Abstract
Background and Purpose
Stroke is one of the most common neurological manifestations of infective endocarditis. The use of intravenous tissue plasminogen activator (t-PA) in the management of acute ischemic stroke is the accepted standard of practice.
Current guidelines for intravenous (IV) t-PA therapy in acute ischemic stroke do not exclude patients with infective endocarditis.
Summary of the Case
We present three patients who received IV t-PA for acute ischemic stroke in the setting of infective endocarditis and developed multifocal intracranial hemorrhage as a complication.
Conclusion
Infective endocarditis related strokes are associated with a higher risk of hemorrhagic complications and our experience suggests that IV t-PA use may potentiate that risk.
Keywords: Stroke, Intracranial hemorrhage, Subarachnoid hemorrhage, Endocarditis, Thrombolysis, t-PA
Case Reports
Patient 1
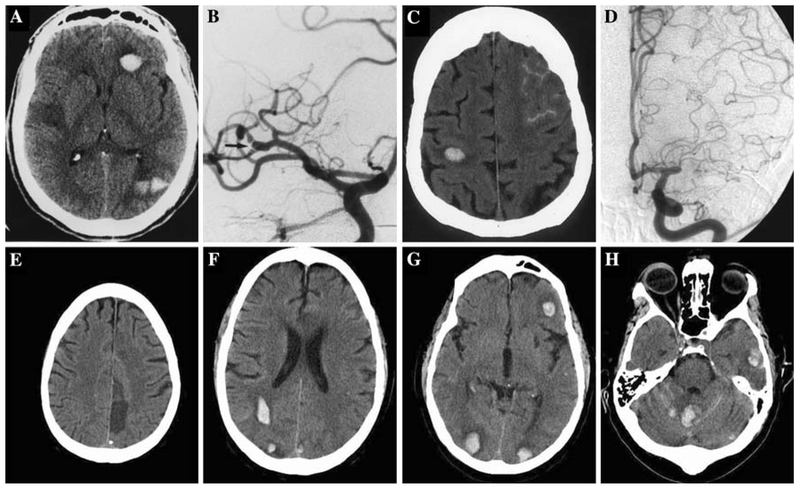
A 46-year-old man with hypertension presented with acute onset left-sided weakness and a left facial droop. His initial National Institutes of Health stroke scale (NIHSS) was 15 and computed tomography (CT) of the head showed no acute abnormalities. He was afebrile with a normal white blood cell count; cardiac examination did not reveal any abnormal heart sounds and there was no evidence of distal emboli. Although the patient had a remote history of intravenous (IV) drug use, he adamantly denied any recent use. With no identifiable contraindications, he received 0.9 mg/kg of IV tissue plasminogen activator (t-PA) 110 min after onset of symptoms. An hour after t-PA infusion, he developed a temperature of 40.1 °C along with vomiting, anisocoria, and downward eye deviation. Emergent head CT showed right middle cerebral artery (MCA) distribution infarction and interval development of cerebellar, occipital, and frontal lobe intracerebral hemorrhage (ICH) (Fig. 1a). He received cryoprecipitate, fresh frozen plasma and was started on broad-spectrum antibiotics. Urine toxicology screen was positive for cocaine metabolites and blood cultures subsequently grew Corynebacterium. Transesophageal echocardiogram (TEE) did not show any signs of valvular vegetations. Cerebral angiogram revealed an area of focal narrowing with post-stenotic dilatation at the proximal right MCA (Fig. 1b) but no evidence of mycotic aneurysm was seen. The patient’s mental status and left hemiparesis improved over the next several days. However, he became acutely obtunded with a dilated right pupil and extensor posturing on hospital day 7. Head CT revealed hemorrhagic transformation of the right MCA infarct with midline shift, uncal herniation, and the patient subsequently died. Autopsy demonstrated endocardial vegetative growths on the mitral and aortic valves but no cerebral mycotic aneurysms were found.
Fig. 1.
a Right middle cerebral artery (MCA) distribution infarction and interval development of cerebellar, occipital and frontal lobe intracerebral hemorrhage (ICH). b Area of focal narrowing with post-stenotic dilatation at the proximal right MCA. c Evolving non-hemorrhagic left proximal MCA infarct, subarachnoid hemorrhage (SAH) over the left frontal lobe, small ICH in the right precentral gyrus, and cerebellum. d Left MCA occlusion with no evidence of mycotic aneurysm. e Chronic left frontal lobe infarct without evidence of hemorrhage. f–h Multiple bilateral cerebral and cerebellar ICH and SAH in the cerebellum
Patient 2
A 65-year-old woman on chronic corticosteroid therapy for presumed cryoglobulinemia presented with acute onset of difficulty speaking and right-sided weakness. Neurological examination revealed global aphasia, left gaze preference, right homonymous hemianopsia, and right hemiplegia with NIHSS of 21. Head CT showed mild left hemisphere sulcal effacement and a hypodense left putamen without evidence of hemorrhage. She was afebrile with a normal white blood cell count; cardiac examination revealed a systolic murmur but no evidence of distal emboli was noted. She received 0.9 mg/kg of IV t-PA 120 min after symptom onset. The following day, her neurological exam was unchanged but she was noted to have a temperature of 38.7°C. Routine 24-h post t-PA head CT showed an evolving non-hemorrhagic left proximal MCA infarct, subarachnoid hemorrhage (SAH) over the left frontal lobe and small ICH in the right precentral gyrus and cerebellum (Fig. 1c). Blood cultures grew Streptococcus viridans and she was started on the appropriate antibiotics. Transthoracic echocardiogram revealed vegetations on both mitral valve leaflets and the aortic valve; cerebral angiogram demonstrated a left MCA occlusion with no evidence of mycotic aneurysm (Fig. 1d). She completed a course of IV antibiotics, showed gradual improvement in neurological symptoms and was transferred to a rehabilitation facility.
Patient 3
A 61-year-old man with a history of hypertension and a recent closed left pubic symphysis fracture presented with acute onset of global aphasia and right hemiparesis with NIHSS of 17. He was afebrile with a normal white blood cell count; cardiac exam was unremarkable and no evidence of distal emboli was seen. Head CT revealed an old infarct in the left frontal lobe without any evidence of hemorrhage (Fig. 1e). He received 0.9 mg/kg of IV t-PA 90 min after onset of symptoms. Given the large size of his stroke, devastating symptoms, and no immediate neurological improvement following IV t-PA, intra-arterial intervention was attempted. However, cerebral angiogram did not reveal any blockage in the major vessels and no mycotic aneurysms were seen. The following day, he developed a temperature of 39.3°C with a decreased level of consciousness. Head CT showed multiple bilateral cerebral and cerebellar ICHs (Fig. 1f–h) and SAH in the cerebellum (Fig. 1h). Blood cultures grew methicillin-sensitive Staphylococcus aureus, TEE showed a small vegetation on the anterior mitral valve leaflet and he was started on the appropriate antibiotics. Subsequently, he developed gangrenous changes of several toes and splinter hemorrhages of the fingernails. His creatinine went up from 0.9 to 1.3 mg/dl. Over the next several days, he showed gradual improvement in neurological symptoms and was transferred to an inpatient rehabilitation facility.
Discussion
Many protocols list infective endocarditis (IE) as a relative contraindication to IV t-PA use but according to the current American Heart Association/American Stroke Association guidelines, IV t-PA administration is not contraindicated in acute ischemic stroke caused by IE [1]. In our case series, three patients that initially lacked sufficient clinical evidence suggestive of IE received IV t-PA for ischemic stroke based on the current guidelines and subsequently developed multifocal ICH and SAH. All three patients ultimately met Duke’s criteria for a diagnosis of IE [2]. This report highlights the plausible additional risk of hemorrhage from IV t-PA use in patients with stroke due to IE.
Successful IV t-PA use has been reported in children with intracardiac vegetations but without any evidence of ischemic stroke [3–5]. To our knowledge, this is the second report in the literature examining IV t-PA use in acute ischemic stroke caused by IE. Junna et al. [6] reported successful use of IV t-PA in a similar setting with improvement in NIHSS from 15 to 4 without intracranial hemorrhage. However, in our experience, all three patients with stroke due to IE developed multifocal ICHs, one of the most feared complications of t-PA use. Intracranial hemorrhage has also been reported in patients receiving IV t-PA for myocardial ischemia due to IE [7, 8]. It is estimated that 2–8% of patients with neurological complications related to IE develop intracranial hemorrhage [9]. Why are patients with IE related stroke more prone to intracranial hemorrhage with IV t-PA use? The exact pathogenesis of intracranial hemorrhage remains unknown. Mycotic aneurysms are thought to be one of the causes, although they are found in less than 3% of the cases [10]. In our case series, no mycotic aneurysms were found by either cerebral angiography or post-mortem pathological examination. Other potential mechanisms include pyogenic arteritis, micro-abscess, hemorrhagic transformation of previous ischemic strokes, immune complex mediated arteritis, and infiltration of meningeal vasculature [10, 11].
The diagnosis of ischemic stroke in patients who present within a 3-h time window is generally made on clinical grounds before administration of thrombolytics. The etiology of the stroke is often not definitively determined until well after the patient has received treatment. In this setting, the diagnosis of IE may be daunting, especially if signs such as fever, leukocytosis, new cardiac murmur, and distal embolic phenomenon are absent. Therefore, one must maintain a high index of clinical suspicion for IE in acute stroke patients with prosthetic cardiac valves, IV drug use, or chronic immunosuppression.
In summary, IE related strokes are associated with a higher risk of hemorrhagic complications and our experience suggests that IV t-PA use may potentiate that risk. Although we believe thrombolytic use to be ill advised in endocarditis-associated ischemic stroke, there is little clinical evidence to guide alternative therapy in the acute setting. There may be a role for interventional therapy but this needs to be further investigated as there is little firm literature supporting its use in this setting.
Contributor Information
Parita Bhuva, Department of Neurology, Baylor College of Medicine, 6550 Fannin, Suite 1801, Houston, TX 77030, USA.
Sheng-Han Kuo, Department of Neurology, Baylor College of Medicine, 6550 Fannin, Suite 1801, Houston, TX 77030, USA.
J. Claude Hemphill, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
George A. Lopez, Department of Neurology, Baylor College of Medicine, 6550 Fannin, Suite 1801, Houston, TX 77030, USA, glopez@bcm.tmc.edu
References
- 1.Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–711. [DOI] [PubMed] [Google Scholar]
- 2.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–9. [DOI] [PubMed] [Google Scholar]
- 3.Gunes AM, Bostan OM, Baytan B, Semizel E. Treatment of infective endocarditis with recombinant tissue plasminogen activator. Pediatr Blood Cancer. 2008;50(1):132–4. [DOI] [PubMed] [Google Scholar]
- 4.Levitas A, Zucker N, Zalzstein E, Sofer S, Kapelushnik J, Marks KA. Successful treatment of infective endocarditis with recombinant tissue plasminogen activator. J Pediatr. 2003;143(5):649–52. [DOI] [PubMed] [Google Scholar]
- 5.Marks KA, Zucker N, Kapelushnik J, Karplus M, Levitas A. Infective endocarditis successfully treated in extremely low birth weight infants with recombinant tissue plasminogen activator. Pediatrics. 2002;109(1):153–8. [DOI] [PubMed] [Google Scholar]
- 6.Junna M, Lin CC, Espinosa RE, Rabinstein AA. Successful intravenous thrombolysis in ischemic stroke caused by infective endocarditis. Neurocrit Care. 2007;6(2):117–20. [DOI] [PubMed] [Google Scholar]
- 7.Di Salvo TG, Tatter SB, O’Gara PT, Nielsen GP, DeSanctis RW. Fatal intracerebral hemorrhage following thrombolytic therapy of embolic myocardial infarction in unsuspected infective endocarditis. Clin Cardiol. 1994;17(6):340–4. [DOI] [PubMed] [Google Scholar]
- 8.Hunter AJ, Girard DE. Thrombolytics in infectious endocarditis associated myocardial infarction. J Emerg Med. 2001;21(4):401–6. [DOI] [PubMed] [Google Scholar]
- 9.Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160(18):2781–7. [DOI] [PubMed] [Google Scholar]
- 10.Hart RG, Kagan-Hallet K, Joerns SE. Mechanisms of intracranial hemorrhage in infective endocarditis. Stroke. 1987;18(6):1048–56. [DOI] [PubMed] [Google Scholar]
- 11.Masuda J, Yutani C, Waki R, Ogata J, Kuriyama Y, Yamaguchi T. Histopathological analysis of the mechanisms of intracranial hemorrhage complicating infective endocarditis. Stroke. 1992;23(6):843–50. [DOI] [PubMed] [Google Scholar]