Abstract
Maintenance of homeostasis and immune protection rely on the coordinated action of different physiological systems. Bidirectional communication between the immune system and physiological systems is required to sense and restore any disruption of equilibrium. Recent transcriptomic analyses of innate lymphoid cells (ILCs) from different tissues have revealed that ILCs express a large array of receptors involved in the recognition of neuropeptides, hormones and metabolic signals. ILCs rapidly secrete effector cytokines that are central in the development and activation of early immune responses, but they also constitutively secrete mediators that are important for tissue homeostasis. To achieve these functions effectively, ILCs integrate intrinsic and extrinsic signals that modulate their constitutive and induced activity. Disruption of the regulation of ILCs by physiological regulators leads to altered immune responses with harmful consequences for the organism. An understanding of these complex interactions between the immune system and physiological mediators is crucial to decipher the events leading to the protective versus pathological effects of these cells.
Keywords: innate lymphoid cells, immunity, immune protection, homeostasis
Subject terms: Innate lymphoid cells, Mucosal immunology, Neuroimmunology
Introduction: sensing of pathological and physiological signals
Life consists of the maintenance of a dynamic equilibrium through regulation of key variables within an acceptable range. These variables include blood pH, osmolarity and glucose levels; sodium, calcium and oxygen concentrations; and body temperature. This equilibrium is constantly challenged by intrinsic and extrinsic stressors. Stress is defined as a state of threatened homeostasis, and reestablishment of this homeostasis is achieved through complex interactions of the endocrine system, autonomic nervous system and immune systems.1 When stress cannot be handled by homeostatic mechanisms, it leads to an inflammatory response; this response is generally triggered by pathogens, toxins and allergens. Inflammation is the last response2 engaged in an organism to maintain and defend homeostasis when all other physiological regulators have failed. Defensive inflammatory responses need to be tightly controlled to be directed towards noxious stimuli without damaging host tissue. This control is achieved by constant communication between different physiological systems that are responsible for both sensing disruptions in equilibrium and restoring equilibrium. Dysfunctions in this communication can lead to the development of diseases due to chronic inflammation caused by uncontrolled homeostasis-restoring responses.
The discovery of innate lymphoid cells (ILCs) has forced immunologists to rethink the immunological architecture that provides immune protection. Indeed, ILCs occupy a particular place in the landscape of immunology. ILCs reside within tissues, and their activity is not modulated by antigen-specific receptors but by a complex mixture of cytokines, alarmins and physiological signals derived from their microenvironment.
ILCs were initially classified into three main subsets based on the cytokines and transcription factors they express: group 1 ILCs (ILC1s), group 2 ILCs (ILC2s) and group 3 ILCs (ILC3s) are associated with T helper 1 (Th1), Th2 and Th17 functions, respectively. In contrast, natural killer (NK) cells are analogous to innate CD8+ cytotoxic T cells. The nomenclature has been recently extended to include five groups; NK cells and lymphoid tissue-inducer cells (LTis) have been added as distinct subsets that were previously categorized within the ILC1 and ILC3 groups, respectively.3 This distinction is based on the evidence that each of these subsets have distinct developmental pathways.4–9
ILCs seed tissues during embryonic stages, developing from progenitors derived from the fetal liver and fetal gut.10 Experiments using parabiont mice suggest that ILCs mainly reside within these tissues and self-renew in the adults from local progenitors without substantial replenishment from bone marrow progenitors, with exception in the case of NK cells.11 However, recent studies suggest that ILCs can be mobilized from bone marrow,12–14 suggesting that ILC trafficking is more dynamic than previously thought.
Once in the tissues, each ILC subset appears to be highly conditioned by the microenvironment and harbor distinct properties in different tissue settings. Analysis at the single-cell level of ILCs from different tissues has highlighted the important heterogeneity of ILC subsets residing within tissues.15,16 These cells display a unique set of receptors encoded by specific transcriptomic signatures imprinted in a tissue-specific manner.15 This adaptability highlights how ILCs sense local endogenous signals that regulate their specification by controlling the expression of distinct patterns of activating/inhibitory receptors. The intimate crosstalk between ILCs and their microenvironment, the strategic localization of ILCs and the constitutive activity of ILCs within peripheral tissues have expanded our view of the role of these cells in health and disease. Their activities go beyond simple reactions to pathogen invasion and inflammation, extending to anticipation and maintenance of the homeostasis of the tissue itself. In this review, we propose that ILCs integrate physiological signals that modulate their constitutive activity in a tissue- and time-specific manner. This regulation involves the specific expression of receptors that are able to sense neuropeptides, hormones and metabolic signals. ILCs appear to be particularly sensitive to such regulation and may represent a link between the immune system and other physiological systems found in organisms. We further propose that this physiological regulation of ILCs is critical to maintaining tissue homeostasis and deregulation of these physiological mechanisms may explain the protective versus pathological properties of these cells. Thus, ILC dysregulation may lead to chronic inflammation, metabolic diseases or tissue damage.
Dysregulated ILC responses are linked to chronic inflammation
ILCs rapidly respond to host- and pathogen-derived signals and are the innate sources of interferon-γ (IFN-γ) (ILC1s); interleukin (IL)-4, IL-5, IL-9 and IL-13 (ILC2s); and IL-17 and IL-22 (ILC3s). ILCs also maintain tissue homeostasis by contributing to tissue remodeling and wound healing through the secretion of amphiregulin17 and IL-22.18
ILC1s
ILC1s are mainly involved in early protection against viruses19 and bacteria.6,20 ILC1s reside in many organs, including the liver, uterus, skin, salivary glands and gut.21 In contrast to NK cells that express integrin α2 (CD49b), ILC1s express high levels of integrin α1 (CD49a), CD200r1, CD103 or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) depending on the tissue they reside (reviewed in ref. 21). While NK cells are cytotoxic cells, ILC1s have reduced cytotoxic activity but produce more cytokines such as IFN-γ, tumor necrosis factor (TNF) and granulocyte-macrophage colony-stimulating factor (GM-CSF).19 Dysregulation of ILC1 and NK cell responses in adipose tissue leads to the development of metabolic disorder and obesity.22
ILC2s
ILC2s play important roles in allergies and anti-parasite immunity.23 ILC2s can be activated by epithelial stress signals such as IL-25 and IL-33.24 In the lung, they are critical for tissue repair and protection through their secretion of amphiregulin,17 which promotes mucus production and tight junctions that limit inflammation and restore barrier integrity. Disruption of ILC2 functions in the lung is associated with asthma and airway hypersensitivity,25 while in adipose tissue, such disruption results in increased insulin resistance and propensity towards obesity.26 ILC2s can be divided into two main subsets: (i) natural ILC2s (nILC2s), which reside within tissue, and (ii) inflammatory ILC2s (iILC2s), which can be mobilized from blood during chronic inflammation.12,14 nILC2s are KLRG1intThy1highST2+, while iILC2s are characterized by their KLRG1hiThy1lowST2− expression profile.12
ILC3s
ILC3s are greatly enriched in the intestine. Their capacity to produce IL-22 promotes colonization of the intestine by beneficial commensal bacteria that protect against intestinal inflammation.27,28 Perturbed ILC3 responses are associated with impaired capacity to maintain epithelial defenses and mucosal barrier function, resulting in disastrous consequences.29 ILC3s are divided into three subsets: (i) LTi cells, which are required for the generation of lymph nodes during embryogenesis, and (ii) NKp46+ and (iii) NKp46− subsets, which are essential for protection of the intestinal mucosa.30 ILC3s depend on the transcription factor Rorγt for their development and produce a variety of cytokines, including IL-17A, IL-17F, IL-22 and GM-CSF, essential for immune protection.
Neuroregulation of ILC responses
Both the nervous and immune systems share common features in their organization, structure and communication. Cells from both systems are spread throughout the body, sense environmental changes, generate memory and interact with other cell populations through the secretion of polypeptides. Importantly, both neurons and immune cells express receptors to sense cytokines and neurotransmitters, allowing direct communication between the two systems. Receptors for IL-6, transforming growth factor-β (TGF-β), IL-1-β and TNF are found in the brain and enteric neurons;31–33 conversely, immune cells express receptors for neuropeptides, as we will discuss further in this section of the review. The strategic positioning of ILCs at mucosal surfaces allows them to be some of the first responders to environmental insults. ILCs are mainly found at mucosal barrier surfaces and appear to be in close contact with neurons and glial cells of the autonomic nervous system (ANS). The ANS is divided into (i) the sympathetic system that predominates during emergency “fight-or-flight” reactions and during exercise to prepare the body for strenuous physical activity and (ii) the parasympathetic system, which prevails during resting conditions, regulating basic body functions such as digestion as well as energy conservation and storage. The expression of specific neuroregulators and neurotrophic factors by the nervous system enables spatiotemporal regulation of immune cells. Disruption of the communication loop between the central nervous system (CNS) and ILCs results in inappropriate responses to infectious and homeostatic cues.
Neuromedin U
Neuromedin U (NMU) is a neuropeptide expressed in many different types of neurons, including cholinergic, noncholinergic and sensory neurons. NMU is widely distributed in the body, but the highest concentration is detected in the gastrointestinal tract, particularly the duodenum and jejunum. In the lamina propria, NMU is specifically expressed in cholinergic neurons.34 The physiological roles of NMU are numerous and include regulation of food intake35 and gastric acid secretion as well as smooth muscle contraction and blood pressure regulation. NMU signals through two receptors. Nmur1 is more abundantly expressed in peripheral than in central tissues, particularly the gastrointestinal tract, whereas Nmur2 is mainly found in the CNS. Among immune cells, NMUR1 is specifically expressed on ILC2s.34,36,37 Mucosal neurons express NMU and modulate ILC2 activity through this pathway (Fig. 1). NMU can induce the production of IL-5 and IL-13 by ILC2s through extracellular signal-regulated kinase-1/2 (ERK1/2) kinase activation and calcium-influx-dependent activation that lead to the translocation of the nuclear factor of activated T cells (NFAT).36 Infection by the parasitic helminth Nippostrongylus brasiliensis induces the secretion of NMU by cholinergic neurons via MyD88 activation, which then promotes the secretion of IL-5, IL-13 and amphiregulin by ILC2s.34,36 In the absence of NMUR signaling, type 2 responses are impaired, resulting in poor control of worm infection. Interestingly, treatment of wild-type mice with the recombinant neuropeptide NmU23 accelerates the ability of the mice to clear parasitic helminth infection.
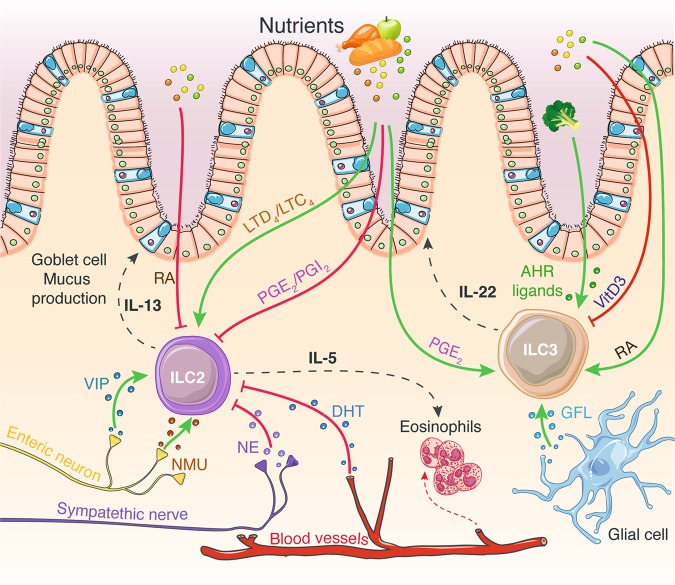
Fig. 1.
ILC functions are regulated through extensive integration of dietary-derived metabolites and neuroendocrine signals. Enteric neurons that produce VIP and NMU stimulate ILC2 IL-5 and IL-13 secretion, leading to eosinophil recruitment and mucus production by goblet cells. Norepinephrine secreted by the sympathetic nervous system inhibits ILC2 function. DHT inhibits ILC2 proliferation and cytokine production. Glial cell GFL secretion induces ILC3 IL-22 production. Dietary-derived metabolites influence ILC functions. These include vitamins A and D, AHR ligands, prostaglandins and leukotrienes. While prostaglandins negatively regulate ILC2 functions, these lipid-derived molecules induce ILC3 IL-22 secretion. In contrast, leukotrienes impede ILC2 cytokine release. Vitamins have opposite effects on ILC2s and ILC3s. While RA inhibits ILC2 activity, it promotes ILC3 IL-22 production. Vitamin D is a negative regulator of ILC3 functions. Finally, AHR ligands are potent inducers of ILC3 IL-22 secretion. The green and red arrows indicate positive and negative regulators of ILC functions, respectively. ILC innate lymphoid cell, IL interleukin, VIP vasoactive intestinal peptide, NMU neuromedin U, GFL glial-derived neurotrophic factor family of ligands, RA retinoic acid (vitamin A), VitD3 vitamin D3, PG prostaglandin, LT leukotriene, AHR aryl hydrocarbon receptor, DHT dihydrotestosterone
ILC2s are also important mediators of allergic reactions, and NMU can mediate allergen-induced eosinophilia and mast cell activation in the lung mucosa.38 Synergistic effects of the alarmin IL-25 and NMU on ILC2s promote the proliferation of transcriptionally active inflammatory ILC2s, leading to marked amplification of IL-5 and IL-13 production and resulting in exacerbated allergic lung inflammation.37 Administration of IL-25 and NMU in vivo results in enhanced histopathological evidence of allergic inflammation and increases lung and bronchoalveolar lavage fluid eosinophilia. Upon house dust mite (HDM) challenge, Nmur1-deficient mice exhibit marked reductions in ILC2 numbers compared to control mice and demonstrate decreased type 2 allergic airway inflammation, consistent with reductions in ST2+ inflammatory ILC2s.37 Because of its action on smooth muscles, NMU can coordinate the expulsion of invading pathogens and allergens by coordinating the contraction of the muscularis mucosae and the promotion of type 2 immunity by ILC2s to generate optimal immune responses to such threats.
Substance P
Substance P (SP) is a key player in nociception and is released upon stimulation of nociceptors to generate neurogenic inflammation, which is characterized by increased capillary permeability and vasodilatation. SP signals through the neurokinin receptors NK1R, NK2R and NK3R and has been shown to regulate cell migration and proliferation. SP also plays a proinflammatory role by stimulating the innate immune system, such as by activating monocytes, eosinophils, neutrophils and mast cells.39
Studies on the effects of SP on ILCs have been limited to NK cells with contradictory results. Early works reported that SP enhanced cytotoxicity,40,41 while a more recent study showed an inhibitory role of SP in human NK cells associated with reduced cytotoxicity, mobilization of intracellular calcium, degranulation and chemotaxis.42 In mice, SP induces IL-18 production by NK cells, and inhibition of SP signaling through NK1R reduces IFN-γ and IL-18 production during Pseudomonas aeruginosa infection, leading to poor control of the bacterial infection in the cornea.43 These findings, however, indicate that there is a regulatory role for SP in NK cells, warranting further study to explore the potential roles of other arms of the innate immune system in contributing to the modulation of NK cell activity by SP.
Regulation of ILCs by the sympathetic and parasympathetic pathways
Epinephrine and norepinephrine are released by the sympathetic nervous system, which is responsible for preparing the body for physical and mental activities. These signaling molecules engage β1- and β2-adrenergic receptors (β2AR), which are commonly found on bronchial smooth muscles and cardiac muscles. Therefore, modulating β-adrenergic receptor signaling is a common pharmacological strategy for the treatment of asthma44 and cardiovascular conditions.45 NK cell cytotoxicity and IFN-γ, granzyme-B and perforin expression are reduced when β2AR is stimulated.46,47 Similar to other catecholamines, norepinephrine is released in a pattern following the circadian rhythm and has been shown to regulate circadian oscillations in NK cell cytolytic activity. Increased levels of norepinephrine in the spleen during the light phase (the resting phase in rodents) are correlated with inhibition of granzyme-B and perforin production. In contrast, during the dark phase (the active phase in rodents) when the levels of norepinephrine are reduced, granzyme-B and perforin transcript levels are increased.48
Norepinephrine also exerts an inhibitory effect on ILC2 proliferation and function by binding to β2AR.49 After N. brasiliensis infection, mice lacking β2AR demonstrated increased ILC2 infiltration in the small intestine and increased production of IL-13. β2AR deficiency leads to increased eosinophilia and goblet cell hyperplasia, resulting in better control of worm burdens. In contrast, stimulation of β2AR with the agonist clenbuterol has opposite effects, inhibiting ILC2 effector functions and consequently increasing worm burden.49
Through the release of acetylcholine via the vagus nerve, the parasympathetic nervous system also plays an important role in controlling inflammatory responses. Upon inflammation, the IL-1 released by innate cells activates the vagus nerve, which leads to negative feedback control of inflammation through the release of acetylcholine. Vagotomy is associated with exacerbation of the TNF response after endotoxin shock, while activation of the cholinergic pathway inhibits the release of proinflammatory cytokines by macrophages.50,51 Vagal disruption also induces dysregulation of ILC3s in the peritoneum and changes in lipid mediator profiles, particularly the levels of protectin conjugates in tissue regeneration 1 (PCTR1).52 PCTR1 is a pro-resolving mediator that exerts potent tissue regenerative effects by enhancing macrophage recruitment and phagocytosis and reducing leukocyte infiltration and inflammation.53 ILC3s produce PCTR1 in response to acetylcholine, and disruption of the vagus nerve delays the resolution of infectious inflammation.52 Collectively, these studies reveal how the intimate connection between the nervous system and the innate immune system provides a fine-tuned balance between the acute responses necessary to protect the host and those necessary to prevent excessive inflammation leading to chronic pathologic inflammation. Neuronal control can therefore affect the development and function of innate immune cells, resulting in macroscopic changes of the gut microbiota. These findings prompt further studies to unravel the underlying molecular mechanisms and potentially find different indications for current therapeutics.
Vasoactive intestinal peptide
Vasoactive intestinal peptide (VIP) acts as a neurotransmitter and is expressed in neurons found in the lung and gut.54 VIP is a potent vasodilator but is also involved in other physiological processes, including coordination of gastrointestinal motility and mucus and enzyme secretions in response to feeding and synchronization of the central circadian rhythm.55 In the immune system, VIP is known to promote Th2 and T regulatory cell differentiation.56,57 Release of VIP in the gut and lung stimulates ILC2s through VIP receptor type 2 (VPAC2) to induce IL-5 production. The cyclic release of VIP in response to feeding induces a rhythmic production of IL-5 by ILC2s. This circadian expression of IL-5 is detectable in the blood circulation and appears to regulate systemic eosinophil numbers.58
Interestingly, lung nociceptors can sense IL-5 released by activated immune cells, which, in turn, induces the production of VIP. If uncontrolled, this inflammatory signaling loop can lead to the development of allergic inflammation.59 In allergic airway inflammatory models, ablation of NaV1.8+ nociceptor signaling in mice reduces the infiltration and activation of lung-resident ILC2s and Th2 cells, reducing bronchial hyperresponsiveness during early and peak allergic airway inflammation. Consequently, infiltration of eosinophils and macrophages is also reduced in the lungs and bronchoalveolar lavage (BAL) fluid in NaV1.8+ deficient mice compared to mice with intact nociceptors. Mice treated with a VPAC2 antagonist exhibit inhibition of ILC2 activation and production of the type 2 cytokines IL-5 and IL-13.59 This bidirectional communication between nociceptors and ILC2s may represent a mechanism to prime and enhance type 2 immune responses; however, in the absence of a negative feedback loop, the uncontrolled release of IL-5 and VIP may contribute to the development of allergic airway inflammation.
Calcitonin gene-related protein
The neuropeptides calcitonin gene-related protein (CGRP) and γ-aminobutyric acid (GABA) are secreted by pulmonary neuroendocrine cells (PNECs). PNECs and the closely associated lung-resident ILC2s are preferentially found at the lung airway branch points. This is a strategically advantageous location for PNECs and ILCs to sense and respond to noxious environmental stimuli, as computation modeling has shown that over time, particles accumulate at lung branch points.60 Through the release of CGRP, PNECs directly orchestrate and amplify ILC2-mediated type 2 airway inflammation in response to environmental allergens.61 CGRP increases the capacity of ILC2s to produce IL-5 in the presence of the alarmin IL-33 in vitro, while GABA has no effect. Deletion of the CGRP receptor in CalcrlKO mice reduces immune cell infiltration after HDM challenge. Similarly, mice that have no PNECs (AsclCKO) that are challenged with ovalbumin peptide allergen display impaired type 2 immune responses, including defective goblet cell hyperplasia and eosinophil and immune cell recruitment and infiltration. In AsclCKO mice, the levels of CGRP and GABA are strongly reduced.61 Disruption of GABA synthesis in PNECs does not directly affect the immune response but is essential to promote goblet cell hyperplasia and mucus and IL-13 overproduction during allergic airway inflammation.62 Asthmatic patients have elevated numbers of PNECs, suggesting that future therapeutics altering CGRP signaling in ILC2s may be viable agents for treatment of allergic asthma symptoms. Additionally, future studies on similar neuroendocrine cells in other organs and tissues, such as the intestinal mucosa, may help us to gain further insight into the neuroendocrinological regulation of ILCs and how tissue specificity can be established.
Neurotrophic factors and the RET receptor
The gastrointestinal tract is a highly innervated system under the influence of a whole separate arm of the ANS called the enteric nervous system. Therefore, it is unsurprising that nervous control can also directly influence cells of the immune system to maintain homeostasis. ILC3s respond to neurotrophic factors released by associated mucosal glial cells in response to the commensal gut flora (Fig. 1).63 Glial cells surround neurons and modulate their activity, migration and development. Glial cells in the small intestine are able to sense commensal products and alarmins.63 These cells secrete neurotrophic factor family ligands (GFL) that bind to the tyrosine kinase receptor (RET) expressed at the surface of ILC3s. In a gut organoid model cultured in the presence of GFL, RET signaling in ILC3s was found to be able to directly control the constitutive expression of IL-22 via downstream phosphorylation of STAT3 (signal transducer and activator of transcription 3), ERK, AKT and p38. When the neurotrophic receptor RET is deleted in enteric ILC3s, IL-22 production by ILC3s is decreased both under steady-state conditions and during Citrobacter rodentium infection or epithelial irritation with dextran sodium sulfate (DSS) water. Mice without RET show increased propensity towards gut inflammation that leads to altered microbial communities.63 These findings demonstrate the direct influence of enteric glial cells on ILC3 functions and how glial-derived ligands facilitate the maintenance of gut homeostasis and its involvement in gut defense.
Endocrine regulation of ILCs
All corticosteroids and sex hormones are synthesized from cholesterol through successive enzymatic reactions. Glucocorticoids are produced in the adrenal cortex, testosterone is produced in the Leydig cells of the testes, and estrogen is produced in the ovaries. These small lipophilic molecules can diffuse through the membranes of cells and bind to their receptors in the cytosol. In the absence of ligands, hormone receptors are associated with chaperone proteins that prevent their nuclear translocation. Ligand binding induces the dissociation of the receptors from the chaperone complexes and allows their translocation into the nucleus. In the nucleus, these receptors directly regulate the transcription of target genes with or without interacting with other transcription regulators upon binding to their hormone response elements.
Glucocorticoids
The hypothalamic–pituitary–adrenal (HPA) axis is a crucial neuroendocrine regulatory axis for coordinating and responding to environmental cues. It controls the rhythmic secretion of glucocorticoids (GCs), which are released through the blood stream into the periphery. GCs have potent immunosuppressive and anti-inflammatory effects that enable maintenance of physiological homeostasis and host integrity, especially during pathogen elimination, inflammation resolution and tissue repair. Consequently, adrenalectomy, which prevents GC secretion, results in increased levels of IL-12, IFN-γ, TNF, and IL-6 in mice in response to murine cytomegalovirus (MCMV) infection, rendering these mice more susceptible to viral infection than intact mice.64 This increased susceptibility is not due to a lack of viral control but rather than a dysregulation of virus-elicited cytokines. GCs play an immunoregulatory role by inducing apoptosis of T helper cells, B cells, macrophages, NK cells, dendritic cells, neutrophils and eosinophils, consequently downregulating the secretion of proinflammatory cytokines such as IFN-γ, IL-1α, IL-6 and IL-12 and promoting the production of immunosuppressive cytokines such as IL-10 and TGF-β.65 ILCs do not escape from such regulation.66,67 GCs endogenously produced after stimulation of the HPA axis can inhibit ILC1s and NK cell functions during both bacterial67 and viral infections,66 thus preventing immunopathologies without impairing immune responses against infections.
When glucocorticoid receptor is deleted from NKp46-expressing cells, mice show exacerbated induction of septic shock by the endotoxin lipopolysaccharide (LPS), as the affected cells are unable to control the production of inflammatory cytokines such as IFN-γ, which in turn impedes IL-10-dependent immune tolerance. Similarly, GCs produced during MCMV infection induce the expression of the checkpoint inhibitor programmed death-1 (PD-1/CD279) specifically on splenic, but not hepatic, NK cells.66 This induction of PD-1 inhibits the production of IFN-γ and represents an essential regulatory mechanism for protection against harmful inflammation. The tissue specificity of GC signaling on NK cells and ILC1s is interesting because it reveals how the nervous system can regulate the spatiotemporal reactivity of immune cells. This dynamic regulation is critical for the return to homeostasis. Systemic inhibition of IFN-γ production would be deleterious for antiviral responses occurring in the liver, but uncontrolled IFN-γ production would be harmful to the organism.
Sex hormones
Differences in the susceptibility, development and outcomes of infectious diseases and chronic inflammation observed between males and females have suggested an important immunoregulatory role of sex hormones. Despite the fact that the effects of sex hormones on immune cells are dependent on cell type, dose and exposure timing, androgens have generally been described as mainly anti-inflammatory, while estrogens are considered proinflammatory.
Estrogens
Estrogens can mediate their effects through direct binding to estrogen receptor (ER) α and β or through nongenomic action via modulation of intracellular signaling pathways, such as the phosphoinositide 3-kinase (PI3K)/AKT and ERK pathways; intracellular calcium mobilization; cyclic adenosine monophosphate (cAMP); and phospholipase C activation.68 Expression of one or both ER isoforms has been reported in almost all immune cell types.69 Contradictory roles have been attributed to estrogens in NK cell functions. While some researchers found that estrogens enhance the cytotoxicity of the human NK-like cell line in vitro,70 others described that estrogens reduce NK cell activity, including cytotoxicity, cytokine production and proliferation.71,72 These discrepancies could be explained by the concentrations of estrogens used in such in vitro studies, which are often much higher than the physiological concentrations measured in females even during the last month of pregnancy. While NK cell cytotoxicity is not different between ovariectomized mice and control mice, administration of estrogens in vivo decreases NK cell cytotoxicity in ovariectomized mice. However, in the absence of appropriate models in which ER can be specifically deleted in NK cells, the direct influence of estrogens on these cells will be complicated to elucidate.
Uterine ILC2s uniquely express ERα, while ILC2s in other tissues do not.73 In the absence of estrogen signaling (in mice deficient for ERα and β), ILC2 populations are significantly decreased in the uterus, while ILC2 populations in other tissues remain unaffected.74 A similar phenotype is observed when mice are ovariectomized; the number of uterine ILC2s is increased in mice exposed to estrogens compared to mice exposed to placebo pellets. Lung ILC2s are not modulated by estrogen administration. The specific expression of ER in uterine ILC2s may explain why ILC2s from other tissues are not modulated by the presence of estrogens. Currently, the consequences of such tissue-specific regulation of uterine ILC2s on the homeostasis of the uterus or during gestation are not understood.
Androgens
Androgens, such as testosterone and dihydrotestosterone, mediate their effects via the androgen receptor (AR), which can act, like ER, through genomic and nongenomic pathways. Sex differences in the susceptibility to develop asthma75 have been described, and we recently revealed the role of androgen signaling in ILC2s as a key mechanism that would explain these differences found between men and women. Both the proportion and total number of ILC2s are greater in female mice than in male mice.74 This trend has also been reported in asthmatic patients. Women have more ILC2s in the blood than men.76 In mice, we showed that ILC2 progenitors specifically express AR but not ER. Mechanistically, AR signaling directly inhibits the differentiation and proliferation of mature ILC2s in a cell-intrinsic manner.74 Compared to control conditions, treatment of ILC2 progenitors with dihydrotestosterone (DHT) reduces the development of ILC2s, while incubation with flutamide, an inhibitor of AR signaling, increases the number of ILC2s. Production of the type 2 cytokines IL-5 and IL-13 is reduced in males compared to females and when ILC2s are stimulated in the presence of testosterone. This inhibitory effect of androgen signaling on ILC2s appears to protect males from developing allergic airway inflammation, as male mice have less immune cell infiltration and IgE production in the lungs than female mice when challenged with HDM, IL-33 or Alternaria alternata.74,76
ILC regulation by dietary-derived metabolites
Mucosal surfaces, in particular those of the gastrointestinal tract, are constantly exposed to food- and microbiota-derived metabolites and micronutrients. Recent studies have highlighted how dietary-derived metabolites, such as vitamins A and D, aryl hydrocarbon receptor (Ahr) ligands and lipids influence local immunity and gut homeostasis. Located at the frontlines of the gut mucosa, ILCs of the immune system are well equipped to sense these molecules through the expression of specific receptors. These receptors include vitamin D receptor (VDR), retinoic acid receptor-α (RARα), Ahr and several prostaglandin (PG) and leukotriene (LT) receptors. The activation of these specific receptors upon ligand binding markedly impacts ILC maintenance and functions but also directly regulates ILC metabolism, revealing a mechanism of fine control between the immune system and its environment.
Vitamins A and D
Recent studies have shed light on the critical roles of vitamins A and D and their active forms, retinoic acid (RA) and 1,25-dihydroxyvitamin D3, respectively, on gut-resident immune cells.77,78 RA is an essential fat-soluble vitamin that is of absolute necessity for many biological and physiological processes. It is required during embryogenic development, acting as an essential regulator of tissue differentiation and growth.78 Vitamin D is derived from a precursor molecule present in the skin that requires ultraviolet B radiation for its synthesis. Then, vitamin D is further metabolized into its active biological form, 1,25-dihydroxyvitamin D3 (VitD3),78 which is critical for bone mineralization and calcium homeostasis, among other processes.79 RA and VitD3 bind to the retinoic acid receptor and vitamin D receptor, respectively, two nuclear receptors that are both highly expressed in all intestinal ILCs. Upon ligand binding, both receptors heterodimerize to a common nuclear receptor, RXR, before binding to their response elements and initiating cell-specific DNA transcriptional responses. Although they share the same nuclear receptor, the RXR, VDR–RXR and RAR–RXR complexes have different and opposing effects on ILC functions.
RA is shown to regulate LTi cell maturation during embryogenic development by modulating RORγt expression, which subsequently controls lymph node and Peyer patch formation but also influences immune fitness in adulthood.80 In adulthood, RA regulates ILC3 functions and the capacity of ILCs to express IL-2281,82 through direct binding of the RAR–RXR complex to the il22 and Rorc promotor regions, which subsequently promotes their transcription (Fig. 1).80,81 Exogenous supplementation of RA induces gut ILC3 proliferation and accumulation, increases protection against C. rodentium infection and reduces gut inflammation in DSS-induced colitis. Deficiency in vitamin A is associated with significant reductions in the expression of the important antimicrobial peptide RegIIIγ by goblet cells, resulting in compromised immunity to acute bacterial infection.82 Interestingly, a lack of vitamin A increases ILC2 numbers and IL-13 production, enhancing protection against Trichuris muris, a nematode parasite. RA is also a regulator of the capacity of ILCs to home to the intestine. RA induces the upregulation of the homing molecules CCR7, CCR9 and α4β7 at the surface of ILC1s and ILC3s but not ILC2s.83 Upon RA stimulation, ILC1s and ILC3s downregulate CCR7 and upregulate CCR9 and α4β7 expression, allowing cells to egress from the mesenteric lymph nodes and migrate to the gut. In CCR9-deficient mice, ILC3s accumulated in the periphery despite being stimulated with RA. While CCR7 is critical for the migration of ILC3s to the mesenteric lymph nodes, CCR9 and integrin α4β7 expression is mandatory for short-term ILC3 gut homing and long-term residence in the small intestine. The differential regulation of ILC homing subsets in response to RA can explain why ILC3 numbers, but not ILC2 numbers, are reduced in previous studies.81,82 Why such important differences have been observed between ILC2s and ILC3s in response to RA has not yet been elucidated. Investigation of the molecular events and major cellular modifications after RAR–RXR activation in ILCs is critical to answer such questions. Furthermore, in humans, RA is known to facilitate the conversion of ILC1s into ILC3s by promoting RORγt expression.84
Conversely, VDR-deficient mice are resistant to C. rodentium infection; this effect is associated with increases in IL-22-producing ILC3s, increased expression of RegIIIγ in epithelial cells and modification of the gut microbiota composition.85 VitD3 is a negative regulator of the IL-23R pathway and can induce a cytokine shift in human ILC3s, inducing the IL-6 production and decreasing IL-22, IFN-γ and GM-CSF secretion in vitro.86 Patients suffering from inflammatory bowel disease have less VitD3 in their plasma than healthy volunteers or patients in remission.86 This finding suggests that VitD3 is potentially an important regulator of the pro- versus anti-inflammatory balance controlling the cytokine programs of gut-resident ILC3s, acting as a rheostat of mucosal homeostasis. In humans, stimulation of ILC2s and ILC3s with RA increases cytokine production and homing molecule expression, while addition of VitD3 reduces RA-dependent effects, revealing opposing roles of these two vitamins on ILC functions.87 Taken together, these studies reveal a fine control of gut ILC migration, maintenance and function by RA and VitD3 and indicate that vitamin deficiencies broadly modify ILC behavior, immune responses and gut homeostasis.
Aryl hydrocarbon receptor ligands
Aryl hydrocarbon receptor (AHR) belongs to the superfamily of nuclear receptors. AHR is activated by aromatic hydrocarbons that are naturally found in fruits and vegetables. These include indoles that are directly found in cruciferous vegetables or are obtained following tryptophan degradation by the gut microbiota.88,89 The binding of these ligands to AHR induces its translocation from the cytoplasm to the nucleus to regulate the transcription of its target genes.89 The first evidence of the impact of Ahr on the immune system emerged with the development of Ahr-null mice. The primary study revealed a profound reduction in T and B cells in the spleen and peripheral lymph nodes of these mice.90 Gut-resident ILCs highly express AHR, allowing them to actively sense their environment and act as wardens of the gut mucosa. A lack of AHR is associated with reduced ILC3 numbers and decreased ILC3-derived IL-22 (Fig. 1).91,92 AHR directly binds the Il22 promoter and acts in concert with RORγt to induce Il22 expression in ILC3s.93 Loss of Ahr expression does not impact LTi formation and accumulation in the fetal intestine in mouse embryos; rather, Ahr loss influences the maintenance of gut ILC3s in adulthood through increased apoptosis associated with decreased expression of the prosurvival molecules Bcl2 and Bcl2l1 in these cells.93 These results reveal a network that critically regulates both the maintenance and functions of these cells. However, the regulated pathways might differ between ILC3 subsets given that Ahr deficiency differentially impacts LTi populations and other ILC3 populations.
The regulation of AHR signaling is tightly controlled because prolonged activation by ligands that resist metabolic clearance or excessive AHR ligand degradation can both have detrimental effects. The clearance of ARH ligands involves the cytochrome P4501 (CYP1) family of enzymes. Constitutive expression of CYP1 enzymes results in strong reductions in the AHR ligands available and leads to loss of enteric ILC3s and Th17 cells.94 Consequently, constitutive expression of Cyp1a and loss of Ahr have similar consequences when mice are infected with C. rodentium, leading to increased susceptibility associated with reduced production of IL-22.92–94 Excessive clearance of AHR ligands in constitutive Cyp1a-expressing mice can be counterbalanced by increased intake of AHR ligands in the diet, resulting in restoration of immune protection against bacterial infections.94 Such findings highlight how the availability of AHR ligands in mucosal tissue regulates the activity of ILC3s during homeostasis and under inflammatory conditions.
In humans, AHR is a major transcription factor participating in the maintenance of ILC3s. Ahr expression in ILC3s (Rorc+Ahr+Tbx21−Eomes−) prevents cells from transdifferentiating into NK cells (Rorc−Ahr−Tbx21+Eomes+) under IL-15 and IL-1β stimulation.95 In contrast, disruption of Ahr expression in ILC3s with specific short hairpin RNA or inhibition with AHR antagonists in vitro induces transdifferentiation of ILC3s into NK cells, which is associated with acquired IFN-γ expression and killing functions.95
As mentioned before, one of the major sources of AHR ligands is tryptophan catabolism by the gut microbiota. It has been well demonstrated that modification of eating habits directly influences the gut microbiota composition.96 This subsequently impacts the availability of Ahr inducers and modulates ILC3 maintenance and functions; notably, beneficial changes provide resistance against colonization of the fungus Candida albicans and promote mucosal protection against inflammation.97,98 Interestingly, gestation-specific gut colonization of pregnant germ-free mice has been shown to favor ILC3 development and long-term ILC3 maintenance in offspring as well as to positively regulate IL-22 production in these cells.99 Importantly, these effects rely on maternal microbiota-derived molecules, many of which have in fact been identified as precursors or natural Ahr ligands (e.g., kynurenine).99
Collectively, these studies demonstrate, once again, the importance of crosstalk between the gut microbiota and innate immune mechanisms in the regulation and maintenance of gut homeostasis. This crosstalk is mediated by Ahr expression and activation and is finely controlled by both AHR ligand availability and feedback loop mechanisms.
Lipids
Lipids are essential components of cells that provide precursor molecules for cell membrane formation or fuel cells to support their functions. Lipid mediators obtained from the catabolism of food-derived lipids or from the recycling of lipid cell membrane components markedly modulate immune responses, playing both proinflammatory and anti-inflammatory roles to maintain body homeostasis.100 They have recently been shown to be critical players in modulating ILC functions. Most of these mediators come from the catabolism of arachidonic acid, which gives rise to different products. These include prostaglandins (D2, E2 and I2) and leukotrienes (B4, C4, D4 and E4), among others. These products are obtained following the action of specific enzymes.100 PGs and LTs signal through specific receptors that are expressed in all ILC subsets. Activation of these receptors conveys specific immune responses and regulates ILC migration, proliferation and cytokine production.
Leukotrienes
Lung ILC2s highly express one LT receptor, cysteinyl leukotriene receptor 1 (CysLTR1).101,102 ILC2 stimulation by LTD4 induces rapid and robust calcium influx, which leads to the secretion of IL-4, IL-5, and IL-13 in a CysLT1R-dependent manner. LTC4 and LTD4 administered in vivo act synergistically with IL-33 to activate lung ILC2s through the NFAT pathway, promoting lung ILC2 proliferation, IL-5 expression and lung eosinophilia.102 CysLT1R-deficient mice infected with N. brasiliensis or A. alternata species display reduced ILC2 lung accumulation, IL-5 and IL-13 production and proliferation.101–103 In humans, ILC2 IL-13 production is also increased upon LT stimulation. Furthermore, the expression of IL-33/IL-25 receptors is augmented, which allows ILC2s to be more responsive to these cytokines.104 Altogether, these studies highlight the role of LTs in ILC2 biology and their impact on lung homeostasis. Given the essential role of ILC2s in the development of allergic reactions, it would be interesting to specifically target this LT-CysLTR1 pathway to modulate ILC2-mediated proinflammatory activity and potentially reduce asthma symptoms and disease severity.102,105,106 To date, only CysLTR1 has been identified as a potent regulator of ILC functions that is mainly involved in promoting allergy and lung inflammation. Given the expression levels of other LT receptors in ILCs, especially gut-resident ILCs, further investigations are warranted to determine the roles of these lipid mediators on ILC maintenance and functions.
Prostaglandins
PGs differentially modulate ILC activity. While PGE2 and PGI2 signaling inhibits ILC functions,107,108 PGD2 signaling promotes ILC2 activation and ILC2 migration to and accumulation in inflamed lungs.109–111
PGD2 signaling
The PGD2 receptor, CRTH2, was first identified in Th2 cells.112 It is now used to differentiate human ILC2s from other ILC subsets.113 During allergic reactions, mast cells and myeloid cells are potent producers of PGD2,114 which is found in elevated concentrations in the BAL of patients with severe asthma.115 PGD2 induces the production of large amounts of proinflammatory cytokines, such as IL-4, IL-5, IL-13, IL-8, GM-CSF, CSF-1 and TNF-α, by human ILC2s. Production of these cytokines is further increased by administration of LTE4, demonstrating the potential synergy between different lipid mediators.104 Accumulation of PGD2 in the lungs amplifies type 2 immunity synergistically with IL-33/IL-25 stimulation, subsequently enhancing ILC2 IL-13 production and chemotaxis.110,116 Murine ILC2s also express CRTH2 (Gpr44), and its activation enhances ILC2 migration and functions but does not influence ILC2 development.109 During inflammation, CRTH2-deficient ILC2s fail to produce IL-4 and IL-13 and to accumulate in inflamed lungs.109 Therefore, the PGD2-CRTH2 pathway represents an additional layer of ILC2 regulation that can modulate lung allergic inflammation.
PGI2 signaling
PGI2 is recognized by prostacyclin receptor (IP), which is almost exclusively expressed on ILC2s.108 PGI2 is a negative regulator of ILC2 functions.108 Incubation with cicaprost (a PGI2 analog) inhibits ILC2 proliferation and cytokine production by ILC2s when ILC2s are stimulated with IL-33.108 IP-deficient mice challenged intranasally with A. alternata extracts have increased ILC2 lung accumulation together with enhanced IL-5 and IL-13 expression, resulting in increased eosinophil infiltration and lung inflammation.
PGE2 signaling
PGE2 signals through the PGE2 receptor family. PGE2 receptor 2 (EP2) is mainly expressed by ILC3s, while PGE2 receptor 4 (EP4) is more commonly expressed across multiple subsets of ILCs, such as ILC1s, NK cells and ILC2s. PGE2 attenuates IL-33-induced ILC2 proliferation and cytokine production in mice107 and humans.117 During A. alternata infection, deletion of EP4 exacerbates lung inflammation. This effect is associated with increased IL-5 and IL-13 production by ILC2s, leading to enhanced eosinophil recruitment. These studies reveal how the PGE2-EP2/4 pathway negatively regulates ILC2 activity.
IL-22 production by ILC3s also appears to be influenced by PGE2. Inhibition of PGE2 synthesis with indomethacin leads to the development of systemic inflammation induced by LPS due to the release of important levels of TNFα and IL-6 in the circulation, resulting in translocation of gut bacteria and accumulation of neutrophils in the peritoneal cavity.118 These effects can be prevented by treating mice with EP4 agonists. PGE2 contributes to systemic inflammation by regulating the homeostatic production of IL-22 by ILC3s (Fig. 1). Reduced synthesis of PGE2 inhibits the IL-22–IL-22R signaling pathway in intestinal epithelial cells, leading to the downregulation of critical proteins involved in mucosal integrity, such as RegIIIβ, RegIIIγ, Fut2, mucins and molecules forming tight junctions.118
Overall, these lipid mediators exhibit similar or opposite roles in ILC functions, depending on the pathways triggered. LTs and PGD2 promote ILC2 cytokine expression and cell proliferation, whereas PGI2 and PGE2 limit ILC2 activation. Little is known about the impact of these lipid mediators on ILC3 functions, with the exception of PGE2, which appears to promote IL-22 production by ILC3s to confer intestinal barrier protection and reduce systemic inflammation in sepsis. As all these lipid mediators are derived from the catabolism of arachidonic acid, a promising strategy may be to modulate different enzymatic pathways to enhance or inhibit ILC functions according to clinical needs. In asthma, for example, many new drugs have been developed to target final products such as IL-5 or IL-13;106 however, modulating upstream events or the catabolism of arachidonic acid and its derived products would allow us to broadly dampen disease severity by targeting several pathways and cell types at the same time (e.g., IL-5, IL-13, ILC2s and Th2 cells). To date, numerous FDA (Food and Drug Administration)-approved compounds have been implicated in the regulation of these pathways, and the feasibility of using such therapeutic agents to improve asthma could be quickly evaluated.119–121
Conclusion
This review highlights the intense crosstalk between the neuroendocrine system and different ILC populations at mucosal surfaces and discusses how these interactions participate in the maintenance of tissue homeostasis and the induction of appropriate responses to infections. We are, however, only at the beginning of our investigations about how ILCs integrate these signals to fine-tune the immune response to prevent immunopathology without impairing infection control.
The complexity of the effects of physiological regulators on the immune system is due to their broad impacts involving a large number of targets, including several regulatory loops, and their potential indirect effects. Because of synergistic or antagonistic effects among factors, the ultimate consequences of multiple physiological mediators on immune cells can be ascertained not through addition of the effects of the different hormones, neuropeptides and metabolites but rather through multiplication of their effects (Fig. 1). Thorough study of the direct and indirect effects of physiological regulators requires the development of complex models, such as new models in which a mediator or its receptor can be deleted in a spatiotemporal manner. With recent developments in single-cell genomic technologies that facilitate whole-transcriptome analysis and knockout mouse generation together with advancements in microscopy techniques, it has begun to be possible to tease apart, in molecular detail, the crosstalk between the body’s multiple organ systems and immune systems in vivo.
Understanding the interactions among these systems will provide great insight into how disruption within one or more of these compartments influences each of the other systems and how outcomes, such as the ability of the host to regulate inflammation, disease protection and wound healing, are affected. This approach could also provide us with an important opportunity to manipulate immune responses using physiological molecule-based strategies that may be less harmful than synthetic drug-based strategies.
Acknowledgements
The authors apologize to all investigators whose works were not cited in this article due to space limitations. We are grateful to Kylie Luong for helpful comments and preparation of the manuscript. The figure was drawn using Servier Medical Art (https://smart.servier.com) and modified by the authors under the terms of the Creative Commons Attribution 3.0 Unported License. C.S. was supported by grants and fellowships from the National Health and Medical Research Council (NHMRC) of Australia (APP1165443) and the Australian Research Career Development Fellowship (APP1123000). N.J. was supported by Grant 1163990 awarded through the 2018 Priority-driven Collaborative Cancer Research Scheme and cofunded by Cancer Australia and Cure Cancer. This work was made possible through the Victorian State Government Operational Infrastructure Support and the Australian Government NHMRC IRIIS.
Competing interests
The authors declare no competing interests.
References
- 1.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 2.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Ishizuka IE, et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat. Immunol. 2016;17:269–276. doi: 10.1038/ni.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klose CSN, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Seillet C, et al. Differential requirement for Nfil3 during NK cell development. J. Immunol. 2014;192:2667–2676. doi: 10.4049/jimmunol.1302605. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, et al. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature. 2016;539:102–106. doi: 10.1038/nature20105. [DOI] [PubMed] [Google Scholar]
- 9.Seillet C, et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 2016;17:436–447. doi: 10.1016/j.celrep.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Bando JK, Liang HE, Locksley RM. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat. Immunol. 2015;16:153–160. doi: 10.1038/ni.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stier MT, et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J. Exp. Med. 2018;215:263–281. doi: 10.1084/jem.20170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359:114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricardo-Gonzalez RR, et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 2018;10:1093–1099. doi: 10.1038/s41590-018-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gury-BenAri M, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166:1231–1246.e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawa S, et al. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 19.Weizman OE, et al. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171:795–808 e712. doi: 10.1016/j.cell.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abt MC, et al. Innate immune defenses mediated by two ILC subsets are critical for protection against acute Clostridium difficile infection. Cell Host Microbe. 2015;18:27–37. doi: 10.1016/j.chom.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao Y, Huntington ND, Belz GT, Seillet C. Type 1 innate lymphoid cell biology: lessons learnt from natural killer cells. Front Immunol. 2016;7:426. doi: 10.3389/fimmu.2016.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulenouar S, et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity. 2017;46:273–286. doi: 10.1016/j.immuni.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seillet C, Belz GT, Mielke LA. Complexity of cytokine network regulation of innate lymphoid cells in protective immunity. Cytokine. 2014;70:1–10. doi: 10.1016/j.cyto.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behnsen J, et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity. 2014;40:262–273. doi: 10.1016/j.immuni.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickard JM, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh-Takayama N, et al. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 2014;41:776–788. doi: 10.1016/j.immuni.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Rankin LC, et al. The transcription factor T-bet is essential for the development of NKp46(+) innate lymphocytes via the Notch pathway. Nat. Immunol. 2013;14:389–395. doi: 10.1038/ni.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci. Lett. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- 32.Neumann H, et al. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J. Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gougeon PY, et al. The pro-inflammatory cytokines IL-1beta and TNFalpha are neurotrophic for enteric neurons. J. Neurosci. 2013;33:3339–3351. doi: 10.1523/JNEUROSCI.3564-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klose CSN, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549:282–286. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard AD, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406:70–74. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- 36.Cardoso V, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–281. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallrapp A, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriyama M, et al. The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J. Exp. Med. 2005;202:217–224. doi: 10.1084/jem.20050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mashaghi A, et al. Neuropeptide substance P and the immune response. Cell Mol. Life Sci. 2016;73:4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feistritzer C, et al. Natural killer cell functions mediated by the neuropeptide substance P. Regul. Pept. 2003;116:119–126. doi: 10.1016/s0167-0115(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 41.Lang K, Drell TL, Niggemann B, Zanker KS, Entschladen F. Neurotransmitters regulate the migration and cytotoxicity in natural killer cells. Immunol. Lett. 2003;90:165–172. doi: 10.1016/j.imlet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Monaco-Shawver L, et al. Substance P inhibits natural killer cell cytotoxicity through the neurokinin-1 receptor. J. Leukoc. Biol. 2011;89:113–125. doi: 10.1189/jlb.0410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-gamma production and resistance to Pseudomonas aeruginosa infection. Eur. J. Immunol. 2005;35:1567–1575. doi: 10.1002/eji.200425902. [DOI] [PubMed] [Google Scholar]
- 44.Sears MR, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 45.Milano CA, et al. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 46.Sun Z, et al. Norepinephrine inhibits the cytotoxicity of NK92MI cells via the beta2adrenoceptor/cAMP/PKA/pCREB signaling pathway. Mol. Med. Rep. 2018;17:8530–8535. doi: 10.3892/mmr.2018.8872. [DOI] [PubMed] [Google Scholar]
- 47.De Lorenzo BH, de Oliveira Marchioro L, Greco CR, Suchecki D. Sleep-deprivation reduces NK cell number and function mediated by beta-adrenergic signalling. Psychoneuroendocrinology. 2015;57:134–143. doi: 10.1016/j.psyneuen.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Logan RW, Arjona A, Sarkar DK. Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain Behav. Immun. 2011;25:101–109. doi: 10.1016/j.bbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriyama S, et al. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 51.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 52.Dalli J, Colas RA, Arnardottir H, Serhan CN. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity. 2017;46:92–105. doi: 10.1016/j.immuni.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramon S, et al. The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am. J. Pathol. 2016;186:962–973. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Said SI, Rosenberg RN. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976;192:907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- 55.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim H, Barrow P, Foster N. Transcriptional modulation by VIP: a rational target against inflammatory disease. Clin. Epigenetics. 2011;2:213–222. doi: 10.1007/s13148-011-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delgado M, Ganea D. Vasoactive intestinal peptide: a neuropeptide with pleiotropic immune functions. Amino Acids. 2013;45:25–39. doi: 10.1007/s00726-011-1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talbot S, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoegger MJ, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sui Pengfei, Wiesner Darin L., Xu Jinhao, Zhang Yan, Lee Jinwoo, Van Dyken Steven, Lashua Amber, Yu Chuyue, Klein Bruce S., Locksley Richard M., Deutsch Gail, Sun Xin. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360(6393):eaan8546. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang YY, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat. Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 63.Ibiza S, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J. Immunol. 1999;162:3527–3533. [PubMed] [Google Scholar]
- 65.Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. 2018;9:1332. doi: 10.3389/fimmu.2018.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quatrini L, et al. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat. Immunol. 2018;19:954–962. doi: 10.1038/s41590-018-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quatrini L, et al. Host resistance to endotoxic shock requires the neuroendocrine regulation of group 1 innate lymphoid cells. J. Exp. Med. 2017;214:3531–3541. doi: 10.1084/jem.20171048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 70.Sorachi K, Kumagai S, Sugita M, Yodoi J, Imura H. Enhancing effect of 17 beta-estradiol on human NK cell activity. Immunol. Lett. 1993;36:31–35. doi: 10.1016/0165-2478(93)90065-a. [DOI] [PubMed] [Google Scholar]
- 71.Souza SS, et al. Influence of menstrual cycle on NK activity. J. Reprod. Immunol. 2001;50:151–159. doi: 10.1016/s0165-0378(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 72.Hao S, Li P, Zhao J, Hu Y, Hou Y. 17beta-estradiol suppresses cytotoxicity and proliferative capacity of murine splenic NK1.1+ cells. Cell. Mol. Immunol. 2008;5:357–364. doi: 10.1038/cmi.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartemes K, Chen CC, Iijima K, Drake L, Kita H. IL-33-responsive group 2 innate lymphoid cells are regulated by female sex hormones in the uterus. J. Immunol. 2018;200:229–236. doi: 10.4049/jimmunol.1602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laffont S, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 2017;214:1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cephus JY, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21:2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Czarnewski, P., Das, S., Parigi, S. M. & Villablanca, E. J. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients9, 68 (2017). [DOI] [PMC free article] [PubMed]
- 78.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol. Cell Endocrinol. 2017;453:68–78. doi: 10.1016/j.mce.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 80.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mielke LA, et al. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spencer SP, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim MH, Taparowsky EJ, Kim CH. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity. 2015;43:107–119. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernink JH, et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 85.Chen J, Waddell A, Lin YD, Cantorna MT. Dysbiosis caused by vitamin D receptor deficiency confers colonization resistance to Citrobacter rodentium through modulation of innate lymphoid cells. Mucosal Immunol. 2015;8:618–626. doi: 10.1038/mi.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Konya V, et al. Vitamin D downregulates the IL-23 receptor pathway in human mucosal group 3 innate lymphoid cells. J. Allergy Clin. Immunol. 2018;141:279–292. doi: 10.1016/j.jaci.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 87.Ruiter B, Patil SU, Shreffler WG. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin. Exp. Allergy. 2015;45:1214–1225. doi: 10.1111/cea.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 89.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev. Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 90.Fernandez-Salguero P, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 91.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2011;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 93.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schiering C, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes T, et al. The transcription factor AHR prevents the differentiation of a stage 3 innate lymphoid cell subset to natural killer cells. Cell Rep. 2014;8:150–162. doi: 10.1016/j.celrep.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zelante T, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Sanos SL, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomez de Aguero M, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev. Pharmacol. Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 101.Doherty TA, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Moltke J, et al. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J. Exp. Med. 2017;214:27–37. doi: 10.1084/jem.20161274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lund SJ, et al. Leukotriene C4 potentiates IL-33-induced group 2 innate lymphoid cell activation and lung inflammation. J. Immunol. 2017;199:1096–1104. doi: 10.4049/jimmunol.1601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salimi M, et al. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J. Allergy Clin. Immunol. 2017;140:1090–1100 e1011. doi: 10.1016/j.jaci.2016.12.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yokomizo T, Nakamura M, Shimizu T. Leukotriene receptors as potential therapeutic targets. J. Clin. Invest. 2018;128:2691–2701. doi: 10.1172/JCI97946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim AS, Doherty TA. New and emerging therapies for asthma. Ann. Allergy Asthma Immunol. 2016;116:14–17. doi: 10.1016/j.anai.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, et al. Prostaglandin E2 inhibits group 2 innate lymphoid cell activation and allergic airway inflammation through E-prostanoid 4-cyclic adenosine monophosphate signaling. Front Immunol. 2018;9:501. doi: 10.3389/fimmu.2018.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou W, et al. Prostaglandin I2 signaling and inhibition of group 2 innate lymphoid cell responses. Am. J. Respir. Crit. Care Med. 2016;193:31–42. doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wojno ED, et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8:1313–1323. doi: 10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J. Allergy Clin. Immunol. 2014;133:899–901 e893. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xue L, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nagata K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J. Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- 113.Mjosberg JM, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 114.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 115.Fajt ML, et al. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J. Allergy Clin. Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barnig C, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maric J, et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J. Allergy Clin. Immunol. 2018;141:1761–1773 e1766. doi: 10.1016/j.jaci.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duffin R, et al. Prostaglandin E(2) constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351:1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Claar D, Hartert TV, Peebles RS., Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev. Respir. Med. 2015;9:55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rumzhum NN, Ammit AJ. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin. Exp. Allergy. 2016;46:397–410. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 121.Hara S. Prostaglandin terminal synthases as novel therapeutic targets. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:703–723. doi: 10.2183/pjab.93.044. [DOI] [PMC free article] [PubMed] [Google Scholar]