Abstract
F-652 is a recombinant fusion protein consisting of two human interleukin-22 (IL-22) molecules linked to an immunoglobulin constant region (IgG2-Fc). IL-22 plays critical roles in promoting tissue repair and suppressing bacterial infection. The safety, pharmacokinetics (PK), tolerability, and biomarkers of F-652 were evaluated following a single dose in healthy male volunteers in a randomized, double-blind, placebo-controlled study. Following single-dose subcutaneous (SC) injection of F-652 at 2.0 µg/kg into healthy subjects, six out of six subjects experienced delayed injection site reactions, which presented as erythematous and/or discoid eczematous lesions 10 to 17 days post-dosing. F-652 was then administered to the healthy subjects via an intravenous (IV) infusion at 2.0, 10, 30, and 45 µg/kg. No severe adverse event (SAE) was observed during the study. Among the IV-dosed cohorts, eye and skin treatment emergent adverse events (TEAEs) were observed in the 30 and 45 µg/kg cohorts. F-652 IV dosing resulted in linear increases in Cmax and AUC(0–t), and the T1/2 ranged from 39.4 to 206 h in the cohorts. An IV injection of F-652 induced dose-dependent increases in serum marker serum amyloid A, C-reactive protein, and FIB, and decreased serum triglycerides. The serum levels of 36 common pro-inflammatory cytokines/chemokines were not altered by the treatment of F-652 at 45 μg/kg. In conclusion, IV administration of F-652 to healthy male volunteers is safe and well-tolerated and demonstrates favorable PK and pharmacodynamic properties. These results warrant further clinical development of F-652 to treat inflammatory diseases.
Keywords: Interleukin-22, F-652, Pharmacokinetics, Pharmacodynamics, Safety
Introduction
Interleukin-22 (IL-22), a member of the IL-10 cytokine family, is produced by T cell subsets, including T helper 22 (Th22), Th1, and Th17 cells, and subsets of innate lymphoid cells.1 IL-22 was first identified as an IL-10-related T-cell-derived inducible factor (IL-TIF) produced by IL-9-stimulated T lymphoma cells.2, 3 The activation of IL-22 gene expression requires the ligand-dependent transcription factor aryl hydrocarbon receptor (AHR).4 IL-22 signals through a distinct class 2 receptor (IL-22 R) composed of the subunits IL-22 RA1 and IL-10 R2. The IL-22 RA1 subunit is also used by the IL-20 and IL-24 receptors, while the IL-10 R2 subunit is used by IL-10 and many other cytokines receptors, including those of IL-26 and IL-28.5 Similar to other members of the IL-10 family, IL-22 uses the Janus-signal transducer and activator of transcription (Jak-STAT) signal transduction pathway, thus inducing the phosphorylation of the kinases Jak1 and Tyk2 to mainly activate STAT3, with the activation of STAT1 and STAT5 to a lesser extent.6–9 IL-22RA1 is not detected in the immune or hematopoietic cells,10 suggesting there may not be a direct effect on the hematopoietic and immune systems. IL-22 R is mainly expressed on epithelial cells, such as skin keratinocytes, as well as pancreatic acinar cells, hepatocytes, and the lung, kidney, and colon epithelium.11–13 The protective effect against epithelial injury was first reported in the liver in 2004,14, 15 and then, many ensuing studies strongly suggested that IL-22/IL-22 R signaling has high potential clinical relevance in protecting against damage to multiple organs, including the lung, kidney, pancreas, and gut.1 In mouse models of intestinal homeostasis and colitis, an inflammasome pathway involving Absent in Melanoma 2 (AIM2), interleukin-18, IL-22 binding protein (IL-22BP), and STAT3 was demonstrated to regulate IL-22 functions to prevent dysbiosis and to promote tissue repair.16
Natural human IL-22 protein consists of 179 amino acids, with a predicted 33-amino acid signal peptide at the N-terminal. Recombinant human IL-22 (rhIL-22), expressed in Escherichia coli, has two disulfide bonds between amino acids 40–132 and amino acids 89–178.17 In addition, the predicted N-linked glycosylation sites in rhIL-22 protein are at amino acids 54, 68, and 97,17, 18 and the rhIL-22 made in E. coli forms a dimer structure in solution.19 It is postulated that the rhIL-22 dimer forms a tetramer first with two IL-22 RA1 molecules before binding to IL-10R2.19 The half-life of the rhIL-22 made in E. coli was less than 2 h in animals (data not shown), thus limiting its clinical application. We developed F-652, a recombinant fusion protein consisting of two human IL-22 molecules linked to the human IgG2 constant region (IgG2-Fc). The F-652 IL-22 dimer mimics the dimeric binding of IL-22 to IL-22RA1, and the IgG2-Fc extends the serum half-life. F-652 is expressed in the CHO cell line and is intended for clinical applications. We completed a thorough pre-clinical evaluation of F-652. The pre-clinical study results of F-652 will be reported elsewhere. In this communication, we report the results of a phase I clinical study of F-652 in healthy male volunteers. The aim of the study was to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics as well as the potential biomarkers after F-652 administration.
Materials and methods
Study drug
The recombinant human IL-22-Fc fusion protein (F-652) was manufactured by Generon Corporation (Shanghai), Ltd. (Shanghai, China). F-652 was expressed in CHO cells and produced in a serum-free and chemically defined medium, followed by a series of purification steps. F-652 was manufactured according to the Chinese State Food and Drug Administration (SFDA): Good manufacturing practice for pharmaceutical products, 1998, and followed the US FDA’s Guidance for Industry “cGMP for Phase 1 Investigational Drugs”. The test procedure and acceptance criteria followed the ICH Guideline, Q6B, 1999. The F-652 drug product was provided as a sterile, lyophilized white cake in 2-mL clear glass single-use vials. F-652 was stable for 36 months when kept at 3–8 °C. The F-652 clinical lot (52–1201) showed in vitro bioactivity in the COLO 205 cell assay, with 114±18% of a reference standard (with EC50 at 185±35 ng/mL, n = 10). The purity of F-652 showed >98% using reverse-phase high-performance liquid chromatography (RP-HPLC) and >99% using size exclusion HPLC (SE-HPLC) analysis, with an endotoxin level at <0.05 EU/mg protein. The F-652 drug product was reconstituted by using 1 mL water for injection (WFI) and was further diluted with saline to make the subcutaneous (SC) or intravenous (IV) dosing solutions based on the body weight of the study subjects. In the IV dosing study, F-652 was infused over approximately 60 min.
Study management
This study was conducted at the Nucleus Network (5th Floor Burnet Tower, AMREP Precinct, 89 Commercial Rd, Melbourne, Australia) in accordance with the principles stated in the Declaration of Helsinki and the Good Clinical Practice (GCP) guidelines described in the International Committee for Harmonisation (ICH, CPMP/ICH/135/95) and European Community (Australian New Zealand Clinical Trails Registry number: ACTRN12612000713897). All the subjects were provided with written informed consent before entering the study.
Study design
This study was a phase I, first-in-human, randomized, double blind, dose escalation study to evaluate the safety and PK of F-652 in healthy male volunteers. Recruitment of male subjects in first-in-human phase I studies is a common practice. The primary objective of the study was to evaluate the safety and tolerability of a single dose of 2.0 μg/kg F-652 or placebo by SC administration and 2.0, 10, 30, and 45 μg/kg F-652 or placebo by IV administration. The starting dose of 2.0 μg/kg was determined using the human equivalent dose (HED) conversion from pre-clinical minimal efficacy doses in mice (3 μg/kg) and monkeys (30 μg/kg). The no observed adverse effect levels (NOAELs) in rats (1000 μg/kg) and monkeys (750 μg/kg) provided an estimation that F-562 should be safe up to 75 μg/kg. The secondary objective of the study was to characterize the PK profile of single doses of F-652. The exploratory objective of the study was to identify the potential serum biomarkers after F-652 administration.
Forty healthy adult subjects were randomized and assigned to seven sequential dose cohorts of F-652 (2.0 µg/kg SC or 2.0, 10, 30, and 45 µg/kg IV infusion) or placebo. Each cohort of eight volunteers was randomly assigned to receive either a single dose of F-652 or placebo at a ratio of 3:1. The decision to escalate the dose was made based on review of the blinded data of adverse events, safety laboratory results, blood pressure, heart rate, and 12-lead ECG.
The PK blood sampling time points for the F-652 SC dosing cohort were at pre-dose, 1, 2, 4, 8, 16, 24, and 36 h and 3, 4, 5, 6, 8, 11, 15, and 22 days after the F-652 administration. For the IV dosing cohorts, the PK blood sampling time points were at pre-dose, 5 and 30 min, 1, 2, 4, 8, and 24 h, and 3, 4, 5, 6, 8, 11, 15, and 22 days after the F-652 dosing. The serum samples for the PK analysis were stored at −60~–80 °C until they were assayed.
Sample analysis
Hematology
The hematological parameters analyzed included hemoglobin (Hb), hematocrit, red blood cell (RBC), white blood cell (WBC), platelet count, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and differential blood count (neutrophils, lymphocytes, monocytes, basophils, and eosinophils).
Clinical chemistry
The serum chemistry parameters included the following: electrolytes: sodium, potassium, chloride, and calcium; liver function: serum glutamic oxaloacetic transaminase (AST/SGOT), serum glutamic pyruvic transaminase (ALT/SGPT), gamma-glutamyl transferase (GGT), alkaline phosphatase (AKP), total bilirubin, direct bilirubin, total protein, albumin, globulins, and A/G ratio (albumin–globulin); renal function: creatinine, blood urea nitrogen, and uric acid; pancreas function: lipase and amylase; lipids: triglyceride (TG), HDL-cholesterol, LDL-cholesterol, and total cholesterol; other enzymes and proteins: glucose, lactate dehydrogenase (LDH), and creatine kinase (CK); coagulation: prothrombin time (PT), International Normalized Ratio (INR), activated partial thromboplastin time (APTT), and fibrinogen (FIB); immunological tests: C-reactive protein (CRP), IgG, IgM, IgA, IgE, and IgD; and urinalysis: pH, glucose, ketones, leukocytes, blood/hemoglobin, protein, nitrite, urobilinogen, and bilirubin.
The pharmacodynamics and the toxicological marker analysis were performed including serum amyloid A-1 (SAA-1), CRP, TG, FIB, serum glutamic oxaloacetic transaminase (AST/SGOT), and serum glutamic pyruvic transaminase (ALT/SGPT).
F-652 bioanalysis
The levels of F-652 in human serum samples were measured using a sensitive commercial ELISA kit (Legend Max Human IL22 ELISA Kit, Biolegend, Cat # 434507, CA, USA). The assay protocol followed the supplier’s recommendation. The Legend Max ELISA plate was pre-coated with an anti-human IL-22 polyclonal antibody and incubated with the test serum samples, which was followed by antibody detection (biotin-conjugated human IL-22 polyclonal antibody), Avidin-HRP and HRP substrate. The ELISA assay was stopped, and the optical density (OD) was measured. A four-parameter curve fitting was applied to analyze the F-652 concentration in the test serum samples.
The assay validation followed US FDA guidance, including the assay specificity, selectivity, linearity, detection limit, precision, accuracy, and sample storage stability. The standard (F-652) and quality control samples (9, 72, and 168 ng/mL, denoted low, medium, and high, respectively) were prepared with a final assay matrix of 33% serum. The linearity range of the bioanalytical method was from 1.5 to 196 ng/mL. The lower limit of quantification (LLOQ) of the assay was 3.0 ng/mL for the human serum samples. The intra-assay precision of the quality control samples (9, 72 and 168 ng/mL, denoted low, medium and high, respectively) was from 1.3 to 11.2% with an accuracy rate of 91.4 to 117.6%, and the inter-assay precision was from 7.4 to 8.9% with an accuracy rate of 101.4 to 107.6%.
Serum cytokine assay
The serum cytokine levels in healthy human subjects following the IV administration of F-652 were assayed using a Human Proteome Profiler TM Array assay (HPPA, Human Cytokine Array Panel A, R&D Systems, Cat. # ARY005, Lot # 1327628). The HPPA assay detects 36 inflammatory cytokines or chemokines that are commonly associated with the inflammatory response in humans, including C5/C5a, CD154, G-CSF, GM-CSF, CXCL1, CCL1, CD54, INF-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-16, IL-17, IL-17E, IL-23, IL-27, IL-32α, CXCL10, CXCL11, CCL2, MIF, MIP-1α, MIP-1β, Serpin E1, CCL5, CXCL12, TNF-α, and sTREM-1. The assay procedure followed the protocol provided by the manufacturer. Briefly, the selected capture antibodies were spotted in duplicate on nitrocellulose membranes. The cell culture supernatants or serum samples were diluted and mixed with a cocktail of biotinylated detection antibodies. The sample/antibody mixture was then incubated with the Human Cytokine Array Panel A membrane. Cytokine/detection antibody complex was then bound onto the membrane through its cognate immobilized capture antibody. Following a wash to remove the unbound material, Streptavidin-HRP and chemiluminescent detection reagents were added sequentially. Chemiluminescence was measured in proportion to the amount of cytokine expressed by using an Odyssey CLx Imaging System (LI-COR), and the pixel densities were analyzed by Quantity One (BIO-RAD) software.
Data analysis
The PK parameters were estimated using the standard non-compartmental analytical procedures (WinNonlin v 5.2, Pharsight Corporation, USA). The actual times of sample collection were used in the estimation of the PK parameters. The Cmax (the maximum observed serum concentration over the sampling period) and Tmax (the time at which Cmax was observed) were observed directly from the data. The area under the serum concentration vs. time curve (AUC) was determined by the linear trapezoidal rule, where AUC(0–last) was the AUC from time zero until the last blood collection point and AUC(0–inf) was the AUC extrapolated to infinity. The apparent clearance (CL) was calculated as Dose/AUC(0–inf). The mean residence time (MRT) was calculated as AUMC/AUC, where AUMC is the area under the moment curve. MRT(0–inf) was extrapolated to infinity, while MRT(0–t) was derived from the area calculations up to and including the last measured time point.
Results
Demographic characteristics
Forty subjects were randomized into different treatment groups in the current study. All the subjects received a single dose of F-652 (2 μg/kg SC, 2, 10, 30, and 45 μg/kg IV) or placebo. All the subjects completed the study as planned. The demographic data for the treatment cohorts are summarized in Table 1. The demographic data showed that the different F-652 dose groups were relatively well-matched with regard to age, weight, height, and body mass index (BMI). All the subjects were male, as required per the protocol, and were a mixture of Asian and White.
Table 1.
Summary of demographic characteristics
| 2.0 µg/kg SC (N = 6) |
Placebo SC (N = 2) |
2.0 µg/kg IV (N = 6) |
10 µg/kg IV (N = 6) | 30 µg/kg IV (N = 6) | 45 µg/kg IV (N = 6) | Placebo IV (N = 8) | |
|---|---|---|---|---|---|---|---|
| Mean age (years)a | 27.2 (3.1) | 33.5 (13.4) | 28.0 (8.4) | 25.0 (3.7) | 25.2 (4.2) | 25.3 (1.8) | 23.9 (3.0) |
| Gender | |||||||
| Male | 6 (100.0%) | 2 (100.0%) | 6 (100.0%) | 6 (100.0%) | 6 (100.0%) | 6 (100.0%) | 8 (100.0%) |
| Race | |||||||
| Asian | 3 (50.0%) | 1 (50.0%) | 0 | 0 | 0 | 1 (16.7%) | 1 (12.5%) |
| White | 3 (50.0%) | 1 (50.0%) | 6 (100.0%) | 6 (100.0%) | 6 (100.0%) | 5 (83.3%) | 7 (87.5%) |
| Ethnicity | |||||||
| Hispanic/Latino | 1 (16.7%) | 0 | 0 | 0 | 1 (16.7%) | 2 (33.3%) | 0 (0.0%) |
| Non-Hispanic/Latino | 5 (83.3%) | 2 (100.0%) | 6 (100.0%) | 6 (100.0%) | 5 (83.3%) | 4 (66.7%) | 8 (100.0%) |
| Smoking habits | |||||||
| Current smoker | 2 (33.3%) | 0 | 1 (16.7%) | 1 (16.7%) | 0 | 2 (33.3%) | 3 (37.5%) |
| Ex-smoker | 0 | 0 | 3 (50.0%) | 3 (50.0%) | 2 (33.3%) | 1 (16.7%) | 2 (25.0%) |
| Never smoked | 4 (66.7%) | 2 (100.0%) | 2 (33.3%) | 2 (33.3%) | 4 (66.7%) | 3 (50.0%) | 3 (37.5%) |
| Body weight (kg)a | 65.52 (10.83) | 66.95 (20.15) | 80.77 (10.73) | 73.17 (8.14) | 73.83 (10.78) | 71.88 (7.84) | 75.33 (8.56) |
| Height (cm)a | 172.7 (10.5) | 165.0 (2.8) | 182.2 (4.2) | 177.0 (5.5) | 179.5 (6.2) | 174.3 (8.5) | 177.0 (4.5) |
| BMI (kg/m²)a | 21.82 (1.13) | 24.45 (6.58) | 24.28 (2.50) | 23.35 (2.15) | 22.82 (2.14) | 23.67 (1.97) | 24.01 (2.13) |
BMI body mass index
aMean (SD)
Treatment emergent adverse events (TEAEs)
A summary of the TEAEs is listed in Table 2. A summary of the severity for the TEAEs by treatment group is listed in Table 3. A total of 41 TEAEs were reported for 23 Subjects (57.5%). All the TEAEs were mild or moderate in severity. There were no severe or life-threatening AEs reported. The most frequently reported TEAE was dry skin (12 events by 12 subjects). Delayed injection site reactions (ISRs) in subjects after F-652 SC dosing were observed at the injection sites 10 to 17 days after the administration in 6/6 subjects that received F-652, but none of the subjects that received the placebo developed ISRs. The ISRs were presented as dry skin and erythematous and/or discoid eczematous lesions of F-652. A skin biopsy was performed in one subject and indicated minor superficial perivascular lymphocytic cuffing, epithelial hyperplasia, and patchy parakeratosis. These changes were non-specific and consistent with the drug administration, where the high concentration of F-652 at the injection site could have activated keratinocytes and/or subepithelial myofibroblast, a known target for IL-22 (the active portion of F-652), thus triggering the ISRs. The TEAEs observed following the SC dosing were considered drug-related delayed ISRs. Thus, the study design was amended to change to the IV infusion dosing route. There was an increase in the number and severity of TEAEs observed with the SC dosing when compared to the IV dosing at the 2 μg/kg dose, indicating that IV dosing was better tolerated than SC dosing. No skin reaction was observed at 2.0 or 10 μg/kg. An increase in the number and severity of TEAEs at the higher IV doses of F-652 (30 and 45 μg/kg), compared to the placebo, was seen in the study. Ten subjects had mild to moderate skin reactions, including itchy and dry skin, erythema, and eye pruritus. Two subjects, at 45 μg/kg, had erythema and were treated with a local steroid cream. The symptoms disappeared 4 days after the treatment.
Table 2.
Summary of treatment emergent adverse events (TEAEs)
| System Organ Class | 2.0 μg/kg SC (N = 6) S (%) E |
Placebo SC (N = 2) S (%) E |
2.0 μg/kg IV (N = 6) S (%) E | 10 μg/kg IV (N = 6) S (%) E |
30 μg/kg IV (N = 6) S (%) E |
45 μg/kg IV (N = 6) S (%) E |
Placebo IV (N = 8) S (%) E |
|---|---|---|---|---|---|---|---|
| Subjects with at least one TEAE | 6 (100.0%) 7 | 0 (0.0%) 0 | 1 (16.7%) 2 | 3 (50.0%) 4 | 4 (66.7%) 10 | 6 (100.0%)13 | 3 (37.5%) 5 |
| Eye disorders (eye pruritus) | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 3 (50.0%) 3 | 0 (0.0%) 0 |
| Gastrointestinal disorders | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (12.5%) 1 |
| Dry mouth | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Tooth disorder | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Toothache | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (12.5%) 1 |
| General disorders and administration site conditions | 2 (33.3%) 2 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Application site dryness | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Application site erythema | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Chills | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Vessel puncture site hematoma | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Infections and infestations (URTI) | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 1 (16.7%) 1 | 0 (0.0%) 0 | 1 (12.5%) 1 |
| Injury, poisoning, and procedural complications (IRR) | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Musculoskeletal and connective tissue disorders (back pain) | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 1 (16.7%) 1 | 0 (0.0%) 0 |
| Nervous system disorders | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 1 (16.7%) 1 | 0 (0.0%) 0 | 1 (16.7%) 1 | 2 (25.0%) 2 |
| Headache | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Lethargy | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (12.5%) 1 |
| Parasthesia | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (12.5%) 1 |
| Somnolence | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 |
| Skin and subcutaneous tissue disorders | 4 (66.7%) 4 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 4 (66.7%) 5 | 6 (100.0%) 8 | 1 (12.5%) 1 |
| Dermatitis allergic | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Dry skin | 2 (33.3%) 2 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 4 (66.7%) 4 | 6 (100.0%) 6 | 0 (0.0%) 0 |
| Eczema nummular | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Erythema | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
| Hyperhidrosis | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (12.5%) 1 |
| Rash erythematous | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 2 (33.3%) 2 | 0 (0.0%) 0 |
| Solar lentigo | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (16.7%) 1 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 |
URTI upper respiratory track infection, IRR infusion-related reaction, S number of subjects, E number of events
Table 3.
Summary of TEAE severity by treatment group
| 2.0 µg/kg SC (N = 6) S (%) E |
Placebo SC (N = 2) S (%) E |
2.0 µg/kg IV (N = 6) S (%) E |
10 µg/kg IV (N = 6) S (%) E |
30 µg/kg IV (N = 6) S (%) E |
45 µg/kg IV (N = 6) S (%) E |
Placebo IV (N = 8) S (%) E |
|
|---|---|---|---|---|---|---|---|
| Subjects with at least one TEAE | 6 (100.0%) 7 | 0 | 1 (16.7%) 2 | 3 (50.0%) 4 | 4 (66.7%) 10 | 6 (100.0%) 13 | 3 (37.5%) 5 |
| Mild | 4 (66.7%) 4 | 0 | 1 (16.7%) 2 | 3 (50.0%) 3 | 3 (50.0%) 5 | 2 (33.3%) 3 | 3 (37.5%) 5 |
| Moderate | 3 (50.0%) 3 | 0 | 0 | 1 (16.7%) 1 | 4 (66.7%) 5 | 5 (83.3%) 10 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Life threatening | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The data are presented as S (%) E, where S is the number of subjects, % is the percentage of subjects, and E is the number of events
The review of the hematology, biochemistry, coagulation and urinalysis, vital signs, and ECG data suggested that no changes over time were noted following the administration of any IV dose of F-652. We did not observe any diarrhea symptoms during the study. No marked changes in the levels of serum ALT and AST, amylase and lipase, and creatinine and blood urine nitrogen were observed. No immunological reaction, such as an increase in serum inflammatory cytokines and chemokines (see below), was observed during the study for any treatment of F-652. F-652 administered to healthy male subjects as a single IV infusion at a dose level up to 20 μg/kg was safe and well-tolerated. For the 30 and 45 μg/kg cohorts, F-652 caused some discomfort, including dry skin and eye pruritus. Except for mild to moderate skin reactions, no other adverse events were observed. An additional dose escalation was terminated due to the discomfort of the erythematous rash and itchiness in the healthy subjects. The maximum tolerance dose (MTD) for ISRs was established at 30 μg/kg in the study.
We did not perform an immunogenicity study in this trial. However, we found a low frequency of anti-F652 antibodies in a phase 1 study in China (data not shown). These antibodies cannot neutralize the biological function of F-652 in a cell-based assay.
Pharmacokinetics
The PK of F-652 after IV infusion was evaluated in four cohorts of 32 healthy male volunteers at doses of 2, 10, 30, or 45 μg/kg by IV infusion. The concentration of F-652 was analyzed using an ELISA assay, which detects the IL-22 portion of the F-652 molecule. No serum F-652 (IL-22) was detected in the placebo cohort or in the subjects before the dosing.
After the IV infusion of F-652 at the 2–45 μg/kg dose range, both the Cmax and exposure (AUC0–last) increased with the increasing doses of F-652 (Table 4). These two parameters and the dose levels demonstrated linear relationships (Fig. 1). The serum concentrations of F-652 declined in an exponential manner over time (Fig. 2). The T1/2 ranged from 25.5 to 55.0 h for the 2 μg/kg cohort, from 89.8 to 138 h for the 10 μg/kg cohort, from 136 to 209 h for the 30 μg/kg cohort, and from 153 to 275 h for the 45 μg/kg cohort. The CL ranged from 1.82 to 3.35 mL/h/kg and appeared to decrease with the increasing doses. The mechanism underlining the decreased CL at the higher doses is unclear but could be related to the receptor internalization and recycling of F-652. The volume of distribution (Vz) apparently increased with the elevation of the dose.
Table 4.
Pharmacokinetic parameters of F-652 in healthy men by IV infusion
| F-652 µg/kg | Cmax (ng/mL) | T1/2 (h) | MRT0–last (h) | AUC(0–last) (h × ng/mL) | AUC(0–24 h) (h × ng/mL) | CL (mL/hr/kg) | Vz (mL/kg) |
|---|---|---|---|---|---|---|---|
| 2.0 | 15.5 (4.08) | 39.4 (25.5–55.0) | 22.6 (6.15) | 437 (178) | 247 (65.4) | 3.35 (0.94) | 177 (41) |
| 10 | 62.3 (7.21) | 108 (89.8–138) | 86.4 (20.1) | 4150 (1060) | 1050 (125) | 2.15 (0.45) | 330 (61) |
| 30 | 176 (8.79) | 161 (136–209) | 139 (18.3) | 15,400 (2420) | 3230 (254) | 1.82 (0.31) | 419 (71) |
| 45 | 247 (39.5) | 206 (153–275) | 130 (9.07 | 18,000 (2610) | 4340 (737) | 2.26 (0.42) | 654 (77) |
The data are shown as the mean (SD), except for the T1/2 (range)Please check if the change made in the units of the parameter AUC in Table 4 are ok.OK. Thank you.
Fig. 1.
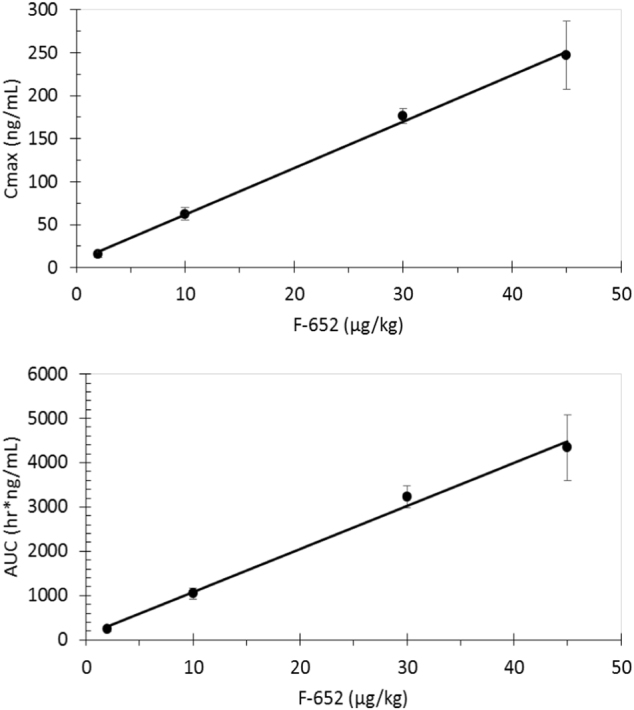
The linear relationships between the F-652 dose and Cmax (R=0.9981) and the F-652 dose and AUC(0–24 h) (R=0.9938)
Fig. 2.
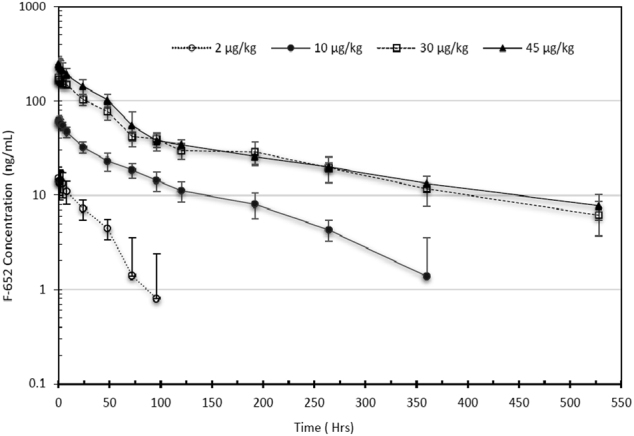
Serum F-652 levels in healthy men after IV infusion. Solid circle (F-652 2.0 μg/kg); open circle (F-652 10 μg/kg); solid triangle (F-652 30 μg/kg); open triangle (F-652 45 μg/kg). The error bars indicate the SD
Pharmacodynamics effects
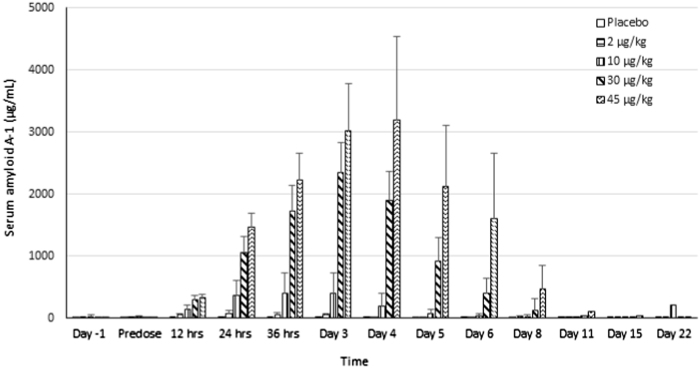
In the pre-clinical studies, F-652 induced a dose-dependent increase in serum amyloid A (SAA) levels in mice and monkeys, increased serum FIB level in monkeys, and decreased serum TG levels in mice and rats (unpublished data). The current study explored the potential serum biomarkers reflecting the biological activities of F-652 in humans based on the pre-clinical findings. The biological serum markers identified with the most potential to follow the biological response after the administration of F-652 were SAA (Fig. 3), CRP (Fig. 4), FIB (Fig. 5), and TG (Fig. 6). Dose-dependent increases in these serum markers were observed following F-652 dosing compared to placebo, commencing at 12 to 24 h post-dose. The changes were evident in the higher dose groups (30 and 45 μg/kg). A significant increase in the CRP levels was observed in the 30 and 45 μg/kg dosing cohorts. A significant decrease in the serum TG levels was observed in the 30 and 45 μg/kg dosing cohorts compared to the pre-dose levels and the placebo cohort.
Fig. 3.
Effect of F-652 on the serum levels of amyloid A-1 (µg/mL) in healthy subjects. The different dose groups are indicated
Fig. 4.
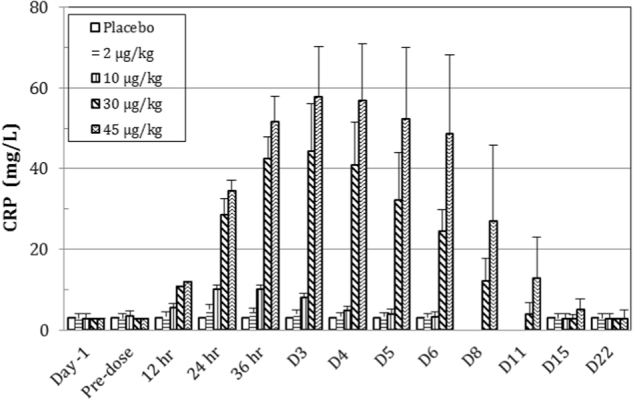
Effect of F-652 on the serum levels of C-reactive protein (CRP, mg/L) in healthy subjects. The different dose groups are indicated
Fig. 5.
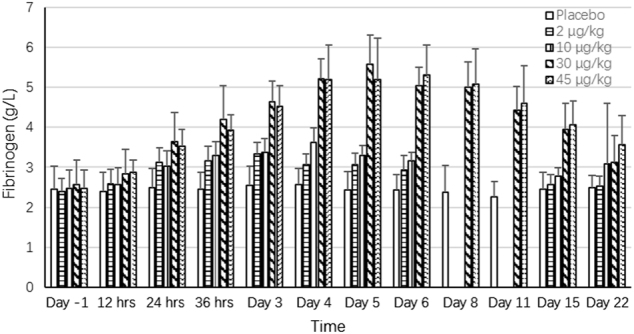
Effect of F-652 on the serum levels of fibrinogen (g/L) in healthy subjects. The different dose groups are indicated
Fig. 6.
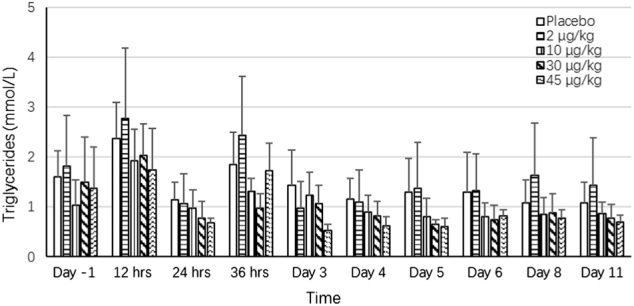
Effects of F-652 on the serum levels of triglycerides (mmol/L) in healthy subjects. The different dose groups are indicated
The most striking biological marker was SAA. In the 2.0 μg/kg F-652 cohort, the mean SAA levels peaked at 24 h, with a 13.7-fold increase compared to the pre-dose level and a 14.2-fold increase compared to the placebo cohort with a mean Cmax at 15.5±4.08 ng/mL (N = 6). In the 45 μg/kg cohort, the mean SAA levels peaked at 72 h with a 320-fold increase compared to the pre-dose level and a 515-fold increase compared to the placebo cohort with a mean Cmax at 247±39.5 ng/mL (N = 6). These results demonstrated the potent bioactivity of F-652 to induce SAA in humans, suggesting that SAA could serve as a potential biomarker for further clinical development of F-652. The correction of these potential serum biomarkers with the disease indications remains to be further explored.
Cytokine induction
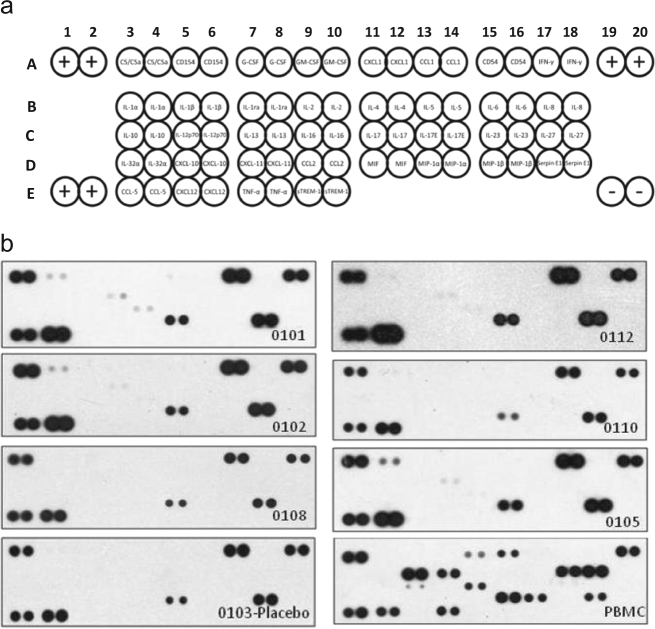
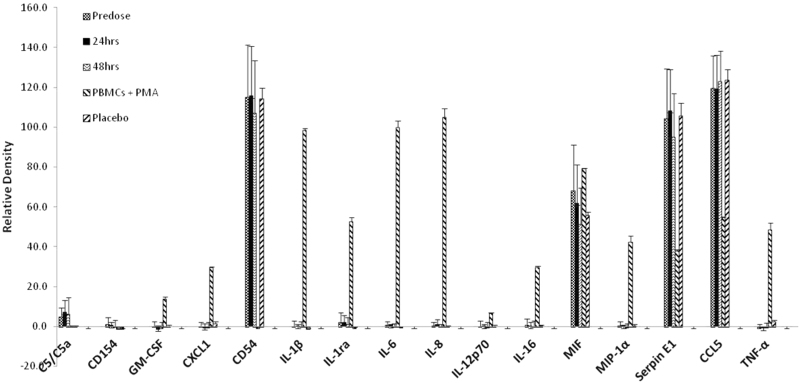
IL-22 is also considered a pro-inflammatory cytokine.20 The results of a cDNA microarray analysis showed that IL-22 induces inflammatory cytokine expression in human myofibroblasts.21 To evaluate if the administration of F-652 induces inflammatory cytokines in humans, a human Proteome Profiler TM Array (HPPA) assay (R&D systems) was conducted in the serum samples collected from subjects after F-652 dosing. The HPPA assay can detect 36 inflammatory cytokines or chemokines that are commonly associated with inflammatory responses in humans (Materials and Methods, Fig. 7a). The serum samples collected from six subjects (subjects 0101, 0102, 0108, 0112, 0110, and 0105) in the highest F-652 dose cohort (45 μg/kg) at pre-dose, 24 and 48 h after dosing were analyzed using the HPPA assay. The serum samples collected from one placebo (subject 0103) were also analyzed in parallel in the assay as a placebo control. The positive control for the assay was derived from human peripheral blood mononuclear cells (PBMC) stimulated with phorbol myristate acetate (PMA) at 50 ng/mL for 24 h. It is well-established that PMA induces the expression of inflammatory cytokines in PBMC. The results of HPPA assay at 48 h after dosing are shown in Fig. 7b. The positive cytokines that were detected in the HPPA assay are summarized in Fig. 8.
Fig. 7.
Changes in the cytokine levels in healthy subjects dosed with F-652 at 48 h after dosing. Panel a shows the human cytokine array coordinates (R&D systems). Each cytokine is shown in duplicate. The positive controls for the detection assay are shown in A1,A2, E1,E2, A19, and A20. The negative controls are shown in E19 and E20. Panel b shows the results of subjects 0101, 0102, 0108, 0112, 0110, and 0105 treated with F-652 45 μg/kg at 48 h after dosing. Subject 0103 was from the placebo group. PBMC shows the results of PBMC from a healthy donor treated with 50 ng/mL PMA at 24 h in vitro
Fig. 8.
Relative density of the cytokine levels in healthy subjects dosed with F-652 at 24 and 48 h after IV dosing. In PBMC treated with PMA for 24 h, a significant induction of inflammatory cytokines was used as a positive control. The cytokines that tested negative in the human cytokine array assay are not shown in the figure
As expected, PBMC treated with PMA showed a significant elevation in cytokine levels in the cell culture supernatant, including GM-CSF, CXCL1, IL-1β, IL-1ra, IL-6, IL-8, IL-12p70, MIF, MIP-1α, Serpin E1, CCL5, and TNF-α. Among the 36 cytokines/chemokines, most were undetectable in the placebo subject as well as in six subjects at pre-dose, except for five cytokines, including CD54, MIF, Serpin E1, CCL5, and C5/C5a, which were detectable in these subjects. At 24 and 48 h after F-652 dosing, none of these 36 cytokines/chemokines in the serum samples were altered.
Discussion
Based on the safety profile of SC administration of F-652 in pre-clinical studies in SD rats, cynomolgus monkeys, rabbits, and guinea pigs (data not published), the SC route of administration was selected for the clinical study. The first cohort of F-652 SC dosing was completed with no marked adverse effects noted. In this first cohort, six subjects received 2.0 μg/kg of F-652, and two subjects received placebo. No adverse or laboratory abnormalities were observed, although there were delayed ISRs in the subjects after F-652 SC dosing, which were observed 10 days after F-652 dosing; none of the subjects that received the placebo developed ISRs. ISRs or rashes were noted in 6/6 subjects receiving F-652 starting from day 10 after dosing. Therefore, further dose escalation by SC injection was suspended.
The delayed ISRs found in healthy men dosed with F-652 by SC injection were unexpected. This finding contrasted with the results from all the pre-clinical safety studies, in which it was demonstrated that the SC injection of a single dose or multiple doses of F-652 did not reveal ISRs in SD rats and in cynomolgus monkeys. For example, in the 13-week repeated dose monkey toxicity study, F-652 at a dose of 750 μg/kg did not induce ISRs (unpublished data). In the local irritation studies conducted in New Zealand rabbits with a 7-day repeat IV, intramuscular (IM), and SC using the F-652 drug product, no significant irritation effect was noted at the sites of injection (unpublished data). Therefore, the underlining mechanism of the delayed ISRs following the SC dosing in human subjects remains to be further investigated. A toxicology study in pig, with skin that more resembles human skin, could result in observations that allow the study of F-652-related ISR.
IL-22 induces an acute phase response by targeting hepatocytes.22 Here, we also confirmed that F-652 is biologically active in humans at very low concentrations, as evidenced by the elevated serum levels of acute phase response proteins. When dosed at 2.0 μg/kg by IV infusion, with a Cmax at 15.5±4.08 ng/mL, SAA increased 13.7-fold compared to the pre-dose levels. In the high-dose cohort, F-652 was dosed at 45 μg/kg, with Cmax at 247±39 ng/mL, and SAA increased, with the mean SAA levels peaking at 72 h. The SAA increased by 320-fold when compared to the pre-dose level, and there was a 515-fold increase compared to the placebo cohort. Furthermore, the serum CRP levels reached 57.0±17.6 mg/L at 72 h after dosing, while the levels of CRP in the pre-dose and placebo groups were below the lower limit of quantitation (<3.0 mg/L). The correlation between CRP and SAA was very good, indicating that the regulation of these two markers is closely related. For the regulation of TG, Yang et al. reported that dosing mIL-22 to C57BL/6 resulted in a decrease in the hepatic gene expression of enzymes related to TG synthesis and lipogenesis.23 Therefore, the regulation of TG by IL-22 in humans is likely driven by IL-22 changing the levels of TG synthesis and lipogenesis enzymes and factors.
An additional noteworthy finding from the current study was that the IV injection of F-652 did not alter any hematological and immunological parameters we measured, nor did it elevate the common inflammatory cytokines/chemokines. These findings are interesting because IL-22 is implicated in autoimmune diseases and is reported to induce cytokines and chemokines in pre-clinical studies. For example, IL-22 is upregulated in several different human chronic inflammatory conditions, including psoriasis, inflammatory bowel disease (IBD), and rheumatoid arthritis (RA).10, 21 The upregulation of IL-22 is clearly correlated with disease severity, but it is not clear if IL-22 is a cause of the inflammation and/or is a result of it. In addition, expression analyses in human colonic sub-epithelial myofibroblasts using cDNA microarray revealed that IL-22 increases the messenger RNA (mRNA) expression of inflammatory cytokines (IL-6, IL-8, IL-11, and leukemia inhibitory factor (LIF)), chemokines, and matrix metalloproteinases.21 Furthermore, IL-22 induces the activation of nuclear factor (NF-kB) and activating protein-1 (AP-1) within 1 h, and the blockade of NF-kB and AP-1 activation markedly reduces IL-22 induction of IL-6, IL-8, IL-11, and LIF mRNA.21 This is supported by the fact that MAP-kinase inhibitors (PD98059, U0216, and SB202190) significantly reduce IL-22-induced cytokine secretion.21 In animal studies, the systemic administration of IL-22 protein (rmIL-22, 25 µg/mouse, IP) or utilizing adenoviral-mediated delivery shows that IL-22-modulated factors affect coagulation, such as FIB levels and platelet numbers.22 In addition, the injection of adenovirus IL-22 induces thymic atrophy, body weight loss, and renal proximal tubule metabolic activity changes, as well as hepatic biochemical changes, causing increases in the levels of FIB, CXCL1, and SAA-1. Some of these effects are caused by non-physiopathological concentrations of IL-22 because the injection of adenovirus IL-22 in mice results in 35,000–95,000 pg/ml of IL-22 in the serum.22 In contrast, IL-22 transgenic mice with circulating IL-22 levels from 600 to 6000 pg/mL have elevated acute phase response proteins but do not have significantly elevated inflammatory cytokines.24 The results obtained from the current clinical studies suggest that the administration of rhIL-22 in humans induced an acute phase response but did not elevate systemic inflammatory responses.
In alcohol-fed mice, treatment with rIL-22 protein reduces hepatic steatosis and hepatic TG levels with elevated serum TG levels.25 However, the treatment with rIL-22 does not affect serum TG levels in obese mice.23 Similarly, treatment with IL-6, which activates the same STAT3 signaling pathway in hepatocytes, also reduces hepatic TG with elevated serum TG levels in mice.26 One of the mechanisms by which IL-22 or IL-6 reduces hepatic TG with elevating serum TG is through IL-22/IL-6 stimulating hepatocytes to release TG into the circulation. In contrast, serum TG levels were markedly decreased in a dose-dependent manner in humans treated with 30 and 45 μg/kg. At the present time, the mechanisms by which IL-22 reduces serum TG levels in humans remain unknown, and further studies are required to explore these mechanisms.
In conclusion, F-652 was well-tolerated in the first-in-man study following IV dosing. F-652 demonstrated potent bioactivities, as evidenced by serum markers with a long half-life. No evidence of the induction of pro-inflammatory cytokines/chemokines was identified. Different from the conventional anti-inflammation new drug development approaches targeting the inflammation pathways and immune suppression, F-652 could be a first-in-class drug that plays an important protective role in promoting the survival and regeneration of tissues under immunological attack. This unique mechanism of action that targets the tissue cells but not the immune cells could either act alone or as an add-on therapy for multiple indications with unmet medical needs. Two phase IIa clinical trials have been initiated. One study is “A Phase IIa Study of recombinant human IL-22 IgG2-Fc (F-652) in Combination with Systemic Corticosteroids for the Treatment of Newly Diagnosed Grade II-IV Lower Gastrointestinal Acute Graft-vs.-Host Disease in Hematopoietic Stem Cell Transplantation Recipients”, which was led by the Memorial Sloan Kettering Cancer Center. In addition, the second study is “An Open-Label, Cohort Dose Escalation Study to Assess the Safety and Efficacy of F-652 in Patients with Alcoholic Hepatitis”, which was led by the Mayo clinic. The clinical results from the ongoing trials will help researchers understand the biology of IL-22 in human disease settings.
Acknowledgements
The authors thank Dr. Bin Gao and Dr. Ada Kung for their review and comments and Dr. Stewart Leung for organizing and editing the manuscript. This project is supported by funding from the Generon (Shanghai) Corporation.
Competing interests
X.Q.Y., K.Y.T., C.H., C.X., Y.L.H., Z.H.H., H.L., Z.S.X., H.Y.C., Y.P.W., Y.T., and L.F.X. are employees of the Generon (Shanghai) Corporation, which is involved in the development of F-652. J.L. was sponsored by Generon and has no potential conflict of interest.
References
- 1.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug. Discov. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 2.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl Acad. Sci. USA. 2000;97:10144–10149. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie MH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 4.Alam MS, et al. Notch signaling drives IL-22 secretion in CD4+T cells by stimulating the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA. 2010;107:5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pestka S, et al. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 6.Bleicher L, et al. Crystal structure of the IL-22/IL-22R1 complex and its implications for the IL-22 signaling mechanism. FEBS Lett. 2008;582:2985–2992. doi: 10.1016/j.febslet.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 7.Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Lejeune D, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 9.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin. Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 11.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J. Gastroenterol. Hepatol. 2012;27(Suppl. 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J. Gastroenterol. Hepatol. 2013;28(Suppl. 1):56–60. doi: 10.1111/jgh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int. Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 14.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell. Mol. Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- 15.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 16.Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell. Mol. Immunol. 2017;14:127–142. doi: 10.1038/cmi.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagem RA, et al. Crystal structure of recombinant human interleukin-22. Structure. 2002;10:1051–1062. doi: 10.1016/S0969-2126(02)00797-9. [DOI] [PubMed] [Google Scholar]
- 18.Nagem RA, Ferreira Junior JR, Dumoutier L, Renauld JC, Polikarpov I. Interleukin-22 and its crystal structure. Vitam. Horm. 2006;74:77–103. doi: 10.1016/S0083-6729(06)74004-3. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira Neto M, et al. Interleukin-22 forms dimers that are recognized by two interleukin-22R1 receptor chains. Biophys. J. 2008;94:1754–1765. doi: 10.1529/biophysj.107.112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andoh A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 22.Liang SC, et al. IL-22 induces an acute-phase response. J. Immunol. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol. 2010;53:339–347. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Park O, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ki SH, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong F, et al. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]