Abstract
Background
Reports show that ultrasound-targeted microbubble destruction (UTMD) is a promising method of gene therapy, and metadherin (MTDH) is related to the development of breast cancer. Thus, we investigated the role of MTDH in breast cancer and compared the effect of suppressing MTDH by shRNA using liposome, UTMD, or the combination of these 2 methods.
Material/Methods
Graphing of survival curves of MTDH was analyzed by bioinformatics. UTMD was conducted using an ultrasonic therapeutic apparatus. Cell counting kit-8 (CCK-8) assay was used to measure cell viability. Migration and invasion rates were measured by wound healing test and Transwell invasion assay, respectively. The expression of MTDH, E-cadherin, metastasis-associated protein-1 (MTA-1), matrix metalloproteinase (MMP)-2, and MMP-9 were measured by Western blot and qPCR.
Results
The prognosis of breast cancer can be decreased by the high expression of MTDH, and elevated expression of MTDH was discovered in MCF-7, MCF-10A, and T47D cell lines. UTMD combined with liposome is most efficient in transfecting shRNA, clearly suppressing the expression of MTDH and thereby decreasing cell viability, migration, invasion rate, and epithelial- mesenchymal transition (EMT) processes in the MCF-7 cell line.
Conclusions
UTMD combined with liposome could be used as a more efficient way to transfect shRNA into cells to suppress the expression of MTDH and thus lead to the downregulation of proliferation, migration, and EMT processes of the MCF-7 cell line, showing the potential for use in gene therapy.
MeSH Keywords: 14-3-3 Proteins, Cell Proliferation, Neoplasm Metastasis, Transfection
Background
In recent years, the incidence of breast cancer has been increasing year by year, and has become the most common malignant tumor in women [1]. Although clinical research and treatment have made great progress, breast cancer is still the leading cause cancer-related death around the world, and recurrence and metastasis are the main causes of death in patients with advanced breast cancer [1]. Early detection and treatment are important, and it is also important to identify factors influencing the occurrence and metastasis of breast cancer and to develop effective treatments to improve the prognosis of patients.
Metadherin (MTDH) is an oncogene closely related to the occurrence and development of various tumors [2]. Brown et al. found that the MTDH gene promotes lung metastasis in breast cancer cells; however, the inhibition of MTDH expression by MTDH antibody or siRNA interference can effectively reduce the probability of lung metastasis of breast cancer, and it might be associated with the decreased adhesion between tumor cells and endothelial cells [3]. In liver cancer cells, upregulation of MTDH can increase the invasion and migration ability of tumor cells, and downregulation of MTDH can reverse epithelial-mesenchymal transition (EMT) and reduce the invasion and migration capacity of tumor cells [4]. Li et al. reported that miR-153 can inhibit EMT by targeting MTDH in human breast cancer [5]. Thus, MTDH appears to be a promising target for the treatment of breast cancer.
RNAi technology is one of the new tools for gene therapy, which can effectively block the expression of specific genes in the body and induce gene silencing in the process of cell proliferation, with low toxicity [6]. SiRNA and shRNA are fragments commonly used in RNAi technology [7]. The silencing effect of siRNA is transient, while the shRNA binding with gene vectors can be more stable in transfection, leading to the decomposition of target gene mRNA and thus achieving the therapeutic effect of sustained inhibition of the target gene [8]. Lipofectamine 2000 is a cationic liposome transfection reagent commonly used in research, but its effectiveness needs to be improved [9,10]. Ultrasound-targeted microbubble destruction (UTMD) has developed rapidly in recent years and is a new method for gene therapy that shows promise for wide use in cell biology [11]. It can increase the ability of cells to uptake genes by producing sonoporation, which temporarily elevates the permeability of cells and capillaries [12]. However, this method has poor loading capacity because the microbubbles cannot carry sufficient numbers of genes [13,14].
Given these advantage and disadvantage of liposome transfection and UTMD, we hypothesized that the combination of these 2 methods would result in better transfection. Thus, we performed the present study to assess the role of MTDH in breast cancer cell lines, as well as to compare the effect of suppressing on MTDH by shRNA using the methods of liposome, UTMD, or the combination of these 2 and the changes in cell viability, wound closure rate, invasion rate, and genes promoting EMT. Our findings may provide a promising method which could be considered for wide use in gene therapy for breast cancer in the future.
Material and Methods
Bioinformatics analysis
The MTDH expression data of 818 breast cancer patients were downloaded from the Cancer Genome Atlas (TCGA). The data were analyzed by R language to assess the effects of different MTDH expression levels on the survival of breast cancer patients.
Preparation of plasmids and cell culture
The interference shRNA plasmid (pGPU6) for MTDH and negative control (NC) plasmid were synthesized by Shanghai Genepharma Company (Genepharma, Shanghai, China). The Hs 578Bst cell line is from normal human breast cells and was used as a control group, and MCF-7, MCF-10A, and T47D cell lines are from human breast cancer cells. All cell lines above were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The T47D cell line was cultured in RPMI-1640 medium (Thermo Fisher, Waltham, USA), and the Hs 578Bst, MCF-7, and MCF-10A cell lines were cultured in DMEM (Thermo Fisher, Waltham, USA). We added 10% fetal bovine serum (HyClone, USA) to each medium at 37 with 5% CO2. The cells were subcultured every 3 days.
Grouping and transfection
MCF-7 cells were randomly separated into 4 groups: blank control (BC), negative control (NC) + UTMD (MB), UTMD (MB), Liposome (Lipo), and MB + Lipo groups. The liquid volume was based on the single volume of 24-well plates, and each group was repeated 4 times. According to the directions of the Lipofectamine™ 2000 (LF2000, Thermo Fisher, Waltham, USA) transfection kit, the plasmid/liposome mixture was prepared at the proportion of 1 ug plasmid to 2 uL LF2000. The microbubble suspension was prepared according to the SonoVue (Bracco, Shanghai, China) instructions, and the plasmid/microbubble mixture was prepared at the proportion of 1 ug plasmid to 50 uL microbubble suspension. Further, 500 uL DMEM was added to the cells of the BC group. The mixture of negative plasmid/microbubble suspension was added to the cells of the NC + MB group and the total volume was replenished with DMEM to 500 uL. The positive plasmid/microbubble suspension was added to the cells of the MB group and the total volume was replenished with DMEM to 500 uL. The positive plasmid/liposome mixture was added to the cells of the Lipo group and the total volume was replenished with DMEM to 500 uL. The microbubble suspension/liposome mixture was added to the cells of the MB + Lipo group and the total volume was replenished with DMEM to 500 uL. An ultrasonic probe was placed under the culture plate of the NC + MB group, MB group, and MB + Lipo group. After 30 min of power-on preheating of the ultrasonic therapeutic apparatus (Fysiomed, Doornstraat, Belgium), these cells were subjected to ultrasonic waves, and the parameters were set as 1 MHz for frequency, 0.75 w/cm2 for sound intensity, and the time was set for 45 s.
Cell viability assay
Cell vitality was assessed using the Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich, Shanghai, China). The assay was done when the cells were cultured for 24 and 48 h. Procedures were performed according to the manufacturer’s instructions, and cells were incubated at 37°C in 96-well plates for 1 h. Then, the optical density (OD) value for each well was measured at 450 nm wavelength on a microplate reader (Multiskan, Thermo, USA).
Transwell invasion assay
We used 8-μm Transwell chambers (BD Biosciences, CA, USA) to measure the invasion rate of cells. Cells were collected when cell density reached 5×104 cells, and we then added 200 μL serum-free medium to the top chamber. We then added 200 uL medium with 10% FBS to the bottom chamber to serve as a chemoattractant, and then the cells were incubated for 24 h at 37°C. After that, cells that did not pass through the pores were carefully removed. Cells that had invaded into the bottom chamber were fixed and stained with 0.5% crystal violet (Sigma-Aldrich, Shanghai, China) for 30 min. Cells were then counted under an inverted microscope (Olympus IX71, Tokyo, Japan) and photographed at 200× magnification.
Wound healing test
Cells were cultured in 6-well plates when the density reached 2.5×104/cm2. A 50-μL pipette tip was used to make a 0.5-cm gap on the confluent monolayer of cells. Medium was removed from the plate after 24 h of culturing and the plate was washed with PBS 2 times. Medium was then added to the plates for continuing culture. At the same observation site, the healing of the scratch of the cells between each group was compared at 0 h and 48 h. The distance between edges of the gaps was used to calculate the rate of cell migration. Three different regions were visualized and images were taken with an inverted microscope (Olympus IX71, Tokyo, Japan).
Extraction and assessment of total proteins
Total proteins of the cells were extracted by RIPA buffer (Thermo Fisher, Waltham, USA). After the adding of RIPA buffer to cells, samples were kept on ice for 5 min. Lysate was then gathered and centrifuged at 14 000 g at 4°C for 15 min. Supernatant was then transferred to a new tube. Five standard wells were established, according to the Pierce™ BCA Protein Assay Kit (Thermo Fisher, Waltham, USA) instructions, and standard protein liquid was diluted to 1, 0.5, 0.25, 0.125, and 0.0625 g/mL and added to the wells of a 96-well plates. We also added 2 uL of samples to each well. According to the number of samples, the BCA reagent was prepared following the manufacturer’s instructions. We added 200 uL BCA reagent to each well and then the plate was placed in a 37°C incubator for 30 min. The plate was then put in the microplate reader (Multiskan, Thermo, Waltham, USA), and the absorbance at 562 nm wavelength of each well was recorded. In EXCEL program, the standard curve was drawn with standard absorbance as abscissa and protein concentration as ordinate. The concentration of the total proteins was calculated according to the standard curve.
Western blot analysis
Total proteins were boiled for 5 min and mixed with loading buffer (Bio-Rad, Shanghai, China) and then loaded onto SDS-polyacrylamide gel for electrophoresis. The SDS-PAGE was put into the electrophoresis apparatus with 100 V for 2 h and then transferred to a PVDF membrane. The membrane was blocked in 5% non-fat milk with PBST (Solarbio Life Sciences, Beijing, China) at room temperature for 2 h. Anti-LYRIC (MTDH) antibody (ab227981, Abcam, San Francisco, USA, 1: 2000, 64 kDa), Anti-E-Cadherin antibody (ab15148, Abcam, San Francisco, USA, 1: 2000, 120 kDa), Anti-MTA1 antibody (ab71153, Abcam, San Francisco, USA, 1: 2000, 81 kDa), anti-matrix metalloproteinase (MMP) −2 antibody (ab37150, Abcam, San Francisco, USA, 1: 2000, 72 kDa), anti-MMP-9 antibody (ab73734, Abcam, San Francisco, USA, 1: 2000, 95 kDa), and anti-GAPDH antibody (ab181602, Abcam, San Francisco, USA, 1: 2000, 36 kDa) were used separately to detect the target proteins with shaking overnight. The membranes were then washed with PBST 3 times with shaking. The membrane was probed with the secondary antibody IgG H&L (HRP) (ab6721, Abcam, San Francisco, USA, 1: 2000) for 1 h at room temperature. Pierce™ ECL plus Western blotting substrate (Thermo Fisher, Waltham, USA) was used to detect the blots and images were taken for analysis.
MRNA expression assessment by qPCR
Total RNA was extracted using Trizol reagent (Thermo Fisher, Waltham, USA) and the OD 260/OD 280 of extracted RNA was 1.8–2.2.ABI High Capacity cDNA Reverse Transcription Kit (Thermo Fisher, Waltham, USA) was then used to obtain cDNA. Reaction system was prepared on icebox, based on primers (Table 1) and templates in each tube. qPCR was performed on a qPCR machine (CFX96 Touch™, Bio-Rad, CA). The reaction system was as follows: Premix Taq 25 uL, cDNA template 2 uL, upstream primer (20 uM) 1 uL, downstream primer (20 uM) 1 uL, and total volume was made to 50 uL with distilled water. The PCR procedure was as follows: 94°C for 5 min, 94°C for 30 s, 56°C for 30 s, and 72°C for 3 min, for 40 cycles. Data were analyzed by ABI SDS version 2.3 software.
Table 1.
Primers used in quantitative PCR.
| Genes | Primers |
|---|---|
| MTDH | (F)5′-CAAATGGGCGGACTGTTGAA-3′ (R)5′-GCTGCTGTCGTTTCTCTCTG-3′ |
| E-cadherin | (F)5′-TTTGAAGATTGCACCGGTCG-3′ (R)5′-CAGCGTGACTTTGGTGGAAA-3′ |
| MTA-1 | (F)5′-CTACGACCCACAGCAGAAGA-3′ (R)5′-TGGTCGATCTGCTTGTCTGT-3′ |
| MMP-2 | (F)5′-ACCACAGCCAACTACGATGA-3′ (R)5′-GGTGCCAAGGTCAATGTCAG-3′ |
| MMP-9 | (F)5′-CTCTGGAGGTTCGACGTGAA-3′ (R)5′-TCAACTCACTCCGGGAACTC-3′ |
| GAPDH | (F)5′-ATGGTGAAGGTCGGTGTGAA-3′ (R)5′-TGGAAGATGGTGATGGGCTT-3′ |
Statistical analysis
Data were analyzed using SPSS 13.0 software. All data are shown as the mean ±SD. One-way analysis of variance was used to compare multiple groups. The overall survival rate was analyzed by log rank test. P<0.05 was considered to indicate statistical significance.
Results
High expression of MTDH worsens prognosis of breast cancer
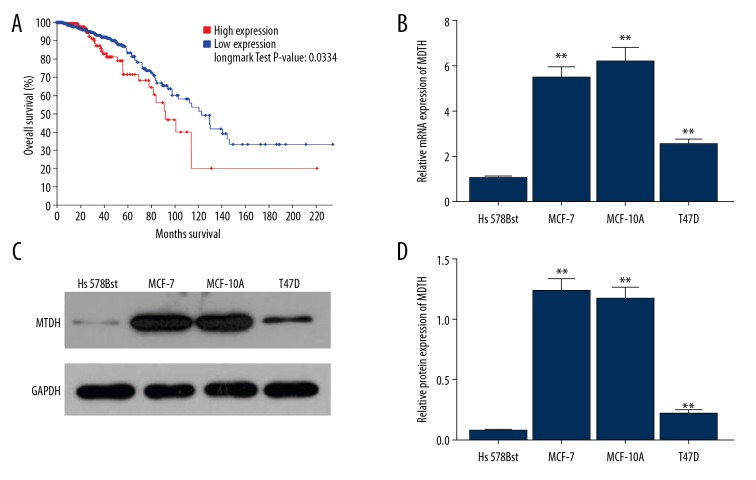
To investigate whether MTDH influences the prognosis of patients with breast cancer, we downloaded the data of 818 cases with breast cancer from TCGA, Cell, 2015, and obtained the overall survival curve of the cases with high or low expression of MTDH, and compared them (Figure 1A). The result showed that the overall survival curve of cases with high expression of MTDH was nearly under the curve of cases with low expression of MTDH, and the log rank test P value was 0.0334, which is less than the standard α=0.05, indicating that high expression of MTDH worsens prognosis of breast cancer.
Figure 1.
The expression of MTDH in Hs 578Bst, MCF-7, MCF-10A, and T47D cell lines and its overall survival curve in breast cancer. (A) The overall survival curve of the cases with high or low expression of MTDH. (B) Relative mRNA expression of MTDH in the cell lines. (C) The results of Western blot of the expression of MTDH protein in the cell lines. (D) Relative protein expression of MTDH in the cell lines. Bars indicate means ±SD. ** P<0.01 vs. Hs 578Bst cell line.
The expression of MTDH was elevated in MCF-7, MCF-10A, and T47D cell lines
To assess whether the expression of MTDH was increased in breast cancer, we used the MCF-7, MCF-10A, and T47D cell lines as representatives of breast cancer, and measured the expression of MTDH by qPCR and Western blot analysis. We found that mRNA and protein levels of MTDH were higher than in the normal mammary gland cell line Hs 578Bst (Figure 1B–1D, ** P<0.01), suggesting that the expression of MTDH is elevated in breast cancer patients.
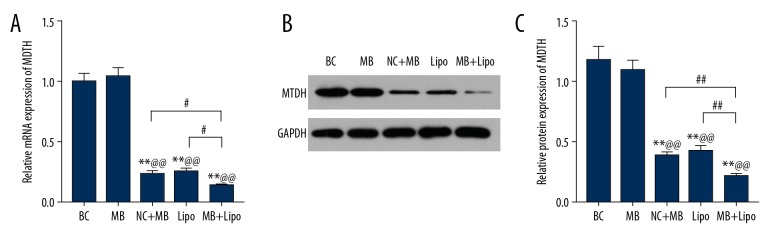
UTMD combined with liposome is more efficient in transfecting shRNA to suppress the expression of MTDH
To explore whether UTMD combined with liposome exhibits greater efficiency of transfecting shRNA to suppress MTDH, we compared the expression of MTDH by qPCR and Western blot analysis in BC, NC + MB, MB, Lipo, and MB + Lipo groups. At transcriptional and translational levels, the data showed that there was no significant difference between BC and NC + MB groups in the expression of MTDH, suggesting that microbubbles and negative plasmids cannot affect the expression of MTDH by themselves. importantly, the expression of MTDH in MB, Lipo, and MB + Lipo groups were distinctly suppressed, and was lowest in the MB + Lipo group. However, there was no significant difference between the MB and Lipo groups in the expression of MTDH (Figure 2A–2C, ** P<0.01, ## P<0.01, ^^ P<0.01, # P<0.05), showing that microbubbles or liposomes or the combination of these 2 transfection methods can all transfect cells with shRNA suppressing MTDH and worked functionally, but the combination of UTMD combined with liposome was more efficient in the transfection.
Figure 2.
The expression of MTDH in BC, NC + MB, MB, Lipo, and MB + Lipo groups. (A) Relative mRNA expression of MTDH in each group. (B) The results of Western blot of the expression of MTDH protein in each group. (C) Relative protein expression of MTDH in each group. Bars indicate means ±SD. ** P<0.01 vs. BC group; @@ P<0.01 vs. NC + MB group; ## P<0.01 and # P<0.05 vs. MB or Lipo group.
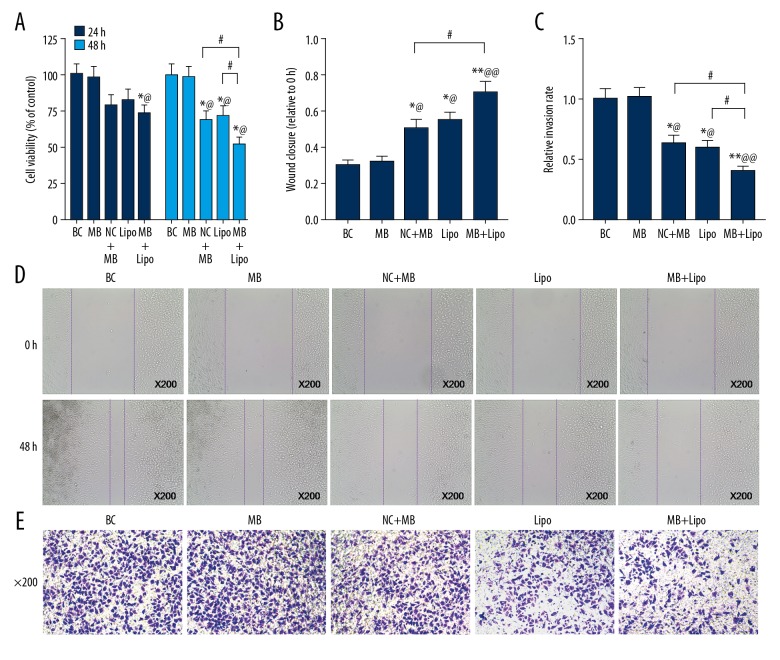
UTMD combined with liposome was more efficient in transfecting shRNA to suppress MTDH, thus decreasing cell viability, migration, and invasion rates
To determine whether the cell viability, migration, and invasion rates were influenced, we measured the cell viability by CCK-8, migration by wound healing test, and invasion by Transwell invasion assay. As expected, cell viability in the MB + Lipo group was lower than in the BC or NC + MB group at 24 h after transfection, and it was significantly decreased in MB, Lipo, and MB + Lipo groups after 48 h, and it was clearly lowest in the MB + Lipo group (Figure 3A, * P<0.05, ^ P<0.05, # P<0.05). The wound closure rate was increased in MB, Lipo, and MB + Lipo groups, but it was much higher in the MB + Lipo group than in the MB group (Figure 3B, 3D, ** P<0.01, ^^ P<0.01, * P<0.05, ^ P<0.05, # P<0.05). The relative invasion rate was lower in MB, Lipo, and MB + Lipo groups, and it was lowest in the MB + Lipo group (Figure 3C, 3E, ** P<0.01, ^^ P<0.01, * P<0.05, ^ P<0.05, # P<0.05). We found no significant difference between MB and Lipo groups in cell viability, wound closure, or relative invasion rates. Our findings suggest that microbubbles or liposomes can both transfect shRNA suppressing MTDH into cells and thereby decrease the cell viability, but the combination of these 2 methods is more efficient.
Figure 3.
The cell viability, wound closure, and relative invasion rate in BC, NC + MB, MB, Lipo, and MB + Lipo groups. (A) The cell viability in each group. (B) Wound closure in each group. (C) Relative invasion rate in each group. (D) The images showing the results of wound healing test in each group (×200). (E) The images of the cells in the bottom chamber after transwell test in each group (×200). Bars indicate means ±SD. ** P<0.01 and * P<0.05 vs. BC group; @@ P<0.01 and @ P<0.05 vs. NC + MB group; # P<0.05 vs. MB or Lipo group.
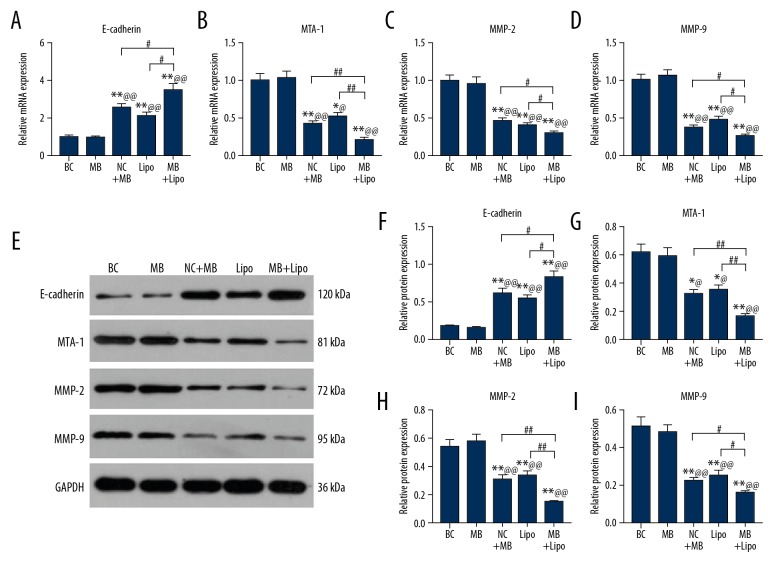
UTMD combined with liposome is more efficient in transfecting shRNA to suppress MTDH and thereby downregulate the EMT process
To investigate whether EMT was promoted, we measured the expression of E-cadherin, MTA-1, MMP-2, and MMP-9, which can reflect the EMT process by qPCR and Western blot. The expression of E-cadherin was elevated in the MB, Lipo, and MB + Lipo groups and it was the highest in the MB + Lipo group (Figure 4A, ** P<0.01, ^^ P<0.01, # P<0.05). The expression of MTA-1, MMP-2, and MMP-9 were decreased in the MB, Lipo, and MB + Lipo groups and they were the lowest in the MB + Lipo group (Figure 4B–4E, ** P<0.01, ^^ P<0.01, ## P<0.01, * P<0.05, ^ P<0.05, # P<0.05). The expressions of these 4 molecules demonstrated the same trends at transcriptional and translational levels (Figure 4F–4I, ** P<0.01, ^^ P<0.01, ## P<0.01, * P<0.05, ^ P<0.05, # P<0.05). Our results suggest that the EMT process is depressed by the transfection of shRNA suppressing MTDH with microbubbles or liposomes, but the combination of these 2 transfection methods is most effective.
Figure 4.
The expression of E-cadherin, MTA-1, MMP-2, and MMP-9 in BC, NC + MB, MB, Lipo, and MB + Lipo groups. (A) Relative mRNA expression of E-cadherin in each group. (B) Relative mRNA expression of MTA-1 in each group. (C) Relative mRNA expression of MMP-2 in each group. (D) Relative mRNA expression of MMP-9 in each group. (E) The results of Western blot in each group. (F) Relative protein expression of E-cadherin in each group. (G) Relative protein expression of MTA-1 in each group. (H) Relative protein expression of MMP-2 in each group. (I) Relative protein expression of MMP-9 in each group. Bars indicate means ±SD. ** P<0.01 and * P<0.05 vs. BC group; @@ P<0.01 and @ P<0.05 vs. NC + MB group; ## P<0.01 and # P<0.05 vs. MB or Lipo group.
Discussion
In this study we analyzed the overall survival curve of breast cancer patients with high or low expression of MTDH, observed the expression of MTDH in MCF-7, MCF-10A, and T47D cell lines of breast cancer as representatives. We compared the efficiency of the transfection of the shRNA, which can suppress MTDH, and the consequences on cell viability, wound closure rate, invasion rate, and genes promoting EMT by microbubbles, liposomes, and the combination of these 2 methods. The results of the study revealed that UTMD combined with liposome could be used to improve the transfection system, raising the efficiency of transfection in cell biology, or as promising therapeutic method in gene therapy.
MTDH was previously reported to lead to poor prognosis of breast cancer [15]. After investigation of the overall survival curve of the cases with high or low expression of MTDH, and the expression of MTDH in 4 cell lines of breast cancer, we found that the cases with high expression of MTDH had worse prognosis and the expression of MTDH was clearly elevated in these 4 cell lines. These results verify the results reported by other researchers [15,16], and further prove that MTDH is elevated in breast cancer patients and might be a factor causing poor prognosis of breast cancer patients.
We found that the expression of MTDH was suppressed by microbubbles or liposomes alone that carried shRNA; however, the combination of the e methods suppressed MTDH even more efficiently. The barriers to the entry and expression of exogenous DNAs are mainly the cell membrane and nuclear membrane, and the most important rate-limiting steps liposome-mediated gene transfection are the dissociation of DNAs from inclusion bodies and the entry of DNA into the nucleus [17]. The cavitation effect created by UTMD is the main mechanism of gene transfection mediated by UTMD combined with liposome, and is also the main reason for the enhancement of efficiency of liposome transfection [18]. Thus, the explanation of the elevated efficiency by UTMD combined with liposome may be as follows. Firstly, liposome should have a good encapsulation rate for plasmid DNAs, but some of them might be still outside the liposome [18]. Secondly, only a small part of DNA can be released from the inclusion body after transfection, but the strong effect of cavitation might play a critical role in promoting movement of DNA out of the inclusion body [18]. Thirdly, as a result of the cell’s clearance of exogenous substances, part of the DNAs is excluded by cells through the exocytosis, and only a small part of the DNA can enter the nucleus[19]. If the cell’s exocytosis could be effectively inhibited, the transfection efficiency of liposome could be increased [19].The strong driving forces of cavitation such as shock wave and jet could inhibit cellular exocytosis to some extent [19]. Feril et al. found that the transfection rate of microbubbles with ultrasound was highest when the exocytosis was the strongest (about 2 h after transfection of liposome) [19]. Lastly, small DNA molecules (<500 bp) can enter the nucleus through the nuclear pore [20]. Except for the mitotic phase of the cell, the macromolecule DNA mainly enters the nucleus through active transport of the nuclear pore complex, which has low efficiency [20], perhaps because the nuclear membrane was broken and the exogenous DNAs in the cytoplasm could easily enter the nucleus at the phase of mitosis [20]. In addition, nuclear membranes have similar structures to cell membranes, and cavitation effects could also increase the permeability of nuclear membranes [21].
Proliferation, migration, and invasion rates are critical pathological features of tumor cells and are strongly associated with prognosis of patients with cancer [22]. Our results showed that the proliferation of cells and relative invasion rate were decreased by all 3 methods (MB, Lipo, MB + Lipo), but they were most reduced by the method of UTMD combined with liposome. The results of wound closure assay revealed that the migration rate was reduced using MB, Lipo, and MB + Lipo methods, but it was lowest with use of the MB method alone. Zhou et al. reported that miR-630 suppresses breast cancer progression by targeting MTDH, thus leading to downregulation of proliferation [23].Liu et al. also revealed that miR-26a could suppress tumor proliferation and metastasis by targeting MTDH in triple-negative breast cancer [24]. Thus, we assumed that the proliferation, migration, and invasion rates could be depressed due to the suppression of MTDH by shRNA, and the method of UTMD combined with liposome could raise the efficiency of transfection of shRNA into cells, showing more effectiveness on the suppression of MTDH.
EMT is a process whereby epithelial cells lose their polarity and adhesion, and acquire characteristics of invasive and metastatic mesenchymal stem cells [25].The occurrence of EMT and the separation of primary tumor cells are key to the metastasis of tumor cells [26]. The abnormal expression of E-cadherin is the most important initial step in EMT, while the abnormal expression of E-cadherin interferes with the strength of the adhesion connections between cells and promotes cell separation and escape from the primary tumor site [26,25]. In 1993, Oka et al. found that the lymph node metastasis rate of breast cancer patients with negative E-cadherin expression was significantly higher than that of those with positive expression, and the prognosis was significantly worse than in those with positive expression [27]. MTA1 is a tumor metastasis-related gene associated with the degree of infiltration and lymph node metastasis of many tumors [28]. It has been proved that it can lead to the downregulation of E-cadherin and thereby promote EMT [29].Thorne et al. found that expression level of the MTA1 gene in the infiltrative LCC1 cell line was twice than that of the non-invasive MCF27 cell line [30]. This suggests that the mRNA expression level of the MTA1 gene is related to its invasion and metastasis potential. The MPs family is a kind of protease with similar structure, which can degrade the extracellular matrix and is related to the ability to break through the barrier in the infiltration of tumor cells [31]. MMP-2 can specifically degrade collagen type IV and V, which are the major components of the basal membrane, and it plays an important role in tumor invasion and metastasis [32]. MMP-9, on the other hand, can degrade a variety of matrix components and destroy the basement membrane, leading to infiltration and metastasis of cancer cells to other parts of the human body [33]. MMP-2 and MMP-9 are now considered to be strongly connected with the EMT process, leading to metastasis of cancers [34]. Li et al. proved that MMP-2 and MMP-9 are highly expressed in breast cancer tissues and are closely related to lymph node metastasis and tumor staging [35]. Thus, we chose E-cadherin, MTA-1, MMP-2, and MMP-9 as markers to measure the EMT process in MCF-7 cell lines. As shown in the results, the expression of E-cadherin was elevated after the transfection by MB, Lipo, or MB + Lipo methods, but it was the highest by means of MB + Lipo. Simultaneously, the expressions of MTA-1, MMP-2, and MMP-9 were clearly decreased by the methods of MB, Lipo, or MB + Lipo, but they were the lowest using MB + Lipo. Given the evidence detailed above, it appears that the EMT process in the MCF-7 cell line is inhibited by suppressing shRNA to MTDH, and the effectiveness of the suppression can be reduced by raising the efficiency of transfection by use of UTMD combined with liposome.
Conclusions
In conclusion, UTMD combined with liposome could be used as a more efficient way to transfect shRNA into cells to suppress the expression of MTDH and thereby lead to the downregulation of proliferation, migration, and EMT process of MCF-7 cell lines.
Footnotes
Source of support: Departmental sources
Conflict of Interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Li J, Wang Z, et al. Metadherin is a novel prognostic marker for bladder cancer progression and overall patient survival. Asia Pac J Clin Oncol. 2012;8(3):e42–48. doi: 10.1111/j.1743-7563.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5(4):365–74. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhu K, Dai Z, Pan Q, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–302. doi: 10.1158/1078-0432.CCR-11-1327. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Zhai L, Zhao C, Lv S. MiR-153 inhibits epithelial-mesenchymal transition by targeting metadherin in human breast cancer. Breast Cancer Res Treat. 2015;150(3):501–9. doi: 10.1007/s10549-015-3346-y. [DOI] [PubMed] [Google Scholar]
- 6.Salvi A, Arici B, Alghisi A, et al. RNA interference against urokinase in hepatocellular carcinoma xenografts in nude mice. Tumour Biol. 2007;28(1):16–26. doi: 10.1159/000097699. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Zhu F, Jiang Y, et al. siRNA targeting survivin inhibits the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep. 2013;29(3):1183–88. doi: 10.3892/or.2012.2196. [DOI] [PubMed] [Google Scholar]
- 8.Li JM, Zhao MX, Su H, et al. Multifunctional quantum-dot-based siRNA delivery for HPV18 E6 gene silence and intracellular imaging. Biomaterials. 2011;32(31):7978–87. doi: 10.1016/j.biomaterials.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Zhi D, Zhang S, Qureshi F, et al. Synthesis and biological activity of carbamate-linked cationic lipids for gene delivery in vitro. Bioorg Med Chem Lett. 2012;22(11):3837–41. doi: 10.1016/j.bmcl.2012.01.097. [DOI] [PubMed] [Google Scholar]
- 10.Li QH, Jin WN, Zhang HM, et al. [Comparison of effect between homologous recombinant gene knockout and siRNA gene silence in cell lines]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(1):122–26. [ub Chinese] [PubMed] [Google Scholar]
- 11.Reslan L, Mestas JL, Herveau S, et al. Transfection of cells in suspension by ultrasound cavitation. J Control Release. 2010;142(2):251–58. doi: 10.1016/j.jconrel.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Un K, Kawakami S, Suzuki R, et al. Enhanced transfection efficiency into macrophages and dendritic cells by a combination method using mannosylated lipoplexes and bubble liposomes with ultrasound exposure. Hum Gene Ther. 2010;21(1):65–74. doi: 10.1089/hum.2009.106. [DOI] [PubMed] [Google Scholar]
- 13.Carson AR, McTiernan CF, Lavery L, et al. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res. 2012;72(23):6191–99. doi: 10.1158/0008-5472.CAN-11-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Yang L, Tian H, et al. [Enhancement of gene transfection efficiency and therapeutic effect of ultrasound-targeted microbubble destruction in vivo with cationic microbubble]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(2):228–36. doi: 10.7507/1002-1892.201706058. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokunaga E, Nakashima Y, Yamashita N, et al. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer. 2014;21(3):341–49. doi: 10.1007/s12282-012-0398-2. [DOI] [PubMed] [Google Scholar]
- 16.Hu G, Chong RA, Yang Q, et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15(1):9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostarelos K, Miller AD. Synthetic, self-assembly ABCD nanoparticles; A structural paradigm for viable synthetic non-viral vectors. Chem Soc Rev. 2005;34(11):970–94. doi: 10.1039/b307062j. [DOI] [PubMed] [Google Scholar]
- 18.Xie A, Belcik T, Qi Y, et al. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc Imaging. 2012;5(12):1253–62. doi: 10.1016/j.jcmg.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feril LB, Jr, Ogawa R, Kobayashi H, et al. Ultrasound enhances liposome-mediated gene transfection. Ultrason Sonochem. 2005;12(6):489–93. doi: 10.1016/j.ultsonch.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Trotman LC, Mosberger N, Fornerod M, et al. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nature Cell Biol. 2001;3(12):1092–100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- 21.Afadzi M, Strand SP, Nilssen EA, et al. Mechanisms of the ultrasound-mediated intracellular delivery of liposomes and dextrans. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(1):21–33. doi: 10.1109/TUFFC.2013.2534. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Song Y. LINC00473 predicts poor prognosis and regulates cell migration and invasion in gastric cancer. Biomed Pharmacother. 2018;107:1–6. doi: 10.1016/j.biopha.2018.07.061. [DOI] [PubMed] [Google Scholar]
- 23.Zhou CX, Wang CL, Yu AL, et al. MiR-630 suppresses breast cancer progression by targeting metadherin. Oncotarget. 2016;7(2):1288–99. doi: 10.18632/oncotarget.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Tang H, Chen B, et al. miR-26a suppresses tumour proliferation and metastasis by targeting metadherin in triple negative breast cancer. Cancer Lett. 2015;357(1):384–92. doi: 10.1016/j.canlet.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Memni H, Macherki Y, Klayech Z, et al. E-cadherin genetic variants predict survival outcome in breast cancer patients. J Transl Med. 2016;14(1):320. doi: 10.1186/s12967-016-1077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Sun T, Yuan Q, et al. The expressions of NEDD9 and E-cadherin correlate with metastasis and poor prognosis in triple-negative breast cancer patients. Onco Targets Ther. 2016;9:5751–59. doi: 10.2147/OTT.S113768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oka H, Shiozaki H, Kobayashi K, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53(7):1696–701. [PubMed] [Google Scholar]
- 28.Fujita N, Jaye DL, Kajita M, et al. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113(2):207–19. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 29.Mazumdar A, Wang RA, Mishra SK, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3(1):30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- 30.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci. 2004;117(Pt 3):373–80. doi: 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 31.Lukaszewicz-Zajac M, Mroczko B, Kornhuber J, Lewczuk P. Matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in the tumors of central nervous system (CNS) J Neural Transm (Vienna) 2014;121(5):469–77. doi: 10.1007/s00702-013-1143-5. [DOI] [PubMed] [Google Scholar]
- 32.Cao WH, Liu HM, Liu X, et al. Relaxin enhances in-vitro invasiveness of breast cancer cell lines by upregulation of S100A4/MMPs signaling. Eur Rev Med Pharmacol Sci. 2013;17(5):609–17. [PubMed] [Google Scholar]
- 33.Farina AR, Cappabianca L, DeSantis G, et al. Thioredoxin stimulates MMP-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes MMP-9 dependent invasion in human MDA-MB-231 breast cancer cells. FEBS Lett. 2011;585(20):3328–36. doi: 10.1016/j.febslet.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Lee AY, Fan CC, Chen YA, et al. Curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-E-cadherin pathway. Integr Cancer Ther. 2015;14(5):484–90. doi: 10.1177/1534735415588930. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Qiu Z, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol Lett. 2017;14(5):5865–70. doi: 10.3892/ol.2017.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]