Abstract
In the present study, a systematic review was conducted to evaluate the biomaterials and their effectiveness for bone augmentation in implant dentistry. The databases of Cochrane Library, Google Scholar, PubMed (National Center for Biotechnology Information), and Scopus were searched for published studies between 2006 and March 30, 2018. We only included clinical studies in this research. Due to a lack of quantitative evidence and the vast heterogeneity of the biomaterials, implant surgery sites, implant types, follow-up periods, and various implant placement techniques (1-stage or 2-stage), we could not manage to do a meta-analysis on the 13 included studies. Several techniques can result in vertical bone augmentation. Complications can be seen in vertical bone augmentation and especially in the autogenous bone grafting; however, some biomaterials showed promising results to be practical substitutes for autogenous bone. Bio-Oss and beta-tricalcium phosphate are our second-level candidates for vertical bone augmentation due to their promising clinical results with the least infection and immunologic response risk. The gold standard, however, remains the autogenous bone graft. Further clinical studies in the future with exact report of bone measures are needed to develop new comparisons and quantitative analyses.
Key words: Biomaterial, dental implant, osteoconduction, osteogenesis, osteoinduction, vertical bone augmentation
INTRODUCTION
Augmenting alveolar bone tissue around the dental implants is of great concern due to its critical role in the long-term treatment success.[1] We focused on the vertical alveolar ridge augmentation technique for this study. Due to an increase in peri-implantitis conditions in the past decade, it is crucial to provide the best bone augmenting biomaterial to accomplish the best treatment results. Tissue engineering is one of the most critical and expanding fields, which mainly cooperates with regenerative medicine and has indicated a remarkable potential in clinical dental practice applications. Biomaterials are one of the three basics in tissue engineering, namely cells, scaffolds/biomaterials, and growth/differentiation factors.[2,3,4,5] Considering their role, many biomaterials have been applied and suggested to use as an alternative to the autogenous bone which is still the gold standard for bone augmentation.[6] Aside from autogenous, xenogenic, and allogenic grafting materials, other natural and synthetic biomaterials have also been playing critical roles in dental clinical cases.[7] Till today, different types of these biomaterials have established their practical roles in dental clinics mainly based on their ease of application and predictable results. To decide which biomaterial to choose, we should consider some factors to mimic the autogenous bone structure, e.g., crystal structure, micro- and macroporosity, and intercrystalline spaces.[8] Chemical, physical, and mechanical properties of the scaffolds should be as similar as possible to that of a natural bone structure.[9,10] A good bone substituting scaffold should be settled by the resident bone cells or undifferentiated mesenchymal cells.[11,12,13] Various biomaterials have been applied into the bone defects using different surgical techniques. Autogenic, allogenic, xenogenic, and synthetic biomaterials are currently on-the-board options for a dental bone grafting process. Lack of immunological responses and a high-volume augmented bone can be considered as the main advantages of autogenic grafts, while they showed a higher infection rate. Other natural biomaterials such as xenogenic grafts can also be encouraged due to their low-content inflammatory reactions and high longevity.[14] Synthetic biomaterials such as bioactive glasses are also another promising choice for bone augmentation considering their notable neosynthetized bone and low amount of residual graft. We retrieved relevant studies about alveolar bone augmentation in implant dentistry and systematically reviewed them based on the PRISMA protocol. This study aimed to systematically review the biomaterials and their effectiveness for bone augmentation in implant dentistry.
METHODS
Searching and selection of studies
We have searched four databases of Google Scholar, PubMed, Scopus, and Cochrane Library with the keywords, “Biomaterials,” “Bone Augmentation,” and “Dental Implant.” Searching query was modified for each database if needed to achieve most relevant studies. Then, we collected data, based on the relevance to the study topic and the main objective. Any conflicts between the authors were resolved by abstract and full-text reading to determine the criteria which were used in the studies. Twenty-one studies were chosen according to the title skimming and abstract screening, and then the references of these studies were manually searched and checked in Google Scholar. After removing duplicates, we added the relevant ones based on the title and abstract screening. Only clinical trials and case reports were included; the exclusion criteria were as follows: studies which included patients with any systemic disease (e.g., diabetes, cancer, and angina pectoris) and patients older than 65 years of age or younger than 15 years, studies with implant surface modification interventions or maxillary sinus lifting or sinus floor augmentation procedures, non-English language studies, and those reflecting information from before 2006. In the final step, inclusion was according to a consensus between the two authors and 13 studies were chosen for data extraction.
Risk of bias and quality of studies
Both authors independently evaluated the risk of bias for the studies using the Cochrane Collaboration's tool for clinical trials named as grades of recommendation, assessment, development, and evaluation (GRADE) [Supplementary Table 1]. Furthermore, the complications, blinding, source of funding, sample size, and the inclusion and exclusion criteria were assessed for each study. The risk of bias was determined based on these evaluations as “low,” “moderate,” or “high.” Conflicts between the authors were resolved by a consensus. Finally, the overall quality of each study was defined as “high” or “moderate” using the GRADEpro online service. Also, the “importance” of each study was determined by a consensus between authors, based on all of the evaluations in a range from 1 to 9 as defined in the GRADE protocol. The importance of studies was reported as “not important,” “important,” or “critical” according to their related scores.
Supplementary Table 1.
Quality assessment of the included studies
| Quality assessment |
№ of participants in each group |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of implant abutments | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | biomaterials | placebo | Relative (95% CI) | Absolute (95% CI) | ||
| b-TCP/autologous BMMNCs (follow up: 12 months) | ||||||||||||
| 17 | Case series | not serious | not serious | not serious | not serious | No blinding, relative low sample size | 3 | - | - | 68.48% | High | IMPORTANT |
| Bioactive glass (follow up: 24 months) | ||||||||||||
| 5 | Case series | not serious | not serious | not serious | not serious | no blinding, reporting bias risk, low sample size | 3 | - | - | - | Moderate | NOT IMPORTANT |
| xenograft material/bone fragments from traumatic site (GBR) (follow up: range 6 months to 48 months) | ||||||||||||
| 10 | Case series | not serious | not serious | not serious | not serious | No blinging, relative low sample size | 3 | - | - | - | Moderate | IMPORTANT |
| Prehydrated Corticocancellous Bone Graft (GBR) without autogenous bone (follow up: 24 months) | ||||||||||||
| 1 | Case report | not serious | not serious | not serious | not serious | No blinding, low sample size | 1 | - | - | - | Moderate | NOT IMPORTANT |
| collagen sponge/nonresorbable high-density PTFE membrane (follow up: 12 months) | ||||||||||||
| 2 | Case report | not serious | not serious | not serious | not serious | No blinding, low sample size | 1 | - | - | 49.3% | Moderate | NOT IMPORTANT |
| Titanium Mesh/Combination of Autogenous Bone and Anorganic Bovine Bone (follow up: 24 months) | ||||||||||||
| 44 | Case series | not serious | not serious | not serious | not serious | No blinding | 16 | - | - | 28.27% | Moderate | CRITICAL |
| Flexible Heterologous Cortical Bone Sheet (follow up: 36 months) | ||||||||||||
| 49 | Case series | not serious | not serious | not serious | not serious | No blinding | 18 | - | - | - | Moderate | CRITICAL |
| Deproteinized Bovine Bone Mineral/free gingival graft (follow up: 6 months) | ||||||||||||
| 1 | Case report | not serious | not serious | not serious | not serious | No blinding, low sample size | 1 | - | - | - | Low | NOT IMPORTANT |
| Iliac crest vs. bovine anorganic bone (follow up: 16 months) | ||||||||||||
| 38 | randomized clinical trial | not serious | not serious | not serious | not serious | - | 5 | 5 | - | MD 1.51 mm higher (7.07 lower to 10.09 higher) |
High | CRITICAL |
| Xenogenic bone blocks (Bio-Oss) (follow up: 9 months) | ||||||||||||
| 18 | Case series | not serious | not serious | not serious | not serious | No blinding | 9 | - | - | 50.5% | High | IMPORTANT |
| Autogenous demineralized dentin matrix from extracted tooth vs anorganic bovine bone (follow up: 6 months) | ||||||||||||
| 33 | randomized clinical trial | not serious | not serious | not serious | not serious | - | 21 | 12 | - | mean 5.38 mm higher (2.65 higher to 4.75 higher) |
High | CRITICAL |
| Assessment of vertical ridge augmentation in anterior aesthetic zone using onlay xenografts with titanium mesh versus the inlay bone grafting technique: A randomized clinical trial | ||||||||||||
| 40 | randomized clinical trial | not serious | not serious | not serious | not serious | - | 8 | 8 | - | mean percentage of vertical bone gain: 20.7% in control group and 31.6% in study group | High | CRITICAL |
| Long-term outcomes of implants placed after vertical alveolar ridge augmentation in partially edentulous patients: a 10-year prospective clinical study | ||||||||||||
| 82 | prospective clinical study | not serious | not serious | not serious | not serious | - | 41 | - | - | - | Moderate | NOT IMPORTANT |
Measures of treatment effect
The mean vertical bone augmentation at implant sites and peri-implant marginal bone losses were reported as we did not get enough statistical data to calculate the standard error. The unit of analysis to determine the study quality was the number of implant abutments. Within final studies, we did not find necessary data for the analysis; thus, we sent E-mails to the electronic links or E-mail addresses provided in the studies, but we did not get any response back from them. In the other six studies, weighted mean differences and standard deviations with 95% confidence intervals were used for each study to express the effect measures on continuous outcomes (i.e., vertical bone augmentation and peri-implant marginal bone loss).
Software and applications
The Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. of Cochrane Library was used to create the flow diagram of searching and selecting the studies. The GRADEpro online service was used to create the study quality table. The tables of quantitative and qualitative analysis were created by excel software (Microsoft, Redmond, Washington, 2016), and all of the references were inputted by Endnote (Version X7, Thomson reuters, Canada).
RESULTS
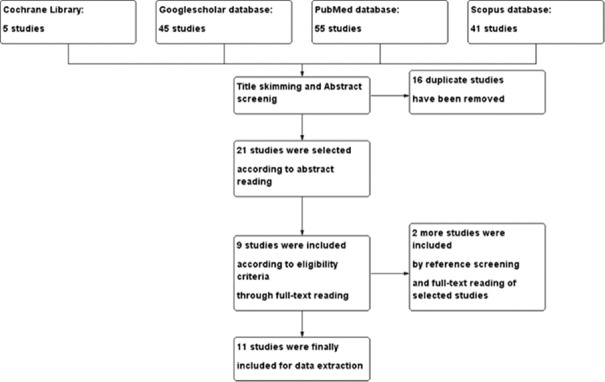
The search results and the number of chosen studies in each step are shown in Figure 1.
Figure 1.
Study selection flow chart
Some qualitative [Supplementary Table 2] and quantitative [Supplementary Table 3] data were extracted.
Supplementary Table 2.
Qualitative evaluation of the included studies
| Author/date | Pieri et al. 2008 | Pang et al. 2016 | Bulgin and Hodzic 2015 | Aimetti et al. 2017 | Cucchi and Ghensi 2014 | Cechetti et al. 2014 | Ludovichetti et al. 2011 | Felice et al. 2009 | Gatti et al. 2014 | Li et al. 2013 | Kim and Leem 2014 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomaterials | Anorganic bovine bone and autogenous bone (30:70), micro-mesh | Autogenous demineralized dentin matrix (AutoBT), Bio-Oss | BMMNCs, OSferion (β-TCP) granules | Collagen sponge, high-density d-PTFE membrane | Corticocancelous porcine-derived bone, d-PTFE membrane (without autogenous bone) | Deproteinized bovine bone, free gingival graft | Flexible cortical equine bone sheet (without GBR membrane) | Iliac crest bone versus bovine anorganic bone | PerioGlas (bioactive glass) mixed with autogenous bone | Xenogenous bone block (Bio-Oss) onlay graft | Xenograft material and bone fragments from traumatic site, resorbable collagen membrane or titanium mesh |
| Study design | Case series | Prospective randomized clinical trial | Case series | Case report | Case report | Case report | Case series | Randomized clinical trial | Case series | Case series | Case series |
| Study sample | 16 patients, 44 implant abutments | AutoBT in 21 sites of 15 patients, Bio-Oss in 12 sites of 9 patients | 3 patients, 17 implant abutments | 1 patient, no implant abutment | 1 patient, 1 implant abutment | 1 patient, 1 implant abutment | 18 patients, 49 implant abutments | 10 patients, 20 implant abutments | 3 patients, 5 implant abutments | 9 patients, 18 implant abutments | 3 patients, 10 implant abutments |
PTFE: Polytetrafluoroethylene, β-TCP: Beta-tricalcium phosphate, GBR: Guided bone regeneration
Supplementary Table 3.
Quantitative evaluation of the included studies
| Biomaterials | Treatment success and failures | Posttreatment evaluation method | Vertical bone augmentation | Bone loss after treatment | Reference |
|---|---|---|---|---|---|
| Anorganic bovine bone and autogenous bone (30:70), micro-mesh as the GBR membrane | One of the implants become exposed and was removed, all of the implants were retained after 2 years (100% survival rate and 91.3% success rate) | Clinical examination and CT at baseline and 8 to 9 months after the bone grafting, clinical examinations and PA radiographs at every 6 months till 2 years after the prosthetic loading | Mean vertical bone augmentation equals to 3.71 mm (SD=1.24 mm) | Mean bone resorption around the implants equals to 1.37 mm (SD=0.32 mm), 3 implants showed bone resorption >2 mm | Pieri et al., 2008 |
| Autogenous demineralized dentin matrix (AutoBT), Bio-Oss | No infection or wound dehiscence, ISQ of AutoBT-grafted sites equals to 72.80 (SD=10.81), ISQ of Bio-Oss-grafted sites equals to 70.0 (SD=12.86) | Clinical probing through resin template from graft placement till 6 months postoperative, CT and histomorphometric analysis 6 months after the bone grafting, panoramic radiography after implant placement | 5.38 mm in AutoBT (SD=2.65 mm), 6.56 in Bio-Oss (SD=3.54 mm) at 6 months postextraction | Not mentioned | Pang et al., 2016 |
| BMMNCs (autologous cell source) andOSferion (ß-TCP) granules | No adverse tissue reaction, infection or delayed healing, good peri-implant health within 12 months after bone graft | Panoramic radiography at 1 and 12 monthsafter operation | 15.3 mm mean bone augmentationat 12 months | Not mentioned, all patients maintained good peri-implant health and oral hygiene | Bulgin and Hodzic, 2015 |
| Collagen sponge, high-density d-PTFE membrane | No signs of infection, preserved keratinized tissue, no implant complications | CBCT at 12 months after the bone grafting, clinical examination, histologic analysis, and CT at 12 months after implant placement | The overall mean percentage of newly formed bone equals to 49.3% (SD=4.7%) | No implant was placed | Aimetti, et al., 2017 |
| Corticocancelous porcine-derived bone and d-PTFE membrane (without autogenous bone) | Uneventfully healing with no clinical signs of soft-tissue inflammation, no recession, and no membrane exposure, complete maintenance of peri-implant without any signs of bone resorption | Biopsy and histomorphometric analysis (optical microscope) at 9 months after the bone grafting, follow-up PA radiographs at 1, 12, and 24 months after prosthesis delivery (15 days after implant abutment surgery) | Adequate bone for implant placement and to support the functional loading of the implant | Complete maintenance of bone level and no signs of bone resorption in all of the follow-ups | Cucchi and Ghensi, 2014 |
| Deproteinized bovine bone and free gingival graft | Successful implant placement and favorablesoft tissue preservation | Radiographic evaluation on Tc scans and PA radiographs3 months after the bone grafting, clinical examination of implantat 6 months after implant placement | Adequate bone volume in height and in width, allowing an implant placement | Notmentioned | Cechetti et al., 2014 |
| Flexible cortical equine bone sheet (without GBR membrane) | All of the implant abutments were judged to be successful throughout the study (Albrektsson and Zarb criteria) | Panoramic radiography and clinical evaluation after implant placement at 1 week, 1 month, 6 months and then yearly for at least 3 years | Adequate bone volume to reconstruct the correct ridge profile and to ensure successful implant outcomes | Not mentioned, stable PD at all of the follow-ups (2-3 mm) | Ludovichetti et al., 2011 |
| Iliac crest bonevs. bovine anorganic bone | Two implants could not be placed in one patient at the autogenous bone group (graft failure),1 implant in the Bio-Oss group failed after loading, after implant loading, one peri-implantitis occurred at the autogenous bone group | Clinical and radiographic examination at 3 and 6 weeks and 3 months after the bone grafting, biopsy and histological analysis at 4 months after the bone grafting, PA radiographs at the implant placement time, and at 1 year after that | 31.2% in autogenous bone (SD=6.9%), 27.3% in Bio-Oss (SD=7%), at 4 months after the bone grafting | 0.82 mm peri-implant marginal bone loss in autogenous bone (SD=0.59), 0.59 mm peri-implant marginal bone loss in Bio-Oss (SD=0.4) | Felice et al., 2009 |
| PerioGlas® (Bioactive glass) and autogenous bone | All of the implant abutments were reliable and lasting throughout the study | Panoramic radiography immediate postoperative, and at 6, 12, 18, and 24 months after bone graft, a biopsy at 6 months after the bone grafting and then histomorphometric analysis and SEM microscopy, clinical examination of implant abutments at follow-up sessions | Adequate bone volume tosupport the implants placement | Maintained bone volume during all of the follow-ups | Gatti, et al., 2014 |
| Xenogenous bone block (Bio-Oss) onlay graft | No inflammation, no implant complications | Clinical examination and panoramic radiographs at 1 day, 1 month, and 6 months after the bone grafting, bone tissue segments harvested and histological analysis at 9 months after the bone graft, CTs at 6 months after the bone grafting, PA radiographs at 12 months after placing the final prosthesis | The level of bone augmentation was measured in height ranged from 4.1 to 6.0 mm | 0.5 mm peri-implant marginal bone loss (SD=1.00 mm) | Li et al. 2013 |
| Xenograft material and bone fragments from traumatic site, resorbable collagen membrane or titanium mesh as the GBR membrane | Successful placement of all the implant abutments, and progressed through the follow-up periods without complications | Cases 1, 2, and 3: CBCT at 6 months after the bone grafting; Cases 1 and 2: panoramic radiograph and clinical examination at 4 and 1 year (s) after implant placement, respectively | Adequate bone volume for implant placement | No specific bone resorption during all of the follow-ups | Kim and Leem, 2014 |
CT: Computed tomography, PA: Posteroanterior, PD: Progressive disease, SD: Standard deviations, GBR: Guided bone regeneration, CBCT: Cone-beam computed tomography, ISQ: Implant stability quotient
The risk of bias in the included studies was determined by Cochrane's GRADEpro online tool [Supplementary Table 1]. Vertical bone augmentation was considered as the first continuous outcome and the second continuous outcome was peri-implant marginal bone loss. Due to a lack of evidence, the measurement of effect size and heterogeneity assessment was not accomplished and no meta-analysis could be done.
DISCUSSION
We aimed to systematically review the biomaterials and their effectiveness for bone augmentation in implant dentistry. Between the included studies, three articles have used autogenous bone fragments. Autogenous bone grafts exhibit three main features as being osteogenic, osteoconductive, and osteoinductive.[7,15]
Iliac crest bone and bovine anorganic bone were used in two different groups of patients in a randomized controlled trial. The residual graft in the xenograft group (bovine bone) was significantly more than the autogenous bone. The main advantage of the xenograft over the autogenous graft was reported as its less invasiveness.[16] Also, a mixture of autogenous bone and anorganic bovine bone in association with micro-titanium mesh were used for bone augmentation in another case series.[17]
We observed that a mixed xenograft material (Bio-Oss) with autogenous bone and a collagen or titanium mesh membrane as a part of GBR technique can provide an adequate bone augmentation during 6 months after grafting without any specific bone resorption in the follow-up periods.[18]
Bio-Oss was the most common material being used in our data and showed some promising results comparable to autogenous bone grafts in every study.[19] Some of the best characteristics featured about this material can be listed as follows: adequate new bone formation, low reabsorption rate, osteoconductive characteristics, and compensation for the natural bone resorption caused by remodeling.[19] Bio-Oss has also been applicated in sinus floor elevation,[20] extraction socket filling,[21] and treatment of periodontal defects.[22]
Another randomized clinical trial has used autogenous demineralized dentin matrix (AutoBT) from the extracted tooth in comparison with anorganic bovine bone (Bio-Oss) for bone augmentation. Their work showed that AutoBT exhibits osteoconductivity and biocompatibility comparable to Bio-Oss.[23]
Beta-tricalcium phosphate (β-TCP) scaffold materials are eminent as bone substitutes according to their biocompatibility, practically extensive availability, ease of sterilization, long shelf life, and low infection risk.[24] β-TCP exhibits a good balance among absorption, degradation, and new bone formation and can also sustain its structural stability by discharging a large quantity of calcium (Ca2+) and sulfate (SO42−) ions, which are crucial inorganic salts for new bone formation.[25,26]
β-TCP granule-scaffolds with sizes of 1 mm and 1–2.5 mm can also improve the proliferation of BMSCs and promote the expression of osteogenic genes and osteogenesis-related proteins.[12]
Two case series studies had used β-TCP and bioactive glass as the filling biomaterials. Autologous bone marrow-derived mononuclear cells (BMMNCs) were combined with β-TCP, and the role of BMMNCs in reducing early absorption of β-TCP alloplasts in the implant sites was asserted.[24] Bioactive glass provided adequate bone height for implant placement without any complications for implant stability and peri-implant tissue health.[27] The most important aspect here was the “osteostimulation” effect of bioactive glass.[14,28]
Our data also showed the effectiveness of xenogeneic biomaterials alone to augment the bone defects. Porcine-derived bone and flexible equine bone sheets without membranes have also yielded insufficient bone augmentation for implant placement with no significant resorption of the graft material during a 3-year follow-up period.[29,30]
Cecchetti et al.[31] showed enough bone preservation after tooth extraction using deproteinized bovine bone mineral to conduct an implant-supported treatment.
The limitations of our systematic review were the heterogeneity in the implant sizes, the different timing of implant placement, the technique of placement (1-stage or 2-stage), and also lack of studies using a single type of scaffold to specifically evaluate its effect. The included studies have used different antibiotic regimens before and after bone grafting for their patients which could possibly affect the bone augmentation results. Various sites of implant placement and different characteristics of bone regions in the maxilla and mandible were the most important limiting factors in our study, and we did not sort our results based on the implant placement locations due to their wide heterogeneity.
CONCLUSIONS
Several biomaterials have been used for bone augmentation in implant dentistry, but there are not enough predictable results to show one or more of them as an alternative to the autogenous bone. In general, after the autogenous grafts, we can introduce the Bio-Oss and β-TCP as the most trusted and widely used biomaterials in the xenogenic and synthetic biomaterial categories of grafting materials in dentistry, respectively. These two can give predictable, sustainable, and adequate new bone formation with the least infection rates in implant placement cases, which is the current goal of vertical bone augmentation in dentistry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Atala A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 2004;7:15–31. doi: 10.1089/154916804323105053. [DOI] [PubMed] [Google Scholar]
- 2.Athanasiou KA, Zhu C, Lanctot DR, Agrawal CM, Wang X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000;6:361–81. doi: 10.1089/107632700418083. [DOI] [PubMed] [Google Scholar]
- 3.Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P, et al. Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 2009;4:CD003607. doi: 10.1002/14651858.CD003607.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42:323–37. doi: 10.1007/s10439-013-0859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu YC, Nie H, Ho ML, Wang CK, Wang CH. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng. 2008;99:996–1006. doi: 10.1002/bit.21648. [DOI] [PubMed] [Google Scholar]
- 6.Raghoebar GM, Batenburg RH, Vissink A, Reintsema H. Augmentation of localized defects of the anterior maxillary ridge with autogenous bone before insertion of implants. J Oral Maxillofac Surg. 1996;54:1180–5. doi: 10.1016/s0278-2391(96)90346-8. [DOI] [PubMed] [Google Scholar]
- 7.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 8.Luz GM, Mano JF. Mineralized structures in nature: Examples and inspirations for the design of new composite materials and biomaterials. Compos Sci Technol. 2010;70:1777–88. [Google Scholar]
- 9.O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95. [Google Scholar]
- 10.Bra-nemark P-I, Zarb GA, Albrektsson T, Rosen HM. Tissue-integrated prostheses.osseointegration in clinical dentistry. Philadelphia, Pensylvania, United States: LWW; 1986. p. 499. [Google Scholar]
- 11.Jensen SS, Aaboe M, Pinholt EM, Hjørting-Hansen E, Melsen F, Ruyter IE, et al. Tissue reaction and material characteristics of four bone substitutes. Int J Oral Maxillofac Implants. 1996;11:55–66. [PubMed] [Google Scholar]
- 12.Zerbo IR, Bronckers AL, de Lange GL, van Beek GJ, Burger EH. Histology of human alveolar bone regeneration with a porous tricalcium phosphate. A report of two cases. Clin Oral Implants Res. 2001;12:379–84. doi: 10.1034/j.1600-0501.2001.012004379.x. [DOI] [PubMed] [Google Scholar]
- 13.Bignon A, Chouteau J, Chevalier J, Fantozzi G, Carret JP, Chavassieux P, et al. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med. 2003;14:1089–97. doi: 10.1023/b:jmsm.0000004006.90399.b4. [DOI] [PubMed] [Google Scholar]
- 14.Murphy W, Black J, Hastings GW. Handbook of biomaterial properties. 2 ed. Verlag New York: Springer; 2016. [Google Scholar]
- 15.Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002;13:103–11. doi: 10.1034/j.1600-0501.2002.130113.x. [DOI] [PubMed] [Google Scholar]
- 16.Felice P, Marchetti C, Iezzi G, Piattelli A, Worthington H, Pellegrino G, et al. Vertical ridge augmentation of the atrophic posterior mandible with interpositional bloc grafts: Bone from the iliac crest vs. bovine anorganic bone. Clinical and histological results up to one year after loading from a randomized-controlled clinical trial. Clin Oral Implants Res. 2009;20:1386–93. doi: 10.1111/j.1600-0501.2009.01765.x. [DOI] [PubMed] [Google Scholar]
- 17.Pieri F, Corinaldesi G, Fini M, Aldini NN, Giardino R, Marchetti C, et al. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J Periodontol. 2008;79:2093–103. doi: 10.1902/jop.2008.080061. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Leem DH. Post traumatic immediate GBR: Alveolar ridge preservation after a comminuted fracture of the anterior maxilla. Dent Traumatol. 2015;31:156–9. doi: 10.1111/edt.12144. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel AK, Donath K. BIO-OSS – A resorbable bone substitute? J Long Term Eff Med Implants. 1998;8:201–9. [PubMed] [Google Scholar]
- 20.Valentini P, Abensur D, Densari D, Graziani JN, Hämmerle C. Histological evaluation of bio-oss in a 2-stage sinus floor elevation and implantation procedure. A human case report. Clin Oral Implants Res. 1998;9:59–64. doi: 10.1034/j.1600-0501.1998.090108.x. [DOI] [PubMed] [Google Scholar]
- 21.Araújo M, Linder E, Wennström J, Lindhe J. The influence of bio-oss collagen on healing of an extraction socket: An experimental study in the dog. Int J Periodontics Restorative Dent. 2008;28:123–35. [PubMed] [Google Scholar]
- 22.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with bio-oss and bio-gide. Int J Periodontics Restorative Dent. 1998;18:321–31. [PubMed] [Google Scholar]
- 23.Pang KM, Um IW, Kim YK, Woo JM, Kim SM, Lee JH, et al. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin Oral Implants Res. 2017;28:809–15. doi: 10.1111/clr.12885. [DOI] [PubMed] [Google Scholar]
- 24.Bulgin D, Hodzic E. Autologous bone marrow-derived mononuclear cells combined with β-TCP for maxillary bone augmentation in implantation procedures. J Craniofac Surg. 2012;23:1728–32. doi: 10.1097/SCS.0b013e31826cf177. [DOI] [PubMed] [Google Scholar]
- 25.Pilliar RM, Filiaggi MJ, Wells JD, Grynpas MD, Kandel RA. Porous calcium polyphosphate scaffolds for bone substitute applications –In vitro characterization. Biomaterials. 2001;22:963–72. doi: 10.1016/s0142-9612(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 26.Uzeda MJ, de Brito Resende RF, Sartoretto SC, Alves AT, Granjeiro JM, Calasans-Maia MD, et al. Randomized clinical trial for the biological evaluation of two nanostructured biphasic calcium phosphate biomaterials as a bone substitute. Clin Implant Dent Relat Res. 2017;19:802–11. doi: 10.1111/cid.12516. [DOI] [PubMed] [Google Scholar]
- 27.Nan K, Sun S, Li Y, Chen H, Wu T, Lu F, et al. Ectopic osteogenic ability of calcium phosphate scaffolds cultured with osteoblasts. J Biomed Mater Res A. 2010;93:464–8. doi: 10.1002/jbm.a.32526. [DOI] [PubMed] [Google Scholar]
- 28.Gatti AM, Simonetti LA, Monari E, Guidi S, Greenspan D. Bone augmentation with bioactive glass in three cases of dental implant placement. J Biomater Appl. 2006;20:325–39. doi: 10.1177/0885328206054534. [DOI] [PubMed] [Google Scholar]
- 29.Cucchi A, Ghensi P. Vertical guided bone regeneration using titanium-reinforced d-PTFE membrane and prehydrated corticocancellous bone graft. Open Dent J. 2014;8:194–200. doi: 10.2174/1874210601408010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludovichetti M, Di Stefano DA, Pagnutti S, Vaccari E, Ludovichetti FS, Celletti R, et al. Vertical ridge augmentation using a flexible heterologous cortical bone sheet: Three-year follow-up. Int J Periodontics Restorative Dent. 2011;31:401–7. [PubMed] [Google Scholar]
- 31.Cecchetti F, Germano F, Bartuli FN, Arcuri L, Spuntarelli M. Simplified type 3 implant placement, after alveolar ridge preservation: A case study. Oral Implantol (Rome) 2014;7:80–5. [PMC free article] [PubMed] [Google Scholar]