Abstract
Memory impairment progressing to dementia is the main clinical symptom of Alzheimer's disease (AD). AD is characterized histologically by the presence of β-amyloid (Aβ) plaques and neurofibrillary tangles in specific brain regions. Although Aβ derived from the Aβ precursor protein (β-APP) is believed to play a central etiological role in AD, it is not clear whether soluble and/or fibrillar forms are responsible for the memory deficit. We have generated and previously described mice expressing human wild-type β-APP751 isoform in neurons. These transgenic mice recapitulate early histopathological features of AD and form Aβ deposits but no plaques. Here we describe a specific and progressive learning and memory impairment in these animals. In the Morris water maze, a spatial memory task sensitive to hippocampal damage, one pedigree already showed significant differences in acquisition in 3-month-old mice that increased in severity with age and were expressed clearly in 6-month- and 2-year-old animals. The second transgenic pedigree displayed a milder impairment with a later age of onset. Performance deficits significantly decreased during the 6 days of training in young but not in aged transgenic animals. Both pedigrees of the transgenic mice differed from wild-type mice by less expressed increase of escape latencies after the platform position had been changed in the reversal experiment and by failure to prefer the goal quadrant in probe trials. Both pedigrees performed at wild-type level in a number of other tests (open field exploration and passive and active place avoidance). The results suggest that plaque formation is not a necessary condition for the neuronal β-APP751 transgene-induced memory impairment, which may be caused by β-APP overexpression, isoform misexpression, or elevated soluble Aβ.
Alzheimer's disease (AD) is a progressive dementia characterized clinically by a specific pattern of memory impairment followed by more global cognitive deficits. Diagnosis is confirmed histologically by the presence of β-amyloid (Aβ) containing plaques and of neurofibrillary tangles. Aβ, derived from the β-amyloid precursor protein (β-APP), plays a central etiological role in the disease (reviewed in ref. 1). A number of mutations in the β-APP gene are linked to familial AD (FAD) in an autosomal dominant fashion with complete penetrance, and a large collection of presenilin-1 and -2 mutations linked to FAD all are associated with elevation of Aβs 1–42 (2). Extracellular β-APP and large derivatives generated by an α-secretase activity cleaving a site within the Aβ domain have been shown to have neuroprotective and other effects in vitro and in vivo (3–6), whereas intraneuronal β-APP has been shown to inhibit heme oxygenase-2, which produces large amounts of bilirubin, a major endogenous antioxidant in the brain (7). Aβ constitutes a collection of peptides of 39–43 residues in length that can assume a number of oligomeric and aggregated forms. Aβ has been shown to be neurotoxic in vivo and in vitro (8–11). However, whether fibrillar forms such as those found in plaques, soluble forms, or intracellular peptides are more deleterious remains controversial. Similarly, whether Aβ plaques are a pathogenic entity composed of toxic amyloid, are merely benign, or are even a beneficial mechanism for sequestering Aβ is unresolved (12–16). Recent elegant studies by Janus et al. (17) and Morgan et al. (18) using Aβ vaccination strategy demonstrated that reduction in brain amyloid burden alleviates certain learning impairments in animal models of AD, suggesting that Aβ plaques are a factor contributing to cognitive deficits in AD.
A growing set of β-APP-overexpressing mice have been generated as models for aspects of AD (19–22). A number of these, all of which express high levels of FAD β-APP in neurons, form abundant Aβ plaques. These mice have proven to be impressive models for Aβ deposition in that the anatomical pattern and microanatomy of the plaques recapitulates those in AD patients. β-APP mice expressing wild-type (WT) human sequence, rodent sequence with or without FAD mutations, or lower levels of the transgenic product do not form plaques. We have generated and previously described mice expressing lower levels of human WT β-APP751 in neurons (neuron-specific enolase:β-APP751; ref. 23). Histopathological features of early AD are recapitulated in these animals (24, 25). The mice do not form plaques but do have Aβ deposits that follow the anatomic selectivity seen in AD and resemble the early AD-like deposition that is observed in early adult Down's syndrome (DS, trisomy 21) brain. Indeed, individuals with DS overexpress β-APP, the gene of which is located on chromosome 21, at levels similar to the low-expressing transgenic mice (23, 26). Because individuals with DS develop AD in middle age, pathological changes seen in young-adult DS provide a view of early AD (27, 28). Neither neurofibrillary tangles nor the overt neuronal losses that are major observations in the human disease have been observed in any of the β-APP transgenic mice, although neuritic and synaptic changes are observed (29, 30).
The cardinal feature of AD is dementia, beginning with memory impairment early in the disease course. Clearly, testing clinical relevance of observed histological and biochemical correlates of the disease as well as testing candidate therapeutic approaches require a model of the behavioral aspects of AD. Success in reproducing behavioral aspects of AD has proved to be more difficult than obtaining a deposition model (19, 21). A number of factors contribute. These factors include the inherent difficulty of accurate and specific measurement of a behavioral endpoint, strong effects of parental strain, and mixed backgrounds used in many of the β-APP transgenic lines (21, 31). Although a variety of behavioral changes have been described in the plaque forming β-APP-overexpressing mice, severe impairments appear well before plaques (refs. 16, 32, and 33, see also ref. 13).
Here we describe a specific and progressive learning and memory impairment in the neuron-specific enolase:β-APP751 mice. The profile and selectivity of the memory disturbance reproduces our previous findings in aged mice from one of these pedigrees (34). We now report that the deficit is evident already in young mice and increases in an age-dependent manner. It was highly expressed in one pedigree and in a similar but milder and later-appearing form in another one. Our results further confirm that plaque formation is not necessary for memory impairment in the β-APP-overexpression models. β-APP elevation, isoform misexpression, soluble Aβ, or intracellular peptides may cause the behavioral deficit.
Materials and Methods
Transgenic Mice.
The generation of transgenic mice carrying human β-APP751 cDNA under the control of the rat neuron-specific enolase promoter is described elsewhere (23). Male homozygous transgenic mice originating from two different founder mice (F10 and F15 pedigrees) were used. JU mice of the parental inbred strain served as WT controls. Mice were 3 (n = 12 WT; n = 13 F10; n = 11 F15), 6 (n = 8 WT; n = 9 F10; n = 16 F15), or 24 months old (n = 10 WT; n = 7 F10; n = 8 F15) at the start of the behavioral experiments. Animals were housed in groups (2–5 mice per cage) in temperature- and humidity-controlled rooms with ad libitum access to food and water. All experiments were carried out blinded with respect to the genetic status of mice.
Behavioral Testing.
Morris water maze (MWM).
The spatial learning abilities of mice were assessed in the MWM task (35) modified for use in mice. The water maze consisted of a metal circular pool (diameter, 80 cm; height, 30 cm), the upper part of which was surrounded by a 40-cm-high Perspex wall and filled with water (25°C) in which a circular escape platform (11 cm in diameter) was hidden 0.5 cm below the surface of the 25-cm-deep water. The maze was located in an experimental room rich in environmental cues. Mice were trained in six daily sessions consisting of four trials that were started from four cardinal points of the compass. Mice were given 60 s to find the escape platform in the center of the south-west quadrant of the pool, and if the mouse did not find the platform within this limit, it was guided onto it. All animals were allowed to rest on the platform for 15–20 s. Trajectories were monitored with a computerized tracking system (36), and swim paths and latencies to locate the platform were evaluated. Because there was a high positive correlation between the swim paths lengths and escape latencies and no sensorimotor impairment was observed, only the latter values were used for evaluation of the animals' performance. Asymptotic latency was taken as the average latency on days 4–6 when no significant difference between days occurred. Probe trials were run as fifth trials on days 4 and 6, during which the platform was removed from the maze and the mice were allowed to swim freely for 60 s before they were removed from the pool. The percentage of time spent in the quadrant where the platform was in previous trials was calculated and compared with the percentage of time spent in other quadrants of the maze. A reversal experiment was made on day 7 when the platform was placed into the opposite side of the pool (north-east quadrant), and the mice were given six trials to find it.
Dry arena tests.
Adaptation of these tests for mice has been described in detail (37). Briefly, dry arena tests consisted of 10 min of open field exploration on a metal circular arena (80 cm in diameter surrounded by a 40-cm-high Perspex wall) on day 1. The most visited 60° segment of the arena was used on the following days as the punished segment (PS), the entering of which was punished by a puff of compressed air passing over the segment from the periphery to the center of the arena. The first session was followed on day 2 by 10 min of passive avoidance (PA) training on a stable arena. This 10-min PA session was repeated on day 3. Two hours later the mice received the first 10-min active avoidance (AA) training on an arena rotating at 1 revolution per minute. In AA sessions, the mouse was punished by an air puff whenever it either actively entered the PS of the arena or allowed to be passively transported into it by the rotation of the arena. Two 10-min AA sessions separated by a 2-hour interval were administered on day 4, and the fourth AA session concluded the experiment on day 5. The PS was stable in respect to the room frame in avoidance tests. Time to the first entrance to the PS, the longest time between two subsequent entrances into the PS, total number of entrances into the PS, total time and path length spent in the PS, as well as overall total path length were evaluated. Both passive (rotation of the arena) and active (walking, running) movements contributed to the path length measured in the coordinate system of the room.
Statistical analysis.
The analysis was performed with the STATISTICA software (StatSoft, Tulsa, OK). The data from Figs. 1–3 were evaluated by two-way ANOVA with repeated measures on one factor followed by Newman–Keuls posthoc tests and individual contrasts where appropriate. The differences between means were accepted as significant at the P < 0.05 level.
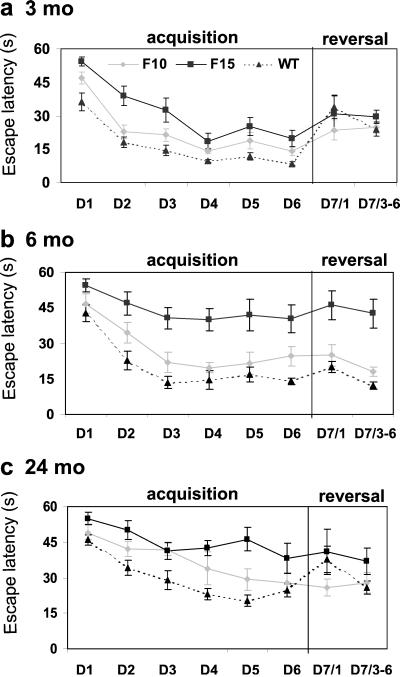
Figure 1.
Learning curves showing the average latency (±SEM) to find the platform in the water maze during the acquisition (days 1–6) and reversal (day 7) experiments. Note that day 7 shows separately latency of the first reversal trial and the average latency of reversal trials 3–6. The graphs represent data from 3- (a), 6- (b), and 24-month-old (c) mice.
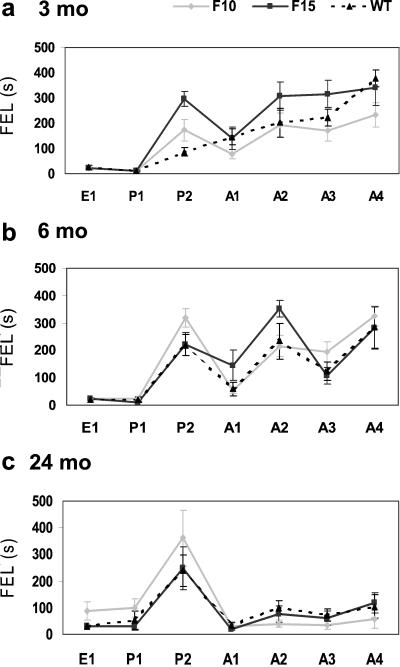
Figure 3.
The FEL (mean ± SEM) into the prohibited sector of the arena (ordinate) during successive sessions of the dry maze tests (abscissa) administered to the F15 (dotted line), F10 (full line), and WT (dashed line) mice aged 3, 6, and 24 months. E, exploration; P1 and P2, first and second passive place-avoidance sessions, respectively; A1-A4, first to fourth active place-avoidance sessions, respectively.
Results
MWM.
Acquisition.
The ability of mice to acquire, process, and recall spatial information was assessed in the modified MWM test by using escape latency as an indicator of learning. Age-dependent learning and memory deficits were revealed in both pedigrees of the β-APP751 transgenic mice, but the impairment was more severe and reached statistical significance only in the F15 pedigree.
The performance of the 3-month-old mice significantly improved over the 6 training days in all groups (Fig. 1a), although the between-group differences were clear at this early age already. Two-way ANOVA rendered significant main effects of groups F(2,33) = 18.91, P < 0.01, days F(5,165) = 60.44, P < 0.01, and no significant interaction F(10,165) = 0.87, P = 0.56. Post hoc tests showed that the F15 pedigree had significantly longer escape latencies than the WT mice on days 1–6. The respective asymptotic levels on days 4–6 were 24.2 and 9.7 s, P < 0.01. The asymptotic performance of the F10 pedigree was significantly different from F15 (P < 0.01) but not from WT mice (P = 0.07; Fig. 1a).
The learning impairment of the F15 mice was expressed clearly at the age of 6 months. Two-way ANOVA revealed significant main effects of groups F(2,30) = 24.25, P < 0.01, days F(5, 150) = 23.31, P < 0.01 and not significant interaction F(10,150) = 1.01, P = 0.45. Post hoc tests showed that escape latencies of the F15 pedigree were not reduced significantly during the 6 days of training (asymptotic level, 40.8 s) and were different from the latencies of the WT mice on days 1–6 and of the F10 mice on days 2–6. On neither day were the F10 mice significantly different from WT mice (Fig. 1b).
Although essentially similar results were obtained in the 24-month-old F15 mice as in 6-month-old animals, the F10 pedigree did not differ from either the F15 or the WT mice, assuming an intermediate position between them (see Fig. 1 b and c). Two-way ANOVA of the results obtained in 24-month-old animals (Fig. 1c) revealed significant main effects of groups F(2,22) = 10.40, P < 0.01, days F(5,110) = 22.77, P < 0.01, as well as significant interaction F(10,110) = 1.96, P = 0.04. Post hoc tests showed that escape latencies of the F15 pedigree did not improve during the training and remained significantly above the corresponding WT values on days 2–5 and were only marginally nonsignificant on day 6, P = 0.06. The learning curve was slowed down markedly in the F10 pedigree, the latencies of which differed neither from those of the F15 pedigree on days 1, 2, 3, 4, and 6 nor from the WT latencies on all days. The performance of the F10 pedigree gradually improved, and their escape latencies on days 5 and 6 were significantly shorter than on day 1 and approached those of the WT mice.
Asymptotic latency (expressed as average latency on days 4–6) increased with age from 9.7 ± 0.8 s in the 3-month-old to 21.2 ± 2.2 s in the 24-month-old WT mice. This increase was much steeper in the F15 mice (from 24.2 ± 3.9 to 40.9 ± 4.3 s) than in WT mice, but this was not the case in the F10 mice (from 15.9 ± 1.6 to 27.4 ± 4.0 s). It should be noted that even though no statistical difference between F10 and WT mice was observed at any age, the mean values of the escape latencies of F10 pedigree were higher compared with WT mice on days 1–6 at all ages studied.
Reversal trials.
The reversal training was used to distinguish goal-directed navigation from search strategy. With the platform moved to the opposite quadrant of the pool, it was expected that escape latency of the first trial would increase in previously well performing animals but remain unchanged in the impaired mice.
The results were evaluated by two-way ANOVA comprising the mean escape latencies on day 6, the first reversal trial on day 7, and reversal trials 3–6 on day 7. Analysis of the reversal results in 3-month-old (Fig. 1a) and older (Fig. 1 b and c) mice indicated that the escape latency of the first reversal trial was significantly longer than the mean escape latency of day 6 and mean of the last four trials on day 7 only in the WT mice. F10 and F15 mice did not show a similar increase of the escape latency on the first reversal trial, indicating impaired performance of the transgenic mice. In contrast, WT mice showed significantly increased reversal latencies in all examined age groups, even against the 20-s asymptotic latencies of the 24-month-old mice (P < 0.05). This result shows that the age-related increase of escape latencies in the WT mice does not reflect substitution of place navigation by search strategy, i.e., by swimming over a circular trajectory traversing all possible target positions. The latter strategy is not affected by the changed position of the platform, which interferes with well trained place navigation.
Probe trials.
Search of the hidden platform can be terminated by chance contact with the target. Therefore, goal-directed search pattern can be better distinguished in probe trials when the animals are allowed to search the platform for 60 s in an empty maze. Because later stages of the 1-min search can be influenced by a failure to find the goal at the expected location during the first half of the probe trial, the percentage of time spent in different quadrants of the pool during the first 30 s was used to assess the effectiveness of the animals' search of the position of the goal.
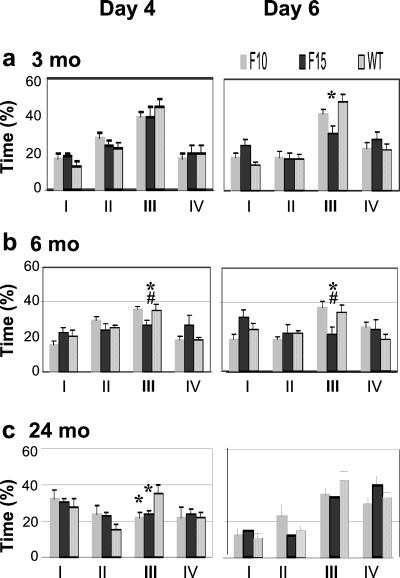
Probe trial experiments revealed learning deficits in the F15 pedigree at all ages studied, whereas spatial learning abilities of the F10 pedigree were found to be impaired only at 24 months of age.
At 3 months of age, the groups did not differ significantly from each other on day 4, but only the WT and F10 mice spent significantly more than 25% of search time in the target quadrant (Fig. 2a). WT mice spent significantly more time in the goal quadrant than the age-matched F15 mice on day 6 [48% compared with 31%, F(2,33) = 4.86, P < 0.05].
Figure 2.
Percentage of time (±SEM) spent in each quadrant in probe trials on days 4 and 6 of the water-maze training. Average time in the opposite (I), adjacent left (II), goal (III), and adjacent right (IV) quadrants is shown for 3- (a), 6- (b), and 24-month-old (c) mice in probe trials on both training days. The symbols indicate significantly decreased (P < 0.05) preference for the goal quadrant compared with the WT (*) and the F10 pedigree (#).
The 6-month-old F15 mice spent significantly less time in the goal quadrant than the F10 and WT mice on both days 4 and 6 [day 4: F(2,29) = 4.59, P < 0.05; day 6: F(2,30) = 4.56, P < 0.05]. No significant difference was found between the F10 and WT mice (Fig. 2b).
The 24-month-old transgenic pedigrees spent significantly less time in the goal quadrant than WT controls [F(2,21) = 3.80, P < 0.05] in the first probe trial. The second probe trial on day 6 revealed no differences between groups. All groups spent more than 25% of time in the goal quadrant, but the difference was significant only in the WT mice (Fig. 2c).
Dry Arena.
The dry arena tests were used to examine the spatial memory of the mice under conditions differing from the MWM test. While in the MWM task the animals learn to display aversively motivated place preferences for small, safe places in the maze; in the dry arena tasks they learn to avoid a punished place (37). In the PA task the mice learn to inhibit a locomotor response leading to punishment. In our study, the mice learn to refrain from visiting a particular segment of the stable arena. In the AA task the mice learn to avoid the same segment of the rotating arena not only by not visiting it but also by not allowing to be passively transported into it. The dry arena tests thus differ from the MWM by the character of the learned response, the relative size of the safe and punished surface, and continuous versus distributed learning.
Although the dry arena tests were influenced differentially by age (passive place avoidance improved, whereas active place avoidance deteriorated with age), no significant differences between the WT and transgenic mice could be detected in any of the parameters studied.
In the initial exploration test WT mice walked ≈20 m during 10 min. Activity was distributed rather uniformly over the whole surface of the arena, and the latency of the first entrance (FEL) into the future prohibited sector was 25 s. Similar results were obtained in the transgenic groups at all ages examined (Fig. 3).
FEL remained unchanged in the first session (P1) of the subsequent PA training but increased in the second session (P2) to 90 s in the youngest and 240 s in the oldest WT mice. Fig. 3 indicates that the PA strength increased with age and was expressed best in the 6- and 24-month-old mice. Two-way ANOVA of the P2 data (group x age) showed significant main effects of age [F(2,79) = 3.79, P < 0.05] and groups [F(2,79) = 3.54, P < 0.05], and significant interaction [F(4,79) = 2.86, P < 0.05]. The only significant between-group difference obtained in post hoc tests indicated that PA was more expressed in the 3-month-old F15 mice than in the WT mice (P < 0.05).
Transition from PA to AA was smooth in the 3-month-old mice, which continued to avoid the room frame sector punished on stable arena even when the arena started to rotate and different segments of the rotating arena floor were passing through this sector. Rotation elicited a sudden decrease of FEL from 220 to 60 s in the 6-month-old and from 250 to 35 s in the 24-month-old WT mice, indicating that these animals solved the PA task by avoiding the punished area of the floor rather than a certain position in the room. Although the FEL of the 6-month-old mice improved in the fourth AA session to 280 s, it reached only 105 s in the 24-month-old WT mice, which seemed unable to master the task. Two-way ANOVA of the A1 data showed a significant main effect of age [F(2,79) = 5.77, P < 0.01] but not of groups [F(2,79) = 1.50, P = 0.22] and no significant interaction [F(4,79) = 1.04, P = 0.39]. Essentially similar results were obtained by ANOVA of the A2-A4 data (Fig. 3).
Discussion
This study demonstrates that neuronal overexpression of human β-APP751 isoform at the levels observed in DS patients (26, 23) results in specific spatial learning and memory deficits that are age-related and observed in more than one pedigree. The previous study on 6- and 12-month-old F10 pedigree of β-APP751 transgenic mice has shown that the mice do not have gross motor, physiological, or behavioral impairments that could confound the interpretation of the data obtained in the learning paradigms (34). Most importantly, our transgenic animal model does not develop amyloid plaques (24, 25), suggesting that increased levels of soluble Aβ40/42, abnormal isoform expression in neurons, neuronal or secreted β-APP holoprotein, or its proteolytic fragments other than Aβ40/42 are involved in development of cognitive deficits. Several possible mechanisms for β-APP-induced behavioral deficits exist. Liu et al. (38) reported that in the hippocampus, nanomolar concentrations of Aβ40 and Aβ42 block the response of α7-containing nicotinic receptors, which are thought to mediate synaptic currents (39, 40) and modulate transmitter release (41, 42), thereby contributing to spatial memory and working memory formation. In addition, the β-APP holoprotein inhibits heme oxygenase-produced carbon monoxide (7), which has been reported to alter long-term potentiation (43–45). The role of β-APP holoprotein is supported also by findings showing increased mRNA for β-APP751 and β-APP770 in the brains of aged rats with spatial memory deficit but not in rats without impairments (46). Finally, our animal model shows an age-related increase of extracellular amyloid deposits associated occasionally with phosphorylated tau and even local gliosis (24), suggesting that inflammatory mechanisms, which may interfere also with learning and memory functions (47, 48), are activated in β-APP-overexpressing mice.
Previous studies with transgenic mouse lines expressing mutated β-APP have demonstrated that the development of at least some of the cognitive deficits correlates with accumulation of amyloid plaques during aging (33). A strong support for the relationship between the plaques and behavioral deficits comes from the elegant studies by Janus et al. (17) and Morgan et al. (18), who demonstrated that Aβ peptide vaccination, which reduced amyloid burden in the brain without changes in the levels of β-APP holoprotein or Aβ40 and Aβ42, reduced behavioral impairment in animal models of AD. However, the reversal of the cognitive dysfunctions was not complete. The APP751 transgenic mice of the present study have no amyloid plaques even at very advanced (22 months) age but develop sparsely distributed, small Aβ42-immunoreactive Aβ deposits in the hippocampus, cortex, and amygdala during aging (24). In addition, our preliminary ELISA assays for brain Aβ in the 24-month-old β-APP751 mice have shown concentrations that are 2,000 times lower than in 6-month-old β-APP/presenilin-1 bigenic mice, which lack both amyloid plaques and cognitive deficits (M.K., unpublished observation; J. Puoliväli, personal communication). Altogether, our findings raise a possibility that neither extracellular amyloid plaques nor substantially increased levels of soluble Aβ are required for age-related impairment of cognitive functions caused by aberrant expression of β-APP751 in these mice and possibly in patients with DS and AD.
Several previous studies have demonstrated that cognitive deficits such as impairment in Y-maze alternation, eight-arm radial maze, circular platform, and visible platform test of the water maze can be demonstrated before the appearance of amyloid plaques. These observations were made in transgenic mice overexpressing mutant β-APP with or without coexpression of mutant presenilin-1 (13, 16, 32, 49–52). Because the mice carrying mutated β-APP transgenes have elevated brain β-APP and Aβ levels throughout their adulthood, elevated neuronal β-APP expression may be responsible, at least partially, for the learning deficits in these mice. The finding that transgenic mice overexpressing FAD-linked M146L or L286V variants of presenilin-1 have a modest and selective increase in brain Aβ42 levels but no cognitive deficits (53) also supports our notion that APP protein may have a role in the development of cognitive dysfunctions.
In our previous study of the F10 pedigree β-APP751 mice, longer water maze acquisition latencies were detected in transgenic mice at the age of 6 and 12 months, whereas in probe trials only the 12-month-old transgenic mice spent significantly less time in the goal quadrant compared with the WT mice (34). In the same study a similar age-dependent deficit of F10 mice was seen in a spontaneous alternation test, which involves working memory. Our present results confirm the progressive development of learning and memory deficits in the F10 pedigree and demonstrates similar but more severe impairments in another (F15) pedigree with an earlier onset. Because the expression level of the transgene is comparable in both pedigrees (23), the difference in the severity of the cognitive impairment might be caused by differential insertion of the transgene into the genome or to a small difference in expression levels. Overall, slightly different time courses of cognitive impairment in F10 and F15 pedigrees suggest that, in general, conclusions from behavioral studies using only one transgenic line should be drawn with caution.
Although spatial memory tested in the MWM showed marked deterioration in the transgenic mice, no corresponding deficit was found in the passive and active place-avoidance tests on dry arena. This result shows that β-APP overexpression did not elicit large-scale dementia but resulted in rather limited cognitive failure disrupting some but sparing other forms of spatial memory.
Performance of WT mice deteriorated with increasing age in the MWM and active place-avoidance task but not in the passive place-avoidance task. This may be because of the fact that substratal idiothesis plays an important role in the latter task but is almost negligible in the MWM and active place-avoidance task, which mainly depend on visually mediated allothesis (54). If β-APP overexpression interferes mainly with allothetic navigation, it may disturb MWM but leave passive place-avoidance unaffected. The greater resistance to the β-APP overexpression in the active place avoidance on the dry arena than in the water maze may be caused by the lesser demands of the task and higher level of training. Consistent with this observation, F10 mice performed well in simple memory tests, and impairment was detected in assays of increasing complexity (34). Further analysis of the dissociation of the β-APP effect on the MWM task and on the dry arena AA may shed more light on the mechanism of the underlying deficit.
In summary, our present results confirm that mild overexpression of β-APP in neurons confers an age-dependent, progressive, and specific memory impairment independent of plaque formation. Clear differences in age of onset and severity of the behavioral phenotype in two pedigrees expressing the transgene at similar levels underscores the need to study multiple transgenic lines.
Acknowledgments
We are grateful to Fred H. Gage for helpful suggestions and critical reading of the manuscript and to Yu. Kaminsky and A. Zahalka for software and hardware support of the experiments. The research was supported by Grant Agency of the Czech Republic Grant 309/00/1656, the Saastamoinen Foundation (Finland), and Scios, Inc.
Abbreviations
- AD
Alzheimer's disease
- Aβ
β-amyloid
- β-APP
Aβ precursor protein
- FAD
familial AD
- DS
Down's syndrome
- WT
wild type
- MWM
Morris water maze
- PS
punished segment
- PA
passive avoidance
- AA
active avoidance
- FEL
latency of the first entrance
References
- 1.Selkoe D. Nature (London) 1999;399,Suppl.:23–31. [Google Scholar]
- 2.Lendon C L, Ashall F, Goate A M. J Am Med Assoc. 1997;277:825–831. [PubMed] [Google Scholar]
- 3.Mattson M P, Cheng B, Culwell A R, Esch F S, Lieberburg I, Rydell R E. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 4.Milward E A, Papadopoulos R, Fuller S J, Moir R D, Small D, Beyreuther K, Masters C. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 5.Saitoh T, Sundsmo M, Roch J M, Kimura N, Cole G, Schubert D, Oltersdorf T, Schenk D B. Cell. 1989;58:615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Swintosky V L, Pettigrew L C, Craddock S D, Culwell A R, Rydell R E, Mattson M P. J Neurochem. 1994;63:781–784. doi: 10.1046/j.1471-4159.1994.63020781.x. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Doré S, Ferris C D, Tomita T, Sawa A, Wolosker H, Borchelt D, Iwatsubo T, Kim S-H, Snyder S. Neuron. 2000;28:461–473. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 8.Pike C J, Burkick D, Walencewicz A J, Glabe C G, Cotman C W. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKee A C, Kowall N W, Schumacher J S, Beal M F. Amyloid Int J Exp Clin Invest. 1998;5:1–9. doi: 10.3109/13506129809007283. [DOI] [PubMed] [Google Scholar]
- 10.Emere M, Geula C, Ransil B J, Mesulam M-M. Neurobiol Aging. 1992;13:553–339. doi: 10.1016/0197-4580(92)90055-3. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo A, Yankner B A. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartley D M, Walsh D M, Ye C P, Diehl T, Vasquez S, Vassilev P M, Teplow D B, Selkoe D J. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 14.Giulian D, Haverkamp L J, Li J, Karshin W L, Yu J, Tom D, Li X, Kirkpatrick J B. Neurochem Int. 1995;27:119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- 15.Campbell A. Med Hypotheses. 2001;56:388–391. doi: 10.1054/mehy.2000.1212. [DOI] [PubMed] [Google Scholar]
- 16.Dodart J-C, Meziane H, Mathis C, Ungerer A, Bales K R, Paul S M. Behav Neurosci. 1999;111:982–990. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- 17.Janus C, Pearson J, McLaurin J, Mathews P M, Jiang Y, Schmidt S D, Chishti M A, Horne P, Heslin D, Westaway D. Nature (London) 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 18.Morgan D, Diamond D M, Gottschall P E, Ugen K E, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Arendash G W. Nature (London) 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 19.Duff K. Curr Opin Biotechnol. 1998;9:561–564. doi: 10.1016/s0958-1669(98)80132-8. [DOI] [PubMed] [Google Scholar]
- 20.Price D L, Tanzi R E, Borchelt D R, Sisodia S S. Annu Rev Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- 21.Higgins L S. Mol Med Today. 1999;5:274–276. doi: 10.1016/s1357-4310(99)01465-3. [DOI] [PubMed] [Google Scholar]
- 22.Janus C, Chishti M A, Westaway D. Biochim Biophys Acta. 2000;1502:63–75. doi: 10.1016/s0925-4439(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 23.Quon D, Wang Y, Marian Scardina J, Murakami K, Cordell B. Nature (London) 1991;352:239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- 24.Higgins L S, Holtzman D M, Rabin J, Mobley W C, Cordell B. Ann Neurol. 1994;35:598–607. doi: 10.1002/ana.410350514. [DOI] [PubMed] [Google Scholar]
- 25.Higgins L S, Rodems J M, Catalano R, Quon D, Cordell B. Proc Natl Acad Sci USA. 1995;92:4402–4403. doi: 10.1073/pnas.92.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neve R L, Finch E A, Dawes L R. Neuron. 1988;1:669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- 27.Motte J, Williams R S. Acta Neuropathol. 1989;77:535–546. doi: 10.1007/BF00687256. [DOI] [PubMed] [Google Scholar]
- 28.Burger P C, Vogel F S. Am J Pathol. 1973;73:457–476. [PMC free article] [PubMed] [Google Scholar]
- 29.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Zhao J. Nature (London) 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 30.Irizarry M C, Soriano F, McNamara M, Page K J, Schenk D, Games D, Hyman B T. J Neurosci. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao K. Prog Brain Res. 1998;117:335–341. doi: 10.1016/s0079-6123(08)64026-1. [DOI] [PubMed] [Google Scholar]
- 32.Holcomb L, Gordon M N, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Duff K. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Chen K S, Knox J, Inglis J, Bernard A, Martin S J, Justice A, McConlogue L, Games D, Morris R G. Nature (London) 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 34.Moran P M, Higgins L S, Cordell B, Moser P C. Proc Natl Acad Sci USA. 1995;93:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris R J. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaminsky Y, Krekule I. Physiol Res. 1997;46:223–231. [PubMed] [Google Scholar]
- 37.Cimadevilla J M, Fenton A A, Bures J. Brain Res Bull. 2001;54:559–563. doi: 10.1016/s0361-9230(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 38.Liu Q, Kawai H, Berg D. Proc Natl Acad Sci USA. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray R, Rajan A S, Radcliffe K A, Yakehiro M, Dani J A. Nature (London) 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z W, Coggan J S, Berg D K. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- 41.Levin E D, Bettegowda C, Blosser J, Gordon J. Behav Pharmacol. 1999;10:675–880. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Meyer E M, Tay E T, Papke R L, Meyers C, Huang G L, de Fiebre C M. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- 43.Alkadhi K A, Al-Hijailan R S, Malik K, Hogan Y H. J Neurosci. 2001;21:3515–3520. doi: 10.1523/JNEUROSCI.21-10-03515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibuki K, Kimura S, Wakatsuki H. Eur J Neurosci. 2001;13:609–616. doi: 10.1046/j.0953-816x.2000.01413.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhuo M, Laitinen J T, Li X C, Hawkins R D. Learn Mem. 1998;5:467–480. [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins G A, Oyler G A, Neve R L, Chen K S, Gage F H. Proc Natl Acad Sci USA. 1990;87:3032–3036. doi: 10.1073/pnas.87.8.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiore M, Angelucci F, Alleva E, Branchi I, Probert L, Aloe L. Behav Brain Res. 2000;112:165–175. doi: 10.1016/s0166-4328(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 48.Gold L H, Heyser C J, Roberts A J, Henriksen S J, Steffensen S C, Siggins G R, Bellinger F P, Chiang C S, Powell H C, Campbell I L. Adv Exp Med Biol. 1996;402:199–205. doi: 10.1007/978-1-4613-0407-4_26. [DOI] [PubMed] [Google Scholar]
- 49.Arendash G W, King D L, Gordon M N, Morgan D, Hatcher J M, Hope C E, Diamond D M. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 50.Chapman P F, White G L, Jones M W, Cooper-Blacketer D, Marshall V J, Irizarry M, Younkin L, Good M A, Bliss T V, Hsiao K K. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 51.Holcomb L A, Gordon M N, Jantzen P, Hsiao K, Duff K, Morgan D. Behav Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- 52.King D L, Arendash G W, Crawford F, Sterk T, Menendez J, Mullan M J. Behav Brain Res. 1999;103:145–162. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 53.Janus C, D'Amelio S, Amitay O, Chishti M A, Strome R, Fraser P, Carlson G A, Roder J C, St. George-Hyslop P, Westaway D. Neurobiol Aging. 2000;21:541–549. doi: 10.1016/s0197-4580(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 54.Cimadevilla J M, Fenton A A, Bures J. Neurosci Lett. 2000;285:53–56. doi: 10.1016/s0304-3940(00)01019-3. [DOI] [PubMed] [Google Scholar]