Abstract
Developments in understanding bee responses to habitat loss indicate that body size is a trait with important consequences for conservation. Stingless bees (Hymenoptera, Apidae, Meliponini) are a diverse group of eusocial bees providing pollination services in tropical landscapes, exhibiting a large range in body size across species. We tested the effects of deforestation on the body sizes of stingless bee communities by using museum specimens and revisiting a previous effort that sampled stingless bee communities across varying levels of deforestation at 183 sites in Rondônia, Brazil, in 1996–1997. Body size measurements (intertegular distance) from 72 species collected were included as dependent variables in response to forest area, forest edge, and connectivity of forest patches at several spatial scales. We find that stingless bee body size is negatively related to forest cover: mean community body size was larger in areas with greater amounts of deforestation, and smaller in areas with less deforestation. Second, stingless bee species richness was positively associated with forest edge regardless of body size. Lastly, we find that as forest patch isolation increased, the stingless bee community body size also increased. These findings support hypotheses that small stingless bee species might be more negatively affected by deforestation, adding to the growing body of evidence that stingless bees require areas of intact forest in near proximity to other forest patches to conserve these diverse pollinator communities.
Keywords: body size, stingless bees, Brazil, deforestation, intertegular distance
Habitat loss and decreased connectivity of suitable habitat have been associated with declines in wild pollinator diversity (Ricketts et al. 2008, Winfree et al. 2009). In tropical regions, deforestation has been related to a decrease in species richness of native wild bees (Brosi et al. 2007, Brown and Oliveira 2013); however, some species seem less affected by forest loss or disturbance (Klein et al. 2002, Pioker-Hara et al. 2014, Giannini et al. 2015). In efforts to parse these responses, studies have included traits such as diet breadth, nesting requirements, foraging behavior, and intraspecific and interspecific variation in body size (Winfree et al. 2007, Bommarco et al. 2010, Williams et al. 2010, Lichtenberg et al. 2017).
Body size is an important trait to consider in understanding wild bee responses to land use change. Foraging range is positively related to body size of bees, with larger species capable of foraging greater distances (Araújo et al. 2004, Greenleaf et al. 2007). Bees are central place foragers and must collect nest resources within the limitations of their foraging ranges (Michener 2000), which may present greater challenges for smaller species as resources become locally scarce or increasingly disconnected. Including life history traits and body size of bees in analyses have provided insight regarding patterns of wild bees across landscapes with varying amounts of habitat loss, with some indications that smaller-bodied, social species are particularly sensitive (Jauker et al. 2013); however, these patterns are not always consistent. Williams et al. (2010) did not find body size to be a reliable predictor of bee responses to land use change, but habitat isolation negatively affected aboveground nesting and social species while ground nesting species responded more negatively to soil tillage. At the intraspecific level, Renauld et al. (2016) found that the average body size of a solitary ground-nesting bee species (Andrena nasonii Robertson) decreased as the percent of agricultural land increased. In contrast, Warzecha et al. (2016) found that medium-sized Andrena spp. increased in body size with fragmentation with no discernable patterns for larger- and smaller-sized species. Bee body size has important implications for dispersal, as well as pollination efficiency (Stout 2000); therefore, additional work is needed to better understand how bee body size responses may vary in response to land use changes.
Stingless bees (Hymenoptera: Apidae: Meliponini) are a species rich group of eusocial bees and are ecologically important as pollinators in tropical systems (Vit et al. 2013). In addition, they make important economic contributions via the pollination of many tropical crops, including coffee (Ricketts et al. 2004), and they exhibit a great range of interspecific variation in body size (Araújo et al. 2004), colony size (Michener 2000), and foraging behaviors (Lichtenberg et al. 2017) across several hundred described species (Michener 2000, Pedro 2014). Studies examining how land use change driven by human activities affects stingless bee communities have found complicated responses. In general, forest area is positively associated with increased species richness (Brosi 2009, Brown and Oliveira 2013), with changes in the species observed at forest edges versus open areas (Brosi et al. 2007, 2008; Lichtenberg 2017). Eltz et al. (2002) found that in northern Borneo stingless bee nest densities tended to be higher in sites located in close proximity to mangroves and plantations than in continuous forests, benefitting from collection of nonforest pollen resources. While many studies have examined the impact of land use change on stingless bees (Brosi 2009, Frankie et al. 2009 and references therein; Vit et al. 2013), few have addressed trait-based responses to land use change.
This analysis tests the hypothesis that stingless bee communities respond differently to deforestation depending on body size and measures of habitat loss and fragmentation. In the present study, we measured the body size of 72 stingless bee species collected in Rondônia, Brazil in 1996–1997 using museum specimens and a data set which included a major systematic inventory of stingless bees (Brown and Oliveira 2013). Specifically, we examined how body size in stingless bee communities is related to forest habitat fragmentation, as measured by total forest area, the amount of forest edge, and distance of forest patches to nearest patches (i.e., isolation) at several spatial scales surrounding sample points where bees were collected. Because body size is related to foraging distance (Greenleaf et al. 2007), we predict that smaller species will be more negatively affected by deforestation and increasing forest patch distance, and that these relationships will differ by landscape scale (Lichtenberg 2017). Further, because many stingless bee species depend on forests for nesting but may also forage outside of the forest, we predict a positive relationship between richness and forest edge regardless of body size (Eltz et al. 2002).
Methods
Study Area
This study took place in the state of Rondônia, Brazil, which has undergone heavy deforestation due to agricultural expansion since the 1970s (Frohn and Hao 2006). Sample points occurred across varying levels of deforestation, which is positively correlated with the length of time of modern human settlement (Brown and Albrecht 2001). One hundred eighty-three locations were sampled across the state from September 1996 to September 1997. To avoid resampling bees from the same colony, sampling locations were a minimum distance of 1.5 km apart.
Bee Collections and Identifications
Bees were collected using a standardized method in which three sublocations (when possible, one forested and two nonforested) per location were sampled. Within each sublocation, three collectors each located a bush 50 m apart (parallel to the nearest forest edge located 250–500 m away) and then sprayed 15 pumps of a 1:1 mixture of honey and water on 0.25 m surface area of the bush and waited 60 m to attract and capture arriving bees (after methods of Wille 1962). All bee species collected at each sublocation per location were combined for the purpose of this analysis. Because a nest’s distance from the sampling areas could influence the number of foraging nestmates that come to the bait, and because stingless bee species are eusocial and differ in their recruitment to resources, we based our analyses on presence rather than abundance of each species in our samples. Previous work has examined the influence of sublocations (i.e., forest and nonforest) on bee species richness and foraging behavior (Brown and Oliveira 2013, Brown et al. 2016). A full description of the sampling protocol can be found in Brown and Oliveira (2013).
The species collected by Brown and Oliveira (2013) included those individuals identified to species level by the late Dr. João M. F. Camargo and Dr. Sílvia R. M. Pedro at the University of Sao Paulo-Ribeirao Preto, with additional representatives from the study region located in the Snow Entomological Museum at the University of Kansas, Lawrence, KS.
We assessed body size by measuring the intertegular distance (ITD) of each species following the method of Cane (1987) by measuring the shortest distance between the tegulae using an Olympus SZ60 stereo microscope. We chose to use ITD as our body size measurement due to its correlation with stingless bee wing dimensions (Araújo et al. 2004) and foraging distance (Greenleaf et al. 2007). To account for possible differences in intraspecific variation, we measured five individuals of each species (all females) collected from the study region whenever possible. We divided all samples bee species into two categories, large and small. We used 1.44 mm, the median ITD of all species sampled, as the dividing point; ‘Small’ bees had ITDs lower than the median (0.60–1.44 mm) and ‘Large’ bees had ITDs greater than the median (1.45–3.81 mm) (Supp Table 1 [online only]; Supp Fig. 1 [online only]). At each collection location, species were scored as ‘present’ or ‘absent’. To make comparisons with other studies examining bee body size responses to disturbance, we included both the species richness of size classes and the mean body size of the community as response variables in our analyses.
Forest Parameters
In this study, deforestation is characterized from several aspects, including forest area and connectivity, patch isolation and increased fragmentation. The forest parameters—such as forest area, forest edge, and average distance of a forest patch to its nearest neighboring forest patch—that characterize deforestation properties were computed based on geo-referenced data with the aid of GIS (Geographic Information System, ArcGIS, ESRI, Redlands, California). The geographic coordinates of sample locations were generated using GPS (see Brown and Oliveira 2013 for full description of field methods). Vegetation coverage information in 1997 was collected from PRODES (Amazon Deforestation Calculation Program) from INPE (National Institute of Space Research) (Câmara et al. 2006) with 30-m spatial resolution. The sample locations were imposed on the vegetation layer depicting forest and nonforest cover in ArcGIS.
Our study aims to investigate how body size reflects the response of bee communities to deforestation at different spatial levels. The forest parameters and models were generated and constructed at three scales (radii of 500, 1,000, and 1,500 m) surrounding each study site. At each scale the total forest area, total forest edge, and distance between forest patches were computed using FRAGSTATS software (McGarigal et al. 2012). We classified areas of human disturbance based on Brown and Olivera (2013) and Fearnside (1989), where ‘new’ refers to land that was deforested from 1981 to 1996, ‘old’ refers to deforestation that took place prior to 1980, and ‘protected’ includes those areas that were under preservation during the time the collections took place.
Statistical Analysis
Before examining whether body size of stingless bee species is related to the landscape variables of interest, a Mantel test was performed to check for spatial autocorrelation among sites using the dist function in R version 3.2.2 (R Development Core Team 2015). Based on these results, we accept the null hypothesis that the two matrices (Site and Species) are not related due to geographic location (P = 0.4155).
To investigate associations between forest landscape variables and stingless bee species richness, we used generalized linear models with a Poisson distribution at each scale (500, 1,000, 1,500 m) surrounding sample points; landscape variables include forest area (ha), total edge (m), and average forest patch to nearest forest patch distance (m) as a measure of forest patch isolation (Tables 1, 3, and 5). Response variables include total species richness, and species richness within each bee’s size category (‘Small’, ‘Large’). To examine the response of the mean community ITD, we used a linear regression with the lm function in R. Finally, we categorized sample sites according to of the length of time since settlement to visualize the average ITD of those areas due to the positive relationship between settlement and deforestation (Brown and Oliveira 2013). Visual inspection of residual plots did not reveal obvious deviations from homoscedasticity or normality. All statistical analyses were carried out using R version 3.2.2 (R Development Core Team 2015).
Table 1.
Results from generalized linear models of total stingless bee species richness and the species richness of two body size (ITD) categories (small = <1.44 mm, large = >1.44 mm) against forest cover at 500, 1,000, and 1,500 m radii of sample points
| Group | Distance (m) | Coefficient | SE | z-value | df | P-value |
|---|---|---|---|---|---|---|
| Species richness (all) | 500 | 0.0020 | 0.0008 | 2.381 | 1, 180 | 0.017 |
| 1,000 | 0.0005 | 0.0002 | 2.192 | 1, 180 | 0.028 | |
| 1,500 | 1.961e-04 | 8.578e-05 | 1.982 | 1, 180 | 0.047 | |
| Species richness (small) | 500 | 0.0014 | 0.0011 | 1.217 | 1, 174 | 0.224 |
| 1,000 | 0.0004 | 0.0003 | 1.404 | 1, 174 | 0.160 | |
| 1,500 | 0.0001 | 0.0001 | 1.268 | 1, 174 | 0.205 | |
| Species richness (large) | 500 | −0.0001 | 0.0012 | −0.055 | 1, 174 | 0.956 |
| 1,000 | −3.038e-05 | 3.054e-04 | −0.143 | 1, 174 | 0.887 | |
| 1,500 | −2.157e-06 | 1.375e-04 | −0.076 | 1, 174 | 0.939 |
Table 3.
Results from generalized linear models of stingless bee species richness and the species richness of two body size (ITD) categories (small = <1.44 mm, large = >1.44 mm) against total forest edge at 500, 1,000, and 1,500 m radii of sample points
| Group | Distance (m) | Coefficient | SE | z-value | df | P-value |
|---|---|---|---|---|---|---|
| Species richness (all) | 500 | 1.685e-04 | 1.712e-05 | 9.244 | 1, 162 | <0.001 |
| 1,000 | 5.674e-05 | 5.947e-06 | 10.75 | 1, 176 | <0.001 | |
| 1,500 | 2.884e-05 | 3.056e-06 | 10.31 | 1, 177 | <0.001 | |
| Species richness (small) | 500 | 0.0002 | 2.640e-04 | 6.540 | 1, 159 | <0.001 |
| 1,000 | 6.143e-05 | 9.187e-06 | 6.853 | 1, 174 | <0.001 | |
| 1,500 | 3.197e-05 | 4.719e-06 | 6.775 | 1, 174 | <0.001 | |
| Species richness (large) | 500 | 1.297e-04 | 2.906e-05 | 4.463 | 1, 159 | <0.001 |
| 1,000 | 5.338e-05 | 9.596e-06 | 5.801 | 1, 174 | <0.001 | |
| 1,500 | 2.590e-05 | 4.930e-06 | 5.253 | 1, 174 | <0.001 |
Table 5.
Results from generalized linear models of stingless bee species richness and the species richness of two body size (ITD) categories (small = <1.44 mm, large = >1.44 mm) against average forest patch to nearest forest patch distance at 500, 1,000, and 1,500 m radii of sample points
| Group | Distance (m) | Coefficient | SE | z-value | df | P-value |
|---|---|---|---|---|---|---|
| Species richness (all) | 500 | 0.0006 | 0.0002 | 3.24 | 1, 162 | 0.001 |
| 1,000 | −0.0004 | 0.0001 | −2.707 | 1, 77 | 0.006 | |
| 1,500 | −0.0003 | 0.0001 | −0.607 | 1, 177 | 0.544 | |
| Species richness (small) | 500 | 0.0007 | 0.0003 | 2.181 | 1, 159 | 0.029 |
| 1,000 | −0.0005 | 0.0002 | −2.567 | 1, 75 | 0.010 | |
| 1,500 | −0.0001 | 0.0001 | −0.971 | 1, 174 | 0.331 | |
| Species richness (large) | 500 | 0.0009 | 0.0003 | 2.91 | 1, 159 | 0.003 |
| 1,000 | −8.553e-05 | 1.734e-04 | −0.493 | 1, 75 | 0.622 | |
| 1,500 | 4.224e-05 | 1.162e-04 | 0.364 | 1, 174 | 0.716 |
Results
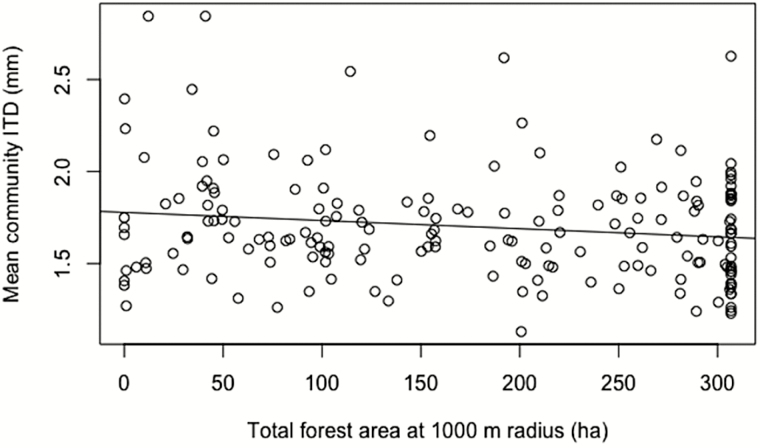
In total, we measured the ITD of 72 stingless bee species (range of individuals per species = 1–5, mean individuals per species = 4.7; Supp Table 1 [online only]). Total stingless bee species richness was positively related to forest area at the 500, 1,000, and 1,500 m scales, and it was not significant when placed into small and large size categories (Table 1). Mean community ITD was negatively related to forest area at 500 m (F(1, 164) = 6.171, P = 0.014), 1,000 m (F(1, 179) = 4.741, P = 0.031, Fig. 1) and nearly significant at 1,500 m (Table 2).
Fig. 1.
Single regression of mean community ITD against total forest area at 1,000 m radius (R2 = 0.02, df = 1, 179, P = 0.031).
Table 2.
Results from linear models of mean community body size (ITD) against forest cover at 500, 1,000, and 1,500 m radii of sample points
| Group | Distance (m) | Coefficient | SE | F-statistic | df | P-value |
|---|---|---|---|---|---|---|
| Mean body size of community (mean ITD) | 500 | −0.0021 | 0.0008 | 6.171 | 1, 164 | 0.014 |
| 1,000 | −0.0004 | 0.0002 | 4.741 | 1, 179 | 0.031 | |
| 1,500 | −1.673e-04 | 9.165e-05 | 3.384 | 1, 179 | 0.069 |
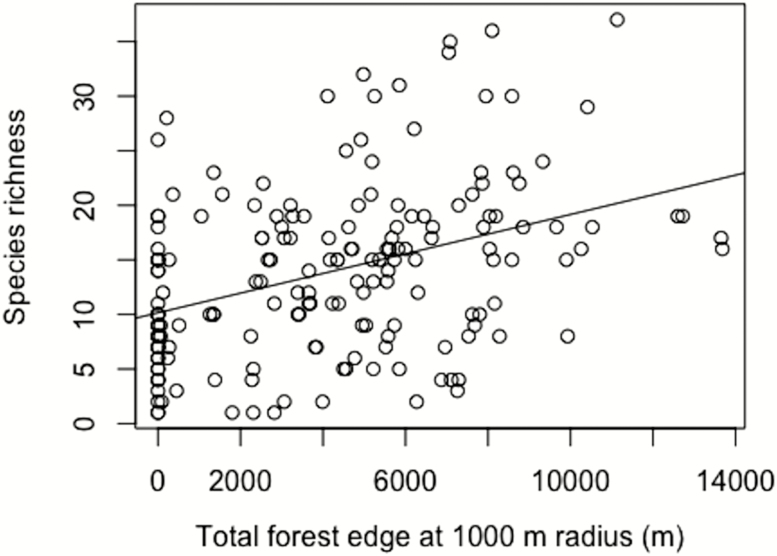
Stingless bee species richness was positively related to amount of forest edge at all scales (Table 3; P < 0.001, Fig. 2), and also for both categories of body sizes (‘Small’; P < 0.001; ‘Large’; P < 0.001). Mean community ITD was not significantly related to total forest edge at any scale surrounding sample points (Table 4).
Fig. 2.
Single regression of total species richness against forest edge at 1,000 m radius (R2 = 0.14, df = 1, 176, P < 0.001).
Table 4.
Results from linear models of mean community body size (ITD) against total forest edge at 500, 1,000, and 1,500 m radii of sample points
| Group | Distance (m) | Coefficient | SE | F-statistic | df | P-value |
|---|---|---|---|---|---|---|
| Mean body size of community (mean ITD) | 500 | 1.163e-05 | 1.845e-05 | 0.0253 | 1, 164 | 0.873 |
| 1,000 | 3.156e-06 | 6.818e-06 | 0.3879 | 1, 179 | 0.534 | |
| 1,500 | 5.179e-07 | 3.421e-06 | 0.0138 | 1, 179 | 0.906 |
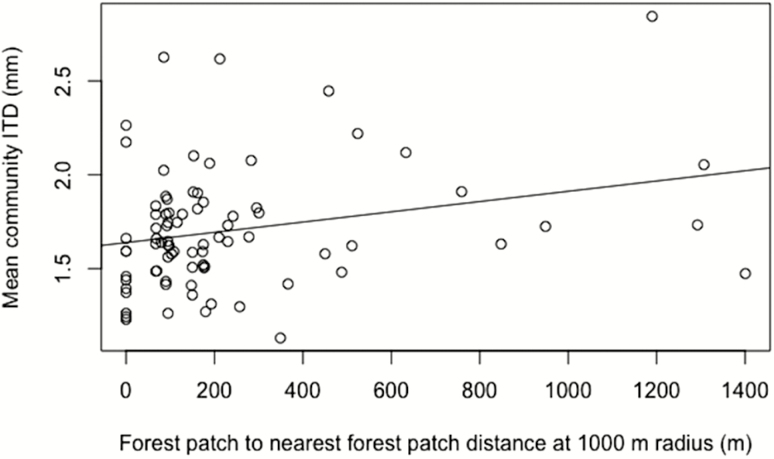
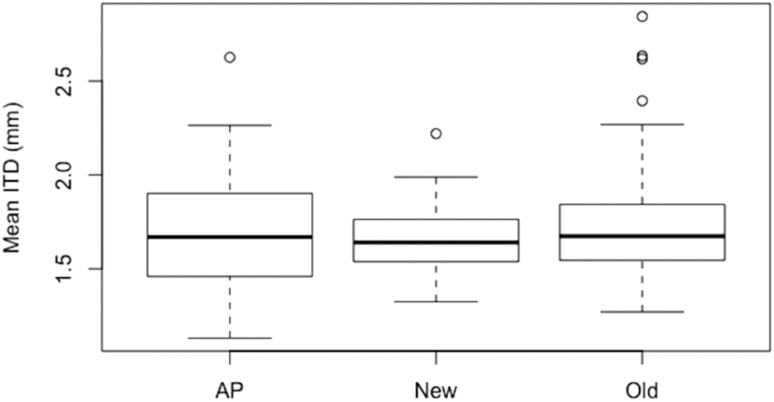
Stingless bee species richness was positively related to average forest patch distance at 500 and 1,000 m, but not at 1,500 m (Table 5). Species richness of ‘Small’ bees was positively related to patch distance at 500 and 1,000 m (P < 0.029), but not 1,500 m (Table 5). Species richness of the ‘Large’ bee category was positively related to patch distance at 500 m (P < 0.004), but not for the other distances. Mean community ITD was positively related to patch distance at 500 and 1,000 m but not at 1,500 m (Table 6; Fig. 3). We found no significant differences in the mean community ITD when grouped by time since settlement (‘old’, ‘new’, and ‘protected’) (Fig. 4).
Table 6.
Results from linear models of mean community body size (ITD) against average forest patch to nearest forest patch distance at 500, 1,000, and 1,500 m radii of sample points
| Group | Distance (m) | Coefficient | SE | F-statistic | df | P-value |
|---|---|---|---|---|---|---|
| Mean body size of community (mean ITD) | 500 | 0.0005 | 0.0002 | 6.661 | 1, 163 | 0.011 |
| 1,000 | 0.0003 | 0.0001 | 5.669 | 1, 79 | 0.019 | |
| 1,500 | −4.285e-06 | 8.097e-05 | 0.0101 | 1, 179 | 0.958 |
Fig. 3.
Single regression of mean community ITD against forest patch to nearest forest patch distance at 1,000 m radius (R2 = 0.05, df = 1, 79, P = 0.019).
Fig. 4.
Box plot of mean community ITD by settlement type (AP = preservation area; new = deforested from 1981 to 1997; old = areas deforested prior to 1980).
Discussion
The total species richness of stingless bees was positively related to forest area at all scales, which agrees with earlier findings examining this relationship (Brown and Oliveira 2013). When divided into two body size categories (i.e., ‘Small’ and ‘Large’), the relationship of species richness to forest area was nonsignificant. Interestingly, however, when examining the mean ITD of the stingless bee community, there was a significant negative relationship between body size and forest area. Bee body size responses to disturbance or habitat loss have been analyzed using the species richness or abundance individuals within a specified size class (Cane et al. 2006, Bommarco et al. 2010, Hopfenmüller et al. 2014), or by taking the average body size of individuals or species in a community (Steffan-Dewenter and Tscharntke 1999, Jauker et al. 2013). We include both approaches to make comparisons to other findings. The distribution in body sizes across all species included in our study was unimodal; thus, no clear division of body size classes separated smaller-sized from larger-sized species (Supp Table 1 [online only]; Supp Fig. 1 [online only]). Focusing on mean ITD community response to deforestation supports observations that smaller-sized bees may be more susceptible to the effects of deforestation (Araújo et al. 2004, Kambach et al. 2012, Lichtenberg 2017).
In our study, forest edge was significantly related to total species richness, and species richness of both small- and large-sized stingless bee species. The direction of the relationship was positive for all groups, indicating that increased amounts of forest edge support a greater richness of stingless bees regardless of body size. Others have found the presence of edge to be beneficial for bees (Eltz et al. 2002), with some observations that bees respond differently in relation to forest edges (Brosi et al. 2008) and disturbance (Kambach et al. 2012).
The severity of fragmentation and amount of natural habitat remaining differently affects wild bees (Winfree et al. 2009). The site history and landscape composition may influence stingless bee responses to disturbance events. Kambuch et al. (2012) found increased species richness in areas that were fire degraded when compared to intact forest interiors. Areas that have been deforested may provide some added foraging opportunities for those species able to access the resources (Eltz et al. 2002). However, the beneficial aspects of disturbance to stingless bees should be considered with a clear understanding of the species needs for survival including availability of suitable nesting and foraging resources.
Few studies have examined the effects of connectivity on wild bees across fragmented habitats in tropical forest systems. In a Costa Rican landscape largely converted for agricultural production, Brosi et al. (2008) did not find forest fragment isolation to significantly affect bee richness or abundance; however, there were marked differences in the community composition based on landscape attributes with stingless bees comprising the majority (75%) of bees sampled in forest interiors and less frequent (50%) in adjacent pasture areas. Similarly, Calvillo et al. (2010) did not find forest fragment connectivity to be significantly related to bee richness or diversity; however, they found overall increased species richness and diversity in relation to fragment size. In our study, stingless bees had mixed responses to forest patch connectivity. The total species richness was positively related to forest inter-patch distance at 500 m, but negatively related at 1,000 m. A similar trend was found for the small bee category, and large bee species responded positively at 500 m, but there were no other distances with a significant relationship. Because smaller-sized bees have shorter foraging distances (Greenleaf et al. 2007), it is likely that the smaller stingless bee species are negatively affected by increasing forest patch distances in fragmented landscapes. For example, Araújo et al. (2004) estimated that larger stingless bee species are capable of foraging over distances up to 2 km, while smaller species foraged at a range of 621–951 m. Larger-bodied stingless bees have been found more frequently in pasture areas in Costa Rica (Lichtenberg et al. 2017), which may be due to the ability of larger bees to forage greater distances in search of resources. Our study found the mean community body size increased with increasing forest patch distances, supporting findings that larger species are present more in areas with greater forest patch distances compared to fewer smaller-sized species.
These results support our prediction that smaller bee species may rely on large forested areas and forest patches that are closely connected. Body size influences foraging range in many bee species; in addition, stingless bees initiate new colonies by moving resources from the maternal nest site to a newly established nest (Roubik 2006, Vit et al. 2013) which may place additional limits on smaller species. However, it must be noted that some larger stingless bee species in the genus Melipona have also been found to be sensitive to deforestation or disturbance (Brown and Albrecht 2001, Pioker-Hara et al. 2014).
Adult bee body size is related to the quantity of resources consumed during the larval growth period (Johnson 1988) and temperature (Radmacher and Strohm 2009). Some work examining the effects of larval food intake on Melipona adult worker body sizes have found that less food results in weaker colonies with smaller workers, but that smaller individuals had higher pollen load carrying capacities (Ramalho et al. 1998). Additionally, Kuhn-Neto et al. (2009) found that larger Melipona workers foraged and recruited at significantly greater distances than smaller workers. Our study did not examine intraspecific differences in stingless bee body sizes across the range of deforestation; rather, we used an average ITD measurement to represent each species within the communities sampled. We observed a range of ITD across species (min = 0.66 mm, max = 3.81 mm) with some variation within species (Supp Table 1 [online only]). Stingless bees are capable of adjusting their body size and corbiculae during periods of resource scarcity (Veiga et al. 2013) which may provide some flexibility to disturbance events.
Stingless bees are central place foragers (Stephens and Krebs 1986) and must collect their resources within a foraging distance that is related to their body size (Roubik and Aluja 1983, Araújo et al. 2004, Kuhn-Neto et al. 2009). While our models suggest that body size is an important trait to include when aiming to understand the effects of deforestation, there are many other factors to consider. For example, foraging strategies may also influence stingless bee community responses, with generalist species being more sensitive to forest loss (Lichtenberg et al. 2017). Literature examining bee body size generally supports the conclusion that heritability of body size within bee species is low (Tepedino et al. 1984, Pignata and Diniz-Filho 1996), emphasizing the need for resources to be available within the foraging ranges of bees to ensure their persistence in modified landscapes.
Our study provides important insights into the body size responses of a highly diverse and important community of bees native to the Brazilian Amazon forest. While we find stingless bees respond positively to increased forest edge, we highlight that smaller bees favor larger areas of forest located in close proximity to other forest patches; therefore, the ability of these bees to tolerate increasing levels of deforestation may be limited.
Supplementary Material
Acknowledgments
We give thanks to the following institutions and people for the use of specimens included in the body size analysis: Dr. Michael Engel, Dr. Zack Falin, and Ms. Jennifer Thomas at the University of Kansas, Division of Entomology, Biodiversity Institute. D.M.M. and C.P.B. received summer scholarship support from the University of Kansas Entomology Endowment. The authors wish to thank Drs. João M. F. Camargo (in memoriam) and Sílvia R. M. Pedro for identification of the species; to José Amilcar Tavares for his technical assistance with the collection, and to Sandro Boina and José Aparecido Vieira for their field and lab assistance during the project. We also thank Denise Perpich who designed and managed the database of the project. We are grateful to Caio Márcio Vasconcellos Cordeiro de Almeida and Francisco Antônio Neto for providing laboratory space in Ouro Preto. The fieldwork for this study was funded by Tecnosolo and DHV Consultants, and it was conducted as part of the Second Approximation of the Socio Economic-Ecological Zoning of the State of Rondônia. We greatly appreciate the comments of the anonymous reviewers. All errors remain ours. Contribution of authors: Project design: D.M.M., J.C.B., D.R.S.; data collection: J.C.B. (field work), D.M.M. (measurements), C.P.B. (measurements), D.S. (geospatial data); data analysis: D.M.M.; paper writing: D.M.M., C.P.B., D.R.S.
References Cited
- Araújo E. D., Costa M., Chaud-Netto J., and Fowler H. G.. . 2004. Body size and flight distance in stingless bees (Hymenoptera: Meliponini): inference of flight range and possible ecological implications. Braz. J. Biol. 64: 563–568. [DOI] [PubMed] [Google Scholar]
- Bommarco R., Biesmeijer J. C., Meyer B., Potts S. G., Pöyry J., Roberts S. P., Steffan-Dewenter I., and Ockinger E.. . 2010. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. R. Soc. Lond. B Biol. Sci. 277: 2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosi B. J. 2009. The complex responses of social stingless bees (Apidae: Meliponini) to tropical deforestation. Forest Ecol. Mgt. 258: 1830–1837. [Google Scholar]
- Brosi B. J., Daily G. C., and Ehrlich P. R.. . 2007. Bee community shifts with landscape context in a tropical countryside. Ecol. Appl. 17: 418–430. [DOI] [PubMed] [Google Scholar]
- Brosi B. J., Daily G. C., Shih T. M., Oviedo F., and Durán G.. . 2008. The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45: 773–783. [Google Scholar]
- Brown J. C., and Albrecht C.. . 2001. The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondônia, Brazil. J. Biogeog. 28: 623–634. [Google Scholar]
- Brown J. C., and Oliveira M. L.. . 2013. The impact of agricultural colonization and deforestation on stingless bee (Apidae: Meliponini) composition and richness in Rondônia, Brazil. Apidologie. 45: 172–188. [Google Scholar]
- Brown J. C., Mayes D., and Bhatta C.. . 2016. Observations of Africanized honey bee Apis mellifera scutellata absence and presence within and outside forests across Rondônia, Brazil. Insectes Soc. 63: 603–607. [Google Scholar]
- Calvillo L. M., Meléndez Ramírez V., Parra-Tabla V., and Navarro J.. . 2010. Bee diversity in a fragmented landscape of the Mexican neotropic. J. Insect Cons. 14: 323–334. [Google Scholar]
- Câmara G., Valeriano D. D. M., Soares D. de M., and Vianei J.. . 2006. Metodologia para o Cálculo da Taxa Anual de Desmatamento na Amazônia Legal. INPE, São José dos Campos, Brazil. [Google Scholar]
- Cane J. H. 1987. Estimation of bee size using intertegular span (Apoidea). J. Kansas Entomol. Soc. 6: 145–147. [Google Scholar]
- Cane J. H., Minckley R. L., Kervin L. J., Roulston T. H., and Williams N. M.. . 2006. Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 16: 632–644. [DOI] [PubMed] [Google Scholar]
- Eltz T., Brühl C. A., van der Kaars S., and Linsenmair E. K.. . 2002. Determinants of stingless bee nest density in lowland dipterocarp forests of Sabah, Malaysia. Oecologia. 131: 27–34. [DOI] [PubMed] [Google Scholar]
- Fearnside P. M. 1989. A ocupação humana de Rondônia: impactos, limites e planejamento. Assessoria Editorial e Divulgação Científica, Brasília, Brazil. [Google Scholar]
- Frankie G. W., Rizzardi M., Bradleigh Vinson S., and Griswold T. L.. . 2009. Decline in bee diversity and abundance from 1972–2004 on a flowering leguminous tree, Andira inermis in Costa Rica at the interface of disturbed dry forest and the urban environment. J. Kansas Entomol. Soc. 8: 21–20. [Google Scholar]
- Frohn R. C., and Hao Y.. . 2006. Landscape metric performance in analyzing two decades of deforestation in the Amazon Basin of Rondonia, Brazil. Rem. Sens. Environ. 100: 237–251. [Google Scholar]
- Giannini T. C., Garibaldi L. A., Acosta A. L., Silva J. S., Maia K. P., Saraiva A. M., Guimarães P. R., and Kleinert A. M.. . 2015. Native and non-native supergeneralist bee species have different effects on plant-bee networks. PLoS One. 10: e0137198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf S. S., Williams N. M., Winfree R., and Kremen C.. . 2007. Bee foraging ranges and their relationship to body size. Oecologia. 153: 589–596. [DOI] [PubMed] [Google Scholar]
- Hopfenmüller S., Steffan-Dewenter I., and Holzschuh A.. . 2014. Trait-specific responses of wild bee communities to landscape composition, configuration and local factors. PLoS One. 9: e104439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauker B., Krauss J., Jauker F., and Steffan-Dewenter I.. . 2013. Linking life history traits to pollinator loss in fragmented calcareous grasslands. Landsc. Ecol. 28: 107–120. [Google Scholar]
- Johnson M. D. 1988. The relationship of provision weight to adult weight and sex ratio in the solitary bee, Ceratina calcarata. Ecol. Entomol. 13: 165–170. [Google Scholar]
- Kambach S., Guerra F., Beck S., Hensen I., and Schleuning M.. . 2012. Human-induced disturbance alters pollinator communities in tropical mountain forests. Diversity. 5: 1–14. [Google Scholar]
- Klein A. M., Steffan-Dewenter I., Buchori D., and Tscharntke T.. . 2002. Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Cons. Biol. 16: 1003–1014. [Google Scholar]
- Kuhn-Neto B., Contrera F. A. L., Castro M. S., and Nieh J. C.. . 2009. Long distance foraging and recruitment by a stingless bee, Melipona mandacaia. Apidologie. 40: 472–480. [Google Scholar]
- Lichtenberg E. M., Mendenhall C. D., and Brosi B.. . 2017. Foraging traits modulate stingless bee community disassembly under forest loss. J. Anim. Ecol. 86: 1404–1416. [DOI] [PubMed] [Google Scholar]
- McGarigal K., Cushman S. A., and Ene E.. . 2012. FRAGSTATS v4: spatial pattern analysis program for categorical and continuous maps. Computer software program produced by the authors at the University of Massachusetts, Amherst, MA: http://www.umass.edu/landeco/research/fragstats/fragstats.html [Google Scholar]
- Michener C. D. 2000. The bees of the world. Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Pedro S. R. M. 2014. The stingless bee fauna of Brazil (Hymenoptera: Apidae). Sociobiology. 61: 348–354. [Google Scholar]
- Pignata M. I. B., and Diniz-Filho J. A. F.. . 1996. Phylogenetic autocorrelation and evolutionary constraints in worker body size of some neotropical stingless bees (Hymenoptera: Apidae). Heredity. 76: 222–228. [Google Scholar]
- Pioker-Hara F. C., Drummond M. S., and Kleinert A. M. P.. . 2014. The influence of the loss of Brazilian savanna vegetation on the occurrence of stingless bee nests (Hymenoptera: Apidae: Meliponini). Sociobiology. 61: 393–400. [Google Scholar]
- R Development Core Team 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Radmacher S., and Strohm E.. . 2009. Factors affecting offspring body size in the solitary bee Osmia bicornis (Hymenoptera, Megachilidae). Apidologie. 41: 169–177. [Google Scholar]
- Ramalho M., Imperatriz-Fonseca V. L., and Giannini T. C.. . 1998. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie. 29: 221–228. [Google Scholar]
- Renauld M., Hutchinson A., Loeb G., Poveda K., and Connelly H.. . 2016. Landscape simplification constrains adult size in a native ground-nesting bee. PLoS One. 11: e0150946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts T. H., Daily G. C., Ehrlich P. R., and Michener C. D.. . 2004. Economic value of tropical forest to coffee production. Proc. Natl. Acad. Sci. USA. 101: 12579–12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts T. H., Regetz J., Steffan-Dewenter I., Cunningham S. A., Kremen C., Bogdanski A., Gemmill-Herren B., Greenleaf S. S., Klein A. M., Mayfield M. M., . et al. 2008. Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 11: 499–515. [DOI] [PubMed] [Google Scholar]
- Roubik D. W. 2006. Stingless bee nesting biology. Apidologie. 37: 124–143. [Google Scholar]
- Roubik, D. W. M. Aluja. 1983. Flight ranges of Melipona and Trigona in tropical forest. J. Kansas Entomol. Soc. 56: 217–222. [Google Scholar]
- Steffan-Dewenter I., and Tscharntke T.. . 1999. Effects of habitat isolation on pollinator communities and seed set. Oecologia. 121: 432–440. [DOI] [PubMed] [Google Scholar]
- Stephens, D. W. J. R. Krebs. 1986. Foraging Theory. Princeton University Press, Princeton, New Jersey. [Google Scholar]
- Stout J. C. 2000. Does size matter? Bumblebee behavior and the pollination of Cytisus scoparius L. (Fabaceae). Apidologie. 31: 129–139. [Google Scholar]
- Tepedino V. J., Thompson R., and Torchio P. F.. . 1984. Heritability for size in the Megachilid bee Osmia lignaria propinqua Cresson. Apidologie. 15: 83–88. [Google Scholar]
- Veiga J. C., Menezes C., Venturieri G. C., and Contrera F. A. L.. . 2013. The bigger, the smaller: relationship between body size and food stores in the stingless bee Melipona flavolineata. Apidologie. 44: 324–333. [Google Scholar]
- Vit P., Pedro S. R. M., and Roubik D.. . 2013. Pot honey: a legacy of stingless bees. Springer, New York, NY. [Google Scholar]
- Warzecha D., Diekötter T., Wolter V., and Jauker F.. . 2016. Intraspecific body size increases with habitat fragmentation in wild bee pollinators. Landsc. Ecol. 31: 1449–1455. [Google Scholar]
- Wille A. 1962. A technique for collecting stingless bees under jungle conditions. Insectes Soc. 9: 291–293. [Google Scholar]
- Williams N. M., Crone E. E., Roulston T. H., Minckley R. L., Packer L., and Potts S. G.. . 2010. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 143: 2280–2291. [Google Scholar]
- Winfree R., Griswold T., and Kremen C.. . 2007. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 21: 213–223. [DOI] [PubMed] [Google Scholar]
- Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., and Aizen M. A.. . 2009. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology. 90: 2068–2076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.