Abstract
The signaling lymphocyte activation molecule (SLAM) family of receptors (SFRs) are ubiquitously expressed on immune cells, and they regulate multiple immune events by recruiting SH2 (Src homology 2) domain-containing SAP family adapters, including SAP and its homologs, Ewing’s sarcoma-associated transcript 2 (EAT-2) and EAT-2 related transducer (ERT). In human patients with X-linked lymphoproliferative (XLP) disease, which is caused by SAP mutations, SFRs alternatively bind other inhibitory SH2 domain-containing molecules to suppress immune cell activation and development. NK cells express multiple SFRs and all SAP family adapters. In recent decades, SFRs have been found to be critical for enhancing NK cell activation in response to abnormal hematopoietic cells in SAP-family-intact NK cells; however, SFRs might suppress NK cell activation in SAP-family-deficient mice or patients with XLP1. In this paper, we review how these two distinct SFR signaling pathways orchestrate NK cell activation and inhibition and highlight the importance of SFR regulation of NK cell biology and their physiological status and pathological relevance in patients with XLP1.
Keywords: SLAM, SAP, NK cells, immune signaling
Subject terms: NK cells, Bone marrow transplantation
Introduction
Natural killer (NK) cells play an essential role in innate defenses against “unwanted” allogeneic bone marrow transplants or autologous cells undergoing various forms of stress, such as transformed tumorous cells and virus-infected cells. NK cells kill these cells via a sequential process, including recognition, formation of a conjugate and synapse, polarization and targeted killing. One of the most important steps is discrimination between self cells and nonself or modified-self cells, which is mediated by NK cell receptors. In contrast with adaptive T cells and B cells, NK cells have no antigen-specific TCRs or BCRs that enable this recognition. There are two distinct types of NK cell receptors, activating receptors and inhibitory receptors. Whether NK cells eliminate these abnormal cells largely depends on the signaling balance originating from these cell receptors.
The mouse inhibitory Ly49 family and the human killer inhibitory receptors (KIRs), as well as NKG2A located in the NK gene complex in both mice and humans, represent the major NK cell inhibitory receptors.1–6 They can specifically sense the presence of MHC class I (MHC-I) molecules, which are considered self-cell markers.7 MHC-I molecules on normal self-cells can sufficiently elicit inhibitory signaling leading to NK cell inactivation. However, the “unwanted” cells usually lose or express mismatched MHC-I and thus fail to elicit sufficient inhibitory signals. Under “missing-self” status, these cells become susceptible to NK cell-mediated lysis. However, it remains unclear whether the absence of self-MHC-I molecules is sufficient for NK cell activation. NK cells also bear many activating receptors, such as CD16, natural killer gene 2D (NKG2D), natural cytotoxicity receptors, and activating KIRs in humans (Ly49D and Ly49H in mice).2,8,9 They execute NK cell recognition in a “modified self” manner, by which they detect stress-inducible ligands unduly expressed on tumorous or virus-infected cells.2–4 After engagement with their respective ligands, these receptors initiate various activating signaling through noncovalent association with transmembrane immunoreceptor tyrosine-based activation motif (ITAs)-bearing signaling adapters, such as FcRγ, CD3ζ, DNAX-activating protein of 12 kD (DAP12), and DAP10 that harbor a YxxM motif.2,8–10
Signaling lymphocytic activation molecule (SLAM) family of receptors (SFRs) are also expressed on NK cells, and they are usually self-ligands and mediate homotypic interactions of NK cells between hematopoietic cells. SFRs are characterized by the presence of two or more immunoreceptor tyrosine-based switch motifs (ITSMs) in their cytoplasmic domain, and they regulate multiple immune events by recruiting SH2 domain-containing SAP family adapters, including SAP and its homologs EAT-2 and ERT. ERT has been found to be a nonfunctional pseudogene in humans.11,12 SFRs alternatively bind other inhibitory molecules with the SH2 domain to suppress immune cell activation and development.11–17 The great importance of the SLAM-SAP family in immune regulation was highlighted by the discovery that mutations of Sh2d1a1, which encodes for SAP, were identified in human patients with X-linked lymphoproliferative (XLP)-1 disease, which is a rare primary immunodeficiency disease affecting approximately 1–2 per 1 million males.12,18,19 A key feature of XLP1 is the exquisite sensitivity of affected individuals to disease induced by EBV infection.20–23 These patients develop a severe immunodeficiency syndrome that includes impaired NK-T cell development and humoral immunity.21,23–27 NK cells express multiple SLAM family receptors and all SAP family adapters.11,13,28–30 NK cells from SAP-deficient patients display impaired NK cell cytotoxicity to hematopoietic cell lines.17,31–33 During recent decades, SFRs have been revealed to be critical for enhancing NK cell activation in response to abnormal hematopoietic cells in SAP-family-intact NK cells; however, SFRs might suppress NK cell activation in SAP-family-deficient NK cells.17,31–34 As a consequence, SAP-deficient patients are susceptible to lymphoma partly due to compromised NK cell activation. Here, we review how these SFR signaling pathways orchestrate activation of mouse NK cells, which highlights the great importance of SFR regulation of NK cell biology and their physiological status and pathological relevance in patients with XLP1.
Expression of SLAM-family receptors and SAP family adapters during NK cell development
SFRs include seven members: SLAM, CD48, Ly9, 2B4, CD84, and Ly108 [in mice, but natural killer T and B-cell antigen (NTB-A) in humans] and CD2-like receptor activating cytotoxic cells (CRACC).11–13,35 The genes encoding SFRs are all located within the same locus on chromosome 1 in humans and mice.12,13,36 Although this gene cluster is likely generated from duplication of a common ancestor gene and these receptors have the same origin, they are structurally and functionally distinct.12,25,36,37
With the exception of CD48, which is a glycosyl-phosphatidylinositol-anchored protein, SFRs are type I transmembrane proteins, with an extracellular segment followed by a single transmembrane region and a cytoplasmic domain. The extracellular segment is characterized by an N-terminal Ig V-like domain and a membrane-proximal C2 domain. SFRs recognize themselves, in other words, they are self-ligands, with the exception that 2B4 binds CD48. The binding specificity of SFRs is determined by the extracellular IgV domain.37–39 In contrast with immune receptors usually bearing ITAM or immunoreceptor tyrosine-based inhibitory motifs (ITIMs), SFRs are characterized by the presence of one or more ITSMs in their cytoplasmic domain, which is also observed in the coreceptor programmed death 1 (PD1).11,12,40
SFRs transmit immune signaling via coupling of Src homology 2 (SH2) domain-containing adapters, including SAP, EAT-2 and ERT.41–44 The Sh2d1a gene encoding SAP is positioned on the chromosome X, whereas the gene encoding EAT-2 and ERT are colocalized near the SFR gene cluster. The SAP family adapters consist almost entirely of an SH2 domain followed by a short carboxy-terminal tail. SFRs bind with SAP family adapters through their ITSMs. The structural properties of SFRs determine their binding specificity with SAP family adapters. It is not clear whether all SFRs are able to recruit all three adapters. In fact, CRACC binds EAT-2 and ERT but not SAP.44
SFRs are detectable only on hematopoietic lineage cells. SLAM-family members, such as SLAM, 2B4, and CD48, are differentially expressed among distinct progenitor cells in mice. Long-term hematopoietic stem cells (HSCs) express only SLAM, defined as SLAM+2B4–CD48– cells, whereas multipotent progenitors (MPPs) lose SLAM and acquire 2B4 expression and are defined as SLAM–2B4+CD48– cells.45,46 Common lymphoid progenitors (CLPs) that are SLAM–2B4+CD48+ start to acquire CD48 expression. Moreover, heterogeneous HSCs and MPPs can be subdivided into a hierarchy of functionally distinct subpopulations based on their expression of Ly9.45 Although the combinatorial expression of SFRs is a putative marker to precisely distinguish hematopoietic progenitors, whether SFRs play any physiological roles is still unclear. At a minimum, mice with a combined deficiency of SFRs appear to have an intact composition of hematopoietic progenitors (unpublished data). Thus, SFRs behave as makers for distinguishing HSCs and other progenitors.
NK cell development occurs in specialized BM niches. Nearly all SFRs are expressed on mouse NK cells, but these receptors display a dynamic expression profile along with NK cell maturation. For example, a high proportion of NK cell progenitors express both SLAM and Ly108 when NK cells are committed.28,29 During NK cell differentiation, the presence of these two SFRs gradually decreases. SLAM disappears at the terminal stage. Interestingly, Ly108 expression is highly associated with NK cell development and education; Ly108-positive NK cells are mostly “licensed” as Ly49C/I+ in the B6 background.28,29 In addition to 2B4, Ly9, and CD84, CRACC maintains a high level of expression throughout all stages.28,29 It is also expressed on activated CD8+ T cells and B cells.12 Notably, CRACC is highly expressed on multiple myeloid cells, and thus has become a potential target for treatment of this malignancy.47–49 Although SFRs are widely expressed and dynamically altered during NK cell differentiation, there is no strong evidence suggesting which receptors are involved in regulation of NK cell differentiation.28,44 Individual deletion of Ly108 does not disturb NK cell development, suggesting a redundant role of SFRs.28 This possibility is supported by the recent finding that a combined deficiency of SFRs leads to a mild change in NK cell differentiation.29,50
SAP family adapters are all expressed in mouse NK cells. These adapters also undergo temporal changes in their expression during NK cell development. SAP expression is lowest in NK cell precursors and gradually increases during NK cell maturation.28,30,51,52 In contrast, a preponderance of EAT-2 over SAP is seen in immature NK cells, but a predominance of SAP over EAT-2 is observed in more differentiated NK cells.30,51 Despite this, mice lacking all SAP family adapters preserve intact NK cell differentiation.14,24,25,34,43 Thus, SFRs likely regulate NK cell differentiation through SAP-independent signaling, which needs to be further elucidated. Furthermore, deletion of SAP family adapters also fails to rescue the slightly altered NK cell differentiation in SFR-deficient mice, excluding the possibility of alternative signaling of SAP family signaling in NK cell maturation.29
SLAM-family receptors are hematopoietic cell-specific NK cell activating receptors
Allogeneic bone marrow transplantation is a potentially curative treatment for a substantial proportion of patients with hematologic malignancies, including intermediate and high-risk acute myeloid leukemia (AML), recurrent chronic myeloid leukemia (CML), multiple myeloma, and lymphoma. This therapeutic effect is partly due to the action of NK cells.53–55 Data based on clinical studies also indicate that adoptive transfer of ex vivo activated NK cells has some beneficial effects on hematopoietic malignancies.56,57 Rejection of parental allogeneic bone marrow was reported in a phenomenon called “hybrid resistance”, which refers to the failure of first-generation hybrid mice to accept semiallogeneic parental bone marrow but not other non-hematopoietic grafts.58–60 NK cells are experimentally verified to be responsible for the rejection of bone marrow via missing-self reactivity.61,62 Based on the theory of NK cell licensing, self-MHC-I molecule is essential for NK cell functional competence, likely through engagement with self-MHC-specific inhibitory receptors. Because F1 offspring mice carry heterozygote MHC-I alleles that are parentally inherited, two distinct NK cell subsets are presumably separately licensed by paternal and maternal MHC-I. As a result, the functional NK cells that are licensed by maternal MHC-I will reject MHC-I mismatched paternal BM transplants but not MHC-I matched maternal BM transplants, and vice versa.4,62 However, these findings raise another important question, namely, why NK cells preferentially kill hematopoietic cells under missing-self conditions. Although NK cells can kill primary MHC-I-deficient hematopoietic cells, which presumably lack any endogenous ligands for NK cell activation. This result raises two possibilities: the absence of self-MHC-I is sufficient for NK cell activation, or NK cells likely bear self-specific activating receptors that can specifically recognize hematopoietic cells.
Most NK cell experts typically disagree with the first possibility, and they have tried to find an activating receptor that executes NK cell rejection of hematopoietic cells. As a result, some NK cell activating receptors, such as Ly49D and NKG2D, were revealed to contribute to allogeneic bone marrow rejection and “hybrid resistance”.63–66 In hybrid resistance, Ly49D and NKG2D are necessary only for F1 (BALB/c×C57BL/6) NK cell-mediated rejection of BM from BALB/c but not C57BL/6 donors.63–65 Blockade of NKG2D can also diminish NK-mediated rejection of hematopoietic cells. Because the identity and tissue-specificity of those ligands for NKG2D or Ly49D are not clear, particularly on primary healthy cells, these findings cannot fully explain the specificity of hybrid resistance.
In patients with XLP1, the ability of NK cells to kill hematopoietic cells is highly impaired. As a result, over 30% of these patients suffer from lymphoma following EBV infection, but not other non-hematopoietic tumors. The incidence of lymphoma is most likely correlated with NK cell and CD8+ cytotoxic T lymphocytes dysfunction.17,31–33,67,68 Mouse studies further validate these data from humans; SAP-deficient NK cells fail to eliminate hematopoietic cells.14,28,34,69 Thus, SAP is critical for the NK cell response towards hematopoietic cells. In mice lacking all three SAP family adapters, NK cells nearly lose their ability to kill a range of hematopoietic cells, including tumor cell lines, MHC-I-deficient RMA-S cells, YAC-1 cells (which overexpress ligands for mouse NKG2D), and primary MHC-I-deficient hematopoietic cells.34 Intriguingly, SAP-family-deficient NK cells preserve their intact ability to kill non-hematopoietic cells.28,34,69 Therefore, these data demonstrate that SAP is compulsory for NK cell recognition of hematopoietic cells. Due to the position of the Sh2d1a gene on the X chromosome, SAP-deficient F1 hybrid mice can be obtained. SAP-deficient F1 hybrid mice on the BALB/c×B6 background show a pronounced defect in NK cell rejection of parental hematopoietic cells.29 Thus, SAP is also required for NK cell-mediated “hybrid resistance”.
SAP is mainly recruited downstream of SFR signaling. The determination of NK cell specificity to kill hematopoietic cells via SAP family adapters indicates that SFRs are key activating receptors specific to hematopoietic cells. Notably, SFRs are strictly detectable on hematopoietic cells, including HSCs, lymphocytes, and myeloid cells. Donor hematopoietic cells from mice lacking SFRs significantly increase the resistance to killing by wild-type NK cells.29 However, disassociation of SFR engagement between NK cells and hematopoietic targets only moderately influences NK cell elimination. This experiment implies that other NK cell activating receptors may also be involved. Considering that SFRs are hematopoietic-specific and endogenously expressed on NK cells, these receptors may represent the first self-specific activating receptors for NK cell recognition under “missing self” conditions. The existence of these receptors also verifies the hypothesis that the absence of self-MHC-I is not sufficient for full activation of NK cells.
Much effort has been employed to increase the success of bone marrow transplantation. Blockade of host NK cell activation may facilitate engraftment. The discovery that the SLAM family acts as self-specific activating receptors and accounts for the preference of NK cells to kill hematopoietic grafts may bring a glimmer hope for therapeutic interference with bone marrow rejection. Through ectopic expression of SFR on nonhematopoietic B16 cells, multiple SFRs, including 2B4, Ly9, CRACC, and Ly108, can elicit activation of NK cells, at least in mice. Determination of the key SLAM family members that carry out NK cell recognition of hematopoietic cells needs further investigation.
Although SFRs are self-specific activating receptors during the NK cell effector process, genetic deletion of all SFR members leads to elevated NK cell responsiveness to hematopoietic cells.29 A possible explanation is that chronic engagement of SFRs between NK cells and other hematopoietic cells may desensitize NK cell responsiveness. This suggests that in addition to self-specific inhibitory receptors, endogenous NK cell activating receptors may also regulate NK cell education, a process of NK cell functional acquisition.70–74
SAP-dependent SLAM family signaling in NK cell activation
NK cells express multiple SFR members and all SAP family adapters, and thus, engagement of SFRs between NK cells and hematopoietic targets trigger very complicated signaling, which in turn gives rise to distinct NK cell responses. SAP is highly homologous to EAT-2 and ERT, but they transmit divergent downstream signaling. The downstream molecules that bind to SFRs usually transmit signaling through SAP, EAT-2, ERT, and other SH2-domain-containing phosphatases.
An essential role of SAP in NK cell activation has been found in human XLP1 patients and SAP-deficient mice. The engagement of SFRs on mature NK cells induces tyrosine phosphorylation of ITSMs via Src family kinases. Phosphorylated ITSMs binds the SAP protein. Apart from CRACC, all other SFRs, including 2B4, Ly108 in mice (NTB-A in humans), Ly9 and CD84, are able to recruit SAP.11–13 Nevertheless, only triggering 2B4, Ly108, or Ly9 potentiates NK cell cytotoxicity, whereas CD84 promotes NK cell activation to a lesser extent in mice. Thus far, 2B4 is one of the most well studied SFRs in NK cell activation, and its activity is highly dependent on SAP, which also contributes to human NTB-A-triggered NK cell activation. Less is known about the SAP-dependence of Ly9 signaling activity.
2B4 is expressed on virtually all NK cells and contains four ITSMs in its cytoplasmic tail.16,75 Its engagement results in the formation of lipid rafts, where tyrosine phosphorylation of its ITSM domain is likely initiated by the enzyme Csk.76,77 This is an early and essential step in 2B4-mediated NK cell activation. The phosphorylated ITSM of 2B4 recruits at most SAP, or other SH2 domain-containing signaling molecules, to trigger downstream signals.14–17,69 Although SAP is able to bind to all four ITSMs of 2B4, the first ITSM is sufficient for 2B4-mediated NK cell activation, and the second ITSM may contribute to SAP-dependent 2B4 signaling.16,75
SAP is an SH2 domain-only containing adapter, and it must recruit other kinases to facilitate SFR signaling. In contrast with EAT-2/ERT, SAP has no signaling activity mediated by its short cytoplasmic tail. SAP has to recruit Fyn, a Src family protein tyrosine kinase (PTK), to 2B4.14,16,69,75 Biochemical studies have revealed a direct interaction between arginine 78 (R78) in the SH2 domain of SAP and the Src homology 3 (SH3) domain of Fyn.14,75,78,79 The recruitment of Fyn kinase promotes full phosphorylation of other ITSMs of 2B4, which results in positive feedback, enhancing SAP binding with Fyn. In addition to the Fyn kinase, SAP can also bind with other SH3-domain-containing molecules, such as Pak-interacting exchange factor (PIX), the adapter Nck, and protein kinase C-θ (PKC-θ), through the same R78 residue.14,16,69,80–82
Because 2B4-triggered cytotoxicity and cytokine production are compromised in both SAP-deficient and Fyn-deficient mice, the SAP-Fyn interaction must be critical for 2B4-mediated NK cell activation.14,69 This hypothesis was further approved by SAPR78A mutant mice, in which SAP cannot bind with Fyn.14 R78A mutant NK cells also show less killing activity against hematopoietic cells, such as MHC-I-missing RMA-S. However, compared with SAP-deficient NK cells, SAP R78ANK cells have only mild defects.14 This interesting finding not only indicates that SAP-Fyn binding is critical for NK cell activation but also suggests that SFRs can transmit other SAP-independent signaling pathways, which are most likely to be inhibitory.
2B4 promotes NK cell activation by inducing SAP-Fyn binding (Fig. 1a). Biochemically, 2B4 engagement can evoke tyrosine phosphorylation of Vav-1, an exchange factor that promotes cytoskeleton reorganization and formation of lytic synapses.14,75,83–85 Deficiency of the family of Vav proteins, including Vav-1, −2, and −3, severely compromises NK cell activity.86 2B4 crosslinking-induced Vav-1 phosphorylation is highly dependent on SAP and Fyn kinase.14,75 Importantly, Vav-1 activation is also minimal when SAP-Fyn binding is disrupted in SAPR78A NK cells.14 Consequently, the abovementioned activating signaling cascade augments the affinity of the adhesion molecule LFA-1 for intercellular adhesion molecule-1 (ICAM-1), leading to durable conjugation between NK cells and hematopoietic target cells.14,87–89 To this end, SFRs, such as 2B4, act as activating receptors by recruiting SAP and triggering Fyn-Vav-1-mediated adhesion signaling to facilitate NK cell formation of stable conjugation with hematopoietic cells. In addition to Fyn, other signaling molecules, such as PLCγ, calcium mobilization, and MAPK pathway, can also promote 2B4-mediated NK cell activation.14,90,91
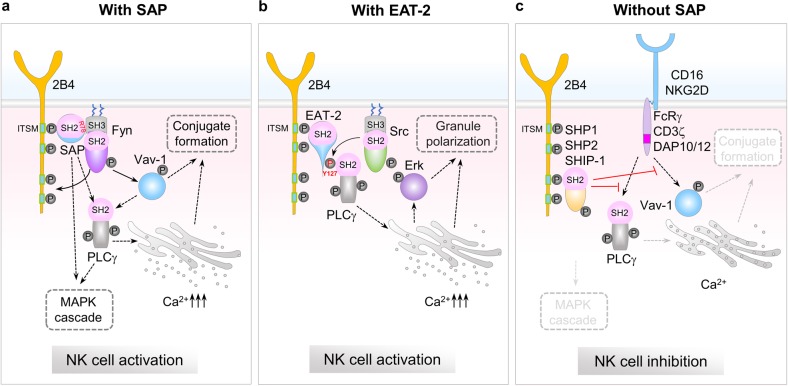
Fig. 1.
SAP-family-dependent and -independent SFR signaling in NK cell activation and inhibition. Engagement of SFRs (2B4 is shown as an example) induces tyrosine phosphorylation of cytoplasmic ITSMs, likely by Src family kinases. SH2 domain-containing proteins, SAP family adapters, or other phosphatases then bind to the phosphorylated ITSM of SFR through their SH2 domain. a SAP-dependent SFR signaling. Through arginine 78 (R78) in the SH2 domain, SAP binds to the SH3 domain of Fyn. Fyn subsequently promotes full phosphorylation of other ITSMs in SFRs, further enhancing SAP binding with Fyn. Fyn triggers Vav-1 phosphorylation and PLCγ-mediated Ca2+ flux directly or indirectly, leading to augmented NK cell conjugate formation. b EAT-2-dependent SFR signaling. EAT-2 coupling enables phosphorylation of the tyrosine in its C-terminal tail by a Src family kinase. EAT-2 associates with PLCγ through a direct interaction between phosphorylated Tyr127 in the C-terminal tail of EAT-2 and the N-terminal SH2 domain of PLCγ, which evokes calcium flux and Erk activation, leading to NK cell granule polarization. c SAP-family-independent SFR signaling. In the absence of SAP family adapters, SFRs preferentially bind to other SH2 domain-containing inhibitory molecules, such as SHP-1, SHP-2, and SHIP-1, which strongly suppress CD16- or NKG2D-mediated activating signaling
Although 2B4 predominantly triggers NK cell activation in a significant manner through SAP-mediated enhancement of conjugate formation, 2B4 also utilizes another mechanism to initiate intracellular signaling. Suppression of either NKp46 or the downstream ITAM-containing protein FcεRIγ substantially reduces 2B4-initiated cytotoxicity of human NK cells.92,93 NK cells from FcεRIγ, CD3ζ, and DAP12 triple deficient NK cells have a compromised cytolysis ability against CD48-bearing target cells.94 Moreover, a physical interaction of CD3ζ with SAP and 2B4 has been delineated.93 In addition, the cytoplasmic adapter 3BP2 is directly associated with the fourth phosphorylated ITSM of 2B4 in human NK cells.95–97 These data suggest that 2B4-initiated NK cell activation may utilize other adapters, thus updating the prevailing assumption that SAP-dependent SFR signaling is critical for NK cell activation. The involvement of other adapters in SFR-mediated NK cell activation needs to be validated as either direct or indirect binding.
EAT-2-dependent SLAM family signaling in NK cell activation
EAT-2 and ERT have very similar amino acid sequences. Distinct from SAP, both EAT-2 and ERT bear a short cytoplasmic tail, which possesses two tyrosine residues.12,30,42–44,98 Although EAT-2 and ERT do not share the same binding site as SAP for Fyn in their intracellular domain, they can be recruited to SFRs by binding of their SH2 domain to the phosphorylated tyrosine of the SFR cytoplasmic domain.42 This coupling enables phosphorylation of one or two tyrosine residues in their C-terminal tails, linking SFRs to downstream signals. In mice, the C-terminal tail of EAT-2 encompasses two tyrosine residues, Tyr120 and Tyr127, whereas the human EAT-2 contains only a single C-terminal Tyr127.30,43 The capacity of EAT-2 to promote NK cell activation by SFRs requires the C-terminal Tyr127 in both species (Fig. 1b). Despite this, compared with SAP-deficient NK cells, NK cells deficient in both EAT-2 and ERT show only minor defects in NK cell activation in response to hematopoietic cells.34,43 Thus, SAP-mediated SFR signaling may dominate NK cell activation. However, simultaneous deletion of all SAP family adapters might severely compromise the NK cell-mediated rejection of allogeneic hematopoietic cells.34
EAT-2 and ERT were first identified to be associated with 2B4.43 EAT-2 serves a stimulatory function in 2B4-evoked NK cell activation. When the 2B4 ITSM tyrosine is phosphorylated by the Src family kinase Fyn and to a lesser extent by Lck, Src, and Lyn, EAT-2 directly associates with PLCγ in human NK cells, and this coupling is mediated by a direct interaction between the phosphorylated Tyr127 in the C-terminal tail of EAT-2 and the N-terminal SH2 domain of PLCγ, which supports 2B4-triggered PLCγ tyrosine phosphorylation, calcium fluxes and then Erk activation30,98,99 (Fig. 1b). This interaction also provokes a small increase in c-Cbl tyrosine phosphorylation, which likely creates a negative feedback network to terminate the function of EAT-2.85,98 Unlike SAP, EAT-2 cannot evoke Fyn or Lck tyrosine phosphorylation, EAT-2 also cannot trigger activation of PI3K and the downstream protein Akt.30 However, the effect is only limited to an enhancement of calcium flux triggered by CD16 in mouse NK cells. Although EAT-2 is dispensable for conjugate formation, the process critical for SAP-mediated NK cell activation, it promotes granule polarization towards the NK cell synapse to accelerate the effector phase of NK cell activation.30 This effect is Tyr127-dependent and is generally mediated by the ability of EAT-2 to enhance calcium flux. Mutation of Tyr127 decreases NK cell activation.
CRACC is the only SLAM family member that maintains an activating function in NK cells of XLP1 patients, partly due to its binding specificity with EAT-2.44,100 In addition, the action of EAT-2 in CRACC-induced NK cell activation is well-documented. Mouse CRACC contains three tyrosine residues, Tyr261, Tyr266, and Tyr281, in its cytoplasmic domain, whereas human CRACC does not contain the second tyrosine residue, Tyr266.44 The third tyrosine (Tyr281) specifically interacts with EAT-2 and is responsible for the activating activity of CRACC, whereas Tyr261 accounts for its inhibitory effect when EAT-2 is absent in mouse NK cells. EAT-2 binds to phosphorylated Tyr281 in CRACC through its SH2 domain, linking CRACC to downstream effectors.44 Whether CRACC tyrosine phosphorylation is dependent on EAT-2/ERT is controversial.
In human NK cells, EAT-2 seems to induce tyrosine phosphorylation of CRACC. The two tyrosine residues located in a short sequence in the carboxy-terminal portion of the SH2 domain of EAT-2 facilitate the coupling of CRACC to downstream effector proteins. This indicates that EAT-2 is involved in more distal events in the CRACC signaling pathway. Ligation of CRACC profoundly phosphorylates PLCγ1, PLCγ2, PI3K and c-Cbl and to a minor extent, Vav-1 and SHIP-1.44,98 EAT-2 binding induces activation of the PLCγ and PI3K signaling pathways, triggering calcium flux and CRACC-mediated NK cell cytotoxicity.44,98
In addition to 2B4 and CRACC, EAT-2 can also link human NTB-A to downstream activation signaling. The homophilic interaction of NTB-A triggers its tyrosine phosphorylation by Src family kinases. The cytoplasmic tail of NTB-A contains three tyrosine residues, Tyr273, Tyr284, and Tyr319, and the second and third tyrosine are embedded within the consensus sequence of ITSM.101 The second tyrosine specifically recruits EAT-2, which is essential and sufficient for NTB-A-mediated NK cell cytotoxicity, while the third tyrosine contributes to the full activation of NK cells mediated by NTB-A, likely through associating with SAP.101 NTB-A primarily stimulates NK cell activation by enhancing the activity of PLCγ and PI3K and, to a lesser extent, the MEK kinase pathway.101 Actin reorganization and Src kinases are also involved in the generation of NTB-A-mediated NK effector functions.
SAP-family-independent SLAM family signaling in NK cell inhibition
The severe defect in NK cell response to hematopoietic cells in SAP-family-deficient NK cells seemingly demonstrates an important role for SAP-family-dependent SFR signaling in NK cell activation. However, several studies suggest that SFRs can also employ other SH2 domain molecules when SAP family adapters are absent from NK cells. First, SFRs can induce tyrosine phosphorylation of other SH2 domain-containing molecules in SAP-family-deficient NK cells.14–17 Second, compared with SAP-deficient mice, NK cell activity is only mildly impaired by blocking SAP-dependent activating signaling in SAPR78A mutant mice.14 Finally, but importantly, the removal of SFRs on SAP-family-deficient NK cells can completely rescue the severe defect of NK cell rejection of MHC-I-deficient hematopoietic cells.29 On the basis of these clues, SFRs not only can transmit the activating signaling for NK cell activation but also deliver certain inhibitory signals for NK cell inhibition in the absence of SAP family members. In other words, SAP family adapters mediate the switch-of-function through two distinct activities, active signaling molecules and natural blockers.
NK cell activity is severely impaired in SAP- but not EAT-2/ERT-deficient mice; however, ectopic expression of individual SFRs strongly mitigates the activation of EAT-2/ERT-lacking NK cells while only slightly affecting SAP-deficient NK cells.34 Thus, NK cell dysfunction caused by SAP deficiency is likely due to the loss of SFR-mediated activating signaling and the gain of inhibitory signaling. However, EAT-2 and ERT most likely function as “natural blockers” to prevent the binding of inhibitory molecules to SFRs.42 This probably explains the discrepancy between mice deficient in SAP and those deficient in EAT-2/ERT.
NK cells express multiple SFRs. Except for CD84, most of these SFRs are activating. Ectopic expression of CRACC, Ly9 and CD48 can enhance NK cell cytotoxicity towards nonhematopoietic B16 cells and IFN-γ production.29,34 However, when SAP family adapters are absent, the overexpression of individual SFRs not only fails to elicit NK cell activation but also strongly suppresses NK cell activation caused by other “induced self” stimuli.34 Although CD84 is not an activating receptor on NK cells, the engagement of CD84 can still pronouncedly dampen NK cell cytotoxicity.34,102 Thus, all SFRs appear to be inhibitory in SAP-family-deficient NK cells. As a result, deletion of SFRs either on donor hematopoietic cells or on recipient NK cells can overcome the severe defect of SAP-deficient NK cell killing towards hematopoietic cells under “missing-self” status.29 This finding raises many critical questions. First, if SFRs are self-specific activating receptors, how can mice with a combined deficiency in all SFRs and SAP family adapters reject allogenic hematopoietic cells? SFR deficiency only partially affects NK cell elimination of MHC-I-deficient hematopoietic cells, suggesting the presence of other NK cell activating receptors, which most likely compensate for the deficiency in SFRs. Second, which SFR member(s) contributes to the severely defective NK cell activation in patients with XLP1 is unknown. To answer this question, individual deletions of each SFR member will be useful in determining the key inhibitory SFR(s) in SAP-deficient NK cells and will aid in the treatment of NK cell dysfunction in XLP1 by enabling blockade of certain SFRs.
Genetic and biochemical analyses provide strong evidence supporting the inhibitory identity of SFRs in SAP-family-deficient NK cells. Many inhibitory SH2-domain-containing molecules, including protein phosphatase SHP1 and SHP2, the lipid phosphatase SHIP1, and Csk, can be alternatively recruited to certain phosphorylated ITSMs of SFRs.14–17 SAP family adapters likely prevent their binding to SFRs either through competing with the same ITSMs or through occupancy hindrance (Fig. 1c).
Many indirect studies support the notion that these SH2-domain phosphatases are critical for NK cell inhibition induced by SFR. 2B4 is a well-documented SFR member that induces NK cell inhibition. Functionally, 2B4-mediated inhibition of NK cell cytotoxicity is robustly elevated in SAP-deficient NK cells.14,17 Biochemically, the engagement of 2B4 induces tyrosine phosphorylation of SHIP-1.14,16,75 In contrast with the first two ITSMs, which have specificity for SAP binding, the phosphorylated third ITSM of 2B4 can additionally recruit several proteins, such as SHIP-1, SHP-1, and SHP-2.14-17 Genetic deletion of SHIP1 significantly abolishes 2B4-mediated inhibition of calcium flux and Erk phosphorylation induced by BCR crosslinking, at least in the chicken DT40 cell line.14 This is the first genetic evidence showing that SFRs directly couple with other inhibitory molecules to exert their inhibitory activity. In fact, combined deletion of SHP-1 and SHP-2 in DT40 cells can also slightly relieve 2B4-induced inhibition.14 Thus, SFRs likely couple multiple SH2-domain-containing proteins to suppress various activating signaling pathways via NK cell receptors, such as CD16 and NKG2D. However, because mice lacking SHIP-1 or SHP-1 have severe defects in NK cell development or functional acquisition, it is still not clear whether SFR-mediated inhibitory molecules are involved in NK cell biology. Limited study suggests that this SAP-family-independent signaling mediated by SLAMF6 participates in mouse NK cell education.28
Concluding remarks
Hematopoietic cell-specific SFRs act as self-specific endogenous activating receptors, endowing NK cell specificity for control of “unwanted” hematopoietic cellular targets. The presence of SAP family adapters can switch the features of SFRs to generate inhibitory or activating behavior, and thus, they strictly determine this important action mediated by SFRs. SAP promotes SFR-mediated NK cell activation occurs via a combined mechanism: SAP coupling of SFRs to Fyn through R78 in the SH2 domain, which triggers Vav-1 phosphorylation leading to augmented NK cell conjugate formation; SAP can also function as a natural blocker to prevent recruitment of SH2 domain-containing inhibitory molecules. EAT-2 has two distinct roles in NK cell activation: EAT-2 functions through Y127-dependent recruitment of PLCγ, which evokes calcium flux and Erk activation, and EAT-2 can also prevent SFR binding with SH2 domain-containing inhibitory molecules. In the absence of SAP, inhibitory molecules, such as SHIP-1, link with SFRs to restrain NK cell activation, which occurs in NK cells from SAP-mutated patients. Thus, studies of NK cells in SAP deficiency have shed substantial light on the requirement of the SLAM-SAP families for NK cell immunosurveillance in abnormal hematopoietic cells. This knowledge, in turn, has the potential to be translated into novel treatments for not only XLP1 patients but also individuals suffering from viral infection-related diseases and hematopoietic tumors.
Acknowledgements
Research in Dong’s lab was supported by the Natural Science Foundation of China (to Z.D., 81725007, 31830027, and 31821003), National Key Research and Development Program (2018YFC1003900 to Z.D), Beijing Natural Science Foundation (5172018 to Z.D.), the Postdoctoral Innovation Talent Support Program of China (to S.C., BX201700134) and the China Postdoctoral Science Foundation grant (to S.C., 2017M620051).
Competing interests
The authors declare no competing interests.
Contributor Information
Shasha Chen, Phone: +86-10-62798536, Email: css0206@mail.tsinghua.edu.cn.
Zhongjun Dong, Phone: +86-10-62798536, Email: dongzj@mail.tsinghua.edu.cn.
References
- 1.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–372. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Hilton HG, Parham P. Missing or altered self: human NK cell receptors that recognize HLA-C. Immunogenetics. 2017;69:567–579. doi: 10.1007/s00251-017-1001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 8.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat. Immunol. 2002;3:807–813. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- 10.Rosen DB, et al. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J. Immunol. 2004;173:2470–2478. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z, Veillette A. How do SAP family deficiencies compromise immunity? Trends Immunol. 2010;31:295–302. doi: 10.1016/j.it.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 13.Veillette A. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol. Rev. 2006;214:22–34. doi: 10.1111/j.1600-065X.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z, et al. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity. 2012;36:974–985. doi: 10.1016/j.immuni.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Tangye SG, et al. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J. Immunol. 1999;162:6981–6985. [PubMed] [Google Scholar]
- 16.Eissmann P, et al. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- 17.Parolini S, et al. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J. Exp. Med. 2000;192:337–346. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffey AJ, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 19.Nichols KE, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl Acad. Sci. USA. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada S, et al. Immune deficiency in the X-linked lymphoproliferative syndrome. I. Epstein-Barr virus-specific defects. J. Immunol. 1982;129:2532–2535. [PubMed] [Google Scholar]
- 21.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 22.Bar RS, et al. Fatal infectious mononucleosis in a family. N. Engl. J. Med. 1974;290:363–367. doi: 10.1056/NEJM197402142900704. [DOI] [PubMed] [Google Scholar]
- 23.Provisor AJ, Iacuone JJ, Chilcote RR, Neiburger RG, Crussi FG. Acquired agammaglobulinemia after a life-threatening illness with clinical and laboratory features of infectious mononucleosis in three related male children. N. Engl. J. Med. 1975;293:62–65. doi: 10.1056/NEJM197507102930202. [DOI] [PubMed] [Google Scholar]
- 24.Nichols KE, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma CS, et al. Selective generation of functional somatically mutated IgM + CD27 + , but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J. Clin. Invest. 2006;116:322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 28.Wu N, et al. A hematopoietic cell-driven mechanism involving SLAMF6 receptor, SAP adaptors and SHP-1 phosphatase regulates NK cell education. Nat. Immunol. 2016;17:387–396. doi: 10.1038/ni.3369. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, et al. The self-specific activation receptor SLAM family is critical for NK cell education. Immunity. 2016;45:292–304. doi: 10.1016/j.immuni.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Quintero LA, et al. EAT-2, a SAP-like adaptor, controls NK cell activation through phospholipase Cgamma, Ca++, and Erk, leading to granule polarization. J. Exp. Med. 2014;211:727–742. doi: 10.1084/jem.20132038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benoit L, Wang X, Pabst HF, Dutz J, Tan R. Defective NK cell activation in X-linked lymphoproliferative disease. J. Immunol. 2000;165:3549–3553. doi: 10.4049/jimmunol.165.7.3549. [DOI] [PubMed] [Google Scholar]
- 32.Bottino C, et al. NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 2001;194:235–246. doi: 10.1084/jem.194.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima H, et al. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur. J. Immunol. 2000;30:3309–3318. doi: 10.1002/1521-4141(200011)30:11<3309::AID-IMMU3309>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Dong Z, et al. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat. Immunol. 2009;10:973–980. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 35.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat. Rev. Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 36.Kingsmore SF, Souryal CA, Watson ML, Patel DD, Seldin MF. Physical and genetic linkage of the genes encoding Ly-9 and CD48 on mouse and human chromosomes 1. Immunogenetics. 1995;42:59–62. doi: 10.1007/BF00164988. [DOI] [PubMed] [Google Scholar]
- 37.Velikovsky CA, et al. Structure of natural killer receptor 2B4 bound to CD48 reveals basis for heterophilic recognition in signaling lymphocyte activation molecule family. Immunity. 2007;27:572–584. doi: 10.1016/j.immuni.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ames JB, Vyas V, Lusin JD, Mariuzza R. NMR structure of the natural killer cell receptor 2B4 (CD244): implications for ligand recognition. Biochemistry. 2005;44:6416–6423. doi: 10.1021/bi050139s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao E, et al. NTB-A receptor crystal structure: insights into homophilic interactions in the signaling lymphocytic activation molecule receptor family. Immunity. 2006;25:559–570. doi: 10.1016/j.immuni.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 41.Sayos J, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 42.Morra M, et al. Structural basis for the interaction of the free SH2 domain EAT-2 with SLAM receptors in hematopoietic cells. EMBO J. 2001;20:5840–5852. doi: 10.1093/emboj/20.21.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roncagalli R, et al. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat. Immunol. 2005;6:1002–1010. doi: 10.1038/ni1242. [DOI] [PubMed] [Google Scholar]
- 44.Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat. Immunol. 2009;10:297–305. doi: 10.1038/ni.1693. [DOI] [PubMed] [Google Scholar]
- 45.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell. Stem. Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Tai YT, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malaer JD, Mathew PA. CS1 (SLAMF7, CD319) is an effective immunotherapeutic target for multiple myeloma. Am. J. Cancer Res. 2017;7:1637–1641. [PMC free article] [PubMed] [Google Scholar]
- 49.Tai YT, et al. CS1 promotes multiple myeloma cell adhesion, clonogenic growth, and tumorigenicity via c-maf-mediated interactions with bone marrow stromal cells. Blood. 2009;113:4309–4318. doi: 10.1182/blood-2008-10-183772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Guo H, et al. Deletion of Slam locus in mice reveals inhibitory role of SLAM family in NK cell responses regulated by cytokines and LFA-1. J. Exp. Med. 2016;213:2187–2207. doi: 10.1084/jem.20160552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KM, et al. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivori S, et al. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc. Natl Acad. Sci. USA. 2002;99:4526–4531. doi: 10.1073/pnas.072065999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall SA, Devine S, Vasu S. The who, how and why: allogeneic transplant for acute myeloid leukemia in patients older than 60 years. Blood Rev. 2017;31:362–369. doi: 10.1016/j.blre.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Copelan EA. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 55.Giralt S, Bishop MR. Principles and overview of allogeneic hematopoietic stem cell transplantation. Cancer Treat. Res. 2009;144:1–21. doi: 10.1007/978-0-387-78580-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin. Cancer Res. 2014;20:3390–3400. doi: 10.1158/1078-0432.CCR-13-1766. [DOI] [PubMed] [Google Scholar]
- 57.Bachanova V, Miller JS. NK cells in therapy of cancer. Crit. Rev. Oncog. 2014;19:133–141. doi: 10.1615/critrevoncog.2014011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahr Benedikt, Pilat Nina, Granofszky Nicolas, Wiletel Mario, Muckenhuber Moritz, Maschke Svenja, Hock Karin, Wekerle Thomas. Hybrid resistance to parental bone marrow grafts in nonlethally irradiated mice. American Journal of Transplantation. 2018;19(2):591–596. doi: 10.1111/ajt.15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J. Exp. Med. 1971;134:1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cudkowicz G, Stimpfling JH. Deficient growth of C57bl marrow cells transplanted in F1 hybrid mice. Association with the histocompatibility-2 locus. Immunology. 1964;7:291–306. [PMC free article] [PubMed] [Google Scholar]
- 61.Bix M, et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 62.Raulet DH. Bone marrow cell rejection, MHC, NK cells, and missing self recognition: ain’t that peculiar (with apologies to marvin gaye) J. Immunol. 2015;195:2923–2925. doi: 10.4049/jimmunol.1501804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat. Immunol. 2005;6:938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamby K, et al. NK cells rapidly reject allogeneic bone marrow in the spleen through a perforin- and Ly49D-dependent, but NKG2D-independent mechanism. Am. J. Transplant. 2007;7:1884–1896. doi: 10.1111/j.1600-6143.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 65.Beilke JN, Benjamin J, Lanier LL. The requirement for NKG2D in NK cell-mediated rejection of parental bone marrow grafts is determined by MHC class I expressed by the graft recipient. Blood. 2010;116:5208–5216. doi: 10.1182/blood-2010-05-285031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.George TC, Ortaldo JR, Lemieux S, Kumar V, Bennett M. Tolerance and alloreactivity of the Ly49D subset of murine NK cells. J. Immunol. 1999;163:1859–1867. [PubMed] [Google Scholar]
- 67.Dupre L, et al. SAP controls the cytolytic activity of CD8 + T cells against EBV-infected cells. Blood. 2005;105:4383–4389. doi: 10.1182/blood-2004-08-3269. [DOI] [PubMed] [Google Scholar]
- 68.Sharifi R, et al. SAP mediates specific cytotoxic T-cell functions in X-linked lymphoproliferative disease. Blood. 2004;103:3821–3827. doi: 10.1182/blood-2003-09-3359. [DOI] [PubMed] [Google Scholar]
- 69.Bloch-Queyrat C, et al. Regulation of natural cytotoxicity by the adaptor SAP and the Src-related kinase Fyn. J. Exp. Med. 2005;202:181–192. doi: 10.1084/jem.20050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 72.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115:1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 74.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 75.Chen R, et al. Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors. Mol. Cell. Biol. 2004;24:5144–5156. doi: 10.1128/MCB.24.12.5144-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watzl C, Long EO. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J. Exp. Med. 2003;197:77–85. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meinke S, Watzl C. NK cell cytotoxicity mediated by 2B4 and NTB-A is dependent on SAP acting downstream of receptor phosphorylation. Front. Immunol. 2013;4:3. doi: 10.3389/fimmu.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan B, et al. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 79.Latour S, et al. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signaling in immune regulation. Nat. Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 80.Cannons JL, et al. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. J. Immunol. 2010;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu C, et al. The X-linked lymphoproliferative disease gene product SAP associates with PAK-interacting exchange factor and participates in T cell activation. Proc. Natl Acad. Sci. USA. 2006;103:14447–14452. doi: 10.1073/pnas.0606624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li C, Schibli D, Li SS. The XLP syndrome protein SAP interacts with SH3 proteins to regulate T cell signaling and proliferation. Cell Signal. 2009;21:111–119. doi: 10.1016/j.cellsig.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 2004;16:1–11. doi: 10.1016/s0898-6568(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 84.Swat W, Fujikawa K. The Vav family: at the crossroads of signaling pathways. Immunol. Res. 2005;32:259–265. doi: 10.1385/IR:32:1-3:259. [DOI] [PubMed] [Google Scholar]
- 85.Kim HS, Das A, Gross CC, Bryceson YT, Long EO. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity. 2010;32:175–186. doi: 10.1016/j.immuni.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cella M, et al. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J. Exp. Med. 2004;200:817–823. doi: 10.1084/jem.20031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J. Exp. Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urlaub D, Hofer K, Muller ML, Watzl C. LFA-1 activation in NK cells and their subsets: influence of receptors, maturation, and cytokine stimulation. J. Immunol. 2017;198:1944–1951. doi: 10.4049/jimmunol.1601004. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann SC, Cohnen A, Ludwig T, Watzl C. 2B4 engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy. J. Immunol. 2011;186:2757–2764. doi: 10.4049/jimmunol.1002867. [DOI] [PubMed] [Google Scholar]
- 90.Chuang SS, Kumaresan PR, Mathew PA. 2B4 (CD244)-mediated activation of cytotoxicity and IFN-gamma release in human NK cells involves distinct pathways. J. Immunol. 2001;167:6210–6216. doi: 10.4049/jimmunol.167.11.6210. [DOI] [PubMed] [Google Scholar]
- 91.Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc. Natl Acad. Sci. USA. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sivori S, et al. 2B4 functions as a co-receptor in human NK cell activation. Eur. J. Immunol. 2000;30:787–793. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 93.Bida AT, et al. 2B4 utilizes ITAM-containing receptor complexes to initiate intracellular signaling and cytolysis. Mol. Immunol. 2011;48:1149–1159. doi: 10.1016/j.molimm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiesa S, et al. Multiplicity and plasticity of natural killer cell signaling pathways. Blood. 2006;107:2364–2372. doi: 10.1182/blood-2005-08-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saborit-Villarroya I, et al. The adaptor protein 3BP2 binds human CD244 and links this receptor to Vav signaling, ERK activation, and NK cell killing. J. Immunol. 2005;175:4226–4235. doi: 10.4049/jimmunol.175.7.4226. [DOI] [PubMed] [Google Scholar]
- 96.Jevremovic D, Billadeau DD, Schoon RA, Dick CJ, Leibson PJ. Regulation of NK cell-mediated cytotoxicity by the adaptor protein 3BP2. J. Immunol. 2001;166:7219–7228. doi: 10.4049/jimmunol.166.12.7219. [DOI] [PubMed] [Google Scholar]
- 97.Saborit-Villarroya I, et al. The adaptor 3BP2 activates CD244-mediated cytotoxicity in PKC- and SAP-dependent mechanisms. Mol. Immunol. 2008;45:3446–3453. doi: 10.1016/j.molimm.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 98.Tassi I, Colonna M. The cytotoxicity receptor CRACC (CS-1) recruits EAT-2 and activates the PI3K and phospholipase Cgamma signaling pathways in human NK cells. J. Immunol. 2005;175:7996–8002. doi: 10.4049/jimmunol.175.12.7996. [DOI] [PubMed] [Google Scholar]
- 99.Clarkson NG, Brown MH. Inhibition and activation by CD244 depends on CD2 and phospholipase C-gamma1. J. Biol. Chem. 2009;284:24725–24734. doi: 10.1074/jbc.M109.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J. Immunol. 2001;167:5517–5521. doi: 10.4049/jimmunol.167.10.5517. [DOI] [PubMed] [Google Scholar]
- 101.Eissmann P, Watzl C. Molecular analysis of NTB-A signaling: a role for EAT-2 in NTB-A-mediated activation of human NK cells. J. Immunol. 2006;177:3170–3177. doi: 10.4049/jimmunol.177.5.3170. [DOI] [PubMed] [Google Scholar]
- 102.Wang N, et al. Cutting edge: the adapters EAT-2A and -2B are positive regulators of CD244- and CD84-dependent NK cell functions in the C57BL/6 mouse. J. Immunol. 2010;185:5683–5687. doi: 10.4049/jimmunol.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]