Abstract
Purpose:
The objectives were to assess changes in sexual QOL and body image distress over time and to examine longitudinal associations between sexual QOL and body image variables with psychosocial outcomes in a sample of colorectal cancer patients.
Methods:
Participants (N=141) completed a mail-based survey assessing sexual QOL [sexual distress (ISS), treatment impact on sexual function (SFQ), sexual function (FSFI; IIEF)], body image distress (BIS), and psychosocial outcomes [relationship quality (DAS-4), depressive symptoms (CESD-SF), and health-related QOL (HRQOL; FACT-C)]; 88 patients completed 6-month follow-up surveys (62%). Gender and cancer subgroups (male vs. female; rectal vs. colon cancer) were compared and longitudinal models examined associations between sexual QOL and body image variables with psychosocial outcomes over time and by subgroup.
Results:
Impairments in sexual QOL and body image distress were common. Women and patients with rectal cancer reported worse body image distress compared to men (p=.005) and those with colon cancer (p=.03), respectively; compared to patients with colon cancer, those with rectal cancer reported worse treatment impact (p<.001) and marginally worse sexual function and HRQOL (p’s=.05). At 6-month follow-up, body image distress decreased (p=.02), while sexual QOL was stable (e.g., 58% classified as dysfunctional at both time points, p=.13). For most sexual and body image predictors, worse impairment was associated with worse psychosocial outcomes over time. Several significant gender and cancer subgroup effects were found.
Conclusions:
Sexual QOL and body image are compromised after colorectal cancer and tend to remain impaired if unaddressed. Sexual concerns should be addressed early to limit broader-reaching psychosocial effects.
Keywords: Colorectal Cancer, Sexuality, Sexual Function, Relationship Quality, Quality of Life, Body Image
Structured Abstract:
Findings from the current study have implications for the clinical care of colorectal cancer patients by suggesting that sexual and body image problems should be queried and managed early in patients’ care in order to limit potential damaging long-term effects such problems may have on patients’ intimate relationships and overall well-being. The study findings can inform future research by highlighting the importance of assessing multiple dimensions of colorectal cancer patients’ sexual QOL and body image in survey studies as well as the utility of longitudinal methods in understanding the impact of sexual problems on psychosocial health over time, and by suggesting directions for future interventions addressing patients’ sexual QOL and body image. Sexual QOL is of importance to the growing number of individuals facing long-term survivorship after a diagnosis of colorectal cancer.
Background
Colorectal cancers are the third most commonly diagnosed cancers in the U.S [1]. Among the multiple negative side effects of colorectal cancer surgeries and treatments, changes to patients’ sexual function and intimate relationships are common [2, 3]. Sexual complaints reported by colorectal cancer survivors include physical concerns (e.g., erectile dysfunction; vaginal dryness), often due to radiation and pelvic surgery, emotional or motivational changes (e.g., low sexual desire), relational challenges (e.g., loss of intimacy) [4], and body image changes that can impact sexual relationships [5–7]. As survivorship extends for many colorectal cancer survivors, sexual quality of life (QOL) is increasingly recognized as important to understand and address clinically [8].
Although research examining the impact of colorectal cancer on patients’ sexual outcomes has been increasing in recent years, there continue to be important gaps. For example, much of the research focuses on sexual function [4] whereas other important components of patients’ sexual experiences, such as sexual distress or the perceived impact of the cancer treatment on sexual function, are less well understood [9]. Such data could add granularity to our understanding of sexual QOL in this population and help to clarify potential intervention targets. Further, with a few notable exceptions [10, 11], much of the research examining sexual outcomes in colorectal cancer has been cross-sectional [6, 12, 13], making it difficult to determine whether sexual QOL or body image remains stable or changes over time after colorectal cancer. Given that sexual problems are known to persist for many cancer survivors, research is needed to examine whether such changes occur and, if so, whether these changes are associated longitudinally with important psychosocial outcomes, such as relationship quality, mood, or health-related QOL (HRQOL).
In an effort to fill these gaps, using self-report data obtained from a prospective longitudinal study conducted in 141 colorectal patients, the objectives of the current study were to: 1) assess sexual QOL (i.e., sexual distress, treatment impact on sexual function, and sexual function status as either functional or dysfunctional) and body image distress, 2) examine whether sexual QOL and body image distress are stable over a 6 month time period; and 3) evaluate whether worse sexual QOL and body image distress are longitudinally associated with worse psychosocial outcomes (i.e., relationship quality, depressive symptoms, HRQOL). In addressing these objectives, we also examined whether these associations would change over time, or would differ by gender or cancer site (colon versus rectal). Informed by prior research examining sexual outcomes for colorectal cancer survivors [2, 4, 9, 14, 15] and potential differences in such outcomes by gender or cancer site [9, 16], we hypothesized that: 1) sexual QOL and body image would be impaired across both genders and cancer sites but would be worse in women and patients with rectal cancer versus men and those with colon cancer, respectively, 2) sexual QOL would be largely stable over time, and 3) worse sexual QOL and body image distress would be associated with worse psychosocial outcomes.
Methods
Participants
Adult men and women older than age 21 with a diagnosis of colorectal cancer who had been treated at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center were eligible.
Procedure
Participants were recruited to participate in a prospective study of sexual QOL in individuals diagnosed and treated for colorectal cancer through the clinic or through mailings to Johns Hopkins tumor registry patients (diagnosed within the past 5 years). Of the 258 surveys administered, 143 (55%) were returned, two of which were excluded due to incorrect diagnosis. Thus, a final sample of 141 individuals was available for analysis at baseline; 57% were recruited in the clinic (n = 80); the rest (n = 61) were recruited through mailings. A mail-based paper survey was administered assessing a range of sexual, relationship, and physical and mental health outcomes at baseline and at six months (mean duration of follow-up in weeks = 28.3; SD = 6.5). The majority of participants completed follow-up surveys (n = 88; 62%; mean follow-up = 28.3 weeks; SD = .84). Of the participants who did not complete follow-up surveys, 22 entered a separate study pilot testing a couple-based intervention, 23 were lost to follow-up, and 8 died. Institutional Review Board approval was obtained and patients provided informed consent through completing the baseline survey.
Measures
Predictor Variables
Sexual Distress.
The Index of Sexual Satisfaction (ISS) [17] consists of 25 gender-neutral items assessing the degree of discord in a couple’s sexual relationship [18]. This measure was originally developed as a tool to assess improvement in psychological treatments for couples seeking clinical help for sexual problems [17]. The ISS has shown responsivity to a sexual QOL intervention conducted with colorectal cancer patients and their partners [18]. Sample items include “I feel that my sex life is lacking in quality” and “My partner does not want sex when I do.” Positively worded items (e.g., “I feel that our sex life really adds a lot to our relationship”) are reverse-scored such that higher scores indicate greater sexual distress.
Treatment Impact on Sexual Function.
The Treatment Impact subscale of the Sexual Function Questionnaire (SFQ) [19] consists of five questions assessing the impact of the patient’s medical condition (in this case “colorectal cancer or its treatment”) on aspects of the patient’s sex life (i.e., desire, arousal, orgasm, overall impact, and degree of adjustment to sexual difficulties). Higher scores indicate worse impact.
Sexual Function.
Established cut-off scores on gold standard multidimensional measures of sexual function for women (Female Sexual Function Index; FSFI; cut-off score of 26.55) [20, 21] and men (International Index of Erectile Function; IIEF; cut-off score of 25) [22, 23] were used to characterize patients in either the dysfunctional or functional range (0 = functional; 1 = dysfunctional), which facilitates cross-gender analyses.
Body Image Distress.
Body image distress was assessed using the Body Image Scale (BIS) [24], a 10-item scale developed for use in cancer patients that assesses body image changes and distress due to cancer and its treatment. Higher scores indicate worse distress.
Outcome Variables
Dyadic Adjustment.
The 4-item version of the Dyadic Adjustment Scale (DAS) [25] was used to measure relationship quality [26–28]. Higher scores signify better relationship quality.
Depressive Symptoms.
The Center for Epidemiologic Studies Depression Scale−Short Form (CESD-SF) is a 10-item, widely used self-report scale designed to measure symptoms of depression [29]. Higher scores indicate more severe depressive symptoms.
Health-Related Quality of Life (HRQOL).
The total score [30] of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) [31] was used to assess HRQOL, using items from four subscales (Physical Well-being, Emotional Well-being, Social Well-being, and Functional Well-Being) and a Colorectal Cancer Specific (CCS) module. In order to limit overlap with the sexual QOL variables, the item assessing sexual satisfaction (Social Well-Being Item #7) was removed. Higher scores indicate better HRQOL.
Covariates
Demographic and Medical Characteristics.
Socio-demographic characteristics were obtained through self-report. Medical variables were determined through self-report and medical chart review.
Statistical Methods
First, descriptive statistics were conducted on baseline socio-demographic and medical variables. Next, baseline socio-demographic characteristics (i.e., age, marital/partnered status, education, race/ethnicity) and medical characteristics (i.e., metastatic disease, currently receiving active treatment, and time since diagnosis) were compared by cancer site (colon versus rectal) and gender using 2-sample t-tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. In univariate analyses, study outcomes were compared across cancer site and gender using 2-sample t-tests or Chi-square tests. Pearson correlation coefficients were calculated to assess associations among study variables at baseline. We then conducted paired t-tests or McNemar tests on sexual QOL scores, as appropriate, to examine changes from baseline to 6-month follow-up. As in prior similar studies [5, 14], available data from those who self-identified as partnered (i.e., married or cohabiting) and as unpartnered (i.e., not married or cohabiting) individuals were used in analyses. Prior to longitudinal linear mixed models, the outcome variables were assessed for normality; depressive symptoms was negatively skewed and thus log-transformed for all mixed model analyses to improve the distribution. Other outcome measures were not transformed. To examine whether sexual QOL variables were associated with psychosocial outcomes and whether these associations changed over time, linear mixed-effects regressions were used including a random intercept to account for within-subject correlation, time of assessment (baseline or follow-up), and time from diagnosis to baseline.1 The decision to include gender and colon versus rectal cancer site in the models was made based on prior research showing them to be significantly associated with sexual and/or psychosocial outcomes in colorectal cancer [9, 16, 32]. All two-way and three-way interactions between study predictors of gender, time of assessment, and cancer site were evaluated. Analyses were performed using SPSS 22.0 (IBM Corp. 2014) and Stata Version 12.1 (StataCorp, 2011). Statistical significance was considered at the level of p < .05, two-tailed.
Results
Sample Characteristics
Baseline characteristics of the study sample are shown in Table 1. Overall, the sample was predominantly male, Caucasian, partnered, and highly educated. The majority was diagnosed with colon cancer as opposed to rectal cancer and had undergone surgery and/or chemotherapy, with fewer having undergone radiation therapy or pelvic surgery specifically. Half of the sample had metastatic disease and slightly less than half was currently receiving active treatment at baseline. Forty-three individuals had a history of ostomy use. A greater proportion of patients with rectal cancer (97%) were partnered compared to those with colon cancer (82%), χ2 = 5.4, p = .02. No other significant differences by either cancer site or by gender were found in other socio-demographic characteristics, and no differences in medical characteristics were found.
Table 1.
Demographic and Clinical Characteristics for the Study Sample (N=141)
| Variable | N (%) |
|---|---|
| Age: Mean ± SD, y | 57.7 ± 13.2 |
| Female Gender | 59 (41.8) |
| Education | |
| Less than Bachelor’s Degree | 46 (32.6) |
| Bachelor’s Degree or Advanced Degree | 95 (67.4) |
| Marital Status | |
| Married or cohabiting | 121 (85.8) |
| Ethnicity | |
| Caucasian | 117 (83.0) |
| African American | 11 (7.8) |
| Asian | 9 (6.4) |
| Other | 4 (2.8) |
| Tumor Site | |
| Colon | 104 (73.8) |
| Rectum | 37 (26.2) |
| Disease Stage at Survey | |
| I – II | 37 (26.2) |
| III | 34 (24.1) |
| IV | 70 (49.6) |
| Currently receiving treatment | 56 (39.7) |
| Length of time since diagnosis (months) | 31.5 ± 23.0 |
| Treatment received | |
| Surgery | 132 (93.6) |
| Chemotherapy | 106 (75.2) |
| Radiation | 45 (31.9) |
| Pelvic Surgery | 50 (35.5) |
| Ostomy Status | |
| Never had ostomy | 98 (69.5) |
| Past ostomy | 18 (12.8) |
| Current ostomy | 25 (17.7) |
Baseline Scores on Sexual and Psychosocial Variables
Baseline scores on sexual and psychosocial variables are shown by cancer site and gender in Table 2. The majority of the sample fell into the dysfunctional range of sexual function. At baseline, compared to men, women reported worse body image distress (p = .005) but did not differ from men on sexual QOL variables. Compared to those with colon cancer, patients with rectal cancer reported significantly worse treatment impact on sexual function (p < .001) and body image distress (p = .03). Rectal cancer patients were marginally more likely to be classified as sexually dysfunctional (p = .049), and reported marginally poorer HRQOL (p=.046) compared to colon cancer patients. No other differences were found.2
Table 2.
Baseline Scores on Study Variables by Cancer Site and Gender
| Colon Cancer | Rectal Cancer | Men | Women | |||
|---|---|---|---|---|---|---|
| Variable | Mean (SD) | p | Mean (SD) | p | ||
| Treatment Impact on Sexual Function | 2.25 (1.04) | 3.12 (.85) | <.001 | 2.47 (1.04) | 2.57 (1.12) | .64 |
| Sexual Distress | 31.76 (18.76) | 30.87 (19.85) | .83 | 30.23 (17.65) | 33.74 (21.32) | .39 |
| Male Sexual Function | 43.20 (24.11) | 32.12 (23.55) | .06 | -- | -- | -- |
| Female Sexual Function | 16.65 (11.26) | 12.95 (10.58) | .34 | -- | -- | -- |
| Body Image Distress | 6.70 (7.39) | 9.85 (7.21) | .03 | 6.03 (6.85) | 9.62 (7.79) | .005 |
| Relationship Quality | 16.46 (3.08) | 16.94 (2.96) | .43 | 16.58 (3.06) | 16.64 (3.04) | .92 |
| Depressive Symptoms | 6.51 (6.09) | 6.93 (5.60) | .70 | 6.18 (5.50) | 7.24 (6.53) | .31 |
| Quality of Life | 104.57 (18.49) | 97.73 (17.08) | .05 | 104.65 (17.33) | 100.10 (19.42) | .16 |
| Level of Sexual Concerns | N (%) | -- | N (%) | -- | ||
| Sexual Function Status, Dysfunctional | 51 (62.2%) | 29 (80.6%) | .05 | 49 (64.5%) | 31 (73.8%) | .30 |
The available cases and actual ranges of scores on study measures were: Treatment Impact on Sexual Function [n=113; .40–4.88]; Sexual Distress [n=106; 0–82.67]; Female Sexual Function [n=42; 2–32.80]; Male Sexual Function [n=76; 5–75]; Body Image Distress [n=141; 0–30]; Relationship Quality [n=117; 6–21]; Depressive Symptoms [n=140; 0–25]; Quality of Life [n=137; 54–132].
Correlations among Study Variables
Correlations among study variables at baseline were conducted separately for men and women (see Table 3). Overall, sexual QOL variables were inversely related to the psychosocial outcomes, and correlations were similar across genders. Strong correlations were found between worse sexual distress and poorer relationship quality, and between worse body image distress and more severe depressive symptoms and worse quality of life.
Table 3.
Correlation Coefficients for Domains of Functioning at Baseline by Gender
| Measures | (1) | (2) | (3) | (4) | (5) | (6) | (7) |
|---|---|---|---|---|---|---|---|
| (1) Treatment Impact on Sexual Function | -- | .53** | −.62*** | .50** | −.10 | .43** | −.53*** |
| (2) Sexual Distress | .40** | -- | −.65*** | .27 | −.64*** | .48** | −.33* |
| (3) Sexual Function | −.54*** | −.28 | -- | −.11 | .29+ | −.35* | .29+ |
| (4) Body Image Distress | .40*** | .35** | −.05 | -- | −.18 | .51*** | −.59*** |
| (5) Relationship Quality | −.07 | −.59*** | .03 | −.30* | -- | −.53*** | .38* |
| (6) Depressive Symptoms | .24* | .40** | −.05 | .64*** | −.16 | -- | −.80*** |
| (7) Quality of Life | −.40** | −.31* | .18 | −.60*** | .17 | −.76*** | -- |
p<.05
p<.01
p<.001
Correlation coefficients above the diagonal line are for female participants; below the diagonal line are for male patients.
Stability of Sexual QOL Variables and Body Image Distress over Time
With the exception of body image distress, which decreased significantly from baseline (M = 7.25; SD = 7.75) to follow-up (M = 5.99; SD = 6.78, p = .02), sexual QOL was stable (i.e., did not change) over time. Comparisons of sexual function status at baseline and follow-up were not significant (p = .13), indicating no change in the proportions classified as sexually dysfunctional; 58.1% and 32.8% were classified as either dysfunctional or functional, respectively, at both time points. Similarly, neither treatment impact on sexual function nor sexual distress changed significantly from baseline (M = 2.32, SD = 1.10; M = 30.64, SD = 19.04, respectively) to 6 month follow-up (M = 2.26, SD = .98, p=.43; M = 30.76, SD = 17.75, p = .93, respectively).
Relationships between Sexual QOL Variables Multiple Domains of Psychosocial Function3
Significant interactions are depicted graphically in Figure 1. Main effects of the covariates of cancer site, gender, and time since diagnosis were not significant in any of the models.
Figure 1.
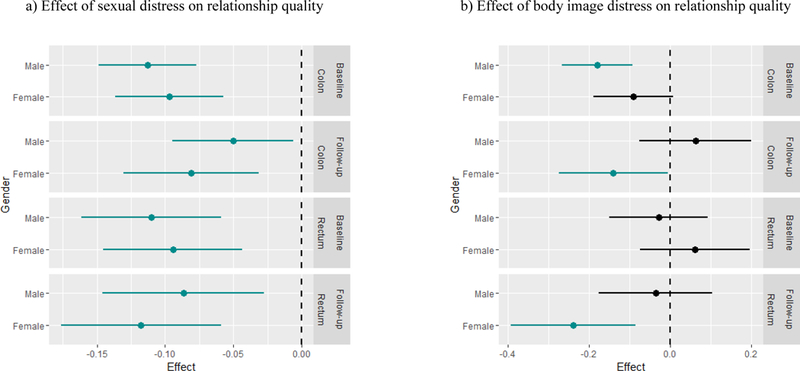
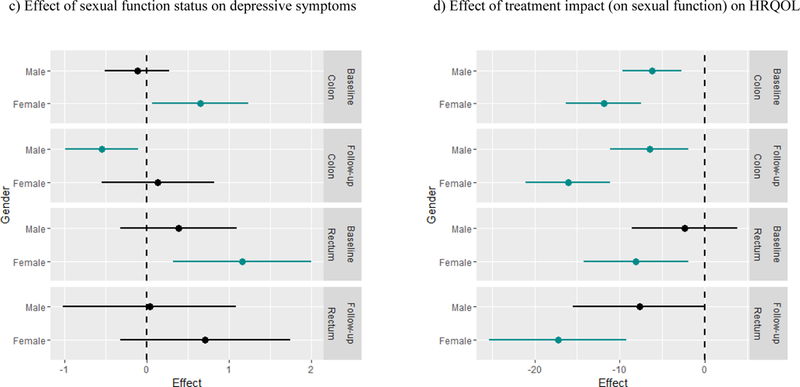
Effects of a 1 unit increase in predictors on outcomes Significant interactions are depicted graphically in Figure 1 as effects of a 1 unit increase in the predictor on the outcome by each cancer site and gender subgroup according to time point (baseline or follow-up). Model-based estimates for effects within subgroups are shown with respective confidence intervals, with green lines representing significant subgroup effects (i.e., subgroups with effects significantly different from 0).
Relationship Quality.
As shown in Table 4, we found a significant main effect of sexual distress (β = −.11, CI −.15, −.08, p < .001) indicating that worse sexual distress was associated with poorer relationship quality. As shown in Figure 1a, the interaction between time of assessment and sexual distress (β = .06, CI .02, .11, p = .005) indicates that the association between worse sexual distress and poorer relationship quality was attenuated at follow-up compared to baseline. The effect of sexual distress on relationship quality did not differ by gender or cancer site.
Table 4.
Summary of Main Effects and Significant Interaction Effects from Longitudinal Linear Mixed Regression Models
| Psychosocial Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Relationship Quality | Depressive Symptoms | HRQOL | |||||||
| Sexual QOL Predictors | β [CI] | P value | Significant interactions (p<.05) | β [CI] | P value | Significant Interactions (p<.05) | β [CI] | P value | Significant Interactions (p<.05) |
| Treatment Impact on Sexual Function (TI) | −.47 [−1.11, .17] | .15 | -- | .26 [.07, .44] | .008 | -- | −6.19 [−9.65, −2.72] | <.001 | 1. TI x gender |
| Sexual Distress (SD) | −.11 [−.15, −.08] | <.001 | 1. SD x time | .02 [.01, .03] | <.001 | -- | −.31 [−.55, −.06] | .02 | -- |
| Sexual Function Status (SFS) | .21 [−1.19, 1.61] | .77 | -- | −.12 [−.51, .28] | .56 | 1. SFS x gender | .03 [−8.2, 8.25] | .99 | -- |
| Body Image Distress (BID) | −.18 [−.27, −.09] | <.001 | 1. Time x gender 2. BID x time 3. BID x cancer site 4. BID x cancer site x time 5. BID x time x gender |
.07 [.05, .10] | <.001 | -- | −1.66 [−2.17, −1.16] | <.001 | -- |
Note: Standardized coefficients (β) and confidence intervals (CI) are shown for main effects. Significant P values are bolded.
We also found a significant main effect (see Table 4) of body image distress, indicating that greater body image distress was associated with poorer relationship quality (β = −.18, CI −.27, −.09, p < .001). The specific subgroup effects (i.e., combinations of main and interaction effects) were significant for male colon cancer patients at baseline, and for female colon and female rectal cancer patients at follow-up. In the models for treatment impact on sexual function and sexual function status on relationship quality, neither the main effects nor the interaction effects were significant.
Depressive Symptoms.
In three separate models with depressive symptoms as the outcome, there were significant main effects of predictors such that worse sexual QOL or body image distress was each associated with more severe depressive symptoms: treatment impact on sexual function (β = .26, CI .07, .44, p = .008), sexual distress (β = .02, CI .01, .03, p < .001), and body image distress (β = .07, CI .05, .10, p < .001). None of these effects varied over time or across gender or cancer site. While there was no significant main effect found for sexual function status on depressive symptoms, the interaction between sexual function status and gender was significant (β = .77, CI .10, 1.44, p = .02; see Figure 1c), such that the effect of being classified in the dysfunctional range of sexual function on depressive symptoms was consistently greater for women than for men. No other effects emerged as significant.
Health-Related Quality of Life (HRQOL).
There was a significant effect of treatment impact on HRQOL (β = −6.19, CI −9.65, −2.72, p <.001), such that worse treatment impact was associated with poorer HRQOL. Further, there was a significant interaction between treatment impact and gender (β = −5.70, CI −10.82, −.58, p = .03), such that the effect of worse treatment impact on poorer quality of life was more pronounced for women compared to men (see Figure 1d). The effect of treatment impact on sexual function on HRQOL did not vary by time of assessment or by cancer site. The main effect of sexual distress was significant (β = −.31, CI 0.55, −.06, p = .02) suggesting that worse sexual distress was associated with poorer HRQOL, and this effect did not vary over time or across gender or cancer site. Sexual function status did not emerge as significant in the model, nor were any interactions found significant.
Conclusions
Findings of the current study supported the hypothesis that sexual QOL and body image distress would be impaired for patients with colorectal cancer. The impairment was evident across a range of measures assessing distinct dimensions of patients’ sexual health. For example, average scores on the sexual distress measure were comparable to those reported by a clinically distressed sample [17]. Findings suggest that for patients with colorectal cancer, sexual health is impacted in a number of ways, extending beyond function to include distress, perceived impact, and body image distress. The additional hypothesis that sexual and body image outcomes would be worse for women and for patients with rectal cancer was partially supported; women reported worse body image distress compared to men in the study sample, but did not reported worse outcomes on sexual QOL. By contrast, patients with rectal cancer reported impaired sexual QOL and body image distress, as well as worse HRQOL. These findings echo findings of prior research [3, 5, 7, 33] and are likely due in part to receiving treatments (e.g., preoperative radiation therapy, pelvic surgery) known to interfere with physiological sexual response and other domains of physical function [34, 35].
In light of research suggesting that sexual problems can persist for many cancer survivors [14, 15, 36], we hypothesized that sexual QOL and body image distress would be largely stable for the study duration. Findings partially supported these hypotheses. While sexual QOL did not improve over a six month period, by contrast, body image distress decreased significantly over the same period of time. Taken alongside findings of previous studies examining change in sexual outcomes and body image [11, 14, 15], these results suggest potentially different post-treatment trajectories for sexual outcomes compared to body image distress for colorectal cancer survivors, with body image emerging as possibly more amenable to improvement over time as compared to sexual QOL. Findings of significant unexpected time by predictor interactions in several analyses further suggest that studies using longitudinal designs may be preferable to those using cross-sectional designs because the longitudinal design can capture changes in the associations between sexual QOL or body image and psychosocial outcomes that might occur over time.
One of the most striking findings from this study pertained to the significant associations found between most of the sexual QOL variables and body image distress and worse psychosocial outcomes. Though such associations were predicted, they were noteworthy in that they were seen across different and distinct predictor and outcome variables and because they tended to hold after accounting for gender, cancer site, and time since diagnosis. Although the current findings cannot confer causality, the directionality of these associations is consistent with prior research demonstrating negative consequences of cancer-related sexual sequelae on psychosocial outcomes (e.g., QOL, mood) in cancer [14, 37]. The associations found here are also consistent with research evidence of a causal pathway both from sexual dysfunction to worse psychological well-being [38], and, conversely, from improved sexual function to improved mood [39], in non-cancer populations. Also intriguing, the findings of gender interactions in two models (i.e., sexual function on depressive symptoms; treatment impact on HRQOL) suggest that women may be particularly vulnerable to negative effects of worse sexual QOL on their mood and overall well-being. Other researchers have similarly found that women with colorectal cancer reported worse sexual QOL [40] and greater psychological distress [7] compared to men, and that associations between sexual QOL and psychological distress were strongly linked for women [12]. Large well-designed research studies on sexual QOL in women with colorectal cancer could help clarify these associations, yet such studies are challenging to conduct because of low recruitment and inadequate survey completion [15, 18, 41]. Therefore, well-funded multi-site studies are needed.
Clinical Implications
These findings suggest the importance of identifying and addressing sexual concerns and body image distress early in care in order to limit the negative effects on colorectal cancer patients’ intimate relationships and well-being. Further, it is critical that patients be assessed regardless of their gender, age, partnered status, specific cancer site, or treatment status [42]. A simple brief validated sexual concerns screener [43, 44], could be used to identify patients with sexual or body image concerns and lead to timely treatments. Recent work offers guidance to clinicians to assess and refer patients who report sexual and body image concerns once identified [44, 45], and a few small intervention studies offer promising findings for interventions addressing sexual outcomes in samples of patients with colorectal cancer [18, 41]. Interventions are particularly needed to help ensure that the sexual concerns of women treated for cancer are addressed given that compared to men with cancer, women are significantly less likely to have sexual concerns discussed in their care [46].
Study Limitations
This study has several limitations, primary of which is that the findings cannot be interpreted as causal in nature. Another limitation is the heterogeneity of the study sample in their clinical characteristics. We controlled for time from diagnosis and examined subgroups by cancer site and gender, but additional studies should assess sexual outcomes at treatment-specific time points, consider outcomes according to different patient subgroups by stage of disease, and use a longer duration of follow-up. Further, while the study was designed to assess sexual QOL in this colorectal cancer sample, it was not powered to detect modest interaction effects and future studies should be powered to find such effects. Additionally, the study sample was fairly homogenous with respect to race/ethnicity and was highly educated. Finally, sexual orientation was not queried and future studies could consider examine these important outcomes for sexual minority groups. Larger sample sizes and oversampling to ensure diversity could help determine whether these findings would hold for patients from racial/ethnic or sexual minority backgrounds. Despite these limitations, the current study contributes to the research by assessing multiple domains of sexual QOL in colorectal cancer and by examining associations between sexual and psychosocial outcomes over time. Sexual QOL is of importance to the growing number of individuals facing long-term survivorship after a diagnosis of colorectal cancer [8].
Acknowledgements:
This study was supported by American Cancer Society Postdoctoral Fellowship PF-09–154-01-CPPB (Reese). Jennifer Barsky Reese is currently supported by a Mentored Research Scholar Grant (MRSG-14–031-01-CPPB) from the American Cancer Society and by P30CA006927 from the National Cancer Institute.
Footnotes
Compliance with Ethical Standards
Disclosure of potential conflicts of interest: The authors have no relationships that might bias this work or any conflicts of interest to report.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Because age was positively correlated with time since diagnosis at baseline (r=.25, p=.003), to avoid redundancy, age was excluded from longitudinal models. In addition, because a diagnosis of rectal cancer strongly overlapped with ostomy use (p<.001), to avoid redundancy, we selected cancer site for inclusion in the regression analyses.
Given that the association between cancer site and marital/partnered status were significant, we examined whether group differences on sexual QOL variables were due to partnered status differences by re-rerunning comparisons by both cancer site and gender on sexual QOL variables relevant to partnered sexual activity (sexual distress, treatment impact on sexual function, and sexual function) only in the partnered subsample (N=121). Because the findings were similar, data from the total sample were retained for analyses.
Contributor Information
Jennifer Barsky Reese, Cancer Prevention and Control Program, Fox Chase Cancer Center, 333 Cottman Ave., Philadelphia, PA, 19111; 215-214-3223 (Ph); Jennifer.Reese@fccc.edu.
Elizabeth Handorf, Department of Biostatistics, Fox Chase Cancer Center, 333 Cottman Ave., Philadelphia, PA, 19111.
Jennifer A. Haythornthwaite, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, 5501 Hopkins Bayview Circle, Asthma and Allergy Center Suite 100, Baltimore, MD 21224.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA SEER Cancer Statistics Review, 1975–2014, National Cancer Institute Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 2.Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, Macrae HM, Gryfe R, McLeod RS (2005) Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 242: 212–223. 10.1097/01.sla.0000171299.43954.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange MM, Marijnen CA, Maas CP, Putter H, Rutten HJ, Stiggelbout AM, Meershoek-Klein Kranenbarg E, van de Velde CJ (2009) Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer 45: 1578–1588. 10.1016/j.ejca.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 4.Traa M, De Vries J, Roukema J, Den Oudsten B (2012) Sexual (dys) function and the quality of sexual life in patients with colorectal cancer: a systematic review. Ann Oncol 23: 19–27. 10.1093/annonc/mdr133 [DOI] [PubMed] [Google Scholar]
- 5.Reese JB, Finan PH, Haythornthwaite JA, Kadan M, Regan KR, Herman JM, Efron J, Diaz LA Jr., Azad NS (2014) Gastrointestinal ostomies and sexual outcomes: a comparison of colorectal cancer patients by ostomy status. Support Care Cancer 22: 461–468. 10.1007/s00520-013-1998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milbury K, Cohen L, Jenkins R, Skibber J, Schover L (2013) The association between psychosocial and medical factors with long-term sexual dysfunction after treatment for colorectal cancer. Support Care Cancer 21: 793–802. 10.1007/s00520-012-1582-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krouse RS, Herrinton LJ, Grant M, Wendel CS, Green SB, Mohler MJ, Baldwin CM, McMullen CK, Rawl SM, Matayoshi E, Coons SJ, Hornbrook MC (2009) Health-related quality of life among long-term rectal cancer survivors with an ostomy: manifestations by sex. J Clin Oncol 27: 4664–4670. 10.1200/JCO.2008.20.9502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denlinger CS, Barsevick AM (2009) The Challenges of Colorectal Cancer Survivorship. J Natl Compr Canc Netw 7: 883–894. 10.6004/jnccn.2009.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Den Oudsten BL, Traa MJ, Thong MSY, Martijn H, De Hingh IHJT, Bosscha K, van de Poll-Franse LV (2012) Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: A population-based study. Eur J Cancer 48: 3161–3170. 10.1016/j.ejca.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 10.Traa MJ, Braeken J, De Vries J, Roukema JA, Slooter GD, Crolla RM, Borremans MP, Den Oudsten BL (2015) Sexual, marital, and general life functioning in couples coping with colorectal cancer: a dyadic study across time. Psychooncology 24: 1181–1188. 10.1002/pon.3801 [DOI] [PubMed] [Google Scholar]
- 11.da Silva GM, Hull T, Roberts PL, Ruiz DE, Wexner SD, Weiss EG, Nogueras JJ, Daniel N, Bast J, Hammel J, Sands D (2008) The effect of colorectal surgery in female sexual function, body image, self-esteem and general health: a prospective study. Ann Surg 248: 266–272. 10.1097/SLA.0b013e3181820cf4 [DOI] [PubMed] [Google Scholar]
- 12.Philip EJ, Nelson C, Temple L, Carter J, Schover L, Jennings S, Jandorf L, Starr T, Baser R, Duhamel K (2013) Psychological correlates of sexual dysfunction in female rectal and anal cancer survivors: analysis of baseline intervention data. J Sex Med 10: 2539–2548. 10.1111/jsm.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe L, Patel D, Clarke S (2011) The relationship between body image disturbance and distress in colorectal cancer patients with and without stomas. J Psychosom Res 70: 395–402. 10.1016/j.jpsychores.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 14.Reese JB, Shelby RA, Keefe FJ, Porter LS, Abernethy AP (2010) Sexual concerns in cancer patients: a comparison of GI and breast cancer patients. Support Care Cancer 18: 1179–1189. 10.1007/s00520-009-0738-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman JM, Narang AK, Griffith KA, Zalupski MM, Reese JB, Gearhart SL, Azad NS, Chan J, Olsen L, Efron JE, Lawrence TS, Ben-Josef E (2013) The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 85: e15–e19. 10.1016/j.ijrobp.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Fabio F, Koller M, Nascimbeni R, Talarico C, Salerni B (2008) Long-term outcome after colorectal cancer resection. Patients’ self-reported quality of life, sexual dysfunction and surgeons’ awareness of patients’ needs. Tumori 94: 30–35. doi: [DOI] [PubMed] [Google Scholar]
- 17.Hudson WH, Harrison DF, Crosscup PC (1981) A short-form scale to measure sexual discord in dyadic relationships. The Journal of Sex Research 17: 157–174. 10.1080/00224498109551110 [DOI] [Google Scholar]
- 18.Barsky Reese J, Porter LS, Regan KR, Keefe FJ, Azad NS, Diaz LA Jr., Herman JM, Haythornthwaite JA (2014) A randomized pilot trial of a telephone-based couples intervention for physical intimacy and sexual concerns in colorectal cancer. Psychooncology 23: 1005–1013. 10.1002/pon.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syrjala KL, Schroeder TC, Abrams JR, Atkins TZ, Brown WS, Sanders JE, Schubert MA, Heiman JR (2000) Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res 37: 213–225. 10.1080/00224490009552042 [DOI] [Google Scholar]
- 20.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R Jr. (2000) The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 26: 191–208. 10.1080/009262300278597 [DOI] [PubMed] [Google Scholar]
- 21.Wiegel M, Meston C, Rosen R (2005) The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther 31: 1–20. 10.1080/00926230590475206 [DOI] [PubMed] [Google Scholar]
- 22.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A (1997) The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49: 822–830. 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 23.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH (1999) Diagnostic evaluation of the erectile function domain of the international index of erectile function. Urology 54: 346–351. 10.1016/S0090-4295(99)00099-0 [DOI] [PubMed] [Google Scholar]
- 24.Hopwood P, Fletcher I, Lee A, Al Ghazal S (2001) A body image scale for use with cancer patients. Eur J Cancer 37: 189–197. doi: S0959804900003531 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Sabourin S, Valois P, Lussier Y (2005) Development and validation of a brief version of the dyadic adjustment scale with a nonparametric item analysis model Psychol Assess 17: 15–27. 10.1037/1040-3590.17.1.15 [DOI] [PubMed] [Google Scholar]
- 26.Harden JK, Sanda MG, Wei JT, Yarandi H, Hembroff L, Hardy J, Northouse LL, Group PCS (2013) Partners’ long-term appraisal of their caregiving experience, marital satisfaction, sexual satisfaction, and quality of life 2 years after prostate cancer treatment. Cancer Nurs 36: 104–113. 10.1097/NCC.0b013e3182567c03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford A, Fellman B, Urbauer D, Gallegos J, Meaders K, Tung C, Ramondetta L (2015) Assessment of sexual activity and dysfunction in medically underserved women with gynecologic cancers. Gynecol Oncol 139: 134–140. 10.1016/j.ygyno.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reese JB, Porter LS, Somers TJ, Keefe FJ (2012) Pilot feasibility study of a telephone-based couples intervention for physical intimacy and sexual concerns in colorectal cancer. J Sex Marital Ther 38: 402–417. 10.1080/0092623x.2011.606886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andresen EM, Malmgren JA, Carter WB, Patrick DL (1994) Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am J Prev Med 10: 77–84. doi: [PubMed] [Google Scholar]
- 30.Ganesh V, Agarwal A, Popovic M, Cella D, McDonald R, Vuong S, Lam H, Rowbottom L, Chan S, Barakat T, DeAngelis C, Borean M, Chow E, Bottomley A (2016) Comparison of the FACT-C, EORTC QLQ-CR38, and QLQ-CR29 quality of life questionnaires for patients with colorectal cancer: a literature review. Support Care Cancer 24: 3661–3668. 10.1007/s00520-016-3270-7 [DOI] [PubMed] [Google Scholar]
- 31.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D (1999) Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res 8: 181–195. 10.1023/A:1008821826499 [DOI] [PubMed] [Google Scholar]
- 32.Grant M, McMullen CK, Altschuler A, Mohler MJ, Hornbrook MC, Herrinton LJ, Wendel CS, Baldwin CM, Krouse RS (2011) Gender differences in quality of life among long-term colorectal cancer survivors with ostomies. Oncol Nurs Forum 38: 587–596. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benedict C, Philip EJ, Baser RE, Carter J, Schuler TA, Jandorf L, DuHamel K, Nelson C (2016) Body image and sexual function in women after treatment for anal and rectal cancer. Psychooncology 25: 316–323. 10.1002/pon.3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho VP, Lee Y, Stein SL, Temple LKF (2011) Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum 54: 113–125 10.1007/DCR.0b013e3181fb7b82 [DOI] [PubMed] [Google Scholar]
- 35.Ameda K, Kakizaki H, Koyanagi T, Hirakawa K, Kusumi T, Hosokawa M (2005) The long-term voiding function and sexual function after pelvic nerve-sparing radical surgery for rectal cancer. Int J Urol 12: 256–263. 10.1111/j.1442-2042.2005.01026.x [DOI] [PubMed] [Google Scholar]
- 36.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L (1996) Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat 38: 183–199. 10.1007/BF01806673 [DOI] [PubMed] [Google Scholar]
- 37.Levin AO, Carpenter KM, Fowler JM, Brothers BM, Andersen BL, Maxwell GL (2010) Sexual Morbidity Associated With Poorer Psychological Adjustment Among Gynecological Cancer Survivors. Int J Gynecol Cancer 20: 461–470. 10.1111/IGC.0b013e3181d24ce0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen RC, Seidman SN, Menza MA, Shabsigh R, Roose SP, Tseng LJ, Orazem J, Siegel RL (2004) Quality of life, mood, and sexual function: a path analytic model of treatment effects in men with erectile dysfunction and depressive symptoms. Int J Impot Res 16: 334–340. 10.1038/sj.ijir.3901197 [DOI] [PubMed] [Google Scholar]
- 39.Moncada I, Martinez-Jabaloyas JM, Rodriguez-Vela L, Gutierrez PR, Giuliano F, Koskimaki J, Farmer IS, Renedo VP, Schnetzler G (2009) Emotional changes in men treated with sildenafil citrate for erectile dysfunction: a double-blind, placebo-controlled clinical trial. J Sex Med 6: 3469–3477. 10.1111/j.1743-6109.2009.01514.x [DOI] [PubMed] [Google Scholar]
- 40.Sun V, Grant M, Wendel CS, McMullen CK, Bulkley JE, Herrinton LJ, Hornbrook MC, Krouse RS (2016) Sexual function and health-related quality of life in long-term rectal cancer survivors. J Sex Med 13: 1071–1079. 10.1016/j.jsxm.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DuHamel K, Schuler T, Nelson C, Philip E, Temple L, Schover L, Baser RE, Starr TD, Cannon K, Jennings S, Jandorf L, Carter J (2015) The sexual health of female rectal and anal cancer survivors: results of a pilot randomized psycho-educational intervention trial. J Cancer Surviv 10.1007/s11764-015-0501-8 [DOI] [PMC free article] [PubMed]
- 42.Traa MJ, De Vries J, Roukema JA, Rutten HJT, Den Oudsten BL (2014) The sexual health care needs after colorectal cancer: the view of patients, partners, and health care professionals. Support Care Cancer 22: 763–772. 10.1007/s00520-013-2032-z [DOI] [PubMed] [Google Scholar]
- 43.Flynn KE, Lindau ST, Lin L, Reese JB, Jeffery DD, Carter J, Baron SR, Abramsohn EM, Weinfurt KP (2015) Development and validation of a single-item screener for self-reporting sexual problems in U.S. adults. J Gen Intern Med 30: 1468–1475. 10.1007/s11606-015-3333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bober SL, Reese JB, Barbera L, Bradford A, Carpenter KM, Goldfarb S, Carter J (2016) How to ask and what to do: a guide for clinical inquiry and intervention regarding female sexual health after cancer. Current opinion in supportive and palliative care 10: 44–54. 10.1097/spc.0000000000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindau ST, Abramsohn EM, Baron SR, Florendo J, Haefner HK, Jhingran A, Kennedy V, Krane MK, Kushner DM, McComb J, Merritt DF, Park JE, Siston A, Straub M, Streicher L (2016) Physical examination of the female cancer patient with sexual concerns: What oncologists and patients should expect from consultation with a specialist. CA Cancer J Clin 66: 241–263. 10.3322/caac.21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reese JB, Sorice K, Beach MC, Porter LS, Tulsky JA, Daly MB, Lepore SJ (2017) Patient-provider communication about sexual concerns in cancer: a systematic review. J Cancer Surviv 11: 175–188. 10.1007/s11764-016-0577-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


