Abstract
Azelaic acid is an antiacne drug by inhibiting thioredoxin reductase enzyme of Propionibacterium acnes (P. acnes) that affects the inhibition of bacterial DNA synthesis which occurs in the cytoplasm. Azelaic acid must penetrate through the stratum corneum to the sebaceous tissue and into cytoplasm by passing through thick peptidoglycan of P. acnes. Thus, it is necessary to increase the penetration of azelaic acid that formulated based ethosome. This study using thin-layer hydration method forms an ethosomal suspension with variations of concentration ethanol (30%, 35%, and 40%). Antibacterial activity was conducted using broth dilution method to determine minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The antibacterial activity of azelaic acid ethosome cream based was compared with the marketed cream (Zelface® cream). Azelaic acid ethosome with 35% ethanol has given best result with entrapment efficiency of 94.48% ± 0.14%. Antibacterial activity to P. acnes showed that azelaic acid ethosome-based cream was given better activity than marketed cream (Zelface® cream). The value of MIC and MBC of azelaic acid ethosome-based cream was 250 μg/ml while the marketed cream (Zelface® cream) was shown MIC of 250 μg/ml and MBC of 500 μg/ml. This study proved that the azelaic acid ethosome-based cream has better antibacterial activity.
Key words: Antiacne, azelaic acid, cream, ethosome, minimum bactericidal concentration, minimum inhibitory concentration, Propionibacterium acnes
INTRODUCTION
Acne is one of the most common skin diseases with a percentage of occurrence in women being 27% and in men being 34%.[1] Acne formed when sebum production is increased and then the sebum will be broken down by microorganism into free fatty acids so that the colonization of microorganism will be increased.[2,3] Propionibacterium acnes (P. acnes) is a well-known bacteria having major role in acne development.[4,5,6] P. acnes is a Gram-positive anaerobic bacterium that has a thick peptidoglycan. Sebum is used as a nutrient by P. acnes bacteria. P. acnes can induce an inflammatory response by triggering the release of inflammatory cytokines resulting inflammation in the sebaceous follicle.[7] Hence, one of the acne treatment reduces the population of P. acnes.
Azelaic acid is mainly considered an antiacne drug. Azelaic acid has been shown to decrease the population of P. acnes on skin surface and sebaceous follicle.[8,9] Azelaic acid acts as an antibacterial to P. acnes by inhibiting thioredoxin reductase enzyme that affects the inhibition of bacterial DNA synthesis.[10,11] DNA synthesis of P. acnes occurs in the semi-nucleus that found in the cytoplasm. Hence, to reach that part, azelaic acid must be penetrate through the stratum corneum as the main barrier of skin to the sebaceous tissue in the dermis layer and then penetrate again into the cytoplasm by passing through the thick peptidoglycan of P. acnes.
Azelaic acid was used as antiacne drug in conventional topical formulations, i.e. cream and gel. Azelex cream is one of the commercial cream containing azelaic acid. In 1995, Breatnach did a research on penetration test of azelex cream and given result that only 4% of the dosage can penetrate into stratum corneum.[12] In 2004, Esposito et al. proved that the ethosomes increase the penetration of azelaic acid drugs compared with liposomes.[13] Burchacka et al. (2016) also proved that the liposome of azelaic acid effectively inhibits the growth of Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, and Aspergillus brasiliensis fungi.[14] Recent studies of Mistry and Ravikumar demonstrated that the zone of inhibition of the azelaic acid ethosome-based gel is greater than azelaic acid creams and gels in the market.[15]
Based on the data that have been described above, in this research, azelaic acid will be formulated into ethosome and then the azelaic acid ethosome will be formulated into a cream. Azelaic acid ethosome-based cream will be seen antiacne activity against P. acnes using broth dilution method to determine the value of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) compared with marketed cream that are not formulated in lipid bilayer vesicles (Zelface®).
MATERIALS AND METHODS
Chemical and reagents
Azelaic acid (Sigma, US), phospholipon 90 G (90% hydrogenated soy phosphatidylcholine, US), ethanol 96% (Merck, Germany), propylene glycol (Dow Chemical Co.), potassium dihydrogen phosphate (Merck, Germany), hydrochloric acid (Brataco, Indonesia), sodium hydroxide (Brataco, Indonesia), methanol (Merck, Germany), dichloromethane (Merck, Germany), cream base, aqua demineralisata (Brataco, Indonesia), P. acnes stock (Faculty of Medicine, Universitas Indonesia), and AnaeroGen™ compact (Thermo Scientific, United States) were used in this study.
Preparation of azelaic acid ethosomal
The azelaic acid ethosomal was prepared by thin-layer hydration method with various ethanol concentration, i.e., 30%, 35%, and 40%. The formula of azelaic acid ethosome-based cream in this study is listed in Table 1.
Table 1.
Formula of azelaic acid ethosome-based cream
| Material | Concentration (%) |
||
|---|---|---|---|
| ETHO 30 | ETHO 35 | ETHO 40 | |
| Azelaic acid | 10 | 10 | 10 |
| Phospholipon 90 G | 10 | 10 | 10 |
| Ethanol | 30 | 35 | 40 |
| Phosphate buffer pH 7.4 | Ad 100 mL | Ad 100 mL | Ad 100 mL |
ETHO 30: Ethosomal suspensions produced using 30% ethanol
Lipid phase consists of Phospholipon 90 G and aqueous phase consists of azelaic acid, ethanol, and phosphate buffer pH 7.4. The thin layer formed by dissolving lipid phase with dichloromethane and methanol (2:1) using rotary evaporator. Then, the thin layer is stored in the refrigerator with temperature 4°C ± 2°C for 24 h. After that, the thin layer was hydrated using aqueous phase for 50 min until an ethosomal suspension is formed. The ethosomal suspension was characterized to obtain the optimum suspension. The parameters were entrapment efficiency (EE), particle size distribution, potential zeta, polydispersity index, and morphology of vesicular.
Entrapment efficiency test
EE test (%EE) was obtained by indirect method using supernatant. Ethosomal suspension was centrifuged with cellulose acetate filter 0.22 μm for 1.5 h at 9500 rpm to obtain supernatant. The supernatant and total ethosomal suspensions were analyzed for drug entrapped using UV-Vis spectrophotometer. The total ethosomal suspension was dissolved in methanol before measuring the absorbance.
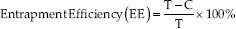
Information:
T.: Total concentration of azelaic acid in ethosome suspension (μg/mL)
C: The concentration of azelaic acid present in the supernatant (μg/mL).
Particle size distribution, polydispersity index, and zeta potential
The particle size, size distribution, and zeta potential of ethosomes were determined by Zeta Potential Analyzer Model Zeta-Phase Analysis Light Scattering (Brookhaven Instruments Co., NY, USA) at 25°C with a scattering angle of 90° after diluted of 10 PL ethosome with 4-mL Milli-Q water. All determinations were performed in triplicate.
Determination of the selected azelaic acid ethosome formula
The formula that gives results high percentage of entrapped drug, nanosized (<200 nm), polydispersity index <0.7, and the zeta potential which is more negative than −30 or more positive than +30 mV was chosen to be incorporated into cream dosage form.
Transmission electron microscope studies
Ethosome morphology of selected formula was analyzed by transmission electron microscope (TEM, JEOL JEM-1400). Samples were shed on a carbon-coated copper grid; the droplet then was dried at room temperature and colored using uranyl acetate solution. After drying, they were viewed under a microscope at 1,000 - 20,000 magnification and the acceleration voltage of 100 kV.
Production of dried ethosomal suspension
The selected ethosomal suspension was dried by freeze-drying method using EYELA FDU-1200 in vacuum at temperature −48.9°C for 2 days.
Preparation of ethosome cream
The dried ethosomal suspension was formulated into a cream. Aqueous phase that consists of glycerin and propylene glycol was dissolved in hot water. In the others, oil phase that consists of stearic acid, cetyl alcohol, isopropyl myristate, glyceryl monostearate, and butylated hydroxytoluene was heated at temperature 75°C. The oil phase and triethanolamine were added to aqueous phase and left to swell for 15 min at 3000 rpm until a homogeneous cream formed. Then, the cream is kept to room temperature. The dried ethosomal suspension was added into the base of cream for 5 min at 100 rpm until the concentration of azelaic acid in cream reaches 20%.
Evaluation cream
Organoleptic evaluation
Azelaic acid ethosome-based cream was inspected visually for their color, homogeneity, consistency, and phase separation.
Azelaic acid content in ethosome-based cream
Specific amount of azelaic acid ethosome-based cream is dissolved in methanol and then ultrasonic for 15 min to increase the solubility. Then, the solution was transferred into a 25-mL volumetric flask and diluted with phosphate buffer pH 6.8 until the volume. The content of azelaic acid was calculated by UV-Vis spectrophotometer.
pH measurement
The pH was measured using a pH meter which was calibrated before use with standard buffer solutions at pH 4 and 7. An electrode was inserted into the azelaic acid ethosome-based cream and measured the value of pH. The average pH was calculated from three replicates.
Viscosity
Viscosity of the azelaic acid ethosome-based cream was determined by Brookfield Viscometer at 25°C using spindle no. 3.
In vitro testing
Antibacterial activity was evaluated using the broth dilution method to determine the value of MIC and MBC. Bacterial suspension was made by diluting some colonies in stock bacterial to brain–heart infusion broth (BHIB). Bacterial suspension of P. acnes was compared visually with 3.0 McFarland Standard.
Testing solution A was made by dissolving azelaic acid ethosome-based cream with dimethyl sulfoxide (DMSO) until the concentration reaches 2000 μg/mL (stock solution A). Testing solution B was made by dissolving Zelface® cream with DMSO until the concentration reaches 2000 μg/mL (stock solution B). From the stock solutions A and B, it made variations of concentration, i.e., 1000, 500, 250, 125, and 62.5 μg/mL.
The specific amount of 5 variation concentration test solution A or B, BHIB, and bacterial suspension was transferred into the test tube and then the solution was mixed by vortex for 10 s. After that, the test tube was placed in anaerobic condition using the anaerobic jar and was incubated at 37°C for 48 h. The value of MIC was determined by checking the turbidity of inoculum suspension compared to positive control (the test tube consists of BHIB and bacterial suspension) and negative control (the test tube consists of BHIB, the test tube consists of BHIB and 1000 μg/mL test solution A, and the test tube consists of BHIB and 1000 μg/mL test solution B). The value of MIC was read as the lowest concentration that showed clear inoculum suspension. The value of MIC was determined by three replicates.
To determine the value of MBC, the test tube that contains 5 variation concentration test solution A or B, BHIB, and bacterial suspension was streak into Brucella agar using ose and then incubated at 37°C for 48 h in anaerobic condition. The value of MBC was read as the lowest concentration without visible growth. The result was compared with positive and negative controls. The value of MBC was determined by three replicates.
RESULTS
Preparation of azelaic acid ethosomes
As described in the method, ethosomal suspensions were spontaneously produced by hydrating thin layer that contains
Phospholipon 90G with aqueous phase containing azelaic acid, ethanol, and phosphate buffer pH 7.4. Ethosomal suspensions were produced with increasing amount of ethanol (30%, 35%, and 40%). In all cases, the use of variations concentration of ethanol resulted white milky suspension with no significant differences in macroscopic aspect. Ethosomal suspensions with 35% ethanol (ETHO 35) were selected to formulate into a cream. The selected formula is an ethosomal suspension that has high percentage EE, particle size distribution <200 nm, polydispersity index <0.7, and zeta potential which is more negative than −30 or more positive than +30 mV [Table 2]. TEM study for ETHO 35 also showed that ETHO 35 has particle size <200 nm [Figure 1].
Table 2.
Characterization of azelaic acid ethosomal
| Formula | Z mean (nm) | Polydispersity index | Zeta potential (mV) | EE (%) |
|---|---|---|---|---|
| ETHO 30 | 283.5±9.08 | 0.502±0.06 | 32.13±7.77 | 90.08±0.13 |
| ETHO 35 | 179.3±2.23 | 0.665±0.02 | −34.87±0.35 | 94.48±0.14 |
| ETHO 40 | 1377±76.06 | 0.975±0.04 | −22.80±2.25 | 92.46±0.30 |
ETHO 30: Ethosomal suspensions produced using 30% ethanol, EE: Entrapment efficiency
Figure 1.
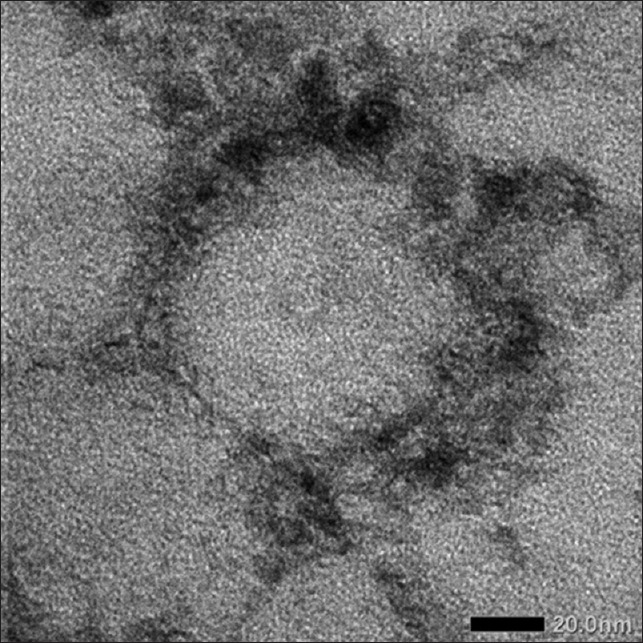
Transmission electron microscope of ETHO 35 at 20,000 magnification
Preparation of ethosomal cream
ETHO 35 was dried by freeze-drying method. The dried ETHO 35 was formulated into a cream. Evaluation of the azelaic acid ethosome-based cream is summarized in Table 3.
Table 3.
Evaluation of azelaic acid ethosome-based cream
| Parameters | Result |
|---|---|
| Organoleptic | White, smooth, and homogeneous |
| pH | 4.90 |
| Phase separation | No evidence |
| Viscosity | 4900 cp |
The final concentration of azelaic acid in azelaic acid ethosome-based cream was 20%. The level of azelaic acid in the final formulations is the same with the level of azelaic acid in market. Azelaic acid concentration in azelaic acid ethosome-based cream was determined by UV-Vis spectrophotometer and summarized in Table 4.
Table 4.
The concentration of azelaic acid in azelaic acid ethosome-based cream
| Theoretical concentrations (µg/mL) | Concentrations actually (µg/mL) | Percentage of concentration | Mean±SD | Percentage of azelaic acid in cream |
|---|---|---|---|---|
| 506.10 | 533.92 | 105.50 | 104.99±0.38 | 21.00±0.08 |
| 530.85 | 104.89 | |||
| 529.31 | 104.59 |
SD: Standard deviation
In vitro testing
Antibacterial activity was evaluated by broth dilution method to determine the value of MIC and MBC. The value of MIC of azelaic acid ethosome-based cream (A) and marketed cream (Zelface®) (B) is summarized in Table 5, and the value of MBC of azelaic acid ethosome-based cream (A) and marketed cream (Zelface®) (B) is summarized in Table 6 and Figure 2.
Table 5.
The value of minimum inhibitory concentration of azelaic acid ethosome-based cream (A) and marketed cream (Zelface®) (B)
| Concentrations (µg/mL) | Turbidity |
|
|---|---|---|
| A | B | |
| 1000 | - | - |
| 500 | - | - |
| 250 | - | - |
| 125 | + | + |
| 62.5 | + | + |
The result of (−) indicates no turbidity and (+) indicates turbidity occurred
Table 6.
The value of minimum bactericidal concentration of azelaic acid ethosome-based cream (A) and marketed cream (Zelface®) (B)
| Concentrations (µg/mL) | Visible growth |
|
|---|---|---|
| A | B | |
| 1000 | - | - |
| 500 | - | - |
| 250 | - | + |
| 125 | + | + |
| 62.5 | + | + |
The result of (−) indicates no bacterial growth and (+) indicates bacterial growth
Figure 2.
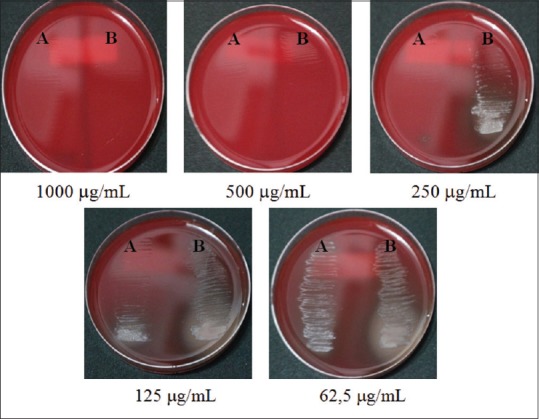
Minimum bactericidal concentration of azelaic acid ethosome-based cream (A) and marketed cream (Zelface®) (B)
From the result of Tables 5 and 6, the value of MIC both of azelaic acid ethosome-based cream and marketed cream (Zelface® cream) is 250 μg/mL. The value of MBC of azelaic acid ethosome-based cream is 250 μg/mL while marketed cream (Zelface® cream) is 500 μg/mL. In concentration 125 μg/mL, visible growth of bacteria in azelaic acid ethosome-based cream is fewer than marketed cream (Zelface® cream).
DISCUSSION
Formulation of azelaic acid ethosomal was successfully prepared, optimized, and characterized. ETHO 35 was selected to formulated into a cream because it has high percentage EE 94.48 ± 0.14, particle size distribution 179.3 ± 2.23 nm, polydispersity inde × 0.665 ± 0.02, and zeta potential −34.87 ± 0.35 mV. Azelaic acid ethosome-based cream has given better antibacterial activity due to the presence of ethanol and phospholipids in the established ethosomal component. Ethanol is known to change the physical properties of bacterial environments and bacterial interactions with their environment. Ethanol can change lipid composition in bacteria, so bacterial growth can be inhibited. In addition, ethanol can also interfere with the integrity of bacterial cell walls making it easy for the ethosome to enter into the bacterial cytoplasmic membrane.[16] Inside the cell walls of Gram-positive bacteria, there is an acid called the teichoic acid. Teichoic acid is hydrophile and can dissolves in ethanol, so it will damage the structure of the bacterial cell wall.[17] Lipids in the ethosome also play an important role in helping the entry of azelaic acid into the bacterial cytoplasmic membrane. Lipids of the ethosome will interact with lipids in the bacterial cytoplasmic membranes by fusion.[18,19,20] Fusion reactions between the ethosomes and bacteria are very rapid and spontaneous based on noncovalent bonds such as van der Waals bonds and hydrophobic interactions so that the inner drug will quickly enter the cytoplasmic membrane, thus reducing efflux pump of the drug.[19] This will cause the concentration of azelaic acid into the cytoplasmic membrane to be optimal and will reduce the effect of the drug. Azelaic acid ethosome-based cream showed better activity to against P. acnes than marketed cream (Zelface® cream) which indicates that azelaic acid ethosome-based cream is more efficient and reliable as antiacne drug than conventional marketed cream. Hence, in this experiment, azelaic acid ethosome-based cream was efficient and reliable as compared to conventional marketed cream based on the antibacterial activity against P. acnes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Universitas Indonesia that funded this research through PITTA Grant 2017. We also thank the technical staff of the Pharmaceutical Research Laboratory, Faculty of Pharmacy Universitas Indonesia (Depok), and Microbiology Laboratory, Faculty of Medical Universitas Indonesia (Salemba), who have assisted in the implementation of this research.
REFERENCES
- 1.Klaus W, Richard A, Dick S. Fitz Patrick's Color Atlas and Sinopsis of Clininal Dermatology. New York: Medical Publishing Division; 2005. [Google Scholar]
- 2.Leyden JJ. Current issues in antimicrobial therapy for the treatment of acne. J Eur Acad Dermatol Venereol. 2001;15(Suppl 3):51–5. doi: 10.1046/j.0926-9959.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 3.West DP, West LE, Musumeci ML, Micali G. Acne vulgaris. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Well BG, Posey LM, editors. Pharmacoterapy: A Pathophysiologic Approach. New York: McGraw-Hill; 2005. [Google Scholar]
- 4.Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–65. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathak R, Kumar R, Kasama N. Staphylococcus epidermidis in human skin microbiome associated with acne: A cause of disease or defence? Res J Biotechnol. 2013;8:78–82. [Google Scholar]
- 6.Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–60. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radji M. Buku Ajar Mikrobiologi: Panduan Mahasiswa Farmasi dan Kedokteran. Jakarta: Penerbit Buku Kedokteran EGC; 2010. [Google Scholar]
- 8.Nguyen QH, Bui TP. Azelaic acid: Pharmacokinetic and pharmacodynamic properties and its therapeutic role in hyperpigmentary disorders and acne. Int J Dermatol. 1995;34:75–84. doi: 10.1111/j.1365-4362.1995.tb03583.x. [DOI] [PubMed] [Google Scholar]
- 9.Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient – A possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321–30. doi: 10.1093/jac/34.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Schallreuter KU, Wood JW. A possible mechanism of action for azelaic acid in the human epidermis. Arch Dermatol Res. 1990;282:168–71. doi: 10.1007/BF00372617. [DOI] [PubMed] [Google Scholar]
- 11.Breatnach AS. Pharmacological properties of azelaic acid. Clin Drug Invest. 1995;10:27–33. [Google Scholar]
- 12.Gollnick H. Azelaic acid-pharmacology, toxicology and mechanisms of action on keratinization in vitro and in vivo. J Dermatol Treat. 1993;4:S3–7. [Google Scholar]
- 13.Esposito E, Menegatti E, Cortesi R. Ethosomes and liposomes as topical vehicles for azelaic acid: A preformulation study. J Cosmet Sci. 2004;55:253–64. [PubMed] [Google Scholar]
- 14.Burchacka E, Potaczek P, Paduszyński P, Karłowicz-Bodalska K, Han T, Han S, et al. New effective azelaic acid liposomal gel formulation of enhanced pharmaceutical bioavailability. Biomed Pharmacother. 2016;83:771–5. doi: 10.1016/j.biopha.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Mistry A, Ravikumar P. Development and evaluation of azelaic acid based ethosomes for topical delivery for the treatment of acne. Indian J Pharm Educ Res. 2016;50:S232–243. [Google Scholar]
- 16.Fletcher M. The effects of methanol, ethanol, propanol and butanol on bacterial attachment to surfaces. J General Microbiol. 1983;129:633–41. [Google Scholar]
- 17.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rukavina Z, Vanić Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics. 2016;8:pii: E18. doi: 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Pornpattananangku D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–94. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 20.Malanovic N, Lohner K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals (Basel) 2016;9:pii: E59. doi: 10.3390/ph9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]


