Abstract
Dental caries, i.e., tooth decay mediated by bacterial activity, is the most widespread chronic disease worldwide. Carious lesions are commonly treated using dental resin composite restorations. However, resin composite restorations are prone to recurrent caries, i.e., reinfection of the surrounding dental hard tissues. Recurrent caries is mainly a consequence of waterborne and/or biofilm-mediated degradation of the tooth-restoration interface through hydrolytic, acidic and/or enzymatic challenges. Here we use amphipathic antimicrobial peptides to directly coat dentin to provide resin composite restorations with a 2-tier protective system, simultaneously exploiting the physicochemical and biological properties of these peptides. Our peptide coatings modulate dentin’s hydrophobicity, impermeabilize it, and are active against multispecies biofilms derived from caries-active individuals. Therefore, the coatings hinder water penetration along the otherwise vulnerable dentin-restoration interface, even after in vitro aging, and increase its resistance against degradation by water, acids, and saliva. Moreover, they do not weaken the resin composite restorations mechanically. The peptide-coated highly-hydrophobic dentin is expected to notably improve service life of resin composite restorations and to enable the development of entirely hydrophobic restorative systems. The peptide coatings were also antimicrobial and thus, they provide a second tier of protection preventing re-infection of tissues in contact with restorations.
Keywords: Antimicrobial Peptide, Hydrophobic Coating, Dentin, Dental Restoration, Recurrent Caries, GL13K
Graphical Abstract
Introduction
Dental caries is a pathologic process caused by bacterial activity that results in localized dental cavities. It is the most widespread chronic disease worldwide. The World Health Organization has estimated that 5,000 million people suffer from dental caries. Nearly 100% of adults and 60–90% of school children have carious lesions [1] and their treatment consumes 5–10% of the healthcare budget in industrialized countries. Thus, preventing and treating dental caries constitutes a major global public health challenge [2, 3].
Filling tooth cavities with direct dental resin composite restorations is the most popular treatment for restoring the esthetics and function of teeth affected by dental caries [4]. However, resin composite restorations have a limited lifespan, which is as short as 4.5 years [5-9]. Every extra surface included in a restoration increases the failure risk by 30-40% [10]. Also, replacement of failed resin composite restorations results in progressively larger cavities and, ultimately, destruction of the tooth structure [11]. Replacement of failed restorations constitutes about 50–70% of all operative dentistry work [12-14], which is the most common procedure in general dentistry [15].
The two main reasons of failure of resin composite restorations are recurrent caries and restoration fracture [16]. Recurrent caries at the margin of an existing restoration [17] is the main cause of failure 3 years or later after its placement [18]. In a typical dental practice, 60% of all restoration replacement is due to recurrent caries [19]. Resin-based bonding agents/adhesives are used to adhere the composite filling to the dental tissues (enamel and dentin). Resin composite restorations are most vulnerable at the dentin/restoration (d/r) interface [20], where gaps can develop over time [21, 22]. These gaps enable passage of bacteria from the oral environment which eventually colonize the exposed dental tissues and initiate recurrent caries [23, 24].
It has been well established that water sorption significantly degrades the d/r interface [25, 26]. However, since dentin is hydrated and intrinsically moist, manufacturers have incorporated increasing concentration of hydrophilic monomers in the bonding systems to increase their infiltration into dentin [27]. The highly hydrophilic adhesive resins can act as semi-permeable membranes that allow intrinsic and extrinsic water exchanges at the d/r interface. Intrinsic water from dentin compromises polymerization of the infiltrating resin, making it more prone to degradation [28]. The result of resin degradation is that the previously resin-infiltrated collagen matrix is exposed and becomes vulnerable to attack by proteolytic enzymes [29]. Additionally, the adhesives cannot displace the free and loosely bound water from the collagen interfibrillar spaces [30, 31]. Intrinsic water that accumulates at the d/r interface results in another internal biodegrading mechanism of resin composite, the so-called nanoleakage [32]. Extrinsically, the semipermeable adhesive resin enables penetration of oral fluids, enzymes, and acidic bacterial products through the d/r interface, which further undermines the restoration and eventually leads to gap formation and recurrent carious lesions [33].
As mentioned above, biofilm accumulated around resin composite restorations can cause recurrent caries by colonizing the exposed dentin surfaces at the compromised interface, which eventually leads to replacement of restorations. Prior to that, the activity of the bacteria can also accelerate degradation of the bulk and interfacial restorative materials, and demineralize tooth tissues. The bacterial production of organic acids lowers the pH of the oral microenvironment, eroding the hydroxyapatite in enamel and dentin, and catalyzing hydrolysis of the adhesive [34]. The latter is also aided by esterases secreted by the bacteria. The resulting exposure of the soft underlying collagenous dentinal matrix allows further infiltration by the pathogenic biofilm [34, 35]. Thus, incorporating antimicrobial agents at the d/r interface might prevent its colonization by biofilm and hinders recurrent caries, even if the d/r interface fails.
There have been several attempts to reduce the hydrolytic degradation of resin composite materials [36] [37] [38] as well as the bacterial burden and bacteria-mediated biodegradation [39] [40] [41] [42] at the d/r interface. However, the prevention of either waterborne or bacteria-mediated degradation have not been completely solved yet. Furthermore, previous strategies have not provided a reliable technology for translation to the clinics because of unrepresentative experimental models or inability to simultaneously address water- and bacteria-mediated degradation of d/r interfaces.
Here, we present a 2-tier protective system for resin composite restorations consisting of amphipathic antimicrobial peptides (AAMPs) used simultaneously as modulators of dentin hydrophobicity and anti-biofilm agents at the d/r interface. The ultimate goal of this biomolecular technology is to increase the degradation resistance and, thus, service life of resin composite restorations by delaying, if not preventing altogether, the occurrence of recurrent caries.
The first tier of protection relies on the selection of strong amphipathic peptides to coat dentin. Amphipathic peptides contain hydrophilic and hydrophobic amino acid residues that, upon molecular arrangement, can be positioned on opposite sides of the molecule. This structural arrangement confers the amphipathic properties to the molecule [43]. This confers dual hydrophobic and hydrophilic characters to the peptide. We hypothesize that by proper organization of the molecules in relation to the dentin surface, the amphipathic peptides can produce a highly-hydrophobic dentin surface at the d/r interface. We further hypothesize that AAMPs-coated hydrophobic dentin will prevent water diffusion along and across the d/r interface, providing protection against degradation of dentin and restorative materials mediated by water and waterborne degrading agents.
The second tier of protection relies on the selection of antimicrobial peptides to coat dentin. Cationic host defense antimicrobial peptides have received strong interest as potentially new antibacterial therapeutics [44], representing an alternative to antibiotics to which bacteria have become adaptive and resistant [45, 46]. Thousands of antimicrobial peptides have been identified and catalogued [47] but only a few hundreds have shown anti-biofilm activity [48]. A much lower number of those have proven effective against pathogenic biofilms related to oral diseases, e.g., periodontitis and peri-implantitis. We hypothesize that AAMPs-coated dentin will hinder cariogenic biofilm growth at the d/r interface.
Noteworthily, many antimicrobial peptides are also amphipathic to a certain degree. This is because their cationic charges and amphipathic conformation allow increased interaction with the negatively charged and lipid-rich bacterial membranes. These peptides then have access to bacterial membranes and intracellular targets [44]. Among the available AAMPs, we have selected for this study the 13-aminoacid GL13K peptide and its D-enantiomer, D-GL13K, to coat dentin surface and test the aforementioned hypothesis. GL13K was derived from a natural sequence of the salivary protein BPIFA2 (previously known as Parotid Secretory Protein, PSP) [49-51]. Both GL13K and D-GL13K contain 31%/54% hydrophilic/phobic residues and have exhibited excellent antibacterial activity against planktonic and biofilm bacteria, covering Gram-positive and Gram-negative representatives [52] [44, 51]. We have reported that GL13K immobilized on metallic surfaces through covalent attachment forms a highly hydrophobic coating with high bactericidal and anti-biofilm activity against oral pathogens and early colonizers of the oral tissues [53, 54]. GL13K also has a favorable toxicity profile [52, 54] with GL13K-coated titanium surfaces showing no cytotoxic effects on osteoblasts and fibroblasts [53]. Meanwhile, the all-D-amino acid version, D-GL13K has been reported to be protease-resistant with notable antimicrobial potency against Enterococcus faecalis and Streptococcus gordonii [51, 52].
Materials and Methods
Peptide Synthesis
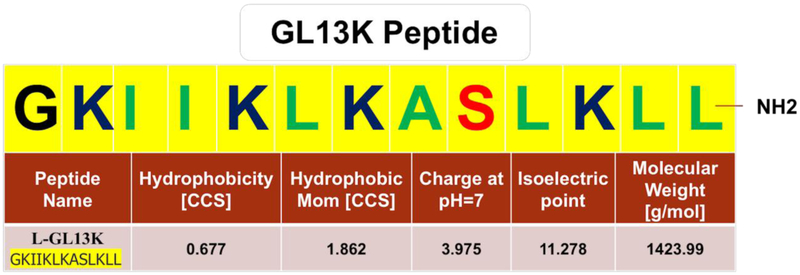
GL13K (GKIIKLKASLKLL-NH2) (Figure 1) and D-GL13K (Gkiiklkaslkll-NH2) peptides were synthesized (purity >98%) by Advanced Automated Peptide Protein Technologies (AAPPTec, Louisville, KY, USA) using solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) chemistry and delivered as lyophilized powder.
Figure 1. Amphipathic antimicrobial peptide, GL13K.
Amino-acid sequence of the GL13K peptide showing hydrophobic (green) and hydrophilic (red) residues, including positively charged amino acids (dark blue). Reported physical and chemical properties of GL13K peptide were obtained from the BaAMPs-Biofilm-active AMPs database (http://www.baamps.it/browse?task=peptide.display&ID=106, last access September 04, 2018).
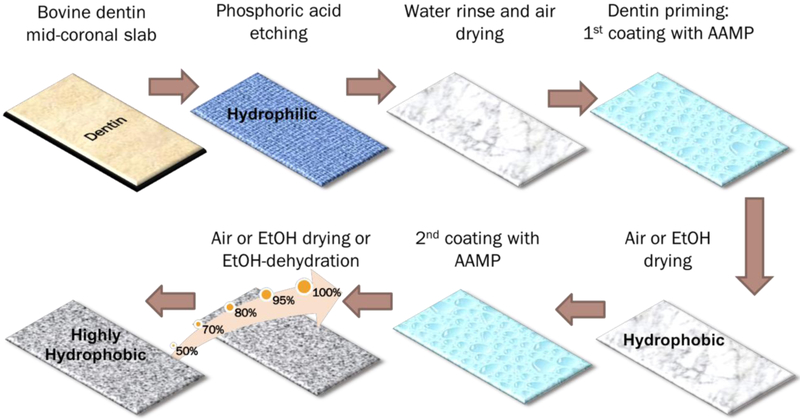
Peptide Coatings on Dentin (Figure 2)
Figure 2. Peptide coatings on dentin.
Schematics of the different procedures for dentin conditioning/coating with amphipathic peptides. See text for detailed description of sample preparation. AAMP: amphipathic antimicrobial peptide; EtOH: absolute ethanol solution.
Bovine incisors were stored in 0.1% thymol solution at 4 °C before being used. Coronal dentin slabs were cut using a diamond saw (Isomet™, Buehler, Lake Bluff, IL, USA). Each crown was split through its mid-dentin region resulting in two dentin-faced slabs lined by either enamel or the pulp chamber (Cut dentin). Then, the dentin slabs were total-etched with 32% phosphoric acid gel (Scotchbond™ Universal Etchant, 3M, St. Paul, MN, USA) for 15 s, rinsed with water for 10 s, and gently air-dried for 10 s (Etched dentin).
GL13K solutions at 1 mg/ml concentration were prepared by re-suspending the lyophilized peptide powder in Na2Co3 buffer solution (pH = 9.5). The dentin slabs were incubated with a thin layer of GL13K solution at 37 °C for 5 min and dried by gentle airstream for 60 s (Single coating) or rinsed with absolute ethanol for 10 s (Single coating-EtOH). Dentin slabs with two coatings of GL13K were obtained by repeating the above procedures on single-coated dentin slabs (Double coatings and Double coatings-EtOH). An alternative method for drying the samples was also tested. Peptide double-coated dentin samples were rinsed with a series of water solutions with increasing ethanol concentration (50%, 70%, 80%, 95% and 100%) for 20 s each (Double coatings-Prog.EtOH). Control samples were Cut dentin, Etched dentin, and dentin slabs treated with Na2CO3 buffer solutions following all of the previously described protocols in the absence of GL13K.
Resistance of Peptide-Coatings on Dentin to Ultrasonication and Saliva Mediated Challenges
Peptide coatings resistance to degradation was determined by ultrasonication and exposure to saliva, respectively. Peptide-coated and control dentin samples were challenged by ultrasonication (S-30, Sonicor INC, West Babylon, NY, USA) in water for 15 min; and by incubation, at 37 °C, in freshly collected saliva on a daily basis for three consecutive days. Unstimulated saliva was collected passively for around 20 min into a conical glass tube on ice. Eating, drinking, and applying oral hygiene procedures were refrained for at least 1 hour prior to saliva collection. Samples exposed to the different challenges were dried by gentle airstream for 60 s before further characterization.
Hydrophobicity of Peptide-coated Dentin
Hydrophobicity of GL13K-coated and control dentin slabs was determined using a contact angle meter (DM-CE1, Kyowa Interface Science, Niiza-City, Japan) before and after ultrasonication and saliva-mediated challenges. Dynamic water contact angles (WCA) were determined using the sessile-drop method. Two μl drops of de-ionized water were dispensed on the tested surfaces and their dynamic wetting was tracked for 21 s at a frequency of 1 Hz. We used the FAMAS software (Kyowa Interface Science, Niiza-City, Japan) to capture water drop images, to detect drop profiles, to measure WCA, and to measure dispensed and remaining water volume on the tested surfaces.
Impermeability and Acid Resistance of Peptide-coated Dentin
We determined the impermeability of peptide-coated dentin by assessing the penetration of a water-based acidic dye through GL13K-coated dentin. We compared the impermeability of peptide-coated dentin to control dentin treated by Na2CO3 buffer. All surfaces of dentin slabs were painted with two layers of acid resistant nail varnish with the exception of the dentin surface that was treated with GL13K peptides (Double coatings-Prog.EtOH) or control buffer solutions. Then, dentin slabs were immersed in cupric sulfate (CuSO4) acidic blue dye (pH=3.4) for 4 hours. The samples were sectioned longitudinally, faciolingually, or transversely and penetration of the blue dye was determined using a Stereo-microscope (MVX10, Olympus, Tokyo, Japan). We also assessed the impermeability of GL13K-coated and control dentin slabs after being challenged with saliva.
Impermeability and Fracture Resistance of Peptide-treated Dentin-composite Discs
Sample preparation and groups tested
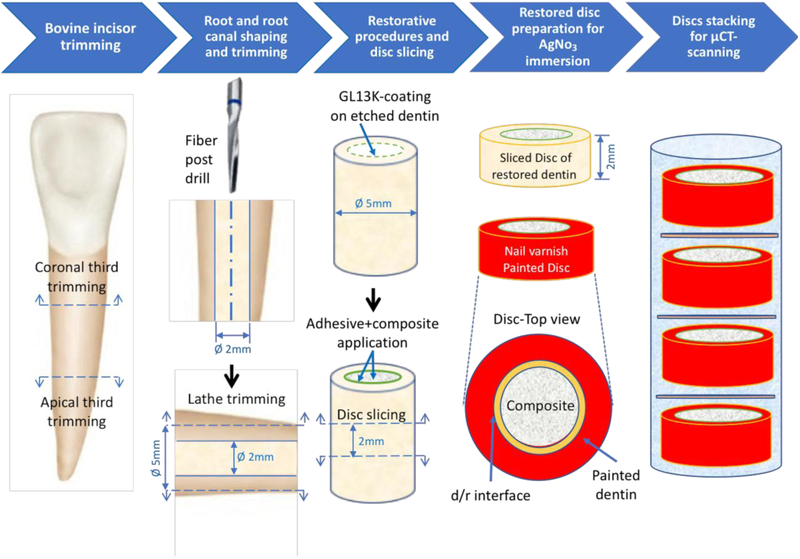
Roots of bovine teeth were used to prepare restored dentin-composite discs (Figure 3). After the crowns and apical thirds of each tooth were cut off, the root canals were enlarged to 2 mm in diameter, using 1.9-mm diameter fiber post drills (RelyX™ Fiber Post, 3M, St. Paul, MN, USA). The outer surface of the roots was trimmed down to 5 mm in diameter using a lathe to remove cementum and external layers of dentin,. The obtained dentin tubes (5 mm in outer diameter and 2 mm in inner diameter) were rinsed and stored in distilled water at 4 °C until the restorative procedures. The radicular dentin tubes were randomly divided into 4 groups (n=7), according to the restorative treatments:
Group C: restored roots using resin Composite with bonding agent. Dentin was not coated with GL13K peptides. Positive control group.
Group CWBA: restored roots using resin Composite Without Bonding Agent. Dentin was not coated with GL13K peptides. Negative control group.
Group C13: restored roots using resin Composite with bonding agent on GL13K-coated dentin. Test group.
Group C13WBA: restored roots using resin Composite Without Bonding Agent on GL13K-coated dentin. Test group.
Figure 3. Method of preparation of restored dentin discs.
The restored discs were used for assessing fracture resistance using the diametral compression test or permeability at the d/r interface using micro-computed tomography (micro-CT, μCT). See text for the detailed description of this method of sample preparation.
All roots were total-etched as described above. Samples in groups C13 and C13WBA were peptide coated following the protocol that produced the most hydrophobic dentin (Double coatings-Prog.EtOH). In samples of groups C and C13, the bonding agent (Scotchbond™ Universal Adhesive, 3M, St. Paul, MN, USA) was scrubbed on the dentin walls for 20 s, dried with gentle air for 5 s, and cured for 10 s using a 1200 mW/cm2 led curing unit (Elipar™ S10, 3M, St. Paul, MN, USA). All samples were finally restored with Filtek™ Z250 Universal Restorative (3M, St. Paul, MN, USA) in 2 mm increments by condensing the composite vertically and against the dentin walls with a plastic dental plugger. The composite was cured for 20 s. The restored dentin cylinders were sliced using a pre-calibrated template into 2-mm thick dentin-composite discs and were stored in distilled water at 4°C until testing.
Impermeability of Dentin/Composite Interfaces
One disc from each root was selected to obtain 7 tooth-independent discs per group, so that we overcome potential tooth dependency concerns [55]. The discs were double painted with a nail polish except for both sides of the composite filling and a 1-mm circumferential perimeter of dentin ensuring complete exposure of the d/r interface (Figure 3). The specimens were submerged in a radiopaque silver nitrate solution (AgNO3, 50% w/w) for 2 hours in a light protected environment and then, thoroughly rinsed.
The volume of dye leakage along d/r interfaces was visualized and quantified using X-ray micro-computed tomography (micro-CT) (XT H 225, Nikon Metrology Inc., Tokyo, Japan). Samples were piled in stacks of 4 for micro-CT scanning (Figure S1) inside a transparent tube mounted on a custom made Teflon™ ring. This ring is used to securely screw the samples to the sample holder inside the micro-CT chamber to avoid sample movement during scanning. Each group of 4 stacked discs was scanned with the following parameters: 95 kV, 105 μA, 720 projections and 4 frames per projection. The 3D spatial reconstructions were done with CT Pro 3D (Nikon Metrology, Brighton, MI, USA). The processed 3D files were visualized with VG Studio MAX 2.1 (Volume Graphics GmbH, Heidelberg, Germany). We specified a region of interest around the interfaces to quantify the leaked dye exclusively within this region. Also, the leaked dye volume along the interface was quantified from top to bottom for comprehensive unbiased evaluation.
We assessed the degradation resistance of peptide coatings and its effects on the impermeability at peptide-coated d/r interfaces. We aged the restored discs for 3 months in water followed by 2,500 thermal cycles (Thermo Neslab EX-7 circulating bath, Marshall Scientific, Hampton, NH, USA) between 5°C-55°C with 30 s dwell time and 5 s transfer time. The aged samples were immersed in the radiopaque dye, scanned, visualized and quantified for volume of leaked dye at the d/r interfaces.
Fracture Resistance of Peptide-treated Dentin-composite Discs
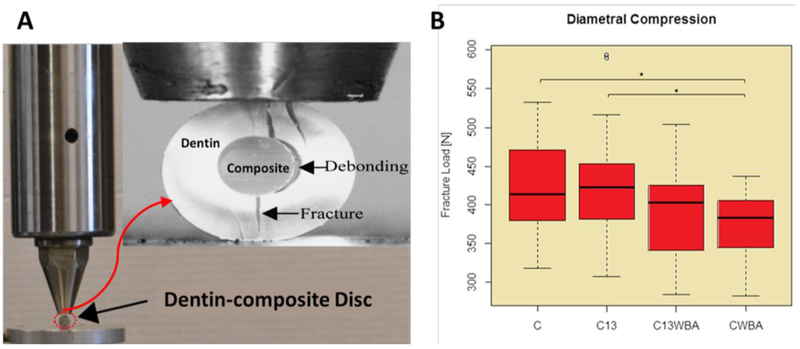
The aforementioned radicular dentin-composite discs (Figure 3) were tested using diametral compression for investigating their fracture resistance with and without GL13K coating. Teeth roots were randomly assigned to be restored according to the previously described four restorative procedures. Twenty-two discs in each group were prepared for assessing their fracture resistance. The diametral compression test was carried out using a Universal Mechanical Test Machine (858 Mini Bionix, MTS, Eden Prairie, MN, USA), with the disc located between two flat and parallel steel components (Figure 6A). The compression load was applied in stroke-control mode at a cross-head speed rate of 0.5 mm/min to fracture the samples. The load and displacement time histories were recorded during the loading process. Most of the discs deformed linearly with increasing load until fracture took place.
Figure 6. Fracture resistance of restored dentin discs.
A) Diametral compression test setting; B) boxplots of fracture loads of the restored dentin discs prepared with different treatments (n=22). C: restored roots using composite with bonding agent; C13: restored roots using composite with bonding agent on GL13K-coated dentin; C13WBA: restored roots using composite without bonding agent on GL13K-coated dentin; CWBA: restored roots using composite without bonding agent. Ends of horizontal bars connect groups with statistically significant different fracture loads (*, p-value < 0.05). Circles denote moderate outliers.
Antimicrobial Potency of Peptides and Peptide-coatings
Peptides and Bacteria tested
For assessing antimicrobial activity of peptides and peptide coatings we used D-GL13K peptides. D-GL13K peptides have demonstrated higher antimicrobial activity and lower bacterial resistance than GL13K peptides [51]. D-GL13K has the same charge and amphipathic properties as GL13K and thus, the two peptides produced coatings with notably similar hydrophobicity (see results section).
Dental clinical plaque samples were collected from 10 caries active subjects following procedures approved by the University of Minnesota Institutional Review Board (Study #1403M48865) and used here by courtesy of Dr. Robert S. Jones. Plaque was immediately transferred to a 10 ml pre-reduced Brain-Heart Infusion medium (BBL Brain Heart Infusion, Becton, Dickinson and Co, Sparks, MD, USA) with no exogenous carbohydrates added. This inoculum was transferred to an anaerobic chamber, and initial stocks of the ten subjects were made after 24 hours of growth as described elsewhere [56].
Biomass adherence was measured for each subject using a microtiter plate biofilm assay with crystal violet (CV) staining. The plaque sample with the highest adherence (data not shown) was selected to assess the antimicrobial and antibiofilm potency of peptides and peptide coatings.
Minimal Inhibitory Concentration (MIC)
Planktonic bacteria were grown anaerobically in modified BHI medium (BHI; RM188, HiMedia, West Chester, PA, USA) (Table S1) overnight. The bacterial suspension was adjusted to optical density, (OD)600 = 0.2±0.01 and then diluted 1:20. D-GL13K was dissolved in 0.01% acetic acid and added to sterile 96-well polypropylene microtiter plates at increasing 2-fold serial dilutions (0-512 μg/ml). Modified BHI medium without peptides was used as a control. Plaque bacteria were inoculated to a final concentration of 5.0×105 CFU/ml per well. The plates were incubated at 37 °C for 24 hours under continuous shaking at 200 rpm. After 24 h of peptide treatment, absorbance at 600 nm was measured using a microtiter plate reader (Bio-Tek Instruments, Winooski, VT, USA). The MIC was determined as the lowest concentration of peptide at which no growth of bacteria was detected [57].
Antibiofilm Potency of D-GL13K in Solution
Five ml of modified BHI medium was inoculated with bacteria, incubated overnight and the final inoculum was adjusted as mentioned above. Sterile 48-well non-treated polystyrene plates were used with modified BHI media, without peptides, as a control. For testing biofilm inhibition potency of peptides in solution, three different D-GL13K concentrations (100 μg/ml, 50 μg/ml, 25 μg/ml; n= 6) were added to the plaque suspension at the beginning of biofilm development, incubated anaerobically at 37°C for 6 days under shaking. After formation of a 2-day-old biofilm, the medium was replaced with fresh media without peptides. The remaining biofilm was stained with 0.1% CV and then, evaluated qualitatively using an inverted fluorescent microscope (Axiovert 40 CFL, ZEISS, Oberkochen, Germany) and a stereo microscope. After dissolving the stained biofilm in 30 % acetic acid, the biomass was quantified using a microtiter plate reader at OD 550 nm.
Antibiofilm Potency of D-GL13K Coatings on Hydroxyapatite Discs
Hydroxyapatite (HA; Clarkson Chromatography, South Williamsport, PA, USA) discs were first autoclaved. Control discs were etched using 32 % phosphoric acid gel for 15 s followed by 10 s water-rinse and 10 s air-dry. Peptide-coated discs were total-etched and then, double coatings were applied at a concentration of 1 mg/ml followed by air-dry for 60 s. Non-treated 24-well polystyrene plates were marked for peptide-coated and control groups (n=12 for each group). In each well, the disc was inoculated with 200 μl of the aforementioned adjusted inoculum and then 1.8 ml of fresh modified BHI media was added. The plate was incubated for 48 h at 37 °C in the anaerobic chamber under shaking. Measurements of remaining bioburden and Live/Dead viability assay were performed to determine the antibiofilm potency of the peptide-coatings.
Qualitative and Quantitative Analysis of the Remaining Bioburden
Each HA disc was washed with 1XPBS by immersion once followed by rinsing to remove the unattached bacteria. The discs were stained with 0.1 % CV solution for 15 min at room temperature. The excess CV satin was washed off with Milli-Q® ultrapure water. For quantitative analysis, the stained biofilm was solubilized in 30 % acetic acid and measured at OD 550 nm using a microtiter plate reader.
Live/Dead Viability Assay
The HA discs were gently submerged into 0.9% NaCl to remove the unattached bacteria. Working solution of fluorescent stains was prepared for Live/Dead assessment (L7012, LIVE/DEAD BacLight Bacterial Viability Kit, ThermoFisher Scientific, Waltham, MA, USA) by adding 3 μl of SYTO® 9 green stain and 3 μl of Propidium iodide red stain to 1 ml of sterilized Milli-Q® ultrapure water. 100 μl of staining solution was added very gently so as not to disturb the biofilm. After 20–30 min in light-protected incubation at room temperature, the discs were examined using 40x water immersion lens with an upright fluorescence microscope (Eclipse E800, Nikon, Tokyo, Japan).
Statistical Analysis
Statistical analysis was performed using R software, version 3.3.2 (64-bit), for statistical computing and graphics (supported by the R Foundation for Statistical Computing) [58]. Parametric statistical tests were applied after assessing data normality using Q-Q plot. Means and standard deviations of WCA as well as quantified biofilm values were calculated and analyzed by two-tailed two-sample t-test. α = 0.05 was used as cutoff for statistical significance. For leaked dye volume and fracture loads, univariate ANOVA and post hoc multiple comparisons were applied to isolate and compare the significant results at a 5% significance level.
Results
Hydrophobicity of Peptide-coated Dentin
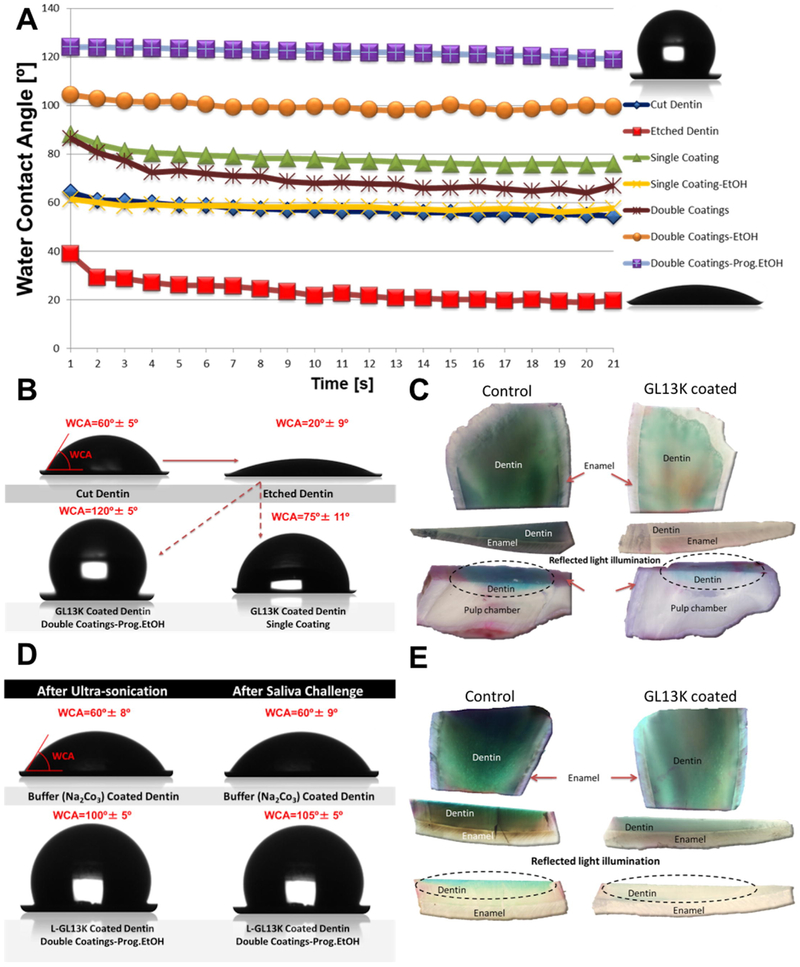
Results on the wettability of etched dentin coated with GL13K are presented in Table 1, Figures 4 A and Figure 4 B. WCA of Cut dentin was 60° ± 5°. Etched dentin became notably hydrophilic with WCA decreasing to 20° ± 5° and ΔWCA being 25° over 21 s. All methods of coating dentin with GL13K resulted in a significant increase in its hydrophobicity and decrease of ΔWCA compared to Etched dentin. WCA values for GL13K-coated dentin varied from 55° ± 9° for Single coating-EtOH to 120° ± 5° for highly hydrophobic Double coatings-Prog.EtOH. Noteworthily, one of the shortest and simplest dentin treatments, Single coating, produced a hydrophobic dentin surface with WCA = 75° ± 11°. When all other treatment conditions were the same, peptide-coated dentin was significantly more hydrophobic than dentin treated with the control buffer solutions (with no peptides). In general, using ethanol as a rinsing/drying agent after peptide coating decreased values of ΔWCA compared to using water. Single and Double coatings of D-GL13K peptides on both bovine and human dentin also produced highly hydrophobic dentin surfaces with WCA ranging 95.9° ± 4.9° - 99.7° ± 6.6° (Table S2).
Table 1: Water contact angles on bovine dentin treated with different conditioning and peptide-coating protocols.
Columns from left to right; Etch: 32% phosphoric acid etched dentin; EtOH: 100% ethanol rinse; 1Coat: 1st peptide coating; 2Coat: 2nd peptide coating; H2O: water rinse; Prog.EtOH: progressive alcohol dehydration (50%, 70%, 80%, 95% and 100% ethanol); WCA±S.D.: final water contact angle ± standard deviation (n=7); Δ WCA: average variation of WCA over time (21s). Rows, from top to bottom; Cut Dentin: freshly cut coronal dentin; Etched Dentin: dentin etched with 32% phosphoric acid; Buffer-1coat- H2O: 1st buffer (Na2CO3) coating followed by water rinse; Buffer-1coat- EtOH: 1st buffer (Na2CO3) coating followed by 100% ethanol rinse; Buffer-2coats-2C-Prog.EtoH: 2nd buffer (Na2CO3) coating followed by progressive alcohol dehydration (50%, 70%, 80%, 95% and 100%); GL13K-1coat-EtOH (Single coating-EtOH): 1st GL13K coating followed by 100% ethanol rinse; GL13K-1coat-H2O (Single coating): 1st GL13K coating followed by water rinse; GL13K-2coat-H2O (Double coatings): double GL13K coating followed by water rinse; GL13K-2coats-EtOH Priming: double GL13K coating preceded by 100% ethanol priming; GL13K-2coats-1C-EtoH: double GL13K coatings separated by 100% ethanol rinse; GL13K-2coats-2C-EtoH (Double coatings-EtOH): double GL13K coatings followed by 100% ethanol rinse; GL13K-2coats-2C-PrEtoH (Double coatings-Prog.EtOH): double GL13K coatings followed by progressive alcohol dehydration (50%, 70%, 80%, 95% and 100%).
| Treatment | Pre-coating (conditioning) |
1st coating | 2nd coating | WCA±S.D. [°] |
Δ WCA [°] |
Image | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Etch | EtOH | 1coat | EtOH | 2coat | H2O | EtOH | Prog.EtOH | ||||
| Cut Dentin | 60±5 | 6 | |||||||||
| Etched Dentin | X | 20±9 | 25 | ||||||||
| Buffer-1coat- H2O | X | X | X | 45±11 | 20 | ||||||
| Buffer-1coat- EtOH | X | X | X | 45±10 | 5 | ||||||
| Buffer-2coats-2C-Prog.EtoH | X | X | X | X | 60±12 | 18 |  |
||||
| GL13K-1coat-EtOH (Single coating-EtOH) | X | X | X | 55±9 | 5 |  |
|||||
| GL13K-1coat-H2O (Single coating) | X | X | X | 75±12 | 15 |  |
|||||
| GL13K-2coat-H2O (Double coatings) | X | X | X | X | 70±11 | 17 |  |
||||
| GL13K-2coats-EtOH Priming | X | X | X | X | 80±8 | 12 |  |
||||
| GL13K-2coats-1C-EtoH | X | X | X | X | 90±8 | 8 |  |
||||
| GL13K-2coats-2C-EtoH (Double coatings-EtOH) | X | X | X | X | 100±8 | 12 |  |
||||
| GL13K-2coats-2C-PrEtoH (Double coatings-Prog.EtOH) | X | X | X | X | 120±5 | 4 |  |
||||
Figure 4. Hydrophobic dentin and its impermeability before and after chemical, mechanical and saliva-mediated challenges.
A) Dynamic water contact angles (WCA, average value, n=7) for cut and etched bovine dentin (controls) and dentin treated with amphipathic peptides following different protocols (see materials and methods section for description of the different dentin treatments); B) sessile water drop images and final WCA (average ± standard deviation, n=7) on bovine dentin before (cut and etched) and after peptide treatments; C) dentin impermeability: copper sulfate acidic dye penetration at the superficial and pulp overlaying dentin visualized using transmitted or reflected light illumination; D) sessile water drop images and final WCA (average ± standard deviation, n=5) on buffer treated and peptide coated bovine dentin, before and after ultrasonication and saliva challenges, E) dentin impermeability after saliva-mediated challenges: copper sulfate dye penetration through dentin visualized using transmitted or reflected light illumination.
Impermeability of Peptide-coated Dentin and Resistance to Ultrasonication, Acid, and Saliva-mediated challenges
We evaluated acidic blue dye penetration through dentin on peptide-coated and control buffer-treated (without peptides) dentin samples. The dye thoroughly penetrated through the surface into deeper areas of control dentin compared to peptide-coated dentin. The dye penetration was hindered in peptide-coated dentin superficially and deeply close to the pulp chamber, where dentinal tissue has large dentinal tubules (Figure 4 C).
Dentin hydrophobicity was maintained after GL13K coatings were challenged by ultrasonication in water for 15 min, WCA = 100° ± 5°; and exposure to fresh saliva for three days, WCA = 105° ± 5° (Figure 4 D and Table S3). Also, peptide-coated dentin maintained its resistance to acidic dye penetration (Figure 4 E).
Impermeability of the Peptide-coated Dentin/Composite Interface
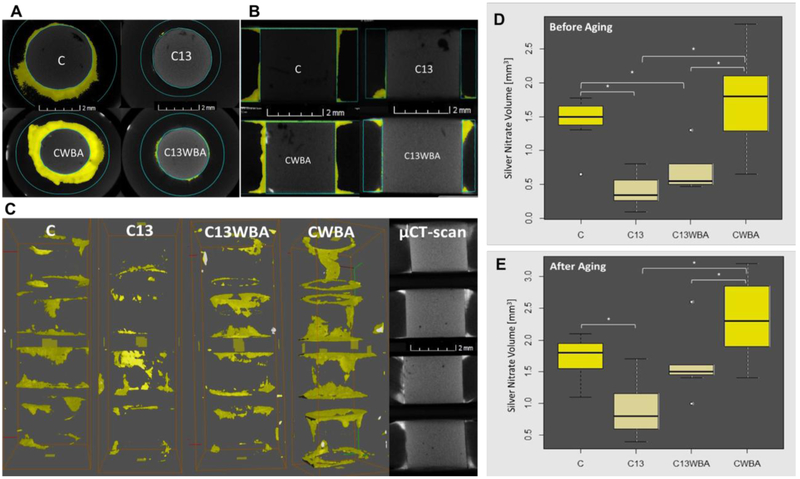
We quantified the volume of radiopaque dye that leaked through d/r interfaces using micro-CT, before and after aging in water for 3 months and 2,500 thermal cycles (Figure 5). Visual evaluation was conducted from the top view (Figure 5 A), side view (Figure 5 B) as well as the 3D rendering (Figure 5 C) of restored discs. Leaking of the dye was notably hindered in the peptide-coated d/r interfaces (C13, C13WBA) compared to control non-coated d/r interfaces (C, CWBA). Before aging, the volume of leaked dye through the highly hydrophobic GL13K-coated d/r interfaces was significantly lower than through non-coated d/r interfaces for both types of restorations; i.e., restorations with (C vs C13, p-value = 0.0009) and without (C13WBA vs CWBA, p-value = 0.0008) application of dental adhesive/bonging agent (Figure 5 D). Aged GL13K-coated d/r interfaces also significantly reduced the volume of leaked dye compared to non-coated d/r interfaces in both types of restorations with (C vs C13, p-value = 0.02) and without (C13WBA vs CWBA, p-value = 0.04) bonding agent (Figure 5 E).
Figure 5. Micro-CT analysis of impermeability at the dentin-composite interface.
(A) Top view and (B) side view of representative restored bovine dentin discs for each tested group showing silver nitrate (AgNO3) dye leakage (converted to yellow for easier visualization); (C) one representative stack of four restored dentin discs for each tested group showing the 3D rendering of the dye that leaked through dentin/composite interfaces and reconstruction of the micro-CT scans for one representative CWBA whole stack (last column). Quantification of the penetrated AgNO3 volume along dentin-composite interface (n=7) before (D) and (E) after aging by water storage and thermal cycling. C: (restored roots using composite with bonding agent; C13: restored roots using composite with bonding agent on GL13K-coated dentin; C13WBA: restored roots using composite without bonding agent on GL13K-coated dentin; CWBA: restored roots using composite without bonding agent. Ends of horizontal bars connect groups with statistically significant different silver nitrate penetration volume (*, p-value < 0.05). Circles denote moderate outliers.
Fracture Resistance of Peptide-coated Dentin-composite Discs
We assessed the fracture resistance of restored dentin discs with and without GL13K coatings using the diametral compression test (Figure 6). Fracture loads for restored discs with and without bonding agents were not significantly different between peptide-coated and non-coated discs (C vs C13, p-value = 0.91 and CWBA vs C13WBA, p-value =0 .81) (Figure 6 B). GL13K-peptide coated restorations with bonding agent, C13 had the highest fracture loads (427 N ± 72 N) of all restored disc groups. Notably, restorations prepared without bonding agent did not significantly reduce fracture loads for the discs with hydrophobic peptide coated dentin (C13 vs C13WBA, p-value = 0.21) but significantly reduced fracture loads for the discs without peptide coatings (C vs CWBA, p-value = 0.04).
Antibiofilm Potency of Peptides and Peptide coatings
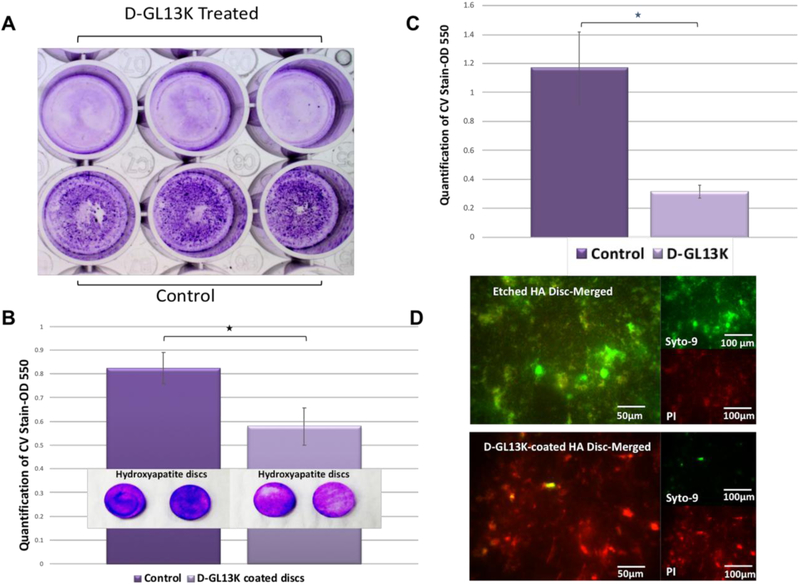
We assessed the MICs of D-GL13K against dental plaque samples from caries active individuals by measuring the absorbance at 600 nm after 24 hours of peptide treatment. Consistently, D-GL13K inhibited the bacterial growth down to a concentration of 1 μg/ml. We also assessed the antibiofilm potency of D-GL13K against the multispecies biofilm grown on microtiter plates (Figure 7 A). The results showed that the solution of 25 μg/ml D-GL13K significantly reduced plaque biofilm bio-volume (n=16, p-value = 0.004) by near 4-fold after 144 hours of treatment (Figures 7 A and B). The reduction of the remaining bioburden between test and control groups was also statistically significant after peptide immobilization, 1mg/ml D-GL13K, on HA discs (D-GL13K-coated HA discs) (n=6, p-value=0.014) (Figure 7 C). Likewise, live and dead viability assay showed notable differences between D-GL13K-coated discs and controls (Figure 7 D). The images showed that most of biofilm cells on control discs were alive (green) whereas most of cells were dead (red) on D-GL13K-coated discs (Figure 7 D).
Figure 7. Antibiofilm potency of D-GL13K against multispecies biofilm derived from cariogenic dental plaque.
A) Stereomicroscope image of 6-day-old plaque biofilm covering the microtiter plate and stained with crystal violet dye without (bottom) or with (top) 25 mg/ml peptide treatment. B) Quantification of biofilm biovolume shown in A), C) Qualitative and quantitative assessments of the remaining bioburden on HA discs with (right) and without (left) D-GL13K coating, D) Merged live (green) and dead (red) viability assay images of 48-hour multispecies biofilm grown on HA discs with (bottom image) and without (top image) D-GL13K coating. Merged images showing bacteria stained with both SYTO-9 (live bacteria, green) and PI (dead bacteria, red). * denotes statistically significant differences (p-value < 0.05) between groups connected by horizontal bars.
Discussion
Dental caries is a highly prevalent infectious disease that is widely treated with resin composite restorations. These restorations currently have limited lifespan, mainly due to the occurrence of recurrent caries [18]. Intrinsic and extrinsic water, together with waterborne agents such as acids and enzymes, degrade materials at the d/r interface leading to formation of interfacial microgaps [20]. Once the microgaps form, bacteria can colonize and form a biofilm at the d/r interface and eventually re-infect the dentinal tissues. Other investigators have attempted to improve the performance of resin composite restorations by either preventing their degradation [36] [37] [38] or providing them with antimicrobial properties [39] [40] [41] [42], but strategies that confer simultaneous protection against both material degradation and bacterial reinfection have not been explored to date. Our hypothesis was that coating/priming dentin with amphipathic and antimicrobial molecules, such as AAMPs, provides the d/r interface with a 2-tier protective system; i.e., it hinders water penetration and, thus, waterborne degradation and confers antibacterial potency to the coated tissue. Here, we successfully developed hydrophobic dentin made of AAMPs coatings and proved their antibacterial properties against multispecies biofilms.
1st Tier of Protection: Hydrophobic and Impermeable GL13K-coated Dentin
The intrinsic high hydrophilicity of dentin has driven the necessary development of hydrophilic resin primers/adhesives that properly interact with etched dentin to obtain a mechanically sound dentin-restoration bonding. However, the water affinity of these resins leads to fast degradation of the materials forming the d/r bonding interface. Thus, developing methods to obtain hydrophobic dentin surfaces that would hinder this degradation and will enable the use of hydrophobic restorative resins is needed.
Etching is required in the procedures of resin composite restorations for enhancing resin infiltration into the network of partially-demineralized collagen fibers to form the so-called hybrid layer. After etching dentin was highly hydrophilic (Figure 4 A and B, Table 1) as dentin contains water and the structure of the etched dentin is highly porous with high surface area. We recorded high ΔWCA for etched dentin due to both its affinity for water and water absorption into the nano- and microporous structure of the etched partially-demineralized dentin.
All GL13K coating protocols on etched dentin produced hydrophobic dentin surfaces (Table 1, Table S2). GL13K peptides are cationic, amphipathic, and fold to secondary structures with increasingly ordered conformations in solutions with increasing pH [44]. The cationic polar groups in a GL13K molecule are free amines located in four lysines distributed along the amino acid sequence and an additional amine in the N-terminus of the peptide. These groups have high affinity for the hydrophilic/polar and negatively-charged etched dentin [59]. The strong interaction of the amine groups with dentin can favor the stable amphipathic conformation of the molecules by organizing and exposing non-charged apolar amino acids at the dentin/air interface; i.e., away from the polar and charged dentin surface. The authors have previously shown transformation of GL13K from unordered to ß-sheet and α-helix conformations when the peptides interacted with an analogous substrate; i.e., negatively-charged bacterial model membranes [44]. Here, the end result of these cooperative polar and electrostatic interactions between dentin and GL13K peptides was the formation of a stable hydrophobic coating on etched dentin. Additionally, ΔWCA for GL13K-coated dentin were significantly smaller than for etched dentin. This suggested that GL13K peptides were thoroughly and homogeneously distributed on dentin so that the peptides not only imparted hydrophobicity to the tissue but also hindered penetration of water through the dentin surface and into its porous structure. However, the time for reaching stability of the WCA was not the same among the different hydrophobic coating protocols, which might indicate relevant effects of the number of coatings and/or the rinsing method on the initial stability of the peptides in these coatings.
We tested different protocols for coating dentin with GL13K peptides to obtain the highest hydrophobicity (Table 1). Our hypothesis was that substituting water with absolute ethanol to rinse GL13K-coated dentin produces a quicker and more efficient dehydration of the tissue that results in higher WCA and lower ΔWCA. Using ethanol instead of water reduced ΔWCA, but did not increase WCA. This was consistent with the collapse and shrinkage of the collagen fibers in demineralized dentin upon alcohol rinsing [60] that might have reduced dentin porosity and number of GL13K molecules exposed at the dentin-air interface. Double coatings were further applied to increase the number of peptide molecules exposed at the dentin-air interface, which resulted in increasing dentin hydrophobicity. Finally, we tested a progressive dehydration rinsing protocol that applied a series of solutions with increasing ethanol concentration. This protocol had the purpose of suspending the demineralized GL13K-coated collagen matrix in its dehydrated fully-extended state [36, 60], maximizing the peptide-coated tissue exposed at the dentin-air interface. The Double coatings-Prog.Et-OH protocol produced the most hydrophobic dentin with WCA = 120° ± 5° and ΔWCA = 4°. The high hydrophobicity was a result of combining the peptides with an extended collagen matrix as this high hydrophobicity was not obtained in control groups exposed to the buffer solution with no peptides and rinsed using the same progressive dehydration protocol, WCA=60° ± 12 and ΔWCA = 18°. Although applying a double 5 min coating with progressive dehydration alcohol rinse is not clinically feasible, it provided a reference for the maximum dentin hydrophobicity displayed with GL13K peptides. Notably, a single 5 min GL13K coating with water rinse also produced a hydrophobic dentin with WCA = 75 ° ± 5° and ΔWCA = 15°. Further, we produced highly hydrophobic bovine and human dentin with a single 1 min coating of D-GL13K, the all D-amino acid enantiomer of GL13K, WCA ~ 100° and AWCA = 5° (Table S2). The authors have recently demonstrated that D-GL13K not only has a higher antimicrobial potency than GL13K but also has a higher propensity to transform into ordered supramolecular structures [61]. The differences in the molecular structure between GL13K and D-GL13K that affect transformation and activity of these peptides are still to be discerned, but the use of D-GL13K peptides might ease the translation of this innovative dentin priming protocol to the clinical scenario.
Hydrophobic dentin was impermeable to acidic dye penetration (Figure 4C). This is relevant because dental caries progression is catalyzed by acidogenic bacteria. The acids produced by these bacteria demineralize dental hard tissues and degrade restorative resins [62]. The GL13K-coated dentin was significantly impermeable on dentin adjacent to both enamel and the pulp chamber, i.e., irrespective of the diameter of the dentinal tubules. It is worth noting that dentin tubules are wider in bovine teeth than in human teeth [63]. The impermeability of GL13K-coated dentin might also be of relevance to prevent elution of irritant monomers to the pulp chamber, which can provoke severe post-operative sensitivity with potential irreversible pulpitis [64-66]. This is one of the main limitations to treat deep cavities with resin composite restorations. Hydrophobicity of GL13K-coated dentin was preserved after being challenged by ultrasonication (Figure 1B, and Table S3). This indicated that the peptides remained adsorbed to the surface of dentin after the mechanical challenge. Hydrophobicity and impermeability of GL13K-coated dentin was also preserved after successive cycles of exposure to fresh saliva (Figure 1A and E, and Table S3). Unfiltered fresh human saliva is a digestive hydrolytic media with biodegrading components, including proteolytic enzymes, such as esterases and bacterial byproducts that compromise the d/r interface [24]. Our results support that hydrophobic dentin hindered interactions of waterborne degradative agents at the d/r interface. The water-repellent property of the hydrophobic surface is its main feature for providing this protective mechanism against biodegradation.
2nd Tier of Protection: Antimicrobial D-GL13K Coatings
Inhibiting biofilm formation at the d/r interface is a major strategy to prevent recurrent caries around the margins of resin composite restorations. We verified the antimicrobial and antibiofilm activity of D-GL13K peptide and coatings made of these peptides against clinically-relevant bacterial communities.
The diversity of the oral microflora is high with more than 700 identified species [67, 68]. Management of this diversity to prevent oral infectious diseases is challenging. D-GL13K had very low MIC = 1 μg/ml against planktonic oral multispecies communities derived from active-caries individuals. However, bacteria primarily form biofilms when growing on surfaces or at air-liquid interfaces [69];[70];[71]. The transition from a planktonic to a biofilm structure provides enhanced resistance to the bacterial community against antimicrobials and host defense mechanisms [72] [70, 73]. Consequently, the authors focused on testing the anti-biofilm potency of D-GL13K against multispecies biofilms, a clinically-relevant challenge for resin composite restorations. Our results strongly supported the potency of D-GL13K against multispecies biofilms. Growth of 6-day-old biofilms was significantly inhibited when we treated them with 25 μg/ml D-GL13K solutions (Figure 7 A and B).
However, potent antimicrobials lose most, if not all, their bactericidal and antibiofilm activity when they are immobilized or exposed at the material/air interface. This is because the amount of molecules available for interacting with bacteria can be notably reduced. Moreover, the substantivity of the molecules can be compromised due to their limited mobility or specific conformation/orientation [39]. Here, D-GL13K peptides immobilized on etched HA discs retained potent antibiofilm activity as the remaining bioburden was significantly reduced when compared to non-coated control discs (Figure 7 C). Moreover, most of the biofilm on the peptide-coated discs was composed of dead bacteria (Figure 7 D). These results confirmed our previous findings where coatings of immobilized GL13K on titanium surfaces were notably effective against single-species Gram-positive and Gram-negative bacterial biofilms [53] [54]. Here, as in the case of titanium, our peptide solution had pH=9.5, which is higher than the point of zero charge of HA, 6.8-8.5 [74] but lower than the isoelectric point of the peptide, 11.2 (Figure 1). Thus, electrostatic attraction between D-GL13K molecules and the HA surface is expected as HA surface was negatively charged and D-GL13K peptides were positively charged. The molecule-surface physical attraction promoted thorough recruitment of peptides to the surface as well as their strong retention on it [75], which favored the antibiofilm potency of the D-GL13K coatings.
GL13K-coated Dentin/Composite Interfaces
In a clinical setting, the use of GL13K coatings on dentin should be complemented with the use of restorative components of resin composite restorations; i.e., the bonding agent and resin composite. Here, radicular dentin discs (Figure 3) were used to test our new technology under clinical simulative conditions for d/r interface impermeability, resistance to degradation and fracture resistance of the restoration. The authors previously validated these radicular dentin-composite discs as an ex-vivo model for assessing microleakage along the d/r interface [76] and bond strength of the restoration [77] and adapted these methods to test hypotheses here.
GL13K coatings on dentin significantly resisted penetration of the radiopaque AgNO3 dye along the d/r interfaces of the restored discs with and without an adhesive layer, and after aging (Figure 5). We used a universal adhesive with pH = 2.7 [78], but its acidity did not interfere with or notably degrade the peptide layer. The impermeability of the d/r interface in resin composite restorations without adhesive layer is a remarkable property of our hydrophobic dentin that suggests a thorough and homogeneous coverage of peptide molecules on the dentin surface. We aged the restored dentin discs via water storage and thermocycling by adapting recommendations from the Academy of Dental Materials [55]. Thermocycling is a commonly used aging method [79] [80] [81] as it simulates the thermal changes in the oral cavity resulted from eating, drinking, and breathing [82]. Three months of storage in water is considered a mid-term aging period and ADM recommends a minimum of 10,000 thermal cycles, which others have correlated to 1 year of clinical function [82]; however, our dentin samples didn’t have the peripheral enamel seal, which provides a crucial protection for improving the resin-dentin bond durability [83, 84]. Thus, the aging process we applied here by combining 3 month storage in water + 2,500 thermocycles may have been a demanding in vitro challenge. The high hydrophobicity of the GL13K-coating can have a self-protective effect to prevent its degradation as the peptide degradation would also be mediated by water and waterborne degradative agents. Although the microtensile bond strength test is commonly used in the literature and recommended by the Academy of Dental Materials [54], the diametral compression test on restored dentin discs can also be used to assess interfacial strength of dental restorations. It has the advantages of zero premature failure, simple testing procedures, a consistent failure mode, and reduced variation in the measurements with respect to the more traditional shear and tensile bond strength tests [77]. Still, the diametral compression test should be combined with additional experimental techniques, such as digital image correlation [85] to accurately discern the point of interfacial de-bonding. Thus, we limit the discussion of our results to the analysis of the fracture resistance of the tested restored discs. The application of a peptide hydrophobic coating on dentin did not reduce restored-dentin disc strength (Figure 6) compared to restored discs without the peptide coating. Indeed, the highest average fracture resistance was for the GL13K-treated discs. This was a somehow unexpected result as interaction of the hydrophilic adhesive with the hydrophobic dentin could have not been favored and thus, bond strength at the d/r interface could have been reduced. Some components in the formulation of commercial universal adhesives, as the one we used in our experiments, are amphipathic molecules that may favor hydrophobic interactions with the GL13K coating before adhesive curing. However, further analysis would be needed to validate our approach for enamel and coronal dentin.
The application of the restorative materials on GL13K-coated dentin did not notably remove and/or degrade the peptide coating and did not reduce the fracture resistance of restored dentin discs. Moreover, aging of the restorations did not prevent the peptides from displaying their hydrophobic and protective properties. The impermeability of the hydrophobic d/r interface, before and after aging, supports our hypothesis that interaction of water and waterborne agents with resin composite materials can be markedly limited and extended over time, which soundly supports the potential of this technology to expand the longevity of resin composite restorations. However, our study has been limited to assess the properties of the new peptide coatings using only one adhesive system. We chose a universal adhesive for our work here for its functional versatility to match our different coating protocols and varying humidity conditions. Still, studying other adhesive systems, especially highly hydrophobic formulations, would be valuable to expand and optimize this new technology.
Conclusions
We obtained a highly hydrophobic dentin by directly priming/coating dentin with an antimicrobial and amphipathic peptide, GL13K. We demonstrated that the hydrophobic coating resisted in vitro hydrolytic, mechanical, thermal, acidic, and enzymatic modes of degradation. The GL13K coatings were also antimicrobial and are expected to increase the durability of resin composite restorations with a 2-tier protective system for preventing degradation at the d/r interface by waterborne agents and preventing re-infection of tissues in contact with restorations. Analysis of the distribution pattern of peptide coatings, improvement of the recruitment and stability of the molecules on dentin, and assessment of other functional properties before and after aging protocols should further support the potential translation of this technology to the clinical field.
Supplementary Material
We present a technology using designer peptides to treat the most prevalent chronic disease worldwide; dental caries. Specifically, we used antimicrobial amphipathic peptides to coat dentin with the goal of increasing the service life of the restorative materials used to treat dental caries, which is nowadays 5 years in average. Water and waterborne agents (enzymes, acids) degrade restorative materials and enable re-infection at the dentin/restoration interface. Our peptide coatings will hinder degradation of the restoration as they produced highly hydrophobic and antimicrobial dentin/material interfaces. We anticipate a high technological and economic impact of our technology as it can notably reduce the lifelong dental bill of patients worldwide. Our findings can enable the development of restorations with all-hydrophobic and so, more protective components.
Acknowledgements
The authors acknowledge Professor Jorge Perdigao, Professor Sven Gorr, and Dr. Brian N. Holmes, University of Minnesota, for constructive discussions regarding the clinical validity of the methods presented here, the differences in peptide activity between L- and D-GL13K peptides, and the effects of rising methods on hydrophobicity of the peptide coatings, respectively. The authors also acknowledge Dr. Tamer Mansour, University of California-Davis, for generating the R-code used to analyze the mechanical testing data in a reproducible manner; and Professor Robert S. Jones, University of Minnesota, for facilitating the use of microbilogy facilities and donating the plaque samples. The authors also acknowledge Dr. Carola Carrera, Ms. Ruoqiong Chen and Ms. Julia Nikrad, University of Minnesota, for technical assistance with the micro-CT experiments and the antimicrobial potency analysis. This research study was supported by the National Institute for Dental and Craniofacial Research of the National Institutes of Health [grant number R01DE026117 to C.A. and R90DE023058 to D.G.M.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding bodies had no role in study design, the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Footnotes
Competing financial interests
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome
Data availability
The raw/processed data required to reproduce these findings will be made available on request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Health Organization, Oral Health Fact Sheet (2012), 2012. http://www.who.int/mediacentre/factsheets/fs318/en/.
- [2].Wold Health Organization, Sugars and dental caries, 2017. http://www.who.int/oral_health/publications/sugars-dental-caries-keyfacts/en/.
- [3].World Health Organization, Dental Diseases and Oral Health, 2003. http://www.who.int/oral_health/publications/en/orh_fact_sheet.pdf.
- [4].Benjamin RM., Oral Health: The Silent Epidemic, Public Health Rep 125(2) (2010) 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burke FJ, Wilson NH, Cheung SW, Mjor IA, Influence of patient factors on age of restorations at failure and reasons for their placement and replacement, J Dent 29(5) (2001) 317–24. [DOI] [PubMed] [Google Scholar]
- [6].Wilson NH, Burke FJ, Mjor IA, Reasons for placement and replacement of restorations of direct restorative materials by a selected group of practitioners in the United Kingdom, Quintessence Int 28(4) (1997) 245–8. [PubMed] [Google Scholar]
- [7].NIDCR, URL available at http://www.nidcr.nih.gov/Research/ResearchPriorities/StrategicPlan, Strategic Plan 2009–2013. [PubMed]
- [8].Forss H, Widstrom E, From amalgam to composite: selection of restorative materials and restoration longevity in Finland, Acta Odontol Scand 59(2) (2001) 57–62. [DOI] [PubMed] [Google Scholar]
- [9].Rho YJ, Namgung C, Jin BH, Lim BS, Cho BH, Longevity of direct restorations in stress-bearing posterior cavities: a retrospective study, Oper Dent 38(6) (2013) 572–82. [DOI] [PubMed] [Google Scholar]
- [10].Opdam NJ, van de Sande FH, Bronkhorst E, Cenci MS, Bottenberg P, Pallesen U, Gaengler P, Lindberg A, Huysmans MC, van Dijken JW, Longevity of posterior composite restorations: a systematic review and meta-analysis, J Dent Res 93(10) (2014) 943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brantley CF, Bader JD, Shugars DA, Nesbit SP, Does the cycle of rerestoration lead to larger restorations?, J Am Dent Assoc 126(10) (1995) 1407–13. [DOI] [PubMed] [Google Scholar]
- [12].Mjor IA, Jokstad A, Qvist V, Longevity of posterior restorations, Int Dent J 40(1) (1990) 11–7. [PubMed] [Google Scholar]
- [13].Deligeorgi V, Mjor IA, Wilson NH, An overview of reasons for the placement and replacement of restorations, Prim Dent Care 8(1) (2001) 5–11. [DOI] [PubMed] [Google Scholar]
- [14].Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF, Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies, Crit Rev Oral Biol Med 13(6) (2002) 509–20. [DOI] [PubMed] [Google Scholar]
- [15].Sunnegårdh-Grönberg K, van Dijken JW, Funegard U, Lindberg A, Nilsson M, Selection of dental materials and longevity of replaced restorations in Public Dental Health clinics in northern Sweden, J Dent 37(9) (2009) 673–8. [DOI] [PubMed] [Google Scholar]
- [16].Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, DeRouen TA, Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial, J Am Dent Assoc 138(6) (2007) 775–83. [DOI] [PubMed] [Google Scholar]
- [17].Mjor IA, Toffenetti F, Secondary caries: a literature review with case reports, Quintessence Int 31(3) (2000) 165–79. [PubMed] [Google Scholar]
- [18].Astvaldsdottir A, Dagerhamn J, van Dijken JW, Naimi-Akbar A, Sandborgh-Englund G, Tranaeus S, Nilsson M, Longevity of posterior resin composite restorations in adults - A systematic review, J Dent 43(8) (2015) 934–54. [DOI] [PubMed] [Google Scholar]
- [19].Mo SS, Bao W, Lai GY, Wang J, Li MY, The microfloral analysis of secondary caries biofilm around Class I and Class II composite and amalgam fillings, BMC Infect Dis 10 (2010) 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL, Adhesive/Dentin interface: the weak link in the composite restoration, Ann Biomed Eng 38(6) (2010) 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Waidyasekera K, Nikaido T, Weerasinghe DS, Ichinose S, Tagami J, Reinforcement of dentin in self-etch adhesive technology: a new concept, J Dent 37(8) (2009) 604–9. [DOI] [PubMed] [Google Scholar]
- [22].ADA Council on Scientific Affairs, Direct and indirect restorative materials, 2003. http://www.ncbi.nlm.nih.gov/pubmed/12733780.
- [23].Ferracane JL, Resin composite--state of the art, Dent Mater 27(1) (2011) 29–38. [DOI] [PubMed] [Google Scholar]
- [24].Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y, Biodegradation of resin-dentin interfaces increases bacterial microleakage, J Dent Res 89(9) (2010) 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH, Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity, Biomaterials 26(33) (2005) 6449–59. [DOI] [PubMed] [Google Scholar]
- [26].Hashimoto M, Fujita S, Kaga M, Yawaka Y, Effect of water on bonding of one-bottle self-etching adhesives, Dent Mater J 27(2) (2008) 172–8. [DOI] [PubMed] [Google Scholar]
- [27].Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B, Systematic review of the chemical composition of contemporary dental adhesives, Biomaterials 28(26) (2007) 3757–85. [DOI] [PubMed] [Google Scholar]
- [28].Tay FR, Pashley DH, Have dentin adhesives become too hydrophilic?, J Can Dent Assoc 69(11) (2003) 726–31. [PubMed] [Google Scholar]
- [29].Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S, Collagen degradation by host-derived enzymes during aging, J Dent Res 83(3) (2004) 216–21. [DOI] [PubMed] [Google Scholar]
- [30].Tay FR, Pashley DH, Biomimetic remineralization of resin-bonded acid-etched dentin, J Dent Res 88(8) (2009) 719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, Pashley DH, Tay FR, Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach, Acta Biomater 6(7) (2010) 2740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH, Nanoleakage: leakage within the hybrid layer, Oper Dent 20(1) (1995) 18–25. [PubMed] [Google Scholar]
- [33].Sano H, Microtensile testing, nanoleakage, and biodegradation of resin-dentin bonds, J Dent Res 85(1) (2006) 11–4. [DOI] [PubMed] [Google Scholar]
- [34].Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS, Proteins, pathogens, and failure at the composite-tooth interface, J Dent Res 93(12) (2014) 1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y, Cariogenic bacteria degrade dental resin composites and adhesives, J Dent Res 92(11) (2013) 989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sadek FT, Castellan CS, Braga RR, Mai S, Tjaderhane L, Pashley DH, Tay FR, One-year stability of resin-dentin bonds created with a hydrophobic ethanol-wet bonding technique, Dent Mater 26(4) (2010) 380–6. [DOI] [PubMed] [Google Scholar]
- [37].Sezinando A, Luque-Martinez I, Munoz MA, Reis A, Loguercio AD, Perdigao J, Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives, Dent Mater 31(10) (2015) e236–46. [DOI] [PubMed] [Google Scholar]
- [38].Cao D, Zhang Y, Li Y, Shi X, Gong H, Feng D, Guo X, Shi Z, Zhu S, Cui Z, Fabrication of superhydrophobic coating for preventing microleakage in a dental composite restoration, Mater Sci Eng C Mater Biol Appl 78 (2017) 333–340. [DOI] [PubMed] [Google Scholar]
- [39].Imazato S, Russell RR, McCabe JF, Antibacterial activity of MDPB polymer incorporated in dental resin, J Dent 23(3) (1995) 177–81. [DOI] [PubMed] [Google Scholar]
- [40].Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, Van Meerbeek B, Suzuki K, Takashiba S, Antibacterial effect of bactericide immobilized in resin matrix, Dent Mater 25(4) (2009) 424–30. [DOI] [PubMed] [Google Scholar]
- [41].Cadenaro M, Pashley DH, Marchesi G, Carrilho M, Antoniolli F, Mazzoni A, Tay FR, Di Lenarda R, Breschi L, Influence of chlorhexidine on the degree of conversion and E-modulus of experimental adhesive blends, Dent Mater 25(10) (2009) 1269–74. [DOI] [PubMed] [Google Scholar]
- [42].Xianju Xie LW, Xing Dan, Zhanga Ke, Weirb Michael D., Liue Huaibing, Baia Yuxing, Xub Hockin H.K., Novel dental adhesive with triple benefits ofcalcium phosphate recharge, protein-repellent andantibacterial functions, Dental Materials 33 (2017) 553–563. [DOI] [PubMed] [Google Scholar]
- [43].Brogden KA, Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria?, Nat Rev Microbiol 3(3) (2005) 238–50. [DOI] [PubMed] [Google Scholar]
- [44].Bechinger B, Gorr SU, Antimicrobial Peptides: Mechanisms of Action and Resistance, J Dent Res 96(3) (2017) 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fjell CD, Hiss JA, Hancock RE, Schneider G, Designing antimicrobial peptides: form follows function, Nat Rev Drug Discov 11(1) (2011) 37–51. [DOI] [PubMed] [Google Scholar]
- [46].Hancock RE, Sahl HG, Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies, Nat Biotechnol 24(12) (2006) 1551–7. [DOI] [PubMed] [Google Scholar]
- [47].Wang G, Li X, Wang Z, APD2: the updated antimicrobial peptide database and its application in peptide design, Nucleic Acids Res 37(Database issue) (2009) D933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Di Luca M, Maccari G, Maisetta G, Batoni G, BaAMPs: the database of biofilm-active antimicrobial peptides, Biofouling 31(2) (2015) 193–9. [DOI] [PubMed] [Google Scholar]
- [49].Abdolhosseini M, Nandula SR, Song J, Hirt H, Gorr SU, Lysine substitutions convert a bacterial-agglutinating peptide into a bactericidal peptide that retains anti-lipopolysaccharide activity and low hemolytic activity, Peptides 35(2) (2012) 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gorr SU, Sotsky JB, Shelar AP, Demuth DR, Design of bacteria-agglutinating peptides derived from parotid secretory protein, a member of the bactericidal/permeability increasing-like protein family, Peptides 29(12) (2008) 2118–27. [DOI] [PubMed] [Google Scholar]
- [51].Hirt H, Hall JW, Larson E, Gorr SU, A D-enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii, PLoS One 13(3) (2018) e0194900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hirt H, Gorr SU, Antimicrobial peptide GL13K is effective in reducing biofilms of Pseudomonas aeruginosa, Antimicrob Agents Chemother 57(10) (2013) 4903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Holmberg KV, Abdolhosseini M, Li Y, Chen X, Gorr SU, Aparicio C, Bio-inspired stable antimicrobial peptide coatings for dental applications, Acta Biomater 9(9) (2013) 8224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen X, Hirt H, Li Y, Gorr SU, Aparicio C, Antimicrobial GL13K peptide coatings killed and ruptured the wall of Streptococcus gordonii and prevented formation and growth of biofilms, PLoS One 9(11) (2014) e111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Armstrong S, Breschi L, Ozcan M, Pfefferkom F, Ferrari M, Van Meerbeek B, Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (muTBS) approach, Dent Mater 33(2) (2017) 133–143. [DOI] [PubMed] [Google Scholar]
- [56].Reilly C, Rasmussen K, Selberg T, Stevens J, Jones RS, Biofilm community diversity after exposure to 0.4% stannous fluoride gels, J Appl Microbiol 117(6) (2014) 1798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].de la Fuente-Nunez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock RE, Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide, Antimicrob Agents Chemother 56(5) (2012) 2696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2013. http://www.R-project.org/. [Google Scholar]
- [59].Weerkamp AH, Uyen HM, Busscher HJ, Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva-coated human enamel and dentin, J Dent Res 67(12) (1988) 1483–7. [DOI] [PubMed] [Google Scholar]
- [60].Osorio E, Toledano M, Aguilera FS, Tay FR, Osorio R, Ethanol wet-bonding technique sensitivity assessed by AFM, J Dent Res 89(11) (2010) 1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ye Z, Zhu X, Acosta S, Kumar D, Sang T, Aparicio C, Self-assembly dynamics and antimicrobial activity of all l- and d-amino acid enantiomers of a designer peptide, Nanoscale 11(1) (2018) 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kidd EA, Fejerskov O, What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms, J Dent Res 83 Spec No C (2004) C35–8. [DOI] [PubMed] [Google Scholar]
- [63].Lopes MB, Sinhoreti MA, Gonini A Junior, Consani S, McCabe JF, Comparative study of tubular diameter and quantity for human and bovine dentin at different depths, Braz Dent J 20(4) (2009) 279–83. [DOI] [PubMed] [Google Scholar]
- [64].Hanks CT, Strawn SE, Wataha JC, Craig RG, Cytotoxic effects of resin components on cultured mammalian fibroblasts, J Dent Res 70(11) (1991) 1450–5. [DOI] [PubMed] [Google Scholar]
- [65].Ratanasathien S, Wataha JC, Hanks CT, Dennison JB, Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts, J Dent Res 74(9) (1995) 1602–6. [DOI] [PubMed] [Google Scholar]
- [66].Costa CA, Vaerten MA, Edwards CA, Hanks CT, Cytotoxic effects of current dental adhesive systems on immortalized odontoblast cell line MDPC-23, Dent Mater 15(6) (1999) 434–41. [DOI] [PubMed] [Google Scholar]
- [67].Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG, The human oral microbiome, J Bacteriol 192(19) (2010) 5002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Human Oral Microbiome Database, 2016. http://www.homd.org/.
- [69].Costerton JW, Stewart PS, Greenberg EP, Bacterial biofilms: a common cause of persistent infections, Science 284(5418) (1999) 1318–22. [DOI] [PubMed] [Google Scholar]
- [70].O'Toole G, Kaplan HB, Kolter R, Biofilm formation as microbial development, Annu Rev Microbiol 54 (2000) 49–79. [DOI] [PubMed] [Google Scholar]
- [71].Kostakioti M, Hadjifrangiskou M, Hultgren SJ, Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era, Cold Spring Harb Perspect Med 3(4) (2013) a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].de la Fuente-Nunez C, Reffuveille F, Fernandez L, Hancock RE, Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies, Curr Opin Microbiol 16(5) (2013) 580–9. [DOI] [PubMed] [Google Scholar]
- [73].Van Acker H, Van Dijck P, Coenye T, Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms, Trends Microbiol 22(6) (2014) 326–33. [DOI] [PubMed] [Google Scholar]
- [74].Bell LC, Posner AM, Quirk JP, The point of zero charge of hydroxyapatite and fluorapatite in aqueous solutions., Journal of Colloid and Interface Science 42(2) (1973) 250–261. [Google Scholar]
- [75].Sevilla P, Gil FG, Aparicio C, Relevant properties for immobilizing short peptides on biosurfaces, IRBM 28(5) (2017) 256–65. [Google Scholar]
- [76].Carrera C, Lan C, Escobar-Sanabria D, Li Y, Rudney J, Aparicio C, Fok A, The use of micro-CT with image segmentation to quantify leakage in dental restorations, Dental Materials 31(4) (2015) 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Carrera CA, Chen YC, Li Y, Rudney J, Aparicio C, Fok A, Dentin-composite bond strength measurement using the Brazilian disk test, J Dent 52 (2016) 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Perdigao J, Swift EJ Jr., Universal Adhesives, J Esthet Restor Dent 27(6) (2015) 331–4. [DOI] [PubMed] [Google Scholar]
- [79].Aguilar LT, Rezende NP, Reis A, Loguercio AD, Grande RH, Ballester RY, Singer Jda M, Tensile bond strength of adhesive systems--effects of primer and thermocycling, Pesqui Odontol Bras 16(1) (2002) 37–42. [DOI] [PubMed] [Google Scholar]
- [80].Dos Santos PA, Garcia PP, Palma-Dibb RG, Shear bond strength of adhesive systems to enamel and dentin. Thermocycling influence, J Mater Sci Mater Med 16(8) (2005) 727–32. [DOI] [PubMed] [Google Scholar]
- [81].Yang B, Adelung R, Ludwig K, Bossmann K, Pashley DH, Kern M, Effect of structural change of collagen fibrils on the durability of dentin bonding, Biomaterials 26(24) (2005) 5021–31. [DOI] [PubMed] [Google Scholar]
- [82].Gale MS, Darvell BW, Thermal cycling procedures for laboratory testing of dental restorations, J Dent 27(2) (1999) 89–99. [DOI] [PubMed] [Google Scholar]
- [83].Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RH, Reis A, Durability of enamel bonding using two-step self-etch systems on ground and unground enamel, Oper Dent 33(1) (2008) 79–88. [DOI] [PubMed] [Google Scholar]
- [84].Gamborgi GP, Loguercio AD, Reis A, Influence of enamel border and regional variability on durability of resin-dentin bonds, J Dent 35(5) (2007) 371–6. [DOI] [PubMed] [Google Scholar]
- [85].Huang SH, Lin LS, Rudney J, Jones R, Aparicio C, Lin CP, Fok A, A novel dentin bond strength measurement technique using a composite disk in diametral compression, Acta Biomater 8(4) (2012) 1597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.