MD ‐5.41 CI ‐8.42 to ‐2.41, Analysis 1.4).
1.4. Analysis.
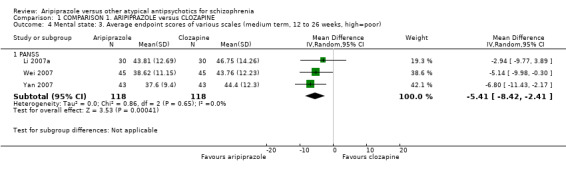
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 4 Mental state: 3. Average endpoint scores of various scales (medium term, 12 to 26 weeks, high=poor).
1.5 Mental state: 4. Average endpoint scores of various scales (skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 1.5.
1.5. Analysis.
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 5 Mental state: 4. Average endpoint scores of various scales (skewed data, high=poor).
| Mental state: 4. Average endpoint scores of various scales (skewed data, high=poor) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Notes |
| BPRS | |||||
| Xiao 2007 | Aripiprazole | 15.33 | 7.96 | 36 | |
| Xiao 2007 | Clozapine | 15.91 | 11.22 | 36 | |
| PANSS | |||||
| Jiang 2009 | Aripiprazole | 53.2 | 13.9 | 40 | |
| Jiang 2009 | Clozapine | 11.2 | 5.9 | 40 | |
| PANSS negative symptom subscale score | |||||
| Jiang 2009 | Aripiprazole | 16.4 | 6.5 | 40 | |
| Jiang 2009 | Clozapine | 11.2 | 5.9 | 40 | |
| Liu 2008 | Aripiprazole | 11.71 | 7.60 | 31 | |
| Liu 2008 | Clozapine | 11.82 | 6.77 | 31 | |
| Xiao 2007 | Aripiprazole | 6.31 | 5.24 | 36 | |
| Xiao 2007 | Clozapine | 13.37 | 5.15 | 36 | |
1.6 Mental state: 5. Specific ‐ average endpoint positive score (PANSS, high = poor)
Overall, there was no difference between groups at both short and medium term (22 RCTs, n = 1523, Analysis 1.6), however there was considerable heterogeneity present (ChI2 = 74.67; df = 21; P = 0.00001; I2 = 72%).
1.6. Analysis.
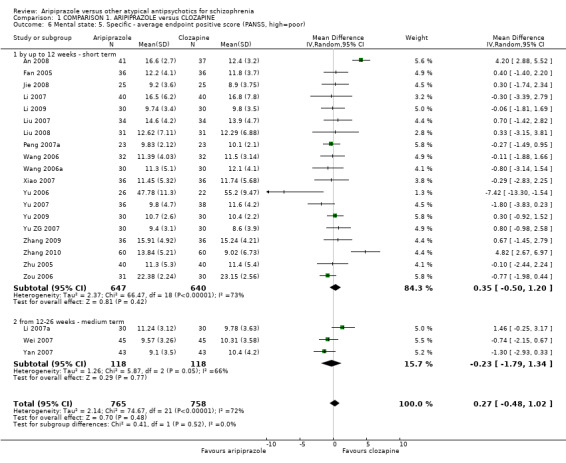
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 6 Mental state: 5. Specific ‐ average endpoint positive score (PANSS, high=poor).
By short term, data indicate no significant difference between groups (19 RCTs, n = 1287), however. considerable heterogeneity was present (ChI2 = 66.47; df = 18; P = 0.00001; I2 = 73%). Again, by the medium term, data indicate no significant difference between groups (3 RCTs, n = 236), however there was considerable heterogeneity present (ChI2 = 5.87; df = 2; P = 0.05; I2 = 66%).
1.7 Mental state: 6. Specific ‐ average endpoint negative score (PANSS, high = poor)
Overall, there was no significant difference between aripiprazole between short term and medium term (23 RCTs, n = 1640), however considerable heterogeneity was present (ChI2 = 114.66; df = 22; P = 0.0001; I2 = 81%).
Short‐term data indicate no significant difference between groups (20 RCTs, n = 1404), however there was considerable heterogeneity present (ChI2 = 51.61; df = 19; P = 0.0001; I2 = 63%). Medium‐term data significantly favoured aripiprazole (3 RCTs, n = 236, MD ‐3.24 CI ‐5.82 to ‐0.67, Analysis 1.7), however with considerable heterogeneity (ChI2 = 15.99; df = 2; P = 0.0003; I2 = 87%)
1.7. Analysis.
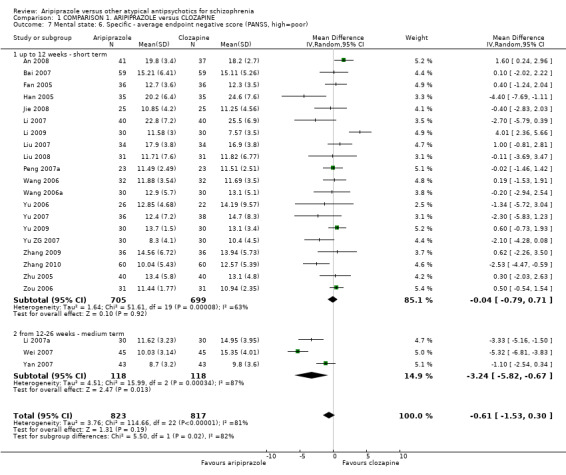
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 7 Mental state: 6. Specific ‐ average endpoint negative score (PANSS, high=poor).
1.8 Mental state: 7. Specific ‐ average endpoint general psychopathological score (PANSS, high = poor )
Overall, data indicate no significant difference between groups (19 RCTs, n = 1330, Analysis 1.8). Data indicate a significant difference (P = 0.02) favouring aripiprazole at medium term (3 RCTs, n = 236 MD ‐1.89 CI ‐3.45 to ‐0.34).
1.8. Analysis.
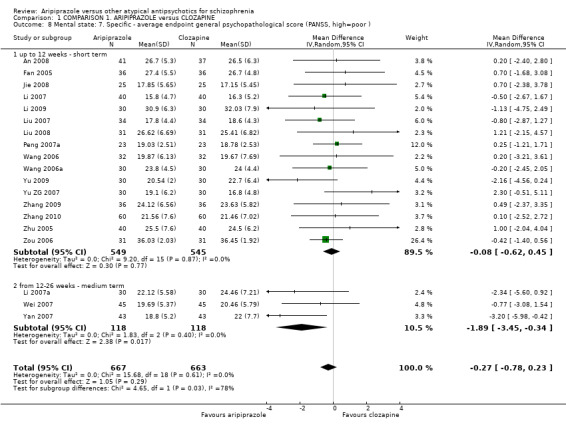
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 8 Mental state: 7. Specific ‐ average endpoint general psychopathological score (PANSS, high=poor ).
1.9 Mental state: 8. Specific ‐ average total score decreased rate (PANSS, low = poor)
1.9.1 By up to 12 weeks
Data in one small study indicate no significant difference between groups (1 RCT, n = 118, Analysis 1.9).
1.9. Analysis.
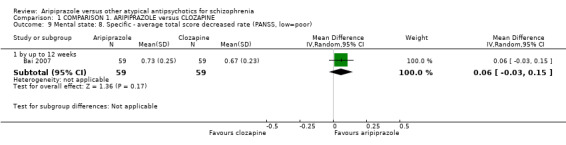
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 9 Mental state: 8. Specific ‐ average total score decreased rate (PANSS, low=poor).
1.10 Mental state: 9. Specific ‐ average positive score decreased rate (PANSS, low = poor)
Short‐term data in one small study indicate no significant difference between groups (1 RCT, n = 118, Analysis 1.10).
1.10. Analysis.
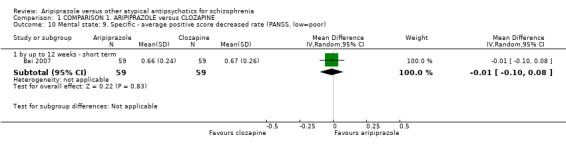
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 10 Mental state: 9. Specific ‐ average positive score decreased rate (PANSS, low=poor).
1.11 Leaving the study early
Data demonstrate no significant difference between groups when leaving the study for any reasons (3 RCTs, n = 240, Analysis 1.11). This was also the case for adverse events (3 RCTs, n = 212) and due to 'economic issues' (1 RCT, n = 120).
1.11. Analysis.
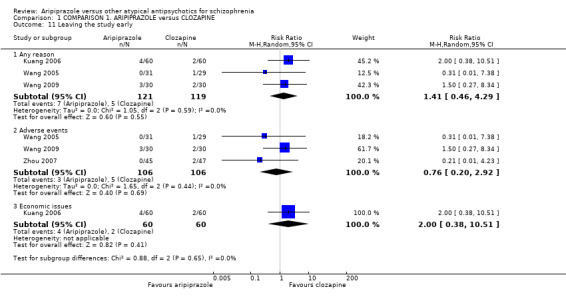
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 11 Leaving the study early.
1.12 Quality of life: 1a. Average scores (short term, up to 12 weeks, WHO‐QOL‐100, low = poor)
Data demonstrate statistical significance (P = 0.0001) in favour of aripiprazole in two small RCTs (n = 132, MD 2.59 CI 1.43 to 3.74, Analysis 1.12). On the physical health component, data demonstrate statistical significance (P = 0.01) in favour of aripiprazole in two small RCTs (n = 132, MD 7.73 CI 1.51 to 13.94), however with considerable heterogeneity present (ChI2 = 6.04; df = 1; P = 0.014; I2 = 83%). Again, mental health component data demonstrate statistical significance (P = 0.0001) in favour of aripiprazole in two small RCTs (n = 132, MD 9.21 CI 4.74 to 13.68), however there was considerable heterogeneity present (ChI2 = 2.31; df = 1; P = 0.13; I2 = 57%). Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of aripiprazole for the spiritual support component (1 RCT, n = 72, MD 6.61 CI 4.25 to 8.97), and for external environment (P = 0.0001) (3 RCTs, n = 248, MD 13.82 CI 8.69 to 18.94) again with considerable heterogeneity present (ChI2 = 7.63; df = 2; P = 0.02; I2 = 74%). Data demonstrate no statistical significance for social function (2 RCTs, n = 132) again with considerable heterogeneity present (ChI2 = 23.23; df = 1; P = 0.00001; I2 = 96%) or independence (1 RCT, n = 72).
1.12. Analysis.
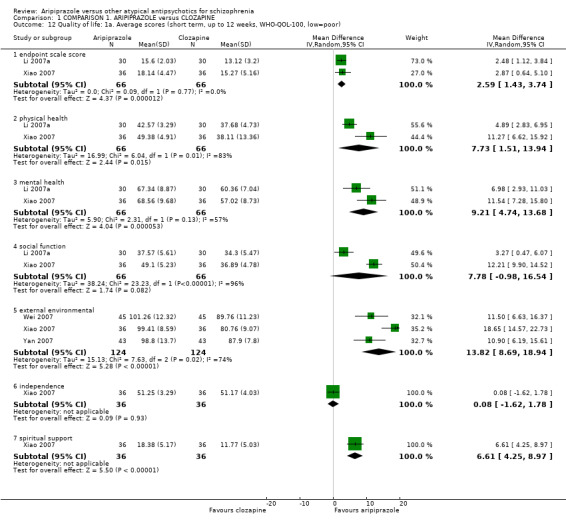
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 12 Quality of life: 1a. Average scores (short term, up to 12 weeks, WHO‐QOL‐100, low=poor).
1.13 Quality of life: 1b. Average scores (medium term, 12 to 24 weeks, WHO‐QOL‐100, low = poor)
The endpoint score demonstrated statistical significance (P = 0.00001) in favour of aripiprazole (2 RCTs, n = 176, MD 2.75 CI 1.98 to 3.53, Analysis 1.13). Data demonstrate statistical significance in the physical health component (P = 0.04) in favour of aripiprazole (3 RCTs, n = 256, MD 4.89 CI 0.22 to 9.56), however with considerable heterogeneity present (ChI2 = 33.90; df = 2; P = 0.00001; I2 = 94%). Mental health data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (3 RCTs, n = 256, MD 7.39 CI 5.26 to 9.53), again with considerable heterogeneity present (ChI2 = 2.6; df = 2; P = 0.27; I2 = 23%). This was also the case for social function with significant favour (P = 0.0008) of aripiprazole (3 RCTs, n = 256, MD 6.68 CI 2.79 to 10.56), again with considerable heterogeneity present (ChI2 = 13.19; df = 2; P = 0.001; I2 = 85%). Data in two small studies demonstrate statistical significance (P = 0.00001) in favour of aripiprazole for the independence component (2 RCTs, n = 176, MD 6.71 CI 4.76 to 8.66). Data demonstrate no significant difference between groups for material life (1 RCT, n = 80) and spiritual support (2 RCTs, n = 176).
1.13. Analysis.
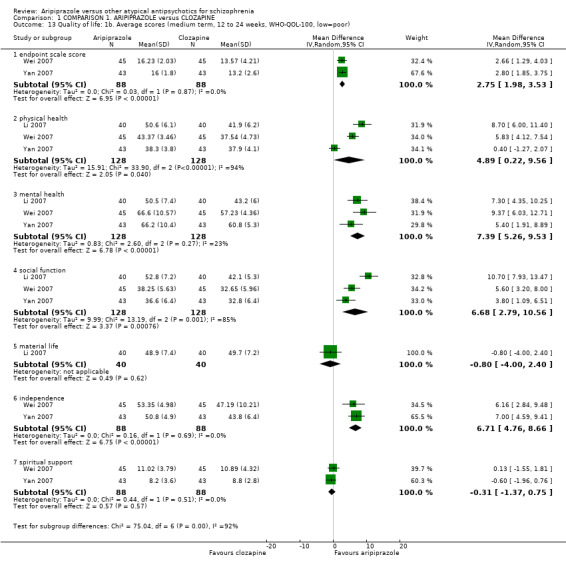
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 13 Quality of life: 1b. Average scores (medium term, 12 to 24 weeks, WHO‐QOL‐100, low=poor).
1.14 Quality of life: 2. Average endpoint general quality of life score (GQOLI ‐ 74, low = poor)
Physical health data from one small RCT demonstrate statistical significance (P = 0.01) in favour of aripiprazole ( n = 120, MD 7.70 CI 2.95 to 12.45, Analysis 1.14). Data for social function from one small RCT demonstrate statistical significance (P = 0.0002) in favour of aripiprazole (n = 120, MD 6.60 CI 3.15 to 10.05). Data from one small RCT demonstrate statistical significance (P = 0.0002) in favour of aripiprazole for environmental area (n = 90, MD 11.50 CI 5.55 to 17.45) and independence (P = 0.0003) in favour of aripiprazole (n = 90, MD 6.16 CI 2.84 to 9.48). No significant difference was found for: total GQOLI score (1 RCT, n = 114); mental health data (1 RCT, n = 120); and material life (1 RCT, n = 120)
1.14. Analysis.
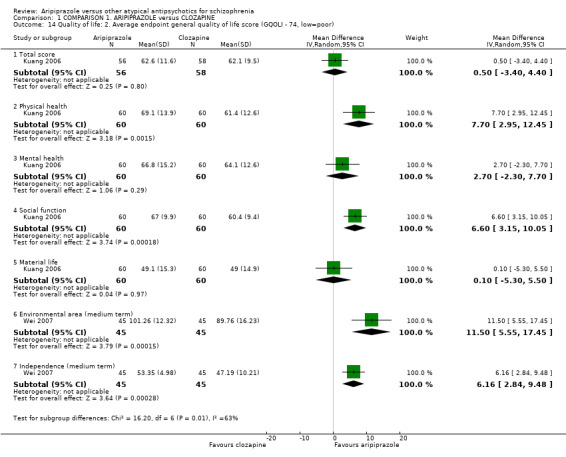
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 14 Quality of life: 2. Average endpoint general quality of life score (GQOLI ‐ 74, low=poor).
1.15 Adverse effects:1. At least one adverse effect, non‐specific
Data demonstrate statistical significance (P = 0.02) in favour of aripiprazole, with more people receiving clozapine experiencing at least one adverse effect (7 RCTs, n = 574, RR 0.67 CI 0.48 to 0.95, Analysis 1.15), however with considerable heterogeneity present (ChI2 = 18.17; df = 7; P = 0.01; I2 = 61%). There was no significant difference between groups in the short term for: epilepsy (1 RCT, n = 60); abnormal liver function (5 RCTs, n = 364); stuffy nose (3 RCTs, n = 258); sweating (1 RCT, n = 74); urinary retention (2 RCTs, n = 132); and urinary incontinence (2 RCTs, n = 120).
1.15. Analysis.
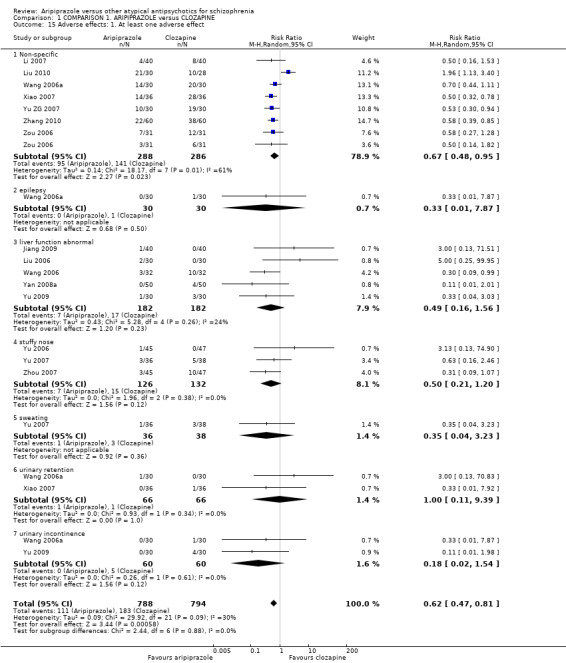
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 15 Adverse effects: 1. At least one adverse effect.
1.16 Adverse effects: 2. Cardiac effects
Overall, less cardiac effects were seen in people receiving aripiprazole compared with clozapine.
Significant results were found in the short term for abnormal ECG (P = 0.00001), with less occurrences found in people receiving aripiprazole (12 RCTs, n = 921, RR 0.38 CI 0.29 to 0.51, Analysis 1.16). Data were also significantly in favour of aripiprazole (P = 0.0003) for short‐term general adverse cardiac events (1 RCT, n = 62, RR 0.32 CI 0.17 to 0.60). QTc prolongation was significantly in favour (P = 0.001) of aripiprazole in both the short (4 RCTs, n = 334, RR 0.16 CI 0.05 to 0.49) and medium (P = 0.03) term (1 RCT, n = 90, RR 0.05 CI 0.00 to 0.79), as well as tachycardia (P = 0.00001) in the short term (15 RCTs, n = 1104, RR 0.29 CI 0.22 to 0.38). There was no significant difference between groups for a short‐term decrease in blood pressure (3 RCTs, n = 194); and medium‐term tachycardia (1 RCT, n = 60).
1.16. Analysis.
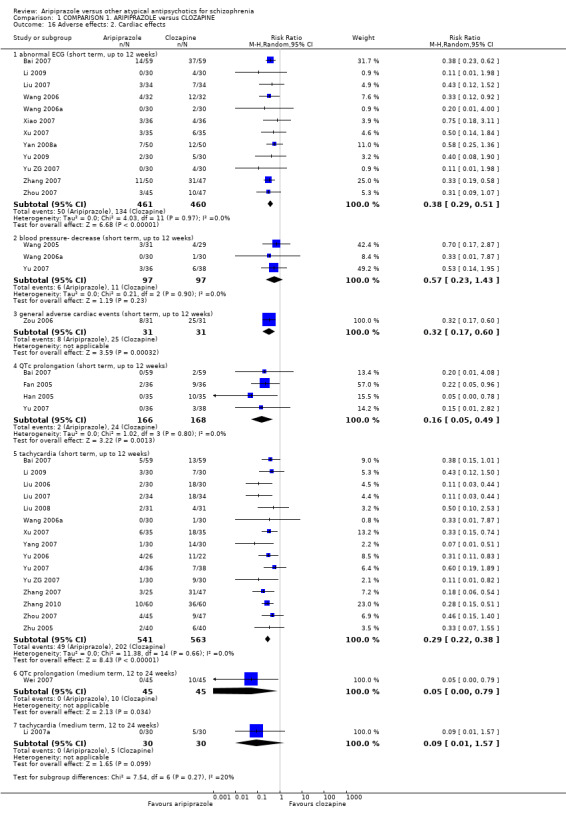
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 16 Adverse effects: 2. Cardiac effects.
1.17 Adverse effects: 3. Central/peripheral nervous system
Data showing a decrease in activity from one small RCT demonstrate statistical significance (P = 0.0002) in favour of aripiprazole at short term (n = 120, RR 0.21 CI 0.10 to 0.48, Analysis 1.17). This was also the case for blurred vision (P = 0.003) (6 RCTs, n = 472, RR 0.29 CI 0.12 to 0.66) with slight heterogeneity present (ChI2 = 9.11; df = 5; P = 0.105; I2 = 45%). Dizziness was also seen significantly more at short term (P = 0.05) in people receiving clozapine (9 RCTs, n = 698, RR 0.60 CI 0.36 to 1.00). Data from one small RCT for general vegetative nervous system adverse reaction demonstrate statistical significance (P = 0.01) in favour of aripiprazole (n = 62, RR 0.48 CI 0.27 to 0.84) by short term. Other short‐term CNS adverse effects that were significantly in favour of aripiprazole include; hyper‐salivation (P = 0.00001) (16 RCTs, n = 1074, RR 0.06 CI 0.03 to 0.10); memory decline (P = 0.04) (1 RCT n = 80, RR 0.13 CI 0.02 to 0.95); sedation (P = 0.02) (n = 80, RR 0.03 CI 0.00 to 0.52); and somnolence (P = 0.00001) (21 RCTs, n = 1492, RR 0.15 CI 0.09 to 0.24), with slight heterogeneity present (ChI2 = 35.6; df = 20; P = 0.02; I2 = 44%).
1.17. Analysis.
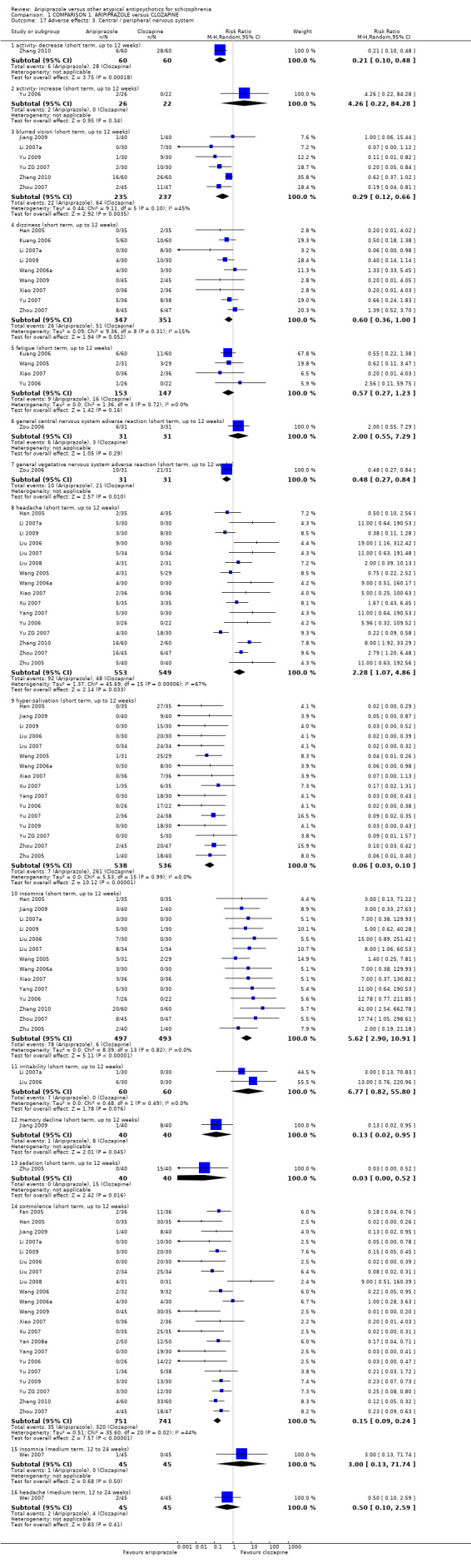
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 17 Adverse effects: 3. Central / peripheral nervous system.
Data significantly favoured clozapine at medium term for: headache (P = 0.03) (16 RCTs, n = 1102, RR 2.28 CI 1.07 to 4.86), however with considerable heterogeneity present (ChI2 = 45.69; df = 15; P = 0.0001; I2 = 67%); and insomnia (P = 0.00001) (14 RCTs, n = 990, RR 5.62 CI 2.90 to 10.91).
There was no difference between groups in the short term for: increase in activity (1 RCT, n = 48); fatigue (4 RCTs, n = 300); general CNS adverse reaction (1 RCT, n = 62); irritability (2 RCTs, n = 120); as well as insomnia at medium term (1 RCT, n = 90); and headache at medium term (1 RCT, n = 90).
1.18 Adverse effects: 4. Extrapyramidal effects
No significant difference was found between groups at short term for the following outcomes: general EPS (8 RCTs, n = 520); tardive dyskinesia (1 RCT, n = 72). For medium‐term outcomes, the following demonstrated no significant difference: akathisia (1 RCT, n = 80); dystonia (1 RCT, n = 80); spasmodic torticollis (1 RCT, n = 80); and tremor (1 RCT, n = 80, Analysis 1.18).
1.18. Analysis.
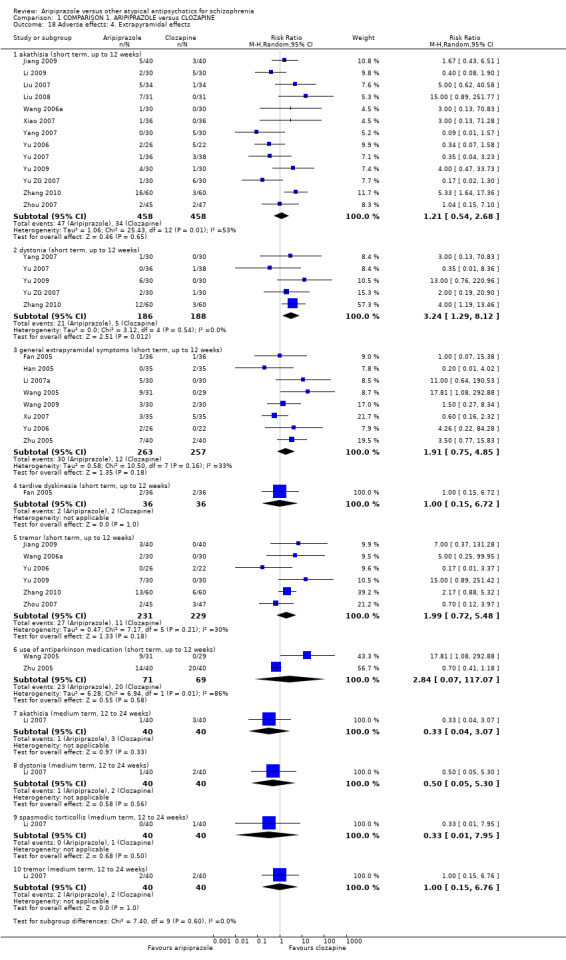
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 18 Adverse effects: 4. Extrapyramidal effects.
The only homogenous data that demonstrate statistical significance (P = 0.01) was in favour of clozapine for the outcome of dystonia at short term (5 RCTs, n = 374, RR 3.24 CI 1.29 to 8.12). All other data either presented no significant difference between groups, or no difference with varying degrees of heterogeneity. The latter include short‐term data for akathisia, with no significant difference between groups (13 RCTs, n = 916, RR 1.21 CI 0.54 to 2.68) with considerable heterogeneity present (ChI2 = 25.43; df = 12; P = 0.01; I2 = 53%). Data for tremor at short term demonstrated no significant difference between groups (6 RCTs, n = 460, RR 1.99 CI 0.72 to 5.48) with slight heterogeneity present (ChI2 = 7.17; df = 5; P = 0.208; I2 = 30%). This was the same for use of antiparkinson medication in the short term (2 RCTs, n = 140, RR 2.84 CI 0.07 to 117.07) with considerable heterogeneity present (ChI2 = 6.94; df = 1; P = 0.008; I2 = 86%).
1.19 Adverse effects: 5. Gastrointestinal
No significant difference was found in the short term for: general gastrointestinal adverse reaction (2 RCTs, n = 130); and indigestion (1 RCT, n = 60). Data for dry mouth at short term also demonstrated no significant difference between groups (4 RCTs, n = 268, RR 0.64 CI 0.08 to 5.38) with substantial heterogeneity present (ChI2 = 13.3; df = 3; P = 0.004; I2 = 77%). This was also true for nausea/vomiting (10 RCTs, n = 790, RR 1.55 CI 0.71 to 3.38) again with heterogeneity present (ChI2 = 17.01; df = 9; P = 0.05; I2 = 47%, Analysis 1.20).
1.20. Analysis.
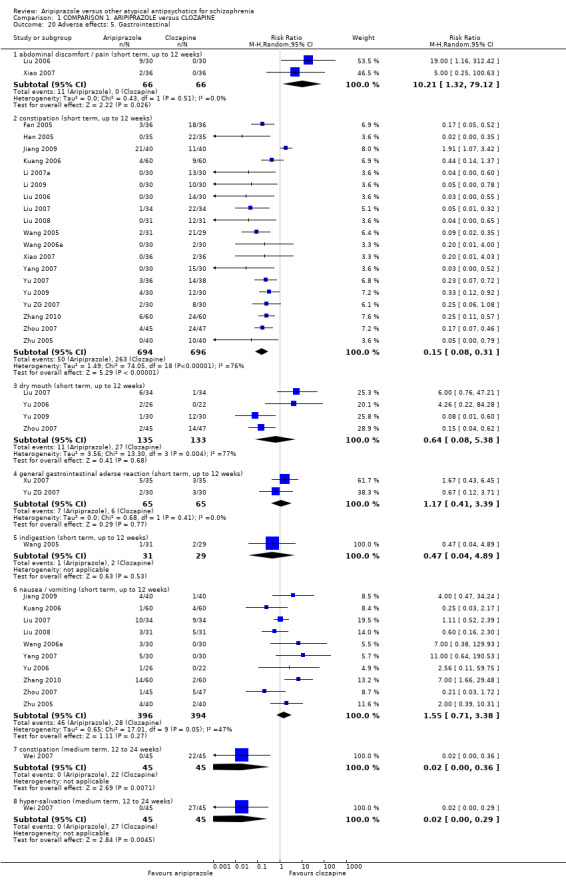
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 20 Adverse effects: 5. Gastrointestinal.
Data were significantly in favour of clozapine for abdominal discomfort or pain at short term (P = 0.03) (2 RCTs. n = 132, RR 10.21 CI 1.32 to 79.12). Short‐term data for constipation demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (19 RCTs, n = 1390, RR 0.15 CI 0.08 to 0.31) with substantial heterogeneity present (ChI2 = 74.05; df = 18; P = 0.0; I2 = 76%). Remaing data for medium‐term outcomes were in favour of aripiprazole, with significantly less instances of constipation (P = 0.007) (1 RCT, n = 90, RR 0.02 CI 0.00 to 0.36) and hyper‐salivation (P = 0.005) (1 RCT, n = 90, RR 0.02 CI 0.00 to 0.29).
1.20 Adverse effects: 6. Haematological
All data for this outcome were homogenous and statistically significant in favour of aripiprazole, including abnormal blood routine in the short term (P = 0.007) (5 RCTs, n = 368, RR 0.16 CI 0.04 to 0.60, Analysis 1.19); and leucopenia in the short term (P = 0.002) (10 RCTs, n = 726, RR 0.21 CI 0.08 to 0.56). Abnormal blood routine in the medium term also demonstrates statistical significance (P = 0.008) in favour of aripiprazole (2 RCTs, n = 152, RR 0.32 CI 0.14 to 0.75).
1.19. Analysis.
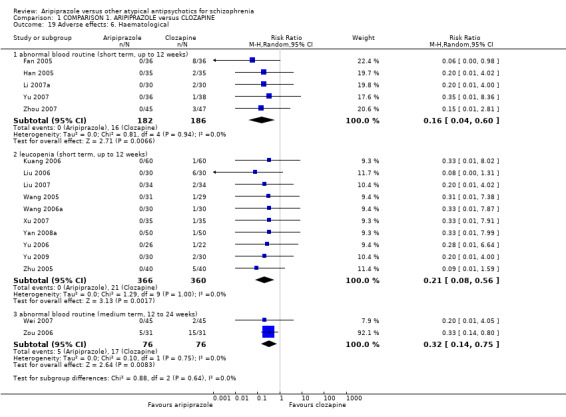
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 19 Adverse effects: 6. Haematological.
1.21 Adverse effects: 7. Hormonal (short term, up to 12 weeks)
Data for lactation or menstrual changes demonstrate statistical significance (P = 0.003) in favour of aripiprazole (3 RCTs, n = 214, RR 0.11 CI 0.03 to 0.47, Analysis 1.21).
1.21. Analysis.
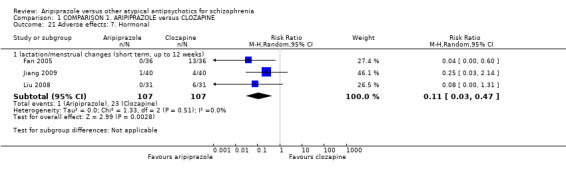
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 21 Adverse effects: 7. Hormonal.
1.22 Adverse effects: 8a. Metabolic ‐ binary measures
1.22.1 Blood glucose ‐ increased (short term, up to 12 weeks)
Data demonstrate statistical significance (P = 0.0002) in favour of aripiprazole (5 RCTs, n = 410, RR 0.12 CI 0.04 to 0.37, Analysis 1.22).
1.22. Analysis.
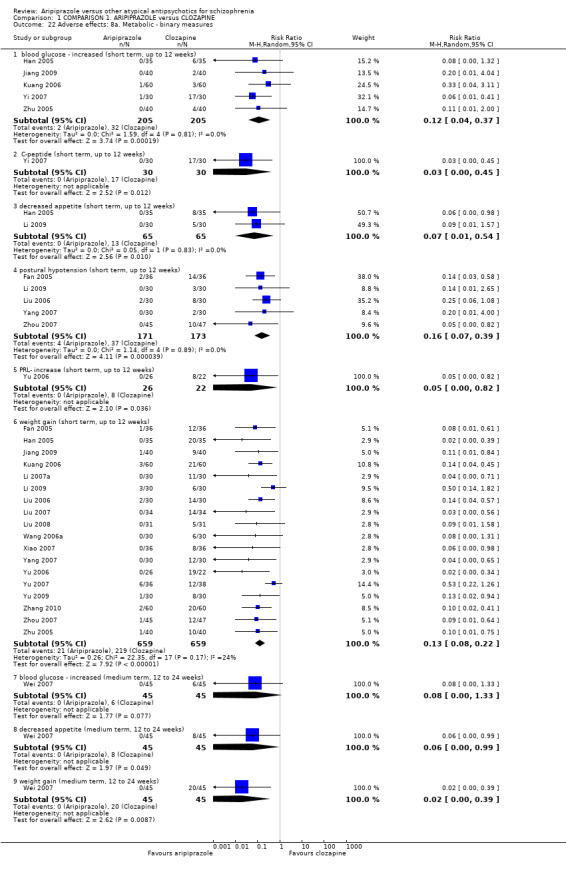
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 22 Adverse effects: 8a. Metabolic ‐ binary measures.
1.22.2 C‐peptide (short term, up to 12 weeks)
Data from one small RCT demonstrate statistical significance (P = 0.01) in favour of aripiprazole (1 RCT, n = 60, RR 0.03 CI 0.00 to 0.45).
1.22.3 Decrease appetite (short term, up to 12 weeks)
Data demonstrate statistical significance (P = 0.01) in favour of aripiprazole (2 RCTs, n = 130, RR 0.07 CI 0.01 to 0.54).
1.22.4 Postural hypotension (short term, up to 12 weeks)
Data demonstrate statistical significance (P = 0.001) in favour of aripiprazole (5 RCTs, n = 344, RR 0.16 CI 0.07 to 0.39).
1.22.5 PRL‐ increase (short term, up to 12 weeks)
Data from one small RCT demonstrate statistical significance (P = 0.04) in favour of aripiprazole (1 RCT, n = 48, RR 0.05 CI 0.00 to 0.82).
1.22.6 Weight gain (short term, up to 12 weeks)
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (18 RCTs, n = 1318, RR 0.13 CI 0.08 to 0.22).
1.22.7 Blood glucose ‐ increased (medium term, 12 to 24 weeks)
Data demonstrate no significant difference between groups (1 RCT, n = 90).
1.22.8 Decreased appetite (medium term, 12 to 24 weeks)
Data from one small RCT demonstrate statistical significance (P = 0.05) in favour of aripiprazole (n = 90, RR 0.06 CI 0.00 to 0.99).
1.22.9 Weight gain (medium term, 12 to 24 weeks)
Data from one small RCT demonstrate statistical significance (P = 0.009) in favour of aripiprazole (n = 90, RR 0.02 CI 0.00 to 0.39, Analysis 1.22).
1.23 Adverse effects: 8b. Metabolic ‐ continuous measures
1.23.1 Blood glucose ‐ FPG in HbA1c normal group (in mmol/L
Data demonstrate no significant difference between groups (1 RCT, n = 36).
1.23.2 Blood glucose ‐ FPG in HbA1c abnormal group (in mmol/L)
Data demonstrate no significant difference between groups (1 RCT, n = 19).
1.23.3 Blood glucose ‐ PBG in HbA1c normal group (in mmol/L)
Data demonstrate no significant difference between groups (1 RCT, n = 36).
1.23.4 Blood glucose ‐ PBG in HbA1c abnormal group (in mmol/L)
Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (1 RCT, n = 19, MD ‐1.90 CI ‐2.63 to ‐1.17).
1.23.5 Blood glucose ‐ FPG average endpoint (in mmol/L)
Data demonstrate statistical significance (P = 0.0009) in favour of aripiprazole (2 RCTs, n = 134, MD ‐0.52 CI ‐0.83 to ‐0.22).
1.23.6 Blood glucose ‐ C‐peptide average endpoint (in mg/dlmmol/L)
Data from one small RCT demonstrate statistical significance (P = 0.05) in favour of aripiprazole (1 RCT, n = 60, MD ‐0.72 CI ‐1.45 to 0.01).
1.23.7 Weight gain ‐ average endpoint level (in kg)
Data demonstrate no significant difference between groups (1 RCT, n = 74, Analysis 1.23).
1.23. Analysis.
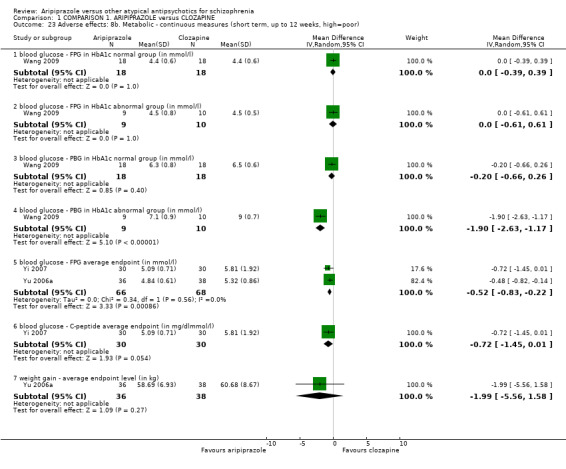
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 23 Adverse effects: 8b. Metabolic ‐ continuous measures (short term, up to 12 weeks, high=poor).
1.24 Cost‐effectiveness analysis (skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 1.24.
1.24. Analysis.
Comparison 1 COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE, Outcome 24 Cost effectiveness analysis (high=poor, data skewed).
| Cost effectiveness analysis (high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| Cost of hospitalisation (in RMB) | |||||
| Liu 2010 | Aripiprazole | 3413.66 | 1815.05 | 30 | |
| Liu 2010 | Clozapine | 4582.00 | 3372.42 | 28 | |
| Cost of drug (in RMB) | |||||
| Liu 2010 | Aripiprazole | 418.13 | 326.43 | 30 | |
| Liu 2010 | Clozapine | 210.39 | 300.32 | 28 | |
| Length of hospitalisation (day) | |||||
| Liu 2010 | Aripiprazole | 33.19 | 16.71 | 30 | |
| Liu 2010 | Clozapine | 49.50 | 30.83 | 28 | |
COMPARISON 2: ARIPIPRAZOLE versus QUETIAPINE
2.1 Global state: 1. No clinically significant response (as defined by original studies)
Data demonstrate no significant difference between groups (12 RCTs, n = 991, Analysis 2.1).
2.1. Analysis.
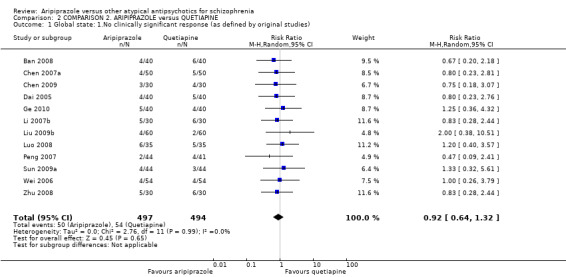
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 1 Global state: 1.No clinically significant response (as defined by original studies).
2.2 Global state: 2 a. Average endpoint total score (short term, up to 12 weeks, high = poor)
Data for the CGI demonstrate no significant difference between groups (1 RCT, n = 80, Analysis 2.2). This was also the case for PANSS (10 RCTs, n = 831), however with considerable heterogeneity present (ChI2 = 21.14; df = 9; P = 0.012; I2 = 57%). Data from the BPRS from one small RCT demonstrate statistical significance (P = 0.007) in favour of aripiprazole (1 RCT, n = 80, MD ‐2.63 CI ‐4.55 to ‐0.71).
2.2. Analysis.
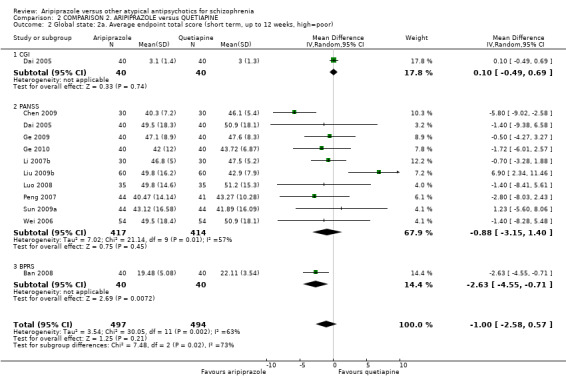
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 2 Global state: 2a. Average endpoint total score (short term, up to 12 weeks, high=poor).
2.3 Global state: 2b. Average endpoint scale score (medium term, 12 to 24 weeks, high = poor)
Data for PANSS demonstrate no significant difference between groups (1 RCT, n = 100, Analysis 2.3).
2.3. Analysis.
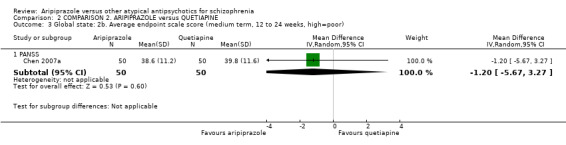
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 3 Global state: 2b. Average endpoint scale score (medium term, 12 to 24 weeks, high=poor).
2.4 Global state: 3. Average endpoint SI score (CGI, high = poor)
2.4.1 Up to 12 weeks ‐ short term
Data demonstrate no significant difference between groups (1 RCT, n = 108, Analysis 2.4).
2.4. Analysis.
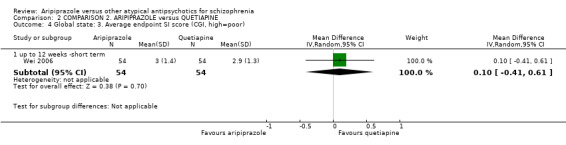
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 4 Global state: 3. Average endpoint SI score (CGI, high=poor).
2.5 Mental state: 2a. Specific ‐ binary outcomes
There was no significant difference between groups in the short term for: agitation (5 RCTs, n = 423); anxiety (2 RCTs, n = 168); and depression (1 RCT, n = 108, Analysis 2.5). This was also the case for agitation at medium term (1 RCT, n = 100).
2.5. Analysis.
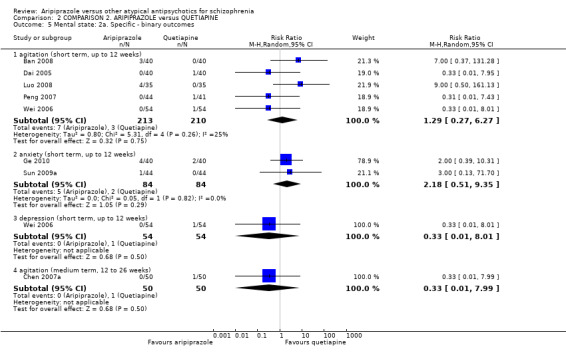
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 5 Mental state: 2a. Specific ‐ binary outcomes.
2.6 Mental state: 3. Specific ‐ average endpoint positive score (PANSS, high = poor)
2.6.1 By up to 12 weeks ‐ short term
Short‐term data demonstrate no significant difference between groups (7 RCTs, n = 583, Analysis 2.6) with substantial heterogeneity present (ChI2 = 20.25; df = 6; P = 0.002; I2 = 70%). Medium term data, again, demonstrate no significant difference between groups (1 RCT, n = 100).
2.6. Analysis.
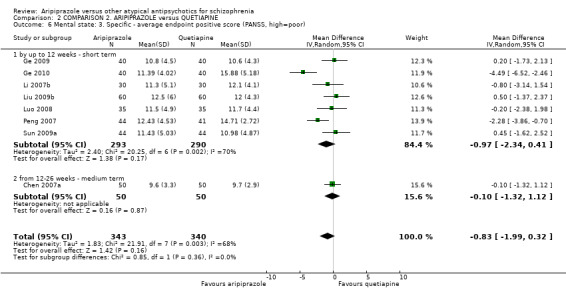
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 6 Mental state: 3. Specific ‐ average endpoint positive score (PANSS, high=poor).
2.7 Mental state: 4. Specific ‐ average endpoint negative score (PANSS, high = poor)
2.7.1 Up to 12 weeks ‐ short term
Short‐term data demonstrate no significant difference between groups using PANSS negative score (6 RCTs, n = 443, Analysis 2.7); this is also the case for medium‐term data (1 RCT, n = 100).
2.7. Analysis.
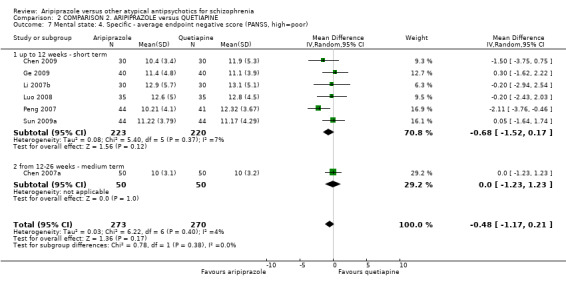
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 7 Mental state: 4. Specific ‐ average endpoint negative score (PANSS, high=poor).
2.8 Mental state: 5. Specific ‐ average endpoint general pathological score (PANSS, high = poor )
2.8.1 Up to 12 weeks ‐ short term
Short‐term data for PANSS pathological score demonstrate no significant difference between groups (10 RCTs, n = 831, Analysis 2.8) with substantial heterogeneity present (ChI2 = 83.29; df = 9; P = 0.00001; I2 = 89%). Medium‐term data also demonstrate no significant difference between groups (1 RCT, n = 100).
2.8. Analysis.
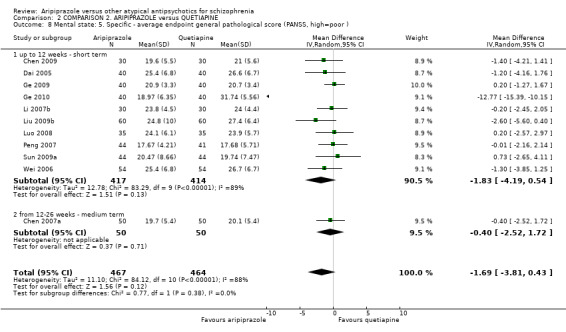
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 8 Mental state: 5. Specific ‐ average endpoint general pathological score (PANSS, high=poor ).
2.9 Mental state: 6. Average scores of various scales (skewed)
All data for the various scales are skewed and are best inspected by viewing Analysis 2.9
2.9. Analysis.
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 9 Mental state: 6. average scores of various scales (high=poor, skewed data).
| Mental state: 6. average scores of various scales (high=poor, skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| BPRS endpoint scale score | |||||
| Zhu 2008 | Aripiprazole | 23.8 | 14.3 | 29 | |
| Zhu 2008 | Quetiapine | 23.9 | 13.7 | 29 | |
| PANSS general pathology subscale score | |||||
| Zhu 2008 | Aripiprazole | 19.4 | 11.7 | 29 | |
| Zhu 2008 | Quetiapine | 19.5 | 11.3 | 29 | |
| PANSS negative subscale score | |||||
| Dai 2005 | Aripiprazole | 11.8 | 7.1 | 40 | |
| Dai 2005 | Quetiapine | 11.7 | 7. 6 | 40 | |
| Ge 2010 | Aripiprazole | 12.88 | 3.54 | 40 | |
| Ge 2010 | Quetiapine | 15.12 | 8.15 | 40 | |
| Wei 2006 | Aripiprazole | 11.9 | 7.2 | 54 | |
| Wei 2006 | Quetiapine | 11.8 | 7.6 | 54 | |
| Zhu 2008 | Aripiprazole | 10.9 | 8.1 | 29 | |
| Zhu 2008 | Quetiapine | 11.4 | 7.4 | 29 | |
| PANSS positive subscale score | |||||
| Chen 2009 | Aripiprazole | 10.1 | 5.1 | 30 | |
| Chen 2009 | Quetiapine | 10.7 | 6.2 | 30 | |
| Dai 2005 | Aripiprazole | 12.3 | 6.9 | 40 | |
| Dai 2005 | Quetiapine | 12.6 | 7.1 | 40 | |
| Wei 2006 | Aripiprazole | 12 .4 | 6.9 | 54 | |
| Wei 2006 | Quetiapine | 12.5 | 7.1 | 54 | |
| Zhu 2008 | Aripiprazole | 12.8 | 8.3 | 29 | |
| Zhu 2008 | Quetiapine | 14.3 | 7.9 | 29 | |
| PANSS total endpoint scale score | |||||
| Zhu 2008 | Aripiprazole | 44.2 | 23.6 | 29 | |
| Zhu 2008 | Quetiapine | 4.38 | 24.2 | 29 | |
2.10 Leaving the study early
All data for leaving the study early showed no significant difference between groups: any reason (2 RCTs, n = 168); because of no effect (1 RCT, n = 108); early discharge (1 RCT, n = 60); early treatment termination (2 RCTs, n = 168); violation of test scheme (1 RCT, n = 108); and withdrawal of informed consent (1 RCT, n = 108, Analysis 2.10).
2.10. Analysis.
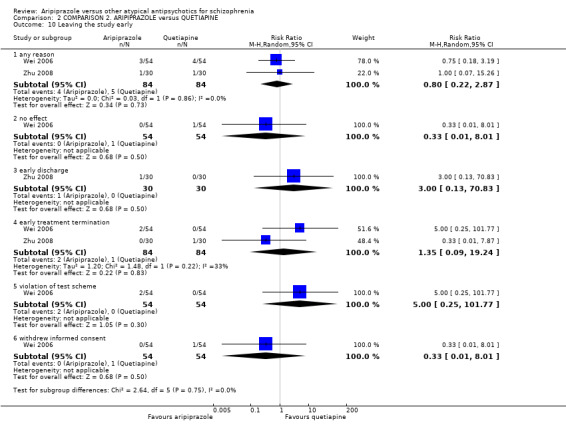
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 10 Leaving the study early.
2.11 Quality of life: Average score (medium term, 12 to 24 weeks, WHO‐QOL‐100, low = poor)
The majority of data from a single study are significantly in favour of aripiprazole for a better outcome using this scale, including; total score (P = 0.0001) (1 RCT, n = 100, MD 2.60 CI 1.31 to 3.89); physical health (P = 0.00001) (1 RCT, n = 100, MD 6.00 CI 4.38 to 7.62); mental health (P = 0.00001) (1 RCT, n = 100, MD 9.10 CI 5.92 to 12.28); social function (P = 0.00001) (1 RCT, n = 100, MD 5.60 CI 3.33 to 7.87); environmental area (P = 0.0001) (1 RCT, n = 100, MD 11.50 CI 5.86 to 17.14); and independence (P = 0.0001) (1 RCT, n = 100, MD 6.20 CI 3.05 to 9.35, Analysis 2.11). Data for spiritual pillar demonstrate no significant difference between groups (1 RCT, n = 100).
2.11. Analysis.
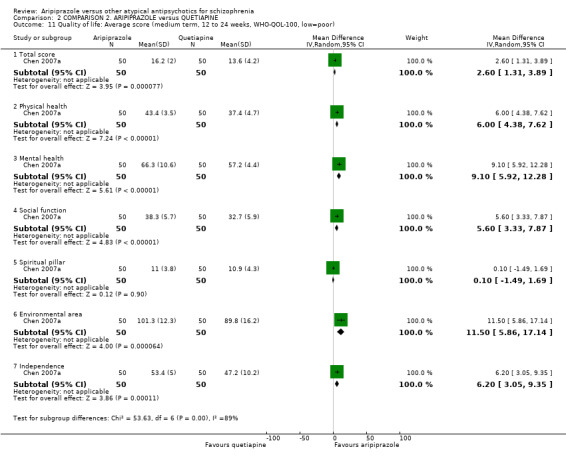
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 11 Quality of life: Average score (medium term, 12 to 24 weeks, WHO‐QOL‐100, low=poor).
2.12 Adverse effects: 1. At least one adverse effect
For non‐specific adverse effects, data demonstrate no significant difference between groups (3 RCTs, n = 258). All other data also demonstrated no significant difference, including: abnormal urinary test results (1 RCT, n = 108); abnormal liver function (8 RCTs, n = 658); stuffy nose (2 RCTs, n = 188); sweating (1 RCT, n = 70); urine routine abnormal (1 RCT, n = 80, Analysis 2.12).
2.12. Analysis.
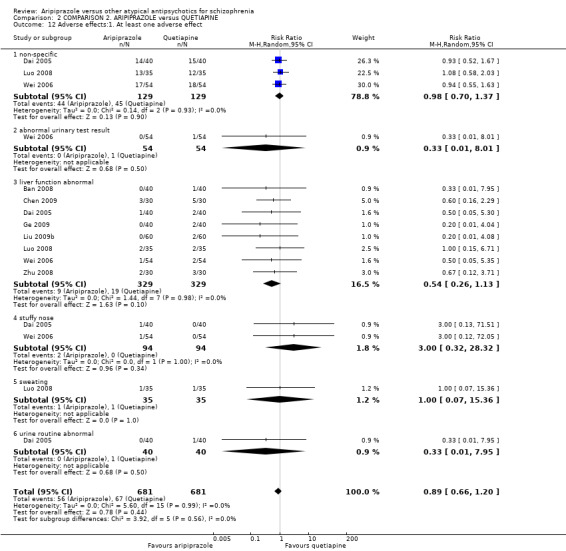
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 12 Adverse effects:1. At least one adverse effect.
2.13 Adverse effects: 2. Cardiac effects (short term, up to 12 weeks)
Data for tachycardia demonstrate statistical significance (P = 0.003) in favour of aripiprazole (8 RCTs, n = 643, RR 0.35 CI 0.18 to 0.69). All other data demonstrate no significant difference between groups: abnormal ECG (6 RCTs, n = 528); decrease in blood pressure (4 RCTs, n = 348); and QTc prolongation (3 RCTs, n = 225, Analysis 2.13).
2.13. Analysis.
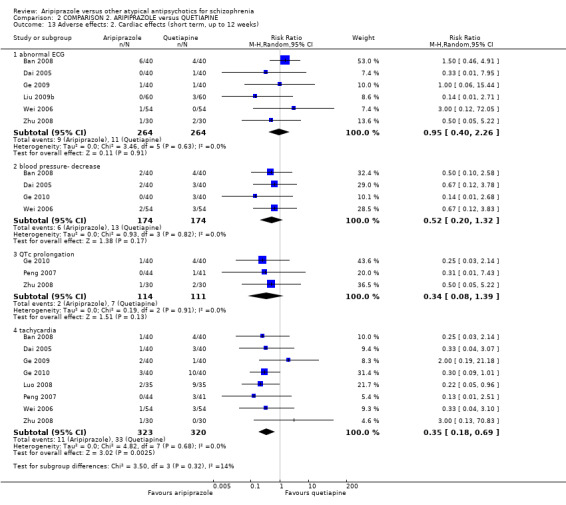
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 13 Adverse effects: 2. Cardiac effects (short term, up to 12 weeks).
2.14 Adverse effects: 3a. Central nervous system
No significant difference was found between groups in the short term for the following outcomes: blurred vision (6 RCTs, n = 521); dizziness (8 RCTs, n = 671); headache (5 RCTs, n = 436) with substantial heterogeneity present (ChI2 = 13.42; df = 4; P = 0.009; I2 = 70%); and insomnia (7 RCTs, n = 591). There was no difference at medium term for: dizziness (1 RCT, n = 100); insomnia (1 RCT, n = 100); or somnolence (1 RCT, n = 100, Analysis 2.14).
2.14. Analysis.
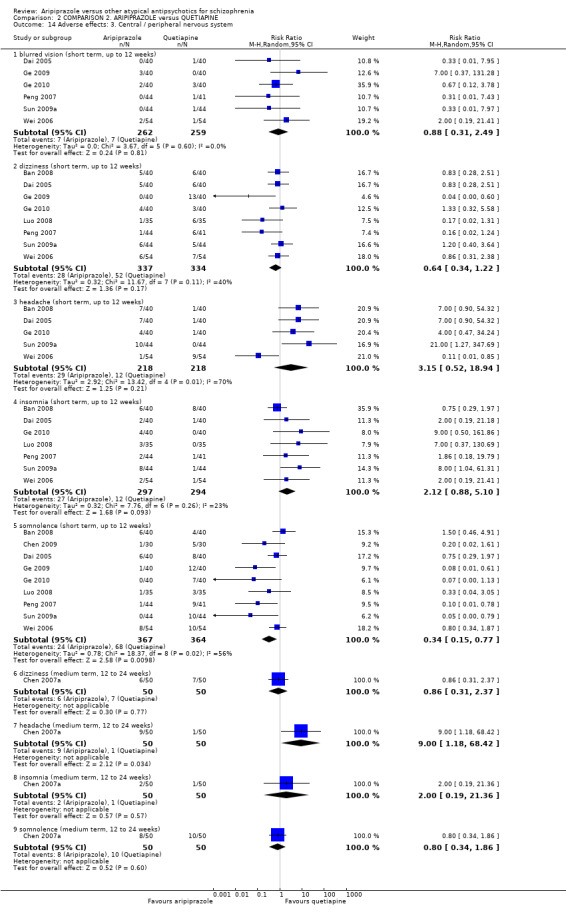
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 14 Adverse effects: 3. Central / peripheral nervous system.
Data at short term for somnolence demonstrate statistical significance (P = 0.01) in favour of aripiprazole (9 RCTs, n = 731, RR 0.34 CI 0.15 to 0.77) with heterogeneity present (ChI2 = 18.37; df = 8; P = 0.019; I2 = 56%). Medium‐term data for headache demonstrate statistical significance (P = 0.03) in favour of quetiapine (1 RCT, n = 100, RR 9.00 CI 1.18 to 48.42).
2.15 Adverse effects: 4. Various extrapyramidal symptoms (short term, up to 12 weeks)
All data demonstrated no significant difference between groups for akathisia (7 RCTs, n = 571, Analysis 2.15); dystonia (2 RCTs, n = 145); general EPS (4 RCTs, n = 348); and tremor (4 RCTs, n = 343).
2.15. Analysis.
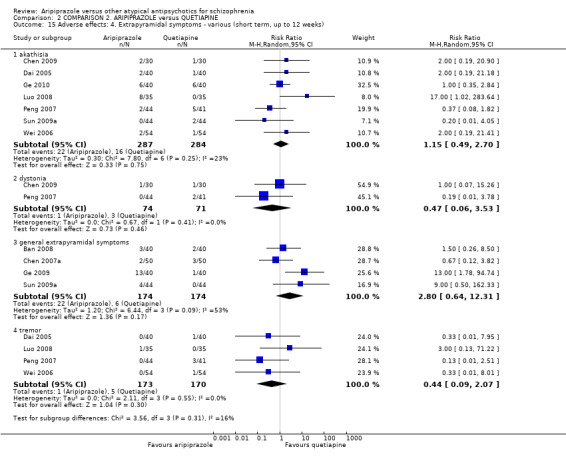
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 15 Adverse effects: 4. Extrapyramidal symptoms ‐ various (short term, up to 12 weeks).
2.16 Adverse effects: 5. Gastrointestinal
Data were significantly in favour of aripiprazole in the shot term from constipation (P = 0.005) (7 RCTs, n = 591, RR 0.38 CI 0.19 to 0.75, Analysis 2.16); dry mouth (P = 0.0007) (7 RCTs, n = 611, RR 0.23 CI 0.10 to 0.53); and in the medium term for dry mouth (P = 0.009) (1 RCT, n = 100, RR 0.11 CI 0.01 to 0.84). Data were significantly in favour of quetiapine in the short term for: nausea/ vomiting (P = 0.004) (7 RCTs, n = 611, RR 2.68 CI 1.36 to 5.26).There was no significant difference at medium term for nausea/vomiting (1 RCT, n = 100).
2.16. Analysis.
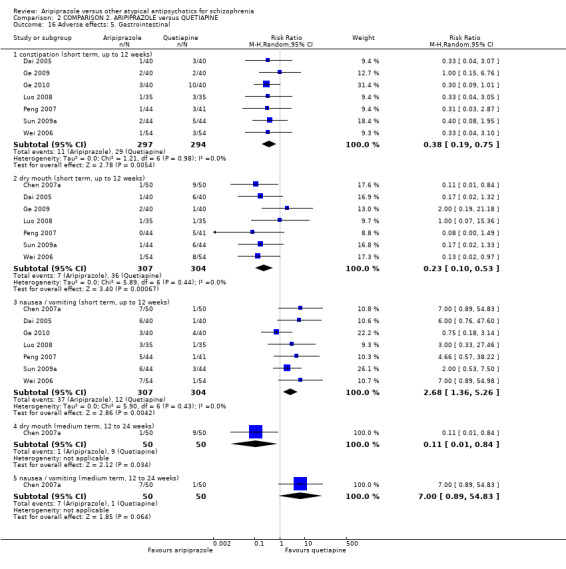
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 16 Adverse effects: 5. Gastrointestinal.
2.17 Adverse effects: 6. Haematological
Data demonstrate no significant difference in the short term for abnormal blood routine (1 RCT, n = 85, Analysis 2.17) and leucopenia (2 RCTs, n = 140).
2.17. Analysis.
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 17 Adverse effects: 6. Haematological.
2.18 Adverse effects: 7. Hormonal ‐ binary measures
2.18.1 Menstrual disorder (short term, up to 12 weeks)
There was no significant difference between groups for menstrual disorder at both short term (6 RCTs, n = 518) and medium term (1 RCT, n = 100, Analysis 2.18).
2.18. Analysis.
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 18 Adverse events: 7. Hormonal.
2.19 Adverse effects: 8a. Metabolic ‐ binary measures
At short term, there was no significant difference between groups for a decrease in appetite (4 RCTs, n = 328) and lactation (5 RCTs, n = 383). Medium data were also not significant for weight gain (1 RCT, n = 100). Data were significantly in favour of aripiprazole in the short term for weight gain (P = 0.01) (10 RCTs, n = 823, RR 0.45 CI 0.24 to 0.85) and at medium term for decreased appetite (P = 0.05) (1 RCT, n = 100, RR 0.13 CI 0.02 to 0.96, Analysis 2.19).
2.19. Analysis.
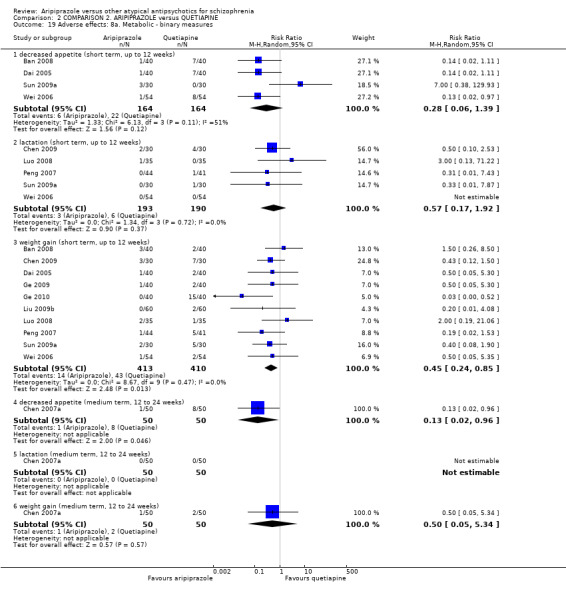
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 19 Adverse effects: 8a. Metabolic ‐ binary measures.
2.20 Adverse effects: 8b.Metabolic ‐ continuous measure
Data from one small RCT demonstrate statistical significance (P = 0.03) in favour of aripiprazole for cholesterol TC average endpoint level (in mmol/L) (1 RCT, n = 180, MD ‐0.19 CI ‐0.36 to ‐0.02, Analysis 2.20). However, all other data demonstrated no significant difference between groups.
2.20. Analysis.
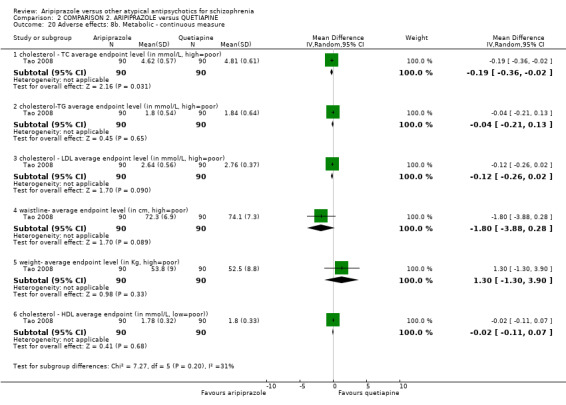
Comparison 2 COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE, Outcome 20 Adverse effects: 8b. Metabolic ‐ continuous measure.
COMPARISON 3: ARIPIPRAZOLE versus RISPERIDONE
3.1 Global state: 1.No clinically significant response (as defined by the original studies)
Data are equivocal at short term, demonstrating no significant difference between groups (80 RCTs, n = 6381, RR 1.08 CI 0.96 to 1.21, Analysis 3.1).
3.1. Analysis.
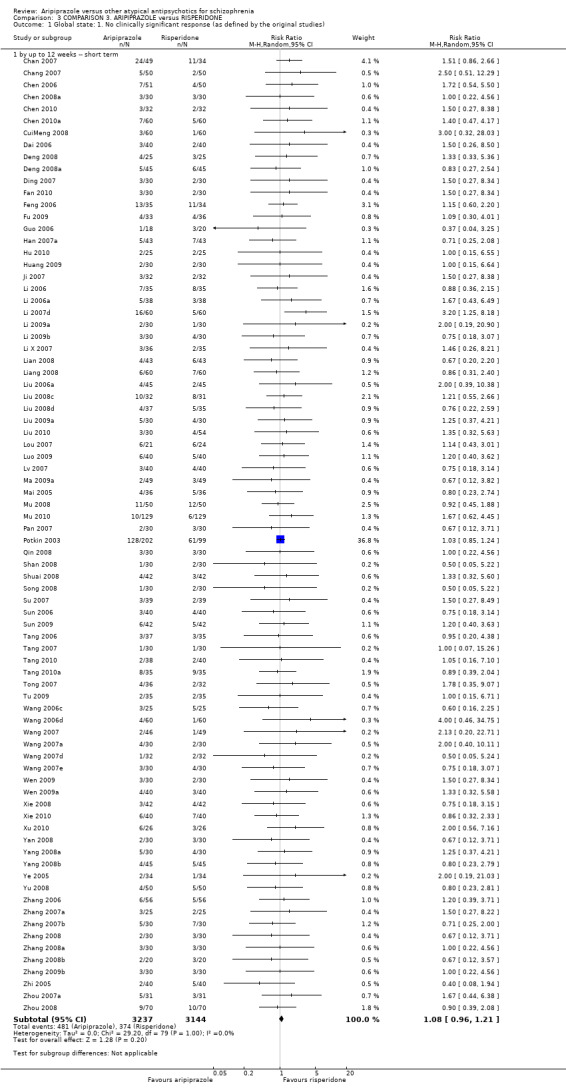
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 1 Global state: 1. No clinically significant response (as defined by the original studies).
3.2 Global state: 2. Average endpoint total score (short term, up to 12 weeks, CGI, high = poor)
Data demonstrate statistical significance (P = 0.006) in favour of risperidone (2 RCTs, n = 196, MD 0.35 CI 0.09 to 0.61, Analysis 3.2).
3.2. Analysis.
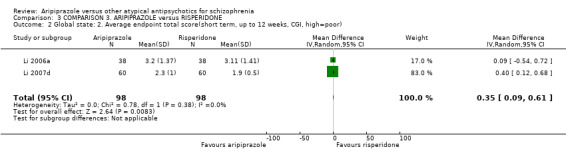
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 2 Global state: 2. Average endpoint total score(short term, up to 12 weeks, CGI, high=poor).
3.3 Global state: 3.Average CGI subscale scores (short term, up to 12 weeks, high = poor)
CGI‐EI data demonstrate statistical significance (P = 0.006) in favour of risperidone (1 RCT, n = 120, MD 0.50 CI 0.14 to 0.86, Analysis 3.3), and CGI‐SI data demonstrate statistical significance (P = 0.006) in favour of risperidone (1 RCT, n = 120, MD 0.40 CI 0.12 to 0.68).
3.3. Analysis.
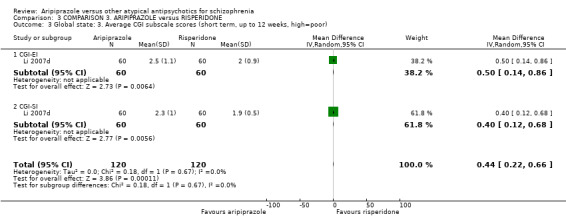
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 3 Global state: 3. Average CGI subscale scores (short term, up to 12 weeks, high=poor).
3.4 Global state average endpoint of various scales (skewed)
All data are skewed and are best inspected by viewing Analysis 3.4.
3.4. Analysis.
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 4 Global state: 4. average endpoint data of various scales (high=poor, data skewed).
| Global state: 4. average endpoint data of various scales (high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| CGI‐GI | |||||
| Chen 2006 | Aripiprazole | 1.7 | 0.9 | 51 | |
| Chen 2006 | Risperidone | 1.6 | 1.2 | 50 | |
| CGI‐SI | |||||
| Chen 2006 | Aripiprazole | 2.5 | 1.4 | 51 | |
| Chen 2006 | Risperidone | 2.3 | 1.2 | 50 | |
| Yang 2008a | Aripiprazole | 2.22 | 1.28 | 30 | |
| Yang 2008a | Risperidone | 2.35 | 1.16 | 30 | |
| CGI | |||||
| Feng 2006 | 1.49 | 1.05 | 35 | ||
| Feng 2006 | 1.44 | 1.2 | 34 | ||
| Zhang 2008 | Aripiprazole | 6.65 | 4.88 | 30 | |
| Zhang 2008 | Risperidone | 7.51 | 5.76 | 30 | |
3.5 Mental state: 1. Specific ‐ binary outcomes (short term, up to 12 weeks)
Data for anxiety demonstrate statistical significance (P = 0.01) in favour of risperidone (9 RCTs, n = 744, RR 1.81 CI 1.12 to 2.94, Analysis 3.5); however all other data are equivocal, demonstrating no difference between groups, including for agitation/excitement (26 RCTs, n = 2038) and irritability (1 RCT, n = 100).
3.5. Analysis.
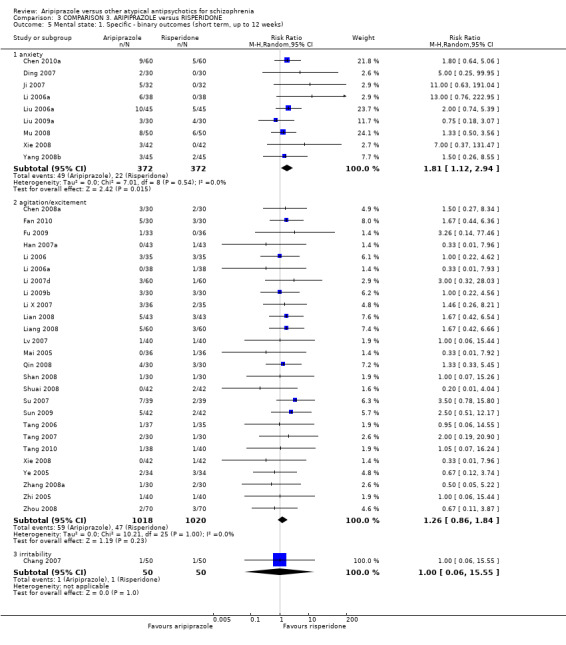
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 5 Mental state: 1. Specific ‐ binary outcomes (short term, up to 12 weeks).
3.6 Mental state: 2a. Average endpoint scale score (short term, up to 12 weeks, high = poor)
Data at short term for BPRS demonstrate statistical significance (P = 0.004) in favour of aripiprazole (5 RCTs, n = 570, MD ‐1.33 CI ‐2.24 to ‐0.42, Analysis 3.6), as were data for PANSS short term which showed a risk reduction by 0.8 on the PANSS scale favouring aripiprazole group (77 RCTs, n = 5733, MD ‐0.80 95% CI ‐1.58 to ‐0.02, Analysis 3.6). However, all other data show no significant difference between groups: PANSS medium term (1 RCT, n = 50) and SANS medium term (1 RCT, n = 50).
3.6. Analysis.
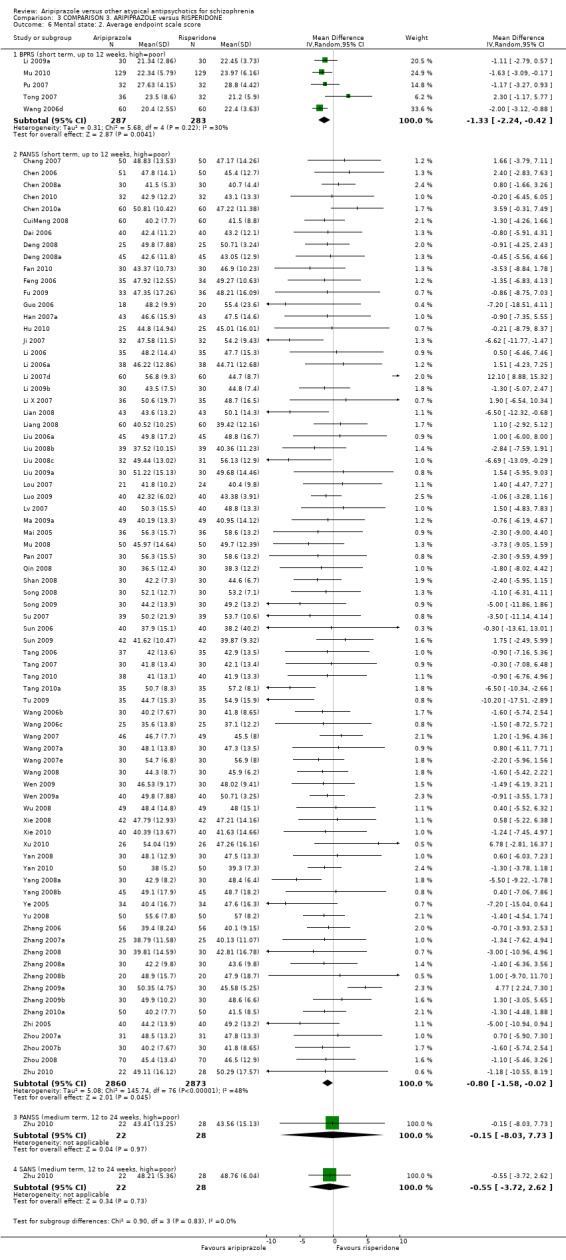
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 6 Mental state: 2. Average endpoint scale score.
3.7 Mental state: 3. Specific ‐ average endpoint positive score (PANSS, high = poor)
Data are equivocal at both short and medium term, demonstrating no significant difference between groups (40 RCTs, n = 3205, MD 0.02 CI ‐0.37 to 0.41, Analysis 3.7).
3.7. Analysis.
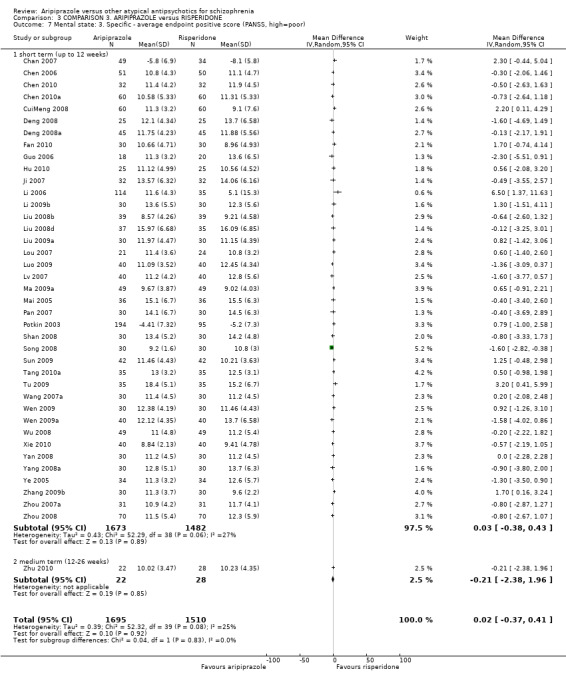
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 7 Mental state: 3. Specific ‐ average endpoint positive score (PANSS, high=poor).
3.8 Mental state: 4. Specific ‐ average endpoint negative score (PANSS, high = poor)
Data demonstrate statistical significance (P = 0.001) in favour of aripiprazole at short term (37 RCTs, n = 2976, MD ‐0.64 CI ‐1.04 to ‐0.25, Analysis 3.8).
3.8. Analysis.
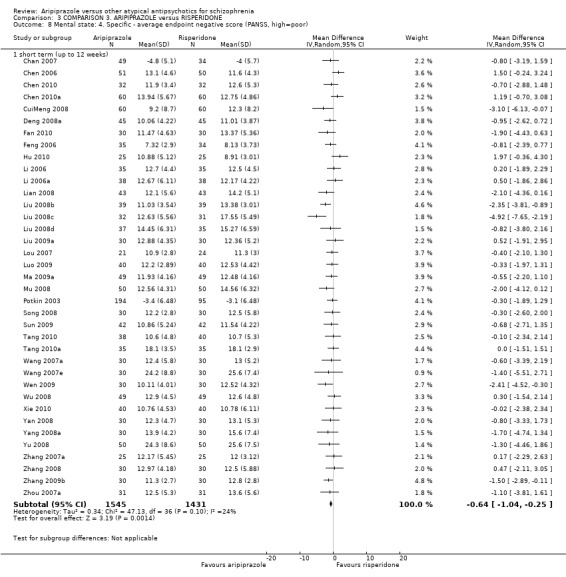
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 8 Mental state: 4. Specific ‐ average endpoint negative score (PANSS, high=poor).
3.9 Mental state: 5. Specific ‐ average endpoint general psychopathological score (PANSS, high = poor)
Data are equivocal at both short and medium term, demonstrating no significant difference between groups (58 RCTs, n = 4243, MD ‐0.25 CI ‐0.71 to 0.20, Analysis 3.9).
3.9. Analysis.
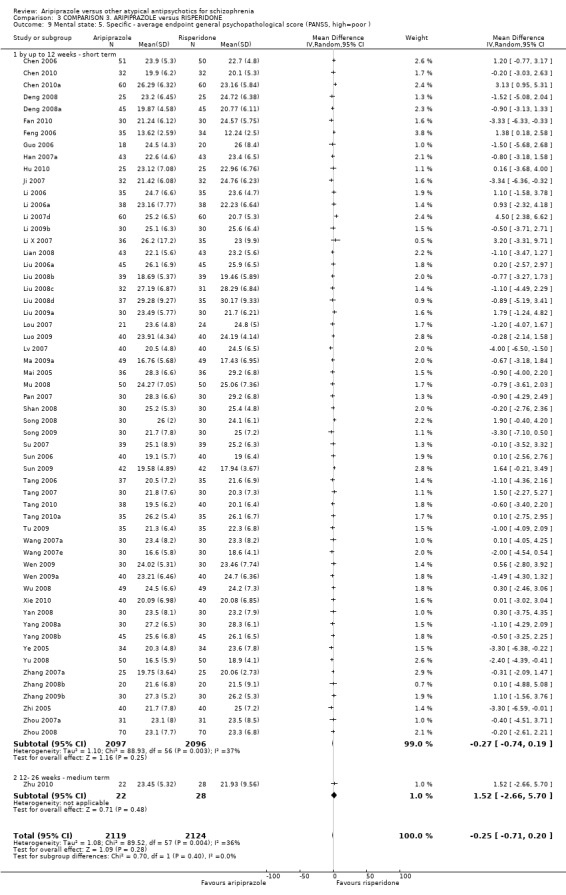
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 9 Mental state: 5. Specific ‐ average endpoint general psychopathological score (PANSS, high=poor ).
3.10 Mental state: 6. PANSS average score decreased rate (short term, up to 12 weeks, low = poor)
Data for total scale score decreased rate demonstrate statistical significance (P = 0.03) in favour of aripiprazole (3 RCTs, n = 219, MD 3.06 CI 0.24 to 5.87, Analysis 3.10). All other data demonstrate no significant difference between groups.
3.10. Analysis.
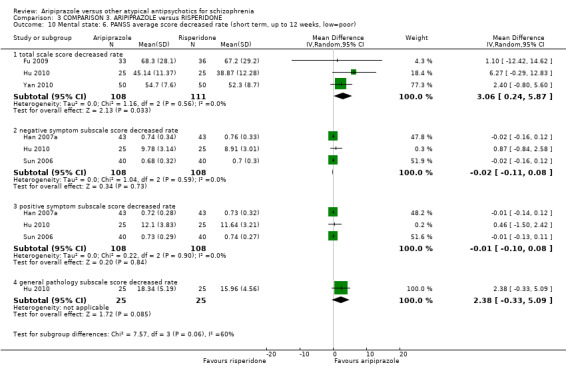
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 10 Mental state: 6. PANSS average score decreased rate (short term, up to 12 weeks, low=poor).
3.11 Mental state: 7. BPRS total score decreased rate (short term, up to 12 weeks, high = poor)
Data demonstrate no significant difference between groups (2 RCTs, n = 132, MD ‐2.97 CI ‐6.61 to 0.67, Analysis 3.11).
3.11. Analysis.
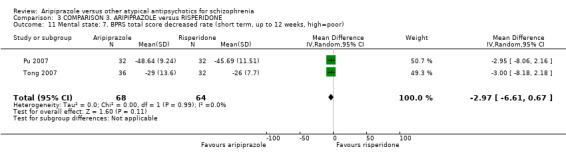
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 11 Mental state: 7. BPRS total score decreased rate (short term, up to 12 weeks, high=poor).
3.12 Mental state: 8. General ‐ average total score (PANSS, high = poor)
Data from two RCTs demonstrate no significant difference between groups at short term (2 RCTs, n = 372, MD 1.50 CI ‐2.96 to 5.96, Analysis 3.12).
3.12. Analysis.
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 12 Mental state: 8. General ‐ average total score (PANSS, high=poor).
3.13 Mental state: 9. Average scores of various scale (skewed)
All data for this outcome are skewed, and are best inspected by viewing Analysis 3.13.
3.13. Analysis.
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 13 Mental state: 9. average scores of various scale (high=poor, skewed data).
| Mental state: 9. average scores of various scale (high=poor, skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| SANS endpoint scale score | |||||
| Tong 2007 | Aripiprazole | 10. 6 | 14. 4 | 36 | |
| Tong 2007 | Risperidone | 14. 2 | 20. 3 | 32 | |
| PANSS general pathology subscale score | |||||
| Chang 2007 | Aripiprazole | 18.65 | 11.64 | 50 | |
| Chang 2007 | Risperidone | 19.15 | 10.95 | 50 | |
| Xie 2008 | Aripiprazole | 19. 24 | 12. 18 | 42 | |
| Xie 2008 | Risperidone | 19. 36 | 10. 71 | 42 | |
| PANSS negative symptom subscale score | |||||
| Chang 2007 | Aripiprazole | 12.04 | 6.46 | 50 | |
| Chang 2007 | Risperidone | 12.08 | 6.56 | 50 | |
| CuiMeng 2008 | Aripiprazole | 9. 2 | 8. 7 | 60 | |
| CuiMeng 2008 | Ziprasidone | 12. 3 | 8. 2 | 60 | |
| Deng 2008 | Aripiprazole | 14.89 | 3.89 | 25 | |
| Deng 2008 | Risperidone | 13.01 | 7.362 | 25 | |
| Guo 2006 | Aripiprazole | 12.4 | 3.9 | 18 | |
| Guo 2006 | Risperidone | 15.8 | 9.2 | 20 | |
| Ji 2007 | Aripiprazole | 12.71 | 4.58 | 32 | |
| Ji 2007 | Risperidone | 15.19 | 9.57 | 32 | |
| Li 2007d | Aripiprazole | 15.9 | 5.8 | 60 | |
| Li 2007d | Risperidone | 12.3 | 6.6 | 60 | |
| Li 2009b | Aripiprazole | 11.1 | 6.2 | 30 | |
| Li 2009b | Risperidone | 11.1 | 6.1 | 30 | |
| Li X 2007 | Aripiprazole | 12.2 | 7.2 | 36 | |
| Li X 2007 | Risperidone | 12.5 | 8.4 | 35 | |
| Liu 2006a | Aripiprazole | 11.3 | 7.4 | 45 | |
| Liu 2006a | Risperidone | 10.8 | 7.7 | 45 | |
| Lv 2007 | Aripiprazole | 11. 5 | 3. 8 | 40 | |
| Lv 2007 | Risperidone | 12. 5 | 6. 8 | 40 | |
| Mai 2005 | Aripiprazole | 12. 9 | 7. 1 | 36 | |
| Mai 2005 | Risperidone | 13. 9 | 6. 1 | 36 | |
| Pan 2007 | Aripiprazole | 11.9 | 7.2 | 30 | |
| Pan 2007 | Risperidone | 13.9 | 6.1 | 30 | |
| Shan 2008 | Aripiprazole | 11.2 | 5.8 | 30 | |
| Shan 2008 | Risperidone | 11.4 | 5.4 | 30 | |
| Song 2009 | Aripiprazole | 11.7 | 6.5 | 30 | |
| Song 2009 | Risperidone | 12.8 | 5.9 | 30 | |
| Su 2007 | Aripiprazole | 11. 4 | 7. 6 | 39 | |
| Su 2007 | Risperidone | 12. 6 | 6. 7 | 39 | |
| Tang 2006 | Aripiprazole | 10.9 | 5.3 | 37 | |
| Tang 2006 | Risperidone | 11.1 | 5.8 | 35 | |
| Tang 2007 | Aripiprazole | 11.9 | 5.7 | 30 | |
| Tang 2007 | Risperidone | 11.2 | 5.8 | 30 | |
| Tu 2009 | Aripiprazole | 11.3 | 6.5 | 35 | |
| Tu 2009 | Risperidone | 13.8 | 6.7 | 33 | |
| Wen 2009a | Aripiprazole | 14.89 | 3.88 | 40 | |
| Wen 2009a | Risperidone | 13.02 | 7.34 | 40 | |
| Xie 2008 | Aripiprazole | 11. 95 | 5. 69 | 42 | |
| Xie 2008 | Risperidone | 12. 32 | 6. 17 | 42 | |
| Xie 2010 | Aripiprazole | 10.76 | 4.53 | 40 | |
| Xie 2010 | Risperidone | 10.78 | 6.11 | 40 | |
| Yang 2008b | Aripiprazole | 12.1 | 6.9 | 45 | |
| Yang 2008b | Risperidone | 11.9 | 7.1 | 45 | |
| Ye 2005a | Aripiprazole | 11. 8 | 4. 3 | 29 | |
| Ye 2005a | Risperidone | 12. 3 | 8. 2 | 29 | |
| Zhang 2008b | Aripiprazole | 9.2 | 8.7 | 20 | |
| Zhang 2008b | Risperidone | 13.5 | 6.9 | 20 | |
| Zhi 2005 | Aripiprazole | 10.7 | 6.5 | 40 | |
| Zhi 2005 | Risperidone | 12.8 | 5.9 | 40 | |
| Zhou 2008 | Aripiprazole | 10. 8 | 5. 5 | 70 | |
| Zhou 2008 | Risperidone | 11. 2 | 5. 7 | 70 | |
| Zhu 2010 | Aripiprazole | 10.40 | 6.57 | 22 | |
| Zhu 2010 | Risperidone | 11.67 | 4.31 | 28 | |
| PANSS positive symptom subscale score | |||||
| Chang 2007 | Aripiprazole | 16.82 | 11.66 | 50 | |
| Chang 2007 | Risperidone | 17.64 | 11.76 | 50 | |
| Chen 2010 | Aripiprazole | 10.58 | 5.33 | 32 | |
| Chen 2010 | Risperidone | 11.31 | 5.33 | 32 | |
| Fan 2010 | Aripiprazole | 10.66 | 4.71 | 30 | |
| Fan 2010 | Risperidone | 8.96 | 4.93 | 30 | |
| Feng 2006 | Aripiprazole | 5.18 | 2.04 | 30 | |
| Feng 2006 | Risperidone | 4.91 | 3.14 | 30 | |
| Hu 2010 | Aripiprazole | 10.56 | 4.52 | 25 | |
| Hu 2010 | Risperidone | 11.16 | 6.21 | 25 | |
| Li 2007a | Aripiprazole | 10.39 | 6.02 | 30 | |
| Li 2007a | Risperidone | 10.31 | 5.11 | 30 | |
| Li 2007d | Aripiprazole | 17.2 | 9.3 | 60 | |
| Li 2007d | Risperidone | 12.1 | 3.4 | 60 | |
| Li X 2007 | Aripiprazole | 12 | 8 | 36 | |
| Li X 2007 | Risperidone | 13.2 | 7.3 | 35 | |
| Lian 2008 | Aripiprazole | 8.9 | 4.1 | 43 | |
| Lian 2008 | Risperidone | 11.5 | 5.9 | 43 | |
| Liu 2006a | Aripiprazole | 12.4 | 7.2 | 45 | |
| Liu 2006a | Risperidone | 12.1 | 7.5 | 45 | |
| Liu 2008c | Aripiprazole | 9. 59 | 3. 17 | 37 | |
| Liu 2008c | Risperidone | 10. 29 | 5. 47 | 35 | |
| Mu 2008 | Aripiprazole | 10. 95 | 5. 07 | 50 | |
| Mu 2008 | Risperidone | 11. 50 | 6. 02 | 50 | |
| Song 2009 | Aripiprazole | 10.7 | 5.3 | 30 | |
| Song 2009 | Risperidone | 11.3 | 5.9 | 30 | |
| Su 2007 | Aripiprazole | 10. 8 | 7. 5 | 39 | |
| Su 2007 | Risperidone | 12. 2 | 6. 8 | 39 | |
| Tang 2006 | Aripiprazole | 10.6 | 5.1 | 37 | |
| Tang 2006 | Risperidone | 11.2 | 6.0 | 35 | |
| Tang 2007 | Aripiprazole | 10.1 | 5.3 | 30 | |
| Tang 2007 | Risperidone | 8.6 | 6.5 | 30 | |
| Tang 2010 | Aripiprazole | 10.2 | 4.1 | 38 | |
| Tang 2010 | Risperidone | 10.6 | 5.8 | 40 | |
| Wang 2007e | Aripiprazole | 9.7 | 5.8 | 30 | |
| Wang 2007e | Risperidone | 10.1 | 5.6 | 30 | |
| Xie 2008 | Aripiprazole | 17. 21 | 10. 97 | 42 | |
| Xie 2008 | Risperidone | 17. 33 | 11. 82 | 42 | |
| Xie 2010 | Aripiprazole | 8.94 | 2.13 | 40 | |
| Xie 2010 | Risperidone | 9.41 | 4.78 | 40 | |
| Yang 2008b | Aripiprazole | 12.2 | 6.8 | 45 | |
| Yang 2008b | Risperidone | 12.3 | 7.1 | 45 | |
| Yu 2008 | Aripiprazole | 9.7 | 5.53 | 50 | |
| Yu 2008 | Risperidone | 10.1 | 5.8 | 50 | |
| Zhang 2007a | Aripiprazole | 8.0 | 2.4 | 25 | |
| Zhang 2007a | Risperidone | 9.31 | 5.12 | 25 | |
| Zhang 2008 | Aripiprazole | 9.77 | 6.78 | 30 | |
| Zhang 2008 | Risperidone | 11.17 | 5.75 | 30 | |
| Zhang 2008b | Aripiprazole | 13.1 | 8.6 | 20 | |
| Zhang 2008b | Risperidone | 9.1 | 7.6 | 20 | |
| Zhi 2005 | Aripiprazole | 10.7 | 5.3 | 40 | |
| Zhi 2005 | Risperidone | 11.3 | 5.9 | 40 | |
3.14 Leaving the study early
All data for leaving the study early demonstrate no significant difference between groups, including leaving for any reason (12 RCTs, n = 1239, Analysis 3.14); progressive disease (2 RCTs, n = 188); incomplete data (1 RCT, n = 180); adverse effects (9 RCTs, n = 1272); and no effect of study drug (5 RCTs, n = 681).
3.14. Analysis.
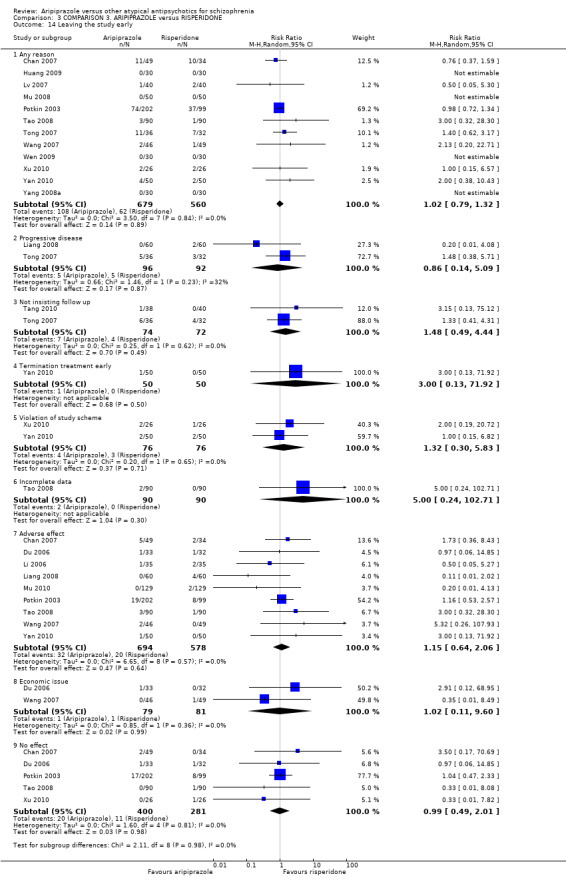
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 14 Leaving the study early.
3.15 Adverse effects: 1. At least one adverse effect, non‐specific
With more non‐specific adverse effects seen with people receiving risperidone, data demonstrate statistical significance (P = 0.0002) in favour of aripiprazole (28 RCTs, n = 2361, RR 0.81 CI 0.73 to 0.91, Analysis 3.15). Generally, data demonstrated no significant difference between groups, including hyper‐salivation (7 RCTs, n = 554), and sweating (4 RCTs, n = 278). Data demonstrate statistical significance (P = 0.003) in favour of aripiprazole for abnormal liver function (29 RCTs, n = 2300, RR 0.63 CI 0.46 to 0.86) and sexual desire change (P = 0.0001) (8 RCTs, n = 614, RR 0.11 CI 0.04 to 0.30).
3.15. Analysis.
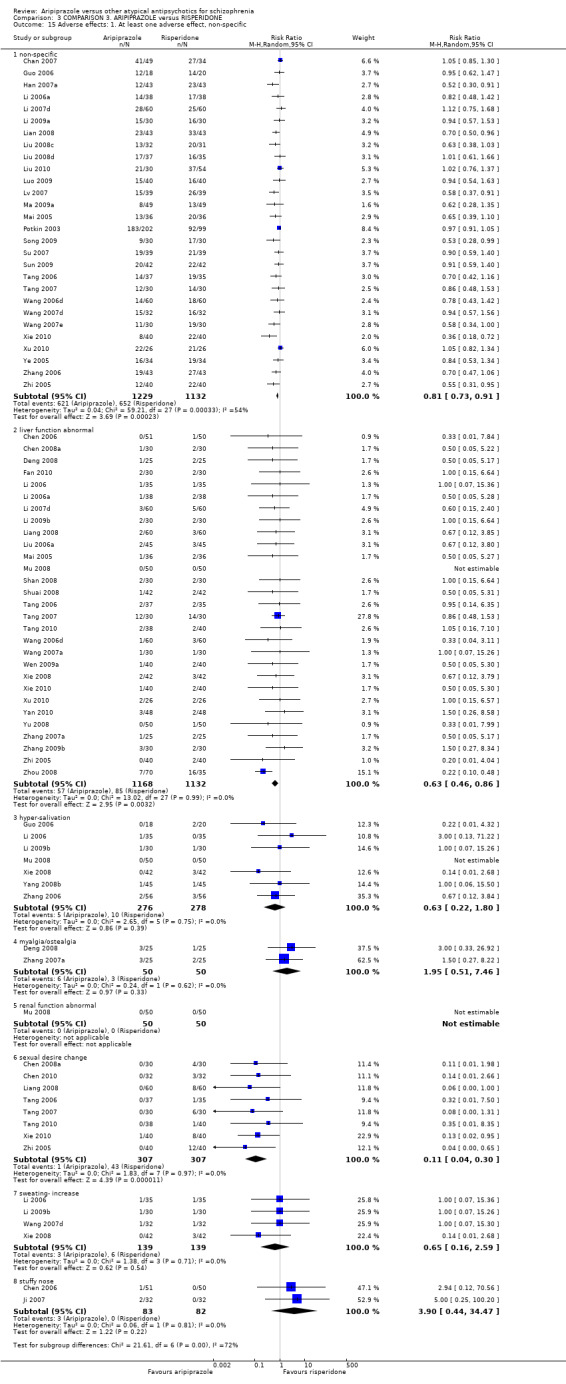
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 15 Adverse effects: 1. At least one adverse effect, non‐specific.
3.16 Adverse effects: 2a.Cardiac effects (short term, up to 12 weeks)
Data for tachycardia demonstrate statistical significance (P = 0.02) in favour of aripiprazole (49 RCTs, n = 3835, RR 0.76 CI 0.61 to 0.96, Analysis 3.16). All other data are equivocal, demonstrating no significant difference between groups.
3.16. Analysis.
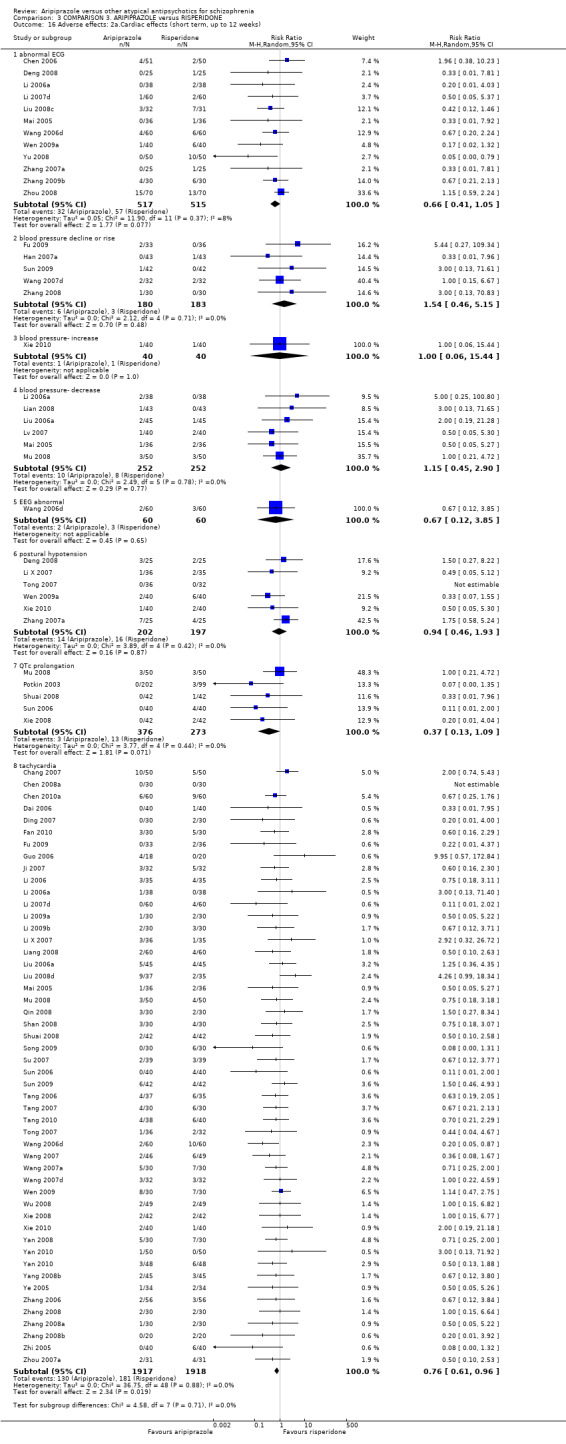
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 16 Adverse effects: 2a.Cardiac effects (short term, up to 12 weeks).
3.17 Adverse effects: 2b. Cardiac ‐ QTc change from baseline (in ms)
Data demonstrate statistical significance (P = 0.005) in favour of aripiprazole (2 RCTs, n = 383, MD ‐7.19 CI ‐12.19 to ‐2.19, Analysis 3.17).
3.17. Analysis.
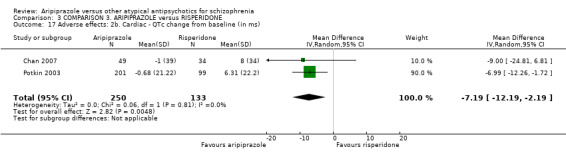
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 17 Adverse effects: 2b. Cardiac ‐ QTc change from baseline (in ms).
3.18 Adverse effects: 3. Central nervous system (short term, up to 12 weeks)
The majority of data are equivocal, demonstrating no difference between groups. Data for headache demonstrate statistical significance (P = 0.01) in favour of risperidone (20 RCTs, n = 1505, RR 1.91 CI 1.31 to 2.78, Analysis 3.18). Data from one small RCT demonstrate statistical significance (P = 0.04) in favour of aripiprazole for decrease in memory, with higher instances seen in people receiving risperidone (1 RCT, n = 60, RR 0.22 CI 0.05 to 0.94).
3.18. Analysis.
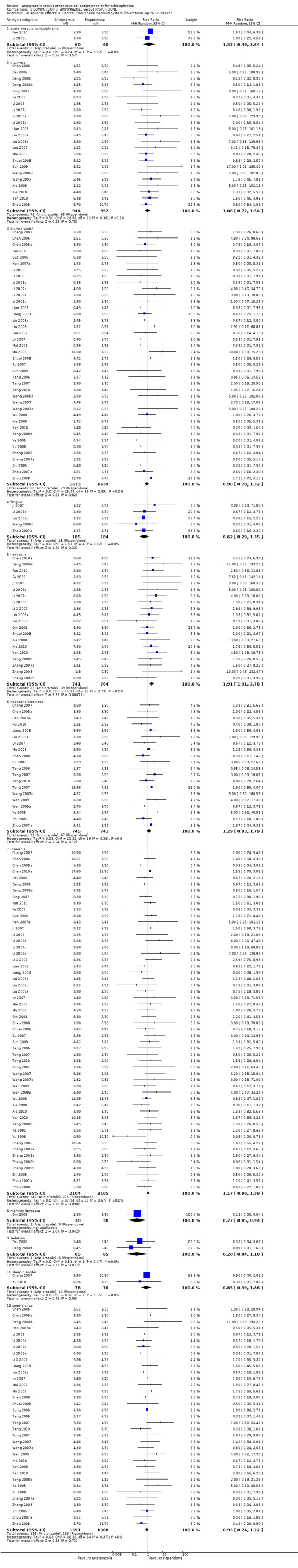
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 18 Adverse effects: 3. Central / peripheral nervous system (short term, up to 12 weeks).
3.19 Adverse effects: 3a. Endocrine ‐ Prolactin ‐ average change (ng/mL)
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (2 RCTs, n = 383, MD ‐54.71 CI ‐60.06 to ‐49.36, Analysis 3.19).
3.19. Analysis.
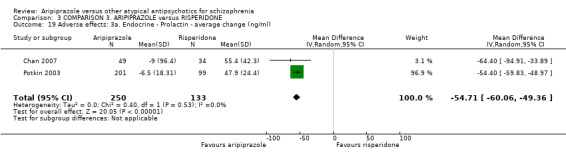
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 19 Adverse effects: 3a. Endocrine ‐ Prolactin ‐ average change (ng/ml).
3.20 Adverse effects: 3b. Endocrine ‐ Prolactin ‐ associated
Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of aripiprazole for abnormally high prolactin value (1 RCT, n = 301, RR 0.04 CI 0.02 to 0.08, Analysis 3.20). Data demonstrate no significant difference between groups for dysmenorrhoea (1 RCT, n = 91).
3.20. Analysis.
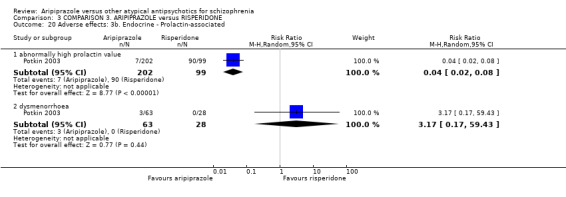
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 20 Adverse effects: 3b. Endocrine ‐ Prolactin‐associated.
3.21 Adverse effects:4.Various extrapyramidal symptoms (short term, up to 12 weeks)
3.21.1 Akathisia
Data demonstrate statistical significance of aripiprazole for: akathisia (P = 0.00001) (42 RCTs, n = 3501, RR 0.60 CI 0.48 to 0.74, Analysis 3.21); tremor (P = 0.00001) (36 RCTs, n = 2799, RR 0.35 CI 0.27 to 0.45); dystonia (P = 0.00001) (32 RCTs, n = 2640, RR 0.35 CI 0.25 to 0.49); and EPS (P = 0.00001) (31 RCTs, n = 2605, RR 0.39 CI 0.31 to 0.50) with slight heterogeneity present (ChI2 = 53.91; df = 30; P = 0.005; I2 = 44%). All other data demonstrate no difference between groups for parkinsonism (1 RCT, n = 301); use of antiparkinson medication (1 RCT, n = 83); tremor (2 RCTs, n = 391).
3.21. Analysis.
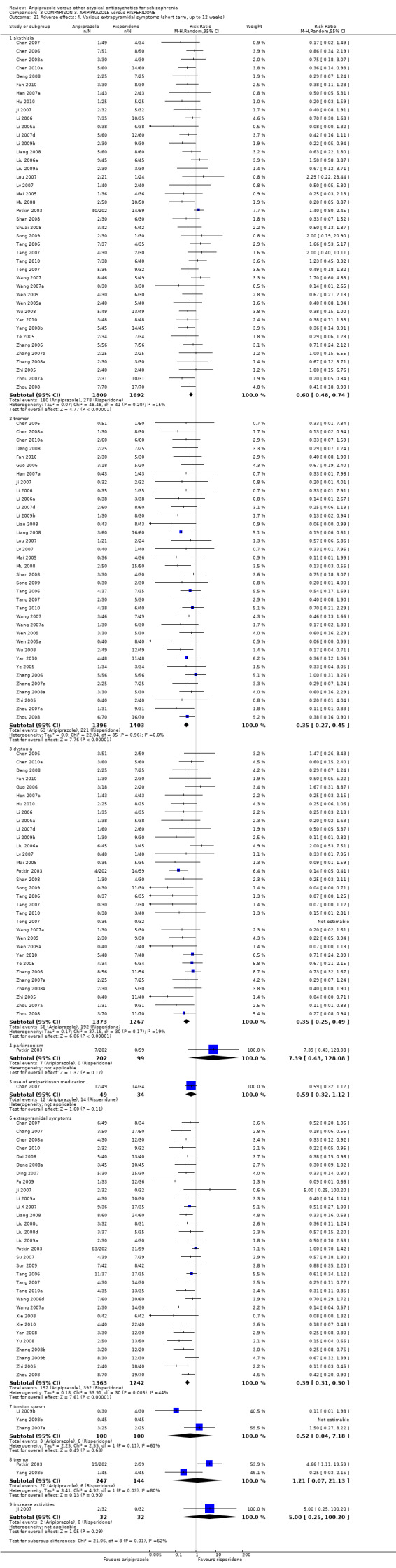
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 21 Adverse effects: 4. Various extrapyramidal symptoms (short term, up to 12 weeks).
3.22 Adverse effects: 4b. Extrapyramidal ‐ average score
3.22.1 Abnormal Involuntary Movement Scale (high = poor)
Data demonstrate no significant difference between groups (2 RCTs, n = 383, MD ‐0.25 CI ‐1.24 to 0.75, Analysis 3.22) with considerable heterogeneity present (ChI2 = 3.09; df = 1; P = 0.079; I2 = 68%).
3.22. Analysis.
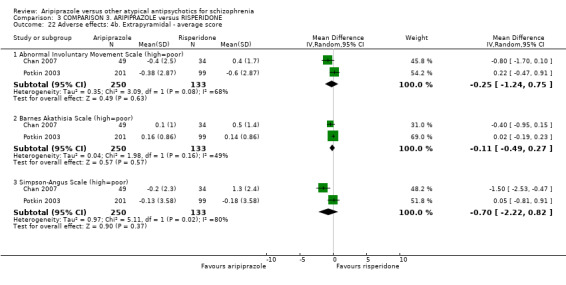
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 22 Adverse effects: 4b. Extrapyramidal ‐ average score.
3.22.2 Barnes Akathisia Scale (high = poor)
Data demonstrate no significant difference between groups (2 RCTs, n = 383, MD ‐0.11 CI ‐0.49 to 0.27) with slight heterogeneity present (ChI2 = 1.98; df = 1; P = 0.16; I2 = 49%).
3.22.3 Simpson‐Angus Scale (high = poor)
Data demonstrate no significant difference between groups (2 RCTs, n = 383, MD ‐0.70 CI ‐2.22 to 0.82) with substantial heterogeneity present (ChI2 = 5.11; df = 1; P = 0.024; I2 = 80%).
3.23 Adverse effects: 5. Gastrointestinal
Data for constipation (27 RCTs, n = 2067) and dry mouth (33 RCTs, n = 2658) are equivocal, demonstrating no significant difference between groups (Analysis 3.23). For nausea and vomiting data demonstrate statistical significance (P = 0.00001) in favour of risperidone (28 RCTs, n = 2180, RR 1.84 CI 1.31 to 2.56).
3.23. Analysis.
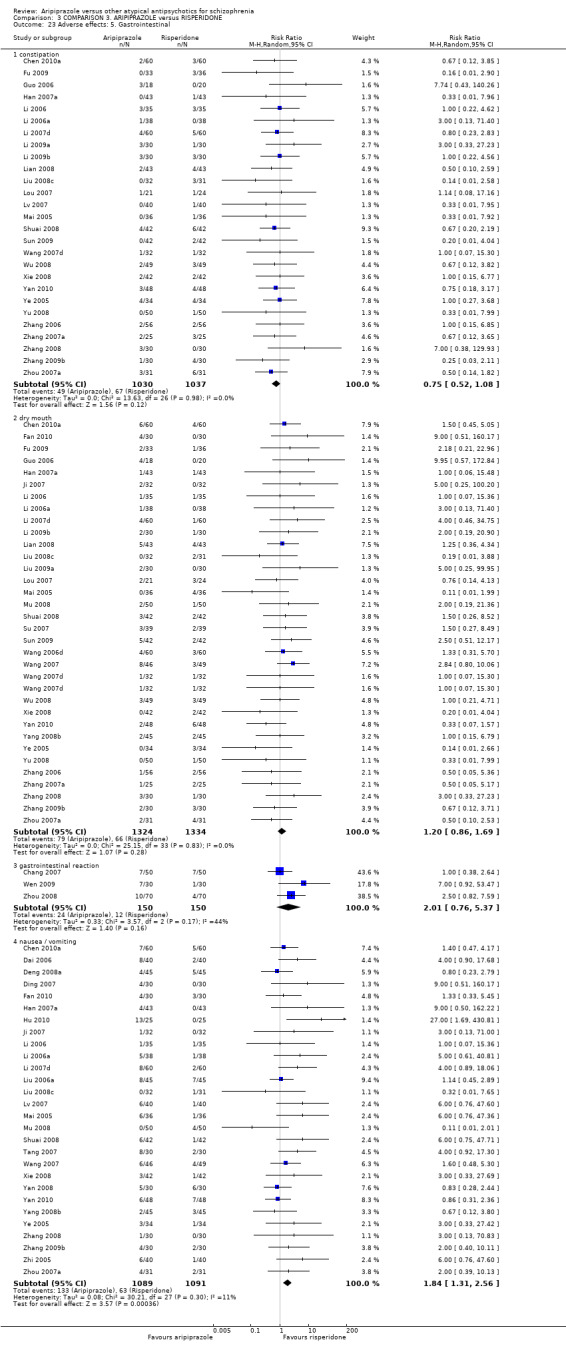
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 23 Adverse effects: 5. Gastrointestinal.
3.24 Adverse effects: 6. Haematological
All data demonstrate no difference between groups for abnormal blood routine (6 RCTs, n = 515, Analysis 3.24); leucopenia (1 RCT, n = 60) and abnormal blood lipids (1 RCT, n = 80).
3.24. Analysis.
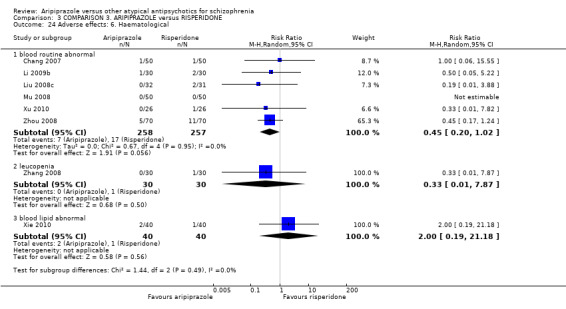
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 24 Adverse effects: 6. Haematological.
3.25 Adverse effects: 7a. Metabolic ‐ binary measures (short term, up to 12 weeks)
3.25.1 Appetite ‐ decrease
Data demonstrate statistical significance (P = 0.05) in favour of aripiprazole (2 RCTs, n = 204, RR 0.26 CI 0.07 to 1.03, Analysis 3.25).
3.25. Analysis.
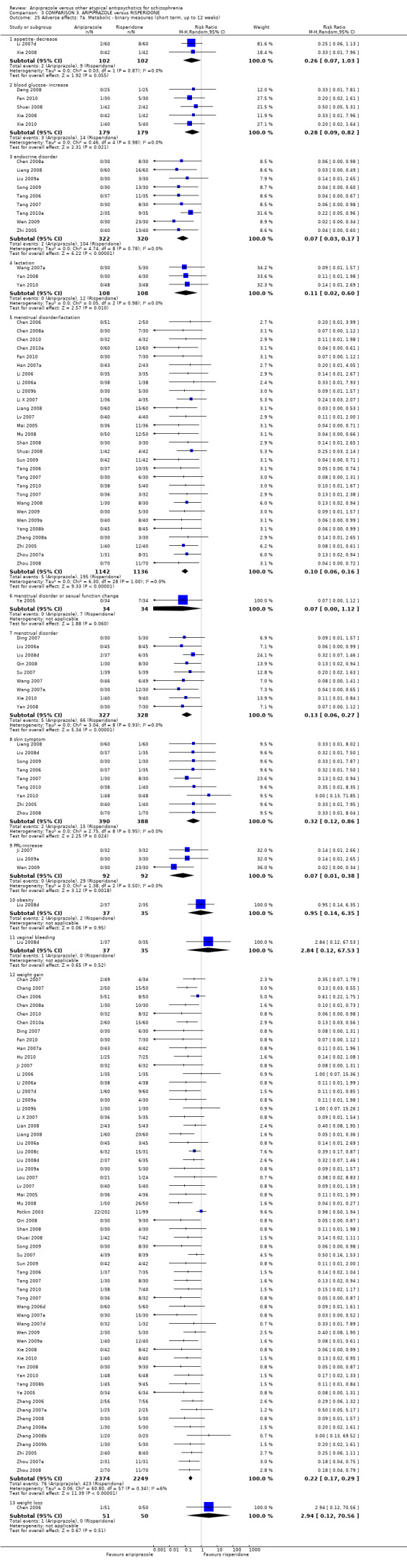
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 25 Adverse effects: 7a. Metabolic ‐ binary measures (short term, up to 12 weeks).
3.25.2 Blood glucose‐ increase
Data demonstrate statistical significance (P = 0.02) in favour of aripiprazole (5 RCTs, n = 358, RR 0.28 CI 0.09 to 0.82).
3.25.3 Endocrine disorder
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (9 RCTs, n = 642, RR 0.07 CI 0.03 to 0.17).
3.25.4 Lactation
Data demonstrate statistical significance (P = 0.01) in favour of aripiprazole (3 RCTs, n = 216, RR 0.11 CI 0.02 to 0.60).
3.25.5 Menstrual disorder/lactation
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (29 RCTs, n = 2278, RR 0.10 CI 0.06 to 0.16).
3.25.6 Menstrual disorder or sexual function change
Data demonstrate no significant difference between groups (1 RCT, n = 68).
3.25.7 Menstrual disorder
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (9 RCTs, n = 655, RR 0.13 CI 0.06 to 0.27).
3.25.8 Skin symptoms
Data demonstrate statistical significance (P = 0.02) in favour of aripiprazole (9 RCTs, n = 778, RR 0.32 CI 0.12 to 0.86).
3.25.9 PRL‐increase
Data demonstrate statistical significance (P = 0.002) in favour of aripiprazole (3 RCTs, n = 184, RR 0.07 CI 0.01 to 0.38).
3.25.10 Obesity
Data demonstrate no significant difference between groups (1 RCT, n = 72).
3.25.11 Vaginal bleeding
Data demonstrate no significant difference between groups (1 RCT, n = 72).
3.25.12 Weight gain
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (58 RCTs, n = 4623, RR 0.22 CI 0.17 to 0.29).
3.25.13 Weight loss
Data demonstrate no significant difference between groups (1 RCT, n = 101)
3.26 Adverse effects: 7b. Metabolic ‐ continuous measures
3.26.1 Endpoint average weight (in kg)
Data demonstrate statistical significance (P = 0.02) in favour of aripiprazole (5 RCTs, n = 465, MD ‐2.30 CI ‐4.17 to ‐0.44, Analysis 3.26), however with substantial heterogeneity present (ChI2 = 37.17; df = 4; P = 0.0; I2 = 89%).
3.26. Analysis.
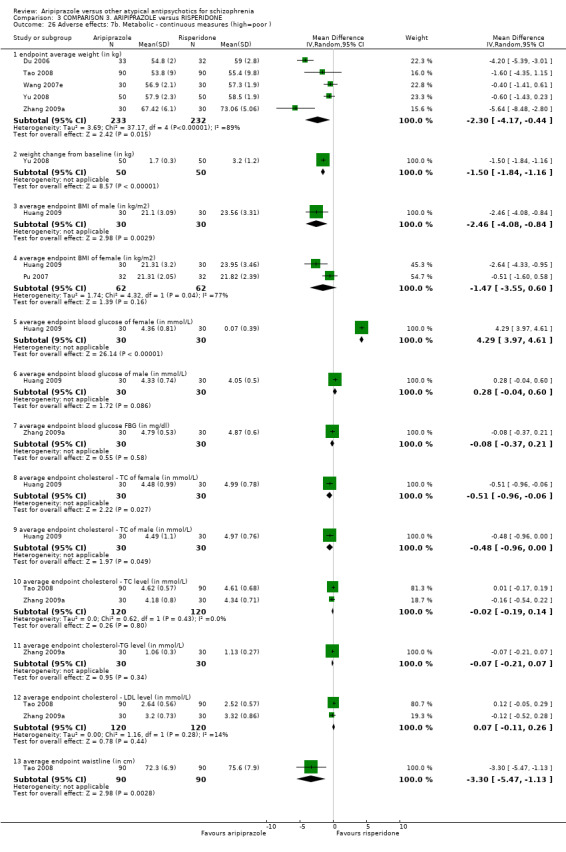
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 26 Adverse effects: 7b. Metabolic ‐ continuous measures (high=poor ).
3.26.2 Weight change from baseline (in kg)
Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (1 RCT, n = 100, MD ‐1.50 CI ‐1.84 to ‐1.16).
3.26.3 Average endpoint BMI of male participants (in kg/m2)
Data from one small RCT demonstrate statistical significance (P = 0.003) in favour of aripiprazole (1 RCT, n = 60, MD ‐2.46 CI ‐4.08 to ‐0.84).
3.26.4 Average endpoint BMI of female participants (in kg/m2)
Data demonstrate no significant difference between groups (2 RCTs, n = 124, MD ‐1.47 CI ‐3.55 to 0.60) with substantial heterogeneity between groups (ChI2 = 4.32; df = 1; P = 0.038; I2 = 77%).
3.26.5 Average endpoint blood glucose of female participants (in mmol/L)
Data demonstrate statistical significance (P = 0.00001) in favour of risperidone (1 RCT, n = 60).
3.26.6 Average endpoint blood glucose of male participants (in mmol/L)
Data demonstrate no significant difference between groups (1 RCT, n = 60).
3.26.7 Average endpoint blood glucose FBG (in mg/dL)
Data demonstrate no significant difference between groups (1 RCT, n = 60).
3.26.8 Average endpoint cholesterol ‐ TC of female participants (in mmol/L)
Data from one small RCT demonstrate statistical significance (P = 0.03) in favour of aripiprazole (1 RCT, n = 60, MD ‐0.51 CI ‐0.96 to ‐0.06).
3.26.9 Average endpoint cholesterol ‐ TC of male participants (in mmol/L)
Data from one small RCT demonstrate statistical significance (P = 0.05) in favour of aripiprazole (1 RCT, n = 60, MD ‐0.48 CI ‐0.96 to 0.00).
3.26.10 Average endpoint cholesterol ‐ TC level (in mmol/L)
Data demonstrate no significant difference between groups (2 RCTs, n = 240).
3.26.11 Average endpoint cholesterol ‐ TG level (in mmol/L)
Data demonstrate no significant difference between groups (1 RCT, n = 60).
3.26.12 Average endpoint cholesterol ‐ LDL level (in mmol/L)
Data demonstrate no significant difference between groups (2 RCTs, n = 240).
3.26.13 Average endpoint waistline (in cm)
Data from one small RCT demonstrate statistical significance (P = 0.003) in favour of aripiprazole (1 RCT, n = 180, MD ‐3.30 CI ‐5.47 to ‐1.13).
3.27. Adverse effect: 7c. Metabolic ‐ continuous measures
Data from one small RCT demonstrate statistical significance (P = 0.01) in favour of aripiprazole for change in cholesterol from baseline (1 RCT, n = 83, MD ‐22.30 CI ‐39.69 to ‐4.91, Analysis 3.27). Remaining data demonstrate no significant difference between groups.
3.27. Analysis.
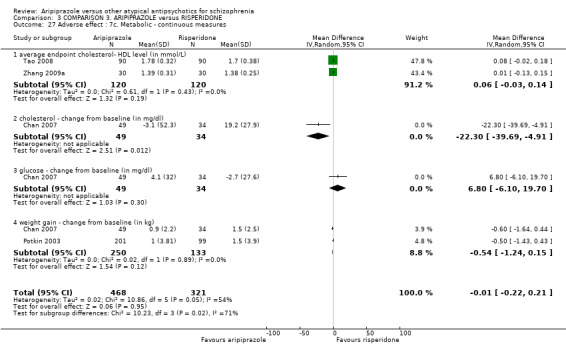
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 27 Adverse effect : 7c. Metabolic ‐ continuous measures.
3.28 Adverse effect: 8. required additional drug combination
3.28.1 Benzodiazepines
Data demonstrate no significant difference between groups (2 RCTs, n = 138, RR 1.07 CI 0.73 to 1.58, Analysis 3.28).
3.28. Analysis.
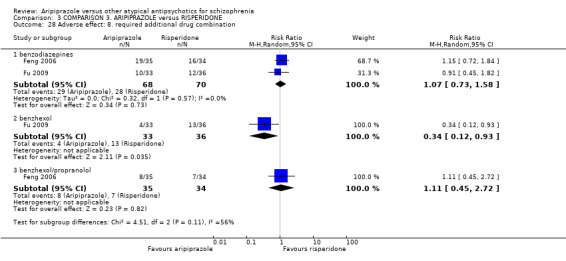
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 28 Adverse effect: 8. required additional drug combination.
3.28.2 Benzhexol
Data from one small RCT demonstrate statistical significance (P = 0.04) in favour of aripiprazole (1 RCT, n = 69, RR 0.34 CI 0.12 to 0.93).
3.28.3 Benzhexol/propranolol
Data demonstrate no significant difference between groups (1 RCT, n = 69, RR 1.11 CI 0.45 to 2.72).
3.29 Adverse effects: 9.TESS score (short term, up to 12 weeks, high = poor)
Data demonstrate statistical significance (P = 0.006) in favour of aripiprazole (4 RCTs, n = 250, MD ‐1.34 CI ‐2.3 to ‐0.39, Analysis 3.29), however with substantial heterogeneity present (ChI2 = 48.54; df = 3; P = 0.00001; I2 = 94%).
3.29. Analysis.
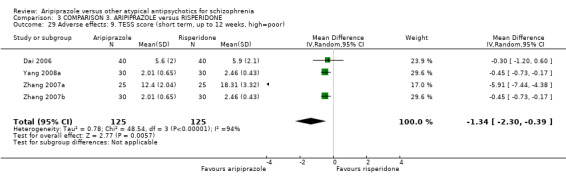
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 29 Adverse effects: 9. TESS score (short term, up to 12 weeks, high=poor).
3.30 Adverse effects: 10. TESS score (skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 3.30.
3.30. Analysis.
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 30 Adverse effects: 10. TESS score (short term, up to 12 weeks, high=poor, data skewed).
| Adverse effects: 10. TESS score (short term, up to 12 weeks, high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| Chen 2006 | Aripiprazole | 0.2 | 0.5 | 51 | |
| Chen 2006 | Risperidone | 0.1 | 0.4 | 50 | |
| CuiMeng 2008 | Aripiprazole | 5.35 | 4.25 | 60 | |
| CuiMeng 2008 | Risperidone | 7.95 | 5.15 | 60 | |
| Mu 2010 | Aripiprazole | 3.59 | 2.08 | 129 | |
| Mu 2010 | Risperidone | 3. 62 | 2.14 | 129 | |
| Qu 2009 | Aripiprazole | 5.35 | 4.2 | 30 | |
| Qu 2009 | Risperidone | 7.95 | 5.1 | 30 | |
| Yan 2010 | Aripiprazole | 3.42 | 2.34 | 50 | |
| Yan 2010 | Risperidone | 4.45 | 1.92 | 50 | |
| Zhang 2010a | Aripiprazole | 5.35 | 4.25 | 50 | |
| Zhang 2010a | Risperidone | 7.95 | 5.15 | 50 | |
| Zhou 2007b | Aripiprazole | 5.35 | 4.25 | 30 | |
| Zhou 2007b | Risperidone | 7.95 | 5.15 | 30 | |
3.31 Adverse effects: 11. weight gain (skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 3.31.
3.31. Analysis.
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 31 Adverse effects: 11. weight gain (in KG, high=poor, data skewed).
| Adverse effects: 11. weight gain (in KG, high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
| Chen 2006 | Aripiprazole | 1.1 | 1.5 | 51 | |
| Chen 2006 | Risperidone | 1.8 | 1.4 | 50 | |
| Tao 2008 | Aripiprazole | 2.06 | 2.67 | 90 | |
| Tao 2008 | Risperidone | 2.73 | 2.43 | 90 | |
| Wang 2007e | Aripiprazole | 0.3 | 1.97 | 30 | |
| Wang 2007e | Risperidone | 1.0 | 3.1 | 30 | |
3.32 Cognitive functioning: 1. Specific ‐ average endpoint total score
3.32.1 Short term, up to 12 weeks, WMS, low = poor
Data demonstrate no significant difference between groups for both WMS (1 RCT, n = 72, Analysis 3.32) and WAIS‐RC (1 RCT, n = 72).
3.32. Analysis.
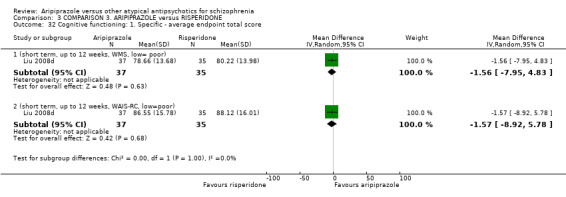
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 32 Cognitive functioning: 1. Specific ‐ average endpoint total score.
3.33 Cost‐effectiveness analysis (skewed)
All data for this outcome are skewed and are best viewed by inspecting Analysis 3.33.
3.33. Analysis.
Comparison 3 COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE, Outcome 33 Cost effectiveness analysis (high=poor, data skewed).
| Cost effectiveness analysis (high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| Cost of hospitalisation (in RMB) | |||||
| Liu 2010 | Aripiprazole | 3413.66 | 1815.05 | 30 | |
| Liu 2010 | Risperidone | 4551.41 | 3024.38 | 54 | |
| Cost of drug (in RMB) | |||||
| Liu 2010 | Aripiprazole | 418.13 | 326.43 | 30 | |
| Liu 2010 | Risperidone | 685.00 | 493.02 | 54 | |
| Length of hospitalisation (day) | |||||
| Liu 2010 | Aripiprazole | 33.19 | 16.71 | 30 | |
| Liu 2010 | Risperidone | 50.84 | 40.85 | 28 | |
COMPARISON 4: ARIPIPRAZOLE versus ZIPRASIDONE
4.1 Global state: 1. No clinically significant response (as defined by the original studies)
Short term data demonstrate no significant difference between groups (6 RCTs, n = 442, RR 0.97 CI 0.62 to 1.52, Analysis 4.1).
4.1. Analysis.
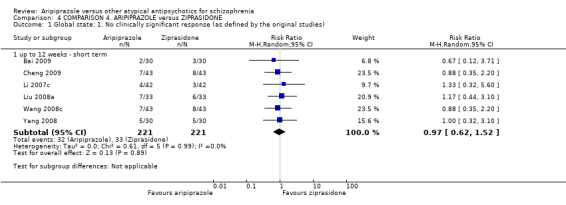
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 1 Global state: 1. No clinically significant response (as defined by the original studies).
4.2 Global state: 2. Average endpoint CGI‐GI score (short term, up to 12 weeks, high = poor)
Data from one small RCT demonstrate statistical significance (P = 0.001) in favour of aripiprazole (1 RCT, n = 86, MD ‐3.93 CI ‐6.32 to ‐1.54, Analysis 4.2).
4.2. Analysis.
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 2 Global state: 2. Average endpoint CGI‐GI score (short term, up to 12 weeks, high=poor).
4.3 Global state: 3. Average change score (CGI‐S, decline = best)
Data demonstrate no significant difference between groups (1 RCT, n = 247, MD ‐0.03 CI ‐0.28 to 0.22, Analysis 4.3).
4.3. Analysis.
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 3 Global state: 3. Average change score (CGI‐S, decline=good).
4.4 Mental state: 1. Average endpoint total score (short term, up to 12 weeks, high = poor)
PANSS data demonstrate no significant difference between groups (7 RCTs, n = 689, Analysis 4.4), neither do BPRS data (1 RCT, n = 247). However, SANS data demonstrate statistical significance (P = 0.02) in favour of aripiprazole (3 RCTs, n = 238, MD ‐1.39 CI ‐2.56 to ‐0.22).
4.4. Analysis.
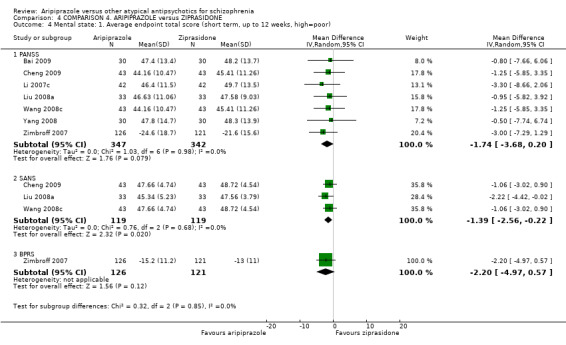
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 4 Mental state: 1. Average endpoint total score (short term, up to 12 weeks, high=poor).
4.5 Mental state: 2. Specific ‐ binary outcomes (up to 12 weeks ‐ short term)
There was no significant difference between groups for anxiety (1 RCT, n = 86) or agitation (2 RCTs, n = 150, Analysis 4.5).
4.5. Analysis.
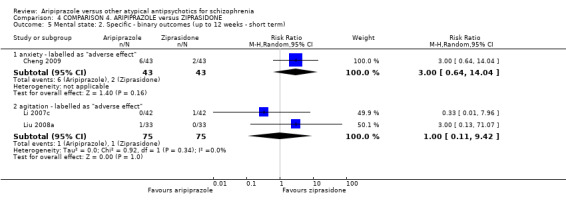
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 5 Mental state: 2. Specific ‐ binary outcomes (up to 12 weeks ‐ short term).
4.6 Mental state: 3. Specific ‐ average endpoint PANSS subscale scores (short term, high = poor)
There was no significant difference between groups for positive symptom score (2 RCTs, n = 146); negative symptom scores (4 RCTs, n = 272); and general pathology scores (5 RCTs, n = 382, Analysis 4.6.)
4.6. Analysis.
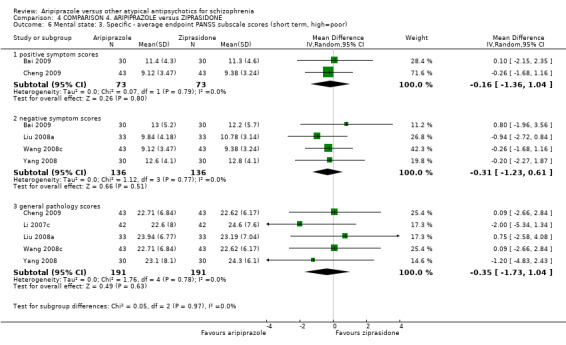
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 6 Mental state: 3. Specific ‐ average endpoint PANSS subscale scores (short term, high=poor).
4.7 Mental state: 4. Average endpoint scores of various scales (skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 4.7.
4.7. Analysis.
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 7 Mental state: endpoint scores of various scales (high=poor, data skewed).
| Mental state: endpoint scores of various scales (high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| PANSS negative symptom subscale score | |||||
| Cheng 2009 | Aripiprazole | 14.56 | 9.51 | 43 | |
| Cheng 2009 | Ziprasidone | 13.12 | 5.16 | 43 | |
| PANSS positive symptom subscale score | |||||
| Liu 2008a | Aripiprazole | 14.21 | 5.45 | 33 | |
| Liu 2008a | Ziprasidone | 12.96 | 8.43 | 33 | |
| Wang 2008c | Aripiprazole | 13.12 | 5.16 | 43 | |
| Wang 2008c | Ziprasidone | 14.56 | 9.51 | 43 | |
| Yang 2008 | Aripiprazole | 11.3 | 5.7 | 30 | |
| Yang 2008 | Ziprasidone | 11.5 | 3.9 | 30 | |
4.8 Leaving the study early
All data for leaving study early were equivocal, demonstrating no significant difference between groups (Analysis 4.8).
4.8. Analysis.
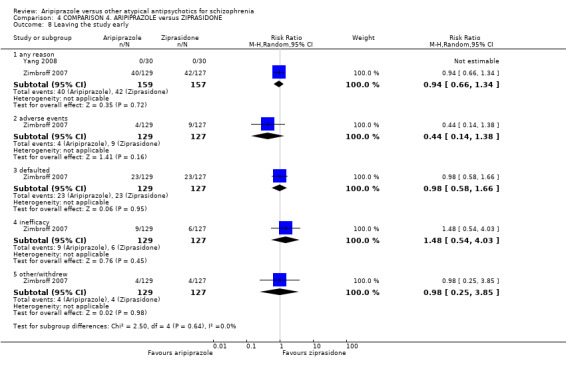
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 8 Leaving the study early.
4.9 Adverse effects:1.At least one adverse effect, non‐specific
Data demonstrate statistical significance (P = 0.0001) in favour of ziprasidone for sexual function change (2 RCTs, n = 172, RR 8.00 CI 2.96 to 21.65, Analysis 4.9); all other data for adverse effects were equivocal.
4.9. Analysis.
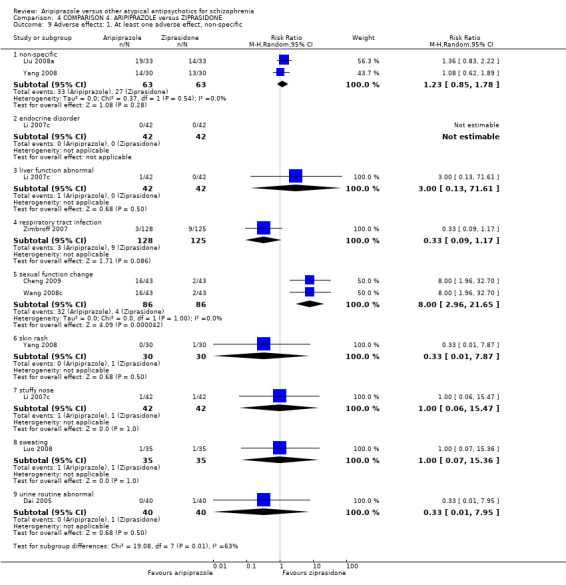
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 9 Adverse effects: 1. At least one adverse effect, non‐specific.
4.10 Adverse effects: 2.Cardiac effects (short term, up to 12 weeks)
All data for cardiac effects are equivocal and demonstrate no difference between groups (Analysis 4.10).
4.10. Analysis.
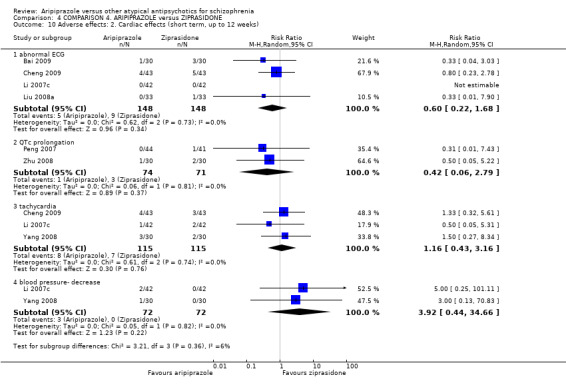
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 10 Adverse effects: 2. Cardiac effects (short term, up to 12 weeks).
4.11 Adverse effects: 3. Central/ peripheral nervous system
Data for dizziness demonstrate statistical significance (P = 0.002) in favour of ziprasidone (5 RCTs, n = 376, RR 3.24 CI 1.57 to 6.70, Analysis 4.11), as well as data for insomnia (P = 0.02) (5 RCTs, n = 382, RR 2.93 CI 1.17 to 7.30), however with slight heterogeneity present (ChI2 = 7.38; df = 4; P = 0.117; I2 = 46%). All other data are equivocal, demonstrating no significant difference between groups, including for headache (2 RCTs, n = 150); blurred vision (1 RCT, n = 84) and somnolence (4 RCTs, n = 296).
4.11. Analysis.
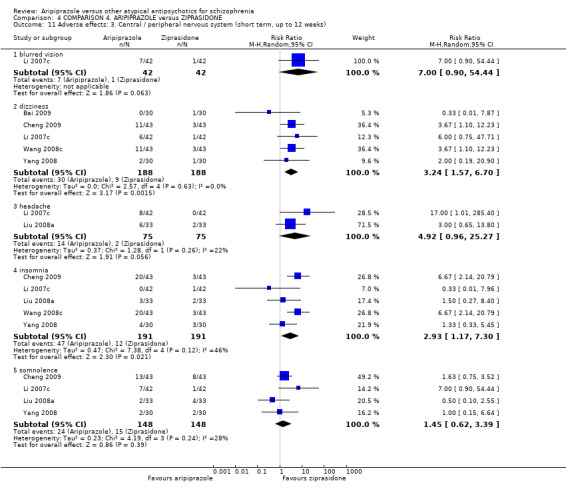
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 11 Adverse effects: 3. Central / peripheral nervous system (short term, up to 12 weeks).
4.12 Adverse effects: 4. Various extrapyramidal symptoms (short term, up to 12 weeks)
All data were equivocal, demonstrating no difference between groups (Analysis 4.12), including dystonia (1 RCT, n = 84); tremor (2 RCTs, n = 152) and use of anti‐Parkinson drugs (2 RCTs, n = 140) with substantial heterogeneity present (ChI2 = 6.94; df = 1; P = 0.008; I2 = 86%).
4.12. Analysis.
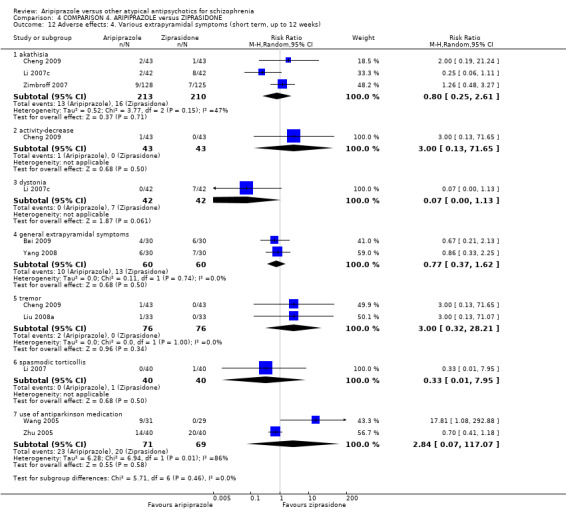
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 12 Adverse effects: 4. Various extrapyramidal symptoms (short term, up to 12 weeks).
4.13 Adverse effects: 5. Gastrointestinal (short term, up to 12 weeks)
Again, all data were equivocal, demonstrating no difference between groups (Analysis 4.13), including: constipation (3 RCTs, n = 230); dry mouth (4 RCTs, n = 296); hyper‐salivation (1 RCT, n = 86) and nausea/vomiting (6 RCTs, n = 442).
4.13. Analysis.
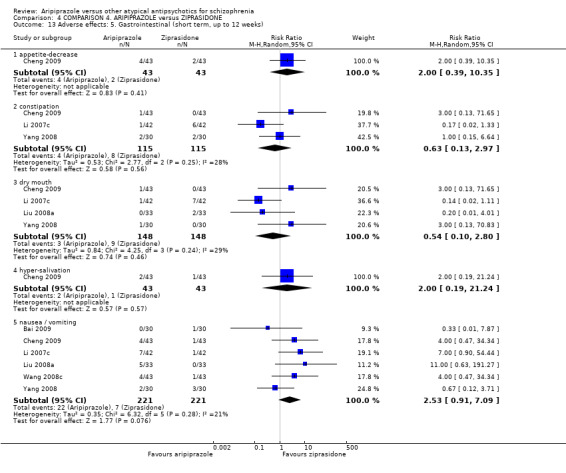
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 13 Adverse effects: 5. Gastrointestinal (short term, up to 12 weeks).
4.14 Adverse effects: 6. Haematological
Data for leucopenia demonstrate no significant difference between groups (2 RCTs, n = 140, Analysis 4.14).
4.14. Analysis.
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 14 Adverse effects: 6. Haematological.
4.15 Adverse effects: 7. Hormonal
Data for menstrual disorder demonstrate no significant difference between groups (6 RCTs, n = 538, Analysis 4.15).
4.15. Analysis.
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 15 Adverse effects: 7. Hormonal.
4.16 Adverse effects: 8a. Metabolic ‐ binary measures
All data were equivocal, demonstrating no difference between groups, including appetite decrease (2 RCTs, n = 152, Analysis 4.16)) with substantial heterogeneity present (ChI2 = 4.04; df = 1; P = 0.045; I2 = 75%); abnormal blood routine (1 RCT, n = 85); lactation (1 RCT, n = 66); and weight gain (3 RCTs, n = 232).
4.16. Analysis.
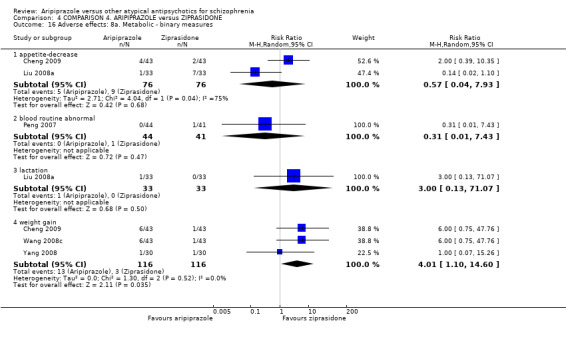
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 16 Adverse effects: 8a. Metabolic ‐ binary measures.
4.17 Adverse effects: 8b. Metabolic ‐ continuous measures
Most data were equivocal, demonstrating no difference between groups; however, data for HDL demonstrate statistical significance (P = 0.04) in favour of aripiprazole (1 RCT, n = 180, MD 0.10 CI 0.01 to 0.19) and data for waistline average endpoint level (in cm) demonstrate statistical significance (P = 0.0004) in favour of aripiprazole (1 RCT, n = 180, MD ‐3.40 CI ‐5.29 to ‐1.51 Analysis 4.17).
4.17. Analysis.
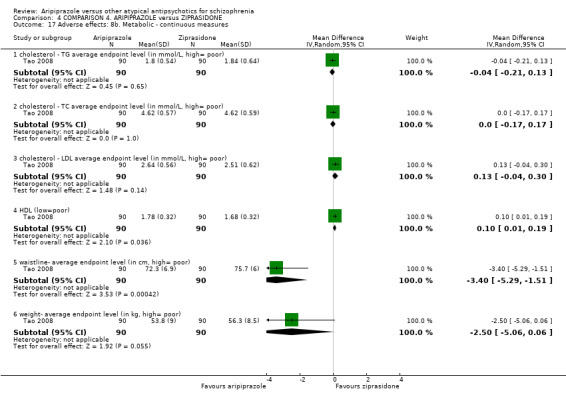
Comparison 4 COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE, Outcome 17 Adverse effects: 8b. Metabolic ‐ continuous measures.
COMPARISON 5: ARIPIPRAZOLE versus OLANZAPINE
5.1 Global state: 1. No clinically significant response (as defined by the original studies)
Data are equivocal at short term (10 RCTs, n = 1422) and medium term (1 RCT, n = 317), demonstrating no significant difference between groups (Analysis 5.1).
5.1. Analysis.
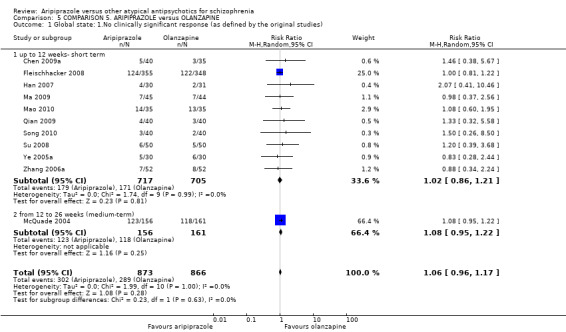
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 1 Global state: 1.No clinically significant response (as defined by the original studies).
5.2 Global state: 2. Not responded (decline in PANSS of 30% or more)
5.2.1 By up to 12 weeks (short term)
Data from one small RCT demonstrate statistical significance (P = 0.04) in favour of olanzapine at short term (1 RCT, n = 566, RR 1.16 CI 1.01 to 1.34, Analysis 5.2) and long term (P = 0.05) (1 RCT, n = 566, RR 1.13 CI 1.0 to 1.27).
5.2. Analysis.
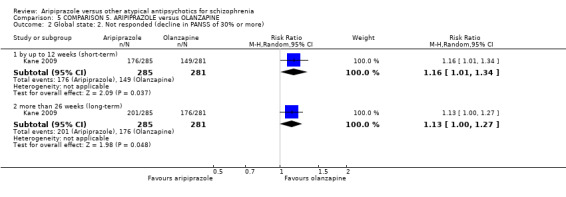
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 2 Global state: 2. Not responded (decline in PANSS of 30% or more).
5.3 Global state: 3. Remission not achieved (as defined in the study)
Data demonstrate no significant difference between groups at short term (1 RCT, n = 566, Analysis 5.3). Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of olanzapine by long term (1 RCT, n = 566, RR 1.38 CI 1.23 to 1.56).
5.3. Analysis.
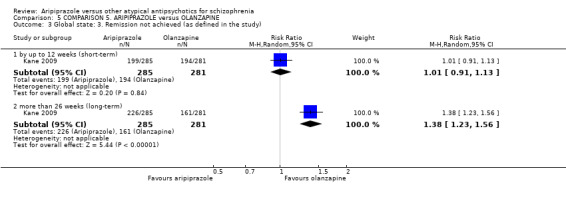
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 3 Global state: 3. Remission not achieved (as defined in the study).
5.4 Global state: 4. Average endpoint EI score (CGI, high = poor)
Data demonstrate no significant difference between groups at short term (2 RCTs, n = 180, Analysis 5.4).
5.4. Analysis.
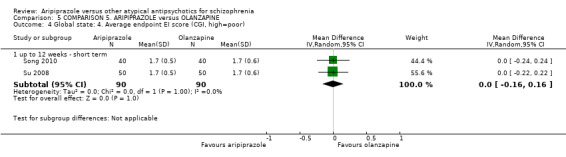
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 4 Global state: 4. Average endpoint EI score (CGI, high=poor).
5.5 Global state: 5. Average endpoint CGI score decreased rate (short term, low = poor)
Data demonstrate no significant difference between groups (1 RCT, n = 60, Analysis 5.5).
5.5. Analysis.
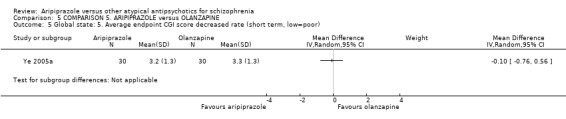
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 5 Global state: 5. Average endpoint CGI score decreased rate (short term, low=poor).
5.6 Global state: 6. Average change score (CGI‐S, decline = best)
Data demonstrate no significant difference between groups at long term (1 RCT, n = 566, Analysis 5.6).
5.6. Analysis.
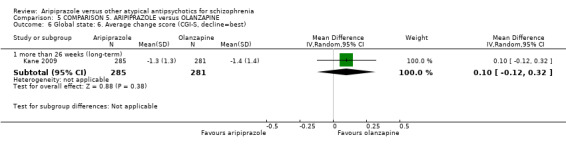
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 6 Global state: 6. Average change score (CGI‐S, decline=best).
5.7 Global state: 7. Improvement (CGI‐I, high = poor)
Data demonstrate no significant difference between groups (1 RCT, n = 566, Analysis 5.7).
5.7. Analysis.
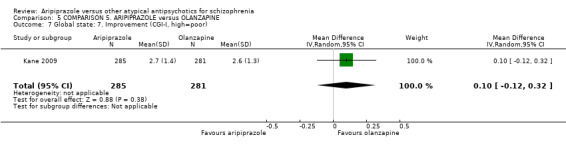
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 7 Global state: 7. Improvement (CGI‐I, high=poor).
5.8 Mental state: 1. General ‐ average total score (PANSS, high = poor)
Data are equivocal, demonstrating no significant difference between groups at short term (11 RCTs, n = 1500, Analysis 5.8) and medium term (2 RCTs, n = 139). Data from one small RCT demonstrate statistical significance (P = 0.04) in favour of olanzapine (1 RCT, n = 566, MD 4.20 CI 0.10 to 8.3) in the long term.
5.8. Analysis.
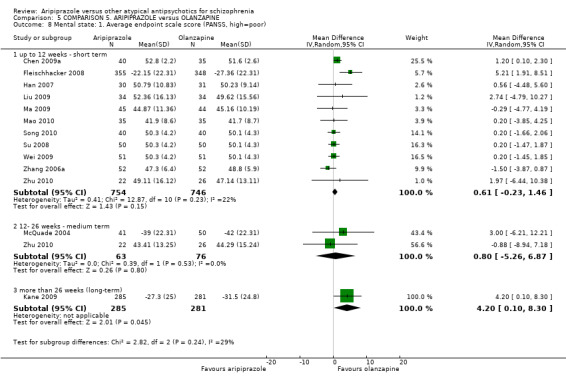
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 8 Mental state: 1. Average endpoint scale score (PANSS, high=poor).
5.9 Mental state: 2. Average endpoint scale score (SANS, high = poor)
Data demonstrate no significant difference between groups at short term (1 RCT, n = 89, Analysis 5.9) and long term (1 RCT, n = 48).
5.9. Analysis.
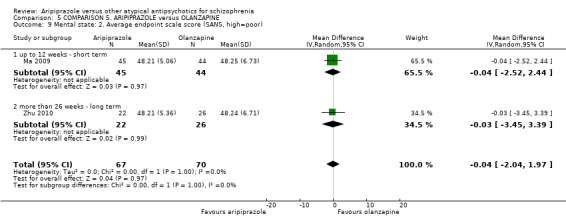
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 9 Mental state: 2. Average endpoint scale score (SANS, high=poor).
5.10 Mental state: 3. average endpoint score (PANSS, skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 5.10.
5.10. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 10 Mental state: 3. average endpoint score (PANSS, high=poor, data skewed).
| Mental state: 3. average endpoint score (PANSS, high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| PANSS total | |||||
| Bai 2007 | Aripiprazole | 11.26 | 5.07 | 59 | |
| Bai 2007 | Olanzapine | 10.24 | 5.85 | 59 | |
| PANSS negative symptom subscale score | |||||
| Han 2007 | Aripiprazole | 12.7 | 3.8 | 30 | |
| Han 2007 | Ziprasidone | 12.6 | 6.8 | 30 | |
| PANSS positive symptom subscale score | |||||
| Han 2007 | 11.3 | 4.3 | 30 | ||
| Han 2007 | 11.5 | 6.7 | 30 | ||
5.11 Mental state: 4. Specific ‐ average endpoint positive score (PANSS, high = poor)
Overall, data from all time frames demonstrate statistical significance (P = 0.01) in favour of olanzapine (7 RCTs, n = 1043, MD 0.71 CI 0.17 to 1.26, Analysis 5.11). Data are equivocal at short term, demonstrating no significant difference between groups (5 RCTs, n = 429) with slight heterogeneity between groups (ChI2 = 6.26; df = 4; P = 0.181; I2 = 36%). Data demonstrate no significant difference at medium term (1 RCT, n = 48) or long term (1 RCT, n = 566).
5.11. Analysis.
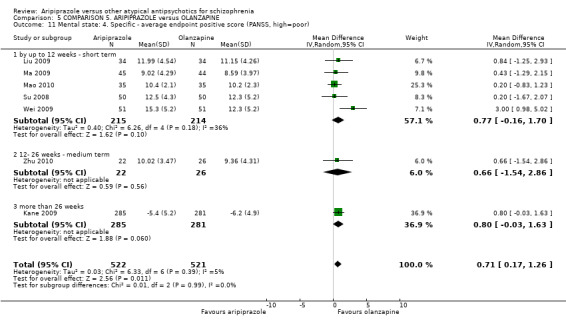
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 11 Mental state: 4. Specific ‐ average endpoint positive score (PANSS, high=poor).
5.12 Mental state: 5. Specific ‐ average endpoint negative subscale score (PANSS, high = poor)
At both short and medium term, data demonstrate no significant difference between group (6 RCTs, n = 967, Analysis 5.12).
5.12. Analysis.
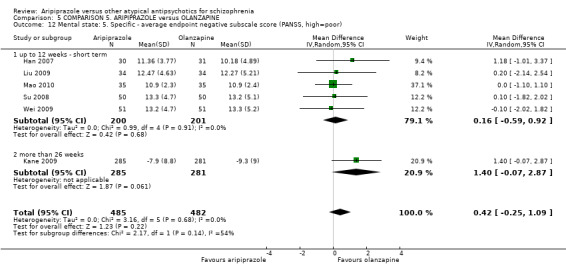
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 12 Mental state: 5. Specific ‐ average endpoint negative subscale score (PANSS, high=poor).
5.13 Mental state: 6. Specific ‐ average endpoint general pathological score (PANSS, high = poor)
At both short and medium term, data demonstrate no significant difference between groups (8 RCTs, n = 642, Analysis 5.13).
5.13. Analysis.
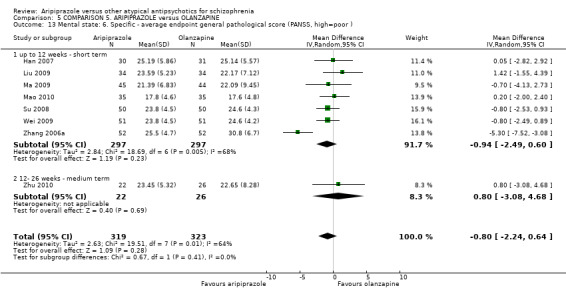
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 13 Mental state: 6. Specific ‐ average endpoint general pathological score (PANSS, high=poor ).
5.14 Mental state: 7. Specific ‐ binary outcomes
5.14.1 Anxiety ‐ labelled as" adverse effect"
Data demonstrate no significant difference between groups for anxiety (2 RCTs, n = 778, Analysis 5.14) or exacerbation of schizophrenia (2 RCTs, n = 778). Data from one small RCT demonstrate statistical significance (P = 0.04) in favour of aripiprazole for depression (1 RCT, n = 566, RR 0.27 CI 0.08 to 0.95).
5.14. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 14 Mental state: 7. Specific ‐ binary outcomes.
5.15 Mental state: 8. Average various scale scores decreased rate (skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 5.15.
5.15. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 15 Mental state: 8. Various scale scores decreased rate (low=poor, data skewed).
| Mental state: 8. Various scale scores decreased rate (low=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
| PANSS positive symptom subscale | |||||
| Ye 2005a | Aripiprazole | 12.8 | 8.4 | 34 | |
| PANSS negative symptom subscale | |||||
| Ye 2005a | Aripiprazole | 10.9 | 8.1 | 34 | |
| PANSS general pathology subscale | |||||
| Ye 2005a | Aripiprazole | 19.4 | 11.8 | 34 | |
| BPRS total | |||||
| Ye 2005a | Aripiprazole | 24.8 | 4.3 | 34 | |
| PANSS total | |||||
| Ye 2005a | Aripiprazole | 44.1 | 23.8 | 34 | |
5.16 Adverse effects: 1. At least one adverse effect
For non‐specific adverse effects, data demonstrate no significant difference between groups (1 RCT, n = 75, Analysis 5.16). For endocrine dyscrasia, data from one small RCT demonstrate statistical significance (P = 0.01) in favour of aripiprazole (1 RCT, n = 80, RR 0.08 CI 0.01 to 0.61); and for high prolactin level (P = 0.001) (1 RCT, n = 317, RR 0.27 CI 0.12 to 0.60). Remaining data are equivocal and demonstrate no difference between groups, including skin reaction (1 RCT, n = 89); and liver function abnormality (5 RCTs, n = 348).
5.16. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 16 Adverse effects: 1. At least one adverse effect.
5.17 Adverse effects: 2. Cardiac effects
All data are equivocal and demonstrate no difference between groups for abnormal ECG (2 RCTs, n = 121); decrease in blood pressure (1 RCT, n = 89); QTc prolongation (3 RCTs, n = 618) and tachycardia (5 RCTs, n = 339).
5.18 Adverse effects: 3a. Cardiac ‐ QTc change from baseline (in ms)
Data from one RCT demonstrate no significant difference between groups (1 RCT, n = 317, Analysis 5.18).
5.18. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 18 Adverse effects: 3a. Cardiac ‐ QTc change from baseline (in ms).
5.19 Adverse effects: 3b. Central/ peripheral nervous system
Data from two RCTs demonstrate statistical significance (P = 0.0001) in favour of aripiprazole for levels of sedation (2 RCTs, n = 883, RR 0.37 CI 0.23 to 0.60) and data from one small RCT demonstrate statistical significance (P = 0.05) in favour of aripiprazole for headache/dizziness (1 RCT, n = 89, RR 0.29 CI 0.09 to 1.00, Analysis 3.19). All other data are equivocal and demonstrate no difference between groups, including dizziness (6 RCTs, n = 1057); blurred vision (1 RCT, n = 75); fatigue (3 RCTs, n = 721) and insomnia (7 RCTs, n = 1141), however with considerable heterogeneity present (ChI2 = 13.87; df = 6; P = 0.031; I2 = 57%).
5.20 Adverse effects: 4. Endocrine ‐ Prolactin ‐ average increase
Data from one small RCT demonstrate statistical significance (P = 0.0001) in favour of aripiprazole (1 RCT, n = 566, MD ‐8.89 CI ‐12.96 to ‐4.82, Analysis 5.20).
5.20. Analysis.
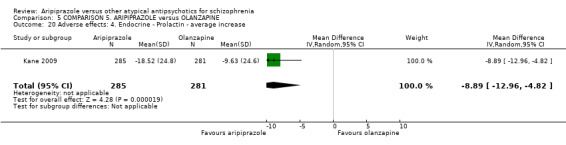
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 20 Adverse effects: 4. Endocrine ‐ Prolactin ‐ average increase.
5.21 Adverse effects: 5. Extrapyramidal ‐ various
All data are equivocal and demonstrate no difference between groups, including: tremor (1 RCT, n = 61); EPS (4 RCTs, n = 667); and parkinsonism (3 RCTs, n = 618). Data for akathisia are equivocal, demonstrating no significant difference between groups (6 RCTs, n = 1320, Analysis 5.21), however with considerable heterogeneity present (ChI2 = 19.45; df = 6; P = 0.003; I2 = 69%).
5.21. Analysis.
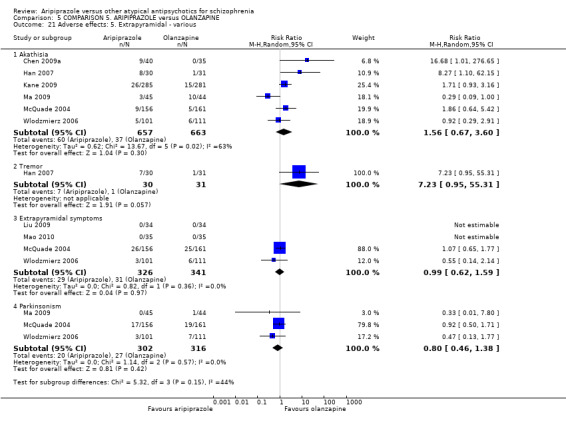
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 21 Adverse effects: 5. Extrapyramidal ‐ various.
5.22 Adverse effects: 6. Gastrointestinal
Data from one small RCT demonstrate statistical significance (P = 0.03) in favour of aripiprazole for abdominal pain (1 RCT, n = 566, RR 2.96 CI 1.09 to 8.03). All remaining data are equivocal and demonstrate no significant difference between groups, including: dry mouth (5 RCTs, n = 854); constipation (6 RCTs, n = 443) and nausea/vomiting (6 RCTs, n = 948, Analysis 5.22).
5.22. Analysis.
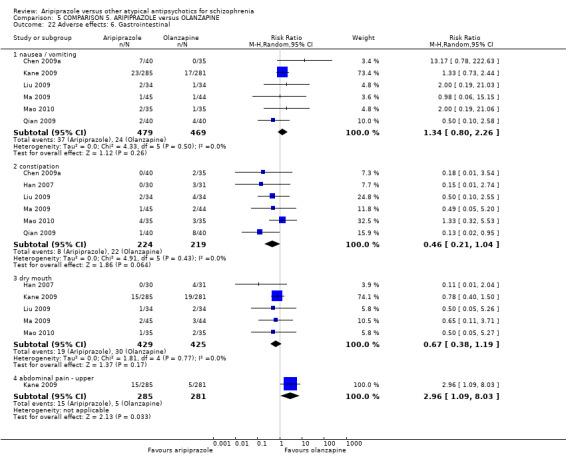
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 22 Adverse effects: 6. Gastrointestinal.
5.23 Adverse effects: 7. Hormonal
5.23.1 Menstrual changes
Data are equivocal and demonstrate no significant difference between groups (3 RCTs, n = 198, Analysis 5.23).
5.23. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 23 Adverse effects: 7. Hormonal.
5.24 Adverse effects: 8a. Metabolic ‐ binary measures
Data demonstrate statistical significance (P = 0.001) in favour of aripiprazole for blood glucose increase (3 RCTs, n = 227, RR 0.12 CI 0.03 to 0.44); for abnormally high cholesterol level (P = 0.0001) (1 RCT, n = 223, RR 0.32 CI 0.19 to 0.54, Analysis 5.24); and weight gain (P = 0.00001) (9 RCTs, n = 1538, RR 0.25 CI 0.15 to 0.43). All remaining data demonstrate no significant difference between groups for appetite increase (2 RCTs, n = 655); lactation (1 RCT, n = 60) and PRL increase (1 RCT, n = 89).
5.24. Analysis.
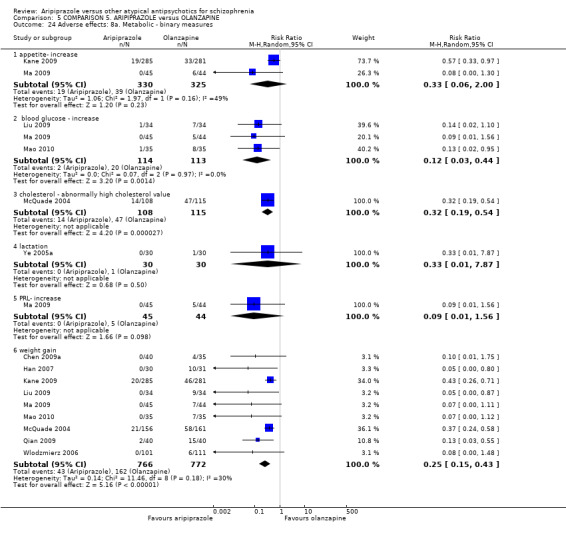
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 24 Adverse effects: 8a. Metabolic ‐ binary measures.
5.25 Adverse effects: 8b. Metabolic ‐ continuous measures (high = poor)
5.25.1 Weight ‐ average endpoint level (in kg)
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (3 RCTs, n = 242, MD ‐7.43 CI ‐9.21 to ‐5.65, Analysis 5.25).
5.25. Analysis.
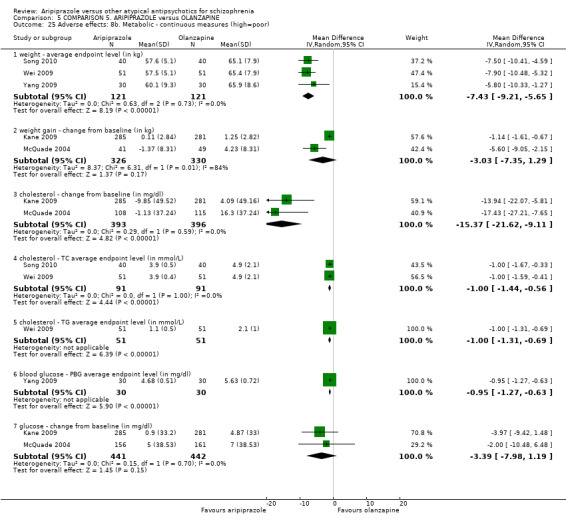
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 25 Adverse effects: 8b. Metabolic ‐ continuous measures (high=poor).
5.25.2 Weight gain ‐ change from baseline (in kg)
Data demonstrate no significant difference between groups (2 RCTs, n = 656), however with substantial heterogeneity present (ChI2 = 6.31; df = 1; P = 0.012; I2 = 84%).
5.25.3 Cholesterol ‐ change from baseline (in mg/dL)
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (2 RCTs, n = 789, MD ‐15.37 CI ‐21.62 to ‐9.11).
5.25.4 Cholesterol ‐ TC average endpoint level (in mmol/L)
Data demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (2 RCTs, n = 182, MD ‐1.00 CI ‐1.44 to ‐0.56).
5.25.5 Cholesterol ‐ TG average endpoint level (in mmol/L
Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (1 RCT, n = 102, MD ‐1.00 CI ‐1.31 to ‐0.69).
5.25.6 Blood glucose ‐ PBG average endpoint level (in mg/dL)
Data from one small RCT demonstrate statistical significance (P = 0.00001) in favour of aripiprazole (1 RCT, n = 60, MD ‐0.95 CI ‐1.27 to ‐0.63).
5.25.7 Glucose ‐ change from baseline (in mg/dl)
Data demonstrate no significant difference between groups (2 RCTs, n = 883).
5.26 Adverse effects: average endpoint scale scores (TESS, skewed)
All data for this outcome are skewed and are best inspected by viewing Analysis 5.26.
5.26. Analysis.
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 26 Adverse effects: 9. Average endpoint scale score (TESS, high=poor, data skewed).
| Adverse effects: 9. Average endpoint scale score (TESS, high=poor, data skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Note |
| Zhang 2006a | Aripiprazole | 4.43 | 4.03 | 52 | |
| Zhang 2006a | Olanzapine | 5.54 | 4.33 | 52 | |
5.27 Leaving the study early
Data for leaving for any reason from one small RCT demonstrate statistical significance (P = 0.002) in favour of olanzapine (9 RCTs, n = 2331, RR 1.15 CI 1.05 to 1.25, Analysis 5.27); statistical significance was also found in inefficacy (P = 0.003) in favour of olanzapine (4 RCTs, n = 1352, RR 1.81 CI 1.23 to 2.67). Remaining reasons for leaving the study early demonstrated no significant difference between groups, including adverse event (4 RCTs, n = 1352); early discharge (1 RCT, n = 60); medication non‐compliance (1 RCT, n = 255); and death (1 RCT, n = 214).
5.27. Analysis.
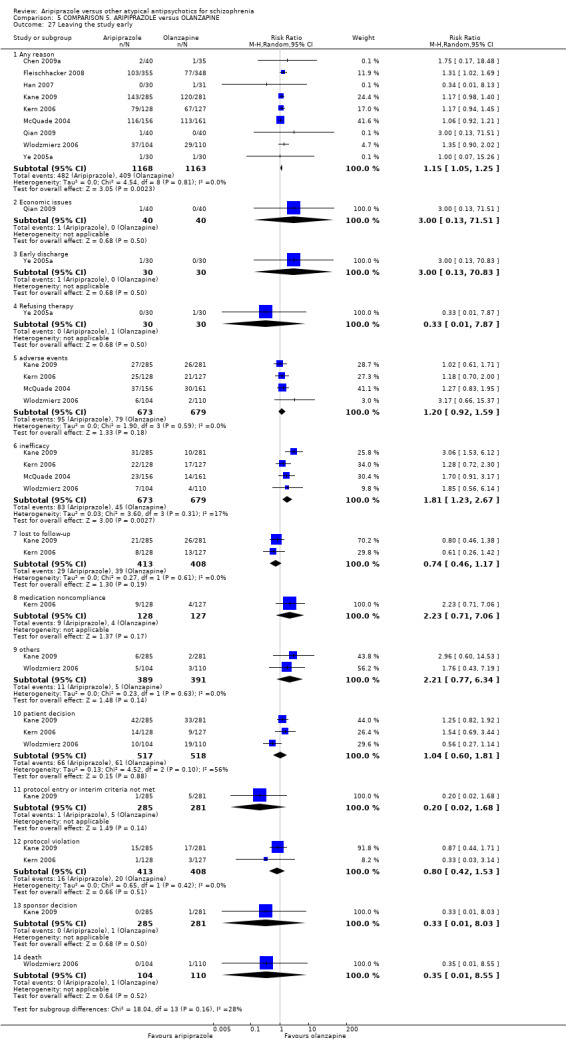
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 27 Leaving the study early.
5.28 Quality of life: 1. Average endpoint general quality of life score (GQOLI‐74, low = poor)
All data are equivocal and demonstrate no difference between groups (Analysis 5.28).
5.28. Analysis.
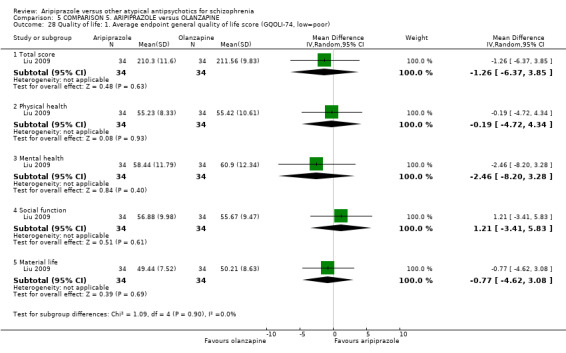
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 28 Quality of life: 1. Average endpoint general quality of life score (GQOLI‐74, low=poor).
COMPARISON 6: ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by five days)
One study was included in this category.
6.1 Global state
An improvement was defined as reduction in the Brief Psychiatric Rating Scale (BPRS). There was no significant difference in the two groups (n = 471, MD ‐0.18, CI ‐0.79 to 0.43, Analysis 6.1).
6.1. Analysis.
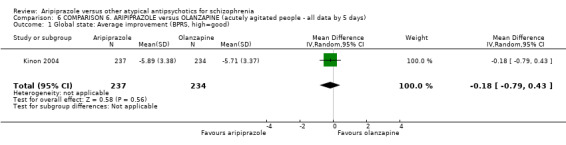
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 1 Global state: Average improvement (BPRS, high=good).
6.2 Mental state
There was no significant difference reported in participants on aripiprazole and olanzapine in agitation level (defined as at least 40% reduction in PANSS‐EC (n = 604, RR 0.92, CI 0.76 to 1.12). No significant difference was noted in agitation (n = 578, RR 0.25, CI 0.05 to 1.17); anxiety (n = 604, RR 0.93, CI 0.40 to 2.17) and exacerbation of schizophrenia (n = 604, RR 5.13, CI 0.25 to 106.49, Analysis 6.2). All such outcomes were labelled as “adverse effects” in the published report.
6.2. Analysis.
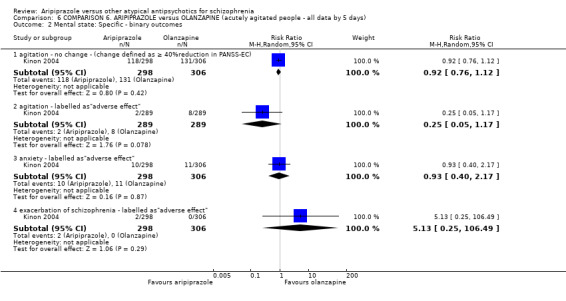
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 2 Mental state: Specific ‐ binary outcomes.
6.3 Leaving the study early
Both groups were similar in the number of participants leaving the study early due to adverse effects (n = 604, RR 0.68 CI 0.12 to 4.07, Analysis 6.3).
6.3. Analysis.
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 3 Leaving the study early.
6.4 Adverse effects
6.4.1 Central nervous system
No significant difference was reported between administering aripiprazole or olanzapine in acutely agitated patients with regards to CNS side effects of dizziness (n = 578, RR 0.29, CI 0.06 to 1.36); sedation (n = 578, RR 0.71, CI 0.23 to 2.22); insomnia (n = 604, RR 1.60, CI 0.87 to 2.94) and somnolence (n = 578, RR 0.14, CI 0.02 to 1.15). Participants on aripiprazole reported more headaches (n = 578, RR 2.43, CI 1.02 to 5.77) and lethargy (n = 578, RR 1.33, CI 0.30 to 5.90, Analysis 6.4).
6.4. Analysis.
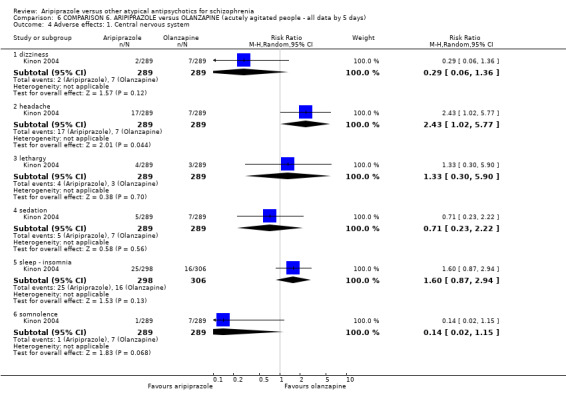
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 4 Adverse effects: 1. Central nervous system.
6.4.2 Endocrine system ‐ increase in average levels of prolactin
People allocated aripiprazole reported significantly lower (P = 0.00001) prolactin level increase compared with participants given olanzapine (n = 604, MD ‐15.76, CI ‐19.18 to ‐12.34, Analysis 6.5).
6.5. Analysis.
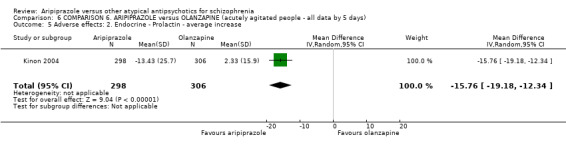
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 5 Adverse effects: 2. Endocrine ‐ Prolactin ‐ average increase.
6.4.3 Extrapyramidal side effects
Participants in both groups experienced similar rates of akathisia (n = 604, RR 1.43, CI 0.79 to 2.56) and parkinsonian symptoms (n = 604, 1 RCT, RR 1.71 CI 0.41 to 7.10, Analysis 6.6).
6.6. Analysis.
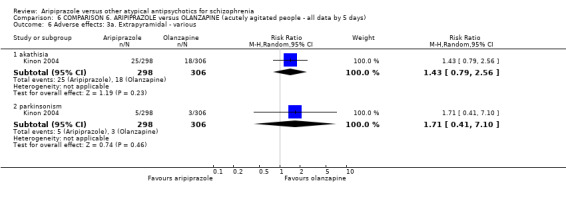
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 6 Adverse effects: 3a. Extrapyramidal ‐ various.
6.4.4 Extrapyramidal symptoms
Extrapyramidal symptoms were also reported by using scales where higher score represents a poor outcome. There was no significant difference between groups when assessed using Barnes Akathisia scale (n = 604, MD 0.07, CI ‐0.24 to 0.38) or Simpson Angus scale (n = 604, MD ‐0.05, CI ‐0.13 to 0.03, Analysis 6.7).
6.7. Analysis.
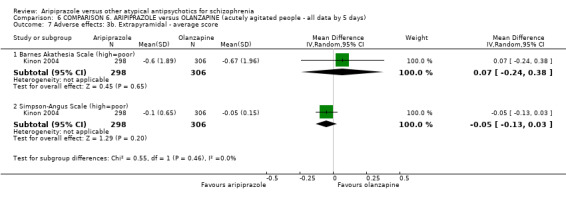
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 7 Adverse effects: 3b. Extrapyramidal ‐ average score.
6.4.5 Gastrointestinal side effects
Both groups reported similar occurrence of dry mouth (n = 578, RR 1.00, CI 0.20 to 4.91) and twice the number of participants on olanzapine reported nausea/dyspepsia (n = 578, RR 0.50, CI 0.09 to 2.71, Analysis 6.8).
6.8. Analysis.
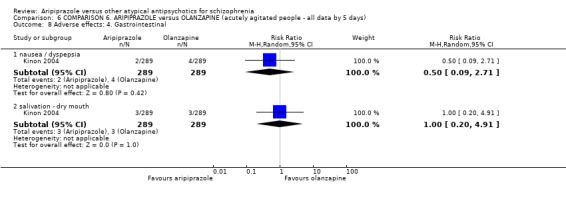
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 8 Adverse effects: 4. Gastrointestinal.
6.4.6 Metabolic side effects‐ increase in triglyceride levels
These results favoured patients on aripiprazole significantly (P = 0.00001) (n = 604, MD ‐35.62, CI ‐49.25 to ‐21.99, Analysis 6.9).
6.9. Analysis.
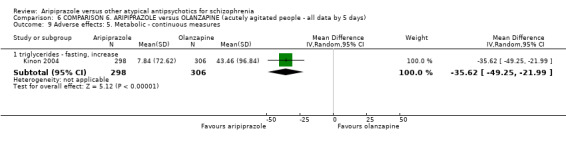
Comparison 6 COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days), Outcome 9 Adverse effects: 5. Metabolic ‐ continuous measures.
COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS
We used data from three studies for this comparison. In all three studies aripiprazole was compared with any one of several new generation antipsychotic drugs.
7.1 Global state: 1. No change as defined by the original study (measured by IAQ)
Kerwin 2007 showed an improvement in global state in energy (n = 523, 1 RCT, RR 0.69 CI 0.56 to 0.84); mood (n = 523, 1 RCT, RR 0.77 CI 0.65 to 0.92); negative symptoms (n = 523, 1 RCT, RR 0.82 CI 0.68 to 0.99); somnolence (n = 523, 1 RCT, RR 0.80 CI 0.69 to 0.93) and weight gain (n = 523, 1 RCT, RR 0.84 CI 0.76 to 0.94) when aripiprazole was used but no significant difference was noted in cognition (n = 523, 1 RCT, Analysis 7.1).
7.1. Analysis.
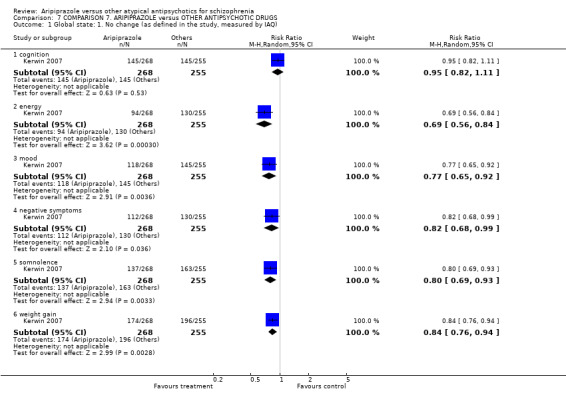
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 1 Global state: 1. No change (as defined in the study, measured by IAQ).
7.2 Global state: 2. Change in sexual dysfunction (measured by ASEX)
No significant difference was seen in the two groups (n = 85, 1 RCT, Analysis 7.2).
7.2. Analysis.
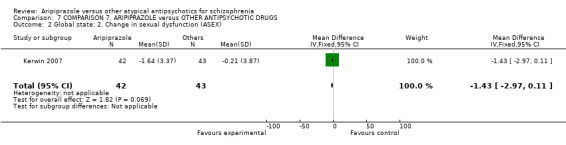
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 2 Global state: 2. Change in sexual dysfunction (ASEX).
7.3 Mental state: 1. Binary outcomes
No significant difference was noted in anxiety (n = 1361, 2 RCTs); "schizophrenia" (n = 548, 1 RCT) and psychotic disorder (n = 2881, 3 RCTs). Participants on aripiprazole experienced slightly less agitation (n = 548, 1 RCT, RR 2.64 CI 0.96 to 7.23, Analysis 7.3).
7.3. Analysis.
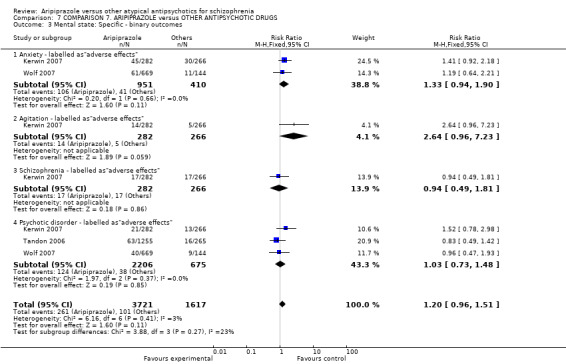
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 3 Mental state: Specific ‐ binary outcomes.
7.4 Medication preference (study medication worse than or equal to previous medication)
People reported aripiprazole being better in the short term (n = 446, 1 RCT, RR 0.67 CI 0.49 to 0.91) and medium term (n = 330, 1 RCT, RR 0.39 CI 0.25 to 0.61). Carers, however, reported no difference in the two groups both in the short (n = 121, 1 RCT) and medium term (n = 80, 1 RCT, Analysis 7.4)
7.4. Analysis.
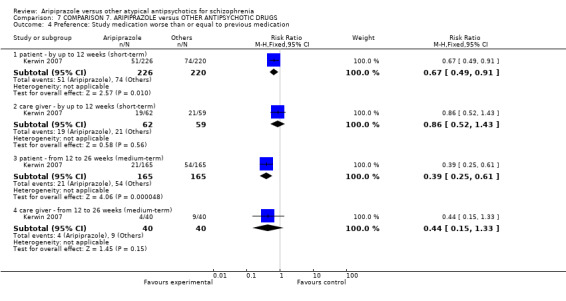
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 4 Preference: Study medication worse than or equal to previous medication.
7.5 Quality of life: 1. Unsatisfactory response (Health dimension scale)
Fewer participants reported an unsatisfactory response on the health dimension scale when using aripiprazole (n = 329, 1 RCT, RR 0.29 CI 0.13 to 0.66, Analysis 7.5).
7.5. Analysis.
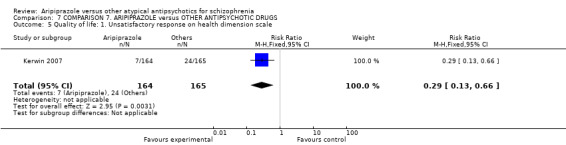
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 5 Quality of life: 1. Unsatisfactory response on health dimension scale.
7.6 Quality of life: 2. Average change in Quality of Life score (high is better)
The results favoured other antipsychotics (n = 326, 1 RCT, MD 6.20 CI 3.08 to 9.32, Analysis 7.6).
7.6. Analysis.
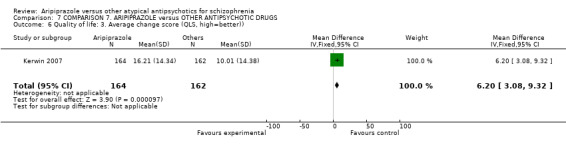
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 6 Quality of life: 3. Average change score (QLS, high=better)).
7.7 Quality of life: 3. Average EQ‐5D utility score (high is better)
No significant difference was noted in the two groups (n = 329, 1 RCT, Analysis 7.7).
7.7. Analysis.
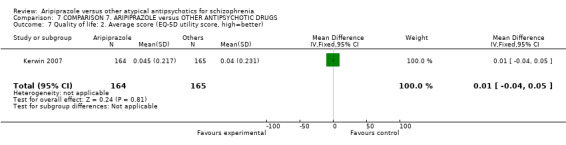
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 7 Quality of life: 2. Average score (EQ‐5D utility score, high=better).
7.8 Quality of life: 4a. Weight‐related score ‐ no meaningful change (measured by Impact of Weight on Quality of Life (IWQoL‐Lite))
No significant difference was noted in the two groups when weight was measured in binary scale (n = 327, 1 RCT, Analysis 7.8).
7.8. Analysis.
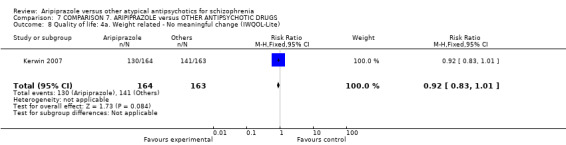
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 8 Quality of life: 4a. Weight related ‐ No meaningful change (IWQOL‐Lite).
7.9 Quality of life: 4b. Weight‐related score (measured by Impact of Weight on Quality of Life (IWQoL‐Lite))
No significant difference was noted in the two groups for average change in weight in the short term (n = 443, 1 RCT) and medium term (n = 328, 1 RCT, Analysis 7.9).
7.9. Analysis.
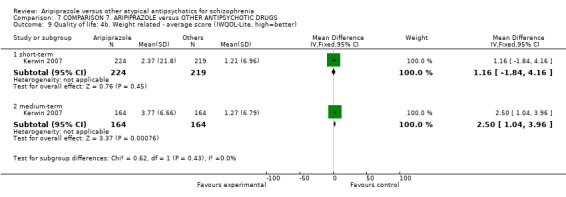
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 9 Quality of life: 4b. Weight related ‐ average score (IWQOL‐Lite, high=better).
7.10 Leaving the study early
No significant difference was noted in the participants who left the study early in the three groups for any reason (n = 2908, 3 RCTs, RR 0.97 CI 0.78 to 1.19); administrative reasons (n = 833, 1 RCT, RR 0.80 CI 0.00 to 1.84); death (n = 555, 1 RCT, RR 0.48 CI 0.04 to 5.23); inefficacy (n = 2908, 3 RCTs, RR 0.94 CI 0.45 to 1.96); lost to follow‐up (n = 1388, 2 RCTs, RR 0.85 CI 0.34 to 2.13); no longer meets criteria (n = 1388, 2 RCTs, RR 0.65 CI 0.16 to 2.63); other (n = 1388, 2 RCTs, RR 0.62 CI 0.10 to 3.80); compliance issues (n = 1388, 2 RCTs, RR 1.10 CI 0.53 to 2.32) and pregnancy (n = 555, 1 RCT, RR 0.95 CI 0.14 to 6.73). More participants in the aripiprazole group left due to adverse events (n = 2908, 3 RCTs, RR 1.40 CI 1.11 to 1.76). Fewer participants in the aripiprazole group withdrew from the study (n = 2908, 3 RCTs, RR 0.48 CI 0.36 to 0.66, Analysis 7.10).
7.10. Analysis.
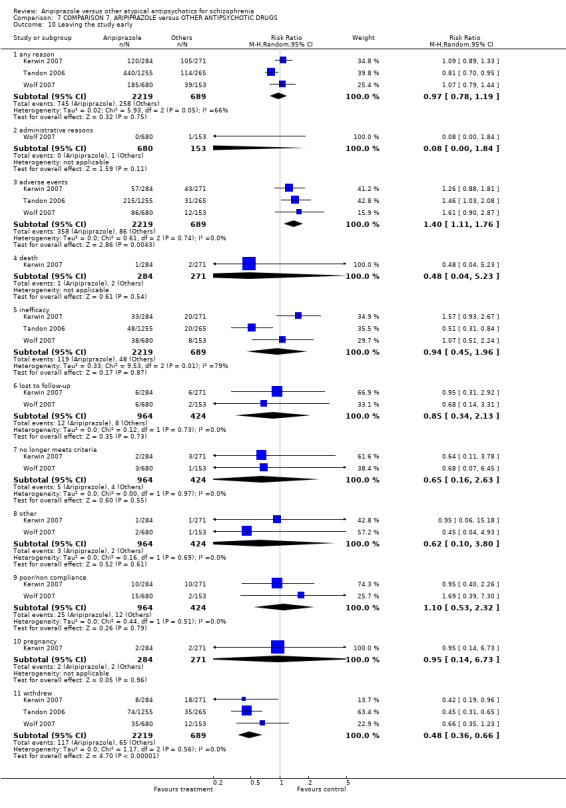
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 10 Leaving the study early.
7.11 Adverse effects ‐ 1. At least one side effect
Participants taking other antipsychotics reported this slightly more (n = 2333, 2 RCTs, RR 1.14 CI 1.05 to 1.23, Analysis 7.11).
7.11. Analysis.
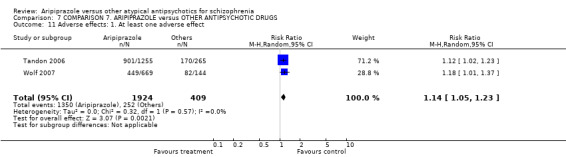
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 11 Adverse effects: 1. At least one adverse effect.
7.12 Adverse effects ‐ 2. Central nervous system
No significant difference was noted in sleep disorder (n = 813, 1 RCT, RR 1.79 CI 0.78 to 4.10) and fatigue (n = 548, 1 RCT, RR 0.59 CI 0.27 to 1.28). A larger number of participants on aripiprazole reported insomnia (n = 2881, 3 RCTs, RR 2.09 CI 1.65 to 2.66) and headache (n = 2881, 3 RCTs, RR 1.47 CI 1.09 to 1.99). Fewer participants on aripiprazole reported somnolence (n = 2881, 3 RCTs, RR 0.52 CI 0.39 to 0.71, Analysis 7.12).
7.12. Analysis.
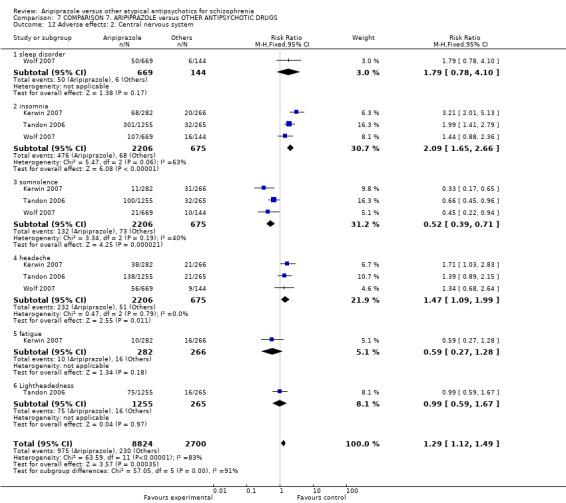
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 12 Adverse effects: 2. Central nervous system.
7.13 Adverse effects ‐ 3a. Endocrine system ‐ increase in prolactin level
Significantly fewer participants on aripiprazole reported an increased prolactin level (n = 548, 1 RCT, RR 0.31 CI 0.23 to 0.41, Analysis 7.13).
7.13. Analysis.
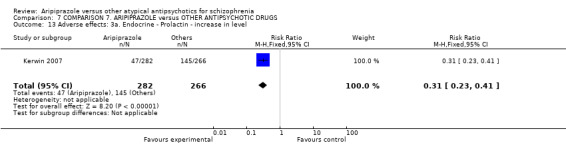
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 13 Adverse effects: 3a. Endocrine ‐ Prolactin ‐ increase in level.
7.14 Adverse effects ‐ 3b. Endocrine system ‐ change in prolactin level from baseline
The results favoured aripiprazole (n = 94, 1 RCT, MD ‐8.60 CI ‐19.14 to 1.94, Analysis 7.14).
7.14. Analysis.
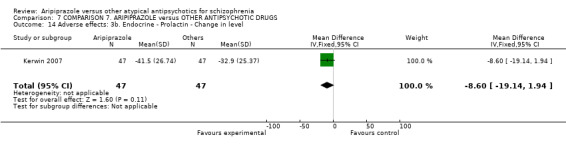
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 14 Adverse effects: 3b. Endocrine ‐ Prolactin ‐ Change in level.
7.15 Adverse effects ‐ 4. Extrapyramidal symptoms (EPS)
The results did not favour any of the comparison groups with a large confidence interval (n = 2881, 3 RCTs, RR 1.02 CI 0.09 to 11.09, Analysis 7.15) and prohibitively high heterogeneity (97%). It is unclear why Tandon 2006 causes this and removal of this study results in homogeneity being restored.
7.15. Analysis.
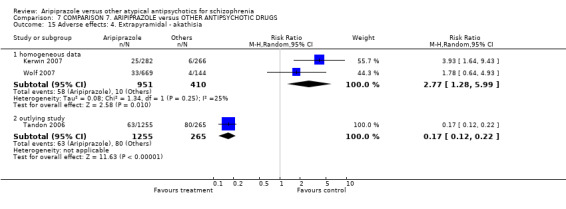
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 15 Adverse effects: 4. Extrapyramidal ‐ akathisia.
7.16 Adverse effects ‐ 5. Gastrointestinal
A significantly larger number of people given aripiprazole reported symptoms of nausea (n = 2881, 3 RCTs, RR 3.13 CI 2.12 to 4.61, Analysis 7.16).
7.16. Analysis.
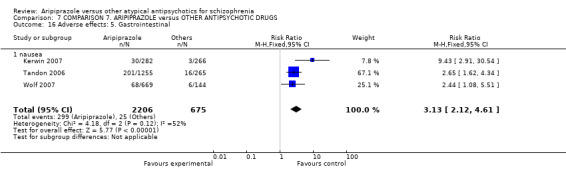
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 16 Adverse effects: 5. Gastrointestinal.
7.17 Adverse effects ‐ 6a. Metabolic ‐ binary measures
Significantly fewer people allocated aripiprazole reported 7% or more weight gain in the medium term (n = 330, 1 RCT, RR 0.35 CI 0.19 to 0.64); average weight gain (n = 548, 1 RCT, RR 0.11 CI 0.03 to 0.37); total cholesterol increase (n = 269, 1 RCT, RR 0.75 CI 0.62 to 0.91) and low‐density lipoprotein (LDL) increase (n = 268, 1 RCT, RR 0.65 CI 0.51 to 0.84). We found no significant difference in either group in high‐density lipoprotein (HDL) increase (n = 269, 1 RCT, RR 0.87 CI 0.61 to 1.22); triglyceride increase (n = 267, 1 RCT, RR O.80 CI 0.64 to 1.00) and increase in fasting glucose (n = 236, 1 RCT, RR 0.95 CI 0.62 to1.47). A greater number of people given aripiprazole reported 7% or more of weight loss in the medium term (n = 330, 1 RCT, RR 1.75 CI 0.97 to 3.19, Analysis 7.17).
7.17. Analysis.
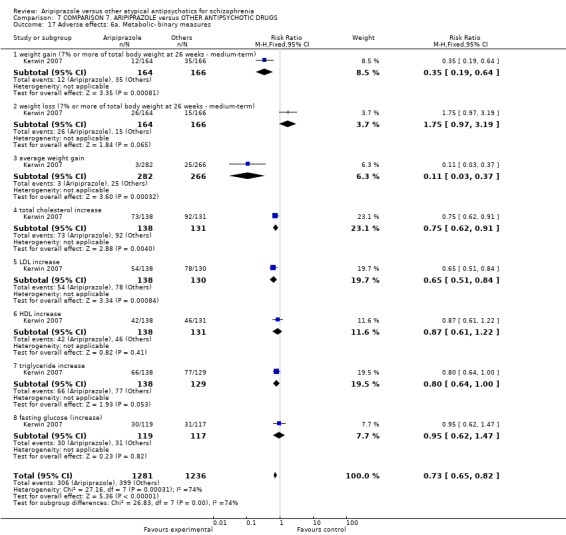
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 17 Adverse effects: 6a. Metabolic‐ binary measures.
7.18 Adverse effects ‐ 6b. Metabolic ‐ continuous measures
Aripiprazole was favoured for no risk of weight gain (n = 537, 1 RCT, MD ‐2.70 CI ‐3.68 to ‐1.72); weight change from baseline (n = 441, 1 RCT, MD short term ‐2.48 CI ‐3.30 to ‐1.66; n = 327, 1 RCT, MD medium term ‐3.74 CI ‐4.65 to ‐2.83); and change in fasting cholesterol (n = 262, 1 RCT, MD ‐9.70 CI ‐16.07 to ‐3.33). There was no difference reported in change in fasting glucose (n = 229, 1 RCT, MD ‐1.90 CI ‐5.78 to 1.98, Analysis 7.18).
7.18. Analysis.
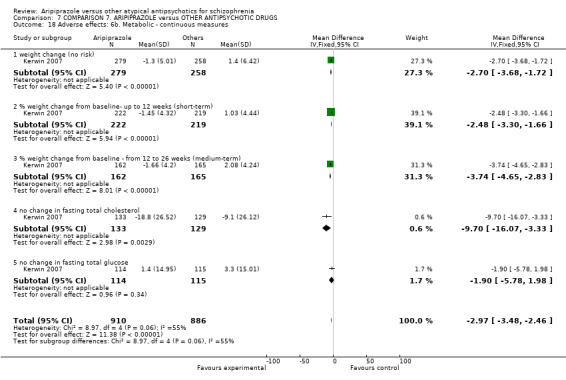
Comparison 7 COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS, Outcome 18 Adverse effects: 6b. Metabolic ‐ continuous measures.
8. SENSITIVITY ANALYSIS
We planned to undertake a sensitivity analysis for the primary outcomes of interest of (i) no clinically important response (as defined by each study); (ii) general functioning; and (iii) clinically important specific adverse effects. No study, however, reported data for the outcome of general functioning. For sensitivity analysis of 'comparator dose', we tested outcome of (i) no clinically important response (as defined by each study); and (ii) general mental state.
8.1 Implication of randomisation
8.1.1 Aripiprazole versus Clozapine
8.1.1.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant differences between groups after removing studies from meta‐analysis that only implied randomisation (n = 322, 4 RCTs, RR 1.04 CI 0.76 to 1.44).
8.1.1.2 Adverse effects ‐ clinically important specific adverse effects
Few studies provided data at medium term for any clinically important adverse effects; for EPS effects, only one study (Li 2007) provided relevant data. This study only implied randomisation, therefore after removing this study from data and analysis, there were no data left to compare. However, at short term, there remained no significant differences between groups after removing studies from meta‐analysis that only implied randomisation for the outcome of 'general EPS' (n = 60, 1 RCT, RR 1.50 CI 0.27 to 8.34). When implied randomised studies were removed from the outcome of akathisia, there was significantly greater instances (P = 0.002) in people receiving aripiprazole (n = 180, 2 RCTs, RR 4.99 CI 1.78 to 14.00).
8.1.2 Aripiprazole versus Quetiapine
8.1.2.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant differences between groups after removing studies from meta‐analysis that only implied randomisation (n = 108, 1 RCT, RR 1.00 CI 0.26 to 3.79).
8.1.2.2 Adverse effects ‐ clinically important specific adverse effects
All adverse data reported are for the short term; there remained no significant differences between groups after removing studies from meta‐analysis that only implied randomisation for the outcome of akathisia (n = 108, 1 RCT, RR 2.00 CI 0.19 to 21.41). This was also the case for tremor (n = 108, 1 RCT, RR 0.33 CI 0.01 to 8.01). Remaining studies in other outcomes of dystonia and general EPS were all implied as randomised; therefore, the removal of these studies left us with no data to compare.
8.1.3 Aripiprazole versus Risperidone
8.1.3.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant differences between groups after removing studies from meta‐analysis that only implied randomisation (n = 703, 7 RCTs, RR 1.29 CI 0.87 to 1.92).
8.1.3.2 Adverse effects ‐ clinically important specific adverse effects
All adverse data reported are for the short term; data were no longer statistically significant in favour of aripiprazole for akathisia after removing studies from the meta‐analysis that only implied randomisation (n = 293, 4 RCTs, RR 0.93 CI 0.45 to 1.90), this was also the case for tremor (n = 210, 3 RCTs, RR 0.61 CI 0.30 to 1.23). Data were still statistically significant (P = 0.003, albeit slightly lower in the level of significance) in favour of aripiprazole for the outcome of dystonia after the removal of implied randomised studies from meta‐analysis (n = 210, 3 RCTs, RR 0.17 CI 0.05 to 0.56), as well as for general EPS (n = 367, 4 RCTs, RR 0.53 CI 0.35 to 0.80). All data for tremor and parkinsonism were from implied randomised studies, which left no data to compare after removal.
8.1.4 Aripiprazole versus Ziprasidone
8.1.4.1 Global state: no clinically important response (as defined by the individual studies)
All studies reporting this outcome were implied as randomised; therefore, there were no data left to compare once they were removed from the meta‐analysis.
8.1.4.2 Adverse effects ‐ clinically important specific adverse effects
All adverse data reported are for the short term; data remained equivocal for akathisia after removing implied randomised studies (n = 253, 1 RCT, RR 1.26 CI 0.48 to 3.27). Data for all other various EPS outcomes, including dystonia, tremor, use of antiparkinson medication and general EPS effects, were reported by implied randomised studies, leaving no data to compare once removed from analysis.
8.1.5 Aripiprazole versus Olanzapine
8.1.5.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant differences between groups after removing studies from meta‐analysis that only implied randomisation (n = 778, 2 RCTs, RR 1.00 CI 0.82 to 1.23).
8.1.5.2 Adverse effects ‐ clinically important specific adverse effects
Data proved statistically significant (P = 0.05) in favour of olanzapine for akathisia after removing implied randomised studies from meta‐analysis (n = 75, 1 RCT, RR 16.68 CI 1.01 to 276.65), while data for remaining extrapyramidal effects (tremor, EPS, parkinsonism) were from implied randomised studies, leaving no data to compare.
8.1.6 Aripiprazole versus Olanzapine (acutely agitated)
The one study included in this comparison only implied randomisation, therefore there were no data left to compare.
8.1.7 Aripiprazole versus Other antipsychotic drugs
8.1.7.1 Global state: no clinically significant response (as defined by the individual studies)
The one study included in this outcome only implied randomisation, therefore there were no data left to compare.
8.1.7.2 Adverse effects ‐ clinically important specific adverse effects
For the outcome of akathisia, after removing the two studies that implied randomisation, only one study (with higher methodological quality) was left to compare (Tandon 2006), which eliminated the heterogeneity present and found statistical significance (P = 0.00001) in favour of aripiprazole over other antipsychotic drugs (n = 1520, 1 RCT, RR 0.17 CI 0.12 to 0.22).
8.2 Assumptions for lost binary data
8.2.1 Aripiprazole versus Clozapine
Only one study for this comparison did not report all data. Yu 2006 reported the loss of n = 5 participants; however it was not clear to which group these participants belonged.
8.2.2 Aripiprazole versus Quetiapine
No included studies reported losses for this comparison.
8.2.3 Aripiprazole versus Risperidone
8.2.3.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no difference in the estimate of effect when completer‐only data were pooled at short term (79 RCTs, n = 6245, RR 1.12 CI 0.98 to 1.28).
8.2.4 Aripiprazole versus Ziprasidone
No included studies reported losses for this comparison.
8.2.5 Aripiprazole versus Olanzapine
8.2.5.1 Global state: no clinically significant response (as defined by the individual studies
There remained no difference in the estimate of effect when completer‐only data were pooled in the short and medium term combined (11 RCTs, n = 1558, RR 1.04 CI 0.93 to 1.16).
8.2.6 Aripiprazole versus Olanzapine (acutely agitated)
The one study included in this comparison did not report losses for this comparison.
8.2.7 Aripiprazole versus Other antipsychotic drugs
No included studies reported losses for this comparison.
8.3 Risk of bias
8.3.1 Aripiprazole versus Clozapine
8.3.1.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant differences between groups after removing studies from meta‐analysis that rated as a 'high' risk on one or more of the risk of bias domains (n = 90, 1 RCT, RR 0.91 CI 0.32 to 2.62).
8.3.1.2 General functioning: no clinically important change in general functioning
No included study reported this outcome.
8.3.1.3 Adverse effects ‐ clinically important specific adverse effects
For extrapyramidal effects, all studies rated as a 'high' risk on one or more of the risk of bias domains, leaving no data to compare for this outcome.
8.3.2 Aripiprazole versus Quetiapine
8.3.2.1 Global state: no clinically significant response (as defined by the individual studies)
All studies rated as a 'high' risk on one or more of the risk of bias domains, leaving no data to compare for this outcome.
8.3.2.2 General functioning: no clinically important change in general functioning
No included study reported this outcome.
8.3.2.3 Adverse effects ‐ clinically important specific adverse effects
All studies rated as a 'high' risk on one or more of the risk of bias domains, leaving no data to compare for this outcome.
8.3.3 Aripiprazole versus Risperidone
8.3.3.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant differences between groups after removing studies from meta‐analysis that rated as a 'high' risk on one or more of the risk of bias domains (n = 465, 6 RCTs, RR 1.27 CI 0.69 to 2.31).
8.3.3.2 General functioning: no clinically important change in general functioning
No included study reported this outcome.
8.3.3.3 Adverse effects ‐ clinically important specific adverse effects
All adverse data reported are for the short term; data were no longer statistically significant in favour of aripiprazole for akathisia after removing studies that rated as a 'high' risk on one or more of the risk of bias domains (n = 161, 2 RCTs, RR 0.59 CI 0.14 to 2.55), this was also the case for tremor (n = 161, 2 RCTs, RR 0.20 CI 0.04 to 1.14), and dystonia (n = 161, 2 RCTs, RR 0.17 CI 0.59 to 4.21). However, data were still statistically significant (P = 0.0001) in favour of aripiprazole for general EPS (n = 284, 4 RCTs, RR 0.29 CI 0.16 to 0.51). All data for tremor and parkinsonism were rated as 'high' risk on one or more of the risk of bias domains, which left no data to compare after removal.
8.3.4 Aripiprazole versus Ziprasidone
All studies providing data for all primary outcomes rated as a 'high' risk on one or more of the risk of bias domains, leaving no data to compare for this outcome in a sensitivity analysis.
8.3.5 Aripiprazole versus Olanzapine
All studies providing data for all primary outcomes rated as a 'high' risk on one or more of the risk of bias domains, leaving no data to compare for this outcome in a sensitivity analysis.
8.3.6 Aripiprazole versus Olanzapine (acutely agitated)
The one study included in this comparison rated as a 'high' risk on one or more of the risk of bias domains, therefore there were no data left to compare.
8.3.7 Aripiprazole versus Other antipsychotic drugs
Again, all studies providing data for all primary outcomes rated as a 'high' risk on one or more of the risk of bias domains, leaving no data to compare for this outcome in a sensitivity analysis.
8.4 Imputed values
No values were imputed for intra‐class correlation coefficients (ICC), since we included no cluster randomised trials in meta‐analysis.
8.5 Skewed data
All skewed data have been presented separately in tables within data and analyses, therefore a sensitivity analysis was not possible via meta‐analysis.
8.6 Comparator dose
Not all studies reported mean doses and standard deviations; therefore, this sensitivity analysis has been undertaken with the dose values made available, taking predominantly ranges into account.
8.6.1 Aripiprazole versus Clozapine
8.6.1.1 Global state: no clinically significant response (as defined by the individual studies)
There remained no significant difference between groups when removing two studies (Li 2007; Yu 2007) that compared doses of aripiprazole that exceeded BNF recommendations to lower doses of clozapine (27 RCTs, n = 1978, RR 1.08 CI 0.88 to 1.32).
8.6.1.2 General mental state (BPRS/ PANSS)
At short term, none of the five studies rating mental state using the BPRS (endpoint scores) used inappropriate comparator doses, therefore there were no differences between results. Using the PANSS at short term, after removing the two studies (Li 2007; Yu 2007) that compared doses of aripiprazole that exceeded BNF recommendations to lower doses of clozapine, there was still no significant difference between groups, (23 RCTs, n = 1484, MD 0.55 CI ‐0.53 to 1.63), however the degree of heterogeneity was reduced from I2 = 64% (P = 0.0001) to I2 = 44% (P = 0.02). At medium term, three studies provided data using PANSS used comparisons within the recommended doses.
8.6.2 Aripiprazole versus Quetiapine
8.6.2.1 Global state: no clinically significant response (as defined by the individual studies)
Three studies exceeded recommended doses of clozapine, each employing a maximum dose of 800 mg daily (Chen 2009; Li 2007b; Luo 2008). There was no difference in the estimate of the effect when these studies were excluded from meta‐analysis (9 RCTs, n = 801, RR 0.91 CI 0.59 to 1.14)
8.6.2.2 General mental state (BPRS/ PANSS)
No studies provided data for general average endpoint scores using either the BPRS or PANSS.
8.6.3 Aripiprazole versus Risperidone
The majority of studies used a range with a maximum of 6 mg daily risperidone, which exceeds the 4 mg BNF recommendation.
8.6.3.1 Global state: no clinically significant response (as defined by the individual studies)
After excluding 70 studies with a range of 1 to 6 mg risperidone, there remained no difference between groups (9 RCTs, n = 727, RR 1.14 CI 0.76 to 1.71).
8.6.3.2 General mental state (BPRS/ PANSS)
For average endpoint mental state in the short term using BPRS, after excluding four of the five studies providing data, there was no longer significant favour for aripiprazole, instead showing no difference between groups (1 RCT, n = 68, MD 2.30 CI ‐1.17 to 5.77) and removing all heterogeneity. For PANSS in the short term, after removing 69 studies that used a higher than recommended dose of risperidone, results still demonstrated no difference (8 RCTs, n = 659, MD ‐0.50 CI ‐2.09 to 1.09s), however all heterogeneity was removed.
8.6.4 Aripiprazole versus Ziprasidone
None of the included studies for the outcomes of interest used inappropriate comparator doses; therefore, there were no studies to exclude.
8.6.5 Aripiprazole versus Olanzapine
None of the included studies for the outcomes of interest used inappropriate comparator doses; therefore, there were no studies to exclude.
8.6.6 Aripiprazole versus Olanzapine (acutely agitated)
The one study included in this comparison did not use any inappropriate comparator doses, therefore there was no difference in results.
8.6.7 Aripiprazole versus Other antipsychotic drugs
Of the three studies included in this outcome, Kerwin 2007 used higher than recommended doses of risperidone and quetiapine; however, this study did not provide data for the outcomes of interest, therefore there were no difference between results.
Discussion
Many trials were sponsored by the manufacturers of aripiprazole or the comparator agent manufacturers. In a blinded analysis of abstracts it has been shown that pharmaceutical companies emphasize positive aspects of their compounds (Heres 2006). Every result, without exception, therefore, must be viewed with this in mind.
Summary of main results
1. COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE
Thirty‐nine studies fell into this category, please see Table 1. Data are generally of poor quality and we found no data on indices including service use or global functioning. Overall, inclusion of new Chinese studies gave us much more data for this comparison. Quality of life outcomes, more often than not, significantly favoured use of aripiprazole over clozapine. However, there was no difference between the use of either drug for the primary outcome of clinically significant response in global state. Largely, people were at significantly less risk of adverse effects when receiving aripiprazole (Analysis 1.15; Analysis 1.16), but greater risk of central nervous system (CNS) adverse effects such as headache and insomnia than people receiving clozapine (Analysis 1.17), Extrapyramidal effects (EPS) were largely equivocal between groups. Data were heterogeneous as regards EPS, which may well be attributable to the differing doses employed between studies ‐ as some used low doses (between 5 to 20 mg aripiprazole versus 5 to 500 mg clozapine) of either drugs, and some used slightly higher (10 to 30 mg aripiprazole versus 50 to 270 mg clozapine). Evidence from 11 studies (n = 732) does suggest that people receiving aripiprazole had a greater risk of experiencing anxiety in the short term (Analysis 1.2).
2. COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE
Fifteen studies provided data for this comparison, please see Table 2; again, the inclusion of new Chinese studies gave us data for this comparison, which was lacking in the previous version of this review. The quality of the data were generally 'low' to 'very low', due to poor outcome reporting and methodological quality. There were, again, no data for service use; only one study reported data using any quality of life scale, which significantly favoured use of aripiprazole in six out of the seven components measured. Further larger scale, high‐quality trials are needed in order to reach any confident conclusions for this outcome. The evidence from 12 included studies (n = 991) suggest no significant difference between the two drugs in terms of clinically significant response. Adverse effect data do suggest a greater risk of tachycardia in people receiving quetiapine (Analysis 2.13), as well as dry mouth, constipation (Analysis 2.16) and weight gain (Analysis 2.19). However, nausea and vomiting was more common in people receiving aripiprazole (Analysis 2.16).
3. COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE
Eighty studies (n = 6381) report data for the primary outcome of clinically significant response for this important comparison. Risperidone, now off‐licence, is inexpensive and widely accessible; aripiprazole is not. Data are, again, of limited quality (randomisation and concealment methods being unclear, Table 3), however, this current update search has provided data that were lacking in the previous version of this review, including global functioning, and economic analyses; however, service use data are still missing. The majority of reported data are short term; adverse effects were selectively reported and studies were often sponsored by pharmaceutical companies.
For global outcomes there were no convincing differences between the two drugs (Analysis 3.1, Analysis 3.2, Analysis 3.3, Analysis 3.4). Binary mental state data were largely equivocal, with the exception of anxiety, which was significantly higher in people receiving aripiprazole (Analysis 3.5). Scale data for mental state favoured aripiprazole using the BPRS and PANSS average endpoint negative score (Analysis 3.6; Analysis 3.8). As regards adverse effects, the only convincing data were for tachycardia (Analysis 3.16) and abnormal liver function (Analysis 3.15) in risperidone. Risperidone also carried greater risk of metabolic adverse effects (Analysis 3.25) including weight gain, menstrual disorder and lactation. General EPS, akathisia, tremor and dystonia were all significantly higher in people receiving risperidone than aripiprazole (Analysis 3.21). Again, headache (Analysis 3.18), and nausea and vomiting were significantly more prominent in people receiving aripiprazole (Analysis 3.23). Both drugs may cause movement disorders and about one third of people allocated aripiprazole used antiparkinsonian drugs (Analysis 3.21).
4. COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE
The addition of new Chinese studies gave us an additional 15 studies to add to the previous one in this comparison. Ziprasidone is a major competitor of aripiprazole but data are of limited quality (Table 4), and there are still no data for service use. Data are all short term (four weeks).
There really does seem to be very little suggestion of differences in global state, attrition (about one third of people left in a matter of a few weeks) and mental state; however, there was greater improvement noted using SANS in people receiving aripiprazole in three RCTs (n = 238).
As regards adverse effects, there were largely no clear differences between groups; however, people receiving aripiprazole were significantly more likely to show signs of dizziness and insomnia (Analysis 4.11) as well as weight gain (Analysis 4.16). There is a need for better trials providing clear information for all interested in the care of people with schizophrenia who have access to these drugs.
5. COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE
Nineteen studies reported data for this comparison, see Table 5. Data are generally of poor quality and we found no data on indices including service use or global functioning. The inclusion of new Chinese studies gave data for quality of life outcomes (one RCT), which were lacking in the previous version of this review. The studies, although randomised and blinded, lack clarity on how both were carried out. Some studies were sponsored by pharmaceutical companies and adverse effects were seemingly selectively reported.
Even with the addition of new studies, global state outcomes show no clear differences between the drugs and the various measures of mental state did not clearly point to an advantage of one drug over another. The only significant findings from PANSS showed greater improvement in positive symptoms over each short, medium and long term combined (Analysis 5.11). Approaching 50% of people left these studies for any reason (Analysis 5.27) which may be a reflection of study design.
Regarding adverse effects, extrapyramidal side effects did not appear to be a major problem. Weight gain appears to be olanzapine’s major shortcoming when compared with aripiprazole (Analysis 5.24). This is important as there were significantly smaller mean increase of cholesterol levels from baseline in the aripiprazole group than in people allocated olanzapine (Analysis 5.25). Few people like gain weight or to be exposed to risks resulting from higher cholesterol levels. Olanzapine seemed to have a higher sedative effect on people in two randomised trials (Analysis 5.19).
5.19. Analysis.
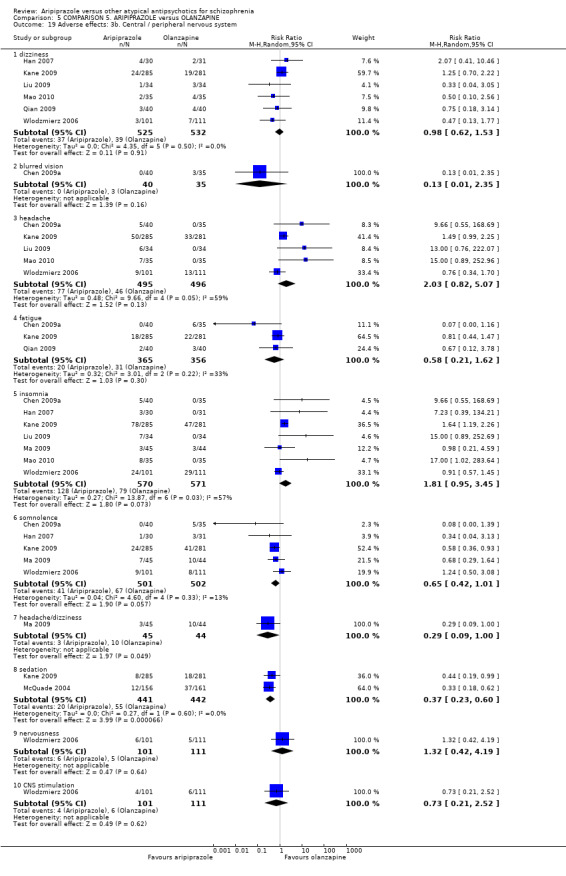
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 19 Adverse effects: 3b. Central / peripheral nervous system.
6. COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (in acutely agitated patients)
These results were based on a single five‐day trial but with nearly 500 participants. This comparison was included to comply with the protocol of this review but, in retrospect, we feel would be best in a separate review and are pleased to see that this is underway (Pagadala 2009). Overall, the results suggest that the two drugs may be of some use in the acute situation but data are few, not of high quality, undertaken by those with a pecuniary interest in the findings, and do not have a comparison group of other more widely used treatments for acute agitation thought due to serious mental illness.
Very few (0.83%) participants left the study early with numbers being similar in both groups. This very low figure may reflect the coercive nature of the treatment and that people were not free to leave. This is an observation and is not meant as criticism as this too may reflect real‐world practice.
7. COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS
The studies were randomised but no concealment methods were clarified. We do think the comparison of aripiprazole with any one of several new generation antipsychotic drugs is practical and reflects real‐world practice. One of the studies reported quality of life measures, which we feel gives a fresh dimension to the comparison. Although some attempts were made to incorporate clinically important issues, the overall quality of evidence remains low.
Many general measures did improve with aripiprazole although there was not any difference for sexual dysfunction. Several measures of mental state did not improve although evidence of limited quality did show some advantage of aripiprazole for negative symptoms. This latter finding is important and should be replicated in a study independent of industry conflicts of interest.
We are not sure why people with schizophrenia reported aripiprazole being preferred when their carers did not (Analysis 7.4). This could be a chance finding. Despite this expression of preference, about one third of participants left the trial early. Perhaps this is reflective of a vote of lack of confidence for the trial design rather than the drug itself.
It is good that quality of life is now recorded as a matter of routine in these studies but not good that it is measured by many scales that are problematic to interpret. Partly as a result of this, we are unsure of the effects of aripiprazole on quality of life compared with several of its competitors.
Aripiprazole is not free of adverse effects but there may be slightly less overall than with the other drugs. Nausea may be a problem (Analysis 7.16) but weight gain less problematic than other drugs.
Overall completeness and applicability of evidence
1. Completeness
Randomised evidence is available for only five out of eight possible comparisons of aripiprazole with other second generation antipsychotic drugs, although three studies compared aripiprazole with other atypical antipsychotics grouped together. Overall, evidence is incomplete. We do not have any good data on the implications of the use of aripiprazole on use of services from trials and we do think that 'admission' is a key outcome for people with schizophrenia. There are also very few economic data, which could be most important in this area.
2. Applicability
In terms of applicability, we highlight that most included studies were 'efficacy' studies. Large and simple, pragmatic, real‐world effectiveness studies are not available, greatly limiting external validity (Thorpe 2009).
Quality of the evidence
All studies were randomised and double blind or open label, but details were rarely presented. It is therefore unclear whether randomisation and blinding, where mentioned, were really appropriately done. The high number of participants leaving studies early (between 30% to 40% overall) must call into question the credibility of the findings (Xia 2007) because, once a person is lost to follow‐up outcomes become a matter of assumption. Three long‐term studies were available but in a chronic, often life‐long, disorder such as schizophrenia it is important that many more participants are followed up for longer. Overall, we considered the quality of evidence to be 'medium' at best with a moderate risk of bias.
Potential biases in the review process
It is possible that some important information may have been excluded during the review process due to human error or information not being available. New data from 162 Chinese studies that were previously awaiting assessment did not substantially change the findings of the first review, however they have given us more comparisons and increased precision whilst not clearly effecting overall quality of data.
Agreements and disagreements with other studies or reviews
An earlier Cochrane review compared aripiprazole with any other antipsychotic drugs (EL‐Sayeh 2006). In this past review authors combined olanzapine and risperidone as one group of 'atypical antipsychotic' drugs. A subsequent review comparing aripiprazole with other atypical antipsychotics was also published (Komossa 2009) where the authors concluded that aripiprazole was not much different from other second generation antipsychotic drugs. This current update again concludes that aripiprazole was similar to other atypical antipsychotic drugs but we have found some evidence of it being better tolerated than its competitors.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
For people with schizophrenia, it may be important to know that aripiprazole may be slightly less effective than olanzapine, but is less associated with adverse effects such as weight gain, cholesterol and prolactin increase and sedation. Aripiprazole's efficacy seems to be similar to that of risperidone, but certain movement disorders, cholesterol and prolactin increase may occur less frequently when taking aripiprazole. Aripiprazole may also be slightly better than ziprasidone with no clear differences apparent in adverse effects. When compared to other atypical antipsychotics as a group, people allocated to aripiprazole preferred its use. However, effects on quality of life were similar and carers found the two groups to be similar.
2. For clinicians
Clinicians should know that randomised evidence of the effects of aripiprazole compared with other second generation antipsychotic drugs is only available for a few drugs. Aripiprazole does not seem dogged by the problems of weight gain and raised prolactin. As with many antipsychotic drugs, it may, however, cause some extrapyramidal effects. More studies are needed to clarify the role of aripiprazole compared to other second generation antipsychotic drugs.
3. For managers/policy makers
Data that are relevant for policy makers such as service utilisation, functioning in society or costs are not available. We therefore find it difficult to make recommendations for decision makers.
Implications for research.
1. General
Outcome reporting remains insufficient in antipsychotic drug trials. Strict adherence to the CONSORT statement (Moher 2001) would make such studies much more informative. Making all data available would set this compound far ahead of its competitors (ALLTRIALS). Increasingly healthcare sub‐specialities are gaining universal agreement on core outcomes to be reported within all trials (COMET). This would greatly reduce disaggregation of data and add to the power of reviews such as this.
2. Specific
2.1 Reviews
Many interesting and potentially informative trials have had to be excluded as they are not of direct relevance to this review. Suggestions for their use within reviews are given in Table 6.
1. Titles for reviews suggested by excluded studies.
| Suggested title/ongoing title | Excluded study | Reference to relevant ongoing Cochrane title* |
| Alpha‐receptor agonists for schizophrenia | Bergman 2007 | Currently no review. |
| Antidepressant augmentation of antipsychotic drugs for schizophrenia | Fawzi 2009 | Whitehead 2002 |
| Aripiprazole augmentation of other antipsychotics for schizophrenia | Bristol‐Myers 2006, Shim 2006 | Maayan 2011 |
| Aripiprazole for people with metabolic syndrome | Colombo 2008 | Mukundan 2010 |
| Aripiprazole versus first generation antipsychotics for schizophrenia | Carson 2000, Mossner 2009, Talbott 2007, Taylor 2007 | Bhattacharjee 2008 |
| Aripiprazole versus placebo for schizophrenia | Carson 2000, Henderson 2009, Mortimer 2004 | Belgamwar 2011 |
| Augmentation of clozapine for schizophrenia | Fleischhacker 2008a, Kim 2006, Ma 2007, Millar 2008, Namey 2006, Remington 2009 | Cipriani 2009 |
| Psychosocial intervention for schizophrenia | Anon 2008 | Unclear which of the many reviews would be relevant. |
| Switching from other antipsychotics to aripiprazole for schizophrenia | Janssen 2005, Lan 2008, Pae 2009a, Schreiner 2009, Takeuchi 2008 | Mukundan 2010 |
* It is possible that these studies could also contribute to other titles not referenced in this column.
2.2 Trials
There is much room for further, better, randomised trials comparing aripiprazole with other second generation antipsychotic drugs. Comparisons with amisulpride, sertindole and zotepine are currently missing. We present a suggestion in Table 7 recognising that much goes into design and conduct of such a large study that cannot be captured in a table.
2. Suggested design of future study.
| Methods | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation. Blindness: double ‐ described and tested. Duration: six months minimum. |
| Participants | Diagnosis: schizophrenia (operational criteria). N = 2700.* Age: any. Gender: both. History: any. |
| Interventions | 1. Aripiprazole: dose ˜ 10‐30 mg/day. N = 300. 2. Amisulpride: dose ˜ 400‐800 mg/day. N = 300. 3. Clozapine: dose ˜ 300‐800 mg/day. N = 300. 4. Olanzapine: dose ˜ 10‐20 mg/day. N = 300. 5. Quetiapine: dose ˜300‐800 mg/day. N = 300. 6. Risperidone: dose ˜ 4‐8 mg/day. N = 300. 7. Sertindole: dose ˜ 12‐24 mg/day. N = 300. 8. Ziprasidone: dose ˜ 120‐160 mg/day. N = 300. 9. Zotepine: dose ˜ 100‐300 mg/day. N = 300. |
| Outcomes | Leaving study early (any reason, adverse events, inefficacy). Service outcomes: hospitalised, time in hospital, attending out patient clinics. Global impression: CGI**, relapse. Mental state: PANSS. Adverse events/effects: UKU, major adverse event/effect. Employment, living independently, family satisfaction, patient satisfaction. |
* power calculation suggested 300/group would allow good chance of showing a 10% difference between groups for primary outcome. ** Primary outcome CGI: Clinical Global Impression Scale PANSS: Positive and Negative Syndrome Scale UKU: Udvalg for kliniske ndersogelser Side Effect Rating Scale ‐side effect rating scale
Feedback
New Feedback, 4 August 2016
Summary
I would like to bring the authors attention to a possible error in their review report. I believe that the a result in Analysis 3.6 (aripiprazole versus risperidone), (Mental state: 2 average endpoint scale), subgroup 2 (PANSS [short term, up to 12 weeks, high=poor]) contain an extra study in error. The study in question, Zhang 2009, appears to compare aripiprazole with clozapine, and not with risperidone. As such, it should not be included in this analysis. For this analysis the authors report no significant difference between aripiprazole and risperidone for PANSS short term (78 RCTs, n=5793, ‐0.69 CI ‐1.49 to 0.11) with considerable heterogeneity (ChI2 = 161.78; df = 76; P=0.00001; I2=53%). However, after excluding Zhang 2009 and replicating the analysis, significant results in favour of aripiprazole are observed (77 RCTs, n = 5733, MD ‐0.80 CI ‐1.58 to ‐0.02, Z = 2.01, P = 0.04) with considerable heterogeneity (ChI2 = 145.74; df = 76; P<0.00001; I2=48%). Conclusions elsewhere in the report may also need reconsideration.
Reply
Thank you for bring this to our attention. Following your query, I have checked through these data and found that Zhang 2009a which has the correct control group, has accidentally been entered twice. For some reason the duplicate entry was added as Zhang 2009. My guess is the software didn't allow the same study to be entered twice in the same outcome, hence the reviewer mistook Zhang 2009 as Zhang 2009a . To rectify the error, I have removed study Zhang 2009 from this outcome. The result does became statistically significant and the results section has changed, but this significance is only marginal, upper CI is ‐0.02. As statistically significant results do not always necessarily have clinical significance, and as this is a marginal change, I feel the overall conclusions of the review should remain the same.
Contributors
Claire Ainsworth: commentator.
Jun Xia: review author.
What's new
| Date | Event | Description |
|---|---|---|
| 18 October 2016 | Feedback has been incorporated | Feedback received and authors have amended data input for a mental state outcome (Analysis 3.6). Data have changed and result is now significant, but overall conclusions of review remain unchanged. See Feedback 1. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 24 October 2013 | New citation required but conclusions have not changed | No overall change to conclusions. |
| 25 June 2013 | New search has been performed | New search run in November 2012. Trials assessed and new data added to review (162 new trials), but no changes to overall conclusions. |
| 16 October 2012 | New citation required but conclusions have not changed | New search and data added, no major changes to conclusions. |
| 24 January 2012 | New search has been performed | This is a major update, eight new trials have been included. |
| 4 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank all members of the Cochrane Schizophrenia Group editorial base for their editorial assistance, and Ben Gray for writing the plain language summary. The Cochrane Schizophrenia Group provides and maintains a standard template of text for the methods section of their reviews. We have used this and adapted it so it is relevant for this review.
We would like to acknowledge and thank Werner Kissling, Heike Hunger, Sandra Schwaz and Franziska Schmid for their contributions in previous versions of this review.
We thank Heather Maxwell for copy editing this review, her skill in this area is greatly appreciated.
Appendices
Appendix 1. Previous searches
1. Update of 2011 We searched the Cochrane Schizophrenia Group Trials Register (November 2011) using the phrase
[ ( (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in title, abstract or index terms of REFERENCE) or ( (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in interventions of STUDY)]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
2. Update of 2007 We searched the Cochrane Schizophrenia Group Trials Register (March 2007) using the phrase:
[ ( (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in title, abstract or index terms of REFERENCE) or ( (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in interventions of STUDY)]
This register is compiled by systematic searches of major databases, handsearches and conference proceedings (see Group Module). The Cochrane Schizophrenia Group Trials Register is maintained on Meerkat 1.5. This version of Meerkat stores references as studies. When an individual reference is selected through a search all references which have been identified as the same study are also selected
3. Initial search
We searched the Cochrane Schizophrenia Group Trials Register (August 2005) using the phrase:
[ (aripiprazol* AND (amisulprid* OR clozapin* OR olanzapin* OR quetiapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in title, abstract or index terms of REFERENCE].
Appendix 2. Previous data collection and analysis
1. Data extraction
We independently extracted data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
2. Management
We extracted the data onto standard simple forms. Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for aripiprazole.
3. Rating scales
A wide range of instruments are available to measure outcomes in mental health studies. These instruments vary in quality and many are not validated or are even ad hoc. It is accepted generally that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure) (Rust 1989). Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore continuous data from rating scales were included only if the measuring instrument had been described in a peer‐reviewed journal. In addition, the following minimum standards for instruments were set: the instrument should either be (a) a self‐report or (b) completed by an independent rater or relative (not the therapist) and (c) the instrument should be a global assessment of an area of functioning.
Assessment of risk of bias in included studies
Again working independently, PK, KK and SL assessed the risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases.
The risk of bias in each domain and overall were assessed and categorised into:
A. Low risk of bias: plausible bias unlikely to seriously alter the results (categorised as 'Yes' in Risk of Bias table) B. High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as 'No' in Risk of Bias table) C. Unclear risk of bias: plausible bias that raises some doubt about the results (categorised as 'Unclear' in Risk of Bias table)
Trials with a high risk of bias (defined as at least three out of five domains were categorised as 'No') or where allocation was clearly not concealed were not included in the review. If the raters disagreed, the final rating was made by consensus with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials are provided, authors of the studies were contacted in order to obtain further information. Non‐concurrence in quality assessment was reported.
Measures of treatment effect
1. Data types
We assessed outcomes using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such as 'little change', 'moderate change' or 'much change') or dichotomous (for example, either 'no important changes' or 'important change' in a person's behaviour) measures. Currently RevMan does not support categorical data so we were unable to analyse this.
2. Dichotomous‐yes/no‐data
We carried out an intention‐to‐treat analysis. Everyone allocated to the intervention were counted, whether they completed the follow‐up or not. It was assumed that those who left early had no change in their outcome. This rule is conservative concerning response to treatment, because it assumes that those discontinuing the studies would not have responded. It is not conservative concerning adverse effects, but we felt that assuming that all those leaving early would have developed adverse effects would overestimate risk. Where possible, efforts were made to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It was generally assumed that if there had been a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005, Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
We calculated the relative risk (RR) and its 95% confidence interval (CI) based on the random‐effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number needed to harm (NNH) as the inverse of the risk difference.
3. Continuous data
3.1 Normal distribution of the data
The meta‐analytic formulas applied by RevMan Analyses (the statistical programme included in RevMan) require a normal distribution of data. The software is robust towards some skew but to which degree of skewness meta‐analytic calculations can still be reliably carried out is unclear. On the other hand, excluding all studies on the basis of estimates of the normal distribution of the data also leads to a bias, because a considerable amount of data may be lost leading to a selection bias. Therefore, we included all studies in the primary analysis. In a sensitivity analysis we excluded potentially skewed data applying the following rules:
a) When a scale started from the finite number zero the standard deviation, when multiplied by two, was more than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, Altman 1996). b) If a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD> (S‐Smin), where S is the mean score and Smin is the minimum score). In large studies (as a cut‐off we used 200 participants) skewed data pose less of a problem. In these cases we entered the data in a synthesis and no sensitivity analysis was applied. d) The rules explained in a) and b) do not apply to change data. The reason is that when continuous data are presented on a scale which includes a possibility of negative values, it is difficult to tell whether data are non‐normally distributed (skewed) or not. This is also the case for change data (endpoint minus baseline). In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALLSTAT electronic statistics mailing list, we presented change data in RevMan Analyses in order to summarise available information. In doing this, it was assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew. Without individual patient data it is impossible to test this assumption. We therefore included change data and did not apply a sensitivity analysis. For continuous outcomes we estimated a mean difference (MD) between groups. MDs were again based on the random‐effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We combined both endpoint data and change data in the analysis because there is no principal statistical reason why endpoint and change data should measure different effects (Higgins 2011). When standard errors instead of standard deviations (SD) were presented, we converted the former to standard deviations. If both were missing we estimated SDs from p‐values or used the average SD of the other studies (Furukawa 2006).
Unit of analysis issues
1. Cluster trials
Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Authors often fail to account for intra class correlation in clustered studies, leading to a unit of analysis error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This can cause Type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra class correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the intra class correlation coefficient (ICC) [Design effect=1+ (m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intra class correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, we will only use data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment groups, if relevant, the additional treatment groups were presented in additional relevant comparisons. Data were not double counted. Where the additional treatment groups were not relevant, these data were not reproduced.
Dealing with missing data
At some degree of loss of follow‐up, data must lose credibility (Xia 2007). Although high rates of premature discontinuation are a major problem in this field, we felt that it was unclear which degree of attrition leads to a high degree of bias. We, therefore, did not exclude outcomes on the basis of the percentage of participants completing them. However we addressed the drop‐out problem in all parts of the review, including the abstract. For this purpose we calculated, presented and commented on frequency statistics (overall rates of leaving the studies early in all studies and comparators pooled and their ranges).
We assumed that the people who discontinued the studies for any reason did not show any response to the treatment.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all the included studies within any comparison to judge for clinical heterogeneity.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I2 statistic
Visual inspection was supplemented using, primarily, the I2statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I2 estimate was greater than or equal to 50% we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2011).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating small‐study effects but are of limited power to detect such effects when there are few studies. We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. We did not undertake a formal test for funnel plot asymmetry.
Data synthesis
Where possible for both dichotomous and continuous data we used the random‐effects model for data synthesis as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We understand that there is no closed argument for preference for use of fixed or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random‐effects does put added weight onto the smaller of the studies ‐ those trials that are most vulnerable to bias.
Subgroup analysis and investigation of heterogeneity
1. Subgroup
We did not anticipate any subgroup analyses.
2. Heterogeneity
If data are clearly heterogeneous we checked that data are correctly extracted and entered and that we had made no unit of analysis errors. If inconsistency was high and clear reasons explaining the heterogeneity were found, we presented the data separately. If not, we commented on the heterogeneity of the data.
Sensitivity analysis
We planned sensitivity analyses a priority for examining the change in the robustness of the sensitivity to including studies with potentially skewed data. A recent report showed that some of the comparisons of atypical antipsychotics may have been biased by using inappropriate comparator doses (Heres 2006). We, therefore, also analysed whether the exclusion of studies with inappropriate comparator doses changed the results of the primary outcome and the general mental state.
Data and analyses
Comparison 1. COMPARISON 1. ARIPIPRAZOLE versus CLOZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. No clinically significant response (as defined by the original studies) | 29 | 2132 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.27] |
| 2 Mental state: 1. Specific ‐ binary outcomes | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 anxiety (short term, up to 12 weeks) | 11 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [1.21, 5.70] |
| 2.2 anxiety (medium term, 12 to 26 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.08] |
| 2.3 agitation (short term, up to 12 weeks) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.18, 19.55] |
| 3 Mental state: 2. Average endpoint scores of various scales (short term, up to 12 weeks, high=poor) | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 BPRS | 5 | 426 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐1.44, 1.00] |
| 3.2 PANSS | 23 | 1638 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.41, 1.22] |
| 3.3 SANS | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐6.27, 1.73] |
| 4 Mental state: 3. Average endpoint scores of various scales (medium term, 12 to 26 weeks, high=poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 PANSS | 3 | 236 | Mean Difference (IV, Random, 95% CI) | ‐5.41 [‐8.42, ‐2.41] |
| 5 Mental state: 4. Average endpoint scores of various scales (skewed data, high=poor) | Other data | No numeric data | ||
| 5.1 BPRS | Other data | No numeric data | ||
| 5.2 PANSS | Other data | No numeric data | ||
| 5.3 PANSS negative symptom subscale score | Other data | No numeric data | ||
| 6 Mental state: 5. Specific ‐ average endpoint positive score (PANSS, high=poor) | 22 | 1523 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.48, 1.02] |
| 6.1 by up to 12 weeks ‐ short term | 19 | 1287 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.50, 1.20] |
| 6.2 from 12‐26 weeks ‐ medium term | 3 | 236 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.79, 1.34] |
| 7 Mental state: 6. Specific ‐ average endpoint negative score (PANSS, high=poor) | 23 | 1640 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.53, 0.30] |
| 7.1 up to 12 weeks ‐ short term | 20 | 1404 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.79, 0.71] |
| 7.2 from 12‐26 weeks ‐ medium term | 3 | 236 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐5.82, ‐0.67] |
| 8 Mental state: 7. Specific ‐ average endpoint general psychopathological score (PANSS, high=poor ) | 19 | 1330 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.78, 0.23] |
| 8.1 up to 12 weeks ‐ short term | 16 | 1094 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.62, 0.45] |
| 8.2 from 12‐26 weeks ‐ medium term | 3 | 236 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐3.45, ‐0.34] |
| 9 Mental state: 8. Specific ‐ average total score decreased rate (PANSS, low=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 by up to 12 weeks | 1 | 118 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.03, 0.15] |
| 10 Mental state: 9. Specific ‐ average positive score decreased rate (PANSS, low=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 by up to 12 weeks ‐ short term | 1 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.08] |
| 11 Leaving the study early | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Any reason | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.46, 4.29] |
| 11.2 Adverse events | 3 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.20, 2.92] |
| 11.3 Economic issues | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.38, 10.51] |
| 12 Quality of life: 1a. Average scores (short term, up to 12 weeks, WHO‐QOL‐100, low=poor) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 endpoint scale score | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 2.59 [1.43, 3.74] |
| 12.2 physical health | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 7.73 [1.51, 13.94] |
| 12.3 mental health | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 9.21 [4.74, 13.68] |
| 12.4 social function | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 7.78 [‐0.98, 16.54] |
| 12.5 external environmental | 3 | 248 | Mean Difference (IV, Random, 95% CI) | 13.82 [8.69, 18.94] |
| 12.6 independence | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐1.62, 1.78] |
| 12.7 spiritual support | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 6.61 [4.25, 8.97] |
| 13 Quality of life: 1b. Average scores (medium term, 12 to 24 weeks, WHO‐QOL‐100, low=poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 endpoint scale score | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 2.75 [1.98, 3.53] |
| 13.2 physical health | 3 | 256 | Mean Difference (IV, Random, 95% CI) | 4.89 [0.22, 9.56] |
| 13.3 mental health | 3 | 256 | Mean Difference (IV, Random, 95% CI) | 7.39 [5.26, 9.53] |
| 13.4 social function | 3 | 256 | Mean Difference (IV, Random, 95% CI) | 6.68 [2.79, 10.56] |
| 13.5 material life | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.00, 2.40] |
| 13.6 independence | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 6.71 [4.76, 8.66] |
| 13.7 spiritual support | 2 | 176 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.37, 0.75] |
| 14 Quality of life: 2. Average endpoint general quality of life score (GQOLI ‐ 74, low=poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Total score | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐3.40, 4.40] |
| 14.2 Physical health | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 7.70 [2.95, 12.45] |
| 14.3 Mental health | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐2.30, 7.70] |
| 14.4 Social function | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 6.60 [3.15, 10.05] |
| 14.5 Material life | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐5.30, 5.50] |
| 14.6 Environmental area (medium term) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 11.5 [5.55, 17.45] |
| 14.7 Independence (medium term) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 6.16 [2.84, 9.48] |
| 15 Adverse effects: 1. At least one adverse effect | 15 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.47, 0.81] |
| 15.1 Non‐specific | 7 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.95] |
| 15.2 epilepsy | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 15.3 liver function abnormal | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.56] |
| 15.4 stuffy nose | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.21, 1.20] |
| 15.5 sweating | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.23] |
| 15.6 urinary retention | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.11, 9.39] |
| 15.7 urinary incontinence | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.54] |
| 16 Adverse effects: 2. Cardiac effects | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 abnormal ECG (short term, up to 12 weeks) | 12 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.29, 0.51] |
| 16.2 blood pressure‐ decrease (short term, up to 12 weeks) | 3 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.23, 1.43] |
| 16.3 general adverse cardiac events (short term, up to 12 weeks) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.60] |
| 16.4 QTc prolongation (short term, up to 12 weeks) | 4 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.05, 0.49] |
| 16.5 tachycardia (short term, up to 12 weeks) | 15 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.22, 0.38] |
| 16.6 QTc prolongation (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.79] |
| 16.7 tachycardia (medium term, 12 to 24 weeks) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.57] |
| 17 Adverse effects: 3. Central / peripheral nervous system | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 activity‐ decrease (short term, up to 12 weeks) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.48] |
| 17.2 activity‐ increase (short term, up to 12 weeks) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 4.26 [0.22, 84.28] |
| 17.3 blurred vision (short term, up to 12 weeks) | 6 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.66] |
| 17.4 dizziness (short term, up to 12 weeks) | 9 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| 17.5 fatigue (short term, up to 12 weeks) | 4 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.23] |
| 17.6 general central nervous system adverse reaction (short term, up to 12 weeks) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.55, 7.29] |
| 17.7 general vegetative nervous system adverse reaction (short term, up to 12 weeks) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.84] |
| 17.8 headache (short term, up to 12 weeks) | 16 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.07, 4.86] |
| 17.9 hyper‐salivation (short term, up to 12 weeks) | 16 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.03, 0.10] |
| 17.10 insomnia (short term, up to 12 weeks) | 14 | 990 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [2.90, 10.91] |
| 17.11 irritability (short term, up to 12 weeks) | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 6.77 [0.82, 55.80] |
| 17.12 memory decline (short term, up to 12 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.95] |
| 17.13 sedation (short term, up to 12 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.52] |
| 17.14 somnolence (short term, up to 12 weeks) | 21 | 1492 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.09, 0.24] |
| 17.15 insomnia (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.74] |
| 17.16 headache (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.59] |
| 18 Adverse effects: 4. Extrapyramidal effects | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 akathisia (short term, up to 12 weeks) | 13 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.54, 2.68] |
| 18.2 dystonia (short term, up to 12 weeks) | 5 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.29, 8.12] |
| 18.3 general extrapyramidal symptoms (short term, up to 12 weeks) | 8 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.75, 4.85] |
| 18.4 tardive dyskinesia (short term, up to 12 weeks) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.72] |
| 18.5 tremor (short term, up to 12 weeks) | 6 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.72, 5.48] |
| 18.6 use of antiparkinson medication (short term, up to 12 weeks) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.07, 117.07] |
| 18.7 akathisia (medium term, 12 to 24 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.07] |
| 18.8 dystonia (medium term, 12 to 24 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.30] |
| 18.9 spasmodic torticollis (medium term, 12 to 24 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
| 18.10 tremor (medium term, 12 to 24 weeks) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.76] |
| 19 Adverse effects: 6. Haematological | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 abnormal blood routine (short term, up to 12 weeks) | 5 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.60] |
| 19.2 leucopenia (short term, up to 12 weeks) | 10 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.56] |
| 19.3 abnormal blood routine (medium term, 12 to 24 weeks) | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.75] |
| 20 Adverse effects: 5. Gastrointestinal | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 abdominal discomfort / pain (short term, up to 12 weeks) | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 10.21 [1.32, 79.12] |
| 20.2 constipation (short term, up to 12 weeks) | 19 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.08, 0.31] |
| 20.3 dry mouth (short term, up to 12 weeks) | 4 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.08, 5.38] |
| 20.4 general gastrointestinal aderse reaction (short term, up to 12 weeks) | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.41, 3.39] |
| 20.5 indigestion (short term, up to 12 weeks) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 4.89] |
| 20.6 nausea / vomiting (short term, up to 12 weeks) | 10 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.71, 3.38] |
| 20.7 constipation (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.36] |
| 20.8 hyper‐salivation (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.29] |
| 21 Adverse effects: 7. Hormonal | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 lactation/menstrual changes (short term, up to 12 weeks) | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.03, 0.47] |
| 22 Adverse effects: 8a. Metabolic ‐ binary measures | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 blood glucose ‐ increased (short term, up to 12 weeks) | 5 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.37] |
| 22.2 C‐peptide (short term, up to 12 weeks) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.45] |
| 22.3 decreased appetite (short term, up to 12 weeks) | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.54] |
| 22.4 postural hypotension (short term, up to 12 weeks) | 5 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.07, 0.39] |
| 22.5 PRL‐ increase (short term, up to 12 weeks) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.82] |
| 22.6 weight gain (short term, up to 12 weeks) | 18 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.08, 0.22] |
| 22.7 blood glucose ‐ increased (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.33] |
| 22.8 decreased appetite (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.99] |
| 22.9 weight gain (medium term, 12 to 24 weeks) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.39] |
| 23 Adverse effects: 8b. Metabolic ‐ continuous measures (short term, up to 12 weeks, high=poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 blood glucose ‐ FPG in HbA1c normal group (in mmol/l) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.39, 0.39] |
| 23.2 blood glucose ‐ FPG in HbA1c abnormal group (in mmol/l) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.61, 0.61] |
| 23.3 blood glucose ‐ PBG in HbA1c normal group (in mmol/l) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.66, 0.26] |
| 23.4 blood glucose ‐ PBG in HbA1c abnormal group (in mmol/l) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.63, ‐1.17] |
| 23.5 blood glucose ‐ FPG average endpoint (in mmol/l) | 2 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.83, ‐0.22] |
| 23.6 blood glucose ‐ C‐peptide average endpoint (in mg/dlmmol/l) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.45, 0.01] |
| 23.7 weight gain ‐ average endpoint level (in kg) | 1 | 74 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐5.56, 1.58] |
| 24 Cost effectiveness analysis (high=poor, data skewed) | Other data | No numeric data | ||
| 24.1 Cost of hospitalisation (in RMB) | Other data | No numeric data | ||
| 24.2 Cost of drug (in RMB) | Other data | No numeric data | ||
| 24.3 Length of hospitalisation (day) | Other data | No numeric data |
Comparison 2. COMPARISON 2. ARIPIPRAZOLE versus QUETIAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1.No clinically significant response (as defined by original studies) | 12 | 991 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.64, 1.32] |
| 2 Global state: 2a. Average endpoint total score (short term, up to 12 weeks, high=poor) | 11 | 991 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.58, 0.57] |
| 2.1 CGI | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.49, 0.69] |
| 2.2 PANSS | 10 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐3.15, 1.40] |
| 2.3 BPRS | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.63 [‐4.55, ‐0.71] |
| 3 Global state: 2b. Average endpoint scale score (medium term, 12 to 24 weeks, high=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 PANSS | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐5.67, 3.27] |
| 4 Global state: 3. Average endpoint SI score (CGI, high=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 up to 12 weeks ‐short term | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.41, 0.61] |
| 5 Mental state: 2a. Specific ‐ binary outcomes | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 agitation (short term, up to 12 weeks) | 5 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.27, 6.27] |
| 5.2 anxiety (short term, up to 12 weeks) | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.51, 9.35] |
| 5.3 depression (short term, up to 12 weeks) | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 5.4 agitation (medium term, 12 to 26 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.99] |
| 6 Mental state: 3. Specific ‐ average endpoint positive score (PANSS, high=poor) | 8 | 683 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.99, 0.32] |
| 6.1 by up to 12 weeks ‐ short term | 7 | 583 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.34, 0.41] |
| 6.2 from 12‐26 weeks ‐ medium term | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.32, 1.12] |
| 7 Mental state: 4. Specific ‐ average endpoint negative score (PANSS, high=poor) | 7 | 543 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.17, 0.21] |
| 7.1 up to 12 weeks ‐ short term | 6 | 443 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.52, 0.17] |
| 7.2 from 12‐26 weeks ‐ medium term | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.23, 1.23] |
| 8 Mental state: 5. Specific ‐ average endpoint general pathological score (PANSS, high=poor ) | 11 | 931 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐3.81, 0.43] |
| 8.1 up to 12 weeks ‐ short term | 10 | 831 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐4.19, 0.54] |
| 8.2 from 12‐26 weeks ‐ medium term | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.52, 1.72] |
| 9 Mental state: 6. average scores of various scales (high=poor, skewed data) | Other data | No numeric data | ||
| 9.1 BPRS endpoint scale score | Other data | No numeric data | ||
| 9.2 PANSS general pathology subscale score | Other data | No numeric data | ||
| 9.3 PANSS negative subscale score | Other data | No numeric data | ||
| 9.4 PANSS positive subscale score | Other data | No numeric data | ||
| 9.5 PANSS total endpoint scale score | Other data | No numeric data | ||
| 10 Leaving the study early | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 any reason | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.22, 2.87] |
| 10.2 no effect | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 10.3 early discharge | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 10.4 early treatment termination | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.09, 19.24] |
| 10.5 violation of test scheme | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 101.77] |
| 10.6 withdrew informed consent | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 11 Quality of life: Average score (medium term, 12 to 24 weeks, WHO‐QOL‐100, low=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Total score | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 2.60 [1.31, 3.89] |
| 11.2 Physical health | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 6.0 [4.38, 7.62] |
| 11.3 Mental health | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 9.10 [5.92, 12.28] |
| 11.4 Social function | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 5.60 [3.33, 7.87] |
| 11.5 Spiritual pillar | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.49, 1.69] |
| 11.6 Environmental area | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 11.5 [5.86, 17.14] |
| 11.7 Independence | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 6.20 [3.05, 9.35] |
| 12 Adverse effects:1. At least one adverse effect | 8 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.20] |
| 12.1 non‐specific | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.37] |
| 12.2 abnormal urinary test result | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 12.3 liver function abnormal | 8 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.13] |
| 12.4 stuffy nose | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 28.32] |
| 12.5 sweating | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.36] |
| 12.6 urine routine abnormal | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
| 13 Adverse effects: 2. Cardiac effects (short term, up to 12 weeks) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 abnormal ECG | 6 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.40, 2.26] |
| 13.2 blood pressure‐ decrease | 4 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.20, 1.32] |
| 13.3 QTc prolongation | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.08, 1.39] |
| 13.4 tachycardia | 8 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.69] |
| 14 Adverse effects: 3. Central / peripheral nervous system | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 blurred vision (short term, up to 12 weeks) | 6 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.31, 2.49] |
| 14.2 dizziness (short term, up to 12 weeks) | 8 | 671 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.22] |
| 14.3 headache (short term, up to 12 weeks) | 5 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.52, 18.94] |
| 14.4 insomnia (short term, up to 12 weeks) | 7 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.88, 5.10] |
| 14.5 somnolence (short term, up to 12 weeks) | 9 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.77] |
| 14.6 dizziness (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.31, 2.37] |
| 14.7 headache (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [1.18, 68.42] |
| 14.8 insomnia (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.36] |
| 14.9 somnolence (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.34, 1.86] |
| 15 Adverse effects: 4. Extrapyramidal symptoms ‐ various (short term, up to 12 weeks) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 akathisia | 7 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.49, 2.70] |
| 15.2 dystonia | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.53] |
| 15.3 general extrapyramidal symptoms | 4 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.64, 12.31] |
| 15.4 tremor | 4 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.07] |
| 16 Adverse effects: 5. Gastrointestinal | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 constipation (short term, up to 12 weeks) | 7 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.75] |
| 16.2 dry mouth (short term, up to 12 weeks) | 7 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.10, 0.53] |
| 16.3 nausea / vomiting (short term, up to 12 weeks) | 7 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [1.36, 5.26] |
| 16.4 dry mouth (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.84] |
| 16.5 nausea / vomiting (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.89, 54.83] |
| 17 Adverse effects: 6. Haematological | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 blood routine abnormal (short term, up to 12 weeks) | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.43] |
| 17.2 leucopenia (short term, up to 12 weeks) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.26] |
| 18 Adverse events: 7. Hormonal | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 menstrual disorder (short term, up to 12 weeks) | 6 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.20, 1.64] |
| 18.2 menstrual disorder (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.34] |
| 19 Adverse effects: 8a. Metabolic ‐ binary measures | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 decreased appetite (short term, up to 12 weeks) | 4 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.06, 1.39] |
| 19.2 lactation (short term, up to 12 weeks) | 5 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.92] |
| 19.3 weight gain (short term, up to 12 weeks) | 10 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.85] |
| 19.4 decreased appetite (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.96] |
| 19.5 lactation (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.6 weight gain (medium term, 12 to 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.34] |
| 20 Adverse effects: 8b. Metabolic ‐ continuous measure | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 cholesterol ‐ TC average endpoint level (in mmol/L, high=poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.36, ‐0.02] |
| 20.2 cholesterol‐TG average endpoint level (in mmol/L, high=poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.21, 0.13] |
| 20.3 cholesterol ‐ LDL average endpoint level (in mmol/L, high=poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.26, 0.02] |
| 20.4 waistline‐ average endpoint level (in cm, high=poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.88, 0.28] |
| 20.5 weight‐ average endpoint level (in Kg, high=poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐1.30, 3.90] |
| 20.6 cholesterol ‐ HDL average endpoint (in mmol/L, low=poor)) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.07] |
Comparison 3. COMPARISON 3. ARIPIPRAZOLE versus RISPERIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. No clinically significant response (as defined by the original studies) | 80 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 by up to 12 weeks ‐‐ short term | 80 | 6381 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.21] |
| 2 Global state: 2. Average endpoint total score(short term, up to 12 weeks, CGI, high=poor) | 2 | 196 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.09, 0.61] |
| 3 Global state: 3. Average CGI subscale scores (short term, up to 12 weeks, high=poor) | 1 | 240 | Mean Difference (IV, Random, 95% CI) | 0.44 [0.22, 0.66] |
| 3.1 CGI‐EI | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.14, 0.86] |
| 3.2 CGI‐SI | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.12, 0.68] |
| 4 Global state: 4. average endpoint data of various scales (high=poor, data skewed) | Other data | No numeric data | ||
| 4.1 CGI‐GI | Other data | No numeric data | ||
| 4.2 CGI‐SI | Other data | No numeric data | ||
| 4.3 CGI | Other data | No numeric data | ||
| 5 Mental state: 1. Specific ‐ binary outcomes (short term, up to 12 weeks) | 34 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 anxiety | 9 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.12, 2.94] |
| 5.2 agitation/excitement | 26 | 2038 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.86, 1.84] |
| 5.3 irritability | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.55] |
| 6 Mental state: 2. Average endpoint scale score | 82 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 BPRS (short term, up to 12 weeks, high=poor) | 5 | 570 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.24, ‐0.42] |
| 6.2 PANSS (short term, up to 12 weeks, high=poor) | 77 | 5733 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.58, ‐0.02] |
| 6.3 PANSS (medium term, 12 to 24 weeks, high=poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐8.03, 7.73] |
| 6.4 SANS (medium term, 12 to 24 weeks, high=poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐3.72, 2.62] |
| 7 Mental state: 3. Specific ‐ average endpoint positive score (PANSS, high=poor) | 40 | 3205 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.37, 0.41] |
| 7.1 short term (up to 12 weeks) | 39 | 3155 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.38, 0.43] |
| 7.2 medium term (12‐26 weeks) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐2.38, 1.96] |
| 8 Mental state: 4. Specific ‐ average endpoint negative score (PANSS, high=poor) | 37 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 short term (up to 12 weeks) | 37 | 2976 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.04, ‐0.25] |
| 9 Mental state: 5. Specific ‐ average endpoint general psychopathological score (PANSS, high=poor ) | 58 | 4243 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.71, 0.20] |
| 9.1 by up to 12 weeks ‐ short term | 57 | 4193 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.74, 0.19] |
| 9.2 12‐ 26 weeks ‐ medium term | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.52 [‐2.66, 5.70] |
| 10 Mental state: 6. PANSS average score decreased rate (short term, up to 12 weeks, low=poor) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 total scale score decreased rate | 3 | 219 | Mean Difference (IV, Random, 95% CI) | 3.06 [0.24, 5.87] |
| 10.2 negative symptom subscale score decreased rate | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.08] |
| 10.3 positive symptom subscale score decreased rate | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.08] |
| 10.4 general pathology subscale score decreased rate | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 2.38 [‐0.33, 5.09] |
| 11 Mental state: 7. BPRS total score decreased rate (short term, up to 12 weeks, high=poor) | 2 | 132 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐6.61, 0.67] |
| 12 Mental state: 8. General ‐ average total score (PANSS, high=poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 up to 12 weeks (short‐term) | 2 | 372 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐2.96, 5.96] |
| 13 Mental state: 9. average scores of various scale (high=poor, skewed data) | Other data | No numeric data | ||
| 13.1 SANS endpoint scale score | Other data | No numeric data | ||
| 13.2 PANSS general pathology subscale score | Other data | No numeric data | ||
| 13.3 PANSS negative symptom subscale score | Other data | No numeric data | ||
| 13.4 PANSS positive symptom subscale score | Other data | No numeric data | ||
| 14 Leaving the study early | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Any reason | 12 | 1239 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 14.2 Progressive disease | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.14, 5.09] |
| 14.3 Not insisting follow up | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.49, 4.44] |
| 14.4 Termination treatment early | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.92] |
| 14.5 Violation of study scheme | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.30, 5.83] |
| 14.6 Incomplete data | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.24, 102.71] |
| 14.7 Adverse effect | 9 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.06] |
| 14.8 Economic issue | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.11, 9.60] |
| 14.9 No effect | 5 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.49, 2.01] |
| 15 Adverse effects: 1. At least one adverse effect, non‐specific | 51 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 non‐specific | 28 | 2361 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.91] |
| 15.2 liver function abnormal | 29 | 2300 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.86] |
| 15.3 hyper‐salivation | 7 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.22, 1.80] |
| 15.4 myalgia/ostealgia | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.51, 7.46] |
| 15.5 renal function abnormal | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 sexual desire change | 8 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.30] |
| 15.7 sweating‐ increase | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.16, 2.59] |
| 15.8 stuffy nose | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 3.90 [0.44, 34.47] |
| 16 Adverse effects: 2a.Cardiac effects (short term, up to 12 weeks) | 61 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 abnormal ECG | 12 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.41, 1.05] |
| 16.2 blood pressure decline or rise | 5 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.46, 5.15] |
| 16.3 blood pressure‐ increase | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.44] |
| 16.4 blood pressure‐ decrease | 6 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.45, 2.90] |
| 16.5 EEG abnormal | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.85] |
| 16.6 postural hypotension | 6 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.46, 1.93] |
| 16.7 QTc prolongation | 5 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.09] |
| 16.8 tachycardia | 49 | 3835 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.96] |
| 17 Adverse effects: 2b. Cardiac ‐ QTc change from baseline (in ms) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐7.19 [‐12.19, ‐2.19] |
| 18 Adverse effects: 3. Central / peripheral nervous system (short term, up to 12 weeks) | 63 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 acute onset of schizophrenia | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.49, 3.64] |
| 18.2 dizziness | 23 | 1896 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.72, 1.54] |
| 18.3 blurred vision | 39 | 3272 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| 18.4 fatigue | 5 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.29, 1.35] |
| 18.5 headache | 20 | 1505 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.31, 2.78] |
| 18.6 headache/dizziness | 20 | 1486 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.93, 1.79] |
| 18.7 insomnia | 54 | 4209 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.98, 1.39] |
| 18.8 memory decrease | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.94] |
| 18.9 sedation | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.18] |
| 18.10 sleep disorder | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.39, 1.86] |
| 18.11 somnolence | 35 | 2779 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.22] |
| 19 Adverse effects: 3a. Endocrine ‐ Prolactin ‐ average change (ng/ml) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐54.71 [‐60.06, ‐49.36] |
| 20 Adverse effects: 3b. Endocrine ‐ Prolactin‐associated | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 abnormally high prolactin value | 1 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.02, 0.08] |
| 20.2 dysmenorrhoea | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [0.17, 59.43] |
| 21 Adverse effects: 4. Various extrapyramidal symptoms (short term, up to 12 weeks) | 64 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 akathisia | 42 | 3501 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.74] |
| 21.2 tremor | 36 | 2799 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.45] |
| 21.3 dystonia | 32 | 2640 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.49] |
| 21.4 parkinsonism | 1 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 7.39 [0.43, 128.08] |
| 21.5 use of antiparkinson medication | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.12] |
| 21.6 extrapyramidal symptoms | 31 | 2605 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.31, 0.50] |
| 21.7 torsion spasm | 3 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.04, 7.18] |
| 21.8 tremor | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.07, 21.13] |
| 21.9 increase activities | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.20] |
| 22 Adverse effects: 4b. Extrapyramidal ‐ average score | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Abnormal Involuntary Movement Scale (high=poor) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.24, 0.75] |
| 22.2 Barnes Akathisia Scale (high=poor) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.49, 0.27] |
| 22.3 Simpson‐Angus Scale (high=poor) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.22, 0.82] |
| 23 Adverse effects: 5. Gastrointestinal | 46 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 constipation | 27 | 2067 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.52, 1.08] |
| 23.2 dry mouth | 33 | 2658 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.86, 1.69] |
| 23.3 gastrointestinal reaction | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.76, 5.37] |
| 23.4 nausea / vomiting | 28 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.31, 2.56] |
| 24 Adverse effects: 6. Haematological | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 blood routine abnormal | 6 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.02] |
| 24.2 leucopenia | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 24.3 blood lipid abnormal | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.18] |
| 25 Adverse effects: 7a. Metabolic ‐ binary measures (short term, up to 12 weeks) | 62 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1 appetite‐ decrease | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.03] |
| 25.2 blood glucose‐ increase | 5 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.09, 0.82] |
| 25.3 endocrine disorder | 9 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.03, 0.17] |
| 25.4 lactation | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.60] |
| 25.5 menstrual disorder/lactation | 29 | 2278 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.06, 0.16] |
| 25.6 menstrual disorder or sexual function change | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.12] |
| 25.7 menstrual disorder | 9 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.06, 0.27] |
| 25.8 skin symptom | 9 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.86] |
| 25.9 PRL‐increase | 3 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.38] |
| 25.10 obesity | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.14, 6.35] |
| 25.11 vaginal bleeding | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.12, 67.53] |
| 25.12 weight gain | 58 | 4623 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.17, 0.29] |
| 25.13 weight loss | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [0.12, 70.56] |
| 26 Adverse effects: 7b. Metabolic ‐ continuous measures (high=poor ) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.1 endpoint average weight (in kg) | 5 | 465 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐4.17, ‐0.44] |
| 26.2 weight change from baseline (in kg) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐1.84, ‐1.16] |
| 26.3 average endpoint BMI of male (in kg/m2) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐4.08, ‐0.84] |
| 26.4 average endpoint BMI of female (in kg/m2) | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐3.55, 0.60] |
| 26.5 average endpoint blood glucose of female (in mmol/L) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.29 [3.97, 4.61] |
| 26.6 average endpoint blood glucose of male (in mmol/L) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.04, 0.60] |
| 26.7 average endpoint blood glucose FBG (in mg/dl) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.37, 0.21] |
| 26.8 average endpoint cholesterol ‐ TC of female (in mmol/L) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.96, ‐0.06] |
| 26.9 average endpoint cholesterol ‐ TC of male (in mmol/L) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.96, ‐0.00] |
| 26.10 average endpoint cholesterol ‐ TC level (in mmol/L) | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.14] |
| 26.11 average endpoint cholesterol‐TG level (in mmol/L) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.21, 0.07] |
| 26.12 average endpoint cholesterol ‐ LDL level (in mmol/L) | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.11, 0.26] |
| 26.13 average endpoint waistline (in cm) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐5.47, ‐1.13] |
| 27 Adverse effect : 7c. Metabolic ‐ continuous measures | 4 | 789 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.21] |
| 27.1 average endpoint cholesterol‐ HDL level (in mmol/L) | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.03, 0.14] |
| 27.2 cholesterol ‐ change from baseline (in mg/dl) | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐22.3 [‐39.69, ‐4.91] |
| 27.3 glucose ‐ change from baseline (in mg/dl) | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 6.8 [‐6.10, 19.70] |
| 27.4 weight gain ‐ change from baseline (in kg) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.24, 0.15] |
| 28 Adverse effect: 8. required additional drug combination | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 28.1 benzodiazepines | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.73, 1.58] |
| 28.2 benzhexol | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.12, 0.93] |
| 28.3 benzhexol/propranolol | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.45, 2.72] |
| 29 Adverse effects: 9. TESS score (short term, up to 12 weeks, high=poor) | 4 | 250 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.30, ‐0.39] |
| 30 Adverse effects: 10. TESS score (short term, up to 12 weeks, high=poor, data skewed) | Other data | No numeric data | ||
| 31 Adverse effects: 11. weight gain (in KG, high=poor, data skewed) | Other data | No numeric data | ||
| 32 Cognitive functioning: 1. Specific ‐ average endpoint total score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 32.1 (short term, up to 12 weeks, WMS, low= poor) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐7.95, 4.83] |
| 32.2 (short term, up to 12 weeks, WAIS‐RC, low=poor) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐8.92, 5.78] |
| 33 Cost effectiveness analysis (high=poor, data skewed) | Other data | No numeric data | ||
| 33.1 Cost of hospitalisation (in RMB) | Other data | No numeric data | ||
| 33.2 Cost of drug (in RMB) | Other data | No numeric data | ||
| 33.3 Length of hospitalisation (day) | Other data | No numeric data |
Comparison 4. COMPARISON 4. ARIPIPRAZOLE versus ZIPRASIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. No clinically significant response (as defined by the original studies) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 up to 12 weeks ‐ short term | 6 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.62, 1.52] |
| 2 Global state: 2. Average endpoint CGI‐GI score (short term, up to 12 weeks, high=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Global state: 3. Average change score (CGI‐S, decline=good) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Mental state: 1. Average endpoint total score (short term, up to 12 weeks, high=poor) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 PANSS | 7 | 689 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐3.68, 0.20] |
| 4.2 SANS | 3 | 238 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.56, ‐0.22] |
| 4.3 BPRS | 1 | 247 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐4.97, 0.57] |
| 5 Mental state: 2. Specific ‐ binary outcomes (up to 12 weeks ‐ short term) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 anxiety ‐ labelled as "adverse effect" | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.64, 14.04] |
| 5.2 agitation ‐ labelled as "adverse effect" | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.11, 9.42] |
| 6 Mental state: 3. Specific ‐ average endpoint PANSS subscale scores (short term, high=poor) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 positive symptom scores | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.36, 1.04] |
| 6.2 negative symptom scores | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.23, 0.61] |
| 6.3 general pathology scores | 5 | 382 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.73, 1.04] |
| 7 Mental state: endpoint scores of various scales (high=poor, data skewed) | Other data | No numeric data | ||
| 7.1 PANSS negative symptom subscale score | Other data | No numeric data | ||
| 7.2 PANSS positive symptom subscale score | Other data | No numeric data | ||
| 8 Leaving the study early | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 any reason | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.66, 1.34] |
| 8.2 adverse events | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.14, 1.38] |
| 8.3 defaulted | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.58, 1.66] |
| 8.4 inefficacy | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.54, 4.03] |
| 8.5 other/withdrew | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.25, 3.85] |
| 9 Adverse effects: 1. At least one adverse effect, non‐specific | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 non‐specific | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.85, 1.78] |
| 9.2 endocrine disorder | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 liver function abnormal | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.61] |
| 9.4 respiratory tract infection | 1 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.17] |
| 9.5 sexual function change | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 8.00 [2.96, 21.65] |
| 9.6 skin rash | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 9.7 stuffy nose | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.47] |
| 9.8 sweating | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.36] |
| 9.9 urine routine abnormal | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
| 10 Adverse effects: 2. Cardiac effects (short term, up to 12 weeks) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 abnormal ECG | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.22, 1.68] |
| 10.2 QTc prolongation | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.06, 2.79] |
| 10.3 tachycardia | 3 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.16] |
| 10.4 blood pressure‐ decrease | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [0.44, 34.66] |
| 11 Adverse effects: 3. Central / peripheral nervous system (short term, up to 12 weeks) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 blurred vision | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.90, 54.44] |
| 11.2 dizziness | 5 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.57, 6.70] |
| 11.3 headache | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 4.92 [0.96, 25.27] |
| 11.4 insomnia | 5 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [1.17, 7.30] |
| 11.5 somnolence | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.62, 3.39] |
| 12 Adverse effects: 4. Various extrapyramidal symptoms (short term, up to 12 weeks) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 akathisia | 3 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.25, 2.61] |
| 12.2 activity‐decrease | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.65] |
| 12.3 dystonia | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] |
| 12.4 general extrapyramidal symptoms | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.62] |
| 12.5 tremor | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 28.21] |
| 12.6 spasmodic torticollis | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
| 12.7 use of antiparkinson medication | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.07, 117.07] |
| 13 Adverse effects: 5. Gastrointestinal (short term, up to 12 weeks) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 appetite‐decrease | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.39, 10.35] |
| 13.2 constipation | 3 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.13, 2.97] |
| 13.3 dry mouth | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.10, 2.80] |
| 13.4 hyper‐salivation | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.24] |
| 13.5 nausea / vomiting | 6 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.91, 7.09] |
| 14 Adverse effects: 6. Haematological | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 leucopenia | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.26] |
| 15 Adverse effects: 7. Hormonal | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 menstrual disorder | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.28, 1.93] |
| 16 Adverse effects: 8a. Metabolic ‐ binary measures | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 appetite‐decrease | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.04, 7.93] |
| 16.2 blood routine abnormal | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.43] |
| 16.3 lactation | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.07] |
| 16.4 weight gain | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 4.01 [1.10, 14.60] |
| 17 Adverse effects: 8b. Metabolic ‐ continuous measures | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 cholesterol ‐ TG average endpoint level (in mmol/L, high= poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.21, 0.13] |
| 17.2 cholesterol ‐ TC average endpoint level (in mmol/L, high= poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 17.3 cholesterol ‐ LDL average endpoint level (in mmol/L, high= poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.30] |
| 17.4 HDL (low=poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.01, 0.19] |
| 17.5 waistline‐ average endpoint level (in cm, high= poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐5.29, ‐1.51] |
| 17.6 weight‐ average endpoint level (in kg, high= poor) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐5.06, 0.06] |
Comparison 5. COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1.No clinically significant response (as defined by the original studies) | 11 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.96, 1.17] |
| 1.1 up to 12 weeks‐ short term | 10 | 1422 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.21] |
| 1.2 from 12 to 26 weeks (medium‐term) | 1 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.22] |
| 2 Global state: 2. Not responded (decline in PANSS of 30% or more) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 by up to 12 weeks (short‐term) | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.01, 1.34] |
| 2.2 more than 26 weeks (long‐term) | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.00, 1.27] |
| 3 Global state: 3. Remission not achieved (as defined in the study) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 by up to 12 weeks (short‐term) | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.13] |
| 3.2 more than 26 weeks (long‐term) | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.23, 1.56] |
| 4 Global state: 4. Average endpoint EI score (CGI, high=poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 up to 12 weeks ‐ short term | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 5 Global state: 5. Average endpoint CGI score decreased rate (short term, low=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Global state: 6. Average change score (CGI‐S, decline=best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 more than 26 weeks (long‐term) | 1 | 566 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.32] |
| 7 Global state: 7. Improvement (CGI‐I, high=poor) | 1 | 566 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.32] |
| 8 Mental state: 1. Average endpoint scale score (PANSS, high=poor) | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 up to 12 weeks ‐ short term | 11 | 1500 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.23, 1.46] |
| 8.2 12‐ 26 weeks ‐ medium term | 2 | 139 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐5.26, 6.87] |
| 8.3 more than 26 weeks (long‐term) | 1 | 566 | Mean Difference (IV, Random, 95% CI) | 4.20 [0.10, 8.30] |
| 9 Mental state: 2. Average endpoint scale score (SANS, high=poor) | 2 | 137 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.04, 1.97] |
| 9.1 up to 12 weeks ‐ short term | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.52, 2.44] |
| 9.2 more than 26 weeks ‐ long term | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐3.45, 3.39] |
| 10 Mental state: 3. average endpoint score (PANSS, high=poor, data skewed) | Other data | No numeric data | ||
| 10.1 PANSS total | Other data | No numeric data | ||
| 10.2 PANSS negative symptom subscale score | Other data | No numeric data | ||
| 10.3 PANSS positive symptom subscale score | Other data | No numeric data | ||
| 11 Mental state: 4. Specific ‐ average endpoint positive score (PANSS, high=poor) | 7 | 1043 | Mean Difference (IV, Random, 95% CI) | 0.71 [0.17, 1.26] |
| 11.1 by up to 12 weeks ‐ short term | 5 | 429 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.16, 1.70] |
| 11.2 12‐ 26 weeks ‐ medium term | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐1.54, 2.86] |
| 11.3 more than 26 weeks | 1 | 566 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.03, 1.63] |
| 12 Mental state: 5. Specific ‐ average endpoint negative subscale score (PANSS, high=poor) | 6 | 967 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.25, 1.09] |
| 12.1 up to 12 weeks ‐ short term | 5 | 401 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.59, 0.92] |
| 12.2 more than 26 weeks | 1 | 566 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐0.07, 2.87] |
| 13 Mental state: 6. Specific ‐ average endpoint general pathological score (PANSS, high=poor ) | 8 | 642 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.24, 0.64] |
| 13.1 up to 12 weeks ‐ short term | 7 | 594 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.49, 0.60] |
| 13.2 12‐ 26 weeks ‐ medium term | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐3.08, 4.68] |
| 14 Mental state: 7. Specific ‐ binary outcomes | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 anxiety ‐ labelled as"adverse effect" | 2 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.79, 1.90] |
| 14.2 depression ‐ labelled as"adverse effect" | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.95] |
| 14.3 exacerbation of schizophrenia ‐ labelled as"adverse effect" | 2 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.50, 1.56] |
| 15 Mental state: 8. Various scale scores decreased rate (low=poor, data skewed) | Other data | No numeric data | ||
| 15.1 PANSS positive symptom subscale | Other data | No numeric data | ||
| 15.2 PANSS negative symptom subscale | Other data | No numeric data | ||
| 15.3 PANSS general pathology subscale | Other data | No numeric data | ||
| 15.4 BPRS total | Other data | No numeric data | ||
| 15.5 PANSS total | Other data | No numeric data | ||
| 16 Adverse effects: 1. At least one adverse effect | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 non‐specific | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.30] |
| 16.2 endocrine dyscrasia | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.61] |
| 16.3 high prolactin level | 1 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 16.4 liver function abnormal | 5 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.07] |
| 16.5 skin adverse reaction | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.41, 6.41] |
| 16.6 sweating‐ increase | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 28.16] |
| 16.7 flu syndrome | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.54] |
| 16.8 respiratory infection | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.50, 4.69] |
| 17 Adverse effects: 2. Cardiac effects | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 abnormal ECG | 2 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.15, 6.29] |
| 17.2 blood pressure‐ decrease | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 17.3 QTc prolongation | 3 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.11, 1.38] |
| 17.4 tachycardia | 5 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.39, 2.08] |
| 18 Adverse effects: 3a. Cardiac ‐ QTc change from baseline (in ms) | 1 | 317 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐9.51, 2.11] |
| 19 Adverse effects: 3b. Central / peripheral nervous system | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 dizziness | 6 | 1057 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.62, 1.53] |
| 19.2 blurred vision | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.35] |
| 19.3 headache | 5 | 991 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.82, 5.07] |
| 19.4 fatigue | 3 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.21, 1.62] |
| 19.5 insomnia | 7 | 1141 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.95, 3.45] |
| 19.6 somnolence | 5 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.42, 1.01] |
| 19.7 headache/dizziness | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.09, 1.00] |
| 19.8 sedation | 2 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.23, 0.60] |
| 19.9 nervousness | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.42, 4.19] |
| 19.10 CNS stimulation | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.21, 2.52] |
| 20 Adverse effects: 4. Endocrine ‐ Prolactin ‐ average increase | 1 | 566 | Mean Difference (IV, Random, 95% CI) | ‐8.89 [‐12.96, ‐4.82] |
| 21 Adverse effects: 5. Extrapyramidal ‐ various | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 Akathisia | 6 | 1320 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.67, 3.60] |
| 21.2 Tremor | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 7.23 [0.95, 55.31] |
| 21.3 Extrapyramidal symptoms | 4 | 667 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.59] |
| 21.4 Parkinsonism | 3 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.38] |
| 22 Adverse effects: 6. Gastrointestinal | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 nausea / vomiting | 6 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.80, 2.26] |
| 22.2 constipation | 6 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.21, 1.04] |
| 22.3 dry mouth | 5 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.19] |
| 22.4 abdominal pain ‐ upper | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [1.09, 8.03] |
| 23 Adverse effects: 7. Hormonal | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 menstrual changes | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.13] |
| 24 Adverse effects: 8a. Metabolic ‐ binary measures | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 appetite‐ increase | 2 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.06, 2.00] |
| 24.2 blood glucose ‐ increase | 3 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.44] |
| 24.3 cholesterol ‐ abnormally high cholesterol value | 1 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.19, 0.54] |
| 24.4 lactation | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 24.5 PRL‐ increase | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.56] |
| 24.6 weight gain | 9 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.43] |
| 25 Adverse effects: 8b. Metabolic ‐ continuous measures (high=poor) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 weight ‐ average endpoint level (in kg) | 3 | 242 | Mean Difference (IV, Random, 95% CI) | ‐7.43 [‐9.21, ‐5.65] |
| 25.2 weight gain ‐ change from baseline (in kg) | 2 | 656 | Mean Difference (IV, Random, 95% CI) | ‐3.03 [‐7.35, 1.29] |
| 25.3 cholesterol ‐ change from baseline (in mg/dl) | 2 | 789 | Mean Difference (IV, Random, 95% CI) | ‐15.37 [‐21.62, ‐9.11] |
| 25.4 cholesterol ‐ TC average endpoint level (in mmol/L) | 2 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.44, ‐0.56] |
| 25.5 cholesterol ‐ TG average endpoint level (in mmol/L) | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.31, ‐0.69] |
| 25.6 blood glucose ‐ PBG average endpoint level (in mg/dl) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.27, ‐0.63] |
| 25.7 glucose ‐ change from baseline (in mg/dl) | 2 | 883 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐7.98, 1.19] |
| 26 Adverse effects: 9. Average endpoint scale score (TESS, high=poor, data skewed) | Other data | No numeric data | ||
| 27 Leaving the study early | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Any reason | 9 | 2331 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.05, 1.25] |
| 27.2 Economic issues | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.51] |
| 27.3 Early discharge | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 27.4 Refusing therapy | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 27.5 adverse events | 4 | 1352 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.59] |
| 27.6 inefficacy | 4 | 1352 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.23, 2.67] |
| 27.7 lost to follow‐up | 2 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.46, 1.17] |
| 27.8 medication noncompliance | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [0.71, 7.06] |
| 27.9 others | 2 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [0.77, 6.34] |
| 27.10 patient decision | 3 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.81] |
| 27.11 protocol entry or interim criteria not met | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.68] |
| 27.12 protocol violation | 2 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.42, 1.53] |
| 27.13 sponsor decision | 1 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.03] |
| 27.14 death | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.55] |
| 28 Quality of life: 1. Average endpoint general quality of life score (GQOLI‐74, low=poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1 Total score | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐6.37, 3.85] |
| 28.2 Physical health | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐4.72, 4.34] |
| 28.3 Mental health | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐8.20, 3.28] |
| 28.4 Social function | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 1.21 [‐3.41, 5.83] |
| 28.5 Material life | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐4.62, 3.08] |
5.17. Analysis.
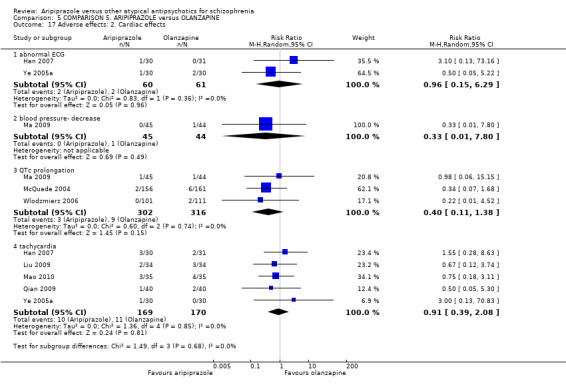
Comparison 5 COMPARISON 5. ARIPIPRAZOLE versus OLANZAPINE, Outcome 17 Adverse effects: 2. Cardiac effects.
Comparison 6. COMPARISON 6. ARIPIPRAZOLE versus OLANZAPINE (acutely agitated people ‐ all data by 5 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Average improvement (BPRS, high=good) | 1 | 471 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.79, 0.43] |
| 2 Mental state: Specific ‐ binary outcomes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 agitation ‐ no change ‐ (change defined as ≥ 40%reduction in PANSS‐EC) | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.12] |
| 2.2 agitation ‐ labelled as"adverse effect" | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.17] |
| 2.3 anxiety ‐ labelled as"adverse effect" | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.40, 2.17] |
| 2.4 exacerbation of schizophrenia ‐ labelled as"adverse effect" | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 5.13 [0.25, 106.49] |
| 3 Leaving the study early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 adverse events | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.12, 4.07] |
| 4 Adverse effects: 1. Central nervous system | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 dizziness | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.36] |
| 4.2 headache | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [1.02, 5.77] |
| 4.3 lethargy | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.30, 5.90] |
| 4.4 sedation | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.23, 2.22] |
| 4.5 sleep ‐ insomnia | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.87, 2.94] |
| 4.6 somnolence | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.15] |
| 5 Adverse effects: 2. Endocrine ‐ Prolactin ‐ average increase | 1 | 604 | Mean Difference (IV, Random, 95% CI) | ‐15.76 [‐19.18, ‐12.34] |
| 6 Adverse effects: 3a. Extrapyramidal ‐ various | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 akathisia | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.79, 2.56] |
| 6.2 parkinsonism | 1 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.41, 7.10] |
| 7 Adverse effects: 3b. Extrapyramidal ‐ average score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Barnes Akathesia Scale (high=poor) | 1 | 604 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.24, 0.38] |
| 7.2 Simpson‐Angus Scale (high=poor) | 1 | 604 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 8 Adverse effects: 4. Gastrointestinal | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 nausea / dyspepsia | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.09, 2.71] |
| 8.2 salivation ‐ dry mouth | 1 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.20, 4.91] |
| 9 Adverse effects: 5. Metabolic ‐ continuous measures | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 triglycerides ‐ fasting, increase | 1 | 604 | Mean Difference (IV, Random, 95% CI) | ‐35.62 [‐49.25, ‐21.99] |
Comparison 7. COMPARISON 7. ARIPIPRAZOLE versus OTHER ANTIPSYCHOTIC DRUGS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. No change (as defined in the study, measured by IAQ) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 cognition | 1 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.11] |
| 1.2 energy | 1 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.84] |
| 1.3 mood | 1 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 1.4 negative symptoms | 1 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 0.99] |
| 1.5 somnolence | 1 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.93] |
| 1.6 weight gain | 1 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.94] |
| 2 Global state: 2. Change in sexual dysfunction (ASEX) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐2.97, 0.11] |
| 3 Mental state: Specific ‐ binary outcomes | 3 | 5338 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.96, 1.51] |
| 3.1 Anxiety ‐ labelled as"adverse effects" | 2 | 1361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.94, 1.90] |
| 3.2 Agitation ‐ labelled as"adverse effects" | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [0.96, 7.23] |
| 3.3 Schizophrenia ‐ labelled as"adverse effects" | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.81] |
| 3.4 Psychotic disorder ‐ labelled as"adverse effects" | 3 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.73, 1.48] |
| 4 Preference: Study medication worse than or equal to previous medication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 patient ‐ by up to 12 weeks (short‐term) | 1 | 446 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.91] |
| 4.2 care giver ‐ by up to 12 weeks (short‐term) | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.52, 1.43] |
| 4.3 patient ‐ from 12 to 26 weeks (medium‐term) | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.25, 0.61] |
| 4.4 care giver ‐ from 12 to 26 weeks (medium‐term) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.15, 1.33] |
| 5 Quality of life: 1. Unsatisfactory response on health dimension scale | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.66] |
| 6 Quality of life: 3. Average change score (QLS, high=better)) | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [3.08, 9.32] |
| 7 Quality of life: 2. Average score (EQ‐5D utility score, high=better) | 1 | 329 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 8 Quality of life: 4a. Weight related ‐ No meaningful change (IWQOL‐Lite) | 1 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| 9 Quality of life: 4b. Weight related ‐ average score (IWQOL‐Lite, high=better) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 short‐term | 1 | 443 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐1.84, 4.16] |
| 9.2 medium‐term | 1 | 328 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [1.04, 3.96] |
| 10 Leaving the study early | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 any reason | 3 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.78, 1.19] |
| 10.2 administrative reasons | 1 | 833 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.84] |
| 10.3 adverse events | 3 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.11, 1.76] |
| 10.4 death | 1 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.23] |
| 10.5 inefficacy | 3 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.45, 1.96] |
| 10.6 lost to follow‐up | 2 | 1388 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.34, 2.13] |
| 10.7 no longer meets criteria | 2 | 1388 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.16, 2.63] |
| 10.8 other | 2 | 1388 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.80] |
| 10.9 poor/non compliance | 2 | 1388 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.53, 2.32] |
| 10.10 pregnancy | 1 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.14, 6.73] |
| 10.11 withdrew | 3 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.36, 0.66] |
| 11 Adverse effects: 1. At least one adverse effect | 2 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.05, 1.23] |
| 12 Adverse effects: 2. Central nervous system | 3 | 11524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.12, 1.49] |
| 12.1 sleep disorder | 1 | 813 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.78, 4.10] |
| 12.2 insomnia | 3 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.65, 2.66] |
| 12.3 somnolence | 3 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.39, 0.71] |
| 12.4 headache | 3 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.09, 1.99] |
| 12.5 fatigue | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.28] |
| 12.6 Lightheadedness | 1 | 1520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.67] |
| 13 Adverse effects: 3a. Endocrine ‐ Prolactin ‐ increase in level | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.41] |
| 14 Adverse effects: 3b. Endocrine ‐ Prolactin ‐ Change in level | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐8.60 [‐19.14, 1.94] |
| 15 Adverse effects: 4. Extrapyramidal ‐ akathisia | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 homogeneous data | 2 | 1361 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [1.28, 5.99] |
| 15.2 outlying study | 1 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.12, 0.22] |
| 16 Adverse effects: 5. Gastrointestinal | 3 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [2.12, 4.61] |
| 16.1 nausea | 3 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [2.12, 4.61] |
| 17 Adverse effects: 6a. Metabolic‐ binary measures | 1 | 2517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.82] |
| 17.1 weight gain (7% or more of total body weight at 26 weeks ‐ medium‐term) | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.19, 0.64] |
| 17.2 weight loss (7% or more of total body weight at 26 weeks ‐ medium‐term) | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.97, 3.19] |
| 17.3 average weight gain | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.37] |
| 17.4 total cholesterol increase | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.62, 0.91] |
| 17.5 LDL increase | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.84] |
| 17.6 HDL increase | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.61, 1.22] |
| 17.7 triglyceride increase | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 1.00] |
| 17.8 fasting glucose (increase) | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.47] |
| 18 Adverse effects: 6b. Metabolic ‐ continuous measures | 1 | 1796 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐3.48, ‐2.46] |
| 18.1 weight change (no risk) | 1 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐3.68, ‐1.72] |
| 18.2 % weight change from baseline‐ up to 12 weeks (short‐term) | 1 | 441 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐3.30, ‐1.66] |
| 18.3 % weight change from baseline ‐ from 12 to 26 weeks (medium‐term) | 1 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐4.65, ‐2.83] |
| 18.4 no change in fasting total cholesterol | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | ‐9.70 [‐16.07, ‐3.33] |
| 18.5 no change in fasting total glucose | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐5.78, 1.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An 2008.
| Methods | Allocation: randomised. No further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. | |
| Participants | Diagnosis:schizophrenia (CCMD‐3). PANSS score of 60 or more. N = 78. Age: not reported. Gender: not reported. History: duration of illness not reported, age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 41. 2. Clozapine: Dose range: 25‐500 mg/day. Mean dose: not reported. N = 37. | |
| Outcomes | Globlal state:PANSS score decreased rate (recovery: ≥ 80%, markedly improved: ≥ 50%, improved: ≥ 20%, no effect: < 20%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no drop‐out. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of use of alprazolam, propranolol and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Bai 2007.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 118.
Age: 20~50 years, aripiprazole group, mean = (32 ± 11) years; clozapine group, mean= (32 ± 13) years. Gender: aripiprazole group: 34 male, 25 female; clozapine group: 38 male, 21 female. History: aripiprazole group: 1~36 months, mean = (22 ± 5) months; clozapine group: 1~36 months, mean = (20 ± 7) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (20 ± 5) mg/day. N = 59. 2. Clozapine: Dose range: 50‐650 mg/day. Mean dose: (450 ± 125) mg/day. N = 59. | |
| Outcomes | Global state: PANSS score decreased rate (markedly improved: > 60%, improved: 40%‐60%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score. Adverse events. Unable to use: Mental state: PANSS anxiety subscale score ‐ unvalidated subscale. Cognitive functioning: PANSS cognitive factor subscale score ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | The outcome did not include missing data. There were no data on other adverse events. This procedure may have missed important data. |
| Other bias | Low risk | None obvious. |
Bai 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (32.1 ± 7.1) years; ziprasidone group: mean = (31.8 ± 7.1) years. Gender: not reported. History: aripiprazole group: mean= (5.5 ± 2.2) years; ziprasidone group: mean = (5.6 ± 2.3) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean= (15.8 ± 5.1) mg/day. N = 30. 2. Ziprasidone: Dose range: 20‐140 mg/day. Mean= (88.7 ± 12.6) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on liver function, insomnia, somnolence, and sleep disorder were missing or incomplete. |
| Other bias | Low risk | None obvious. |
Ban 2008.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 35 or more.
N = 80.
Age: aripiprazole group, 16~45 years. mean= (29 ± 8.8) years; quetiapine group, 18‐45 years, mean = (30 ± 2.7) years. Gender: aripiprazole group: 17 male, 23 female; quetiapine group: 21 male, 19 female. History: aripiprazole group: mean = (1.2 ± 1.0) months; clozapine group: mean = (0.9 ± 1.1) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: (11.8 ± 3.4) mg/day. N = 40. 2. Quetiapine: Dose range: 50‐750 mg/day. Mean dose: (544.8 ± 83.8) mg/day. N = 40. | |
| Outcomes | Global state: no clinically significant improvement. Mental state: BPRS total score. Adverse effects. Unable to use ‐ BPRS anxiety‐depression subscale score, BPRS thought disorder subscale score, BPRS activation subscale score, BPRS hostile subscale score ‐ subscales are unvalidated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on renal function, EEG, use of benzodiazepines and other medication were missing. |
| Other bias | Low risk | None obvious. |
Chan 2007.
| Methods | Allocation: random, permuted block randomisation stratified by centre. Blindness: double, identical capsules. Duration: four weeks. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: (DSM‐IV) schizophrenia (N = 80) or schizoaffective disorder (N = 3), acute relapse. PANSS total score of 60 or more. N = 83. Age: 18‐65 years (mean aripiprazole = 35.2 years, mean risperidone = 35.1 years). Gender: 45 M, 38 F. History: duration of illness not reported, age at onset not reported. Setting: inpatient. | |
| Interventions | 1. Aripiprazole: fixed dose: 15 mg/day. N = 49. 2. Risperidone: fixed dose: 6 mg/day. N = 34. | |
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score, PANSS positive sub score, PANSS negative sub score. Adverse effects: at least one adverse effect, cardiac effects (QTc), extrapyramidal adverse effects (use of antiparkinson medication, extrapyramidal symptoms, AIMS, BAS, SAS), cholesterol increase, glucose elevation, prolactin increase, weight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random, permuted block randomisation stratified by centre. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in adverse effects. This can be a problem for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number of drop‐out = 25%. The LOCF method was used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Only adverse events with an incidence of at least 5% in any treatment group were reported, therefore important side effects may have been missed by this procedure. |
| Other bias | High risk | The study was industry sponsored by the manufacturer of aripiprazole. |
Chang 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 100.
Age: aripiprazole group: 18~48 years. mean= (29.5 ± 11.7) years; risperidone group: 16~60 years, mean = (29.3 ± 10.6) years. Gender: aripiprazole group: 29 male, 21 female; risperidone group: 28 male, 22 female. History: aripiprazole group: 1 month~10 years, mean = (6.4 ± 1.5) years; risperidone group: 1.5 months~13 years, mean = (6.3 ± 1.8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 50. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose:not reported. N = 50. | |
| Outcomes | Globlal state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐ 75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score,PANSS general pathological subscale score, irritability‐ labelled as "adverse effect". Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood glucose, liver function, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Chen 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: two weeks wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 101.
Age: aripiprazole group: 16~60 years. mean = (28.6 ± 12.9) years; risperidone group: 17~59 years, mean = (25.4 ± 7.7) years. Gender: aripiprazole group: 19 male, 32 female; risperidone group: 26 male, 24 female. History: aripiprazole group: 1.4~240 months, mean = (23.3 ± 43.2) months; risperidone group: 1.7~108 months, mean = (23.3 ± 37.1) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean = (15.9 ± 4.0) mg/day. N = 51. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.3 ± 1.1) mg/day. N = 50. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI‐SI score, CGI‐EI score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Chen 2007a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six months. Design: parallel. Location: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 100. Age: not reported. Gender: not reported. History: 1~6 months, mean = (2.8 ± 1.3) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 50. 2. Quetiapine: Dose range: 100‐600 mg/day. Mean dose: not reported. N = 50. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐ 80%, improved: 30%‐50%, no effect: < 30%); CGI‐SI total score. Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. agitation‐labelled as "adverse effect". Life of quality: WHO‐QOL‐100. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, blood glucose, electrolyte, ECG, liver function (deficient data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on blood routine, blood glucose, electrolyte, ECG, liver function were deficient. |
| Other bias | Low risk | None obvious. |
Chen 2008a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (32.5 ± 9.3) years; risperidone group: mean = (33.2 ± 8.0) years. Gender: 60 female. History: aripiprazole group: mean = (3.6 ± 1.3) years; risperidone group: mean = (3. 9 ± 1.5) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean = (18. 6 ±7. 4)) mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4. 3 ±1. 3) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, excitement labelled as "adverse effect". Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on blood routine, blood glucose, use of propranolol, benzodiazepines and other medication were missing. |
| Other bias | Low risk | None obvious. |
Chen 2009.
| Methods | Allocation: randomised, no further details. Blindness: open. Duration: eight weeks. Design: parallel. Location: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60. Age: aripiprazole group: 18~58 years; mean = (25.63 ± 7.9) years; quetiapine group: 18~55 years; mean = (29.4 ± 9.5) years. Gender: aripiprazole group: 18 male, 12 female; quetiapine group: 17 male, 13 female. History: aripiprazole group: 3 months~5 years, mean = (2.39 ± 1.8) years; quetiapine group: 3 months~5 years, mean = (3.2 ± 1.6) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Quetiapine: Dose range: 100‐800 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ Mental state: SANS subscale score ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on EEG, ECG, urine routine, use of benzhexol, benzodiazepine and propranolol were also missing. |
| Other bias | Low risk | None obvious. |
Chen 2009a.
| Methods | Allocation: randomised, random number table. Blindness: unclear. Duration: 3~7 days wash‐out period + six weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more, Excited subscales (PEC) of 14 or more.
N = 75.
Age: aripiprazole group: mean = 38.4 years; olanzapine group: mean = 37.1 years. Gender: aripiprazole group: 25 male, 15 female; olanzapine group: 22 male, 13 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 40. 2. Olanzapine: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐75%, no effect: < 50%). Mental state: PANSS total score. Leaving the study early. Adverse effects. Unable to use ‐ Mental state: PANSS PEC subscale score ‐ unvalidated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% patients were lost to follow up (2/40 vs 1/35), and there were no difference between the two groups. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, ECG, EEG, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Chen 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 64.
Age: aripiprazole group 18~50 years, mean = (22.35 ± 7.15) years; risperidone group 18~49 years, mean = (22.25 ± 9.55) years. Gender: aripiprazole group: 14 male, 18 female; risperidone group: 17 male, 15 female. History: aripiprazole group 1 month~10 years; risperidone group 1 month~9 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (19.4 ± 2.1) mg/day. N = 32. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (2.7 ± 0.7)mg/day. N = 32. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Low riskUnclear riskHigh risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Low riskUnclear riskHigh risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, nausea/vomiting, dizziness, blurred vision, insomnia, constipation, excitement, use of benzodiazepines and other medicines was missing. |
| Other bias | Low risk | None obvious. |
Chen 2010a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 120.
Age: 18~60 years, aripiprazole group mean= (31.8 ±7.9) years; risperidone group mean = (32.5 ± 6.8) years. Gender: aripiprazole group: 24 male, 36 female; risperidone group: 26 male, 34 female. History: aripiprazole group: mean = (11.2 ± 6.1) months; risperidone group: mean= (11.8 ± 6.9) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 60. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 60. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of benzodiazepines and other medicines was missing. |
| Other bias | Low risk | None obvious. |
Cheng 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more, SANS of 60 or more.
N = 86.
Age: aripiprazole group: mean = (29.35 ± 7.6) years; ziprasidone group: mean= (30.28 ± 8.1) years. Gender: aripiprazole group: 25 male, 18 female; ziprasidone group: 26 male, 17 female. History: aripiprazole group: mean = (5.23 ± 2.81) years; ziprasidone group: mean = (5.16 ± 2.6) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean = (11.7 ± 1.2) mg/day. N = 43. 2. Ziprasidone: Dose range: 20‐120 mg/day. Mean = (86.26 ± 10.2) mg/day. N = 43. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. SANS total score score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on CGI‐GI score, TESS score, blood routine, urine routine, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
CuiMeng 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 120.
Age: aripiprazole group: 18~64 years, mean = (25.2 ± 7.5) years; risperidone group: 18~65 years, mean = (25 ± 8) years. Gender: aripiprazole group: 40 male, 20 female; risperidone group: 36 male, 24 female. History: aripiprazole group 1~55 months, mean = (22 ± 12) months; risperidone group 1~55 months, mean = (22 ± 13) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 60. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 60. | |
| Outcomes | Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score. Adverse effects: TESS score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although GQL I ‐ 74 was used to assess Quality of life, no data on score were available. Data on anxiety, somnolence, ECG, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Dai 2005.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more, CGI of 4 or more.
N = 80.
Age: aripiprazole group: 18~58 years. mean = (31 ± 8) years; quetiapine group: 19~57 years, mean = (32 ± 9) years. Gender: aripiprazole group: 20 male, 20 female; quetiapine group: 21 male, 19 female. History: aripiprazole group: 1 month~10 years, mean = (5.9 ± 3) years; clozapine group: 1 month~9 years, mean = (5 ± 3) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: (23.3 ± 3.1) mg/day. N = 40. 2. Quetiapine: Dose range: 75‐700 mg/day. Mean dose: (565 ± 85) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25). CGI average endpoint scale score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Dai 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 80. Age: aripiprazole group: 24~58 years, mean = 31 years; risperidone group: not reported. Gender: aripiprazole group: 28 male, 12 female; risperidone group: 24 male, 16 female. History: aripiprazole group: 1 month~8 years, mean = 6.4 years; risperidone group: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 40. 2. Risperidone: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 40. |
|
| Outcomes | Global state: PANSS score decreased rate (markedly improved: ≥70%, improved: 40%‐70%, no effect: < 40%). Mental state: PANSS total score. Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Deng 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 50.
Age: mean = (63.5 ± 2.3) years; risperidone group: mean = (65.2 ± 2.7) years. Gender: aripiprazole group: 17 male, 8 female; risperidone group: 15 male, 10 female. History: aripiprazole group: mean= (3.7 ± 2.4) years; risperidone group: mean=(5.2 ± 1.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 25. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 25. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: ≥50%, improved: ≥25%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, ESRS score, blood and urine routines, liver function, use of benzodiazepine and anticholinergic medicine were missing. |
| Other bias | Low risk | None obvious. |
Deng 2008a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 90.
Age: aripiprazole group: mean= (25 ± 5. 2) years; risperidone group: mean = (29 ± 4. 2) years. Gender: aripiprazole group: 25 male, 20 female; risperidone group: 23 male, 22 female. History: aripiprazole group: mean = (1.3 ± 1.1) years; risperidone group: mean = (1.5 ± 1.0) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 45. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose: not reported. N = 45. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐74%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzhexol, benzodiazepine and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Ding 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 35 or more.
N = 60.
Age: aripiprazole group: 18~56 years, mean = (28.6 ± 3.7) years; risperidone group: 19~55 years, mean = (28.1 ± 5.6) years. Gender: aripiprazole group: 13 male, 17 female; risperidone group: 14 male, 16 female. History: aripiprazole group: 1 month~3 years, mean = (1.4 ± 0.5) years. risperidone group: 1 month~3 years, mean = (1.3 ± 0.6) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: anxiety ‐ labelled as "adverse effect". Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | Data on BPRS score, TESS score, ECG, EEG were missing. |
| Other bias | Unclear risk | None obvious. |
Du 2006.
| Methods | Allocation: randomised, no further details. Blindness: double. Duration: one week wash‐out period + six weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 65.
Age: aripiprazole group: 18~65 years, mean = (29 ± 9) years; risperidone group: 18~60 years, mean = (31.1 ± 9.7) years. Gender: not reported. History: 1 month ~20 years, mean= (6.8 ± 8.4) years; risperidone group: 1 month ~23 years, mean= (7.3 ± 8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (21 ± 6.2) mg/day. N = 33. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.5 ± 1) mg/day. N = 32. | |
| Outcomes | Adverse effects: level of PRL, weight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Fan 2005.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 72.
Age: aripiprazole group, mean = (32 ± 10.2) years; clozapine group: mean = (31 ± 10.8) years. Gender: aripiprazole group: 19 male, 13 female; clozapine group: 20 male, 12 female. History:aripiprazole group: mean = (6.6 ± 3.3) years; clozapine group: mean = (6.4 ± 3.2 ) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (15.68 ± 6.42) mg/day. N = 36. 2. Clozapine: Dose range: 50‐500 mg/day. Mean = (211 ± 31.82) mg/day. N = 36. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects: central nervous system (somnolence ) extrapyramidal side‐effects, weight gain, postural hypotension, tardive dyskinesia, constipation,endocrine (menstrual disorder), ECG abnormal (Q‐Tc prolongation), change of blood routine. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on laboratory tests (urine routine, glucose, liver function) and ECG were missing. |
| Other bias | Low risk | None obvious. |
Fan 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 19~60 years, mean = (33.5 ± 6.9) years; risperidone group: 18~60 years, mean = (32.7 ± 8.1) years. Gender: aripiprazole group: 17 male, 13 female; risperidone group: 18 male, 12 female. History: aripiprazole group: 1~146 months, mean = (6.3 ± 5.7) years; risperidone group: 1~160 months, mean = (6.9 ± 6.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: (22.5 ± 6.6)mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: (4.5 ± 1.4)mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on blood and urine routine, renal function, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Feng 2006.
| Methods | Allocation: randomised, no further details. Blindness: double. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 69.
Age: 18~55 years. Gender: not reported. History: < 1 year. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (22.68 ± 4.91) mg/day. N = 35. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.94 ± 1.43) mg/day. N = 34. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). CGI score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded evaluation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total proportion of loss to follow‐up was 13.4% (5/35 vs. 4/34), there no no difference between the two groups. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, ECG, liver function and other adverse effects were missing. |
| Other bias | Low risk | None obvious. |
Fleischhacker 2008.
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 52 weeks (first six weeks observed). Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: (DSM‐IV) acute schizophrenia, PANSS of 60 or more. N = 703. Age: 18‐65 years. Gender: M, F. History: duration of illness not reported, age at onset not reported. Setting: in‐ and out‐patient. | |
| Interventions | 1. Aripiprazole: flexible dose. Allowed dose range: 15‐30 mg/day. N = 355. 2. Olanzapine: flexible dose. Allowed dose range: 10‐20 mg/day. N = 348. | |
| Outcomes | Leaving the study early: any reason.
Global state: CGI.
Mental state: PANSS total score, MADRS.
Quality of life/satisfaction with treatment: Quality of Life Enjoyment and Satisfaction Questionnaire, Medication adherence scale.
Adverse effects: open interviews, EPS (SAS, AIMS, BAS), cardiac effects (ECG), weight gain (BMI). Unable to use ‐ Adverse event outcomes (no data, interim report) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomisation using SARA (System for Automated Randomisation). QTONE an automated touch tone system was used. Statistical blocking factor of four was used and stratified by the study centre. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in adverse effects. This can be a problem for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Leaving the study early data within the first six weeks were 25% overall, but data on reason for dropout were not available. The LOCF method was used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Data for the predefined primary outcome are available but secondary outcome measures like 30% PANSS total reduction are missing in the six‐week interim report. Treatment emergent adverse events are hardly addressed in the interim report. |
| Other bias | High risk | The study was industry sponsored by the manufacturer of aripiprazole. Baseline data reporting is insufficient in terms of missing data on history of illness. |
Fu 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 69.
Age: 12~18 years, aripiprazole group: mean = (16.9 ± 3.4) years; risperidone group: mean = (15.6 ± 5.7) years. Gender: aripiprazole group: 20 male, 13 female; risperidone group: 21 male, 15 female. History: aripiprazole group: mean = (4.7 ± 2.9) years; risperidone group: mean= (5.2 ± 3.5) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean = (16.5 ± 3) mg/day. N = 33. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean = (3.6 ± 1) mg/day. N = 36. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: > 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS total score decreased rate, "agitation" labelled as adverse effect. Drug combination. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on blood routine, liver function were incomplete. |
| Other bias | Low risk | None obvious. |
Ge 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80. Age: 18~40 years, aripiprazole group: mean = (22.1 ± 5.9) years; quetiapine group: mean = (24.8 ± 7.2) years. Gender: Not reported. History: aripiprazole group: mean = (1.1 ± 0.7) years; quetiapine group: mean = (1.3 ± 0.7) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Initial dose: 5 mg/day. Mean = (25.5 ± 1.8) mg/day. N = 40. 2. Quetiapine: Initial dose: 100mg/day. Mean = (425 ± 27) mg/day. N = 40. | |
| Outcomes | Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. |
| Other bias | Low risk | None obvious. |
Ge 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80. Age: 16~55 years, aripiprazole group: mean = (30 ± 2.4) years; quetiapine group: mean = (31 ± 3.2) years. Gender: 80 female. History: < 3 years, aripiprazole group: not reported; quetiapine group: 3 months~3 years, mean = (2.05 ± 0.5) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 2.5‐30 mg/day. Mean dose: not reported. N = 40. 2. Quetiapine: Dose range: 50‐600 mg/day. Mean dose: not reported. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score, anxiety‐ labelled as "adverse effect". Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score and use of benzodiazepine were missing. Data on weight‐average endpoint level were incomplete. |
| Other bias | Low risk | None obvious. |
Guo 2006.
| Methods | Allocation: randomised, no further details. Blindness: double. Duration: 3‐7 days wash‐out period + six weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. BPRS of 35 or more.
N = 38.
Age: aripiprazole group: mean = (28.9 ± 7.3) years; risperidone group: mean= (27.7 ± 8.4) years. Gender: aripiprazole group: 11 male, 7 female; risperidone group: 14 male, 6 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 18. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose: not reported. N = 20. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. BPRS total score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, use of benzodiazepines and anticholinergic medicine were missing. |
| Other bias | Low risk | None obvious. |
Han 2005.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 70.
Age: 18~60 years, mean aripiprazole group = (27 ± 6) years; mean clozapine group = (29 ± 8) years. Gender: aripiprazole group: 18 male, 17 female; clozapine group: 19 male, 16 female. History: aripiprazole group, mean = (1.5 ± 2) years; clozapine group: mean = (1.7 ± 2 ) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 35. 2. Clozapine: Dose range: 25‐450 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects: Central nervous system (dizziness, headache,insomnia, somnolence), extrapyramidal side‐effects, gastrointestinal (constipation, salivate), weight gain, blood glucose increase, blood routine abnormal, change of ECG (Q‐T abnormal). Unable to use ‐ Adverse effects: TESS score (no data), EEG, laboratory tests (urine routine, hepatorenal function) and use of Benzodiazepines azoles or Artane (no data). Mental state: PANSS anxiety subscale score ‐ unvalidates subscale. Cognitive function: PANSS cognitive factor subscale score ‐ subscale unvalidated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on laboratory tests (urine routine, hepatorenal function) and EEG were missing. |
| Other bias | Low risk | None obvious. |
Han 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 61.
Age: aripiprazole group: mean = (31.0 ± 10.1) years; olanzapine group: mean = (29.9 ± 9.3) years. Gender: aripiprazole group: 14 male, 16 female; olanzapine group: 14 male, 17 female. History: aripiprazole group: mean = (5.89 ± 1.1) years; olanzapine group: mean= (6.26 ± 1.2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (20.8 ± 3.1) mg/day . N = 30. 2. Olanzapine: Dose range: 5‐20 mg/day. Mean dose = (13.6 ± 2.7) mg/day. N = 31. | |
| Outcomes | Global state: markedly improved, improved, slight improved, no effect. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient was lost to follow‐up in olanzapine. |
| Selective reporting (reporting bias) | High risk | Data on CGI score, TESS total score, blood and urine routine, use of benzodiazepines anticholinergic medicine were missing. |
| Other bias | Low risk | None obvious. |
Han 2007a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 86.
Age: aripiprazole group: 16~61 years. mean = (25.7 ± 7.9) years; risperidone group: 16~62 years, mean = (26.9 ± 8.4) years. Gender: aripiprazole group: 19 male, 24 female; risperidone group: 20 male, 23 female. History: aripiprazole group:1 month~10 years, mean = (4.8 ± 2.6) years; risperidone group: 1 month~9 years, mean = (4.6 ± 2.8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: (15.3 ± 3.2) mg/day. N = 43. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: (3.8 ± 1.6) mg/day. N = 43 | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐75%, no effect: < 50%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Mental state: PANSS subscale score decreased rate ‐ unvalidated subscales. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data of TESS total score, blood routine, blood glucose, electrolyte, ECG, liver function was missing. |
| Other bias | Low risk | None obvious. |
Hu 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 50.
Age: aripiprazole group 18~56 years, mean = (28.9 ± 4.6) years; risperidone group 16~58 years, mean = (30.1 ± 5.2) years. Gender: aripiprazole group: 13 male, 12 female; risperidone group: 12 male, 13 female. History: aripiprazole group: 1 month~5 years, mean = (3.6 ± 2.1) years; risperidone group: 2 months~5.2 years, mean = (3.8 ± 2.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 25. 2. Risperidone: Dose range: 2‐8 mg/day. Mean dose: not reported. N = 25. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, ECG, liver function, blood glucose, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Huang 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score = 50.
N = 60.
Age: 16~ 55 years, aripiprazole group mean = (30.90 ± 11.05) years; risperidone group mean = (29.55 ± 12.35) years. Gender: aripiprazole group: 12 male, 18 female; risperidone group: 13 male, 17 female. History: aripiprazole group: mean = (4.5 ± 6.9) months; risperidone group mean = (5.7 ± 8.7) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose:not reported. N = 30. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Leaving the study early. Adverse effects: the average endpoint score of BMI, blood glucose, TCHO, TG, PRL, LEP. Unable to use ‐ Mental state: PANSS total score (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although PANSS was used to assess mental state, no end data on score were available. Data on use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Ji 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient and patient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 64.
Age: aripiprazole group: 18~58 years, mean = (32.8 ± 6.2) years; risperidone group: 17~59 years, mean = (30.6 ± 7.8) years. Gender: aripiprazole group: 19 male, 13 female; risperidone group: 18 male, 14 female. History: aripiprazole group: 1 month~9 years, mean = (4.2 ± 3.9) years; risperidone group: 1 month~11 years, mean = (5.2 ± 3.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (15 ± 5) mg/ day. N = 32. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.2 ± 1.8) mg/ day. N = 32. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score, anxiety‐labelled as "adverse effect". Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, use of benzodiazepines and anticholinergic medicine were missing. |
| Other bias | Low risk | None obvious. |
Jiang 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: not reported, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 80. Age: Aripiprazole group: 18~56 years, mean = (33 ± 6) years; Clozapine group: 18~57 years, mean = (35 ± 5.7) years. Gender: aripiprazole group: 26 female, 14 male; clozapine group: 27 male, 13 female. History: aripiprazole group: 2~8 years, mean =(5 ± 3.2) years; clozapine group: 2~15years, mean =(5.7 ± 4.1) years. Age at onset not reported. Setting: not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (23.5 ± 1.4) mg/d. N = 40. 2. Clozapine: Dose range: 25‐500 mg/day. Mean = (400 ± 14.2) mg/d. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of use of alprazolam, propranolol and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Jie 2008.
| Methods | Allocation: randomised, random number table. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3), PANSS of 60 or more, SANS of 60 or more. N = 50. Age: aripiprazole group: 18~50 years, mean = (25.7 ± 5.8) years; clozapine group: 18~57 years, mean = (27.85 ± 5.75) years. Gender: aripiprazole group: 16 male, 9 female; clozapine group: 15 male, 10 female. History: aripiprazole group: 1~10 years, mean = (4.15 ± 3.14) years; clozapine group: 1~11years, mean = (4.18 ± 3.15) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean = (13.5 ± .8) mg/d. N = 25. 2. Clozapine: Dose range: 50‐500 mg/day. Mean = (385.5 ± 75.5) mg/d. N = 25. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 80%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects: TESS total score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on somnolence, weight gain, EGG abnormal, salivation, dry mouth, blurred vision (no data) and use of alprazolam, propranolol, anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Kane 2009.
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 28 weeks. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: (DSM‐IV) schizophrenia catatonic (n = 3), disorganised (n = 29), paranoid (n = 464), undifferentiated (n = 62), residual (n = 6), missing (n = 2), PANSS total score of ≥ 75, minimum of ≥ 4 PANSS positive items, score of ≥ 4 on CGI‐S, score of ≥ 3 on CGI‐I. N = 566. Age: 18‐65 years. Mean for males 38 years. Gender: 384 M, 182 F. History: duration of illness not reported, mean (SD) previous episodes 7.7 (8.0). Setting: inpatient and outpatient. | |
| Interventions | 1. Aripiprazole: 15‐ 30 mg/day, N = 285. 2. Olanzapine: 10‐20 mg/day. N = 281. | |
| Outcomes | Leaving the study early. Global state: CGI. Mental state: PANSS total score. Adverse effects: EPSE (SAS, AIMS, BAS), laboratory results‐ lipids, glucose, increase in prolactin level, sedation, weight gain and others. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as death are unlikely to have been much affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF. |
| Selective reporting (reporting bias) | High risk | Only adverse events with an incidence of more than 5% were reported. This procedure may have missed important adverse events. |
| Other bias | High risk | Drug company involvement. |
Kern 2006.
| Methods | Allocation: random, 1:1 ratio. Blindness: open label, no further details. Duration: 26 weeks. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: (DSM‐IV) schizophrenia or schizo‐affective disorder. N = 255. Age: 18‐65 years. Gender: 109 M, 60 F. History: duration of illness not reported. Setting: outpatient. | |
| Interventions | 1. Aripiprazole: 15‐ 30 mg/day, N = 128. 2. Olanzapine: 10‐15 mg/day. N = 127. | |
| Outcomes | Leaving the study early. Unable to use ‐ Cognitive function: CVLT, BVRT‐R, WCST, Trail Making A and B, Verbal Fluency, Letter‐Number Sequencing, Grooved Pegboard Test, CPT‐IP (no usable data). Mental state: PANSS total score (used as co‐variate, no usable data). |
|
| Notes | Study compared the neuro‐cognitive effects of medications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | High risk | Open label. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF. |
| Selective reporting (reporting bias) | High risk | Few neuro‐cognitive tests were mentioned but usable data was not available from them. |
| Other bias | High risk | Drug company involvement. |
Kerwin 2007.
| Methods | Allocation: random, computer generated 1: 1. Blindness: open label. Duration: 26 weeks. Design: parallel, naturalistic. Location: multicentre. | |
| Participants | Diagnosis: schizophrenia (DSM IV). N = 271. Age: 18‐65 years (mean about 38 years). Gender: M and F. History: duration of illness not reported, age at onset not reported. Setting: community or hospital‐based outpatient centre. . |
|
| Interventions | 1. Aripiprazole: 10‐30 mg/day (mean‐18.7 mg). N = 284. 2. Standard care: olanzapine 5‐20 mg/day (mean 12.5 mg); N = 75, risperidone 2‐16 mg/day (mean‐4.6 mg); N = 81, quetiapine100‐800 mg/day (mean‐386.8 mg); N = 110. | |
| Outcomes | IAQ, Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI (data not usable) ASEX. Quality of life: QLS, IWQoL‐Lite, EQ‐5D. Adverse effects: at least one adverse effect, extrapyramidal adverse effects, cholesterol increase, fasting triglycerides abnormalities, glucose elevation, prolactin increase, weight change. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | High risk | Open label. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF. |
| Selective reporting (reporting bias) | High risk | Only adverse events with an incidence of more than 5% were reported. This may have missed important adverse events. Some scales used had no reported usable data. |
| Other bias | High risk | Drug company involvement. |
Kinon 2004.
| Methods | Allocation: random (1: 1), no further details. Blindness: double, no further details. Duration: 5 days. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder or schizophreniform disorder. N = 604. Age 18‐55 years. Gender: male/female. History: duration of illness not reported, acutely ill. Setting: originally inpatient. |
|
| Interventions | 1. Aripiprazole: 15‐30 mg/day, mean 19.26 mg (SD 5.46). N = 298. 2. Olanzapine: 20 mg/day, mean 19.97 mg (SD 0.27). N = 306. | |
| Outcomes | Agitation symptoms: PANSS‐EC. Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score, BPRS. Adverse effects: at least one adverse effect, cardiac effects (QTc), extrapyramidal adverse effects (use of antiparkinson medication, extrapyramidal symptom (BAS, SAS‐modified), cholesterol increase, glucose elevation, prolactin increase, weight, Other measures: GANI, DAI‐10, ACES. | |
| Notes | Lorazepam was used as required throughout the study. Data were not made available for other scales used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random 1:1, no further details available. |
| Allocation concealment (selection bias) | Unclear risk | Not defined. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double. No further details available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The LOCF method was used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Adverse effects were reported if ≥ 1% of patients in either treatment group experienced them. Some scales used had no data published. |
| Other bias | High risk | The study was industry sponsored by the manufacturer of olanzapine. |
Kuang 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3), BPRS of 35 or more. N = 120. Age: aripiprazole group: 18~60 years, mean = (32 ± 9) years, clozapine group: 18~60 years, mean = (31 ± 10) years. Gender: aripiprazole group: 32 male, 24 female; clozapine group: 32 male, 24 female. History: aripiprazole group: mean = (6 ± 2.1) months; clozapine group: mean = (5 ± 2.3) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (20 ± 5) mg/day. N = 60. 2. Clozapine: Dose range: 75‐600 mg/day. Mean = (550 ± 50) mg/day. N = 60. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: BPRS score. Leaving the study early. Quality of life: GQOLI‐74 (material life, physical health, mental health, social function). Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total rate of loss to follow‐up was 5% (6/120), and there was no difference between the two group. All 6 participants dropped out because of economic issue. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on laboratory tests (blood routine, urine routine, glucose, liver function, etc) were missing. |
| Other bias | Low risk | None obvious. |
Li 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 70.
Age: aripiprazole group: 22~43 years, mean = (32.83 ± 9. 42) years; risperidone group: 20~42 years, mean = (33. 64 ± 7. 26) years. Gender: aripiprazole group: 18 male, 17 female; risperidone group: 20 male, 15 female. History: aripiprazole group: 1~3 months, mean= (2.14 ± 0.87) months; risperidone group: 1~3 months, mean= (2.15 ± 0.97) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 35. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. agitation‐labelled as "adverse effect". Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total proportion of loss to follow‐up was 4.29% (1/35: 2/35), and there was no difference between the two groups. All because of adverse effects. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines, benzhexol, and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Li 2006a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 76.
Age: aripiprazole group: 18~59 years. mean = (8. 6 ± 8. 7) years; risperidone group: 20~58 years, mean = (29. 8 ± 9. 3) years. Gender: aripiprazole group: 20 male, 18 female; risperidone group: 21 male, 17 female. History: aripiprazole group: 1 month~13 years, mean = 6.5 years; risperidone group: 2 months~12 years, mean = 7.2 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (18.3 ± 4.2) mg/day. N=38. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.1 ± 1.2) mg/day. N=38. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score, anxiety‐ labelled as "adverse effect", excitement/agitation‐labelled as "adverse effect". Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total proportion of loss to follow‐up was 2.63% (1/38: 0/38), all because of adverse effects. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, renal function, use of benzodiazepines and anticholinergic drug were missing. |
| Other bias | Low risk | None obvious. |
Li 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: not reported, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80.
Age: aripiprazole group: mean = (28.9 ± 13.1) years; clozapine group: mean = (30.2 ± 12.5) years. Gender: aripiprazole group: 22 male, 18 female; clozapine group: 23 male, 17 female. History: aripiprazole group: mean = (7.3 ± 4.1) months; clozapine group: mean = (7.4 ± 4.2) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐60 mg/day. Mean = (21.1 ± 6.6) mg/day. N = 40. 2. Clozapine: Dose range: 75‐500 mg/day. Mean = (301 ± 115.2) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects: extrapyramidal side‐effects (akathisia, tremor, dystonia, spasmodic torticollis). Quality of life: WHO‐QOL‐100. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although ESRS was used to assess adverse effects, no data on score were available. Data on laboratory tests(blood routine, urine routine, glucose, liver function, etc) and ECG were missing. |
| Other bias | Low risk | None obvious. |
Li 2007a.
| Methods | Allocation: random, no further details. Blindness: unclear. Duration: six months. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3), PANSS of 60 or more. N = 60. Age: aripiprazole group: mean = (25.1 ± 6.8) years; clozapine group: mean = (26.4 ± 6.2) years. Gender: aripiprazole group: 13 male, 17 female; clozapine group: 15 male, 15 female. History: 2 years or less. aripiprazole group: mean = (5.7 ± 4.3) months; clozapine group, mean= (6.5 ± 4.8) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 15‐30 mg/day. Mean dose:not reported. N = 30. 2. Clozapine: Dose range: 200‐350 mg/day. Mean dose:not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathology subscale score, anxiety ‐ labelled as "adverse effect''. Quality of life: WHOQOL100. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of anti‐psychotic drugs and benzhexol were missing. |
| Other bias | Low risk | None obvious. |
Li 2007b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60. Age: aripiprazole group: 18~50 years. mean = (24.4 ± 13.9) years; quetiapine group: 18‐48 years, mean = (27.2 ± 8.4) years. Gender: aripiprazole group: 17 male, 13 female; quetiapine group: 17 male, 13 female. History: aripiprazole group: 3 months~5 years, mean= (2.8 ± 2.2) years; quetiapine group: 3 months~5 years, mean= (2.6 ± 2.3) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N=30. 2. Quetiapine: Dose range: 100‐800 mg/day. Mean dose: not reported. N=30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ Adverse effects: TESS total score, menstrual disorder, lactation, weight gain, EEG, ECG, blood routine, urine routine, hepatorenal function, use of benzhexol, benzodiazepine and propranolol (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data onTESS total score, menstrual disorder, lactation, weight gain, EEG, ECG, blood routine, urine routine, hepatorenal function, use of benzhexol, benzodiazepine and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Li 2007c.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 84.
Age: aripiprazole group: 15~62 years. mean = (29.4 ± 11.2) years; ziprasidone group: 18‐60 years, mean = (28.3 ± 10.1) years. Gender: aripiprazole group: 18 male, 24 female; ziprasidone group: 20 male, 22 female. History: aripiprazole group: 1 month~12 years, mean = 5.6 years; ziprasidone group: 2 months~13 years, mean = 6.1 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (25 ± 5) mg/day. N = 42. 2. Ziprasidone: Dose range: 20‐160 mg/day. Mean = (120 ± 40) mg/day. N = 42. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Leaving the study early: no patient. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, EEG, weight, use of alprazolam, propranolol and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, blood routine, EEG, weight, and use of alprazolam, propranolol and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Li 2007d.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: 1~2 weeks wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 120.
Age: aripiprazole group: 16~60 years. mean = (28. 4 ± 9. 1) years; risperidone group: 17~56 years, mean = (29. 6 ± 8. 3) years. Gender: aripiprazole group: 28 male, 32 female; risperidone group: 46 male, 14 female. History: aripiprazole group: mean = (2.3 ± 1.8) months; risperidone group: mean = (2.4 ± 1.6) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 60. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 60. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI‐SI, CGI‐GI. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. agitation‐labelled as "adverse effect". Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, renal function, PRL, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, renal function, PRL, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Li 2009.
| Methods | Allocation: random, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 60. Age: aripiprazole group: 15~50 years, mean = (26. 9 ± 10. 1) years, clozapine group: 15~45 years, mean = (25. 5 ± 9. 3) years. Gender: aripiprazole group: 18 male, 12 female; clozapine group: 20 male, 10 female . History: aripiprazole group: 2 months~10 years, mean = (7.1 ± 5.12) years; clozapine group: 2 months~13 years, mean = (7.8 ± 6.2) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Clozapine: Dose range: 50‐475 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score,PANSS general pathological subscale score. Adverse effects: TESS total score(no data), central nervous system (somnolence, dizziness, headache,insomnia), extrapyramidal side‐effects (akathisia), gastrointestinal (constipation, salivate), weight gain, blood glucose increase, blood routine abnormal, change of ECG, tachycardia, appetite decrease. Unable to use ‐ Adverse effects: Laboratory tests (urine routine, hepatorenal function), use of alprazolam, propranolol and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on laboratory tests (urine routine, hepatorenal function), use of alprazolam, propranolol and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Li 2009a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3), BPRS total score of 20 or more.
N = 60. Age: aripiprazole group: mean = (28.6 ± 6.49) years; risperidone group: mean = (29.13 ± 8.61) years. Gender: aripiprazole group: 16 male, 14 female; risperidone group: 17 male, 13 female. History: aripiprazole group: mean = (2.17 ± 4.82) years; risperidone group: mean = (3.22 ± 6.71) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose:not reported. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose:not reported. N = 30. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: BPRS total score. Adverse effects Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Li 2009b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 15~45 years, mean = (25.3 ± 10.2) years; risperidone group: 17~50 years, mean = (28.3 ± 1.2) years. Gender: aripiprazole group: 14 male, 16 female; risperidone group: 15 male, 15 female. History: aripiprazole group: 1 month~2 years, mean = (1.1 ± 0.8) years; risperidone group: 2 months~2 years, mean = (1.2 ± 0.3) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (25.3 ± 5.1) mg/day. N = 30. 2. Risperidone: Dose range: 2‐6 mg/day. Mean = (3.9 ± 1.7) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, ECG, EPS, use of benzodiazepines, propranolol, and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Li X 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: 3~7 days wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 71.
Age: aripiprazole group: mean = (38.1 ± 8.6) years; risperidone group: mean = (36.3 ± 10.2) years. Gender: aripiprazole group: 25 male, 11 female; risperidone group: 22 male, 13 female. History: aripiprazole group: mean = (6.4 ± 3.9) years; risperidone group: mean = (5.9 ± 4.0) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 36. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. agitation‐labelled as "adverse effect". Adverse effects. Unable to use ‐ Adverse effects: TESS total score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score were missing. |
| Other bias | Low risk | None obvious. |
Lian 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 86.
Age: aripiprazole group: 21~53 years, mean = 38.1 years; risperidone group: 23~56 years, mean = 37.6 years. Gender: aripiprazole group: 27 male, 16 female; risperidone group: 30 male, 13 female. History: aripiprazole group: 2 months~8 years, mean = 4.5 years; risperidone group: 2 months~10 years, mean = 4.6 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 43. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 43. | |
| Outcomes | Global state: PANSS score decreased rate (excellent: ≥ 60%, improved: ≥ 40%, no effect: < 40%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood and urine routines, liver function, ECG (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data of TESS total score, blood and urine routines, liver function, ECG were missing (no data) . |
| Other bias | Low risk | None obvious. |
Liang 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: not reported, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 120.
Age: aripiprazole group 18~60 years, mean = (24. 9 ± 10. 7) years; risperidone group 18~58 years, mean = (26. 5 ± 9. 2) years. Gender: aripiprazole group: 32 male, 28 female; risperidone group: 31 male, 29 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = 18.7 mg/day. N = 60. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean = 4.8 mg/day. N = 60. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score. agitation labelled as "adverse effect". Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, use of benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six patients were lost to follow‐up in risperidone group, four because of adverse effect, two due to progressive disease. No patient was lost to follow‐up in aripiprazole group. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Liu 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS score of 60 or more. N = 60. Age: aripiprazole: 15~45 years, mean = (25.3 ± 10.2) years; clozapine: 17~50 years, mean = (28.3 ± 1.2) years. Gender: aripiprazole group: 13 male, 17 female; clozapine group: 14 male, 16 female . History: aripiprazole group: 1 month ~2 years, mean = (1.1 ± 0.8) years; clozapine group: 2 months ~2 years, mean = (1.2 ± 0.3) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose:not reported. N = 30. 2. Clozapine: Dose range: 50‐500 mg/day. Mean dose:not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score. Adverse effects. Unable to use ‐ Adverse effects: Laboratory tests (urine routine), use of alprazolam, propranolol and anticholinergic medication (no data). |
|
| Notes | Efficacy and side effects of aripiprazole for first‐episode schizophrenia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of benzodiazepines, benzhexol and propranolol medication were also missing. |
| Other bias | Low risk | None obvious. |
Liu 2006a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 90.
Age: aripiprazole group: 18~ 56 years, mean = (28 ± 4.6) years, risperidone group: 18~58 years, mean = (30.1 ± 5) years. Gender: aripiprazole group: 26 male, 19 female; risperidone group: 23 male, 22 female. History: aripiprazole group: 1 month~5 years, mean = (3.6 ± 2.1) years; risperidone group: 1 month~5.2 years, mean = (3.8 ± 2.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 45. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 45. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. agitation/excitement labelled as "adverse effect". Adverse effects. Unable to use ‐ Adverse effects: TESS score, blood routine, blood glucose, use of benzodiazepines, benzhexol (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details.. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score,blood routine, blood glucose, use of benzodiazepines, benzhexol were missing. |
| Other bias | Low risk | None obvious. |
Liu 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS score of 70 or more. N = 68. Age: aripiprazole group: 25~54 years, mean = (35.5 ± 9.8) years; clozapine group: 21~56 years, mean = (33.6 ± 8.9) years. Gender: aripiprazole group: 19 male, 15 female; clozapine group: 21 male, 13 female . History: aripiprazole group: 5.5~ 21 years; clozapine group: 5.3 ~23 years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (18 ± 4.2) mg/day. N = 34. 2. Clozapine: Dose range: 50‐500 mg/day. Mean = (425 ± 5.5) mg/day. N = 34. | |
| Outcomes | Global state: PANSS score decreased rate(recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score,PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score(no data); use of benzodiazepines, benzhexol, or propranolol medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of benzodiazepines, benzhexol and propranolol medication were also missing. |
| Other bias | Low risk | None obvious. |
Liu 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS score of 60 or more. N = 62. Age: aripiprazole group: mean = (26.68 ± 5.69) years; clozapine group: mean = (26.5 ± 5.58) years. Gender: aripiprazole group: 18 male, 13 female; clozapine group: 20 male, 11 female . History: aripiprazole group: 1~12 months, mean = (6.35 ± 6.49) months; clozapine group: 1~12 months, mean = (6.05 ± 5.76) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose:not reported. N = 31. 2. Clozapine: Dose range: 50‐500 mg/day. Mean dose:not reported. N = 31. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on use of benzodiazepines, benzhexol and propranolol medication were also missing. |
| Other bias | Low risk | None obvious. |
Liu 2008a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 66.
Age: aripiprazole group: 17~65 years, mean = (32.5 ± 6.8) years; ziprasidone group: 18~63 years, mean = (30.4 ± 7.7) years. Gender: aripiprazole group: 19 male, 14 female; ziprasidone group: 18 male, 15 female. History: aripiprazole group: 1.5~22 months, mean = (5.1 ± 1.9) months; ziprasidone group: 1~20 months, mean = (4.5 ± 2.1) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (13.12 ± 4.27) mg/day. N = 33. 2. Ziprasidone: Dose range: 20‐80 mg/day. Mean = (38.71 ± 12.63) mg/day. N = 33. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. SANS total score score. Leaving the study early. Adverse effects. Unable to use ‐ Mental state: SANS subscale scores ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details.. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, blood routine, liver function, use of alprazolam, propranolol and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Liu 2008b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 78.
Age: mean = (33 ± 11) years. Gender: 33 male, 45 female. History: 1 month~20 years, mean = (13 ± 8.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (15.4 ± 5.8) mg/day. 2. Risperidone: Dose range: 3‐6mg/day. Mean = (3.8 ±1.5) mg/day. | |
| Outcomes | Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ PANSS subscale scores ‐ unvalidated subscales. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on blood and urine routines, ECG, hepatorenal function, use of benzodiazepines and other medicine were missing. |
| Other bias | Low risk | None obvious. |
Liu 2008c.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 63.
Age: aripiprazole group: mean = (27.5 ± 9.179) years; risperidone group: mean = (28.29 ± 11.25) years. Gender: aripiprazole group: 12 male, 20 female; risperidone group: 14 male, 17 female. History: aripiprazole group: 1~20 years, mean = (4. 31 ± 6. 214) years; risperidone group: 1~29 years, mean = (2.87 ± 2.952) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (21.67 ± 1.12) mg/day. N = 37. 2. Risperidone: Dose range: 1‐6mg/day. Mean = (4.56 ± 1.24) mg/day. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐79%, improved: 30%‐49%, no effect: < 30%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, hepatorenal function, use of benzodiazepines, propranolol and other medicine (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, hepatorenal function, use of benzodiazepines, propranolol and other medicine were missing. |
| Other bias | Low risk | None obvious. |
Liu 2008d.
| Methods | Allocation: randomised, lottery. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 72.
Age: aripiprazole group: 16~49 years, mean = (33.27 ± 8.21) years; risperidone group: 15~42 years, mean = (30.94 ± 8.77) years. Gender: aripiprazole group: 20 male, 17 female; risperidone group: 19 male, 16 female. History: aripiprazole group: 0.25~3 years, mean = (1.54 ± 0.74) years; risperidone group: 0.25~2.5 years, mean = (1.98 ± 0.81) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean= (21.67 ± 1.12) mg/day. N = 37. 2. Risperidone: Dose range: 1‐6 mg/day. Mean= (4.56 ± 1.24) mg/day. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: > 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. WAI‐RC (Wechsler adult intelligence scale) score. WMS (Wechsler memory scale ) score. Adverse effects. Unable to use ‐ Cognitive functioning: WMS subscale scores ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, lottery. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score were missing. |
| Other bias | Low risk | None obvious. |
Liu 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 68.
Age: aripiprazole group: mean = (26.1 ± 7.5) years; olanzapine group: mean = (27.4 ± 8.4) years. Gender: aripiprazole group: 18 male, 16 female; olanzapine group: 17 male, 17 female. History: aripiprazole group: mean = (6.9 ± 3.4) years; olanzapine group: mean = (6.5 ± 3.6) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (19.7 ± 5.3) mg/day. N = 34. 2. Olanzapine: Dose range: 10‐20 mg/day. Mean = (15.4 ± 4.5) mg/day. N = 34. | |
| Outcomes | Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. Quality of life: GQOLI‐ 74 total score, GQOLI‐ 74 material life score, GQOLI‐ 74 physical health score, GQOLI‐ 74 mental health score, GQOLI‐ 74 social function score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and benzhexol were missing. |
| Other bias | Low risk | None obvious. |
Liu 2009a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: not reported. Gender: aripiprazole group: 16 male, 14 female; risperidone group: 17 male, 13 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome were assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Liu 2009b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 120. Age: aripiprazole group:16~60 years. mean = (28.4 ± 7.9) years; quetiapine group: 18~63 years, mean = (29 ± 8.5) years. Gender: aripiprazole group: 28 male, 32 female; quetiapine group: 30 male, 30 female. History: aripiprazole group: mean = (2.3 ± 1.5) years; quetiapine group: mean = (2.3 ± 1.3) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 60. 2. Quetiapine: Dose range: 50‐600 mg/day. Mean dose: not reported. N = 60. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 20%‐50%, no effect: < 20%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, headache, insomnia, somnolence, nausea/vomiting, weight gain, extrapyramidal side‐effects, blood routine, urine routine, hepatorenal function, use of benzodiazepines and propranolol (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | The data on TESS total score, headache, insomnia, somnolence, nausea/vomiting, weight gain, extrapyramidal side‐effects, blood routine, urine routine, hepatorenal function, use of benzodiazepines and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Liu 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: not reported. Design: trial with three arms. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 112.
Age: not reported. Gender: not reported. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (19.83 ± 6.50) mg/day. N = 30.
2. Risperidone: Dose range: 2‐6.5 mg/day. Mean = (3.82 ± 0.86) mg/day. N = 54. 3.Clozapine: Dose range: 150‐500 mg/day. Mean = (265.18 ± 82.59) mg/day. N = 28. |
|
| Outcomes | Global state: PANSS score decreased rate (recovery: > 75%, markedly improved: 50%‐ 5%, improved: 25%‐50%, no effect: < 25%). Adverse effects. Cost‐effect analysis. Unable to use ‐ Adverse effects: TESS total score, ECG, blood glucose and serum lipids, use of benzodiazepines and other medicine (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, ECG, blood glucose and serum lipids, use of benzodiazepines and other medicine were missing. |
| Other bias | Low risk | None obvious. |
Lou 2007.
| Methods | Allocation: randomised, random number table method. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 45.
Age: aripiprazole group: 50~67 years, mean = (56.1 ± 5.9) year, risperidone group: 49~70 years, mean = (54.6 ± 6.1) years. Gender: aripiprazole group: 11 male, 10 female; risperidone group: 11 male, 13 female. History: aripiprazole group:5~14 years, mean = (10.3 ± 2.6) years; risperidone group: 3~12 years, mean = (12.1 ± 2.2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 21. 2. Risperidone: Dose range: 0.5‐4 mg/day. Mean dose: not reported. N = 24. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS score, ECG, use of benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, random number table method. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, ECG, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Luo 2008.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 70. Age: aripiprazole group: mean = (28. 5 ± 8. 2) years; quetiapine group: mean = (27. 1 ± 7. 6) years. Gender: aripiprazole group: 19 male, 16 female; quetiapine group: 18 male, 17 female. History: aripiprazole group: mean =(1.2 ± 0.7) years; quetiapine group: mean =(1.1 ± 0.8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 35. 2. Quetiapine: Dose range: 200‐800 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ Adverse effects: TESS total score (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score were missing. |
| Other bias | Low risk | None obvious. |
Luo 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80.
Age: 18~56 years, aripiprazole group: mean = (31.6 ± 7.8)years; risperidone group: mean = (32.2 ± 7.4)years. Gender: aripiprazole group: 23 male, 17 female; risperidone group: 21 male, 19 female. History: 1 month~26 years, aripiprazole group: mean = (6.2 ± 5.8)years; risperidone group: mean = (6.5 ± 5.6)years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (20.4 ± 3.2)mg/d. N = 40. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.2 ± 2.3)mg/d. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effect. Unable to use ‐ Adverse effects: TESS total score, EPS, tachycardia, menstrual changes, etc. and use of benzhexol, benzodiazepine, propranolol (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, EPS, tachycardia, menstrual changes, etc. and use of benzhexol, benzodiazepine, propranolol were missing. |
| Other bias | Low risk | None obvious. |
Lv 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80; complete study N = 77.
Age: aripiprazole group: 18~ 46 years, mean = (28. 8 ± 7. 50) years, risperidone group: 19~45 years, mean = (28. 6 ± 16. 5) years. Gender: aripiprazole group: 21 male, 18 female; risperidone group: 20 male, 18 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (18. 6 ± 6. 8) mg/day. N = 40. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4. 3 ± 1. 8) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. agitation/excitement labelled as "adverse effect". Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS score, use of benzodiazepines, anticholinergic drug (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total proportion of loss to follow‐up was 7.5% (1/40: 2/40 ), and there was no difference between the two groups. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, use of benzodiazepines, anticholinergic drug were missing. |
| Other bias | Low risk | None obvious. |
Ma 2009.
| Methods | Allocation: randomised, random number table. Blindness: unclear. Duration: 1 week wash‐out period + six weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more, Excited subscales (PEC) of 14 or more.
N = 89.
Age: aripiprazole group: mean = (30.93 ± 8.21) years; olanzapine group: mean = (31.29 ± 7.38) years. Gender: aripiprazole group: 25 male, 15 female; olanzapine group: 22 male, 13 female. History: aripiprazole group: mean = (4.52 ± 3.81) years; olanzapine group: mean = (4.73 ± 4.71) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (19.54 ± 8.35) mg/day. N = 45. 2. Olanzapine: Dose range: 5‐20 mg/day. Mean = (15.36 ± 8.21) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐75%, no effect: < 50%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score,PANSS general psychogenic pathological subscale score. SANS total score. Cognitive function total score. Adverse effects. Unable to use ‐ Mental state: SANS subscale score ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, use of benzodiazepines and anticholinergic medicine were missing. |
| Other bias | Low risk | None obvious. |
Ma 2009a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 98.
Age: 18~55 years, mean = (32 ± 18.79) years. Gender: 43 male, 55 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 49. 2. Risperidone: Dose range: 1‐5 mg/day. Mean dose: not reported. N = 49. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effect. Unable to use ‐ Adverse effects: TESS total score, tremor, dry mouth, dystonia, use of benzhexol, benzodiazepine and propranolol (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data of TESS total score, tremor, dry mouth, dystonia, use of benzhexol, benzodiazepine and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Mai 2005.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 72.
Age: aripiprazole group: 18~60 years, mean = (27. 8 ± 8. 9) year, risperidone group: 17~60 years, mean = (28. 1 ± 8. 5) years. Gender: aripiprazole group: 19 male, 17 female; risperidone group: 18 male, 18 female. History: aripiprazole group:1 month~9 years, mean= (4.7 ± 2.5) years; risperidone group: 1 month~10 years, mean = (4.8 ± 2.9) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (23.2 ± 3.3) mg/day. N = 36. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.1 ± 1.6) mg/day. N = 36. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS score, use of benzodiazepines, anticholinergic drug (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, use of benzodiazepines, anticholinergic drug were missing. |
| Other bias | Low risk | None obvious. |
Mao 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 70.
Age: aripiprazole group: mean = (28.7 ± 9.9) years; olanzapine group: mean = (30.2 ± 8.7) years. Gender: aripiprazole group: 19 male, 16 female; olanzapine group: 18 male, 17 female. History: aripiprazole group: mean = (6 ± 4.8) years; olanzapine group: mean = (6.3 ± 3.2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (20.5 ± 5.2) mg/day. N = 35. 2. Olanzapine: Dose range: 10‐20 mg/day. Mean = (14.5 ± 4.6) mg/day. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychopathological subscale score. Leaving the study early. Adverse effects. Unable to use ‐ Mental state: PANSS cognitive subscale score ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
McQuade 2004.
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 26 weeks. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: (DSM‐IV) schizophrenia disorganised (n = 17), paranoid (n = 271), residual (n = 3) or undifferentiated (n = 26), in acute relapse and hospitalised. PANSS total score of 60 or more. N = 317. Age: > 17 years (mean = 38.4 years). Gender: 229 M, 88 F. History: duration of illness not reported, age at first hospitalisation mean = 24.5 years. Setting: originally inpatient. | |
| Interventions | 1. Aripiprazole: flexible dose. Allowed dose range: 15‐30 mg/day. Mean dose: 25.1 mg/day. N = 156. 2. Olanzapine: flexible dose. Allowed dose range: 10‐20 mg/day. Mean dose: 16.5 mg/day. N = 161. | |
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy.
Global state: CGI.
Mental state: PANSS total score.
Adverse effects. Unable to use ‐ Adverse effects: use of antiparkinson medication (no data), metabolic syndrome (> 70% incomplete). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in adverse effects. This can be a problem for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote:"Because of the high number of participants who discontinued the study (72%) results of analysis by time point are described on the observed case (OC) basis (except for primary outcome), as the last observation‐carried‐forward analysis would have included a large amount of data carried forward from patients who discontinued the study." Due to the high number of participants leaving the study early, the validity is definitely limited. |
| Selective reporting (reporting bias) | High risk | Although inclusion criteria required participants in acute relapse, no data on PANSS positive sub score were available. Data on use of antiparkinson medication were missing. |
| Other bias | High risk | The study was industry sponsored by the manufacturer of aripiprazole. |
Mu 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 100.
Age: 25~45 years. Gender: 100 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (25.2 ± 3.2) mg/day. N = 50. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.8 ± 0.5) mg/day. N = 50. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychopathological subscale score. Lveaing the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Mu 2010.
| Methods | Allocation: Simple randomisation. Blindness: unclear. Duration: one week wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3), BPRS total score of 35 or more.
N = 258, complete study: N=257. Age: aripiprazole group: mean = (38.12 ± 10.34) years; risperidone group: mean = (39.56 ± 10.67) years. Gender: aripiprazole group: 58 male, 71 female; risperidone group: 61 male, 66 female. History: aripiprazole group: mean = (6.92 ± 4.18) years; risperidone group: mean = (7.48 ± 4.37) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (22.5 ± 3.6) mg/day. N = 129. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.7 ± 1.7) mg/day. N = 129. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: BPRS total score. Leaving the study early. Adverse effects. Unable to use ‐ BPRS and TESS subscale scores ‐ unvalidated subscales. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two cases lost in risperidone group because of lactation. |
| Selective reporting (reporting bias) | High risk | Data on blood and urine routine, ECG, liver function, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Pan 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: two weeks wash‐out period and six weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 18~60 years. mean = (26.8 ± 8.7) year, risperidone group: 17~60 years, mean = (27.1 ± 8.5) years. Gender: aripiprazole group: 13 male, 17 female; risperidone group: 12 male, 18 female. History aripiprazole group:1 month~7 years, mean = (4.7 ± 3.5) years; risperidone group: 1 month~8 years, mean = (4.8 ± 3.7) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (25.2 ± 3.3) mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4 ± 1.5) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ Adverse effects (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | The data on adverse effects were missing. |
| Other bias | Low risk | None obvious. |
Peng 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 35 or more.
N = 85.
Age: aripiprazole group: 17~56 years. mean = (31. 7 ± 10. 8) years; quetiapine group, 17~57 years, mean = (29. 5 ± 11. 3) years. Gender: aripiprazole group: 24 male, 20 female; quetiapine group: 21 male, 20 female. History: aripiprazole group: mean = (2.7 ± 1.5) months; clozapine group: mean = (2.7 ± 1.9) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 44. 2. Quetiapine: Dose range: 100‐600 mg/day. Mean dose: not reported. N = 41. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, EEG, liver function, use of benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total rate of lost to follow‐up was 3.5%(3/85), and there was no difference between the two groups (2/44: 1/41). |
| Selective reporting (reporting bias) | High risk | The data on TESS total score, EEG, liver function, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Peng 2007a.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 46.
Age: aripiprazole group: 18~50 years, mean = (22.35 ± 7.15) years; clozapine group: 16~49 years, mean = (23.25 ± 9.75) years. Gender: aripiprazole group: 23 male, 11 female; clozapine group: 23 male, 11 female. History: aripiprazole group: 1~36 months, mean = (22.55 ± 12.45) months; clozapine group: 1~30 months, mean = (22.25 ± 11.25) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 23. 2. Clozapine: Dose range: 50‐400 mg/day. Mean dose: not reported. N = 23 . | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥80%, markedly improved: ≥50%, improved: ≥25%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychopathological subscale score. Adverse events: TESS total score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on somnolence, ECG abnormal, mouth dry, constipation blurred vision, use of benzodiazepines were incomplete. |
| Other bias | Low risk | None obvious. |
Potkin 2003.
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: four weeks. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: (DSM‐IV) schizophrenia (n = 289) or schizoaffective disorder (n = 115), hospitalised due to an acute relapse, response to previous antipsychotic treatment other than clozapine, PANSS of 60 or more. N = 404. Age: 18‐65 years (mean = 38.9 years). Gender: 283 M, 121 F. History: duration of illness not reported, age at onset not reported. Setting: inpatient. | |
| Interventions | 1. Aripiprazole: fixed dose: 20 mg/day. N = 101. 2. Aripiprazole: fixed dose: 30 mg/day. N = 101. 3. Risperidone: fixed dose: 6 mg/day. N = 99. | |
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score, PANSS positive sub score, PANSS negative sub score. Adverse effects. | |
| Notes | Note: There is a placebo group (n = 103), which is not relevant for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in adverse effects. This can be a problem for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall drop out rate was 36.9 %. The LOCF method was used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | For efficacy outcomes there was no standard deviation or standard error indicated which had to be back calculated. Only adverse events with an incidence of more than 5% were reported. This procedure may have missed important adverse events. |
| Other bias | High risk | The study was industry sponsored by the manufacturer of aripiprazole. The study used a fixed dose regimen, where it is difficult to say which comparator doses may be appropriate. |
Pu 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 60.
Age: aripiprazole group: mean = (27.43 ± 7.17) years; risperidone group: mean = (27.06 ± 2.33) years. Gender: aripiprazole group: 32 female; risperidone group: 32 female. History: aripiprazole group: total = (2.2 ± 1.5) years; risperidone group: total = (2.12 ± 1.61) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 15‐30 mg/day. Mean = (19.50 ± 5.14) mg/day. N = 30. 2. Risperidone: Dose range: 3‐6 mg/day. Mean = (4.03 ± 0.87) mg/day. N = 30. | |
| Outcomes | Mental state: BPRS total score, PANSS score decreased rate. Change of BMI, PRL. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Qian 2009.
| Methods | Allocation: randomised, random number table. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS more than 60 years.
N = 80.
Age: aripiprazole group: 16~47 years, mean = (25 ± 3.1) years; olanzapine group: 18~43 years, mean = (26 ± 2.7) years. Gender: 80 female. History: aripiprazole group: mean= (1.2 ± 2.7) years; olanzapine group: mean = (1.3 ± 0.5) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (20 ± 5) mg/day . N = 40. 2. Olanzapine: Dose range: 10‐20 mg/day. Mean = (20 ± 5) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐75%, no effect: < 50%). Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, liver function, blood pressure change, blood glucose change (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient was lost to follow‐up because of economic issues in aripiprazole group. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, liver function, blood pressure change, blood glucose change were missing. |
| Other bias | Low risk | None obvious. |
Qin 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period and eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 21~45 years, mean = (32.1 ± 7.2) years; risperidone group: 24~53 years, mean = (35.2 ± 5.9) years. Gender: aripiprazole group: 18 male, 12 female; risperidone group: 17 male, 13 female. History: aripiprazole group: 5~14 years; risperidone group: 5~16 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, liver function, blood and urine routines, use of benzodiazepines, propranolol, and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, liver function, blood and urine routines, use of benzodiazepines, propranolol, and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Qu 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐5). PANSS of 60 or more.
N = 120.
Age: 19~64 years. aripiprazole group: mean = (31.6 ± 7.8)years; risperidone group: mean = (32.2 ± 7.4)years. Gender: aripiprazole group: 40 male, 20 female; risperidone group: 36 male, 24 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score. Adverse effects. Unable to use ‐ Quality of life: GQOLI ‐ 74 score (no data). Adverse effects: anxiety, EPS, somnolence, ECG, mouth dry, liver function, use of benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on GQOLI ‐ 74 score, anxiety, EPS, somnolence, ECG, anxiety, EPS, somnolence, ECG, mouth dry, liver function, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Shan 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean= (43.37 ± 8.85) years; risperidone group: 17‐59 years, mean = (42.52 ± 9.85) years. Gender: aripiprazole group: 16 male, 14 female; risperidone group: 15 male, 15 female. History: aripiprazole group: mean = (10.46 ± 4.65) years; risperidone group: mean = (11.35 ± 3.28) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: ≥50%, improved: ≥ 30%, no effect: < 30%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and other medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and other medication were missing. |
| Other bias | Low risk | None obvious. |
Shuai 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 84.
Age: aripiprazole group: 19~52 years, mean = (30 ± 6) years; risperidone group: 18~55 years, mean = (32 ± 7) years. Gender: aripiprazole group: 19 male, 23 female; risperidone group: 21 male, 21 female. History: aripiprazole group: 1~9 months, mean = (3.4 ± 2.6) months; risperidone group: 2~9 months, mean = (4.3 ± 2.9) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (15 ± 5) mg/day. N = 42. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.2± 1.8) mg/day. N = 42. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: agitation. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, use of benzodiazepines and other medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, use of benzodiazepines and other medication were missing. |
| Other bias | Low risk | None obvious. |
Song 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more. N = 60. Age: aripiprazole group: 20~60 years, mean = (45.5 ±3.05) years; risperidone group: 19~60 years, mean = (43.7 ± 3.3) years. Gender: 60 female . History: aripiprazole group: mean = (8.4 ± 1.5) years; risperidone group: mean = (8.5 ± 1.4) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (15.2 ± 5.1) mg/day. N = 30. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean = (4.12 ± 1.43) mg/day. N = 30 | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ Adverse effects: (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on adverse effects were missing. |
| Other bias | Low risk | None obvious. |
Song 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 17~62 years; risperidone group: 16~60 years. Gender: aripiprazole group: 10 male, 20 female; risperidone group: 21 male, 9 female. History: aripiprazole group: 1 month~10 years; risperidone group: 1.5 months~13 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐8 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzhexol and benzodiazepines. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score and use of benzhexol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Song 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS more than 60.
N = 80.
Age: aripiprazole group: 19~56 years, mean = (27.6 ± 4.3) years; olanzapine group: 18~58 years, mean = (28.2 ± 3.6) years. Gender: aripiprazole group: 20 male, 20 female; olanzapine group: 20 male, 20 female. History: aripiprazole group: 5 months~4 years, mean = (3.1 ± 1.9) years; olanzapine group: 3 months~4 years, mean = (3.5 ± 1.1) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 40. 2. Olanzapine: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI ‐ SI. Mental state: PANSS total score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, excitement, insomnia, myotonia, akathisia, tremor, use of benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, excitement, insomnia, myotonia, akathisia, tremor, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Su 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + six weeks intervention. Design: parallel. Setting: inpatient and outpatient , China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 78.
Age: aripiprazole group: 18 ~60 years, mean = (27. 6 ± 8. 5) years; risperidone group: 15 ~65 years, mean = (28. 7 ± 8. 4) years. Gender: aripiprazole group: 23 male, 18 female; risperidone group: 25 male, 14 female. History: aripiprazole group: 6 months ~6 years; risperidone group: 1 month ~10 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 39. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 39. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Su 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week washout + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS more than 60.
N = 100.
Age: aripiprazole group: 17~55 years, mean = (28.5 ± 3.5) years; olanzapine group: 18~56 years, mean = (28.2 ± 4.5) years. Gender: aripiprazole group: 20 male, 30 female; olanzapine group: 20 male, 30 female. History: aripiprazole group: 7 months~4 years, mean = (2.5 ± 2.6) years; olanzapine group: 6 months~5 years, mean = (2.5 ± 1.2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 50. 2. Olanzapine: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 50. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: 75≥ %, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). CGI‐SI score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychopathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, lactation, menstrual disorders, excitement, insomnia, myotonia, akathisia, tremor, use of benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, lactation, menstrual disorders, excitement, insomnia, myotonia, akathisia, tremor, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Sun 2006.
| Methods | Allocation: randomised, stratified random. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80.
Age: aripiprazole group: 18~47 years. mean = (27.1 ± 3.4) years; risperidone group: 19~48 years, mean = (26.3 ± 3.8) years. Gender: aripiprazole group: 24 male, 16 female; risperidone group: 23 male, 17 female. History: aripiprazole group: 5~14 months, mean = (7.4 ± 4.6) months; risperidone group: 2~16 months, mean = (7.3 ± 4.7) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 40. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use‐ Mental state: PANSS subscale score decreased rate ‐ unvalidated subscales. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, stratified randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Sun 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 84.
Age: 18~60 years, aripiprazole group: mean = (27.3 ± 6.68) years; risperidone group: mean = (26.8 ± 7.32) years. Gender: aripiprazole group: 23 male, 19 female; risperidone group: 24 male, 18 female. History: < 2 years, aripiprazole group: mean = (1.2 ± 0.77) years; risperidone group: mean = (1.1 ± 0.84) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (19.32 ±6.94) mg/ day. N = 42. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.25 ±1.7) mg/ day. N = 42. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects Unable to use ‐ Adverse effects: TESS total score, use of anticholinergic medicine and benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of anticholinergic medicine and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Sun 2009a.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60. Age: aripiprazole group: 17~50 years. mean = (27. 5 ± 7. 9) years; quetiapine group: 18~51 years, mean = (26. 7 ± 8. 4) years. Gender: aripiprazole group: 23 male, 21 female; quetiapine group: 22 male, 22 female. History: aripiprazole group: mean = (4. 1 ± 2.7) years; quetiapine group: mean = (4. 5± 2. 9) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean= (11. 6±4. 6) mg/day. N=30. 2. Quetiapine: Dose range: 50‐600 mg/day. Mean= (407±65) mg/day. N=30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Unable to use ‐ Adverse effects: TESS total score and use of benzodiazepine (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepine and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Tandon 2006.
| Methods | Allocation: random. Blindness: open label. Duration: 8 weeks. Design: prospective, parallel group. Location: multicentric in USA. | |
| Participants | Diagnosis: Schizophrenia or schizoaffective disorder (DSM IV), required initiation of antipsychotics, antipsychotic switch clinically appropriate. N = 1599. Age: 18‐75 years. History: duration of illness not reported, age at onset not reported. Setting: Outpatients. |
|
| Interventions | 1. Aripiprazole: 10 mg‐30 mg/day (mean 19.9 mg/day); N = 1295. 2. Other antipsychotic: recommended dose; N = 304. | |
| Outcomes | Global state: CGI‐I. Adverse effects: At least one adverse effect, extrapyramidal adverse effects, gastrointestinal disorders, sleep disturbances. Others: POM. | |
| Notes | Primary goal was evaluating the effectiveness of aripiprazole in general psychiatric outpatient practice setting. The other antipsychotic treatment group was primarily included for interpreting the safety data and not for direct comparison. Unable to use data from CGI‐I and POM. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 4:1 computer‐generated randomised schedule using Interactive Voice Response System. |
| Allocation concealment (selection bias) | High risk | Open label. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF method used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Adverse events were reported if they were > 5%. Scales if used were not reported. |
| Other bias | High risk | Industry sponsored. |
Tang 2006.
| Methods | Allocation: simple randomisation. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 72.
Age: aripiprazole group: 18~61 years, mean = (31.3 ± 6.2) years; risperidone group: 16~60 years, mean = (30.8 ± 6.1) years. Gender: aripiprazole group: 37 female; risperidone group: 35 female. History: aripiprazole group: 1 month~4.5 years; risperidone group: 1 month~4.6 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (10 ± 5.22) mg/day. N = 37. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.5 ± 0.81) mg/day. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines, propranolol and benzhexol (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, use of benzodiazepines, propranolol and benzhexol were missing. |
| Other bias | Low risk | None obvious. |
Tang 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 18~63 years, mean = (29.7 ± 12.5) years; risperidone group: 18~62 years, mean = (29.5 ± 11.4) years. Gender: aripiprazole group: 16 male, 14 female; risperidone group: 18 male, 12 female. History: aripiprazole group: mean = (3 ± 1.7) years; risperidone group: mean = (3 ± 2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (23. 3 ± 3. 2) mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4. 6 ± 2. 4) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, EEG, use of benzodiazepines and other medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, EEG, use of benzodiazepines and other medication were missing. |
| Other bias | Low risk | None obvious. |
Tang 2010.
| Methods | Allocation: simple randomisation. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 78.
Age: aripiprazole group: 18~59 years. mean = (28.3 ± 6.2) years; risperidone group: 18~58 years, mean = (27.8 ± 6.1) years. Gender: aripiprazole group: 18 male, 20 female; risperidone group: 19 male, 21 female. History: aripiprazole group: 1 month~4.5 years; risperidone group: 1 month~4.6 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (10 ± 5.22) mg/day. N = 38. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.3 ± 0.81) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. agitation‐labelled as "adverse effect". Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and other medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient lose to follow‐up. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, use of benzodiazepines and other medication were missing. |
| Other bias | Low risk | None obvious. |
Tang 2010a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 70.
Age: 18~65 years, aripiprazole group: mean = (28.5 ± 7.3) years; risperidone group: mean = (30.1 ± 8.9) years. Gender: aripiprazole group: 18 male, 17 female; risperidone group: 20 male, 15 female. History: aripiprazole group: mean = (2.9 ± 2.4) years; risperidone group: mean = (3.3 ± 2.9) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 35. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects Unable to use ‐ Adverse effects: TESS total score, insomnia, anxiety, nausea, endocrine, weight gain, headache, dizziness, use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, insomnia, anxiety, nausea, endocrine, weight gain, headache, dizziness, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Tao 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: four arms intervention. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 70 or more. N = 360. Age: aripiprazole group: mean = (30. 72 ± 11. 59) years; risperidone group: mean = (32. 51 ± 14. 30) years; quetiapine group: mean = (35. 53 ± 14. 23) years; ziprasidone group: mean = (26. 03 ± 7. 23) years. Gender: aripiprazole group: 22 male, 68 female; risperidone group: 43 male, 47 female; quetiapine group: 4 male, 86 female; ziprasidone group: 65 male, 25 female. History: not reported. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose:not reported. N = 90.
2. Risperidone: Dose range: 2‐5 mg/day. Mean dose:not reported. N = 90. 3.Quetiapine: Dose range: 200‐600 mg/day. Mean dose:not reported. N = 90. 4.Ziprasidone: Dose range: 40‐80 mg/day. Mean dose:not reported. N = 90. |
|
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total rate of loss to follow‐up was 4.4%(3/90 vs. 1/90), 1 participant dropped out because of adverse effect, 1 dropped out because of no effect, 2 dropped out because of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Tong 2007.
| Methods | Allocation: randomised, stratified random. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 60 or more.
N = 68; complete study: N = 50.
Age: aripiprazole group: mean = (41 ± 19) years; risperidone group: mean = (36 ± 20) years. Gender: aripiprazole group: 11 male, 14 female; risperidone group: 8 male, 17 female. History: aripiprazole group: mean = (3.4 ± 6.7) months; risperidone group: mean = (2.9 ± 5.7) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean = (17 ± 6) mg/day. N = 36. 2. Risperidone: Dose range: 1‐4 mg/day. Mean = (4 ± 1) mg/day. N = 32. | |
| Outcomes | Global state: GAS score (recovery: > 80%, markedly improved: > 70%, improved: > 60%, no effect: ≦ 60%). Mental state:BPRS total score, SANS total score Adverse effects. Unable to use ‐ Mental state: BPRS & SANS subscale scores are not reported, as these subscale scores are unvalidated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The total rate of lost to follow‐up was 26.5% (18/68, 11/36 vs. 7/32). 8 participants dropped out because of progressive disease, 10 dropped out because the trialists could not insist on follow‐up. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score and use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Tu 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 68.
Age: aripiprazole group: 18˜59 years, mean = (25 ± 11) years; risperidone group: 20˜56 years, mean = (28 ± 10) years. Gender: aripiprazole group: 27 male, 8 female; risperidone group: 23 male, 10 female. History: aripiprazole group: mean = (2.9 ± 4) years; risperidone group: mean = (2.1 ± 3.6) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 35. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose:not reported. N = 33. | |
| Outcomes | Global state: CGI scale: markedly improved, improved, slight improved, no effect. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on adverse effects were missing. |
| Other bias | Low risk | None obvious. |
Wang 2005.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Location: outpatient and inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 60. Age: aripiprazole group: mean = (27 ± 9) years; clozapine group: mean = (28 ± 10) years. Gender: aripiprazole group: 14 male, 17 female; clozapine group: 15 male, 14 female . History: aripiprazole group: 1~11 months, mean = (7.3 ± 4.7) months; clozapine group: 1~12 months, mean = (7.7 ± 4.5) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 31. 2. Clozapine: Dose range: 50‐270.3 mg/day. Mean dose: not reported. N = 29. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect or deteriorate: < 25%). Mental state: PANSS total score. anxiety and agitation, as binary outcomes. Adverse effects. Unable to use ‐ Adverse effects: TESS total score (no data); Laboratory tests (hepatorenal function, glucose, electrolyte) (unclear data), weight and ECG (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient discontinued therapy due to leucopenia. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on ECG and weight were missing. Data on laboratory tests (hepatorenal function, glucose, electrolytes) were unclear. |
| Other bias | Low risk | None obvious. |
Wang 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 64. Age: aripiprazole group: 15~50 years, mean = (24.4 ± 6.8) years; clozapine group: 18~40 years, mean = (25.5 ± 9.3) years. Gender: aripiprazole group: 18 male, 14 female; clozapine group: 21 male, 11 female. History: aripiprazole group: 1~60 months, mean = (24.7 ± 13.9) months; clozapine group: 1~60 months, mean = (25 ± 14) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose:not reported. N=32. 2. Clozapine: Dose range: 50‐500 mg/day. Mean dose:not reported. N=32. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐ 80%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects: TESS total score, central nervous system (somnolence), ECG abnormal, liver function. Unable to use ‐ Adverse effects: gastrointestinal (dry mouth, constipation), blurred vision, weight gain and EEG abnormal (unclear data). blood and urine routine, blood pressure (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on gastrointestinal (dry mouth, constipation), blurred vision, weight gain and EEG abnormal were unclear. Data on blood and urine routine, blood pressure were missing. |
| Other bias | Low risk | None obvious. |
Wang 2006a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 60. Age: aripiprazole group: mean = (38.7 ± 8.47) years; clozapine group: mean = (35.56 ± 3.78) years. Gender: aripiprazole group: 16 male, 14 female; clozapine group: 14 male, 16 female. History: aripiprazole group: 1 month~10 years, mean = (12.6 ± 8.38) months; clozapine group: 3 months~9 years, mean = (12.15 ± 7.22) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose:not reported. N = 30. 2. Clozapine: Dose range: 50‐400 mg/day. Mean dose:not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score,PANSS general psychopathological subscale score. anxiety ‐ labelled as "adverse effect". Adverse effects. Unable to use ‐ Adverse effects: TESS total score, hepatorenal function (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on score were available. Data on hepatorenal function were missing. |
| Other bias | Low risk | None obvious. |
Wang 2006b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 18~60 years. mean = (26.2 ± 7.5) years; risperidone group: 18‐60 years, mean = (27.1 ± 8.5) years. Gender: aripiprazole group: 20 male,10 female; risperidone group: 18 male,12 female. History: aripiprazole group: 1~60 months, mean = (22.4 ± 13.8) months; risperidone group: 1~60 months, mean = (24.9 ± 14) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score. Adverse effect: TESS score. Unable to use ‐ Quality of life: GQOLI‐74 score (no data). Adverse effects: EPS, ECG, liver function, mouth dry (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there was drop‐out. |
| Selective reporting (reporting bias) | High risk | Although GQOLI‐74 was used to assess quality of life, no data on score were available. Data on EPS, ECG, liver function, and mouth dry were missing. |
| Other bias | Low risk | None obvious. |
Wang 2006c.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 50.
Age: aripiprazole group: 25~39 years. mean = (32.5 ± 6.2) years; risperidone group: 20~58 years, mean = (22.5 ± 6.2) years. Gender: aripiprazole group: 8 male, 17 female; risperidone group: 10 male, 15 female. History: aripiprazole group: 5~16 years; risperidone group: 5~18 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 25. 2. Risperidone: Dose range: 0.5‐5 mg/day. Mean dose: not reported. N = 25. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 60%‐79%, improved: 30%‐59%, no effect: < 30%). Mental state: PANSS total score. Unable to use: Adverse effects ‐ the N numbers reported in these outcomes are greater than that of the people randomised. Since we are unable to reach the authors, we are unable to use these data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score were missing. |
| Other bias | Low risk | None obvious. |
Wang 2006d.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 35 or more.
N = 120.
Age: aripiprazole group: 18~45 years, mean = (28.9± 7.9) years; risperidone group: 16‐50 years, mean = (31.53 ± 9.03) years. Gender: aripiprazole group: 32 male, 28female; risperidone group: 46 male, 14 female. History: aripiprazole group: 3 months~10 years, mean = (3.3 ± 4.5) years; risperidone group: 3 months~10 years, mean = (5.03 ± 3.3) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean= 20 mg/day. N = 60. 2. Risperidone: Dose range: 1‐6 mg/day. Mean= 20 mg/day. N = 60. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: BPRS total score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood, urine, and stool routine, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data TESS total score, blood, urine, and stool routine, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Wang 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 35 or more.
N = 95.
Age: aripiprazole group: 16~54 years. mean = (24.4 ± 13.2) years; risperidone group: 13~67 years, mean = (25. 4 ± 14. 6) years. Gender: aripiprazole group: 22 male, 24 female; risperidone group: 23 male, 26 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 46. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 49. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total proportion of drop‐out was 3.15% and the data of the two groups were balanced (2/46: 1/49). Two because of adverse effect, one due to economic issue. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Wang 2007a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (37. 5 ± 9. 8) years; risperidone group: mean = (36. 7 ± 10. 5) years. Gender: 60 female. History: aripiprazole group: 1 month~3 years, mean = (2 ± 1.5) years; risperidone group: 3 months~3 years, mean = (2.3 ± 1.8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychopathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood and urine routine, kidney function, electrolyte, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | Data on TESS total score, blood and urine routine, kidney function, electrolyte, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Wang 2007d.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 64.
Age: aripiprazole group: 16~52 years, mean = (29. 1 ± 3. 2) years; risperidone group: 19~54 years, mean = (28. 3 ± 2. 8) years. Gender: aripiprazole group: 19 male, 13 female; risperidone group: 15 male, 17 female. History: aripiprazole group: 8 months~5 years, mean = (2. 7 ± 1. 1) years; risperidone group: 7 month~6 years, mean = (2. 6 ± 1. 7) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean= (20. 4 ± 3. 5) mg/day. N=32. 2. Risperidone: Dose range: 1‐7 mg/day. Mean= (4. 1 ± 1. 2) mg/day. N=32. | |
| Outcomes | Global state: PANSS score decreased rate (markedly improved: ≥ 60%, improved: 40%‐60%, no effect: < 40%). Adverse effects. Unable to use ‐ Adverse effects: TESS total score (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score were missing. Data on extrapyramidal symptoms were incomplete. |
| Other bias | Low risk | None obvious. |
Wang 2007e.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period and six weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 18~54 years, mean = (29. 4 ± 3. 1) years; risperidone group: 19~53 years, mean = (27. 4 ± 2. 9) years. Gender: aripiprazole group: 17 male, 13 female; risperidone group: 16 male, 14 female. History: aripiprazole group: 1 month~16 years, mean = (2. 7 ± 3. 2) years; risperidone group: 1 month~18 years, mean = (3.1 ± 3.2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (3.1 ± 3.2) mg/day. N = 30. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean = (4.0 ± 1.5) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychopathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, ECG, EPS, use of benzodiazepines, propranolol, and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, ECG, EPS, use of benzodiazepines, propranolol, and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Wang 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: two weeks wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 16~61 years, mean = (30.1 ± 12.5) years; risperidone group: 18~65 years, mean = (32.7 ± 11.3) years. Gender: aripiprazole group: 17 male, 13 female; risperidone group: 16 male, 14 female. History: aripiprazole group: 1 month~410 months, mean = (91.7 ± 115.5) months; risperidone group: 1 month~379 months, mean = (85.7 ± 98.4) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (21.60 ± 6.89) mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (5.25 ± 1.03) mg/day. N = 30. | |
| Outcomes | Mental state: PANSS total score. Adverse effects. Unable to use‐ Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 51%‐74%, improved: 26%‐50%, no effect: ≤25%) ‐ no data reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although PANSS score decreased rate was used to assess effect, no data were available. |
| Other bias | Low risk | None obvious. |
Wang 2008c.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week washout period + eight weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. SANA of 60 or more.
N = 86.
Age: aripiprazole group: mean = (29.4 ± 7.6) years; ziprasidone group: mean = (30.3 ± 8.1) years. Gender: not reported. History: aripiprazole group: mean = (5.5 ± 2.8) years; ziprasidone group: mean = (5.2 ± 2.7) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day, mean = (16.7 ± 1.4) mg/day. N = 43. 2. Ziprasidone: Dose range: 20‐80 mg/day, mean = (46. 3 ±10. 3) mg/day. N = 43. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI‐GI total score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score. SANS total score score. Adverse effects. Unable to use ‐ Mental state: SANS subscale score ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, blood and urine routine, ECG. were missing. |
| Other bias | Low risk | None obvious. |
Wang 2009.
| Methods | Allocation: randomised, stratified random. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3), PANSS total score of 60 or more. N = 60. Age: mean = (35.2 ± 9.7) years. Gender: 36 male, 24 female. History: duration of illness not reported. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 20‐30 mg/day. mean dose: not reported. N = 30. 2. Clozapine: Dose range: 400‐600 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Leaving the study early. Adverse events: Fasting plasma glucose (FPG), glucose tolerance test (OGTT) 2h postprandial blood glucose (PBG) value. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total proportion of drop‐out was 8.3% and the data of the two groups were balanced.The proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | High risk | No data on efficacy were available. Data on other adverse effects were also missing. |
| Other bias | Low risk | None obvious. |
Wei 2006.
| Methods | Allocation: randomised, random number table. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more, CGI of 4 or more.
N = 108; complete study: N = 101.
Age: aripiprazole group: 19~58 years. mean = (32.2 ± 8.8) years; quetiapine group: 18~57 years, mean = (32.1 ± 7.1) years. Gender: 104 female. History: aripiprazole group: 1 month~5 years, mean = (2.8 ± 2.6) years; quetiapine group: 1 month~6 years, mean = (2.7 ± 2.8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Initial dose: 5~10 mg/day. Mean dose: not reported. N = 54. 2. Quetiapine: Initial dose: 75~100 mg/day. Mean dose: not reported. N = 54. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐75%, no effect: < 50%). CGI‐SI subscale score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding on outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, ECG, liver function, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Wei 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six months. Design: parallel. Setting: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 90. Age: aripiprazole group: mean = (25.2 ± 6.1) years; clozapine group: mean = (24.5 ± 7.2) years. Gender: aripiprazole group: 23 male, 22 female; clozapine group: 22 male, 23 female. History: aripiprazole group: mean = (1.4 ± 0.9) years; clozapine group: mean = (1.4 ± 0.8) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 45. 2. Clozapine: Dose range: 50‐450 mg/day. Mean dose: not reported. N = 45. | |
| Outcomes | Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score, anxiety ‐ labelled as "adverse effect". Quality of life: WHO‐QOL‐100. Adverse effects: central nervous system (headache, dizziness, somnolence, insomnia), gastrointestinal (salivate, constipation), ECG(QTc) abnormal, weight gain, appetite decrease, blood glucose increase. Unable to use ‐ Adverse effects: TESS total score, urine routine, hepatorenal function and EEG (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on urine routine, hepatorenal function and EEG were missing. |
| Other bias | Low risk | None obvious. |
Wei 2009.
| Methods | Allocation: randomised, random number table. Blindness: unclear. Duration: one week wash out period + eight weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS more than 60 years.
N = 102.
Age: aripiprazole group: 19~57 years, mean = (28.2 ± 3.9) years; olanzapine group: 20~58 years, mean = (28.4 ± 4.7) years. Gender: 80 female. History: aripiprazole group: 3 months~8 years, mean = (2.4 ± 1.4) years; olanzapine group: 3 months~6 years, mean = (2.1 ± 1.1) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 51. 2. Olanzapine: Dose range: 10‐20 mg/day. Mean dose: not reported. N = 51. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, improved: 50%‐75%, no effect: < 50%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on Global state, TESS total score, excitement or agitation insomnia, somnolence, myotonia, akathisia, tremors, use of benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Wen 2009.
| Methods | Allocation: randomised, random permutation table. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: 18~45 years, mean = (26.2 ± 3.7) years; risperidone group: 19~44 years, mean = (26.3 ± 3.3) years. Gender: 60 female. History: aripiprazole group: 5‐13 weeks, mean = (7.4 ± 4.3) weeks; risperidone group: 4‐17 weeks, mean = (7.2 ± 4.7) weeks. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean =(24.11 ± 6.32) mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.01 ± 1.89) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score, PANSS general psychogenic pathological subscale score. Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutation table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Wen 2009a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80.
Age: aripiprazole group: mean = (44.5 ± 3.0) years; risperidone group: mean = (43.9 ± 3.5) years. Gender: 80 female. History: aripiprazole group: mean = (6.5 ± 1.6) years; risperidone group: mean = (6.9 ± 1.7) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (16.5 ± 5.5) mg/day. N = 40. 2. Risperidone: Dose range: 2‐6 mg/day. Mean = (4.22 ± 1.51) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score,PANSS general pathological subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood and urine routine, renal unction, use of benzodiazepines and anticholinergic medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, renal unction, use of benzodiazepines and anticholinergic medicines were missing. |
| Other bias | Low risk | None obvious. |
Wlodzmierz 2006.
| Methods | Allocation: random, no further details. Blindness: open label. Duration: 52 weeks optional extension to a 26 week multicentre, randomised, double blind placebo controlled trial of aripiprazole. Location: multicentre. | |
| Participants | Diagnosis: schizophrenia ‐chronic stable or acutely psychotic meeting criteria for relapse and had completed at least 2 weeks of double‐blind therapy. N = 214. Gender: M = 116, F = 98. History: duration of illness not reported. Setting: not reported. | |
| Interventions | 1. Aripiprazole: 15‐30 mg/day. Mean dose: 22.0 mg/day. N = 104. 2. Olanzapine: 10‐20 mg/day. Mean dose: 14.2 mg/day. N = 110. | |
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score. Adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised, no further details. |
| Allocation concealment (selection bias) | High risk | Open label. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF method used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Adverse events were reported if they were > 5%. Scales if used were not reported. |
| Other bias | High risk | Industry sponsored. |
Wolf 2007.
| Methods | Allocation: random. Blindness: open label. Duration: 8 weeks. Design: prospective Location: multicentric | |
| Participants | Diagnosis: Schizophrenia (DSM IV), required initiation of antipsychotics, antipsychotic switch clinically appropriate. N = 833. Age: 18‐65 years. History: duration of illness not reported, age at onset not reported. Setting: outpatient. |
|
| Interventions | 1. Aripiprazole: 10 mg‐30 mg/day (mean 19.1 mg/day). N = 680. 2. Other antipsychotic: recommended dose N = 153. | |
| Outcomes | Global state: CGI‐I. Adverse effects: At least one adverse effect, extrapyramidal adverse effects, prolactin‐associated adverse effects. Others: POM, IAQ. | |
| Notes | Primary goal was evaluating the effectiveness of aripiprazole in general psychiatric setting in Europe. Data from patients in UK was not reported. Unable to use data from CGI‐I, POM and IAQ. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 4:1 computer‐generated using Interactive Voice Response System. |
| Allocation concealment (selection bias) | High risk | Open label. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF method used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Adverse events were reported if they were > 5%. Scales if used were not reported. |
| Other bias | High risk | Industry sponsored. |
Wu 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + twelve weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 98.
Age: aripiprazole group: mean = (63.5 ± 4.2) years; risperidone group: mean = (27.8 ± 9.6) years. Gender: not reported. History: aripiprazole group: mean = (26.7 ± 6.2) years; risperidone group: mean = (26.5 ± 6.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 2.5‐30 mg/day. Mean dose: not reported. N = 49. 2. Risperidon: Dose range: 0.5‐6 mg/day. Mean dose: not reported. N = 49. | |
| Outcomes | Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use‐ Adverse effects: TESS score, blood routine, hepatorenal function, electrolyte (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there was drop‐out. |
| Selective reporting (reporting bias) | High risk | The data on TESS score, blood routine, hepatorenal function, electrolyte were missing. |
| Other bias | High risk | None obvious. |
Xiao 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3 and ICD‐ 10). PANSS score of 60 or more. N = 72. Age: aripiprazole: 16~55 years, mean = (29.7 ± 19.6) years; clozapine: 18~58 years, mean = (28.6 ± 18.1) years. Gender: aripiprazole group: 16 male, 20 female; clozapine group: 16 male, 20 female . History: aripiprazole group: 1 months~ 21 years, mean = (5.4 ± 3.4) years; clozapine group: 1.5 months ~20years, mean = (5.5 ± 2.7) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐25 mg/day. Mean dose:not reported. N = 36. 2. Clozapine: Dose range: 50‐450 mg/day. Mean dose:not reported. N = 36. | |
| Outcomes | Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score; BPRS score. Quality of life: WHO‐QOL‐100. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | The data on use of benzhexol, propranolol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Xie 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 84.
Age: 25~40 years. Gender: 84 male. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range:10‐20 mg/day. Mean dose: not reported. N = 42. 2. Risperidon: Dose range: 2‐4 mg/day. Mean dose: not reported. N = 42. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Sexual desire ‐ unvalidated scale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | No imported outcome were missing. |
| Other bias | Low risk | None obvious. |
Xie 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 80.
Age: aripiprazole group 18~65 years, mean = (33.4 ± 9.1) years; risperidone group 18~63 years, mean = (32.4 ± 8.2) years. Gender: aripiprazole group: 22 male, 18 female; risperidone group: 21 male, 19 female. History: aripiprazole group 1~22 months, mean = (13.1 ± 2.1) months; risperidone group 1~23 months, mean = (12.6 ± 3.6) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: (21 ± 10.4) mg/day. N = 40. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: (4 ± 1.6) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on blood routine, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Xu 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS score of 35 or more.
N = 70.
Age: Aripiprazole: mean = (28 ± 6) years; Clozapine: mean = (29 ± 5) years.
Gender: Aripiprazole group: 18 male, 17 female; Clozapine group: 19 male, 16 female .
History: Aripiprazole group: mean = (3.5 ± 1.5) years; Clozapine group: 1.5 months ‐20 years, mean = (3.8 ± 1.4) years. Age at onset not reported. Setting: inpatient. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 35. 2. Clozapine: Dose range: 25‐400 mg/day. Mean dose: not reported. N = 35. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: BPRS total score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzhexol and benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on use of benzhexol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Xu 2010.
| Methods | Allocation: randomised, no further details. Blindness: double. Duration: 3‐7 days wash‐out period + six weeks intervention. Design: parallel. Setting: not reported, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 52.
Age: 18~65 years, aripiprazole group: mean = (35.4 ± 8.8) years; risperidone group: mean= (40.1 ± 13.3) years. Gender: aripiprazole group: 11 male, 15 female; risperidone group: 12 male, 14 female. History: aripiprazole group: mean = (6.5 ± 6.7) years; risperidone group: mean = (8.5 ± 9.4) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 26. 2. Risperidone: Dose range: 2‐6 mg/day. Mean dose: not reported. N = 26. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). CGI‐I, CGI‐S score. Mental state: PANSS total score. Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, Extrapyramidal reaction scale score, BARS score (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total proportion of drop‐out was 7.7% (2/26 vs. 2/26). Aripiprazole group: 1 because of violation of study scheme, 1 because of bad compliance; risperidone group: 1 because of violation of study scheme, 1 because of drug combination; 1 because of effect. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, Extrapyramidal reaction scale score, BARS scores were not reported. |
| Other bias | Low risk | None obvious. |
Yan 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six months. Design: parallel. Location: inpatient and outpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 86, completed study: N = 74. Age: aripiprazole group: mean = (32.5 ± 6.8) years; clozapine group: mean = (28.2 ± 2.8) years. Gender: aripiprazole group: 19 female, 19 male; clozapine group: 18 male, 18 female. History: Not reported. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 15‐30 mg/day. Mean dose:not reported. N = 43. 2. Clozapine: Dose range: 200‐500 mg/day. Mean dose:not reported. N = 43. | |
| Outcomes | Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Quality of life: WHO‐QOL‐100 Unable to use ‐ Adverse effects: TESS total score and use of benzodiazepine (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total proportion of drop‐out was 7.7% (2/26 vs. 2/26). Aripiprazole group: 1 because of violation of study scheme, 1 because of bad compliance; risperidone group: 1 because of violation of study scheme, 1 because of drug combination; 1 because of effect. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, Extrapyramidal reaction scale score, BARS scores were not reported. |
| Other bias | Low risk | None obvious. |
Yan 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (13. 0 ± 2. 3) years; risperidone group: mean = (13. 0 ± 2. 6) years. Gender: aripiprazole group: 14 male, 16 female; risperidone group: 13 male, 17 female. History: aripiprazole group: mean = (10. 6 ± 6. 0) years; risperidone group: mean = (8. 2 ± 5. 2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 2.5‐25 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI‐SI score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Leaving the study early: no patient. Adverse effects. Unable to use‐ Adverse effects: TESS score, blood routine, hepatorenal function, EEG, and weight (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on blood routine, hepatorenal function, EEG, and weight were missing. |
| Other bias | Low risk | None obvious. |
Yan 2008a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: not reported, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3), BPRS total score of 35 or more.
N = 100. Age: 18~56 years, mean = (28.6 ± 11.3) years. Gender: 54 male, 46 female. History: mean = (14.5 ± 6.3) years. Age at onset not reported. Setting: not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: (22.5 ± 7.5) mg/day. N = 50. 2. Clozapine: Initial dose: 25‐50 mg/day. Mean dose:not reported. N = 50. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 25%‐50%, no effect: < 25%). Mental state: BPRS total score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on dizziness, salivate, constipation, weight gain, and renal function were deficient. Data on use of benzodiazepines were also missing. |
| Other bias | Low risk | None obvious. |
Yan 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 100.
Age: aripiprazole group: 18~43 years, mean = (33.40 ± 7.09) years; risperidone group: 21~44 years, mean = (32.17 ± 5.13) years. Gender: aripiprazole group: 27 male, 23 female; risperidone group: 24 male, 26 female. History: aripiprazole group: 1~13 months, mean = (5.94 ± 2.79) months; risperidone group: 1~11 months, mean = (5.46 ± 2.47) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 50. 2. Risperidone: Dose range: 1‐8 mg/day. Mean dose: not reported. N = 50. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐79%, improved: 30%‐49%, no effect: < 30%). Mental state: PANSS total score, PANSS total score decreased rate. Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total proportion of loss to follow up was 6% (4/50 vs. 2/50). Aripiprazole group: 2 cases because of drug combination, 1 case because of adverse effect, 1 case due to family demanded; risperidone group: 2 cases because of drug combination. |
| Selective reporting (reporting bias) | High risk | Data on number of no effect, blood and urine routine, renal function, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Yang 2006.
| Methods | Allocation: randomised, no further detail. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS score of 60 or more.
N = 100*.
Age: Aripiprazole: 20~62 years, mean = (43 ± 22) years; Clozapine: 22~59 years, mean = (40 ± 20) years.
Gender: Aripiprazole group: 29 male, 18 female; Clozapine group: 26 male, 17 female .
History: Aripiprazole group: mean = (25 ± 31) months; Clozapine group: mean = (28.4 ± 33) months. Age at onset not reported. Setting: inpatient. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose:not reported. N = 47. 2. Clozapine: Dose range: 50‐450 mg/day. Mean dose:not reported. N = 43. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). GGI‐SI. Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. anxiety ‐ labelled as "adverse effect". Adverse effects. |
|
| Notes | * There is a discrepancy of 10 people between the number of people selected for the study (100) and the numbers randomised into each group (47:43). Since the report consistently reported the N numbers in each group as 47:43 in the result and discussion sections of the paper, we assume these are the N numbers of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
Yang 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS score of 60 or more. N = 60. Age: not reported. Gender: not reported. History: not reported. | |
| Interventions | 1. Aripiprazole: Started dose: 5 mg/day. Maximum dose: (15 ± 5. 5) mg/day. N = 30. 2. Clozapine: Started dose: 50 mg/day. Maximum dose: (269. 8 ± 132. 5) mg/day. N = 30. | |
| Outcomes | Global state:markedly improved, improved, no effect. Mental state: PANSS total score, anxiety ‐ labelled as "adverse effect". Adverse effects. Unable to use ‐ Adverse effects: TESS total score and hepatorenal function (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there was drop‐out. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score and hepatorenal function were missing. |
| Other bias | Low risk | None obvious. |
Yang 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash‐out period + eight weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (32.7 ± 5. 9) years; ziprasidone group: mean = (31.2 ± 6. 8) years. Gender:aripiprazole group: 20 male, 10 female; ziprasidone group:10 male, 11 female. History: aripiprazole group: mean = (5.5 ± 4.1) years; ziprasidone group: mean = (5.2 ± 3.9) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day, mean dose: not reported. N = 30. 2. Ziprasidone: Dose range: 20‐160 mg/day, mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Leaving the study early. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, RSESE score, blood and urine routine,hepatorenal function, drugs for adverse events were missing. |
| Other bias | Low risk | None obvious. |
Yang 2008a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (28.2 ± 10.3) years; risperidone group: mean = (27.8 ± 9.6) years. Gender: aripiprazole group: 21 male, 9 female; risperidone group: 20 male, 10 female. History: aripiprazole group: mean = (2.6 ± 1.8) years; risperidone group: mean = (2.8 ± 2.0) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean = (16.5 ± 5.5) mg/day. N = 30. 2. Risperidon: Dose range: 1‐5 mg/day. Mean = (3.8 ± 1.6) mg/day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI‐SI score. Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Leaving the study early: no patient. Adverse effects. Unable to use‐ Adverse effects: Blood and urine routine, liver function, ECG, weight and other adverse effect, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Although blood and urine routine, liver function, ECG, weight and other adverse effect, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Yang 2008b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 90.
Age: aripiprazole group: 18~52 years. mean = (26.7± 8.5) years; risperidone group: 18~51 years, mean = (26.2 ± 8.3) years. Gender: aripiprazole group: 45 female; risperidone group: 45 female. History: aripiprazole group: 1~52 months, mean = (15.2 ± 16.7) months; risperidone group: 1~49 months, mean = (14.9 ± 17.1) months. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 45. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 45. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. z Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood routine, liver function, blood glucose, weight, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no drop‐out. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood routine, liver function, blood glucose, weight, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Yang 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 60.
Age: 18~45 years, aripiprazole group: mean = (29.6 ± 7.3) years; olanzapine group: mean = (28.9 ± 6.6) years. Gender: aripiprazole group: 16 male, 14 female; olanzapine group: 15 male, 15 female. History: aripiprazole group: mean = (10.2 ± 5.0) years; olanzapine group: mean = (10.4 ± 5.8) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Initial dose: 10 mg/day. Mean = (20.8 ± 6.2) mg/day. N = 30. 2. Olanzapine: Initial dose: 5 mg/day. Mean = (14.3 ± 5) mg/day. N = 30. | |
| Outcomes | Adverse effects: weight gain, blood glucose. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Ye 2005.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 68.
Age: aripiprazole group: 18~52 years, mean = (28. 6 ± 16. 8) years; risperidone group: 19~55 years, mean = (31.9 ± 15.7) years. Gender: aripiprazole group: 20 male, 14 female; risperidone group: 24 male, 10 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (17.5 ± 6.5) mg/day. N = 34. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.1 ±1.8) mg/day. N = 34. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score, agitation labelled as "adverse effect". Adverse effects. Unable to use‐ Adverse effects: TESS score, blood and urine routine, liver function, blood glucose (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score and blood and urine routine, liver function, blood glucose were missing. |
| Other bias | Low risk | None obvious. |
Ye 2005a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week washout + six weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. BPRS of 40 or more.
N = 60.
Age: aripiprazole group: 18~65 years, mean = (28.3 ± 10.6) years; olanzapine group: 18~56 years, mean = (26.5 ± 11.3) years. Gender: aripiprazole group: 20 male, 10 female; olanzapine group: 19 male, 11 female. History: aripiprazole group: 0.3~18 years, mean = (6.3 ± 6.9) years; olanzapine group: 0.5~15 years, mean = (5.7 ± 6.1) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 30. 2. Olanzapine: Dose range: 5‐10 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: 80≥ %, markedly improved: 60%‐70%, improved: 30%‐59%, no effect: < 30%). CGI score. Mental state: PANSS total decreased score, PANSS positive decreased subscale score, PANSS negative decreased subscale score, PANSS psychopathological decreased subscale score. Leaving the study early. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood and urine routine, weight gain, extrapyramidal effects, constipation, somnolence, use of benzodiazepines and other medicine (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two people were lost to follow‐up (1/30 VS.1/30) in the two groups, one because of early discharge, one due to refusing further therapy. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, weight gain, extrapyramidal effects, constipation, somnolence, use of benzodiazepines and other medicine were missing. |
| Other bias | Low risk | None obvious. |
Yi 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 60. Age: mean = (30.1 ± 9.7) years. Gender: 34 male, 26 female. History: mean = (32 ± 10.5) years. Age at onset: mean = (29.7 ± 9.5) years. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (17.6 ± 4.5) mg/day. N = 30. 2. Clozapine: Dose range: 300‐600 mg/day. Mean = (370 ± 82) mg/day. N = 30. | |
| Outcomes | Adverse events: Fasting plasma glucose (FPG), C‐peptide. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Yu 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: treatment resistant schizophrenia (CCMD‐3). PANSS score of 60 or more. N = 53. Age: aripiprazole group: 18~59 years, mean = (32.8 ± 6.2) years; clozapine group: 17~58 years, mean = (30.6 ± 7.8) years. Gender: aripiprazole group: 17 male, 9 female; clozapine group: 10 male, 12 female . History: aripiprazole group: 1 month˜9 years, mean = (4.2 ± 3.9) years; clozapine group: 1 month~11years, mean = (5.2 ± 3.4) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (15 ± 5) mg/day. N = 26. 2. Clozapine: Dose range: 50‐500 mg/day. Mean = (400 ± 125) mg/day. N = 22. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS positive subscale score, PANSS negative subscale score. Adverse effects. Unable to use ‐ Leaving the study early: 5 people dropped out, but it did not report which group they were from. Adverse effects: TESS total score and hepatorenal function (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five people were excluded from analysis due to incomplete outcome data. It is unclear which group these 5 people belonged to. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score and hepatorenal function were missing. |
| Other bias | Low risk | None obvious. |
Yu 2006a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 74. Age: aripiprazole group: mean = (23.69 ± 11.67) years; clozapine group: mean = (25.61 ± 6.4) years. Gender: aripiprazole group: 18 male, 18 female; clozapine group: 19 male, 19 female . History: aripiprazole group: mean = (7.5 ± 2.3) months; clozapine group: mean = (8.92 ± 3.3) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐40 mg/day. Mean dose: not reported. N = 36. 2. Clozapine: Dose range: 75‐550 mg/day. Mean dose: not reported. N = 38. | |
| Outcomes | Adverse effect: change of ECG and blood glucose. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there was incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Yu 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS score of 60 or more. N = 74. Age: aripiprazole group: mean = (23.69 ± 11.67) years; clozapine group: mean = (25.61 ± 6.4) years. Gender: aripiprazole group: 18 male, 18 female; clozapine group: 19 male, 19 female . History: aripiprazole group: mean = (7.5 ± 2.3) months; clozapine group: mean = (8.92 ± 3.3) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐40 mg/day. Mean dose: not reported. N = 36. 2. Clozapine: Dose range: 75‐550 mg/day. Mean dose: not reported. N = 38. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score. Adverse effect. Unable to use ‐ Adverse effects: TESS total score, liver function and blood glucose (no data). Mental state: BPRS and PANSS subscale scores ‐ unvalidated subscales. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on liver function and blood glucose were missing. |
| Other bias | Low risk | None obvious. |
Yu 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 100.
Age: aripiprazole group: 18~65 years, mean = (36.3 ± 4.1) years; risperidone group: 16~60 years, mean = (34.5 ± 12.7) years. Gender: aripiprazole group: 32 male, 18 female; risperidone group: 30 male, 20 female. History: aripiprazole group: 3 months~10 years, mean = (3.0 ± 1.9) years. risperidone group: 2 months~15 years, mean = (3. 2 ±2. 5) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean= (22.3 ± 3.2) mg/day. N = 50. 2. Risperidone: Dose range: 1‐6 mg/day. Mean= (3.8 ±1.33) mg/day. N = 50. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use‐ Adverse effects: TESS score, blood and urine routine, renal function (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, blood and urine routine, and renal function were missing. |
| Other bias | Low risk | None obvious. |
Yu 2009.
| Methods | Allocation: randomised, stratified random. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more. N = 60. Age: aripiprazole group: 22~57 years, mean = (34. 63 ±9. 86) years; clozapine group: 23~59 years, mean = (9. 49 ± 4.33) years. Gender: aripiprazole group: 19 M, 11 F; clozapine group: 22 M, 8 F. History: aripiprazole group: 5~21 years, mean = (9.15 ± 4.31) years; clozapine group: 5.25~23 years, mean = (9.49 ± 4.33) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (16.75 ± 3.55) mg/day. N = 30. 2. Clozapine: Dose range: 50‐600 mg/day. Mean = (398.33 ± 74.84) mg/day. N = 30. | |
| Outcomes | Mental state: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, EEG, and drugs for adverse events (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on EEG and drugs for adverse events were missing. |
| Other bias | Low risk | None obvious. |
Yu ZG 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more. N = 60. Age: aripiprazole group: 18~54 years, mean = (34. 5 ± 4.7) years; clozapine group: 18~51 years, mean = (33.6 ± 5.2) years. Gender: aripiprazole group: 17 M, 13 F; clozapine group: 18 M, 12 F. History: aripiprazole group: 4 months~6 years; clozapine group: 2 months~4 years . Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (22.1 ± 3.4) mg/ day. N = 30. 2. Clozapine: Dose range: 50‐500 mg/day. Mean = (336 ± 4.7) mg/ day. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: blood routine, urine routine, blood glucose, and hepatorenal function (no data) . |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on blood routine, urine routine, blood glucose,and hepatorenal function were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more. N = 112. Age: aripiprazole group: 15~60 years, mean = (25.7 ± 7.9) years; risperidone group: 15~60 years, mean = (26.1 ± 8.2) years. Gender: aripiprazole group: 30 Male, 26 Female; Risperidon group: 29 Male, 27 Female . History: aripiprazole group: 1 month~10 years, mean = (4.8 ± 2.6) years; risperidone group: 1 month~9 years, mean = (4.6 ± 2.8) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (21.4 ± 3.1) mg/day. N = 56. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.9 ± 1.7) mg/day. N = 56 | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score, PANSS score decreased rate: PANSS positive subscore, PANSS negative subscore, PANSS cognitive score, PANSS excited subscore, PANSS depressed subscore. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzhexol and benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on use of benzhexol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2006a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week washout + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 104.
Age: aripiprazole group: 18~62 years, mean = (28.1 ± 6.3) years; olanzapine group: 19~65 years, mean = (32.6 ± 5.4) years. Gender: aripiprazole group: 29 male, 23 female; olanzapine group: 27 male, 25 female. History: aripiprazole group: mean = (2.8 ± 4.6) years; olanzapine group: mean = (3.5 ± 5.2) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean = (16.53 ± 4.7) mg/day. N = 52. 2. Olanzapine: Dose range: 5‐25 mg/day. Mean = (17.3 ± 6.4) mg/day. N = 52. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: 80≥ %, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS psychopathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on blood and urine routine, liver function, ECG, use of benzodiazepines and benzhexol were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 97.
Age: aripiprazole group: 18~74 years, mean = (31.7 ± 11.6) years; clozapine group: 18~67 years, mean = (34.3 ± 11.4) years. Gender: aripiprazole group: 24 male, 26 female; clozapine group: 23 male, 24 female. History: aripiprazole group: mean = (7.4 ± 6.1) years; clozapine group: mean =(7.4 ± 4.2) months. Age at onset not reported. Setting: inpatient. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose:not reported. N = 50. 2. Clozapine: Dose range: 25‐400 mg/day. Mean dose:not reported. N = 47. | |
| Outcomes | Adverse effect: change of ECG (ECG abnormal, tachycardia and others). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Zhang 2007a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more. N = 50. Age: aripiprazole group: mean = (10.07 ± 2.35) years; risperidone group: mean = (9.37 ± 4.08) years. Gender: aripiprazole group: 11 Male, 14 Female; risperidone group: 10 Male, 15 Female . History: aripiprazole group: mean = (1.64 ± 3.74) months; risperidone group: mean = (1.87 ± 2.96) months. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 25. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 25. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: blood and urine routines, EEG, use of anticholinergic drug and benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on blood and urine routines, EEG, use of anticholinergic drug and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2007b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS of 35 or more.
N = 60.
Age: aripiprazole group: mean = (28.2 ± 10.3) years; risperidone group: mean = (27.8 ± 9.6) years. Gender: aripiprazole group: 20 male, 10 female; risperidone: 20 male, 10 female. History: aripiprazole group: mean= (2.6 ± 10.8) years; risperidone group: mean = (2.8 ± 2.0) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. mean= (16.5 ± 5.5) mg/day.N=30. 2. Risperidone: Dose range: 1‐5mg/day. mean = (3.8 ± 1.6) mg/day. N=30. | |
| Outcomes | Global state: BPRS score decreased rate (recovery: ≥75%, markedly improved: 50%‐75%, improved: 30%‐50%, no effect: < 30%). Mental state: BPRS score. Leaving the study early: no patient. Adverse effect: TESS total score. Unable to use ‐ Various specific adverse effects (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on various specific adverse effects were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week washout period + eight weeks intervention. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (36.3 ± 8.2) years; risperidone group: mean = (37.3 ± 8.7) years. Gender: aripiprazole group: 23 male, 7 female; risperidone: 21 male, 9 female. History: aripiprazole group:1 month‐30 years; risperidone group: 2 months ‐ 29 years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). CGI score. Mental state: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects. Unable to use ‐ Adverse effects: TESS total score(no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although ESRS was used to assess adverse effects, no data on scores were available. |
| Other bias | Low risk | None obvious. |
Zhang 2008a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more. N = 60. Age: aripiprazole group: 18‐58 years, mean = (32.8 ± 6.9) years; risperidone group: 20‐56 years, mean = (33.4 ± 5.8) years. Gender: aripiprazole group: 18 Male, 12 Female; risperidone group: 16 Male, 14 Female . History: aripiprazole group: 1 month‐8 years, mean = (4.8 ± 3.2) years; risperidone group: 2 months‐7 years, mean = (4.6 ± 3.8) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (15 ± 5) mg/day. N = 30. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.5 ± 1.6) mg/day. N = 30 | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood and urine routine, liver function (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although ESRS was used to assess adverse effects, no data on scores were available. |
| Other bias | Low risk | None obvious. |
Zhang 2008b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Location: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 40. Age: aripiprazole group: 18‐58 years, mean = (33.2 ± 7.8) years; risperidone group: 18‐65 years, mean = (34.5 ± 8.7) years. Gender: aripiprazole group: 8 Male, 12 Female; risperidone group: 10 Male, 10 Female . History: aripiprazole group: 1 month‐5 years, mean = (2.8 ± 3.6) years; risperidone group: 2 months‐7 years, mean = (3.7 ± 4.2) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (10 ± 5.22) mg/day. N = 20. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (3.5 ± 0.8) mg/day. N = 20 | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 20%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, endocrine change, laboratory tests (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, endocrine change, laboratory tests were missing. No data of Quality of life GQOLI ‐ 74. |
| Other bias | Low risk | None obvious. |
Zhang 2009.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: twelve weeks. Design: parallel. Setting: not reported, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. Refractory schizophrenia (TRS). N = 72. Age: aripiprazole group: 25‐54 years, mean = 34.4years; clozapine group: 24‐58 years, mean = 33.8 years. Gender: aripiprazole group: 21 female, 15 male; clozapine group: 22 male, 14 female. History: aripiprazole group: 5.8‐26 years, mean = 8.6 years; clozapine group: 5.5‐28 years, mean = 8.9 years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 36. 2. Clozapine: Dose range: 200‐500 mg/day. Mean dose: not reported. N = 36. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: > 75%, markedly improved: 51%‐74%, improved: 26%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS, use of benzhexol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2009a.
| Methods | Allocation: randomised, random number table. Blindness: single. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (31.3 ± 8.7) years; risperidone group: mean = (30.4 ± 8.7) years. Gender: 60 male. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score. Adverse effects: blood glucose and cholesterol. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single, if the blinding was performed well was unknown. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unclear if there were no incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
Zhang 2009b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: one week wash out + eight weeks intervention. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: aripiprazole group: mean = (37 ± 7) years; risperidone group: mean = (36 ± 7) years. Gender: aripiprazole group: 18 male, 12 female; risperidone group: 19 male, 11 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 30. 2. Risperidone: Dose range: 4‐6 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score,PANSS general pathological subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, blood and urine routine, blood glucose, use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, blood and urine routine, blood glucose, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2010.
| Methods | Allocation: Random permutation table. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.Refractory schizophrenia(TRS). N = 120. Age: aripiprazole group 18~55 years, mean= 33 years; clozapine group: 21~53 years, mean = 34 years. Gender: aripiprazole group: 21 female, 15 male; clozapine group: 22 male, 14 female. History: aripiprazole group: 2~16 years, mean = 7 years; clozapine group: 4~19 years, mean = 6 years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 20‐30 mg/day. Mean= (22. 1 ± 5. 4) mg/day. N = 60. 2. Clozapine: Dose range: 300‐600 mg/day. Mean= (354. 5 ± 84. 9) mg/day. N = 60. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects: central nervous system (headache, insomnia, somnolence), gastrointestinal(nausea, constipation), weight gain, extrapyramidal side‐effects (tremor, akathisia), tachycardia. Unable to use ‐ Adverse effects: TESS total score, use of benzhexol and benzodiazepines (no data). The data of at least one adverse effect. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutation table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Although TESS was used to assess adverse effects, no data on scores were available. Data on use of benzhexol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Zhang 2010a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 100.
Age: 19~64 years. Gender: not reported. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 50. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 50. | |
| Outcomes | Mental state: PANSS total score. Adverse effects: TESS total score, Unable to use ‐ Quality of life: GQOLI ‐ 74 Adverse effects: anxiety, EPS, ECG abnormal, mouth dry, liver function, use of benzodiazepines and other medicines. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on GQOLI ‐ 74 score, anxiety, EPS, ECG abnormal, mouth dry, liver function, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Zhao 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 68. Age: aripiprazole group: mean = (24.3 士 9.4) years; quetiapine group: 18‐51 years, mean = (26.7 ± 8.4) years. Gender: male and female History: aripiprazole group: mean = (23 土 13) months; quetiapine group: mean =(4. 5 ± 2.9) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose = not reported. N = 34. 2. Quetiapine: Dose range: 100‐750 mg/day. Mean dose = (465 ± 132) mg/day. N = 34. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score. Unable to use ‐ Mental state: PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | TESS score was measured, but not reported. |
| Other bias | Low risk | None obvious. |
Zhao 2007.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 80. Age: aripiprazole group: 17~62 years, mean = (29.5 ± 11.7) years; risperidone group: 16~60 years, mean = (29.3 ± 10.6) years. Gender: aripiprazole group: 19 Male, 21 Female; risperidone group: 21 Male, 19 Female. History: aripiprazole group: 1 month~10 years, mean =6.4 years; risperidone group: 1.5 months~13 years, mean = 6.3 years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean: not reported. N = 40. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean: not reported. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. agitation labelled as "adverse effect". Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, use of benzodiazepines, benzhexol and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Zhi 2005.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 80. Age: aripiprazole group: 18~35 years, mean = ( 22.0 ± 6.08) years; risperidone group: 18~33 years, mean = (24.56 ± 8.68) years. Gender: 80 female. History: aripiprazole group: 1 month~15 years, mean = (8.42 ± 6.83) years; risperidone group: 1 month~14 years, mean = (8.02 ± 7.32) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = (21.0 ± 6. 17) mg/day. N = 40. 2. Risperidone: Dose range: 1‐6 mg/day. Mean = (4.46 ± 1.03) mg/day. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines, benzhexol and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Zhou 2007.
| Methods | Allocation: Random permutation table. Blindness: unclear. Duration: one week washout period + eight weeks intervention. Design: parallel. Setting: inpatient and outpatient patient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 92,complete study: N = 90. Age: aripiprazole group: mean = (35.2 ± 8.5) years; clozapine group: mean =(34. 3 ±7. 9) years. Gender: aripiprazole group: 20 female, 25 male; clozapine: 23 male, 22 female. History: aripiprazole group: mean = (3.3 ± 2.8) years; clozapine group: mean = (3.4 ± 2.9) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean dose: not reported. N = 45. 2. Clozapine: Dose range: 50‐400 mg/day. Mean dose: not reported. N = 47. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐74%, improved: 25%‐49%, no effect: < 25%). Mental state: PANSS total score. anxiety ‐ labelled as "adverse effect". Leaving the study early. Adverse effects. Unable to use ‐ Mental state: PANSS negative subscore, cognitive factor, affective symptoms (no data). Adverse effects: TESS total score, hepatorenal function, use of benzhexol and benzodiazepines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutation table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS score, PANSS negative subscore, cognitive factor, affective symptoms, hepatorenal function, use of benzhexol and benzodiazepines were missing. |
| Other bias | Low risk | None obvious. |
Zhou 2007a.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 62. Age: aripiprazole group: mean = (37.5 ± 8.1) years; risperidone group: mean = (39.2 ± 10.3) years. Gender: aripiprazole group: 9 Male, 22 Female; risperidone group: 7 Male, 24 Female. History: not reported. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐30 mg/day. Mean = 10.2 mg/day. N = 31. 2. Risperidone: Dose range: 0.5‐6 mg/day. Mean = 3.5 mg/day. N = 31. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. Adverse effects. Unable to use ‐ Adverse effects: TESS total score, use of benzodiazepines and anticholinergic medication (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and anticholinergic medication were missing. |
| Other bias | Low risk | None obvious. |
Zhou 2007b.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more.
N = 60.
Age: 18~60 years. Gender: aripiprazole group: 20 male, 10 female; risperidone group: 18 male, 12 female. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 5‐25 mg/day. Mean dose: not reported. N = 30. 2. Risperidon: Dose range: 1‐4 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score. Adverse effects. Unable to use ‐ Quality of life: GQOLI ‐ 74 (no data). Adverse effects: use of benzodiazepines and other medicines (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on GQOLI ‐ 74 score, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Zhou 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: eight weeks. Design: multi‐centre, parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. N = 140. Age: aripiprazole group: 10~50 years, mean = (28.7 ± 7.8) years; risperidone group: 18~49 years, mean = (28.1 ± 7.8) years. Gender: aripiprazole group: 36 Male, 34 Female; risperidone group: 35 Male, 35 Female. History: aripiprazole group: 1 month~3 years, mean = (3.0 ± 1.4) years; risperidone group: 1 month~3 years, mean = (3.1 ± 2.3) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (22. 4 ± 3. 4) mg/day. N = 70. 2. Risperidone: Dose range: 1‐4 mg/day. Mean dose = (4. 3 ± 1. 7) mg/day. N = 70. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 75%, markedly improved: 50%‐75%, improved: 25%‐50%, no effect: < 25%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. Adverse effects. Unable to use: Adverse effects: TESS total score, EEG, use of benzodiazepines, benzhexol and propranolol (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, EEG, use of benzodiazepines, benzhexol and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Zhu 2005.
| Methods | Allocation: randomised, no further details. Blindness: single. Duration: eight weeks. Design: parallel. Setting: inpatient and outpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). BPRS total score of 40 or more. N = 80. Age: aripiprazole group: 16~57 years, mean = (28.3 ± 10.2) years; clozapine group: 17~58 years, mean = (27.8 ± 8.2) years. Gender: aripiprazole group: 22 M, 18 F; clozapine group: 20 M, 20 F. History: aripiprazole group: 2 months~8 years, mean = (6.2 ± 3.7) years; clozapine group: 3 months~9 years, mean = (6.7 ± 5.8) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐20 mg/day. Mean dose: not reported. N = 40. 2. Clozapine: Dose range: 50‐500 mg/day. Mean dose: not reported. N = 40. | |
| Outcomes | Global state: PANSS score decreased rate (markedly improved: ≥60%, improved: 20%‐59%, no effect: < 20%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. BPRS total score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blind, but whether the blindness was performed well was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were drop‐outs, but specific digital was not obtained. |
| Selective reporting (reporting bias) | High risk | Data on blood and urine routine, liver function were missing. |
| Other bias | Low risk | None obvious. |
Zhu 2008.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. BPRS of 40 or more.
N = 60. Age: aripiprazole group: 18~65 years. mean = (28.3 ± 10.6) years; quetiapine group: 18‐56 years, mean = (26.5 ± 11.3) years. Gender: aripiprazole group: 20 male, 10 female; quetiapine group: 19 male, 11 female. History: aripiprazole group: mean = (6.3 ± 6.9) years; quetiapine group: mean = (5.7 ± 6.1) years. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean dose: not reported. N = 30. 2. Quetiapine: Dose range: 200‐700 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Global state: PANSS score decreased rate (recovery: ≥ 80%, markedly improved: 50%‐80%, improved: 30%‐50%, no effect: < 30%). Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. BPRS total score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total proportion of drop‐out was 3.3% and the data of the two groups were balanced (1/30: 1/30). |
| Selective reporting (reporting bias) | High risk | Data on CGI score, TESS total score, akathisia, tremor, insomnia, constipation, drowsiness sleepiness, weight gain, EEG, blood routine, urine routine, use of benzodiazepine and propranolol were missing. |
| Other bias | Low risk | None obvious. |
Zhu 2010.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six months. Design: trial with three arms. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS of 60 or more. SANS of 60 or more.
N = 81; Number of people completed study: N = 76.
Age: 40~65 years. Gender: not reported. History: not reported. Age at onset not reported. |
|
| Interventions | 1. Aripiprazole: Dose range: 10‐30 mg/day. Mean = (18.3 ± 2.9) mg/day. N: not reported, complete study: N = 22.
2. Risperidone: Dose range: 1‐5 mg/day. Mean = (4.2 ± 0.4) mg/day. N: not reported, complete study: N = 28. 3.Olanzapine: Dose range: 5‐20 mg/day. Mean = (12.6 ± 2.6) mg/day. N: not reported, complete study: N = 26. |
|
| Outcomes | Mental state: PANSS total score, PANSS positive subscale score, PANSS negative subscale score, PANSS general psychogenic pathological subscale score. SANS total score. Adverse effects. Unable to use ‐ BPRS and SANS subscale score ‐ unvalidated subscales. Leaving the study early ‐ unclear from which group people were dropped out. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The total proportion of loss to follow‐up was 6.2% (5 people), but it was unclear proportion in every group. |
| Selective reporting (reporting bias) | High risk | Data on TESS total score, use of benzodiazepines and other medicines were missing. |
| Other bias | Low risk | None obvious. |
Zimbroff 2007.
| Methods | Allocation: random, computer‐generated, centre blocked Blindness: double, dummy administration. Duration: four weeks. Location: multicentre. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N = 256. Age: 18‐70 years. Gender: 169 M, 57 F. History: duration of illness not reported, age at onset not reported, hospitalised > 14 consecutive days prior to screening, PANSS ≥ 80, 4/more on 2+ PANSS positive items, score of ≥ 4 on CGI‐S Setting: inpatient. | |
| Interventions | 1. Aripiprazole: dose 15 mg/day first 14 days, up to 30 mg/day thereafter, mean 21 mg/day (SD 7). N = 129. 2. Ziprasidone: dose 80 mg/day on first 14 days, up to 160 mg/day thereafter mean 149 mg/day ( SD 25): N = 127. | |
| Outcomes | Leaving the study early.
Global state: CGI‐S.
Mental state: BPRS, PANSS.
Adverse effects: system‐specific effects, AIMS, BAS, SAS. Unable to use ‐ Global state: ORDQ. Mental state: CDSS (no numerical data). Adverse effects: SAS (reported as zero difference with P value ‐ not credible data), weight, glucose, blood lipids (median reported with no other data). Adverse effects: AIMS, BAS (P values, and numbers that did allow calculation of SD).* |
|
| Notes | * These data, although possible to report, carried assumptions on numbers of people from which they were derived, identical SDs per group, normality of data on top of other assumptions usually associated with scale‐derived numbers. We feel that these are assumptions too far. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random, computer‐generated, blocked by centre. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, double dummy administration. Whether blinding was successful has not been examined, but both compounds have similar adverse effects. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF method used to account for people leaving the study early. |
| Selective reporting (reporting bias) | High risk | Only adverse events with an incidence of more than 5% were reported. This procedure may have missed important adverse events. CDSS and ORDQ ‐ no numerical data reported because"no statistically significant differences were observed. . ." AIMS, BAS, SAS were portrayed very differently to other scales ‐ allowing computation of data but not giving clear person‐based numbers for the ratings. |
| Other bias | High risk | Random sequence generation (selection bias) |
Zou 2006.
| Methods | Allocation: randomised, no further details. Blindness: unclear. Duration: six weeks. Design: parallel. Setting: inpatient, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). PANSS total score of 60 or more, BPRS total score of 36 or more, TESS total score of 1 or less. N = 62. Age: aripiprazole group: mean = (45.99 ± 6.89) years; clozapine group: mean = (45.81 ± 9.2) years. Gender: aripiprazole group: 20 M, 11 F; clozapine group: 18 M, 13 F. History: aripiprazole group: mean = (16.99 ± 8.64) years; clozapine group: mean = (15.98 ± 3.5) years. Age at onset not reported. | |
| Interventions | 1. Aripiprazole: Dose range: 5‐15 mg/day. Mean dose: not reported. N = 30. 2. Clozapine: Dose range: 25‐400 mg/day. Mean dose: not reported. N = 30. | |
| Outcomes | Mental state: PANSS total score. PANSS positive subscale score, PANSS negative subscale score, PANSS general pathological subscale score. BPRS total score. Adverse effects: TESS scale (poisoning symptoms, laboratory abnormalities, nervous system, autonomic nervous system, cardiovascular system, other). Unable to use: Mental state: BPRS and PANSS subscale scores ‐ unvalidated subscale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome was assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there was drop‐out. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None obvious. |
Diagnostic tools and scales CCMD: Classification of Mental Disorders DSM IV: Diagnostic and Statistical Manual, 4th edition
Rating Scales
Global rating scales: CGI: Clinical Global Impressions. CGI‐S: Clinical Global Impression‐Severity. CGI‐I: Clinical Global Impression‐Improvement ORDQ: Outcome Resourse Discharge Questionnaire ASEX: Arizona Sexual Experience Scale. .
Mental state BPRS: Brief Psychiatric Rating Scale. MADRS: Montgomery‐Asberg Depression Rating Scale. MMSE: Wiing Mini Mental State Examination. PANSS: Positive and Negative Syndrome Scale. PANSS‐EC: Positive and Negative Syndrome Scale‐Excited Component. SANS: Scale for the Assessment of Negative Symptoms. SAPS: Scale for the Assessment of Positive Symptoms. CDSS:Calgary Depression Scale for Schizophrenia Side effects AIMS: Abnormal Involuntary Movement Scale. BAS: Barnes Akathisia Scale. ESRS: Extrapyramidal Syndrome Rating Scale. SAS: Simpson‐Angus Index ‐ for neurological side effects. TESS: Treatment Emergent Symptom Scale UKU: Udvalg for kliniske ndersogelser Side Effect Rating Scale ‐side effect rating scale.
Quality of Life QLS: Quality of Life Scale. IWQoL‐Lite: Impact of Wieght on Quality of Life
Other BMI: body mass index ECG: electrocardiogram EPS: extrapyramidal symptoms LOCF: last observation carried forward SD: standard deviation TG: triglyceride
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ai 2008 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Allen 2007 | Allocation: not randomised, review. |
| Anon 2008 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole + psychosocial intervention vs aripiprazole, not aripiprazole vs other atypical antipsychotics. |
| Bao 2007 | Allocation: randomised. Participants: people with schizophrenia Intervention: aripiprazole + clonazepam vs clozapine |
| Bergman 2007 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole + guanfacine vs aripiprazole, not aripiprazole vs other atypical antipsychotics. |
| Bristol‐Myers 2006 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole as augmenter of atypical antipsychotics, not aripiprazole vs other atypical antipsychotics. |
| Carson 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs haloperidol vs placebo, not aripiprazole vs other atypical antipsychotics. |
| Casey 2003 | Allocation: pooled analysis. |
| Chen 2005 | Allocation: not randomised. |
| Chen 2007 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Chen 2008 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Chen LJ 2010 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Chen YH 2010 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Colombo 2008 | Allocation: randomised. Participants: metabolic syndrome incidence not schizophrenia. Interventions: aripiprazole vs olanzapine. |
| Cornblatt 2002 | Allocation: randomised open label. Participants: people with schizophrenia. Interventions: aripiprazole vs olanzapine. Outcome: No usable data available. |
| Fawzi 2009 | Allocation: randomised open label. Participants: people with schizophrenia. Interventions: aripiprazole vs olanzapine vs olanzapine + clomipramine. Outcome: No usable data available. |
| Fleischhacker 2008a | Allocation: randomised double blind then open label extension. Participants: people with schizophrenia. Interventions: aripiprazole + clozapine vs placebo + clozapine, not aripiprazole vs other atypical antipsychotics. |
| Geng 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Guan 2007 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Han HF 2010 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Hatta 2009 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs olanzapine vs risperidone vs quetiapine. Outcome: No usable data available. |
| Henderson 2009 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs placebo, not aripiprazole vs other atypical antipsychotics. |
| Huang 2008 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Janssen 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: switch treatment with aripiprazole in patients while on risperidone, not aripiprazole vs other atypical antipsychotics. |
| Jiang 2007 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Ju 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Kim 2006 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole + clozapine vs clozapine, not aripiprazole vs other atypical antipsychotics. |
| Kim 2009 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs other atypical antipsychotics. Outcome: No usable data available. |
| Lan 2008 | Allocation: randomised. Participants: people with schizophrenia. Interventions: shift from other antipsychotics to aripiprazole, not aripiprazole vs other drugs. |
| Li 2007e | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Li 2007f | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Li C 2010 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Liang 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs risperidone. Outcome: no usable data available. |
| Liemburg 2011 | Allocation: randomised.
Participants: people with schizophrenia.
Interventions: aripiprazole vs risperidone. Outcome: no usable data available. |
| Lin 2006 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Lin Y 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Liu 2007a | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Liu 2007b | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Liu XH 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Lu 2008 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Lv 2006 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Ma 2007 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole + clozapine vs clozapine, not aripiprazole vs other atypical antipsychotics. |
| Ma 2009b | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Medori 2008 | Allocation: not randomised. Participants: people with schizophrenia. Interventions: risperidone (RLAI) vs quetiapine, not aripiprazole vs other atypical antipsychotics. |
| Millar 2008 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine + placebo vs clozapine + aripiprazole, not aripiprazole vs other drugs. |
| Mortimer 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs placebo, not aripiprazole vs other atypical antipsychotics. |
| Mossner 2009 | Allocation: randomised. Participants: people with schizophrenia. Interventions: risperidone vs haloperidol, not aripiprazole vs other drugs. |
| Namey 2006 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine + placebo vs clozapine + aripiprazole, not aripiprazole vs other drugs. |
| Newcomer 2006 | Allocation: not randomised ‐ pooled analysis. |
| Newcomer 2008 | Allocation: not randomised, pooled analysis. |
| Newcomer 2008c | Allocation: not randomised, pooled analysis. |
| Pae 2009a | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole vs aripiprazole + another antipsychotic (reducing dose over 4 weeks) vs aripiprazole + another antipsychotic (reducing dose over 6 weeks), not aripiprazole alone vs other drugs. |
| Ray 2004 | Allocation: not randomised, cohort study. |
| Remington 2009 | Allocation: randomised. Participants: people with schizophrenia. Interventions: aripiprazole + clozapine vs clozapine + placebo, not aripiprazole alone vs other drugs. |
| Schreiner 2009 | Allocation: randomised. Participants: people with schizophrenia, schizo‐affective disorder. Interventions: switching treatment for patients on oral risperidone, olanzapine and conventional antipsychotics to RLAI vs quetiapine and aripiprazole, not aripiprazole vs other drugs. |
| Shen 2006 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Shim 2006 | Allocation:"double blind", unclear if randomised. Participants: people with schizophrenia. Interventions: aripiprazole + haloperidol vs haloperidol + placebo, not aripiprazole alone vs other drugs. |
| Sun 2006a | Allocation: open lablel controlled clinical trial, not RCT. |
| Sun P 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Takeuchi 2008 | Allocation: randomised. Participants: people with schizophrenia. Interventions: two switching strategies of aripiprazole, not aripiprazole vs other drugs. |
| Talbott 2007 | Allocation: randomised. Participants: people with schizophrenia. Interventions: pooled post‐hoc analysis aripiprazole vs haloperidol and aripiprazole vs olanzapine. |
| Taylor 2007 | Allocation: randomised, open label. Participants: schizophrenia. Interventions: aripiprazole vs olanzapine vs risperidone vs haloperidol vs quetiapine vs ziprasidone, not aripiprazole vs other atypicals. Outcome: no usable data. |
| Wang 2006e | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Wang 2007b | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Wang 2007c | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Watts 2006a | Allocation: randomised, open label. Participants: schizophrenia. Interventions: aripiprazole vs ziprasidone. Outcome: no usable data, study terminated. |
| Xu 2008 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Yang 2007a | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Yang 2007b | Allocation: not randomised. |
| Yu 2007a | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zeng 2006 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhang 2006b | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhang F 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhang RK 2008 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhang XL 2007 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhao GW 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhao JY 2009 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhe 2007 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
| Zhi 2010 | Allocation: not randomised ‐ randomisation allocation according to admission order. |
RCT: randomised controlled trial RLAI: Risperidone Long‐acting Injection vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Wang 2006f.
| Methods | Allocation: randomised (no further description). Blinding: not stated. Duration: 8 weeks. Setting: inpatients, China. |
| Participants | Diagnosis: chronic schizophrenia (CCMD‐3). N = 50. Sex: male and female. Age: 20 to 58 years. History: 5 to 18 years. |
| Interventions | 1. Aripiprazole: 5˜30 mg/day, n = 25*. 2. Risperidone: 0.5˜5 mg/day, n = 25. |
| Outcomes | Global state: curative effect. Mental state: PANSS score. Adverse events*. |
| Notes | N number in various outcomes are inconsistent. We, therefore, decided not to include this trial, until we acquire further clarification from authors. |
Zhao 2006a.
| Methods | Allocation: randomised (no further description). Blinding: not stated. Duration: 8 weeks. Setting: inpatients, China. |
| Participants | Diagnosis: first episode schizophrenia (CCMD‐3). N = 68. Sex: male and female. Age: 24.3 ± 9.4 years in aripiprazole group; 28 ± 10 years in quetiapine group. History: mean˜24 months, SD ˜14 months. |
| Interventions | 1. Aripiprazole: 10˜30 mg/day, n = 34*. 2. Quetiapine: 100˜750 mg/day, n = 34. |
| Outcomes | Global state: curative effect*. Mental state: PANSS score. Adverse events. |
| Notes | * reported N numbers and their corresponding percentage of the same outcome are inconsistent. We, therefore, decided not to include this trial, until we acquire further clarification from authors. |
Zheng XR 2008.
| Methods | Allocation: randomised (no further description). Blinding: not stated. Duration: 8 weeks. Setting: inpatients, China. |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 68. Sex: male and female. Age: 16 to 58 years. History: median˜ 5 months; range 1˜32 months. |
| Interventions | 1. Aripiprazole: 5˜30 mg/day, n = 30*. 2. Risperidone: 1˜5 mg/day, n = 34. |
| Outcomes | Global state: curative effect*. Adverse events*. |
| Notes | The total number of participants (n = 64) is inconsistent with the total number of people randomised (n = 68). We, therefore, decided not to include this trial, until we acquire further clarification from authors. |
陶建青, 2007.
| Methods | Allocation: unclear |
| Participants | Participants: schizophrenia |
| Interventions | Aripiprazole vs Risperidone |
| Outcomes | |
| Notes | No full‐text article. |
CCMD‐3: Chinese Classification of Mental Disorders third version PANSS: Positive And Negative Symptom Scale vs: versus
Characteristics of ongoing studies [ordered by study ID]
Bristol‐Myers 2009.
| Trial name or title | NCT 00857818 |
| Methods | Allocation: random. Blindness: open label. Duration: 16 weeks. Design: parallel. Location: not reported. |
| Participants | Diagnosis: schizophrenia, bipolar 1 disorder. Age 18‐65 years. Gender: both. History: duration of illness not reported. Setting: not reported |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Risperidone. 4. Quetiapine |
| Outcomes | Long‐time effectiveness and tolerability. Global state: CGI, PG‐I. Quality of life: IWQoL ‐Lite. Percent change in fasting lipids from baseline. Change in fasting glucose, levels weight, BMI. Change in fasting non‐ HDL Cholesterol level from baseline. |
| Starting date | April 2009. |
| Contact information | Bristol‐Myers Squibb. |
| Notes | Not open for participant recruitment. |
Eli Lilly 2003.
| Trial name or title | Trial 8047 F1D‐MC‐HGLB |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 28 weeks. Design: parallel. Location: not reported. |
| Participants | Diagnosis: schizophrenia. Age 18‐65 years. Gender: not reported. History: duration of illness not reported. Setting: in‐ and outpatient. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. |
| Outcomes | Long‐time effectiveness and tolerability. Global state: CGI, PG‐I. General Mental State: PANSS. Depression: MADRS. Quality of life: SWN‐S, SF‐36. Cognitive functioning: MOS. Sexual functioning: GISF. Health resource utilisation and resource utilisation costs, hospitalisation time. Treatment‐emergent adverse events: EPS (SAS, BAS, AIMS). Laboratory values. Vital signs. |
| Starting date | October 2003. |
| Contact information | Eli Lilly and Company. |
| Notes |
Essock 2002.
| Trial name or title | NCT 00044655 |
| Methods | Allocation: random, no further details Blindness: single, no further details. Duration: unclear. Design: parallel. Location: multiple. |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder or schizophreniform disorder. Age 18‐65 years. Gender: male/female participants. History: duration of illness not reported. Setting: outpatient. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Risperidone. 4. Quetiapine. 5. Ziprasidone. |
| Outcomes | Efficacy, safety, adverse effects. |
| Starting date | July 2001. |
| Contact information | NIMH. United States Federal Government |
| Notes |
Glorioso 2005.
| Trial name or title | NCT 00245206 ID: R01MH71536 |
| Methods | Allocation: random, no further details Blindness: open label. Duration: 5 years Design: parallel. Location: unclear. |
| Participants | Diagnosis: schizophrenia. Age ≥ 45 years. Gender: male/female participants. History: duration of illness not reported. Setting: not reported. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Risperidone. 4. Quetiapine. |
| Outcomes | Metabolic, cardiovascular and cerebrovascular effects. |
| Starting date | August 2005. |
| Contact information | University of California |
| Notes |
Hanssens 2007.
| Trial name or title | NCT 00508157 CN 138‐489 |
| Methods | Allocation: random, no further details Blindness: open label. Duration: 16 weeks. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age 18‐65 years. Gender: male/female participants. History: duration of illness not reported. Setting: not reported. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Risperidone. 4. Quetiapine. |
| Outcomes | Change from baseline in fasting non‐ HDL cholesterol. |
| Starting date | July 2007. |
| Contact information | Bristol ‐Myers Squibb. |
| Notes |
Johnson 2006.
| Trial name or title | Study ID‐ CR006121 NCT‐ 00299702 |
| Methods | Allocation: random. Blindness: open label. Duration: 2 years. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age 18‐65 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Risperidal Consta. |
| Outcomes | Time to relapse, long‐time effectiveness and tolerability. |
| Starting date | February 2006. |
| Contact information | Johnson and Johnson. |
| Notes |
Lehrer 2008.
| Trial name or title | NCT‐ 00712270 |
| Methods | Allocation: random. Blindness: double blind. Duration: 16 weeks. Design: parallel. |
| Participants | Diagnosis: schizophrenia. Age 18‐55 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Risperidone. |
| Outcomes | Predict differential antipsychotic drug treatment response using PET and MRI. |
| Starting date | April 2005 |
| Contact information | Kettring Health Network |
| Notes |
Lin 2009.
| Trial name or title | NCT‐ 00956189 |
| Methods | Allocation: random. Blindness: single blind. Duration: 1 year. Design: parallel. |
| Participants | Diagnosis: schizophrenia. Age 18‐65 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Amisulpiride. |
| Outcomes | Metabolic profile and clinical efficacy. |
| Starting date | August 2009. |
| Contact information | National Taiwan University Hospital. |
| Notes |
Livingston 2007.
| Trial name or title | NCT 00423878 |
| Methods | Allocation: random. Blindness: single blind. Duration: 6 months. Design: parallel. Location: unclear. |
| Participants | Diagnosis: schizophrenia. Age: 16‐65 years. Gender: male/female participants. History: duration of illness not reported. Setting: not reported. |
| Interventions | 1. Aripiprazole. 2. Risperidone. 3. Quetiapine. 4. Olanzapine. |
| Outcomes | Metabolic effects, adverse events, clinical efficacy and tolerability. |
| Starting date | January 2007. |
| Contact information | |
| Notes |
McCormack 2006.
| Trial name or title | NCT ‐ 00320671 |
| Methods | Allocation: random. Blindness: double blind. Duration: 12 weeks. Design: parallel. Location: unclear. |
| Participants | Diagnosis: schizophrenia. Age: 16‐40 years. Gender: male/female participants. History: duration of illness not reported. Setting: not reported. |
| Interventions | 1. Aripiprazole. 2. Risperidone. |
| Outcomes | Metabolic effects, adverse events, substance use. |
| Starting date | 2005 ‐ December. |
| Contact information | United States Health Authority |
| Notes |
McEvoy 2007.
| Trial name or title | NCT00466310 |
| Methods | Allocation: random. Blindness: open label. Duration: 4 weeks. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age 18‐60 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Risperidone. |
| Outcomes | Metabolic adverse effects. |
| Starting date | February 2007 |
| Contact information | Duke University. |
| Notes |
Montoya 2006.
| Trial name or title | NCT00330863 |
| Methods | Allocation: random. Blindness: open label. Duration: 30 months. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age 18‐65 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Risperidone. 3. Quetiapine. 4. Ziprasidone. 5. Olanzapine. 6. Risperidone microspheres. |
| Outcomes | Time to relapse, long‐time effectiveness and tolerability. |
| Starting date | May 2006 |
| Contact information | United States of America |
| Notes |
Schweiger 2005.
| Trial name or title | NCT‐00205660 |
| Methods | Allocation: random. Blindness: open label. Duration: 12 weeks. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age: 18‐60 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Quetiapine. 4. Risperidone. 5. Ziprasidone. |
| Outcomes | Metabolic adverse effects. Long‐time effectiveness, safety and tolerability. |
| Starting date | February 2005. |
| Contact information | Bristol‐Myers Squibb |
| Notes |
Stroup 2007.
| Trial name or title | NCT‐00423878 |
| Methods | Allocation: random. Blindness: single blind. Duration: 6 months. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age: 18‐65 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Quetiapine. 4. Risperidone. |
| Outcomes | Changes in cholesterol level. Long‐time effectiveness, safety and tolerability. |
| Starting date | January 2007. |
| Contact information | Duke University , U. S. A. |
| Notes |
Swartz 2008b.
| Trial name or title | NCT‐ 00802100 |
| Methods | Allocation: random. Blindness: single blind. Duration: 28‐ 30 weeks. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: schizophrenia. Age: 18‐40 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. 3. Perphenazine. |
| Outcomes | Long‐time effectiveness, safety and tolerability, use other medication to limit side effects. |
| Starting date | December 2008. |
| Contact information | Duke University , U. S. A. |
| Notes |
Vontur 2005.
| Trial name or title | NCT00223418 |
| Methods | Allocation: random. Blindness: open label. Duration: unspecified. Design: parallel. |
| Participants | Diagnosis: schizophrenia. Age 18‐52 years. Gender: male and female participants. History: duration of illness not reported. Setting: not specified. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. |
| Outcomes | Improvement in cognitive functions, long‐time effectiveness and tolerability, social and occupational functioning. |
| Starting date | September 2005. |
| Contact information | United States of America. |
| Notes |
Global rating scales CGI: Clinical Global Impressions PGI‐I: Patient’s Global Impressions of Improvement Mental state: MADRS: Montgomery‐Asberg Depression Rating Scale PANSS: Positive and Negative Syndrome Scale
Depression: MADRS: Montgomery–Åsberg Depression Rating Scale
Sexual functioning: GISF: Global Impressions of Sexual Function
Side effects: AIMS: Abnormal Involuntary Movement Scale BAS: Barnes Akathisia Scale SAS: Simpson‐Angus Index ‐ for neurological side effects
Quality of Life: IWQoL‐Lite: Impact of Wieght on Quality of Life SF‐36: Short form 36 SWN‐S: subjective well‐being Other BMI: body mass index EPS: extrapyramidal symptoms HDL:high‐density lipoprotein MOS: Medical Outcomes Study MRI: magnetic resonance imaging PET: positron emission tomography
Differences between protocol and review
The review was slightly adapted to new formatting and functions available in Review Manager 5, notably the 'Risk of bias' tables. In the first version of this review, we planned to undertake a sensitivity analysis and exclude studies with 'inappropriate comparator doses'; however, this was not defined. In the current review, we have defined this in the Sensitivity analysis section.
Contributions of authors
Tao Suo ‐ study selection (2012 update), data extraction, report writing. Priya Khanna ‐ study selection (2011 update), data extraction, report writing. Katja Komossa ‐ protocol development, searching, study selection, data extraction, report writing. Huai‐xing Ma ‐ study selection (2012 update), data extraction, report writing. Stefan Leucht ‐ protocol development, searching, study selection, data extraction, report writing. Hany George El Sayeh ‐ protocol development, 2011 update ‐ help with write up. Jun Xia ‐ help with study selection (2012 update), data extraction, report writing.
Sources of support
Internal sources
Psychiatrische Klinik, Klinikum rechts der Isar, TU Mü nchen, Freistaat Bayern, Germany.
Nottingham Healthcare NHS Trust, UK.
University of Nottingham, UK.
Cochrane Schizophrenia Group, UK.
External sources
Bundesministerium für Bildung und Forschung, Nr FKZ: 01KG 0606, GZ: GF‐GFKG01100506, Germany.
-
National Institute of Health Research, UK.
This project is supported by the NIHR’s 2012 Cochrane Review Incentive Scheme.
Declarations of interest
Tao Suo ‐ none known. Priya Khanna ‐ none known. Katja Komossa ‐ none known. Huai‐xing Ma ‐ none know.* Stefan Leucht ‐ has received honoraria for lectures from Abbvie, Astra Zeneca, BristolMyersSquibb, ICON, EliLilly, Janssen, Johnson & Johnson, Roche, SanofiAventis, Lundbeck and Pfizer; honoraria for consulting/advisory boards from Roche, EliLilly, Medavante, BristolMyersSquibb, Alkermes, Janssen, Johnson & Johnson and Lundbeck. EliLilly has provided medication for a study with Stefan Leucht as primary investigator. Christine Rummel ‐ has received lecture honoraria and travel grants to attend scientific meetings from AstraZenca, JanssenCilag, EliLilly and Pfizer. Heike Hunger ‐ none known. Sandra Schwarz ‐ none known. Hany George El‐Sayeh ‐ none known. Jun Xia ‐ none known.*
* no conflict of interest but authors work for a professional review company that received an incentive to complete this update (see also Sources of support).
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
An 2008 {published data only}
- Ai C‐Q, Chen S‐M. Comparison study in the treatment of schizophrenia between aripiprazole and clozapine [阿立哌唑与氯氮平治疗精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2008;18(1):53‐4. [Google Scholar]
Bai 2007 {published data only}
- Bai S‐X, Yan L, Ding X‐M. Comparative study on the efficacy of aripiprazole and clozapine in treatment of schizophrenia [阿立哌唑和氯氮平对首发精神分裂症患者疗效及对心电图影响的对照研究]. 中国临床实用医学 2007;1(4):39‐40. [Google Scholar]
Bai 2009 {published data only}
- 白剑文. ziprasidone and aripiprazole in the treatment of schizophrenia] Google Translate [齐拉西酮与阿立哌唑治疗精神分裂症对照研究 []. 青岛医药卫生 2009; Vol. 41, issue 3:199‐200.
Ban 2008 {published data only}
- Ban N, Cui J‐B. Analysis of the efficacy of aripiprazole and quetiapine in the treatment of schizophrenia [阿立哌唑与奎硫平治疗精神分裂症的疗效分析]. Occupation and Health [职业与健康] 2008;24(3):292‐3. [Google Scholar]
Chan 2007 {published data only}
- Chan HY, Lin WW, Lin SK, Hwang TJ, Su TP, Chiang SC, et al. Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: A randomized trial. Journal of Clinical Psychiatry 2007;68(1):29‐36. [DOI] [PubMed] [Google Scholar]
- Hwang TJ, Chan HY, Lin WW, Lin SK, Hwu HG, Cheng MY, et al. Aripiprazole versus risperidone in the treatment of acutely relapsed patients with schizophrenia in Taiwan: a randomized controlled trial. Journal of the European College of Neuropsychopharmacology 2005;14(Suppl 3):S497. [Google Scholar]
Chang 2007 {published data only}
- Chang S. A control study of aripiprazole vs. risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007;13(4):335‐6. [Google Scholar]
Chen 2006 {published data only}
- Chen H, Liu Z, Gong J, Chen H, Hu D, Wang D. Comparative study of therapeutic effects between aripiprazole and risperidone on schizophrenia [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Shanghai Medical and Pharmaceutical Journal [上海医药] 2006;27(9):404‐6. [Google Scholar]
Chen 2007a {published data only}
- 陈飞, 王红艳, 李德咏, 周保卫, 程夫英. Aripiprazole, quetiapine treatment of female episode schizophrenia [阿立哌唑、喹硫平治疗女性首发精神分裂症对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2007; Vol. 20, issue 6:381‐3.
Chen 2008a {published data only}
- 陈震雷, 黄艳. Aripiprazole and risperidone in the treatment of female schizophrenia [阿立哌唑与利培酮治疗女性精神分裂症的对照研究]. Modern Research of Integrated Chinese and Western Medicine [现代中西医结合杂志] 2008;17(35):5454‐5. [Google Scholar]
Chen 2009 {published data only}
- 陈红英, 张郦, 付迎春, 季海峰, 费玥, 黄雅南. Aripiprazole and quetiapine in the treatment of schizophrenia] Google Translate [阿立哌唑和喹硫平治疗精神分裂症的对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2009;22(1):53‐4. [Google Scholar]
Chen 2009a {published data only}
- 陈远岭, 黄文武, 叶鑫武. Comparative study between aripiprazole and olanzapine in the treatment of agitated schizophrenia [阿立哌唑治疗伴激越的精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(4):261‐2. [Google Scholar]
Chen 2010 {published data only}
- 陈平勋, 罗建慈, 刘桃芳. Aripiprazole and risperidone in the treatment of schizophrenia in the clinical control study] Google Translate [阿立哌唑与利培酮治疗精神分裂症的临床对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010; Vol. 22, issue 17:2249‐50.
Chen 2010a {published data only}
- 陈恩民. Aripiprazole and risperidone in the treatment of schizophrenia starting] Google Translate [阿立哌唑与利培酮治疗首发精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(17):2180‐4. [Google Scholar]
Cheng 2009 {published data only}
- 程平, 王天龙, 陈红光, 唐鹏, 周桂明. Ziprasidone and Aripiprazole in the treatment of schizophrenia with predominantly negative symptoms [齐拉西酮与阿立呱唑治疗以阴性症状为主的精神分裂症对照研究]. Journal of the Xianning Medical College [新乡医学院学报] 2009;23(2):106‐8. [Google Scholar]
CuiMeng 2008 {published data only}
- CuiMeng. Compare the aripiprazole with risperidone to treat schizophrenia disease [利培酮与阿立哌唑治疗120例精神分裂症效果对比分析]. Journal of the Mudanjiang Medical College 2008;29(3):20‐2. [Google Scholar]
Dai 2005 {published data only}
- Dai J‐P, Zhao Z‐H, Liu G‐X. Comparison of efficacy and safety of aripiprazole and quetiapine in the treatment of schizophrenia [阿立哌唑與奎硫平治療精神分裂癥療效和安全性比較]. Chinese Journal of Behavioral Medical Science [中国行为医学科学] 2005;14(8):712‐4. [Google Scholar]
Dai 2006 {published data only}
- Dai T. A comparison of schizophrenia with aripiprazole and risperidone [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Journal of the Datong Medical College 2006;26(1):14‐5. [Google Scholar]
Deng 2008 {published data only}
- 邓克文, 张永福, 张海风. Effects were compared aripiprazole in the treatment of elderly schizophrenia [阿立哌唑治疗老年精神分裂症的疗效对比观察]. Sichuan Mental Health [四川精神卫生] 2008;21(1):28‐9. [Google Scholar]
Deng 2008a {published data only}
- 邓华, 赵梅秀. Control study of domestic aripiprazole and risperidone in the treatment of schizophrenia [国产阿立哌唑与利培酮治疗精神分裂症的对照研究]. Journal of the Youjiang Medical College for Nationalities 2008;5:755‐6. [Google Scholar]
Ding 2007 {published data only}
- 丁永红, 周庆海, 邓金英. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. 中国现代医生 2007; Vol. 45, issue 17:87.
Du 2006 {published data only}
- Du B, Zhao S‐M, Tian G‐Q. Study on effects of aripiprazole on serum prolactin level and body weight [阿立哌唑对精神分裂症患者血清催乳素及体重的影响]. Chinese General Practice [中国全科医学] 2006;9(13):1065‐6. [Google Scholar]
Fan 2005 {published data only}
- Fan W‐L, Yu C‐M, Wen J‐S. Efficacy analysis of aripiprazole and clozapine in the treatment of schizophrenia. Journal of North Sichuan Medical College 2005;20(4):377‐9. [Google Scholar]
Fan 2010 {published data only}
- 范文澜, 余成民, 李正发. Aripiprazole and risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑与利培酮治疗精神分裂症的对照研究 []. 西部医学 2010; Vol. 22, issue 6:1051‐2.
Feng 2006 {published data only}
- Feng Y, Yan X, Zhao Z. The clinical study on aripiprazole and risperidone in trea tment of first‐onset schizophrenia [阿立哌唑与利培酮治疗首发精神分裂症患者疗效的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(4):418‐9. [Google Scholar]
Fleischhacker 2008 {published data only}
- BMS. A multicenter, double‐blind, randomized, comparative study of aripiprazole and olanzapine in the treatment of patients with acute schizophrenia. Clinical Study Report CN138003 2005.
Fu 2009 {published data only}
- 傅深省, 朱菇苓, 郭文勇, 刘一呜, 乔胜宇. Comparative study of Aripiprazole with risperidone in the treatment of adolescent patients with first episode schizophrenia [阿立哌唑与利培酮治疗青少年首发精神分裂症对照研究]. Journal of Qiqihar Medical College [齐齐哈尔医学院学报] 2009;30(8):905‐6. [Google Scholar]
Ge 2009 {published data only}
- 袁纯玲. Quetiapine and aripiprazole in the treatment control study of first‐episode schizophrenia] Google Translate [喹硫平和阿立哌唑治疗首发精神分裂症对照研究]. 中国现代药物应用 2009; Vol. 2, issue 3:71.
Ge 2010 {published data only}
- 葛淑君, 王春艳, 朱丹. Aripiprazole and quetiapine in the treatment of women with schizophrenia] Google Translate [阿立哌唑与喹硫平治疗女性精神分裂症对照研究 []. Chinese General Practice [中国全科医学] 2010;13(5):46‐7. [Google Scholar]
Guo 2006 {published data only}
- Guo X‐F, Chen J‐D, Zhao J‐P. A randomized and double‐blind controlled clinical trial of aripiprazole in the treatment of schizophrenia [阿立哌唑治療精神分裂癥的隨機雙盲對照試驗]. Journal of Clinical Research 2006;23(5):716‐8. [Google Scholar]
Han 2005 {published data only}
- Han P, Zhang Y‐H, Zhang C‐Z. Quality of life in schizophrenics treated with aripiprazole or clozapine. Chinese Journal of Rehabilitation Theory and Practice 2006;12(12):995‐6. [Google Scholar]
- Han P, Zhang Y‐H, Zhang Y‐Q. A controlled study of schizophrenia treated with aripiprazole and clozapine. Chinese Journal of Rehabilitation Theory and Practice [中国康复理论与实践] 2005;11(10):853‐4. [Google Scholar]
Han 2007 {published data only}
- Han Y, Li Y, Ren C. Clinical effect of the domestic olanzapine and aripiprazole in the treatment of schizophrenia [国产奥氮平与阿立哌唑治疗精神分裂症的临床研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2007;15(1):28‐9. [Google Scholar]
Han 2007a {published data only}
- 韩雪峰, 范连梅. Aripiprazole and risperidone in the treatment of clinical studies of schizophrenia [阿立哌唑与维思通治疗精神分裂症的临床研究]. Journal of Practical Medical Techniques [实用医技杂志] 2007; Vol. 14, issue 31:4298‐9.
Hu 2010 {published data only}
- 胡亚湘, 崔炳喜, 于浚玫, 崔鹏, 秦颖. Aripiprazole and risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Tianjin Pharmacy 2010;22(1):41‐3. [Google Scholar]
Huang 2009 {published data only}
- 黄河亮, 王桂香. A comparative study in earlier schizophrenic patients treated with aripiprazole and risperidone [阿立哌唑与利培酮治疗早期精神分裂症的疗效和安全性研究]. 中国医药指南 2009;7(16):15‐7. [Google Scholar]
Ji 2007 {published data only}
- Ji D‐C, Yu C‐X, Sun Y‐J, Huang M, Huang Q. A study of aripiprazole and risperidone in treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(4):261‐2. [Google Scholar]
Jiang 2009 {published data only}
- Jiang G, Luo J. Aripiprazole and clozapine in treatment refractory schizophrenia randomized controlled study] Google Translate. Chongqing Medical Journal 2009;38(1):87‐8. [Google Scholar]
Jie 2008 {published data only}
- Jie R, Wang G. Aripiprazole and clozapine treatment of schizophrenia with predominantly negative symptoms. Sichuan Mental Health 2008;21(2):107‐9. [Google Scholar]
Kane 2009 {published data only}
- Ascher‐Svanum H, Stensland MD, Peng X, Faries DE, Stauffer VL, Osuntokun OO, et al. Cost‐effectiveness of olanzapine vs. aripiprazole in the treatment of schizophrenia. Current Medical Research and Opinion 2011;27(1):115‐22. [DOI] [PubMed] [Google Scholar]
- Kane JM, Osuntokun O, Kryzhanovskaya LA, Xu W, Stauffer V, Watson SB. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. Biological Psychiatry 2008;63(7 Suppl):289S. [DOI] [PubMed] [Google Scholar]
- Kane JM, Osuntokun O, Kryzhanovskaya LA, Xu W, Stauffer V, Watson SB. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. Proceedings of the 63rd Annual Convention of the Society of Biological Psychiatry. 2008. [DOI] [PubMed]
- Kane JM, Osuntokun O, Kryzhanovskaya LA, Xu W, Stauffer VL, Watson SB, et al. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. Journal of Clinical Psychiatry 2009;70(4):572‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Osuntokun O, Kane JM, Kryzhanovskaya LA, Xu W, Stauffer V, Watson SB. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):153. [DOI] [PubMed] [Google Scholar]
- Watson SB, Kane JM, Osuntokun O, Kryzhanovskaya LA, Xu W, Stauffer V. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008. [DOI] [PubMed]
Kern 2006 {published data only}
- Kern RS, Green MF, Cornblatt BA, Owen JR, McQuade RD, Carson WH, et al. The neurocognitive effects of aripiprazole: an open‐label comparison with olanzapine. Psychopharmacology 2006;187(3):312‐20. [DOI] [PubMed] [Google Scholar]
Kerwin 2007 {published data only}
- Beuzen JN, L'Italien G, Hanssens L, Marcus RN, McQuade RD, Carson WH. Changes in prolactin levels and arizona sexual experience scale (ASEX) scores among patients switched from risperidone to aripiprazole in the schizophrenia trial of aripiprazole (STAR) study. Schizophrenia Bulletin 2007;33(2):421‐2. [Google Scholar]
- Blonde L, Kan HJ, Gutterman EM, L'Italien GJ, Kim MS, Hanssens L, et al. Predicted risk of diabetes and coronary heart disease in patients with schizophrenia: aripiprazole versus standard of care. Journal of Clinical Psychiatry 2008;69(5):741‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hanssens L, L'Italien G, Marcus RN, McQuade RD, Carson WH, Beuzen JN. Reasons for switching from previous therapy and preference of medication in the large, randomized, naturalistic schizophrenia trial of aripiprazole (STAR). Schizophrenia Bulletin 2007;33(2):432. [Google Scholar]
- Hanssens L, L’ Italien G, McQuade R, Iwamoto T, Pans M. Evaluation of quality of life in community‐treated schizophrenic patients: a naturalistic, open‐label study comparing aripiprazole to standard‐of‐care (Schizophrenia Trial of aripiprazole: STAR study). European Psychiatry 2006;21(Suppl 1):S99. [Google Scholar]
- Kerwin R, Millet B, Herman E, Banki CM, Lublin H, Pans M, et al. A multi centre, randomized, naturalistic, open‐label study between aripiprazole and standard of care in the management of community‐treated schizophrenic patients Schizophrenia Trial of aripiprazole: (STAR) study. European Psychiatry 2007;22(7):433‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kerwin R, Millet B, Herman E, Banki CM, Lublin H, Pans M, et al. A multi centre, randomized, naturalistic, open‐label study between aripiprazole and standard of care in the management of community‐treated schizophrenic patients. Schizophrenic Trial of aripiprazole: (STAR) study. Wiadomosci Psychiatryczne 2008;11(2):105‐19. [DOI] [PubMed] [Google Scholar]
- King D, Knapp M, Thomas P, Razzouk D, Loze JY, Kan HJ, et al. Cost‐effectiveness analysis of aripiprazole vs standard‐of‐care in the management of community‐treated patients with schizophrenia: Star study. Current Medical Research and Opinion 2011;27(2):365‐74. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, Corey‐Lisle PK, Crosby RD, Kan HJ, McQuade RD. Changes in weight and weight‐related quality of life in a multi centre, randomized trial of aripiprazole versus standard of care. European Psychiatry 2008;23(8):561‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- L'Italien G, Hanssens L, Kan HJ, Baker RA, Marcus RN, McQuade RD, et al. Metabolic effects of aripiprazole versus olanzapine, quetiapine, or risperidone (the STAR trial). Schizophrenia Bulletin 2007;33(2):439. [Google Scholar]
- L'Italien GJ, Blonde L, Hanssens L, McQuade R. Prevalence of diabetes/cardiovascular risk factors among schizophrenia patients treated with atypical antipsychotics in a multicenter, randomized, naturalistic, open‐label study (Schizophrenia Trial of aripiprazole: STAR study). Diabetes 2006;55(Suppl 1):A538. [Google Scholar]
- L'ltalien GJ, Hanssens L, Marcus RN, McQuade RD. Evidence of antipsychotic treatment selection based on metabolic risk factors among community‐based schizophrenia patients (The STAR Trial). Proceedings of the 160th Annual Meeting of the American Psychiatric Association; 2007 May 19‐24; San Diego, CA 2007.
- Millet B. A multicenter, randomized, naturalistic, open‐label study between aripiprazole and standard of care in the management of community‐treated schizophrenic patients (Schizophrenia Trial of aripiprazole ‐ STAR). http://www.clinicaltrials.gov 2005. [DOI] [PubMed]
- Taylor D, Hanssens L, Loze J‐Y, Pans M, L'Italien G, Marcus RN. Preference of medicine and patient‐reported quality of life in community‐treated schizophrenic patients receiving aripiprazole vs standard of care: results from the STAR study. European Psychiatry 2008;23(5):336‐43. [DOI] [PubMed] [Google Scholar]
Kinon 2004 {published data only}
- Eli Lilly, Company. Olanzapine versus aripiprazole in the treatment of acutely ill patients with schizophrenia. Eli Lilly and Company Clinical Trial Registry 2004.
- Kinon BJ, Stauffer VL, Kollack‐Walker S, Chen L, Sniadecki J. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. Journal of Clinical Psychopharmacology 2008;28(6):601‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stauffer V, Kinon B, Kollack‐Walker S, Chen L, Sniadecki J. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008. [DOI] [PubMed]
Kuang 2006 {published data only}
- Kuang L‐P. A controlled study on quality of life of schizophrenia treated with aripiprazole and clozapine [阿立哌唑与氯氮平对精神分裂症患者生活质量影响的对照研究]. Medical Journal of Chinese Civil Administration[中国民康医学] 2006;18(9):690‐1. [Google Scholar]
- Kuang L‐P, Xue Z‐M. A controlled study on compliance of schizophrenia treated with aripirazole and clozapine [阿立哌唑与氯氮平对精神分裂症患者治疗依从性的对照研究]. Chinese Journal of Clinical Psychology[中国临床心理学杂志] 2006;14(4):426‐7. [Google Scholar]
Li 2006 {published data only}
- Li G‐R, Lu P‐X, Li Y‐D. A comparative study of aripiprazole and risperidone in the treatment of first‐episode schizophrenia [阿立哌唑与利培酮治疗首发精神分裂症的对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2006;19(3):196‐7. [Google Scholar]
Li 2006a {published data only}
- Li L‐X, Lian H‐T. A comparative study of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2006; Vol. 18, issue 9:723‐4.
Li 2007 {published data only}
- Li M, Wu N, Li Y‐F. Comparative study of influence of aripiprazole on life quality in schizophrenic patients. Chinese Journal of Health Psychology 2007;15(10):956‐7.[MEDLINE:]. [Google Scholar]
Li 2007a {published data only}
- Li Q, Li G, Li G. Effects of aripiprazole and clozapine on life quality of schizophrenics. Journal of Clinical Psychosomatic Diseases 2007;13(5):404‐6.[MEDLINE: ]. [Google Scholar]
- 陈玲, 王俊超. Aripiprazole and clozapine on quality of life of patients with schizophrenia] Google Translate [阿立哌唑与氯氮平对精神分裂症患者生活质量的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(15):1939‐41. [Google Scholar]
Li 2007b {published data only}
- 李美花, 吕伟, 邢秀娟. Aripiprazole and quetiapine in the treatment of schizophrenia [阿立哌唑和喹硫平治疗精神分裂症的对照研究]. 中国医师进修杂志 2007;30(11A):49‐50. [Google Scholar]
Li 2007c {published data only}
- Li H. Comparison of efficacy and safety of aripiprazole and ziprasidone in the treatment of schizophrenia [阿立哌唑和齐拉西酮治疗精神分裂症的临床比较]. International Medicine and Health Guidance News [国际医药卫生导报] 2007;13(11):77‐9. [Google Scholar]
Li 2007d {published data only}
- Li C, Deng L, Feng W. Control studies of domestic aripiprazole vs. risperidone in the treatment of schizophrenia [国产阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007;13(2):100‐2. [Google Scholar]
Li 2009 {published data only}
- 李晓英, 刘琦. Aripiprazole and clozapine treatment of schizophrenia control study of efficacy and safety] Google Translate [阿立哌唑与氯氮平治疗精神分裂症疗效与安全性对照研究]. 甘肃科技 2009;25(16):135‐41. [Google Scholar]
Li 2009a {published data only}
- 李代秀, 陈波, 丁静华. Aripiprazole and risperidone in the treatment of schizophrenia compared] Google Translate [阿立哌唑与利培酮治疗精神分裂症的疗效对比]. Baotou Medical Journal 2009; Vol. 33, issue 1:13‐4.
Li 2009b {published data only}
- 李晓华. Aripiprazole and risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑与利培酮治疗精神分裂症的对照研究]. 中国实用医药 2009;4(18):166‐7. [Google Scholar]
Lian 2008 {published data only}
- 连树奎, 林德颖. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. 中国现代医生 2008; Vol. 46, issue 14:91‐2.
Liang 2008 {published data only}
- 梁佳, 陶建青, 康红. Compliance control study of aripiprazole and risperidone in the treatment of patients with schizophrenia [阿立哌唑与利培酮治疗精神分裂症患者依从性的对照研究]. Guangxi Medical Journal 2008; Vol. 30, issue 1:30‐1.
Liu 2006 {published data only}
- Liu S, Ren C, Song X. Comparative study between aripiprazole and clozapine in the treatment of first‐episode schizophrenia [阿立哌唑与氯氮平治疗首发精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006; Vol. 14, issue 6:658‐9.
Liu 2006a {published data only}
- Liu W, Yang K, Zuo X. A control study on aripiprazole and risperidone in the treatment of schizophrenia [阿立派唑与利培酮治疗精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(4):420‐1. [Google Scholar]
Liu 2007 {published data only}
- Liu B. A control study on aripiprazole vs. clozapine in the treatment of refractory schizophrenia [阿立哌唑与氯氮平治疗难治性精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007;13(6):500‐1, 507. [Google Scholar]
Liu 2008 {published data only}
- Liu J, Ren J, Wang Y. Aripiprazole and clozapine treatment episode schizophrenia. 中国实用医药 2008; Vol. 3, issue 4:55‐7.
Liu 2008a {published data only}
- Liu W, Zhang X. A comparative study of ziprasidone and aripiprazole in the treatment of schizophrenia with predominantly negative symptoms [齐拉西酮与阿立哌唑治疗以阴性症状为主的精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2008; Vol. 16, issue 2:124‐5.
Liu 2008b {published data only}
- 刘从敏. Aripiprazole for schizophrenia patients with psychiatric symptoms and quality of life impact [阿立哌唑对精神分裂症患者的精神症状及生活质量的影响研究]. 中国医药导报 2008;5(19):110‐1. [Google Scholar]
Liu 2008c {published data only}
- 刘伟锋, 张卫敏. Aripiprazole and risperidone in the treatment of refractory schizophrenia Efficacy [阿立哌唑与利培酮治疗难治性精神分裂症疗效观察]. Medical Journal of Chinese People's Health [中国民康医学] 2008; Vol. 20, issue 3:224‐5.
Liu 2008d {published data only}
- 刘峥嵘, 郗晓明, 李丽颖. Aripiprazole and risperidone in the treatment episode schizophrenia [阿立哌唑和利培酮治疗首发精神分裂症对照研究]. Chinese Medical Journal of Metallurgical Industry [中国冶金工业医学杂志] 2008;25(3):276‐7. [Google Scholar]
Liu 2009 {published data only}
- 刘娟, 李洪英. Aripiprazole and olanzapine schizophrenia] Google Translate [阿立哌唑与奥氮平治疗精神分裂症的对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2009; Vol. 22, issue 5:339‐40.
Liu 2009a {published data only}
- 刘志东. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Medical Theory and Practice 2009; Vol. 22, issue 9:1072‐3.
Liu 2009b {published data only}
- 刘昊, 朱怀轩, 赵勤, 谭权. Aripiprazole and quetiapine in the treatment of schizophrenia] Google Translate [阿立哌唑与喹硫平治疗精神分裂症对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2009;22(1):42‐3. [Google Scholar]
Liu 2010 {published data only}
- 刘卉兰, 张香云, 栗克清, 桑文华, 张彦恒, 田桂平, et al. Clozapine, risperidone and aripiprazole adverse drug reactions cost ‐ effectiveness analysis] Google Translate [氯氮平、利培酮和阿立哌唑药物不良反应成本‐效果分析]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2010;18(6):646‐8. [Google Scholar]
Li X 2007 {published data only}
- 李曦, 王萍, 娄涛. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(12):1064, 1067. [Google Scholar]
Lou 2007 {published data only}
- Lou T, Li N, Wu X‐Y, Wang C‐S. Effect of aripiprazole on late‐onset schizophrenia and cognitive function [阿立哌唑治疗晚发精神分裂症的疗效观察及其对认知功能的影响]. Chinese New Drugs Journal [中国新药杂志] 2007;16(20):1709‐12. [Google Scholar]
Luo 2008 {published data only}
- Luo X, An B‐F. Comparison of quetiapine and aripiprazole in the treatment of first episode schizophrenia [奎硫平与阿立哌唑治疗首发精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2008;18(3):191‐2. [Google Scholar]
Luo 2009 {published data only}
- 罗来兴. Aripiprazole and risperidone in the treatment of schizophrenia efficacy analysis [阿立哌唑与利培酮治疗精神分裂症疗效分析]. 当代医学 2009;15(170):101‐2. [Google Scholar]
Lv 2007 {published data only}
- Lv T‐H, Jin C‐X. A comparative study on aripiprazole and risperidone in the treatment of schizophrenic patients [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(5):181‐2. [Google Scholar]
Ma 2009 {published data only}
- 麻显娇, 卢喜金, 邹晓华. Aripiprazole and olanzapine in negative symptoms in schizophrenia] Google Translate [阿立哌唑与奥氮平治疗以阴性症状为主的精神分裂症对照研究]. China Pharmaceuticals [中國藥業] 2009;18(23):59‐60. [Google Scholar]
Ma 2009a {published data only}
- 马忠智, 陈春武. Efficacy of aripiprazole and risperidone in the treatment of early schizophrenia [阿立哌唑与利培酮治疗早期精神分裂症的疗效观察]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2009;17(6):643‐4. [Google Scholar]
Mai 2005 {published data only}
- Mai G‐Y, Cai G‐H, Huang J‐M. Comparative study on the effect of aripiprazole and risperidone on schizophrenia. Chinese Journal of Rehabilitation [中國康復] 2005;20(3):184‐5. [Google Scholar]
Mao 2010 {published data only}
- 毛星, 刘洪秋, 陈玉辉. Olanzapine and aripiprazole in the treatment of refractory schizophrenia control study of 70 cases] Google Translate [奥氮平与阿立哌唑治疗难治性精神分裂症70例的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(11):1365‐6. [Google Scholar]
McQuade 2004 {published data only}
- Abou Gharbia N, McQuade R, Jody D, Kujawa M, Carson W, Iwamoto T, et al. Comparative study of the long‐term effects of aripiprazole and olanzapine treatment on body weight. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
- Blonde L, Ray S, Corey‐Lisle PK, Cislo PR, L'Italien G. The risk of new‐onset type 2 diabetes and coronary heart disease in chronic schizophrenic patients treated with aripiprazole and olanzapine. Journal of the European College of Neuropsychopharmacology 2004;14(Suppl 3):S275. [Google Scholar]
- Jody D, McQuade RD, Kujawa M, Carson W, Iwamoto T, Archibald D, et al. Long‐term weight effects of aripiprazole versus olanzapine. Schizophrenia Research 2004;67(1):187. [Google Scholar]
- McQuade RD, Jody D, Kujawa MJ, Carson WH, Iwamoto T, Archibald DG, et al. Long‐term weight effects of aripiprazole versus olanzapine. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
- McQuade RD, Kujawa M, Saha AR, Ingenito GG, Ali MW, Luo X, et al. Aripiprazole for long‐term maintenance treatment of schizophrenia. Schizophrenia Research 2003;60(1):295. [Google Scholar]
- McQuade RD, Stock E, Marcus R, Jody D, Gharbia NA, Vanveggel S, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double‐blind study. Journal of Clinical Psychiatry 2004;65(Suppl 18):47‐56. [MEDLINE: ] [PubMed] [Google Scholar]
- McQuade RD, Stock E, Marcus R, Jody D, Gharbia NA, Vanveggel S, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double‐blind study. Journal of Clinical Psychiatry 2004;65(Suppl 18):47‐56. [PubMed] [Google Scholar]
- Meyer JM, Rosenblatt LC, Kim E, Baker RA, Whitehead R. The moderating impact of ethnicity on metabolic outcomes during treatment with olanzapine and aripiprazole in patients with schizophrenia. Journal of Clinical Psychiatry 2009;70(3):318‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mu 2008 {published data only}
- 穆德军, 王龙锦, 王巍. Domestic aripiprazole and risperidone in the treatment of female schizophrenia [国产阿立哌唑和利培酮治疗女性精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(23):2781, 2783. [Google Scholar]
Mu 2010 {published data only}
- 沐志强, 杨顺英, 李弼. Aripiprazole and risperidone in the treatment of schizophrenia compared] Google Translate [阿立哌唑与利培酮治疗精神分裂症的疗效对比]. Medical Information 2010;3:641‐3. [Google Scholar]
Pan 2007 {published data only}
- Pan G. A controlled study of aripiprazole and risperdal in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2007;15(4):363‐4. [Google Scholar]
Peng 2007 {published data only}
- Peng X, Huang Y, Liu X‐Y. A clinical analysis of aripiprazole and quetiapine in treatment of first episode schizophrenia [阿立哌唑与奎硫平治疗首发精神分裂症对照分析]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(5):329‐30. [Google Scholar]
Peng 2007a {published data only}
- Peng Z‐Y, Mao W‐L. Comparative study between aripiprazole and clozapine in the treatment of schizophrenia [阿立哌唑与氯氮平治疗精神分裂症对照研究]. Journal of the Gannan Medical College [赣南医学院学报] 2007;27(2):237‐8. [Google Scholar]
Potkin 2003 {published data only}
- Potkin SG, Kujawa M, Carson WH, Saha AR, Ali M, Ingenito G. Aripiprazole and risperidone versus placebo in schizophrenia and schizoaffective disorder. Schizophrenia Research 2003;60:300. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Archives of General Psychiatry 2003;60(7):681‐90. [DOI] [PubMed] [Google Scholar]
Pu 2007 {published data only}
- Pu S‐B, Liang J‐X, Zhang W‐S. The comparison of blood prolactin and body index in the female patients with psychosis treated by aripiprazole and risperidone [阿立派唑与利培酮对女性患者血清催乳素及体型指数影响的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007; Vol. 19, issue 5:163‐4, 7.
Qian 2009 {published data only}
- 钱文胜. Aripiprazole and olanzapine treatment of female control study of patients with schizophrenia] Google Translate [阿立哌唑与奥氮平对女性精神分裂症患者治疗的对照研究]. 兵团医学 2009; Vol. 1, issue 19:28‐9.
Qin 2008 {published data only}
- 秦爱玲, 李敏, 胥爱萍. Aripiprazole and risperidone in the treatment of chronic schizophrenia [阿立哌唑与利培酮治疗慢性精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(4):284, 300. [Google Scholar]
Qu 2009 {published data only}
- 曲万仁. The comparison for effect of treating schizophrenia with aripiprazole and risperidone [阿立哌唑与利培酮治疗120例精神分裂症对比]. Chinese Journal of Ethnomedicine and Ethnopharmacy 2009; Vol. 6:33.
Shan 2008 {published data only}
- Shan H. A controlled study of aripiprazole vs. risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2008;14(2):119‐20, 133. [Google Scholar]
Shuai 2008 {published data only}
- Shuai H. Evaluating the effect and safety of aripirazole and risperidone [阿立哌唑与利培酮疗效及安全性评价]. 中国现代医药杂志 2008;10(2):35‐7. [Google Scholar]
Song 2008 {published data only}
- 宋艳萍. Comparative analysis of aripiprazole and risperidone in the treatment of female schizophrenia [阿立哌唑与利培酮治疗女性精神分裂症的对照分析]. Sichuan Mental Health [四川精神卫生] 2008; Vol. 21, issue 1:34‐5.
Song 2009 {published data only}
- 宋长惠. Aripiprazole Comparative Study of Risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑 利培酮治疗精神分裂症对比研究]. Journal of Medical Forum [医药论坛杂志] 2009;30(19):67‐8. [Google Scholar]
Song 2010 {published data only}
- 宋芳. Olanzapine and aripiprazole in the treatment of schizophrenia] Google Translate [奥氮平与阿立哌唑治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(8):970, 986. [Google Scholar]
Su 2007 {published data only}
- 栗小红. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2007; Vol. 20, issue 5:313‐4.
Su 2008 {published data only}
- Su Q, Huang H‐R, Gao A‐Q, Huang D. A comparative study of olanzapine and aripiprazole in treatment of schizophrenia [奥氮平与阿立哌唑治疗精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2008;18(6):409‐10. [Google Scholar]
Sun 2006 {published data only}
- Sun Q, Guo H, Liu Y. Effect of aripiprazole on coginiton function in patients with schizophrenic [奥派对精神分裂症患者认知功能的影响]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(4):424‐6. [Google Scholar]
Sun 2009 {published data only}
- 孙东, 江琴普, 李建锋, 李薇, 杨老虎. Control study on aripiprazole in the treatment of first‐episode schizophrenia [阿力哌唑治疗首发精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2009; Vol. 17, issue 7:774‐6.
Sun 2009a {published data only}
- 孙德生, 尹焕新. Quetiapine and aripiprazole in the treatment of schizophrenia] Google Translate [奎硫平与阿立哌唑治疗精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2009;17(12):1416‐7. [Google Scholar]
Tandon 2006 {published data only}
- Riera L, Hu R, Stock E, Nyilas M, Torbeyns A, Borian F, et al. Broad effectiveness trial with aripiprazole. Schizophrenia Research 2004;67(1):157. [DOI] [PubMed] [Google Scholar]
- Tandon R, Marcus RN, Stock EG, Riera LC, Kostic D, Pans M, et al. A prospective, multicenter, randomized, parallel‐group, open‐label study of aripiprazole in the management of patients with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With aripiprazole (BETA). Schizophrenia Research 2006;84(1):77‐89. [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang Q, Lai G, Zhang J. Aripiprazole and risperidone in the treatment of female schizophrenics [阿立哌唑和利培酮治疗女性精神分裂症的对照研究]. Shanghai Archives of Psychiatry [上海精神医学] [上海精神醫學] 2006;18(1):30‐2. [Google Scholar]
Tang 2007 {published data only}
- Tang C‐G, Wang R‐C. Comparison of efficacy and safety of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮的疗效和安全性比较]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(17):727‐8. [Google Scholar]
Tang 2010 {published data only}
- Tang Q. Aripiprazole and risperidone in the treatment efficacy of first‐episode schizophrenia] Google Translate [阿立哌唑和利培酮治疗首发精神分裂症的疗效观察]. Chinese Journal of Pharmaco Epidemiology [药物流行病学杂志] 2010; Vol. 19, issue 1:13‐4, 20.
Tang 2010a {published data only}
- 唐静, 陈铁宏. Aripiprazole and risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(17):2243‐4. [Google Scholar]
Tao 2008 {published data only}
- Tao S‐W, Chen Q, Yang C, Pan R‐D, Pan T‐W, Li L, et al. A comparison of the effects on lipid metabolic among four different atypical antipsychotic agents [4种非典型抗精神病药物对精神分裂症患者脂代谢的影响]. Journal of the Youjiang Medical College for Nationalities 2008;6:927‐30. [Google Scholar]
Tong 2007 {published data only}
- Tong J, Yang Z. Control study of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007;13(1):7‐10. [Google Scholar]
Tu 2009 {published data only}
- 涂国和, 胡有平, 周学儿. Aripiprazole (Booz Qing) and risperidone in the treatment of schizophrenia in the clinical control study] Google Translate [阿立哌唑(博思清)与利培酮治疗精神分裂症的临床对照研究]. Jiangxi Medical Journal [江西医药] 2009; Vol. 44, issue 8:799‐800.
Wang 2005 {published data only}
- Wang R, Liu Y, Ning Z. A controlled study of aripiprazole vs clozapine in the treatment of first‐episode schizophrenia. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2005;11(4):301‐2. [Google Scholar]
Wang 2006 {published data only}
- Wang G‐P, Xie R, Pei G‐X, Zhang Y‐L. Comparative study between aripiprazole and clozapine in the treatment of patients with schizophrenia [阿立哌唑的临床应用(一)——阿立哌唑与氯氮平治疗精神分裂症对照研究]. Journal of Clinical Psychiatry 2006;16(1):44‐5. [Google Scholar]
Wang 2006a {published data only}
- Wang Y, Sun F, Zhang Z. Observation of therapeutic effect of aripiprazole in the treatment schizophrenic patients [阿立哌唑治疗精神分裂症的疗效观察]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(4):427‐8. [Google Scholar]
Wang 2006b {published data only}
- Wang G‐P. Effect of aripiprazole on quality of life of schizophrenic patients [阿立哌唑对精神分裂症患者生活质量的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2006; Vol. 16, issue 6:360‐1.
Wang 2006c {published data only}
- Wang H, Wang Y, Lu L. A comparative study between aripiprazole and risperidone in the treatment of chronic schizophrenia [阿立哌唑与利培酮治疗慢性精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(6):650‐1. [Google Scholar]
Wang 2006d {published data only}
- Wang D, Li Y, Feng Y. A controlled study of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2006;12(4):245‐6. [Google Scholar]
Wang 2007 {published data only}
- Wang D. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(17):751. [Google Scholar]
Wang 2007a {published data only}
- Wang L‐Z, Weng J‐Z, Zhuo Z‐M. Comparative study on aripiprazole and risperidone in treatment of female schizophrenia [阿立哌唑与利培酮治疗女性精神分裂症对照研究]. 西部医学 2007; Vol. 19, issue 1:42‐3.
Wang 2007d {published data only}
- 王学滨, 刘宏. A force of aripiprazole and risperidone in the treatment of schizophrenia [阿力哌唑与利培酮治疗精神分裂症对照研究]. 中外健康文摘 [医药学刊] 2007;4(11):44. [Google Scholar]
Wang 2007e {published data only}
- 王国富. Aripiprazole in the treatment of schizophrenia clinical observation [阿立哌唑治疗精神分裂症临床观察]. Journal of the Changzhi Medical College 2007;21(2):110‐1. [Google Scholar]
Wang 2008 {published data only}
- Wang Y‐C, Wang J‐L, Yuan J‐M. A controlled study of effect on schizophrenia serum prolactin level aripiprazole [阿立哌唑对精神分裂症患者血清催乳素的影响]. 中国实用医药 2008;3(21):38‐9. [Google Scholar]
Wang 2008c {published data only}
- 王天龙, 程平, 陈红光, 唐鹏, 周桂明. Ziprasidone treatment of schizophrenia with predominantly negative symptoms [齐拉西酮治疗以阴性症状为主精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2008;18(3):206‐7. [Google Scholar]
Wang 2009 {published data only}
- 王茂萍. New antipsychotic drug aripiprazole on glucose metabolism and glycosylated hemoglobin [新型抗精神病药物阿立哌唑对糖代谢影响及与糖化血红蛋白的关系]. Hebei Medical Journal 2009; Vol. 31, issue 14:1752‐3.
Wei 2006 {published data only}
- Wei S‐Z, Tang Q‐S, Xu Z‐N, Ruan X‐J, Lu B. A randomized, double blind, double dummy parallel controlled study in the female first‐episode schizophrenia treated with aripiprazole and quetiapine [阿立哌唑与奎硫平治疗女性首发精神分裂症的随机、双盲、双模拟平行对照研究]. Chinese Journal of Nervous and Mental Diseases 2006;32(6):511‐7. [Google Scholar]
- 韩树华. Aripiprazole and quetiapine in the treatment of schizophrenia control study of women] Google Translate [阿立哌唑与奎硫平治疗女性首发精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010; Vol. 22, issue 5:511‐6.
Wei 2007 {published data only}
- 魏月玲, 韩鹏, 魏春香. Control study of aripiprazole and clozapine in schizophrenia affect the quality of life in patients with [阿立哌唑与氯氮平对精神分裂症患者生活质量影响的对照研究]. Sichuan Mental Health [四川精神卫生] 2007;20(2):80‐2. [Google Scholar]
Wei 2009 {published data only}
- 魏丽霞, 刘传军, 杨波, 崔宏维. Olanzapine A Leekpai azole treatment of schizophrenia [奥氮平与阿立派唑治疗精神分裂症对照研究]. 中国实用医药 2009;4(16):166‐7. [Google Scholar]
Wen 2009 {published data only}
- 文启琴, 黄远光, 马静山. aripiprazole and risperidone in the treatment of female controlled study of schizophrenia] Google Translate [阿立哌唑与利培酮治疗女性首发精神分裂症的对照观察]. Guangdong Medical Journal [广东医学] 2009;30(6):987‐8. [Google Scholar]
Wen 2009a {published data only}
- 文家松, 夏南, 胡晓丽. Comparison of aripiprazole and risperidone in the treatment of female schizophrenia [阿立哌唑与利培酮治疗女性精神分裂症的对照研究]. Journal of the North Sichuan Medical College 2009;24(4):342‐3. [Google Scholar]
Wlodzmierz 2006 {published data only}
- Sanchez R, Kostic D, Stock E, Torbeyns AF, Kerselaers W, Nyilas M, et al. Aripiprazole vs olanzapine in patients with acutely relapsing or chronic, stable schizophrenia: A 52‐week open‐label extension study. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia Research; 2006 Feb 4‐10; Davos, Switzerland. 2006.
- Wlodzimierz CK, Marcus RN, Torbeyns A, Nyilas M, McQuade RD. Effectiveness of long‐term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52‐week, open‐label comparison with olanzapine. Psychopharmacology 2006;189(2):259‐66. [DOI] [PubMed] [Google Scholar]
Wolf 2007 {published data only}
- Beuzen J‐N, Pans M, Modell S, Hagens P, McQuade R, Iwamato T, et al. Broad effectiveness trial with aripiprazole in Europe Eu‐Beta. Proceedings of the 13th Congress of the Association of European Psychiatrists; 2005 Apr 2‐6; Munich, Germany. 2005.
- Beuzen J‐N, Pans M, Modell S, Hagens P, McQuade R, Iwamoto T, et al. Naturalistic study of aripiprazole treatment. Proceedings of the XIII World Congress of Psychiatry; 2005 Sep 10‐15; Cairo, Egypt. 2005.
- Beuzen J‐N, Schirr K, Pans M, Hagens P, Kostic D, Carson W, et al. Effectiveness of aripiprazole in a naturalistic setting: a European multicenter study. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia Research; 2006 Feb 4‐10; Davos, Switzerland. 2006.
- Wolf J, Janssen F, Lublin H, Salokangas RKR, Allain H, Smeraldi E, et al. A prospective, multi centre, open‐label study of aripiprazole in the management of patients with schizophrenia in psychiatric practice in Europe: Broad Effectiveness Trial with aripiprazole in Europe (EU‐BETA). Current Medical Research and Opinion 2007;23(10):2313‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2008 {published data only}
- Wu S, Liu H‐Y, Huo X‐P. A comparative study of aripiprazole and risperidone in the treamtment of senile schizophrenia [阿立哌唑与利培酮治疗老年期精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(15):1723‐4. [Google Scholar]
Xiao 2007 {published data only}
- Xiao G‐C, Wang D‐P, Huang G‐P. Comparative study on the clinical effect and the life quality of schizophrenic patients treated with aripiprazol and clozepine [阿立哌唑与氯氮平治疗精神分裂症患者疗效及生活质量的对照研究]. 中国医疗前沿 2007; Vol. 1, issue 2:28‐9.
Xie 2008 {published data only}
- 谢新年, 舒人九, 钦桃桃. Aripiprazole and risperidone in the treatment of male schizophrenia efficacy and sexual function [阿立哌唑及利培酮治疗男性精神分裂症的疗效及对性功能的影响]. Hunan Medical Journal 2008; Vol. 25, issue 6:1111‐3.
Xie 2010 {published data only}
- 谢根生, 赵新蕾, 钱国平, 吴玉红, 胡庆芬. Aripiprazole and risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑与利培酮治疗精神分裂症对照研究]. 中国预防医学杂志 2010; Vol. 11, issue 2:190‐2.
Xu 2007 {published data only}
- 徐美勤. Aripiprazole and clozapine treatment of schizophrenia analysis [阿立哌唑与氯氮平治疗精神分裂症对照分析]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007; Vol. 13, issue 6:548‐9.
Xu 2010 {published data only}
- 徐筠, 李朝, 王慧芳, 李华芳. Aripiprazole and risperidone in the treatment of schizophrenia randomized double‐blind controlled study] Google Translate [阿立哌唑与利培酮治疗精神分裂症随机双盲对照研究 []. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2010; Vol. 20, issue 1:35‐7.
Yan 2007 {published data only}
- 阎美英, 刘方莹, 仇爱玫. Aripiprazole impact on the quality of life of patients with schizophrenia [阿立哌唑对精神分裂症病人生活质量影响的研究]. 家庭护士 2007;5(11C):15‐6. [Google Scholar]
Yan 2008 {published data only}
- 闫凤娟, 刘雪峰. Aripiprazole and risperidone in the treatment of children with schizophrenia [阿立哌唑与利培酮治疗儿童精神分裂症对照研究]. 中国医学文摘 [儿科学] 2008;27(6):465‐6. [Google Scholar]
Yan 2008a {published data only}
- 闫宝昌. Aripiprazole and clozapine treatment of refractory schizophrenia [阿立哌唑与氯氮平治疗难治性精神分裂症的对照观察]. Chinese Journal of Misdiagnostics [中国误诊学杂志] 2008;8(18):4304‐5. [Google Scholar]
Yan 2010 {published data only}
- 晏桂萍, 朱志雾, 丁寒春, 杨春霓. Aripiprazole and risperidone in patients with first‐episode schizophrenia comparative efficacy and safety study] Google Translate [阿立哌唑和利培酮对首发分裂症患者疗效及安全性的对照研究]. 中国当代医药 2010; Vol. 17, issue 22:37‐9.
Yang 2006 {published data only}
- Yang L, Yang K, Yao X. A comparative between aripiprazole and clozapine in the treatment of schizophrenia [阿立哌唑与氯氮平治疗精神分裂症的疗效比较]. Sichuan Mental Health [四川精神卫生] 2006;19(2):92‐4. [Google Scholar]
Yang 2007 {published data only}
- Yang T‐R, Song B‐L, Yuan C‐Q. Control study of aripiprazole and clozapine in treatment of patients with schizophrenia [阿立哌唑与氯氮平治疗精神分裂症患者的疗效比较]. 西部医学 2007; Vol. 19, issue 2:234‐5.
Yang 2008 {published data only}
- Yang J, Zhu G‐H, Xie J. A comparative study of ziprasidone and aripiprazole in the treatment of schizophrenia [齐拉西酮与阿立哌唑治疗精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(23):2760‐1. [Google Scholar]
Yang 2008a {published data only}
- Yang X, Deng H, Wen W. Study of aripiprazole in treatment of first‐episode schizophrenia [阿立哌唑治疗首发精神分裂症30例]. China Pharmaceuticals [中國藥業] 2008;17(5):52‐3. [Google Scholar]
Yang 2008b {published data only}
- a杨荣. Aripiprazole in the treatment of female schizophrenia [阿立哌唑治疗女性精神分裂症对照研究]. 中国现代医生 2008;46(13):100‐1. [Google Scholar]
Yang 2009 {published data only}
- 杨春强, 李宝珠. Aripiprazole and olanzapine on body weight, blood glucose control study effects of] Google Translate [阿立哌唑与奥氮平对体质量、血糖影响的对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009; Vol. 19, issue 5:339‐40.
Ye 2005 {published data only}
- Ye X‐R, Xia X‐L. A comparative study between aripiprazole and risperidone in treatment of first‐onset schizophrenia. Medical Journal of National Defending Forces in Southwest China 2005;15(6):616‐8. [Google Scholar]
Ye 2005a {published data only}
- Ye C‐W, Pan D‐J, Ni X‐J, Pan J‐L, Zhu G‐D. Controlled study between aripiprazole and olanzapine on schizophrenia [阿立哌唑与奥氮平治疗精神分裂症的对照研究]. Modern Research of Integrated Chinese and Western Medicine [现代中西医结合杂志] 2005; Vol. 14, issue 24:3199‐200.
Yi 2007 {published data only}
- Yi F, Zhen D L‐L, Wang J‐L, Xu L‐F, Yang G‐P. A comparative study of blood2glucose metabolism between aripiprazole and clozapine in treatment of patientswith schizophrenia [阿立哌唑与氯氮平对精神分裂症患者血糖代谢的影响]. Journal of Clinical Psychological Medicine [临床精神医学杂志] 2007; Vol. 17, issue 4:253.
Yu 2006 {published data only}
- Yu C‐X, Ji D‐C, Yuan H. The study of clinical efficacy of aripiprazole in the treatment for refractory schizophrenia [阿立哌唑治疗难治性精神分裂症的疗效观察]. Shandong Jingshen Yixue 2006;19(4):263‐5. [Google Scholar]
Yu 2006a {published data only}
- Yu L. Effects of aripiprazole vs. clozapine on body weight and blood sugar levels of schizophrenics [阿立哌唑与氯氮平对精神分裂症患者体重及血糖的影响]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2006;12(6):422‐3. [Google Scholar]
Yu 2007 {published data only}
- Yu L, Li B. A control study of aripirazole and clozapine in the treatment of first‐episode schizophrenia [阿立哌唑与氯氮平治疗首发精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007;13(2):115‐6. [Google Scholar]
Yu 2008 {published data only}
- 于永涛. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(19):2267, 2269. [Google Scholar]
Yu 2009 {published data only}
- 余瑞高. Aripiprazole and clozapine in the treatment of chronic schizophrenia] Google Translate [阿立哌唑与氯氮平治疗慢性精神分裂症的对照研究]. Medical Journal of Liaoning 2009;23(4):211‐2. [Google Scholar]
Yu ZG 2007 {published data only}
- 余志刚, 刘金鹏/继龙, 刘军. Controlled clinical studies of aripiprazole and clozapine in the treatment of schizophrenia [阿立哌唑与氯氮平治疗精神分裂症临床对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(12):1066‐7. [Google Scholar]
Zhang 2006 {published data only}
- Zhang Y, Li G, An B. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与维思通治疗精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006; Vol. 14, issue 6:635‐6.
Zhang 2006a {published data only}
- Zhang Y, Gao G, Zhang Q. A comparative study of aripiprazole versus olanzapine in the treatment of chronic schizophrenia [阿立哌唑与奥氮平治疗慢性精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(4):429‐30. [Google Scholar]
Zhang 2007 {published data only}
- Zhang R‐X, Cai Y‐F, Zhao L‐G. A comparative study of EKG changed by aripiprazole and clozapine in the treatment of schizophrenia [阿立哌唑与氯氮平对精神分裂症病人心电图影响的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(12):1016, 1018. [Google Scholar]
Zhang 2007a {published data only}
- Zhang Y, Zhang H, Gu W. A control study of aripiprazole in the treatment of childhood schizophrenia [阿立哌唑治疗儿童精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2007;13(2):122‐4. [Google Scholar]
Zhang 2007b {published data only}
- Zhang Z‐L, Xiao X‐Y, Nie B. Clinical control study of aripiprazole and risperidone in the treatment of first‐ episode schizophrenia [阿立哌唑与利培酮治疗首发精神分裂症对照研究]. Modern Medicine and Health [现代医药卫生] 2007;23(4):494‐5. [Google Scholar]
Zhang 2008 {published data only}
- Zhang J. Comparative investigation on aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的对照分析]. Journal of Qiqihar Medical College [Qiqihar Yixueyuan xuebao][齊齊哈爾醫學院學報] 2008;29(13):1542‐3. [Google Scholar]
Zhang 2008a {published data only}
- 张振东. Controlled clinical observation of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症临床对照观察]. Guangzhou Medical Journal 2008;39(3):52‐3. [Google Scholar]
Zhang 2008b {published data only}
- 张胜利, 盛民生, 李永强, 李振宇. Comparison of the efficacy of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的疗效比较]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(20):2386, 2435. [Google Scholar]
Zhang 2009 {published data only}
- 张艳琦, 于振东. Aripiprazole and clozapine in treatment refractory schizophrenia comparative observation] Google Translate [阿立哌唑与氯氮平治疗难治性精神分裂症疗效比较观察]. People's Military Surgeon [人民军医] 2009; Vol. 52, issue 12:822‐3.
Zhang 2009a {published data only}
- 张东卫, 甘景梨, 高存友, 段惠峰. Aripiprazole and risperidone on glucose and lipid] Google Translate [阿立哌唑与利培酮对血糖、血脂的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(5):343‐5. [Google Scholar]
Zhang 2009b {published data only}
- 张灵娥, 张金光, 张珍. Aripiprazole and risperidone in the treatment of schizophrenia] Google Translate [阿立哌唑与利培酮治疗精神分裂症对照研究]. Journal of Baotou Medical College [包頭醫學院學報] 2009;25(6):41‐2. [Google Scholar]
Zhang 2010 {published data only}
- 张启林, 翟江. Aripiprazole and clozapine in treatment refractory schizophrenia] Google Translate [阿立哌唑与氯氮平治疗难治性精神分裂症的对照研究]. West China Medical Journal [华西医学] 2010; Vol. 25, issue 6:1033‐5.
Zhang 2010a {published data only}
- 张玉清, 王彩萍. Aripiprazole and risperidone in the treatment of schizophrenia comparative analysis of 100 cases] Google Translate [阿立哌唑与利培酮治疗精神分裂症100例的对比分析]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease [实用心脑肺血管病杂志] 2010;18(6):762. [Google Scholar]
Zhao 2006 {published data only}
- Zhao L, Wang M, Wang B. A controlled study of aripiprazole versus quetiapine in the treatment of first‐episode schizophrenia [阿立哌唑与奎的平治疗首发精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(6):663‐4. [Google Scholar]
Zhao 2007 {published data only}
- Zhao S‐M, Du B, Li K‐Q. A comparative study of aripiprazole versus risperdal in patients with schizophrenia [奥派与利培酮治疗精神分裂症临床对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(5):195‐7. [Google Scholar]
Zhi 2005 {published data only}
- Zhi S‐L. Comparison study of aripiprazole and risperidone on schizophrenia. Modern Jorunal of Integrated Traditional Chinese and Western Medicine 2005;14(21):2787‐8. [Google Scholar]
Zhou 2007 {published data only}
- 周平, 刘联琦. Aripiprazole and clozapine treatment of schizophrenia [阿立哌唑与氯氮平治疗精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007; Vol. 17, issue 2:126‐7.
Zhou 2007a {published data only}
- 周伟东. Aripiprazole and risperidone in the treatment of schizophrenia control observation [阿立哌唑与利培酮治疗首发精神分裂症的对照观察]. Journal of the Youjiang Medical College for Nationalities 2007;29(3):384‐5. [Google Scholar]
Zhou 2007b {published data only}
- 周雨林. Aripiprazole impact on the quality of life in patients with schizophrenia [阿立哌唑对精神分裂症患者生活质量的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(5):354, 356. [Google Scholar]
Zhou 2008 {published data only}
- 周勇, 陈雪芳, 林勇, 刘勇. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. 精神医学杂志 2008;21(2):139‐40. [Google Scholar]
Zhu 2005 {published data only}
- Zhu P‐J, Cheng Z‐E, Zhang J, Zhu P‐L, Zhan X‐M. Clinical study of therapeutic effects of aripiprazole in treatment of patients with schizophrenia. Chinese Journal of Clinical Pharmacology and Therapeutics 2005;10(10):1194‐7. [Google Scholar]
- Zhu P‐L, Zhu P‐J, Chen Z‐E, Zhang J, Zhan X‐M, Li Q‐Z. Treatment of 40 cases of schizophrenia with aripiprazole [阿立哌唑治疗精神分裂症40例]. Herald of Medicine [医药导报] [醫藥導報] 2006;25(6):536‐8. [Google Scholar]
Zhu 2008 {published data only}
- 朱满连, 潘大津, 卢舜飞. A Leekpai azole quetiapine controlled study for the treatment of schizophrenia [阿立派唑与喹硫平治疗精神分裂症的对照研究]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine [实用中西医结合临床] 2008;8(1):9‐10. [Google Scholar]
Zhu 2010 {published data only}
- 朱逸溪. Risperidone and olanzapine and aripiprazole on cognitive function in chronic schizophrenia] Google Translate [奥氮平和利培酮及阿立哌唑对慢性精神分裂症认知功能的影响]. 中华临床医师杂志(电子版) 2010; Vol. 4, issue 9:1709‐12.
Zimbroff 2007 {published data only}
- Pfizer. A study comparing the efficacy and safety of ziprasidone and aripiprazole for the treatment of schizophrenia or schizoaffective disorder in hospitalized patients. http://www.clinicaltrials.gov 2008.
- Zimbroff D, Warrington L, Loebel A, Yang R, Siu C. Comparison of ziprasidone and aripiprazole in acutely ill patients with schizophrenia or schizoaffective disorder: A randomized, double‐blind, 4‐week study. International Clinical Psychopharmacology 2007;22(6):363‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zou 2006 {published data only}
- Zou K‐Q, Jiang Q‐L, Wang D. Comparative study on effects of aripiprazole and clozapine [阿立哌唑与氯氮平的临床对照研究]. 中华现代内科学杂志 2006; Vol. 3, issue 1:17‐9.
References to studies excluded from this review
Ai 2008 {published data only}
- Ai C‐Q, Chen S‐M. Comparison study in the treatment of schizophrenia between aripiprazole and clozapine [阿立哌唑与氯氮平治疗精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2008;18(1):53‐4. [Google Scholar]
Allen 2007 {published data only}
- Allen M, Talbott S, Eudicone J, McQuade R, Pikalov A. Similar rates of agitation, anxiety and insomnia in sedating and non‐sedating antipsychotics: evaluating clinical trial results with aripiprazole, haloperidol and olanzapine. Schizophrenia Bulletin 2007;33(2):418. [Google Scholar]
Anon 2008 {published data only}
- Anon. Effectiveness of antipsychotic combination with psychosocial intervention on outcome of patients with schizophrenia. http://www.clinicaltrials.gov 2008.
Bao 2007 {published data only}
- Bao L‐Y, Wang K‐M. The effectiveness of aripiprazole combined with clonazepam treated with agitation in schizophrenia [阿 立 哌 唑 合 用 氯 硝 西 泮 治 疗 精 神 分 裂 症 兴 奋 症 状 疗 效 分 析]. Medical Journal of Chinese People's Health 2007;19(3):198‐200. [Google Scholar]
Bergman 2007 {published data only}
- Bergman M, Friedman JI, Stawarz K, Bowman A, Siever L, Cavagna M, et al. A double blind placebo controlled study of guanfacine adjunctive treatment to atypical antipsychotics for cognitive dysfunction in schizophrenia. http://www.clinicaltrials.gov 2007.
Bristol‐Myers 2006 {published data only}
- Bristol‐Myers Squibb, Otsuka America Pharmaceutical. A multicenter, randomized, double‐blind, placebo‐controlled, 16 week study of aripiprazole used as dual therapy in the treatment of patients with chronic stable schizophrenia or schizoaffective disorder demonstrating an inadequate response to quetiapine or risperidone monotherapy. http://www.clinicaltrials.gov 2006.
Carson 2000 {published data only}
- Carson WH, Kane JM, Ali M, Dunbar GC, Ingenito G. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. European Neuropsychopharmacology 2000;10(Suppl 3):S309. [Google Scholar]
Casey 2003 {published data only}
- Casey D, L'Italien G, Waldeck R, Cislo P, Carson W. Metabolic syndrome comparison between olanzapine, aripiprazole, and placebo. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17– 22; San Francisco, California, USA. 2003.
Chen 2005 {published data only}
- Chen J‐D, Zhao J‐P, Li L‐H, Guo X‐F, Zhai J‐G, Wang C‐Y, et al. A multi centre, randomised and double‐blind controlled trial of aripiprazole in treatment of schizophrenia. Chinese Journal of New Drugs and Clinical Remedies 2005;11:845‐8. [Google Scholar]
Chen 2007 {published data only}
- Chen J, Zhou Y, Zha X‐Y. A comparative study of aripiprazole and clozapine in the treatment of first‐episode schizophrenia [阿立哌唑与氯氮平治疗首发精神分裂症对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2007;20(2):97‐8. [Google Scholar]
Chen 2008 {published data only}
- Chen Q‐S, Li Y‐H, Qu L‐J. A comparative study of aripiprazole and risperidone in the treatment of the first episode schizophrenia [阿立哌唑和利培酮治疗首发精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(5):394‐5. [Google Scholar]
Chen LJ 2010 {published data only}
- 陈莉晶. Aripiprazole and risperidone in the treatment of schizophrenia compared] Google Translate [阿立哌唑与利培酮治疗精神分裂症的对比分析]. 当代医学 2010;16(4):140‐1. [Google Scholar]
Chen YH 2010 {published data only}
- 陈永红. Aripiprazole and risperidone in the treatment of female schizophrenia] Google Translate [阿立哌唑与利培酮治疗女性精神分裂症的对照研究]. Shanxi Clinical Medicine 2010;19(9B):1232‐4. [Google Scholar]
Colombo 2008 {published data only}
- Colombo GL, Caruggi M, Matteo S, Rossi A. An economic evaluation of aripiprazole vs olanzapine adapted to the Italian setting using outcomes of metabolic syndrome and risk for diabetes in patients with schizophrenia. Neuropsychiatric Disease and Treatment 2008;4(5):967‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornblatt 2002 {published data only}
- Cornblatt B, Kern RS, Carson WH, Stock E, Ali M, Ingenito G, et al. Neurocognitive effects of aripiprazole versus olanzapine in patients with stable psychosis. Schizophrenia Research 2002;53(3 Suppl 1):27. [Google Scholar]
Fawzi 2009 {published data only}
- Fawzi M, Fawzi M Jr. Aripiprazole, olanzapine and olanzapine‐clomipramine combination in schizophrenia with obsessive‐compulsive symptoms. World Psychiatry 2009;8(Suppl 1):NRS5.4. [Google Scholar]
Fleischhacker 2008a {published data only}
- Fleischhacker W, Heikkinen ME, Olie JP, Dewaele P, McQuade R, Hennicken D, et al. Long‐term effect on weight of aripiprazole‐clozapine in schizophrenia patients with suboptimal response on clozapine. European Neuropsychopharmacology 2008;18:S447. [Google Scholar]
- Markovic O. A multicenter, comparative, randomized, double‐blind, placebo controlled study on the effect on weight of adjunctive treatment with aripiprazole in patients with schizophrenia. http://www.clinicaltrials.gov 2006.
Geng 2009 {published data only}
- 耿寒松, 刘荣芹, 边辛丑, 苏秀茹, 崔春青, 蒋龙, et al. Comparative study of the effects of ariplprazole and risoeridone on the therapeutic effect and patients’weight in patients with schizophrenia [阿立哌唑与利培酮对精神分裂症疗效与体重的对照研究]. Hebei Medical Journal 2009; Vol. 31, issue 4:407‐9.
Guan 2007 {published data only}
- Guan H‐Y, Wang K‐Q, Jiang L, Geng H‐S, Li Q‐X, Liu Z‐R, et al. A controlled study of efficacy between aripiprazole and risperidone in the treatment of patients with [阿立哌唑与利培酮治疗精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(4):241‐3. [Google Scholar]
Han HF 2010 {published data only}
- 韩惠芬. Second generation of psychiatric drugs 3 cost of the treatment of schizophrenia] Google Translate [3种二代精神病药治疗精神分裂症的成本分析]. Journal of Qiqihar Medical College [Qiqihar Yixueyuan xuebao][齊齊哈爾醫學院學報] 2010; Vol. 31, issue 9:1379‐80.
Hatta 2009 {published data only}
- Hatta K, Sato K, Hamakawa H, Takebayashi H, Kimura N, Ochi S, et al. Effectiveness of second‐generation antipsychotics with acute‐phase schizophrenia. Schizophrenia Research 2009;113(1):49‐55. [DOI] [PubMed] [Google Scholar]
Henderson 2009 {published data only}
- Henderson DC, Fan X, Copeland PM, Borba CP, Freudenreich O, Evins AE, et al. Aripiprazole added to overweight and obese olanzapine‐treated schizophrenia patients. Journal of Clinical Psychopharmacology 2009;29(2):165‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel JJ, Machon DA, Henderson DC. A placebo‐controlled, cross‐over trial of aripiprazole added to obese olanzapine‐treated patients with schizophrenia. http://www.clinicaltrials.gov 2006.
Huang 2008 {published data only}
- Huang X, Du W, Xue S, Tong Z, Jiang Z. Clinical efficacy of aripiprazole and risperidone in treating schizophrenia and their influence [阿立哌唑与利培酮治疗精神分裂症的临床疗效及对血清催乳素的影响]. 药物不良反应杂志 2008;10(2):77‐80. [Google Scholar]
Janssen 2005 {published data only}
- Janssen F. A comparative, randomized, open‐label, multicenter study on the efficacy and safety of switch treatment with aripiprazole in schizophrenic out‐patients who are experiencing insufficient efficacy with risperidone and/or safety and tolerability issues, while on risperidone. http://www.clinicaltrials.gov 2005.
Jiang 2007 {published data only}
- Jiang Y‐H, Shen Z‐X. A comparison between the therapeutic effects of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的疗效比较]. Herald of Medicine [医药导报] [醫藥導報] 2007;26(10):1147‐8. [Google Scholar]
Ju 2009 {published data only}
- 鞠康, 费玥, 季卫东, 高运兰. Comparative analysis of aripiprazole and risperidone in the treatment of schizophrenia negative emotions [阿立哌唑与利培酮治疗精神分裂症负性情绪的比较分析]. Sichuan Mental Health [四川精神卫生] 2009;22(1):39‐40. [Google Scholar]
Kim 2006 {published data only}
Kim 2009 {published data only}
- Kim C‐Y. A multi‐center, randomized, open, treatment‐switching study from orally administered antipsychotic monotherapy in the treatment of chronic schizophrenic and schizoaffective patients. http://www.clinicaltrials.gov 2006.
- Kim CY, Chung S, Lee JN, Kwon JS, Kim DH, Kim CE, et al. A 12‐week, naturalistic switch study of the efficacy and tolerability of aripiprazole in stable outpatients with schizophrenia or schizoaffective disorder. International Clinical Psychopharmacology 2009;24(4):181‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lan 2008 {published data only}
- Lan T, Chiu H, Hu T, Huang H, Wu B, Tuan Y. Prolactin level as an indicator to clinical psychopathological severity in aripiprazole treated schizophrenia. European Neuropsychopharmacology 2008;18:S440. [Google Scholar]
Li 2007e {published data only}
- Li A‐F, Yao J‐X, Liao X‐H. A controlled study of domestic aripiprazole and clozapine in the treatment of refractory schizophrenia [国产阿立哌唑与氯氮平治疗难治性精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(5):165‐7. [Google Scholar]
Li 2007f {published data only}
- Li D, Yu L‐B, Zha Z‐Q. A comparative study on quality of life of female schizophrenics treated with aripiprazole [阿立哌唑对女性精神分裂症患者生活质量的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2007;19(5):170‐2. [Google Scholar]
Liang 2005 {published data only}
- Liang S. A multi‐center, randomized, double‐blind study, comparing with risperidone, to evaluate the efficacy and safety of aripiprazole in the treatment of patients with schizophrenia. http://www.clinicaltrials.gov 2005.
Li C 2010 {published data only}
- 李超, 李慧娥, 刘彩秀. Aripiprazole and clozapine in the treatment of schizophrenia were] Google Translate [阿立哌唑和氯氮平治疗精神分裂症临床对照分析]. Acta Medicinae Sinica [华夏医学] 2010; Vol. 23, issue 4:391‐3.
Liemburg 2011 {published data only}
- Liemburg E, Aleman A, Bous J, Hollander K, Knegtering H. An open randomized pilot trial on the differential effects of aripiprazole versus risperidone on anhedonia and subjective well‐being. Pharmacopsychiatry 2011;44(3):109‐13. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin C. A controlIed study of aripiprazole and risperidone in treatment of first‐episode schizophrenia [博思清与利培酮治疗首发精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006; Vol. 14, issue 6:660‐1.
Lin Y 2009 {published data only}
- 林云, 汪孝魁, 温达民, 黄航. Control study of aripiprazole and clozapine in the treatment of the negative symptoms of schizophrenia [阿立哌唑与氯氮平治疗精神分裂症阴性症状的对照研究]. Sichuan Mental Health [四川精神卫生] 2009; Vol. 22, issue 1:38‐9.
Liu 2007a {published data only}
- Liu G, Cai Y. A comparison study on the efficacy and safety of aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的临床对照研究]. Modern Hospital 2007;7(8):36‐7. [Google Scholar]
Liu 2007b {published data only}
- Liu L, Xinag H. Compared effect and safety analysis of aripiprazole in late‐onset schizophrenia [阿立哌唑治疗晚发性精神分裂症的疗效对照分析]. Sichuan Mental Health [四川精神卫生] 2007;20(3):142‐4. [Google Scholar]
Liu XH 2009 {published data only}
- 刘晓红, 李卫军, 季磊. Clinical comparison of aripiprazole and risperidone in the treatment of female schizophrenia [阿立哌唑与利培酮治疗女性精神分裂症的临床比较]. Sichuan Mental Health [四川精神卫生] 2009; Vol. 22, issue 2:115‐6.
Lu 2008 {published data only}
- 卢艳春, 李扬. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008; Vol. 20, issue 23:2804.
Lv 2006 {published data only}
- Lv X‐M, Jia Z‐L, Liu C‐J. Effect of aripiprazole on body weight and blood glucose in the treatment of first‐episode schizophrenia [阿立哌唑对首发精神分裂症体重及血糖的影响]. Shandong Archives of Psychiatry [山东精神医学] 2006;19(3):181‐2. [Google Scholar]
Ma 2007 {published data only}
- Ma Q, Lian H‐T, Li L‐X. A study of aripiprazole combined with clozapine in refractory schizophrenia [阿 立 哌 唑 合 并 氯 氮 平 治 疗 难 治 性 精 神 分 裂 症]. Medical Journal of Chinese People's Health [中 国 民 康 医 学 ] 2007;19(12):1046, 1048. [Google Scholar]
Ma 2009b {published data only}
- 马闯胜, 马玲, 张淑芳. Comparative study on aripiprazole and risperidone in the treatment of female patients with schizophrenics [阿立哌唑与利培酮治疗女性精神分裂症的对比分析]. 实用药物与临床 2009;12(2):77‐9. [Google Scholar]
Medori 2008 {published data only}
- Medori R, Wapenaar R, Arce R, Rouillon F, Gaebel W, Cordes J, et al. Relapse prevention and effectiveness in schizophrenia of risperidone long‐acting injectable (rlai) versus quetiapine or aripiprazole. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008.
Millar 2008 {published data only}
- Millar H, Felter C, Landsberg W. The effects of aripiprazole in combination with clozapine: patient functioning results from a double‐blind, 16‐week study in patients with schizophrenia (CN138‐170). Journal of Psychopharmacology 2008;22(5):A17. [Google Scholar]
Mortimer 2004 {published data only}
- Mortimer AMP. Aripiprazole is effective for relapse prevention in people with chronic stable schizophrenia. Evidence‐Based Mental Health 2004;7(2):41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mossner 2009 {published data only}
- Mossner R, Schuhmacher A, Kuhn KU, Cvetanovska G, Rujescu D, Zill P, et al. Functional serotonin 1A receptor variant influences treatment response to atypical antipsychotics in schizophrenia. Pharmacogenetics and Genomics 2009;19(1):91‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Namey 2006 {published data only}
- Namey LB, Henderson DC. Aripiprazole for clozapine associated medical morbidity. http://www.clinicaltrials.gov 2006.
Newcomer 2006 {published data only}
- Newcomer JW, L'Italien G, Vester‐Blokland, McQuade RD, Carson WH, Marcus RN. Improvement of non‐hdl cholesterol levels among patients randomized to aripiprazole versus olanzapine. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
Newcomer 2008 {published data only}
- Newcomer JW, Meyer JM, Baker RA, Eudicone JM, Pikalov A, Vester‐Blokland E, et al. Changes in non‐high‐density lipoprotein cholesterol levels and triglyceride/high‐density lipoprotein cholesterol ratios among patients randomized to aripiprazole versus olanzapine. Schizophrenia Research 2008;106(2‐3):300‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcomer 2008c {published data only}
- Newcomer JW, Meyer JM, Baker RA, Eudicone JM, Pikalov A, Vester‐Blokland E, et al. Changes in non‐high‐density lipoprotein cholesterol levels and triglyceride/high‐density lipoprotein cholesterol ratios among patients randomized to aripiprazole versus olanzapine. Schizophrenia Research 2008;106(2‐3):300‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pae 2009a {published data only}
- Pae CU, Serretti A, Chiesa A, Mandelli L, Lee C, Kim J, et al. Immediate versus gradual suspension of previous treatments during switch to aripiprazole: results of a randomized, open label study. European Neuropsychopharmacology 2009;19(8):562‐70. [DOI] [PubMed] [Google Scholar]
Ray 2004 {published data only}
- Ray S, Corey‐Lisle PK, Cislo PR, L'Italien G, Weiden P. An economic evaluation of aripiprazole compared to olanzapine based on metabolic side‐effect profile. Journal of the European College of Neuropsychopharmacology 2004;14(Suppl 3):S279. [Google Scholar]
Remington 2009 {published data only}
- Remington G. Refractory schizophrenia: Adding aripiprazole to clozapine reduces negative but not overall symptoms. Evidence‐Based Mental Health 2009;12(2):51. [DOI] [PubMed] [Google Scholar]
Schreiner 2009 {published data only}
- Schreiner A, Arce R, Eding E, Marques‐Teixeira J, Milanova V, Rancans E, et al. Descriptive analyses of the aripiprazole arm in the risperidone long‐acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Proceedings of the 9th World Congress of Biological Psychiatry; 2009 Jun 28‐Jul 2; Paris, France. 2009. [DOI] [PubMed]
Shen 2006 {published data only}
- Shen J‐Q, Shi J‐F. A controlled study on aripiprazole and risperidone in the treatment of schizophrenic patients [阿立派唑与利培酮治疗门诊首发精神分裂症的临床研究]. Chinese Journal of Modern Applied Pharmacy 2006;23(7):714‐6. [Google Scholar]
Shim 2006 {published data only}
- Shim JC, Kelly DL, Jung DU, Seo YS, Kim YH, Conley RR. Adjunctive treatment with a dopamine partial agonist, aripiprazole for antipsychotic induced hyperprolactinemia. European Neuropsychopharmacology 2006;16(Suppl 4):S405. [DOI] [PubMed] [Google Scholar]
Sun 2006a {published data only}
- Sun F, Dong R, Wang Y. A randomized control study aripiprazole in the treatment of first‐episode schizophrenia [阿立哌唑治疗首发精神分裂症的随机对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(6):642‐3. [Google Scholar]
Sun P 2009 {published data only}
- 孙鹏, 岳英, 张正怡, 张毅蓉, 徐一峰. Quetiapine, aripiprazole treatment efficacy in patients with acute schizophrenia] Google Translate [喹硫平、阿立哌唑治疗急性期精神分裂症患者疗效观察]. Shandong Archives of Psychiatry [山东精神医学] 2009;22(5):381‐2. [Google Scholar]
Takeuchi 2008 {published data only}
- Takeuchi H, Suzuki T, Uchida H, Nakajima S, Nomura K, Kikuchi T, et al. A randomized, open‐label comparison of 2 switching strategies to aripiprazole treatment in patients with schizophrenia: add‐on, wait, and tapering of previous antipsychotics versus add‐on and simultaneous tapering. Journal of Clinical Psychopharmacology 2008;28(5):540‐3. [DOI] [PubMed] [Google Scholar]
Talbott 2007 {published data only}
- Talbott S, Buckley P, Eudicone J, McQuade R, Van‐Tran Q. Akathisia in schizophrenia patients treated with aripiprazole, haloperidol, or olanzapine‐analyses of the first 12 weeks of three double‐blind, long‐term trials. Proceedings of the 11th International Congress on Schizophrenia Research; 2007 Mar 28‐Apr 1; Colorado Springs, Colorado, USA 2007.
Taylor 2007 {published data only}
- Taylor D. Haloperidol, olanzapine and risperidone reduce treatment failure compared to aripiprazole, quetiapine and ziprasidone in acute schizophrenia. Evidence‐Based Mental Health 2007;10(3):76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2006e {published data only}
- Wang X‐Q, Zhou H‐X, Zhang Y‐L, Wang H‐Y, Gao T‐X, Xie P‐L. Clinical study of aripiprazole and risperidone in treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症临床研究]. Journal of Clinical Psychiatry 2006; Vol. 16, issue 2:108‐9.
Wang 2007b {published data only}
- Wang K‐Q, Guan H‐Y, Jiang L, Geng H‐S, Li Q‐X, Liu Z‐R, et al. A controlled study of effect on serum prolactin level between aripiprazole and risperidone [阿立哌唑与利培酮对血清催乳素水平的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007; Vol. 17, issue 3:154‐6.
Wang 2007c {published data only}
- Wang Y, Chen T. Non ‐ classical antipsychotic drugs for schizophrenia: cost ‐ minimization analysis on 3 schemes [3种非经典抗精神病药治疗精神分裂症的最小成本分析]. China Pharmacy [中國藥房] 2007;18(32):2487‐8. [Google Scholar]
Watts 2006a {published data only}
- Watts KL, Wilson H, Tang T. Estimating and reducing the cardiovascular risk of patients with schizophrenia drugs from lipid measures and ischemic electrocardiographic changes. http://www.clinicaltrials.gov 2006.
Xu 2008 {published data only}
- Xu M, Zheng G‐X. Comparative study of aripiprazole and risperidone for schizophrenia [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Jilin Medical Journal 2008;29(19):1629‐30. [Google Scholar]
Yang 2007a {published data only}
- Yang W, Liu X‐Q, Li X‐W. A ramdomized controlled clinical study of aripiprazole in the treatment of childhood schizophrenia [阿立呱唑治疗儿童精神分裂症多中心随机对照研究]. Hunan Medical Journal 2007;24(6):942‐4. [Google Scholar]
Yang 2007b {published data only}
- Yang S, Gao S, Wu X. A control study of aripiprazole vs. clozapine in the treatment of refractory schizophrenia. Journal of Clinical Psychosomatic Diseases 2007;13(1):15‐6. [Google Scholar]
Yu 2007a {published data only}
- Yu L. Comparative study of aripiprazole and risperidone in the treatment of female schizophrenias [阿立哌唑与利培酮治疗女性精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2007;15(10):948‐9. [Google Scholar]
Zeng 2006 {published data only}
- Zeng D‐Z, Chen K‐Q, Hu Z‐D. Influence of aripiprazole on life quality of the patients with schizophrenia [阿立哌唑对精神分裂症患者生活质量的影响]. Chinese Journal of Rehabilitation [中國康復] 2006;21(4):288. [Google Scholar]
Zhang 2006b {published data only}
- Zhang X‐L, Cui Y‐Z, Yang X‐S. Controlled study on aripiprazole in the treatment of schizophrenic patients who has response to clozapine [阿立哌唑替换氯氮平治疗精神分裂症的对照研究]. Medical Journal of Chinese Civil Administration 2006;18(9):753‐4. [Google Scholar]
Zhang F 2009 {published data only}
- 张帆, 熊昆武. Aripiprazole and quetiapine in the treatment control study of first‐episode schizophrenia] Google Translate [阿立哌唑与奎硫平治疗首发精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2009;17(11):299‐300. [Google Scholar]
Zhang RK 2008 {published data only}
- 张仁凯, 付学凯, 王建利, 张春鹏. Aripiprazole and clozapine in the treatment of refractory schizophrenia [阿立哌唑与氯氮平治疗难治性精神分裂症的对照研究]. Journal of Qiqihar Medical College [Qiqihar Yixueyuan xuebao][齊齊哈爾醫學院學報] 2008;29(14):1703‐4. [Google Scholar]
Zhang XL 2007 {published data only}
- 张小聆, 黄献平, 卢友欢. Aripiprazole and risperidone in the treatment of schizophrenia [阿立哌唑与利培酮治疗精神分裂症的对照研究]. Fujian Medical Journal [福建医药杂志] 2007;29(6):117‐8. [Google Scholar]
Zhao GW 2009 {published data only}
- 赵贵文. Aripiprazole and risperidone on serum prolactin levels] Google Translate [阿立哌唑与利培酮对血清催乳素水平的影响 []. Medical Journal of Liaoning 2009;23(4):221‐2. [Google Scholar]
Zhao JY 2009 {published data only}
- 赵晶媛, 郭素芹, 王新友. A control study on effect of domestic aripiprazole and clozapine in treatment of schizophrenia [国产阿立哌唑与氯氮平治疗精神分裂症的疗效及安全性对照研究]. Occupation and Health [职业与健康] 2009; Vol. 25, issue 6:666‐8.
Zhe 2007 {published data only}
- Zhe J, Shi X‐Y, Tan T‐C, Liu W‐X, Tao P‐Y, Wang J, et al. Comparative study of aripiprazole and risperidone in treatment of late onset schizophrenia [阿立哌唑与利培酮治疗晚发精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007; Vol. 17, issue 5:327‐8.
Zhi 2010 {published data only}
- 支胜利. aripiprazole and risperidone in the treatment of schizophrenia compared efficacy] Google Translate [阿立哌唑与利培酮治疗精神分裂症疗效对比 []. China Pharmaceuticals [中國藥業] 2010; Vol. 19, issue 1:52‐3.
References to studies awaiting assessment
Wang 2006f {published data only}
- Wang H, Wang Y, Lu L. A comparative study between aripiprazole and risperidone in the treatment of chronic schizophrenia [阿立哌唑与利培酮治疗慢性精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(6):650‐1. [Google Scholar]
Zhao 2006a {published data only}
- Zhao L, Wang M, Wang B. A controlled study of aripiprazole versus quetiapine in the treatment of first‐episode schizophrenia [阿立哌唑与奎的平治疗首发精神分裂症对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2006;14(6):663‐4. [Google Scholar]
Zheng XR 2008 {published data only}
- 郑孝荣. Aripiprazole in the treatment of schizophrenia, efficacy and safety analysis [阿立哌唑治疗精神分裂症的疗效与安全性分析]. Modern Practical Medicine 2008;20(6):457‐8. [Google Scholar]
陶建青, 2007 {published data only}
- 陶建青, 梁佳, 曾强, 康红. A controlled study on compliance of schizophrenia treated with aripiprazole and risperidone. Proceedings of the 8th National Academic Congress of CSP with the Regional Meeting of the WPA; 2007 Sep 20‐23; Shanghai. 2007:334.
References to ongoing studies
Bristol‐Myers 2009 {published data only}
- Bristol‐Myers Squibb. Metabolic abnormalities ‐ OPT. http://www.clinicaltrials.gov 2009.
Eli Lilly 2003 {published data only}
- Eli Lilly, Company. Study of olanzapine vs aripiprazole in the treatment of schizophrenia. Eli Lilly and Company Clinical Trial Registry 2003.
Essock 2002 {published data only}
- Essock SM, Covell NH, Carbuto M, Bielen K, Jabbour B, Saltz BL, et al. Effectiveness of switching antipsychotic medications. http://www.clinicaltrials.gov 2002.
Glorioso 2005 {published data only}
- Glorioso DK, Jeste DV. Metabolic effects of newer antipsychotics in older patients. http://www.clinicaltrials.gov 2005.
Hanssens 2007 {published data only}
- Hanssens L. A 16‐Week, multicenter, randomized, open‐label study to assess the effects of aripiprazole versus other atypical antipsychotics in the treatment of schizophrenic patients with metabolic syndrome. http://www.clinicaltrials.gov 2007.
Johnson 2006 {published data only}
- Johnson & Johnson Pharmaceutical Research & Development LLC. A 2‐year, prospective, blinded‐rater, open‐label, active‐controlled, multicenter, randomized study of long‐term efficacy and effectiveness comparing risperdal® consta® and abilify® (aripiprazole) in adults with schizophrenia. http://www.clinicaltrials.gov 2006.
Lehrer 2008 {published data only}
- Lehrer DS, Jewell M. Best event schizophrenia trial‐‐a randomized double‐blind trial of aripiprazole and risperidone in schizophrenia. http://www.clinicaltrials.gov 2008.
Lin 2009 {published data only}
- Lin C‐C. Identification and treatment response prediction of antipsychotic‐related metabolic syndrome. http://www.clinicaltrials.gov 2009.
Livingston 2007 {published data only}
- Livingston M, Stroup TS, McEvoy JP, Rojas I, Gamel N, Glick I, et al. Clinical management of metabolic problems in patients with schizophrenia. http://www.clinicaltrials.gov 2007.
McCormack 2006 {published data only}
- McCormack J, Robinson D, Tobias K, Petrides G, Robinson D, Sevy S, et al. Preventing morbidity in first episode schizophrenia, part II. http://www.clinicaltrials.gov 2006.
McEvoy 2007 {published data only}
- McEvoy JP. Metabolic signatures and biomarkers for aripiprazole. http://www.clinicaltrials.gov 2007.
Montoya 2006 {published data only}
- Montoya D, Blizard R, Buckley PF, Holman T, Kerr J, Miller DD, et al. Preventing relapse: oral antipsychotics compared to injectables: evaluating efficacy (PROACTIVE). http://www.clinicaltrials.gov 2006.
Schweiger 2005 {published data only}
- Schweiger J, Flavin K, Rosen B, Spies A, Newcomer JW, Haupt D. Changes in adiposity and metabolic measures during medication switches to aripiprazole from other atypical antipsychotics. http://www.clinicaltrials.gov 2005.
Stroup 2007 {published data only}
- Stroup TS, McEvoy JP, Swartz MS, Hamer RM, Perkins DO, Lieberman JA. Comparison of antipsychotics for metabolic problems (CAMP): a NIMH schizophrenia trials network study. Clinical Schizophrenia and Related Psychoses 2007;1(1):69‐72. [Google Scholar]
Swartz 2008b {published data only}
- Swartz M, Stroup TS, McEvoy JP, Rojas IA. Comparison of optimal antipsychotic treatments for adults with schizophrenia. http://www.clinicaltrials.gov 2008.
Vontur 2005 {published data only}
- Vontur MG, Lopez J, Woolsey MD, Maples NL, Velligan DI. Do patients taking aripiprazole learn more in vocational skills training than patients taking olanzapine?. http://www.clinicaltrials.gov 2005.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2004
- American Psychiatric Association. Practice guidelines for the treatment of patients with schizophrenia. American Journal of Psychiatry. 2nd Edition. American Psychiatric Publishing, Inc, 2004:1‐114. [Google Scholar]
Arnt 1998
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 1998;18:63‐101. [DOI] [PubMed] [Google Scholar]
Barnes 1989
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [DOI] [PubMed] [Google Scholar]
Belgamwar 2011
- Belgamwar Ravindra B, El‐Sayeh Hany George G. Aripiprazole versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD006622.pub2] [DOI] [PubMed] [Google Scholar]
Bhattacharjee 2008
- Bhattacharjee J, El‐Sayeh Hany George G. Aripiprazole versus typical antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006617.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2013
- British National Formulary (BNF). http://www.bnf.org/bnf/index.htm accessed June/July 2013.
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, et al. The problem of therapeutic efficacy indices.3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Chaichan 2008
- Chaichan W. Evaluation of the use of the positive and negative syndrome scale‐excited component as a criterion for administration of p. r. n. medication. Journal of Psychiatric Practice 2008;14(2):105‐13. [PUBMED: 18360196] [DOI] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Boso M, Barbui C. Clozapine combined with different antipsychotic drugs for treatment resistant schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006324.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceeding of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. Cape Town: Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Duggan 2005
- Duggan L, Dardennes R, El‐Dosoky A, Fenton M, Indran S, Rathbone J. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001359.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EL‐Sayeh 2006
- El‐Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004578.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
FDA 2002
- U. S. Federal Drug Administration CDER. Abilify (aripiprazole) tablets, medical review. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm 2002 (accessed 18 August 2006).
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Gaebel 2006
- Gaebel W, Falkai P, Weinmann S, Wobrock T. Treatment guidelines for schizophrenia [Behandlungsleitlinie Schizophrenie]. Steinkopf 2006.
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy U. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health, 1976. [Google Scholar]
Heinrichs 1984
- Heinrichs DW, Hanlon TE, Carpenter WT Jr. The quality of life scale: An instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 1984;10(3):388‐98. [PUBMED: 6474101] [DOI] [PubMed] [Google Scholar]
Heres 2006
- Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine. American Journal of Psychiatry 2006;163:185‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Jelsma 2001
- Jelsma J, Mhundwa K, Weerdt W, Cock P, Chimera J, Chivaura V. The reliability of the Shona version of the EQ‐5D. Central African Journal of Medicine 2001;47(1):8‐13. [PUBMED: 11961858] [DOI] [PubMed] [Google Scholar]
Jones 2006
- Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. Randomized controlled trial of the effect on quality of life. Archives of General Psychiatry 2006;63:1079‐6. [DOI] [PubMed] [Google Scholar]
Kane 1988
- Kane JM, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study Group. Clozapine for the treatment of treatment‐resistant schizophrenia: a double‐blind comparison with chlorpromazine. Archives of General Psychiatry 1988;45:789‐96. [DOI] [PubMed] [Google Scholar]
Kane 1993
- Kane JM. Treatment programme and long‐term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica 1993;46:585‐93. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Kolotkin 2002
- Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life‐lite questionnaire (IWQOL‐lite) in a community sample. Quality of Life Research 2002;11(2):157‐71. [PUBMED: 12018739] [DOI] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2008
- Leucht S, Komossa K, Rummel‐Kluge C, Corves C, Hunger H, Schmid F, et al. A meta‐analysis of head‐to‐head comparisons of second‐generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry 2009;166(2):152‐63. [DOI: 10.1176/appi.ajp.2008.08030368] [DOI] [PubMed] [Google Scholar]
Lieberman 2005
- Liebermann JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209‐23. [DOI] [PubMed] [Google Scholar]
Maayan 2011
- Maayan N, Soares‐Weiser K, Xia J, Adams CE. Antipsychotic combinations for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD009005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Martindale 2013
- Martindale: the complete drug reference. http://www.medicinescomplete.com/mc/martindale/current/ accessed June/ July 2013.
Marvaha 2004
- Marvaha S, Johnson S. Schizophrenia and employment ‐ a review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. [DOI] [PubMed] [Google Scholar]
McGahuey 2000
- McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. Journal of Sex & Marital Therapy 2000;26(1):25‐40. [PUBMED: 10693114] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT Statement: revised recommendations for improving the quality of reports of parallel‐ group randomized trials. JAMA 2001;285:1987‐1. [DOI] [PubMed] [Google Scholar]
Mukundan 2010
- Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD006629.pub2; CD006629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Möller 2000
- Möller HJ. New assessment of atypical antipsychotics [Aktuelle bewertung neuer/atypischer neuroleptika]. Nervenarzt 2000;71:329‐44. [DOI] [PubMed] [Google Scholar]
O'Carroll 2000
- O'Carroll RE, Smith K, Couston M, Cossar JA, Hayes PC. A comparison of the WHOQOL‐100 and the WHOQOL‐BREF in detecting change in quality of life following liver transplantation. Quality of Life Research 2000;9:121‐4. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Pagadala 2009
- Pagadala B, Jayaram Mahesh B, Mitra L. Aripiprazole for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008074] [DOI] [Google Scholar]
Rivas‐Vasquez 2003
- Rivas‐Vasquez RA. Aripiprazole: A novel antipsychotic with dopamine stabilising properties. Professional Psychology 2003;34(1):108‐11. [Google Scholar]
Rust 1989
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989. [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Simpson 1970
- Simpson EN, Angus JWF. A rating scale for extrapyramidal adverse effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. [DOI] [PubMed] [Google Scholar]
Tandon 2005
- Tandon R, Devellis RF, Han J, Li H, Frangou S, Dursun S, et al. Validation of the investigator's assessment questionnaire, a new clinical tool for relative assessment of response to antipsychotics in patients with schizophrenia and schizoaffective disorder. Psychiatry Research 2005;136(2‐3):211‐21. [PUBMED: 16115690] [DOI] [PubMed] [Google Scholar]
Tandon 2008
- Tandon R, Keshavan MS, Nasralllah HA. Schizophrenia, "just the facts"What we know in 2008.2. Epidemiology and etiology. Schizophrenia Research 2008;102:1‐18. [DOI] [PubMed] [Google Scholar]
Thorpe 2009
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic‐explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Journal of Clinical Epidemiology 2009;62(5):464‐75. [PUBMED: 19348971] [DOI] [PubMed] [Google Scholar]
Tsuang 1978
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153‐5. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Whitehead 2002
- Whitehead C, Moss S, Cardno A, Lewis G. Antidepressants for people with both schizophrenia and depression. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002305; PUBMED: 12076447] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001
- WHO. The World Health Report 2001 ‐ Mental Health: New Understanding, New Hope. Geneva: World Health Organization, 2001. [Google Scholar]
Xia 2007
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. The Leeds Outcomes Stakeholders Survey (LOSS) study. Proceedings of the 15th Cochrane Colloquium, Sao Paulo, 23‐27 October 2007. 2007.
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
References to other published versions of this review
Khanna 2013
- Khanna P, Komossa K, Rummel‐Kluge C, Hunger H, Schwarz S, El‐Sayeh HG, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD006569.pub4] [DOI] [PubMed] [Google Scholar]
Komossa 2009
- Komossa K, Rummel‐Kluge C, Schmid F, Hunger H, Schwarz S, El‐Sayeh HG, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006569.pub3; PUBMED: 19821375] [DOI] [PMC free article] [PubMed] [Google Scholar]


