Abstract
Induction of osteopontin (OPN), a well-known pro-inflammatory molecule, has been observed in acetaminophen (APAP)-induced hepatotoxicity. However, the precise cell source for OPN induction and its role during APAP-induced hepatotoxicity has not been fully explored. By employing a hepatotoxic mouse model induced by APAP overdose, we demonstrate that both serum and hepatic OPN levels were significantly elevated in response to APAP treatment. Our in vivo and in vitro studies clearly indicated that the induced expression of hepatic OPN was mainly located in necrosis areas and produced by dying or dead hepatocytes. Functional experiments showed that OPN deficiency protected against the APAP-induced liver injury by inhibiting the toxic APAP metabolism via reducing the expression of the cytochrome P450 family 2 subfamily E member 1 (CYP2E1). Interestingly, this inhibition of CYP2E1 expression did not occur in unfasted Opn−/− mice, but was significant in fasted Opn−/− mice and maintained for 2 hours after APAP challenge in fasted Opn−/− mice. In addition, despite the early protective role of OPN deficiency on APAP-induced hepatotoxicity, OPN deficiency retarded injury resolution by sensitizing hepatocytes to apoptosis and impairing liver regeneration. Finally, we demonstrated that a siRNA-mediated transient hepatic Opn knockdown could sufficiently and significantly protect animals from APAP-induced hepatotoxicity and death. In conclusion, this study clearly defines the cell source of OPN induction in response to APAP treatment, provides a novel insight into the metabolic role of OPN to APAP overdose, and suggests an Opn-targeted therapeutic strategy for the treatment or prevention of APAP-induced hepatotoxicity.
Keywords: acetaminophen, CYP2E1, hepatotoxicity, ketone body, osteopontin
Introduction
Acetaminophen (APAP) is a popular mild analgesic and antipyretic, but acute liver necrosis and rapid death induced by APAP overdose has been reported since 1966.1 In the West, APAP overdose is the main reason for acute liver failure (ALF) related to drug-induced liver injury.2, 3 Thus far, it is well recognized that the formation of N-acetyl-p-benzoquinone imine (NAPQI), a toxic APAP metabolite, is attributed to APAP-induced hepatotoxicity.4, 5 This toxic APAP metabolism reaction is mainly catalyzed by the cytochrome P450 family 2 subfamily E member 1 (CYP2E1).6 Although a substantial amount of NAPQI can be neutralized with glutathione (GSH) in vivo, the residual NAPQI can still react with proteins to form toxic protein adducts. Among the toxic protein adducts, mitochondrial protein adducts are thought to initiate the hepatotoxic cascade by leading to increased oxidant stress in the mitochondria and consequently resulting in mitochondrial dysfunction, hepatocyte necrosis, and the release of damage-associated molecular patterns (DAMPs), such as HMGB1 and mitochondrial DNA (mtDNA).7–11 These released DAMPs subsequently activate inflammatory responses and further amplify the injury of hepatocytes.11–14 Therefore, NAPQI, mitochondrial dysfunction and the inflammatory response are three consecutive detrimental events of APAP-induced hepatotoxicity.
CYP2E1 is a member of the cytochrome P450 superfamily and is conserved across mammalian species.5 As an important detox enzyme, CYP2E1 is essential for metabolizing thousands of endogenous and exogenous chemicals, and its expression is induced in response to a variety of conditions, such as alcohol consumption, diabetes, and obesity, among others.6 Previous studies have indicated that fasting and alcohol use could enhance APAP-induced hepatotoxicity in humans, probably due to conditional CYP2E1 induction.3, 15 It has been well recognized that CYP2E1 is mainly responsible for the toxic metabolic conversion of APAP to NAPQI, as Cyp2e1 knockout mice showed considerable resistance to hepatotoxicity and death during APAP overdose, whereas mice with adenovirus-mediated Cyp2e1 overexpression showed increased susceptibility to APAP-induced hepatotoxicity.5, 16 Therefore, these findings collectively indicate that CYP2E1 could be a therapeutic target for APAP poisoning.
Osteopontin (OPN) is a multifunctional protein involved in various liver diseases, including alcoholic liver diseases, nonalcoholic fatty liver diseases, and hepatocellular carcinoma (HCC).17 In carbon tetrachloride (CCl4)-induced acute liver injury, OPN acts as a pro-inflammatory chemoattractant to promote the infiltration of macrophages and neutrophils into necrotic areas.18 However, in a diethylnitrosamine (DEN)-induced HCC mouse model, OPN has been reported to negatively regulate TLR signaling and the pro-inflammatory response by interacting with MyD88 in Kupffer cells.19 In ischemia/reperfusion (I/R)-induced liver injury, OPN plays a protective role in hepatic injury and inflammation.20 In addition, our recent study indicates that OPN facilitates liver regeneration via interleukin (IL)-6/Stat3 signaling in the model of partial hepatectomy.21 Therefore, OPN is deeply involved in liver injury, inflammation, and regeneration. Previous studies have shown increased OPN expression in mice and patients with APAP overdose,22, 23 but the precise cell source of OPN induction and its role during APAP-induced hepatotoxicity has not been fully explored.
By adopting a hepatotoxic mouse model induced by APAP overdose, we demonstrate the following: (1) dying and dead hepatocytes are the major cell sources responsible for OPN induction in APAP-induced hepatotoxicity; (2) OPN deficiency significantly improves APAP-induced liver injury by inhibiting the toxic APAP metabolism via reducing the expression of CYP2E1; (3) interestingly, this inhibition of CYP2E1 expression does not occur in unfasted Opn−/− mice, but is significant in fasted Opn−/− mice and maintained 2 hours after APAP challenge in fasted Opn−/− mice; (4) despite the early protective role of OPN deficiency on APAP-induced hepatotoxicity, OPN deficiency retards injury resolution by sensitizing hepatocytes to apoptosis and impairing liver regeneration; and (5) a transient hepatic OPN knockdown significantly protects animals from death caused by APAP overdose.
Materials and methods
Animals and treatment
All animal studies were approved by the Institutional Animal Care and Use Committees of Renji Hospital and Shanghai Jiao Tong University. OPN knockout (Opn−/−) mice (B6.Cg-Spp1tm1Blh/J) were purchased from the Jackson Laboratory (Bar Harbor, ME). Male mice aged 8–10 weeks, were fasted for 15–17 hours, and then administered 300 mg/kg or 500 mg/kg (lethal dose) of APAP (Sigma-Aldrich, St. Louis, MO) via intraperitoneal injection. For survival experiments in non-fasted mice, the lethal dose of 750 mg/kg was used.
siRNA design and in vivo transfection
siRNA against OPN and negative control (NC) was purchased from GenePharma (Shanghai, China). The following siOPN sequence was previously described:24 sense, 5′-GCCAUGACCACAUGGACGAdTdT-3′; anti-sense, 5′-dTdTCGGUACUGGUGUACCUGCU-3′. A total of 80 µg of siRNA or NC per mouse was delivered by tail vein injection with Entranster in vivo (Engreen, Beijing, China) according to the manufacturer’s instructions.
Histological analysis and immunohistochemistry
Liver tissues were collected and fixed in 4% paraformaldehyde for at least 24 hours. Then, the liver samples were embedded in paraffin and cut into 5 μm-thick sections. The sections were stained with hematoxylin and eosin using standard procedures. For immunohistochemistry (IHC), the sections were rehydrated, processed for an antigen-unmasking procedure, and then incubated with primary antibodies against mouse OPN (R&D Systems, Minneapolis, MN), mouse Ly-6G (BioLegend, San Diego, CA), mouse Ki67 (Abcam, Cambridge, UK), and mouse cleaved caspase-3 (Cell Signaling, Boston, MA) overnight at 4 °C, followed by horseradish peroxidase-conjugated secondary antibodies. For each stained section, 6–10 images from random fields were taken, and at least three mice per group were subjected to each experiment. Image-Pro Plus 6.0 was used for image analysis of the sections.
Flow cytometry analysis
Liver leukocytes were isolated as we described previously.21 Collected leukocytes were stained with FITC-conjugated anti-CD45, PE-conjugated anti-CD11b, and APC-conjugated anti-Ly-6G (eBioscience, San Diego, CA), and analyzed using a BD LSRFortessa cell analyzer. The purity of the isolated leukocytes was over 90%. The number of neutrophils was calculated by multiplying the percentage of each population from the analyzed data by the total number of leukocytes per liver.
Statistical analysis
Data are expressed as the means ± SEM. Statistical significance was determined using two-tailed, unpaired or paired Student’s t test. Survival curves were compared using the log-rank (Mantel–Cox) test. In all statistical comparisons, a P-value <0.05 was used to indicate a statistically significant difference.
Other methods are included in the supporting materials.
Results
Hepatocytes are the main cell source of increased OPN in wild-type mice in response to APAP overdose
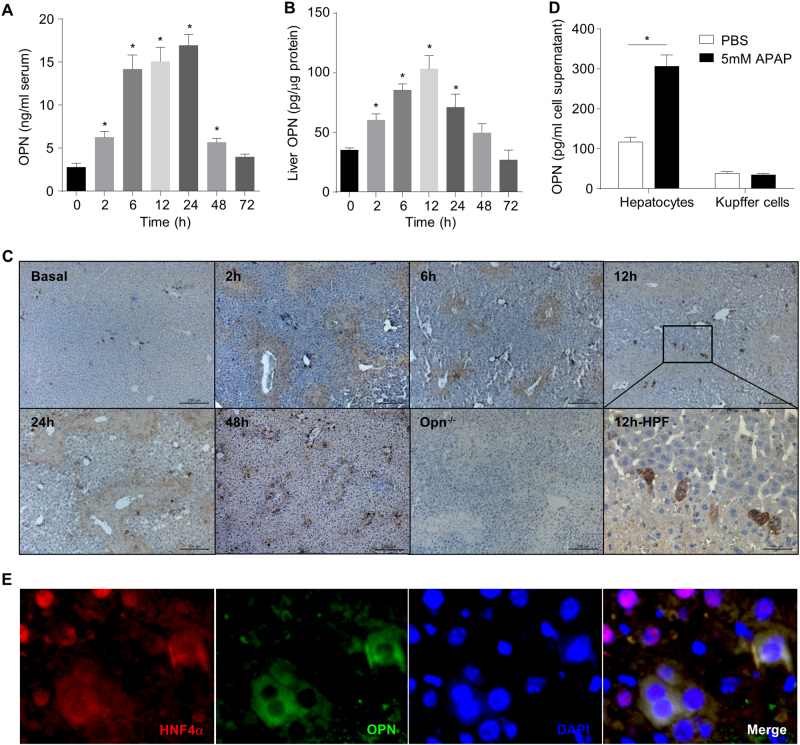
To investigate the role of OPN in APAP-induced hepatotoxicity, we used a classic hepatotoxic mouse model and injected fasted wild-type (WT) mice with a single dose of APAP (i.p., 300 mg/kg). The serum OPN levels increased immediately at 2 h, reached a peak from 6 h to 24 h and declined to the basal level at 72 h after APAP challenge (Fig. 1a). Similarly, the hepatic OPN levels increased at 2 h, reached a peak at 12 h and then declined to the basal level from 48 to 72 h (Fig. 1b). Moreover, IHC analysis showed that the physiological hepatic OPN level was very low and enriched in cells surrounding the biliary ducts (Fig. 1c, basal), whereas the APAP challenge greatly elevated the OPN levels in necrotic areas (Fig. 1c, 2–48 h) and surrounding hepatocytes (Fig. 1c, 12 h; high-power field). The increased OPN signals in necrotic areas are specific because there were no OPN signals detectable in the necrotic areas in Opn−/− mice (Fig. 1c, Opn−/−). To further confirm whether hepatocytes are the major cell source of OPN induction during APAP overdose, primary hepatocytes were isolated from WT mice and treated with APAP in vitro. A significant OPN increase was observed in the culture supernatant (Fig. 1d), suggesting that APAP treatment induced OPN from hepatocytes. Interestingly, a previous study claims that the Kupffer cells are responsible for the OPN induction during APAP overdose, which is discrepant with our findings.22 To resolve this discrepancy, we performed fluorescent double staining with antibodies against OPN and F4/80 (a Kupffer cell marker). Only a few OPN signals (green) overlapped with F4/80 signals (red) (sFig. 1), indicating that Kupffer cells are not the major cell source of OPN induction in response to 12 h of APAP treatment. Moreover, we isolated Kupffer cells and treated them with APAP. No OPN induction in the culture supernatants was detected (Fig. 1d). To further confirm this finding, we performed another fluorescent double staining with antibodies against OPN and HNF4α, a nuclear protein specifically expressed in hepatocytes.25 The majority of the OPN signals (green) overlapped with the HNF4α signals (red) (Fig. 1e), strongly indicating that hepatocytes are the major cell source of APAP-induced OPN expression. It is noteworthy that almost all cells with OPN and HNF4α double staining showed an abnormal cytoplasmic distribution of HNF4α instead of its common nuclear localization, whereas cells without OPN signals showed normal HNF4α signals enriched in the nuclei (Fig. 1e). This striking finding suggests that these OPN and HNF4α double-positive cells may undergo apoptosis or necrosis, further indicating that OPN is induced from dying or dead hepatocytes during APAP treatment.
Fig. 1.
OPN induction and its cell source in response to APAP overdose. Fasted WT mice were subjected to a single dose of APAP. Dynamic serum (a) and hepatic (b) OPN level changes in WT mice after APAP challenge (n = 4–11); (c) immunohistochemistry of OPN in the liver with APAP challenge; representative images are shown (origin magnification ×100, n = 3). Opn−/− is shown as the negative control. Twelve hour-HPF represents the magnified area at 12 h (origin magnification ×400); (d) OPN levels were measured by ELISA in the culture supernatant of primary hepatocytes and Kupffer cells treated either with or without APAP (n = 3); (e) double fluorescent staining of HNF4α and OPN in a liver section 12 h after APAP challenge (origin magnification ×400, n = 3). Data are shown as the means ± SEM, *P < 0.05. HPF high-power field
OPN deficiency decreases APAP-induced liver injury at the early phase
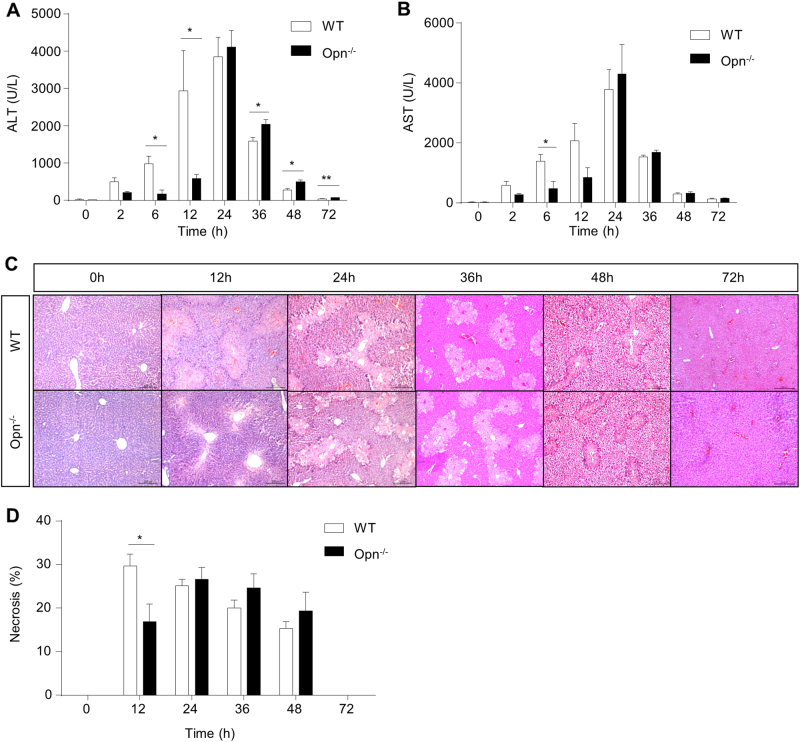
Having demonstrated hepatic OPN induction in WT mice after APAP treatment, we then employed Opn−/− mice to investigate their responses to APAP-induced liver injury. Compared with WT mice, Opn−/− mice were protected against APAP-induced liver injury, as indicated by markedly attenuated alanine aminotransferase (ALT)/aspartate aminotransferase (AST) induction (Fig. 2a, b) and much less hepatic necrosis (Fig. 2c, d) in the first 12 h after APAP treatment. However, these protective effects were abolished at 24 h after APAP challenge in Opn−/− mice (Fig. 2a–d). These findings suggest that OPN deficiency decreases APAP-induced liver injury 12 h after APAP challenge.
Fig. 2.
OPN deficiency decreases APAP-induced liver injury at the early phase. Fasted WT and Opn−/− mice were subjected to a single dose of APAP. Serum levels of ALT (a) and AST (b) at the indicated time points after APAP treatment (n = 3–6). c Representative images of HE staining in liver sections of WT and Opn−/− mice at the indicated time points after APAP challenge (origin magnification ×100). d Quantification of necrotic areas in liver sections by HE staining (n = 3–6). Data are shown as the means ± SEM, *P < 0.05
OPN deficiency attenuates the inflammatory reaction in response to APAP
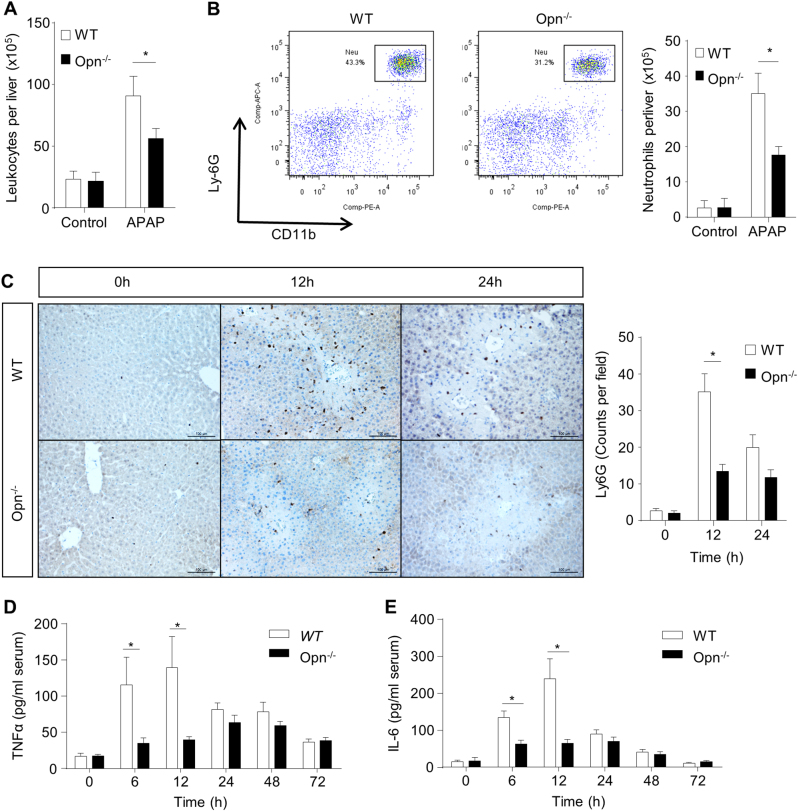
Given that injured hepatocytes can activate inflammatory responses to further amplify liver injury (mainly 12–24 h after APAP),11 we assessed whether OPN deficiency affects inflammation post-APAP treatment. We isolated intrahepatic leukocytes from both genotypes with either 12 h of APAP treatment or no APAP treatment and counted those cells in a hemocytometer. Although the population of intrahepatic leukocytes was increased in both WT and Opn−/− mice 12 h after APAP treatment, the number of intrahepatic leukocytes in Opn−/− mice was significantly less than that in WT mice (Fig. 3a). Flow cytometry analysis of these isolated intrahepatic leukocytes further showed a decreased population of Ly6G+CD11b+ cells (neutrophils) in Opn−/− mice compared to those in WT mice (Fig. 3b). In addition, IHC staining of Ly6G further confirmed less hepatic infiltration of neutrophils in Opn−/− mice within the first 12 h after APAP treatment (Fig. 3c). Corresponding to the reduced hepatic infiltration of leukocytes and neutrophils, significantly less induction of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)α and IL-6, was observed in Opn−/− mice compared to that in WT mice 12 h after APAP treatment (Fig. 3d, e).
Fig. 3.
OPN deficiency attenuates the inflammatory reaction in response to APAP. Fasted WT and Opn−/− mice were subjected to a single dose of APAP. a Hepatic leukocytes of WT and Opn−/− mice after APAP challenge were isolated and counted before or 12 h after APAP challenge (n = 3). b Quantification of Ly6G+CD11b+ neutrophils by flow cytometry and the exact neutrophil number per liver were calculated by multiplying the percentage of neutrophils by the total number of leukocytes (n = 3). c Immunohistochemistry of Ly6G in the liver with APAP challenge. Representative images are shown (origin magnification ×200). Ly-6G-positive cell counts were determined using Image-Pro Plus (n = 3–10). Serum TNFα (d) and IL-6 (e) levels after APAP challenge were determined by ELISA (n = 3–10). Data are shown as the means ± SEM, *P < 0.05
OPN deficiency protects against APAP-induced mitochondrial dysfunction and cytotoxicity both in vivo and in vitro
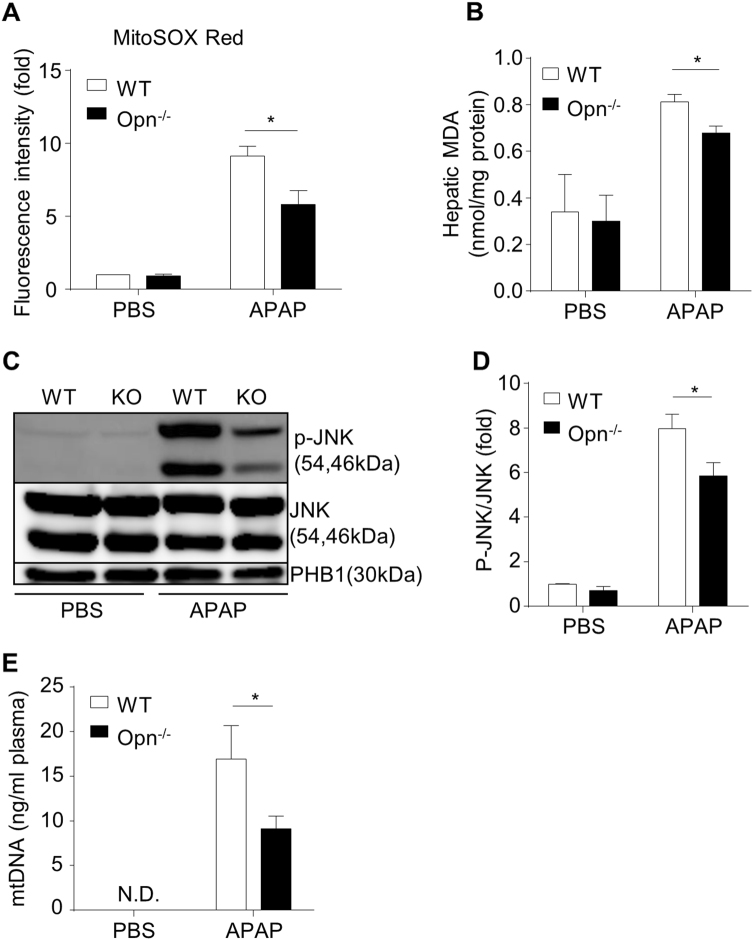
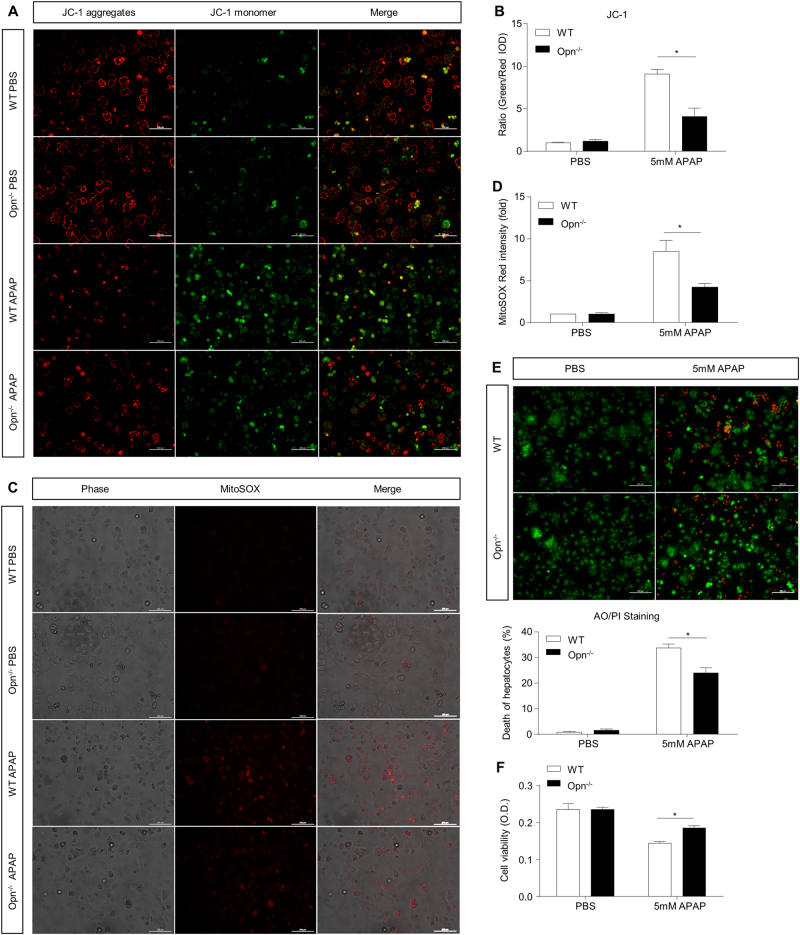
Mitochondrial dysfunction is a detrimental early event (~6 h after APAP) in the pathogenesis of APAP-induced liver injury and is caused by increased mitochondrial reactive oxygen species (ROS), enhanced JNK phosphorylation and its mitochondrial translocation.26–28 To investigate the impact of OPN deficiency on APAP-induced mitochondrial dysfunction, total cellular and mitochondrial ROS were measured by MDA and MitoSOX Red, respectively. Compared to WT mice, Opn−/− mice displayed a significantly weaker ROS induction 6 h after APAP, as demonstrated by reduced MitoSOX Red signals and MDA values (Fig. 4a, b). Accordingly, Opn−/− mice showed significantly less JNK phosphorylation in hepatic mitochondria than that in hepatic mitochondria in WT mice (Fig. 4c, d). Furthermore, compared to WT mice, Opn−/− mice exhibited resistance to APAP-induced mitochondrial dysfunction, as evidenced by a reduced release of plasma mtDNA (Fig. 4e). To further support the concept that OPN deficiency protects against APAP-induced mitochondrial dysfunction, we isolated primary hepatocytes from both genotypes. Mice were starved and treated with 5 mM of APAP or PBS in vitro, and mitochondrial dysfunction was evaluated by measuring the mitochondrial membrane potential ΔΨm (using JC-1 fluorescent dye) and mitochondrial ROS (using MitoSOX Red dye) 6 h after APAP treatment. In vitro APAP treatment caused significant mitochondrial depolarization in both genotypes as indicated by a dramatic increase in the green/red fluorescence intensity ratio; however, Opn null hepatocytes showed less mitochondrial depolarization compared to that in WT mice, as demonstrated by a smaller ratio of green/red fluorescence intensity (Fig. 5a, b). Furthermore, impaired accumulation of mitochondrial ROS was observed in OPN-deficient hepatocytes compared to their WT counterparts after APAP treatment (Fig. 5c, d). In response to the protection against mitochondrial dysfunction, acridine orange and propidium iodide double staining and MTT assays showed that OPN-deficient hepatocytes were more resistant to APAP-induced cytotoxicity than WT hepatocytes 12 h after APAP treatment (Fig. 5e, f). As previous studies demonstrate that enhanced autophagy can antagonize APAP-induced mitochondrial dysfunction and subsequently reduce cellular oxidative stress and cell necrosis,29, 30 we then measured autophagy changes in both WT and Opn−/− mice in this model. Compared to WT mice, Opn−/− mice had very low autophagy levels in the liver after fasting (sFig. 2, 0 h). APAP treatment rapidly and dramatically induced autophagy activity to comparable levels in both genotypes (sFig. 2), indicating that autophagy may not be involved in the protection of APAP-induced mitochondrial dysfunction in Opn−/− mice.
Fig. 4.
In vivo protection against APAP-induced mitochondrial dysfunction in Opn−/− mice. Fasted WT and Opn−/− mice were subjected to 6 h of APAP treatment. a Mitochondrial ROS measured by a MitoSOX Red probe in primary hepatocytes from WT and Opn−/− mice after APAP challenge (n = 3). b Hepatic MDA levels after APAP challenge (n = 4–5). c Western blot analysis of JNK phosphorylation in liver mitochondria after APAP challenge is shown. PHB1 was used as a loading control in mitochondria. d Quantification of the expression ratio of p-JNK to JNK in mitochondria from the livers of WT and Opn−/− mice (n = 3–4). e Plasma mitochondrial DNA levels after APAP challenge (n = 3–5); data are shown as the means ± SEM, *P < 0.05. N.D. not detected
Fig. 5.
In vitro protection against APAP-induced mitochondrial dysfunction in primary hepatocytes with Opn depletion. Isolated primary WT and Opn−/− hepatocytes were starved and subjected to APAP treatment. a Representative images of mitochondrial membrane potentials in primary hepatocytes 6 h after APAP treatment determined using JC-1 (origin magnification ×100); b integrated optical density (IOD) quantification of the ratio of green to red fluorescent intensity (n = 3). c Representative images of mitochondrial ROS in primary hepatocytes 6 h after APAP treatment determined with the MitoSOX Red probe (origin magnification ×100). d Quantification of MitoSOX Red intensity (n = 3). e Representative images of hepatocyte death 12 h after APAP treatment determined with acridine orange and propidium iodide (AO/PI) staining (origin magnification ×100). Quantification of the percentages of PI-stained dead hepatocytes (n = 3). f Hepatocyte viability 12 h after APAP treatment determined using the MTT assay (n = 5). Data are shown as the means ± SEM, *P < 0.05
OPN deficiency inhibits toxic APAP metabolism
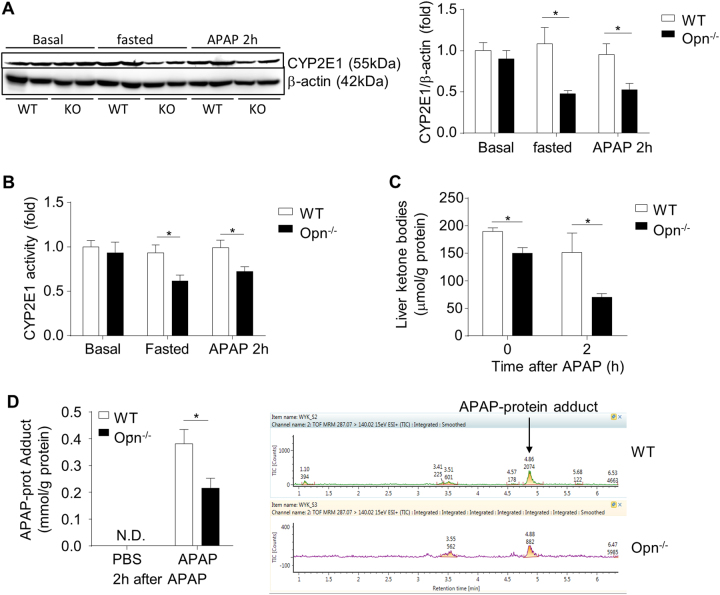
Since our above results showed that OPN deficiency protects against APAP-induced liver injury, inflammation, and mitochondrial dysfunction at the early phase after APAP treatment, we investigated whether Opn deficiency could affect the toxic APAP metabolism, which occurred 6 h after APAP treatment. We examined the expression of hepatic CYP2E1, an enzyme that is mainly responsible for conversion of APAP to the toxic metabolite NAPQI. Western blot showed that OPN deficiency does not affect CYP2E1 expression in regard to physiological conditions (Fig. 6a). Interestingly, CYP2E1 expression was significantly inhibited in fasted Opn−/− mice compared to fasted WT mice, and this inhibition was maintained in fasted Opn−/− mice 2 h after APAP treatment (Fig. 6a). The CYP2E1 enzyme activity data matched the CYP2E1 protein levels (Fig. 6b). Moreover, inhibition of CYP2E1 in Opn−/− mice did not appear to be due to transcriptional regulation of Cyp2e1 because its messenger RNA showed no significant changes in both the fasting and fasting plus APAP groups (sFig. 3). Some early studies indicated that increased ketone bodies can stabilize CYP2E1 but not affect its gene transcription.31, 32 We then examined the hepatic ketone body levels in fasted WT and Opn−/− mice with or without APAP treatment. As shown in Fig. 6c, fasted Opn−/− mice showed significantly less ketone body levels than fasted WT mice, and the difference was also significant 2 h after APAP treatment, suggesting that inhibition of CYP2E1 expression in Opn−/− mice was probably due to reduced hepatic ketone body levels. Ketone bodies are mainly produced by hepatic mitochondria. We then evaluated mitochondrial function by measuring hepatic ATP and complex activities. Under basal conditions, WT and Opn−/− mice showed comparable hepatic ATP levels and complex activities (sFig. 4). However, WT mice exhibited higher levels of hepatic ATP and complex activities than Opn−/− mice after fasting, indicating that WT hepatocytes had better mitochondrial function than Opn−/− hepatocytes. To further assess whether this lower hepatic CYP2E1 level in Opn−/− mice could lead to less NAPQI production, we quantified APAP-protein adducts using high performance liquid chromatography (HPLC) assays. APAP treatment greatly induced APAP-protein adducts in both genotypes, but significantly fewer toxic protein adducts were detected in the liver of Opn−/− mice than in that of WT mice (Fig. 6d). These findings strongly suggest that OPN deficiency inhibits the toxic APAP metabolism by decreasing CYP2E1 expression, resulting in reduced production of NAPQI and subsequent toxic APAP-protein adducts.
Fig. 6.
OPN deficiency inhibits toxic APAP metabolism. a Western blot analysis of the basal CYP2E1 levels in normal mouse livers and CYP2E1 levels in the livers of fasted mice with or without 2 h APAP treatment is shown. β-actin was used as a loading control. Quantification of the hepatic CYP2E1 levels by Image-Pro Plus (n = 4). b Basal CYP2E1 enzyme activity in normal mouse livers and CYP2E1 enzyme activity in the livers of fasted mice with or without 2 h APAP treatment (n = 4); (c) ketone body levels in the liver of fasted mice with or without APAP treatment (n = 4). d HPLC analysis of hepatic APAP-protein adduct levels 2 h after APAP challenge (n = 4). Data are shown as the means ± SEM, *P < 0.05. N.D. not detected
OPN deficiency impairs hepatocyte proliferation and increases apoptosis in response to APAP
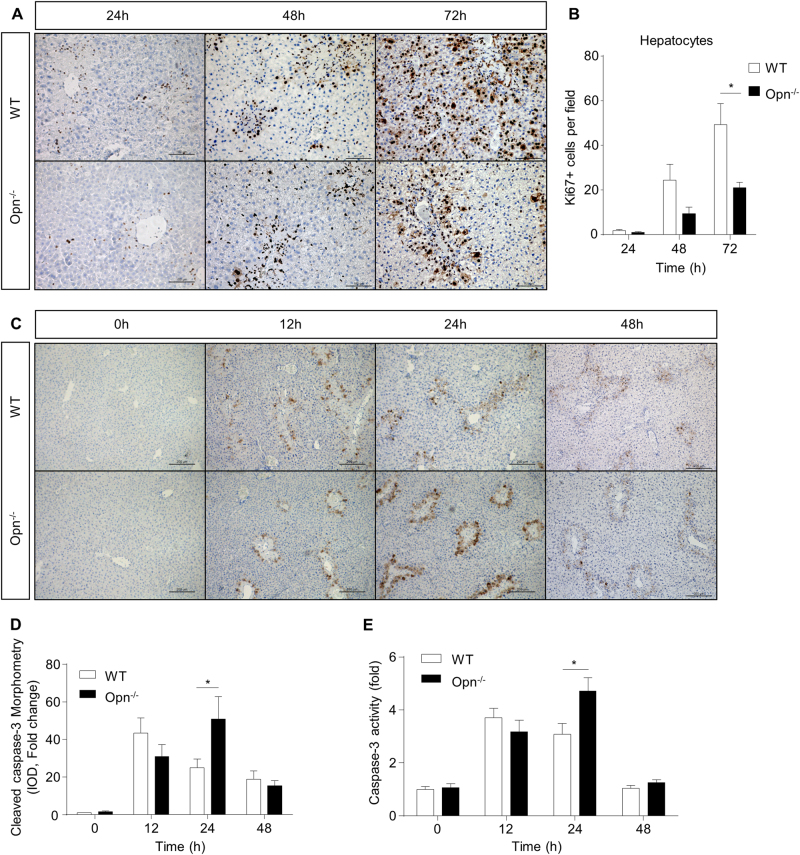
Although OPN deficiency inhibits APAP metabolism and results in early protection against APAP hepatotoxicity, we surprisingly found there was no significant survival advantage in Opn−/− mice compared to that in WT mice during APAP overdose with fasting (sFig. 5). To explore this paradox, we examined whether OPN deficiency could affect liver regeneration after APAP treatment because liver regeneration has been recently recognized as a critical factor for host recovery during APAP overdose, and our recent study demonstrated impaired liver regeneration in Opn−/− mice post-partial hepatectomy.11, 21 Liver repair usually occurred at 24 h after liver injury. As shown in Fig. 7a, b, OPN deficiency significantly inhibited hepatocyte proliferation, as demonstrated by reduced Ki67 staining at 48 h and 72 h after APAP. This proliferation inhibition may be attributed to attenuated STAT3 phosphorylation in Opn−/− mice 12 h after APAP (sFig. 6A). Although necrosis is the most emphasized characteristic of APAP-induced liver injury, apoptosis has recently emerged in APAP-induced liver injury and is strongly correlated with poor outcomes.33 We then assessed hepatic apoptosis by measuring the level of cleaved caspase-3. IHC staining showed increased apoptosis in both genotypes after APAP (Fig. 7c, d). Compared to WT mice, Opn−/− mice showed significantly higher apoptosis levels 24 h after APAP treatment (Fig. 7c, d). In addition, capase-3 activity was significantly higher in Opn−/− mice compared to WT mice 24 h after APAP treatment (Fig. 7e). This increased apoptosis may be because OPN-deficient hepatocytes were more sensitive to TNFα-induced cell death (sFig. 6B). These findings indicate that constant OPN deficiency has detrimental effects on host recovery from APAP overdose by inhibiting liver regeneration and promoting apoptosis.
Fig. 7.
OPN deficiency impairs hepatocyte proliferation and enhances hepatocyte apoptosis. Fasted WT and Opn−/− mice were subjected to a single dose of APAP. a Representative images of IHC of Ki67 in the livers of WT and Opn−/− mice after APAP challenge (origin magnification ×200); (b) Ki67-positive hepatocytes (distinguished by morphology and size of the nucleus) were determined using Image-Pro Plus (n = 4–6). c Representative images of IHC of cleaved caspase-3 in the livers of WT and Opn−/− mice after APAP challenge (origin magnification ×100). d IOD quantification of cleaved caspase-3 was determined using Image-Pro Plus (n = 3–5). e Caspase-3 activity in the livers at the indicated time points after APAP challenge (n = 3-4). Data are shown as the means ± SEM, *P < 0.05
Transient in vivo Opn knockdown protects host against APAP-induced hepatotoxicity
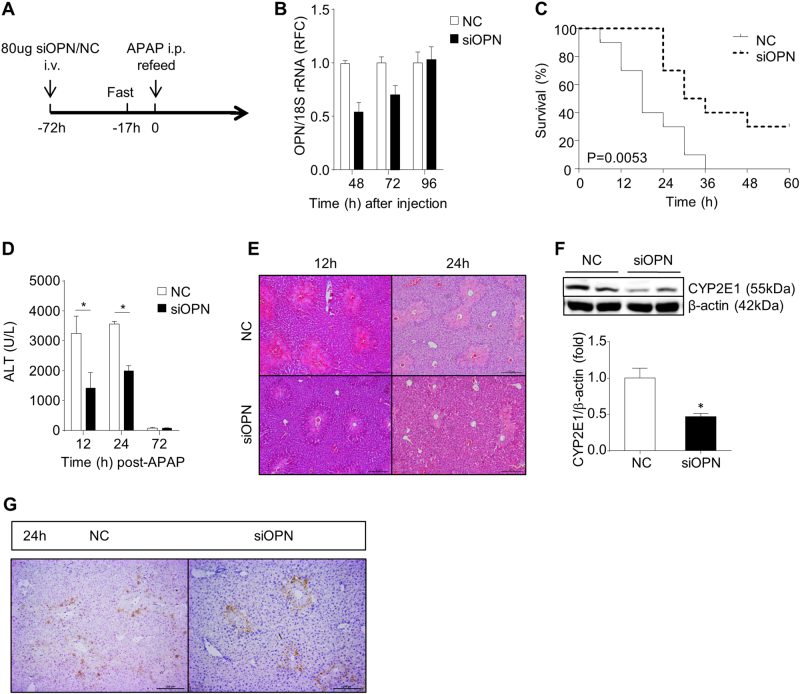
Given that OPN deficiency protects against early liver injury but impairs liver regeneration and enhances apoptosis at the late phase during APAP overdose, we hypothesize that an early transient OPN knockdown will be an appropriate strategy to improve APAP-induced liver injury and host death. To test this hypothesis, we adopted Opn RNAi in hepatotoxicity induced by APAP overdose. A single dose of Opn siRNA or NC siRNA (NC) (80 µg) was administered intravenously to the WT mice 72 h prior to APAP treatment; and before APAP administration, all mice were subjected to fasting for 17 h (Fig. 8a). And efficient hepatic Opn knockdown was confirmed at 48 h and 72 h after the initial siRNA challenge; the OPN level was restored 96 h later (Fig. 8b). As we expected, this early transient Opn knockdown significantly improved host survival after APAP treatment (Fig. 8c). However, an OPN neutralizing antibody could not significantly improve the survival of mice with APAP overdose (sFig. 7). Moreover, we found that transient Opn knockdown failed to improve the survival rate in non-fasted mice that received a lethal APAP overdose (sFig. 8). Consistent with the protective effect in fasted mice, Opn knockdown showed significantly reduced APAP-induced hepatotoxicity, as demonstrated by diminished ALT levels and necrosis areas at 12 and 24 h after APAP treatment (Fig. 8d, e). In response to this reduced hepatotoxicity, siOPN-pretreated mice also exhibited reduced serum IL-6 and TNFα levels and attenuated neutrophil infiltration compared to the NC controls (sFig. 9A and B). Similar observations in Opn−/− mice, siOPN-pretreated mice showed significantly lower CYP2E1 levels than those of NC controls 2 h after APAP challenge (Fig. 8f). Unlike the observations in Opn−/− mice, transient Opn knockdown did not show enhanced apoptosis levels 24 h after APAP treatment (Fig. 8g), whereas liver regeneration was still impaired in mice with transient Opn knockdown (sFig. 9C). Moreover, to exclude the effects of different degrees of liver injury on liver regeneration and confirm the role of OPN in promoting liver regeneration in this APAP model, we treated mice with siOPN or NC immediately after APAP challenge and found that hepatic regeneration of siOPN-treated mice was still impaired (sFig. 10). In summary, these findings implied the therapeutic potential and strategy of OPN in hepatotoxicity induced by APAP overdose.
Fig. 8.
In vivo transient Opn knockdown rescues animals from APAP-induced death. a Schematic of in vivo transient Opn knockdown combined with APAP overdose. b mRNA levels of hepatic Opn in mice at indicated time points after siOPN treatment (n = 2). c Survival was followed for 60 h after administration of a lethal dose of APAP (n = 10). d Serum ALT levels at the indicated time points after APAP treatment (300 mg/kg, i.p, n = 4–5). e H&E staining in liver sections (n = 4–5, origin magnification ×100). f Western blot of the hepatic CYP2E1 levels 2 h after APAP challenge. β-actin was used as a loading control. Quantification of the relative hepatic CYP2E1 levels in NC or siOPN-treated mice (n = 3). g IHC of cleaved caspase-3 in the livers of NC or siOPN-treated mice 24 h after APAP challenge (origin magnification ×100). Data are shown as the means ± SEM, *P < 0.05. NC negative control
Discussion
In the present study, we demonstrated OPN induction in an APAP-induced hepatotoxic mouse model and found that OPN deficiency protects against APAP-induced liver injury at the early stage but staggers injury resolution at the late stage. Mechanistically, OPN deficiency can decrease CYP2E1 expression in fasted mice and, in turn, reduce production of toxic APAP-protein adducts, resulting in protection against APAP-induced hepatotoxicity. Additionally, OPN deficiency retards injury resolution by impairing liver regeneration and enhancing hepatic apoptosis. More importantly, transient Opn knockdown showed therapeutic potential for APAP-induced hepatotoxicity.
Given the matched tendency between serum and hepatic OPN, we supposed that serum OPN mainly came from the liver. In the liver, OPN has been previously reported to be mainly expressed in biliary epithelial cells and activated Kupffer cells.34, 35 However, in our current study, we found that much less OPN was produced by Kupffer cells after APAP. Our in vitro studies confirmed that APAP could not directly induce OPN production in Kupffer cells. We supposed some other secondary signals may contribute to this small amount of OPN production by Kupffer cells. Furthermore, our data clearly defined that dying or dead hepatocytes were the major cell source of APAP-induced OPN induction and that OPN production by hepatocytes was directly induced by APAP, suggesting that OPN may function as a DAMP in APAP-induced liver injury. In fact, hepatocyte-derived OPN induction has been reported in MCD diet-fed or TAA-treated animals.36, 37 DAMP-like OPN might be a potential diagnostic factor in the very early phase of APAP-induced liver failure.
Hepatic infiltration of neutrophils is commonly recognized as a detrimental feature in various liver diseases because infiltrated neutrophils are able to produce excessive ROS and pro-inflammatory cytokines to induce secondary liver injury. However, it has been demonstrated that neutrophil infiltration is not associated with APAP-induced liver injury but is positively correlated with injury resolution, likely due to neutrophil-mediated clearance of cell debris.38 Therefore, the reduced hepatic infiltration of neutrophils in Opn−/− mice may contribute to delayed injury resolution after APAP treatment.
An interesting finding in our current study was that OPN deficiency repressed CYP2E1 expression in fasted mice, but not in unfasted mice. This OPN deficiency was related to CYP2E1 inhibition, probably due to the lower ketone body levels in Opn−/− mice compared to those in WT mice. As ketone bodies were produced by the hepatic mitochondria, the lower ketone bodies in Opn−/− mice might have been attributable to their impaired mitochondrial function after fasting. Yet, how OPN regulates mitochondrial function during fasting remains elusive, and more investigations are necessary.
One possible explanation for the production of ketone bodies following alcoholic consumption and protection of Opn−/− mice against alcohol-induced liver injury39 is that OPN deficiency may inhibit CYP2E1 expression during the process of alcohol metabolism. Given that OPN deficiency may inhibit CYP2E1 expression through regulating ketone body production, OPN-targeted hepatotoxicity prevention may be more suitable for fasting and/or individuals who consume alcohol and are more sensitive to APAP-induced liver injury.
Although constant OPN deficiency protected liver injury at the early stage of APAP treatment, this early protection is apparently not sufficient to reach a survival advantage because OPN-deficient hepatocytes seem to be more susceptible to TNFα-induced cell death. This finding is consistent with a previous study showing that Opn RNAi sensitizes primary and AML12 hepatocytes to TNFα-induced cell death.20 In our current study, the knockdown effect of siOPN was lost at 96 h. Therefore, we treated mice with siOPN 72 h before the APAP challenge, and 24 h after APAP, apoptosis was no longer observed in the livers of siOPN-treated mice, which may contribute to the improved survival of siOPN-treated mice.
In this study, we also used an OPN neutralizing antibody to treat APAP overdose-induced liver failure. However, OPN neutralization did not significantly improve the outcomes, which suggests that intracellular OPN rather than extracellular OPN might play the predominant role in protecting mice from APAP overdose. By adopting the transient Opn knockdown approach based on OPN RNAi, we maintained the beneficial effect of OPN deficiency on protecting against liver injury induced by APAP overdose and avoided the detrimental effects of OPN deficiency on sensitizing hepatocytes to TNFα-induced cell death, which improved host survival against APAP overdose.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81670562 and 31300742 to X.K., 81670598 to Q.X., 81372233 to H.W., and 81673935 to X.S.) and a grant from the Shanghai Municipal Education Commission (Gaofeng Clinical Medicine Grant Support (20171911) to X.K., National Science and Technology major grant (2017ZX10203204-006-005) to X.K., and the Shanghai Health Bureau Key Joint Efforts Foundation (2013ZYJB001) to Q.X.).
Competing interests
The authors declare no competing interests.
Contributor Information
Qiang Xia, Phone: +86 21 34206284, Email: xiaoni-kong@126.com.
Xiaoni Kong, Phone: +86 21 34206284, Email: xiaoni-kong@126.com.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41423-018-0033-z).
References
- 1.Davidson DG, Eastham WN. Acute liver necrosis following overdose of paracetamol. Br. Med. J. 1966;2:497–499. doi: 10.1136/bmj.2.5512.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stravitz RT, Kramer DJ. Management of acute liver failure. Nat. Rev. Gastroenterol. Hepatol. 2009;6:542–553. doi: 10.1038/nrgastro.2009.127. [DOI] [PubMed] [Google Scholar]
- 3.Larson AM, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 4.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl Acad. Sci. USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 6.Novak RF, Woodcroft KJ. The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch. Pharmacol. Res. 2000;23:267–282. doi: 10.1007/BF02975435. [DOI] [PubMed] [Google Scholar]
- 7.Xie Y, et al. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014;279:266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill MR, et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg. Nutr. 2014;3:331–343. doi: 10.3978/j.issn.2304-3881.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch. Toxicol. 2015;89:193–199. doi: 10.1007/s00204-014-1432-2. [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Chao, Feng Jin, Du Jun, Zhuo Zhiyong, Yang Shuo, Zhang Weihong, Wang Weihong, Zhang Shengyuan, Iwakura Yoichiro, Meng Guangxun, Fu Yang-Xin, Hou Baidong, Tang Hong. Macrophage-derived IL-1α promotes sterile inflammation in a mouse model of acetaminophen hepatotoxicity. Cellular & Molecular Immunology. 2017;15(11):973–982. doi: 10.1038/cmi.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaeschke H. Mechanisms of sterile inflammation in acetaminophen hepatotoxicity. Cell. Mol. Immunol. 2018;15:74–75. doi: 10.1038/cmi.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iracheta-Vellve A, Szabo G. IL-1α in acetaminophen toxicity: a sterile danger signal. Cell. Mol. Immunol. 2018;15:284–285. doi: 10.1038/cmi.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA. 1994;272:1845–1850. doi: 10.1001/jama.1994.03520230055038. [DOI] [PubMed] [Google Scholar]
- 16.Bai J. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicol. Sci. 2006;91:365–371. doi: 10.1093/toxsci/kfj165. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y, Jeong S, Xia Q, Kong X. Role of osteopontin in liver diseases. Int. J. Biol. Sci. 2016;12:1121–1128. doi: 10.7150/ijbs.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawashima R, et al. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem. Biophys. Res. Commun. 1999;256:527–531. doi: 10.1006/bbrc.1999.0372. [DOI] [PubMed] [Google Scholar]
- 19.Fan X, et al. Intracellular osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer Res. 2015;75:86–97. doi: 10.1158/0008-5472.CAN-14-0615. [DOI] [PubMed] [Google Scholar]
- 20.Patouraux S, et al. Osteopontin deficiency aggravates hepatic injury induced by ischemia–reperfusion in mice. Cell Death Dis. 2014;5:e1208. doi: 10.1038/cddis.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen Y, et al. Defective initiation of liver regeneration in osteopontin-deficient mice after partial hepatectomy due to insufficient activation of IL-6/Stat3 pathway. Int. J. Biol. Sci. 2015;11:1236–1247. doi: 10.7150/ijbs.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He CY, et al. The dual role of osteopontin in acetaminophen hepatotoxicity. Acta Pharmacol. Sin. 2012;33:1004–1012. doi: 10.1038/aps.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srungaram P, et al. Plasma osteopontin in acute liver failure. Cytokine. 2015;73:270–276. doi: 10.1016/j.cyto.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y, et al. Osteopontin small interfering RNA protects mice from fulminant hepatitis. Human. Gene Ther. 2007;18:1205–1214. doi: 10.1089/hum.2007.069. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Bishop EP, Burke PA. Expression profile analysis of the inflammatory response regulated by hepatocyte nuclear factor 4alpha. BMC Genomics. 2011;12:128. doi: 10.1186/1471-2164-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanawa N, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Ramshesh VK, McGill MR, Jaeschke H, Lemasters JJ. Low dose acetaminophen induces reversible mitochondrial dysfunction associated with transient c-Jun N-terminal kinase activation in mouse liver. Toxicol. Sci. 2016;150:204–215. doi: 10.1093/toxsci/kfv319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellward GD, et al. Hepatic cytochrome P-450j induction in the spontaneously diabetic BB rat. Mol. Pharmacol. 1988;33:140–143. [PubMed] [Google Scholar]
- 32.Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J. Biol. Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- 33.Possamai LA, et al. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure*. Crit. Care Med. 2013;41:2543–2550. doi: 10.1097/CCM.0b013e31829791a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol. Lett. 2014;224:186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, et al. Increased expression of osteopontin in activated Kupffer cells and hepatic macrophages during macrophage migration in Propionibacterium acnes-treated rat liver. J. Gastroenterol. 2000;35:696–701. doi: 10.1007/s005350070049. [DOI] [PubMed] [Google Scholar]
- 36.Sahai A, et al. Roles of phosphatidylinositol 3-kinase and osteopontin in steatosis and aminotransferase release by hepatocytes treated with methionine-choline-deficient medium. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G55–G62. doi: 10.1152/ajpgi.00360.2005. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut. 2014;63:1805–1818. doi: 10.1136/gutjnl-2013-306373. [DOI] [PubMed] [Google Scholar]
- 38.Williams CD, et al. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol. Appl. Pharmacol. 2014;275:122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales-Ibanez O, et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742–1756. doi: 10.1002/hep.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.