Abstract
Background
The pathobiology of rheumatoid arthritis (RA) is similar to that of periodontitis in that proinflammatory cytokines play an important pathologic role. There is evidence to suggest that inhibitors of tumor necrosis factor (TNF) and interleukin-6 (IL-6) receptor for the treatment of RA ameliorated periodontal inflammation. However, no study has evaluated the effect of tofacitinib, an oral Janus kinase inhibitor for the treatment of RA, on periodontitis.
Case presentation
The present report cases are 51- and 43-year-old non-smoking women with RA who demonstrated localized moderate chronic periodontitis. Both cases showed improvement in the periodontal inflammatory condition after 3 months of tofacitinib therapy, although the teeth count and supragingival bacterial plaque level were relatively unchanged. Improvements were also observed in the serum levels of IL-6 in both cases as well as in the serum levels of TNF-α and anti-cyclic citrullinated peptide immunoglobulin G in one case and of rheumatoid factor and matrix metalloproteinase-3 in the other case. Patients who received tofacitinib exhibited an inconsistent clinical response, likely due to the low disease activity of RA at the start of the administration.
Conclusions
These are the first reported cases in which tofacitinib may have a beneficial effect on periodontitis. However, more research is required to understand the relationship between periodontitis and tofacitinib therapy.
Keywords: Rheumatoid arthritis, Periodontitis, Tofacitinib, Proinflammatory cytokines, Case report
Background
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that can cause damage to the cartilage and bone as well as disability [1]. Evidence suggests that RA has an epidemiological, serological, and clinical interrelationship with periodontitis, a chronic inflammatory disease that is characterized by the destruction of the tooth-supporting tissues and is a major cause of tooth loss in adults, through common pathogenic mechanisms [1–4]. One of these mechanisms includes the constitutive overproduction of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), both of which are involved in the pathogenesis of RA and periodontitis [5–7].
Studies have shown that inhibitors of TNF-α and IL-6 receptor not only reduce the signs and symptoms of RA but also ameliorate the periodontal inflammatory conditions in patients with RA [7, 8]. Other studies also suggest the efficacy of targeting intracellular pathways in inhibiting the effects of multiple cytokines [9, 10]. Tofacitinib, an oral small-molecule inhibitor for Janus kinase (JAK) that integrates signals from many cytokines, has been shown to be effective in the treatment of RA [11, 12]. These observations have led to the hypothesis that tofacitinib may also be effective in reducing periodontal inflammation in patients with RA. However, no study has yet documented the effect of tofacitinib on periodontitis.
The aim of the present study was therefore to report the changes in the periodontal inflammatory condition before (baseline) and after 3 months (reassessment) of tofacitinib therapy in two patients with RA.
Case presentation
Case 1: The patient was a 51-year-old non-smoking woman with a 68-month history of RA. Before tofacitinib was administered, she had been treated with prednisolone (PSL, 10 mg/day) and bucillamine (BUC, 200 mg/day) for 13 months and was then switched to receive the recombinant humanized anti-human IL-6 receptor monoclonal antibody tocilizumab (TCZ, 8 mg/kg, every 4 weeks) intravenously. Under this treatment, her disease activity score in 28 joints using C-reactive protein (DAS28-CRP) was well controlled as follows: from 3.8 (the baseline) to 2.5 (after 4 months of treatment). However, TCZ was discontinued after 4 months due to signs of pneumonia in the right lung. She was then switched to the fully humanized anti-TNF-α monoclonal antibody adalimumab (ADA, 40 mg/2 weeks) subcutaneously, which resulted in a well-controlled DAS28-CRP for 34 months as follows: from 3.9 (the baseline) to 1.4 (after 34 months of treatment). She was then transferred to a local rheumatology clinic and showed a similar RA condition for 8 months with 5 mg/day of PSL and 12 mg/week of methotrexate (MTX). However, she returned to visit to the Niigata Rheumatic Center with joint pain and swelling. Her CRP levels were gradually raised and at the last visit of that clinic, it was 3.45 mg/dL. The clinical and laboratory assessments at our rheumatic center revealed DAS28-CRP 4.32 and global visual analogue scale (gVAS) 28, possibly due to the secondary failure of the response to ADA treatment. Two weeks later, we evaluated her periodontal condition and started the administration of tofacitinib (10 mg/day) according to the European League Against Rheumatism recommendations for the management of RA [13]. For some reason, her CRP level was decreased to 0.1 mg/dL, but her gVAS was worsened to 51 (Table 1). The patient had no complications, such as hypertension or systemic viral infections, at baseline.
Table 1.
Patient rheumatologic and serum data at baseline and reassessment
| Parameter | Case 1 | Case 2 | ||
|---|---|---|---|---|
| Baseline | Reassessment | Baseline | Reassessment | |
| Rheumatologic | ||||
| SDAI | 12.3 | 4.5 | 16.0 | 4.0 |
| DAS28-CRP | 2.3 | 2.9 | 2.9 | 2.1 |
| TJC | 2 | 3 | 3 | 1 |
| SJC | 0 | 0 | 4 | 1 |
| gVAS (mm) | 51 | 7 | 45 | 10 |
| Serum | ||||
| RF (IU/mL) | 5.0 | 7.0 | 237.0 | 174.0 |
| anti-CCP IgG (U/mL) | 37.8 | 25.7 | 247.0 | 301.0 |
| CRP (mg/dL) | 0.1 | 0.1 | 0.01 | 0.01 |
| MMP-3 (ng/mL) | 67.7 | 70.0 | 49.9 | 44.3 |
| IL-6 (pg/mL) | 2.2 | 1.5 | 1.9 | 0.9 |
| TNF-α (pg/mL) | 1.2 | 0.0 | 1.0 | 0.9 |
The rheumatologic assessments showed a decrease in the simplified disease activity index (SDAI) and gVAS at reassessment after starting tofacitinib therapy (Table 1). In addition, the laboratory analyses of blood samples showed that the serum levels of anti-cyclic citrullinated peptide (CCP) immunoglobulin G (IgG), TNF-α, and IL-6 were decreased at reassessment compared to the values at baseline (Table 1).
Furthermore, the periodontal assessments indicated that the patient had localized moderate chronic periodontitis at baseline according to the criteria of the Centers for Disease Control and Prevention (CDC)/American Academy of Periodontology (AAP) [14] (Fig. 1a). Tofacitinib therapy reduced periodontal inflammation as indicated by the mean values of the gingival index (GI), probing depth (PD), and clinical attachment level (CAL), as well as the percentage of sites with bleeding on probing (BOP) and of those with PD and CAL of ≥4 mm at reassessment, although the teeth count and supragingival bacterial plaque level as defined by the plaque control record (PCR) were relatively unchanged after tofacitinib therapy (Fig. 1b and Table 2).
Fig. 1.
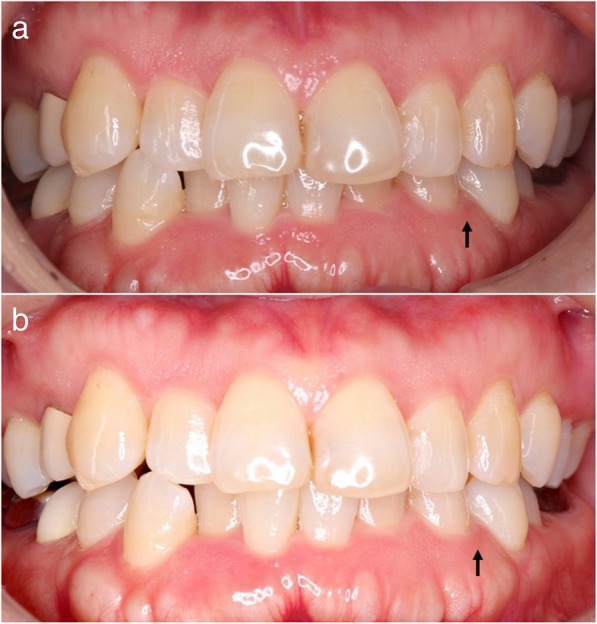
Photographs of case 1, demonstrating localized moderate chronic periodontitis a before (baseline) and b after 3 months (reassessment) of tofacitinib therapy. Improvements were observed in the gingival index (GI), probing depth (PD), and clinical attachment level (CAL) at the periodontitis-affected site with the black arrow at b (reassessment) compared to those at a (baseline), although the supragingival bacterial plaque level as defined by the plaque control record (PCR) was relatively unchanged (baseline to reassessment: 2 to 0 for GI; 4 mm to 2 mm for both PD and CAL)
Table 2.
Patient periodontal data at baseline and reassessment
| Parameter | Case 1 | Case 2 | ||
|---|---|---|---|---|
| Baseline | Reassessment | Baseline | Reassessment | |
| Teeth count | 26 | 26 | 20 | 20 |
| PCR (%) | 11.5 | 12.5 | 57.5 | 55.0 |
| Mean GI | 0.5 | 0.2 | 0.8 | 0.3 |
| BOP (%) | 3.9 | 0.6 | 8.3 | 0.0 |
| Mean PD (mm) | 2.8 | 2.0 | 3.0 | 2.2 |
| PD ≥ 4 mm (%) | 10.3 | 0.0 | 25.0 | 1.7 |
| Mean CAL (mm) | 2.8 | 2.0 | 3.1 | 2.3 |
| CAL ≥ 4 mm (%) | 10.3 | 0.0 | 25.0 | 1.7 |
Case 2: The patient was a 43-year-old non-smoking woman with a 39-month history of RA. Before tofacitinib was administered, she had been treated with MTX (4 mg/week) and BUC (100 mg/day), and the DAS28-CRP was well controlled for 29 months as follows: from 2.0 (the baseline) to 1.2 (after 29 months of treatment). However, because of the lack of a response to the treatment with MTX and BUC, the further administration of tofacitinib (10 mg/day) was started. The patient had no complications, such as diabetes mellitus, hypertension, or systemic viral infections, at baseline.
The rheumatologic assessments showed a decrease in the SDAI, DAS28-CRP, tender joint count (TJC), swollen joint count (SJC), and gVAS at reassessment after starting tofacitinib therapy (Table 1). The laboratory analyses of blood samples showed that the serum levels of rheumatoid factor (RF), matrix metalloproteinase-3 (MMP-3), and IL-6 were decreased at reassessment compared to the values at baseline (Table 1).
Furthermore, the periodontal assessments indicated that the patient had localized moderate chronic periodontitis at baseline according to the criteria of the CDC/AAP [14]. Tofacitinib therapy reduced periodontal inflammation as indicated by the mean values of the GI, PD, and CAL, as well as the percentage of sites with BOP and of those with PD and CAL of ≥4 mm at reassessment, although the teeth count and supragingival bacterial plaque level as defined by the PCR were relatively unchanged after tofacitinib therapy (Table 2).
Discussion
These are the first reported cases in which tofacitinib may have a beneficial effect on periodontitis. Tofacitinib therapy reduced the SDAI, gVAS, and the serum levels of IL-6 in both cases. In particular, tofacitinib has been shown to be efficacious in improving pain as indicated by patient’s assessment [15]. These observations are consistent with the results of other studies that indicated the efficacy of tofacitinib in relieving the rheumatologic condition [11, 12] and showed that tofacitinib was able to suppress IL-6 signaling directly [10]. Improvements were also observed in the serum levels of TNF-α and anti-CCP IgG in one case and in those of RF and MMP-3 in the other case. Patients who received tofacitinib exhibited an inconsistent clinical response, likely due to the low disease activity of RA at the start of the administration.
Notably, both cases also exhibited a decrease in periodontal inflammation, although their teeth count, bacterial plaque level, and RA medication were relatively unchanged after tofacitinib therapy. Rheumatologists and periodontists were blinded regarding the rheumatologic and periodontal conditions as well as the administration of tofacitinib. Corticosteroid and non-steroidal anti-inflammatory drugs have little beneficial effect on periodontitis [16], and the clinical effects of MTX on periodontitis have not been studied. Therefore, the reduction in periodontal inflammation is likely due to the administration of tofacitinib rather than to any changes in periodontitis-related risk factors, such as bacterial plaque and smoking habit. This efficacy of tofacitinib might be due to the suppression of IL-6 signaling [10], which has been shown to be related to the reduction in periodontal inflammation [7, 8, 17, 18]. However, we were unable to evaluate these JAK-mediated signaling of cytokines in periodontal tissue due to the ethical limitations. In addition, improvements in the clinical periodontal conditions resulted in changes in both case definitions from baseline to reassessment (from moderate to no periodontitis for case 1 and from moderate to mild periodontitis for case 2) according to the criteria of the CDC/AAP [14].
Conclusions
The present two cases demonstrated for the first time that tofacitinib may have a beneficial effect on periodontitis. However, more research is required to understand the relationship between periodontitis and tofacitinib therapy.
Acknowledgements
The authors would like to acknowledge all the staff of the Niigata Rheumatic Center for their assistance in investigating these patients.
Funding
The design of the study and collection, analysis, and interpretation of data and in writing the manuscript was supported by Grant-in Aid for Scientific Research (15 K15764) from the Japan Society for the Promotion of Science (JSPS), Tokyo, Japan.
Availability of data and materials
The datasets generated or analyzed during the present study are included within the article and the remaining are available from the corresponding authors on reasonable request.
Abbreviations
- AAP
American Academy of Periodontology
- ADA
Adalimumab
- BOP
Bleeding on probing
- BUC
Bucillamine
- CAL
Clinical attachment level
- CCP
Cyclic citrullinated peptide
- CDC
Centers for Disease Control and Prevention
- DAS28-CRP
Disease activity score in 28 joints using C-reactive protein
- GI
Gingival index
- gVAS
global visual analogue scale
- IgG
Immunoglobulin G
- IL-6
Interleukin-6
- JAK
Janus kinase
- MMP-3
Matrix metalloproteinase-3
- MTX
Methotrexate
- PCR
Plaque control record
- PD
Probing depth
- PSL
Prednisolone
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SDAI
Simplified disease activity index
- SJC
Swollen joint count
- TCZ
Tocilizumab
- TJC
Tender joint count
- TNF
Tumor necrosis factor
Authors’ contributions
TK and SI conceived of and designed the study and wrote the manuscript. TK made the diagnosis of periodontitis and collected periodontal data. SI was involved in the rheumatologic diagnosis, management, and data acquisition. AM, HI, and HY critically reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All patients gave their written informed consent for their record to be published in the present study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tetsuo Kobayashi, Email: kotetsuo@dent.niigata-u.ac.jp.
Satoshi Ito, Email: s-ito@water.ocn.ne.jp.
Akira Murasawa, Email: rasenami@poppy.ocn.ne.jp.
Hajime Ishikawa, Email: med@ra-center.com.
Hiromasa Yoshie, Email: yoshie@dent.niigata-u.ac.jp.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.de Pablo P, Chapple ILC, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 3.Leech MT, Bartold TM. The association between rheumatoid arthritis and periodontitis. Best Pract Res Clin Rheumatol. 2015;29:189–201. doi: 10.1016/j.berh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatic arthritis. Nat Rev Rheumatol. 2017;13:606–620. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- 5.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 6.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Yoshie H. Host responses in the link between periodontitis and rheumatoid arthritis. Curr Oral Health Rep. 2015;2:1–8. doi: 10.1007/s40496-014-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi T, Ito S, Kobayashi D, Kojima A, Shimada A, Narita I, Murasawa A, Nakazono K, Yoshie H. Interleukin-6 receptor inhibitor tocilizumab ameliorates periodontal inflammation in patients with rheumatoid arthritis and periodontitis as well as tumor necrosis factor inhibitors. Clin Exp Dent Res. 2015;1:63–73. doi: 10.1002/cre2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarilina A, Xu K, Chan C, Ivashkiv LB. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum. 2012;64:3856–3866. doi: 10.1002/art.37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, Shurmur R, Singhal AK, Wei N, Rosengren S, Kaplan I, Krishnaswami S, Luo Z, Bradley J, Firestein GS. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signaling in rheumatoid arthritis. Ann Rheum Dis. 2015;74:1311–1316. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Meijide JAG, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz TK, Wallenstein GV, Krishnaswami S, Bradley JD, Wilkinson B, Standard Investigators ORAL. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 12.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF, Start Investigators ORAL. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 13.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykert V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg J-E, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, Mclnnes I, Mysler E, Nash P, Pavelka K, Poór G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 14.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM, Burgos-Vargas R, Wilkinson B, Zerbini CAF, Zwillich SH. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690, 550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, place-controlled trial. Ann Rheum Dis. 2010;69:413–416. doi: 10.1136/ard.2009.108159. [DOI] [PubMed] [Google Scholar]
- 16.Heasman PA, Hughes FJ. Drugs, medications and periodontal disease. Br Dent J. 2014;217:411–419. doi: 10.1038/sj.bdj.2014.905. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Okada M, Ito S, Kobayashi D, Ishida K, Kojima A, Narita I, Murasawa A, Yoshie H. Assessment of interleukin-6 receptor inhibition therapy on periodontal condition in patients with rheumatoid arthritis and chronic periodontitis. J Periodontol. 2014;85:57–67. doi: 10.1902/jop.2013.120696. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Yokoyama T, Ito S, Kobayashi D, Yamagata A, Okada M, Oofusa K, Narita I, Murasawa A, Nakazono K, Yoshie H. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480–1488. doi: 10.1902/jop.2014.140194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the present study are included within the article and the remaining are available from the corresponding authors on reasonable request.


