Summary
Inhibition is fundamental to information processing by neural circuits. In the olfactory bulb (OB), glomeruli are the functional units for odor information coding, but inhibition among glomeruli is poorly characterized. We used two-photon calcium imaging in anesthetized and awake mice to visualize both odorant-evoked excitation and suppression in OB output neurons (mitral and tufted, MT cells). MT cell response polarity mapped uniformly to discrete OB glomeruli, allowing us to analyze how inhibition shapes OB output relative to the glomerular map. Odorants elicited unique patterns of suppression in only a subset of glomeruli in which such suppression could be detected, and excited and suppressed glomeruli were spatially intermingled. Binary mixture experiments revealed that interglomerular inhibition could suppress excitatory mitral cell responses to odorants. These results reveal that inhibitory OB circuits nonlinearly transform odor representations and support a model of selective and nonrandom inhibition among glomerular ensembles.
Introduction
Synaptic inhibition is fundamental to information processing by cortical networks. In sensory systems including vision, somatosensation, and audition, inhibitory circuits impact response features such as the gain, threshold, and selectivity of responses to sensory stimuli (Isaacson and Scanziani, 2011). However, for olfaction - a primary sensory modality for most mammals - little is known about how inhibitory processing shapes odorant responses or how inhibitory circuits are engaged by natural odorant sampling. In the olfactory bulb (OB), the first stage of olfactory processing, multiple inhibitory circuits impact OB output via mitral/tufted (MT) cells (Fukunaga et al., 2014; Shao et al., 2009; Wachowiak and Shipley, 2006). Characterizing these circuits in vitro has led to hypotheses for how inhibition shapes odor coding that include sharpening or decorrelation of odor representations, gain control, filtering weak inputs, temporally shaping MT spike patterns and synchronizing MT spike timing (Cleland and Linster, 2012; Gire and Schoppa, 2009; Najac et al., 2015; Shao et al., 2013). With few exceptions however (Banerjee et al., 2015; Fukunaga et al., 2014; Kato et al., 2013; Yokoi et al., 1995), these hypotheses remain largely untested in vivo.
In the early olfactory system, the functional unit of coding is the glomerulus, which represents sensory input from a single odorant receptor and is the singular site of excitatory input onto MT cells projecting to olfactory cortex. MT cell responses to sensory input are shaped by both intraglomerular inhibitory circuits (i.e., feedforward or recurrent inhibition in the glomerular neuropil) and interglomerular circuits (i.e., lateral inhibition). Intra- and interglomerular circuits appear to have distinct and dissociable roles in shaping MT odor responses (Aungst et al., 2003; Fukunaga et al., 2014; Shao et al., 2012; Shao et al., 2013). However, to fully test predictions for how OB inhibitory circuits impact odor representations, it is critical to relate functional measures of inhibition to odor space and to the glomeruli that represent the functional modules of odor coding at this stage.
In the present study, we used in vivo two-photon GCaMP imaging to visualize sensory-driven inhibition in the OB of awake and anesthetized mice, as reflected in the suppression of activity imaged from MT somata and their apical dendritic tufts. This approach allowed us to characterize how inhibition shapes odor representations at the level of MT output and in the context of the OB glomerular map. We found that odorant-evoked inhibition potently suppresses output from MT cells innervating the same glomerulus and that this suppression is selective for particular glomeruli and odorant-specific. A given odorant excites and suppresses a specific combination of spatially intermingled glomeruli, and MT output from a glomerulus is excited or suppressed by a distinct combination of odorants. Using odorant mixtures, we showed that this suppression is sufficiently strong to gate excitatory responses to other odorants. We further found that odorant-specific MT cell suppression is mediated, at least in part, by interglomerular inhibitory interactions that appear to nonrandomly target glomeruli across the dorsal OB and differentially target deep versus superficial MT cells. These results reveal a previously unseen level of specificity in the impact of inhibitory circuits on OB output patterns and a surprising richness in the representation of odors at the level of glomerular maps.
Results
Mapping mitral/tufted cell excitation and suppression to olfactory bulb glomeruli
We expressed the genetically-encoded calcium reporter GCaMP6f (Chen et al., 2013) selectively in piriform cortex-projecting mitral/tufted (pcMT) cells (see Experimental Procedures), and then imaged evoked fluorescence changes from their apical tufts within dorsal OB glomeruli (Figure 1A). Imaging with two-photon microscopy at relatively low zoom allowed for the visualization of odorant-evoked GCaMP signals in 20-40 glomeruli simultaneously (Supplemental Figure S1) while restricting optical measurements to the glomerular layer.
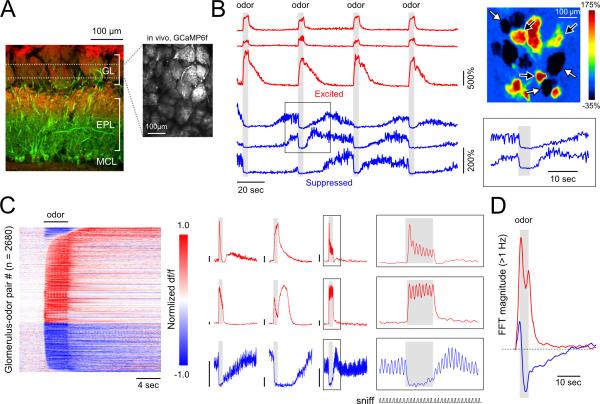
Figure 1. Odorant-evoked excitation and suppression map to discrete OB glomeruli in anesthetized mice.
A. Left: Reference image showing retrograde GCaMP expression in piriform cortex-projecting mitral and tufted (pcMT) cells, in this case expressed in a PCdh21-Cre:Rosa26-tdTomato mouse (to better visualize OB lamina). GCaMP expression was restricted to cells with somata in the mitral cell layer (MCL) and lateral dendrites in the deep ~2/3 of the external plexiform layer (EPL; green). Red label shows tdTomato expression in olfactory sensory neurons in the glomerular layer (GL) as well as in mitral and tufted cells. Right: Two-photon image of GCaMP expression in the dorsal OB in vivo (right).
B. Time series (left) and spatial map (top right) of odorant-evoked fluorescence changes evoked by four presentations of the same odorant. Excited (black arrows, red traces) and suppressed glomeruli (white arrows, blue traces) are intermingled. Traces from suppressed glomeruli show that, during epochs of elevated spontaneous activity, odorants elicit an abrupt decrease in both the intensity and variability of fluorescence (expanded in inset, bottom right) which slowly recovers after odorant offset.
C. Left: Odorant-evoked GCaMP signals across a population of glomerulus-odor pairs sorted by latency separately for excited (upper) and suppressed (lower) cells. Each row represents one glomerulus-odor pair, with time along the horizontal axis. Responses were normalized by their maximum or minimum value for excitatory and suppressive responses respectively. In this plot, responses were categorized as excitatory if there was only a significant excitatory response or both excitatory and suppressive responses. Middle: Example time series from nine glomerulus-odor pairs illustrate the diversity of temporal response patterns. Each trace is the average of 8 trials, with the timing of artificial inhalation and odorant presentation synchronized across trials. Scale bars represent 25% ΔF/F. Right: Expanded view of boxed regions from three glomerulus-odor pairs illustrates inhalation-linked excitatory modulation (top two traces) and suppression of inhalation-linked activity by the odorant (bottom trace). Inhalation coupling is evident in anesthetized, tracheotomized mice in this artificial inhalation paradigm. Lower trace (‘sniff’) indicates sequence of inhalations.
D. Time-course of the summed FFT amplitude in the >1 Hz band across all glomerulus-odor pairs displaying excitatory (red) and suppressive responses (blue). Each point represents amplitude in a 4-sec window centered at the time indicated. Note that the initial downward deflection of the fluorescence time series of suppressed glomeruli causes a brief increase in high frequency power initially. This transient is followed by a sustained period of reduced high-frequency fluctuations.
In the absence of odorant stimulation, many glomeruli showed slow fluctuations in GCaMP6f fluorescence that varied over timescales of seconds, as well as higher-frequency (>1 Hz) fluctuations (Figure 1B,D). The relative power of the high-frequency fluctuations was significantly correlated with the level of the slowly-varying ‘tonic’ fluorescence (r = 0.41, correlation of tonic fluorescence level with its high-frequency variance, p<10−142), consistent with higher tonic fluorescence levels reflecting higher levels of spontaneous spiking or synaptic input to MT cells. Notably, the presence and time-course of these slow fluctuations was glomerulus-specific (e.g., Figure 1B). Some glomeruli also showed fluorescence transients linked to each inhalation, even prior to odorant stimulation (Figure 1C), consistent with prior reports of inhalation-driven sensory input to OB glomeruli (Kato et al., 2012; Wachowiak et al., 2013).
Odorant presentation evoked both increases and decreases in GCaMP6f fluorescence that mapped to discrete glomeruli (Figure 1B). Opposing polarity responses to the same odorant were interspersed among glomeruli within a field of view (Figure 1B, right). Responses often consisted of multiphasic sequences of fluorescence increases and decreases linked to odorant onset, offset and/or inhalation that were specific for a glomerulus-odorant pair and repeatable across presentations (Figure 1B,C). Overall, we observed significant fluorescence decreases in 38.4% of all glomerular odorant responses (n=2680 pairs, 12 mice).
Based on earlier simultaneous recordings of spike rate and GCaMP fluorescence in MT cells (Kato et al., 2012; Wachowiak et al., 2013) and earlier comparisons of calcium signals in the MT soma and apical tuft (Charpak et al., 2001; Debarbieux et al., 2003), the simplest interpretation of these results is that increases and decreases in glomerular GCaMP6f fluorescence reflect increases and decreases, respectively, of ongoing MT cell activity. Consistent with this, glomerular fluorescence decreases were accompanied by a clear suppression of both spontaneous and inhalation-linked transients occurring prior to odorant presentation (Figure 1C), as well as by a marked reduction in the power of spontaneous, higher-frequency fluctuations (Figure 1D).
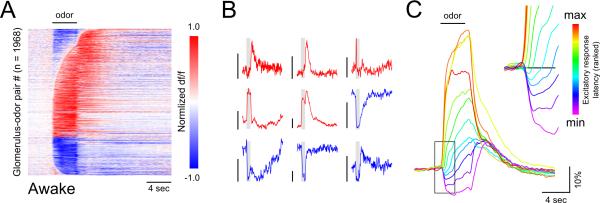
We next measured odorant-evoked glomerular excitation and suppression during wakefulness by imaging glomerular GCaMP6f signals from pcMTs in awake, head-fixed mice. As observed under anesthesia, stimulation during wakefulness yielded odorant-specific excitatory and suppressive responses in discrete, interspersed glomeruli (Figure 2A, B). Odorants evoked net excitatory and suppressive responses with similar probabilities in awake and anesthetized mice (Figure 2A; anesthetized: 38.4%, awake: 35.8%). However, as reported earlier for MT somata (Kato et al., 2012), onset latencies were delayed in awake mice, with approximately 39% of excitatory responses emerging at least one second after the earliest measured response in awake mice, compared to 15% under anesthesia. Notably, longer-latency excitatory responses were preceded by modest suppression of spontaneous GCaMP6 signals (Figure 2A, C). These results suggest that, although glomerulus-specific suppression is prominent in both anesthetized and awake mice, additional suppression impacting the temporal evolution of odorant responses during sustained odorant sampling is engaged during wakefulness.
Figure 2. Suppressive odorant responses are prominent and impact response dynamics among OB glomeruli in awake mice.
A. Response time series across all glomerulus-odorant pairs imaged from pcMTs in glomeruli of awake mice, normalized and plotted as in Figure 1.
B. Example traces from individual glomerulus-odorant pairs in awake mice. Scale bars, 20% dF/F. Diverse temporal patterning persists in the awake state. Inhalation coupling in awake, freely breathing mice is not evident due to trial averaging and occasional bouts of high-frequency sniffing which cannot be resolved, due to the insufficient temporal resolution of GCaMP.
C. Excitatory time series from (A) binned by the latency of the first significant excitatory response, averaged and pseudocolored according to bin. Longer-latency odorant-evoked excitation in pcMT cells is smaller in amplitude and systematically preceded by a period of transient suppression. Following the cessation of odorant application, the average time series of all groups converge to the same trajectory.
Mitral/tufted cell excitation and suppression maps uniformly onto glomeruli
To resolve excitation and suppression at the level of single neurons, we next imaged from MT somata at or near the mitral cell layer in anesthetized mice (Figure 3A, B). In many MT cells, odorants evoked simple fluorescence increases that included inhalation-linked transients, as seen in glomeruli. In other cells, however, responses were more complex and consisted of fluorescence decreases or mixed sequences of increases and decreases that were qualitatively similar to those imaged from glomeruli (Figure 3B, right). The proportion of suppressive responses was moderately smaller than that observed among glomeruli, with 32% of cell-odor pairs (390 pairs, 3 mice) exhibiting a predominate decrease in fluorescence (Figure 3C). The higher proportion of glomeruli showing suppression may follow from the convergence of multiple MT cells onto each glomerulus, with a resulting increase in signal-to-noise ratio arising from averaging across multiple ‘sister’ MT cells. Within a field of view, however, fluorescence increases and decreases (as well as MT somata unresponsive to a given odorant) were interspersed (Figure 3B, left). As with the glomerular signal, somatic fluorescence decreases were typically accompanied by a reduction in the power of high-frequency (> 1 Hz) fluctuations (Figure 3D).
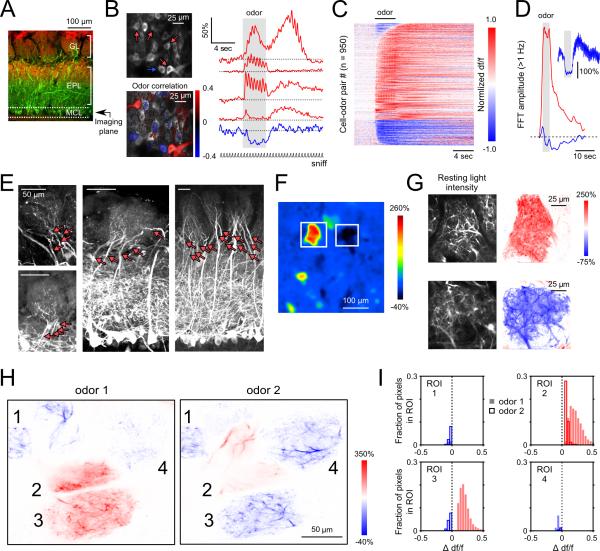
Figure 3. Excitatory and suppressive odorant responses are interspersed among pcMT cell somata and are shared across sister pcMT cells in the same glomerulus.
A. Reference image indicating imaging plane targeting pcMT somata.
B. pcMT cells imaged in vivo (top left) and fluorescence time series (right) from select somata showing distinct temporal patterns including fluorescence increases (red) and decreases (blue). Traces are averages of 4 trials. Excited cells with activity positively correlated with odorant presentation were interspersed with negatively correlated (suppressed) cells (different odorant; lower left). Note that stimulus-activity correlations reveal the polarity of the odorant response but do not quantify similarity between the responses of individual somata.
C. Odorant-evoked GCaMP signals across a population of cell-odor pairs, displayed as in Figs. 1 and 2.
D. Time-course of high-frequency power (summed FFT amplitude > 1 Hz) across all excited (red) and suppressed (blue) somata demonstrates a sustained decrease in high-frequency fluctuations following the initial suppressive transient.
E. Example confocal images from histological sections showing innervation of glomeruli by multiple pcMT cells. Note that fluorescence is comparatively dim in the glomerular layer. All images show native GCaMP fluorescence with no enhancement by immunostaining.
F. GCaMP fluorescence imaged from the glomerular layer in vivo illustrating excitation and suppression in two neighboring glomeruli in response to the same odorant (methyl benzoate).
G. High-resolution imaging from the excited (top) and suppressed (bottom) glomeruli indicated in (F). Left images show individual dendritic processes resolvable within each glomerulus; right images show corresponding pixel-wise df/f maps. All responsive processes within a glomerulus show either a positive (red; excited) or negative (blue; suppressed) response to the odorant.
H. High-resolution response maps showing resolvable dendritic processes within a group of glomeruli. All dendritic processes within a given glomerulus respond with the same polarity, even for glomeruli that respond with different polarity to different odorants (e.g. glomerulus 3). ‘Odor 1’, ethyl butyrate; ‘Odor 2’, 2-hexanone.
I. Histograms of changes in df/f values for every significantly modulated pixel within each glomerulus indicated in (H) for two odors (solid bars and open bars). Glomeruli with intermingled excitatory and suppressive responses were never observed.
How, or whether, MT cell excitation and suppression maps to OB glomeruli – the anatomical units that define odor coding modules within the OB – remains unclear, because MT cells with apical dendrites in the same glomerulus (i.e., ‘sister’ MT cells) may be differentially shaped by inhibitory circuits (Dhawale et al., 2010; Ke et al., 2013). To address this, we imaged at higher magnification to resolve and compare GCaMP6f signals in multiple pcMT dendritic branches within a single glomerulus. Effective retrograde infection of pcMTs results in GCaMP expression in approximately 80% of Tbx21-positive mitral cells (Rothermel et al., 2013); recent studies indicate that individual dorsal OB glomeruli are innervated by 10 – 20 mitral cells (Ke et al., 2013; Sosulski et al., 2011). Thus, we estimate that most imaged glomeruli included dendrites, conservatively, from 5 – 10 pcMT cells. We confirmed glomerular innervation by multiple GCaMP6-expressing pcMT cells in post-hoc histological analysis (Figure 3E) and using high-zoom scans in vivo (Supplemental Figure S2).
To address whether sister pcMT cells show similar or different response polarities, we mapped excitation and suppression across all labeled processes within a glomerulus by calculating, for each responsive pixel, the correlation coefficient between the fluorescence signal extracted from that pixel and the square pulse demarcating the time of odorant presentation (Figure 3F,G). Across 115 glomeruli so examined, nearly all stimulus-related pixels within a glomerulus (i.e., those significantly correlated to the stimulus) had correlation coefficients of the same sign (99.8 ± 0.6% of 71.1 ± 28.8% significantly correlated pixels). Even when different odorants evoked excitatory and suppressive responses in the same glomerulus, excitation or suppression was uniform throughout all responsive processes, with a given odorant evoking responses that were either all positive (for excitatory odorants) or all negative (for suppressive odorants) (Figure 3H,I; see also Supplemental Figure S2). In some cases dendrites within the glomerular neuropil could be putatively assigned to different sister MT cells on the basis of differences in spontaneous activity; in these cases, odorant stimulation nonetheless elicited responses of the same polarity in these cells (Supplemental Figure S2). Uniform response polarities extended to the apical dendrites of sister MT cells visible outside of the glomerular neuropil (Supplemental Figure S2).
Overall, these results suggest that inhibition leading to suppression of ongoing activity targets all pcMT cells innervating the same glomerulus. These results do not rule out the possibility that finer-scale differences in response magnitude or temporal dynamics exist between sister pcMT cells or distinct MT subtypes innervating the same glomerulus - as has been shown with electrophysiological recordings and imaging (Dhawale et al., 2010; Kikuta et al., 2013; Tan et al., 2010). However our data indicate that, at least with respect to response polarity, odorant-evoked pcMT responses map uniformly to their parent glomeruli.
The detection of suppressive responses in a glomerulus requires the presence of spontaneous activity in the neurons innervating it. For each glomerulus-odor pair, we calculated an index of spontaneous activity, calculated as the average df/f value immediately prior to odorant presentation normalized by the threshold used to detect significant responses to odorant stimulation. We found that the probability of detecting a suppressive response in a glomerulus depended strongly on this value, which in turn is dependent upon the number of repetitions of each odorant (Supplemental Figure S3). With eight repetitions, when the mean fluorescence amplitude preceding odorant presentation was at least 2.14 times higher than the significance threshold, the probability of observing a suppressive responses was decreased by less than 5% compared to the probability observed in the 10% of cases with the highest spontaneous activity. Spontaneous activity preceding odorant stimulation was at least this high in 86% of all glomerulus-odor pairs. Overall, we estimate that for our dataset only 3.8% of suppressive responses were not detected due to insufficient spontaneous activity in the MT cells innervating each glomerulus (Supplemental Figure S3) indicating that decreases in activity were reliably identified in this dataset.
Glomerular output maps reveal odorant-specific inhibition of select glomeruli
The ability to, for the first time, map MT response patterns across multiple, directly visualized OB glomeruli allows us to characterize the functional organization of excitation and suppression across the glomerular array in vivo. Perhaps the most striking feature of glomerular MT cell responses was their odorant specificity. Examples of glomerular maps evoked by multiple odorants, imaged at different spatial scales in two preparations, are shown in Figure 4A, B. In both cases, immediately adjacent glomeruli respond with excitation and suppression to different odorants, and other adjacent glomeruli show no detectable response. Excited, suppressed and null-responding glomeruli are highly interspersed. The time-courses of the glomerular MT responses reveal additional diversity in response patterns that extends beyond simple excitation or suppression to include differences in the temporal response to the onset, duration and offset of odorant stimulation (Figure 4C). Glomeruli varied greatly in their selectivity for excitatory versus suppressive responses across the odorant panel, with some glomeruli showing broadly tuned excitation and others showing broadly tuned suppression (Figure 4D, E). Overall, 66% of glomeruli responded with suppression to at least one of the eight odorants tested. The relative distribution of excitatory versus suppressive MT responses appeared qualitatively similar across animals (n = 18 fields of view imaged from 11 mice; Figure 4D and Supplementary Figure S4).
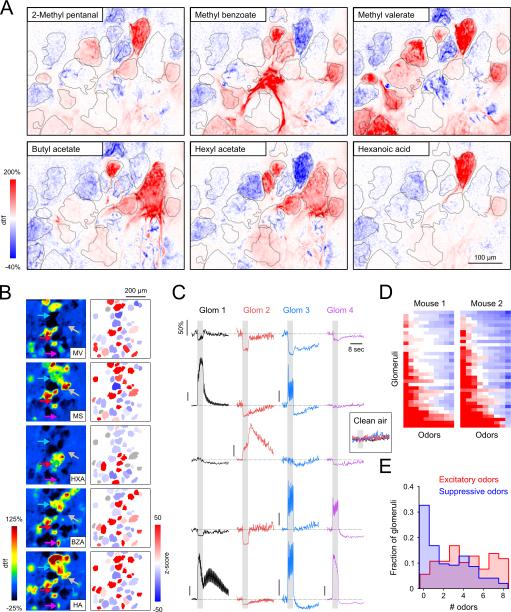
Figure 4. Glomerular response polarities are highly interspersed and odorant-specific.
A. Maps of glomerular MT cell responses (GCaMP6f) evoked by six odorants imaged across the same field of view in an anesthetized mouse. Red and blue indicate fluorescence increases and decreases, respectively. Glomeruli are outlined in gray. Most glomeruli were excited, suppressed, and unresponsive to distinct subsets of the odorant panel.
B. Glomerular activation maps (left) evoked by five odorants known to strongly-activate the dorsal OB, with ROI masks pseudocolored by response z-score (right). In all cases, excited (red), suppressed (blue) and non-responsive (gray) glomeruli are intermingled. MV, methyl valerate; MS, methyl salicylate; HXA, hexanoic acid; BZA, benzaldehyde; HA, hexyl acetate.
C. Time series corresponding to the four glomeruli indicated with arrows in (B) for each of the five odorants. The polarity and time course of each response is glomerulus- and odorant-specific. Responses were abolished when clean air was used a stimulus (inset). Scale bar top left applies to all traces except where indicated (all represent 50% df/f).
D. Response matrix representing the strength and polarity of odor-evoked responses in two different mice. Responses were sorted across rows from most excitation to most suppression left-to-right for ease of visualization. As a result, odorant identity is not preserved across columns. Most glomeruli exhibited excitatory and suppressive responses to subsets of the odorants tested. Odorants not eliciting a detectable response are colored white.
E. Histogram of the number of odorants eliciting excitation and suppression across all glomeruli, assayed with panels of 8 odorants known to strongly activate the dorsal OB.
Functional organization of inhibition among OB glomeruli
How might the specificity in suppression of glomerular output arise from inhibitory OB circuits? Multiple circuits mediate inhibition both within (intraglomerular) and between glomeruli (interglomerular), with distinct predicted impacts on odor representations (Cleland and Linster, 2012; Banerjee et al., 2015). We evaluated the degree to which patterns of glomerular output are consistent with predictions from common interglomerular and intraglomerular circuit models by mapping MT cell excitation and suppression across glomeruli using a panel of odorants, including both structurally similar and divergent compounds that activate glomeruli on the dorsal OB (see Experimental Procedures).
Intensity-dependent intraglomerular inhibition
Odorant-specific suppression of glomerular output could result from a differential sensitivity of MT cell inhibition and excitation to input intensity. OB slice experiments have identified a feedforward inhibitory circuit in which high-sensitivity periglomerular interneurons are recruited at lower input intensities than MT cells, resulting in a predicted suppression of MT output for weak OSN activation and a shift to MT excitation at higher input intensities (Cleland and Linster, 2012; Gire and Schoppa, 2009).
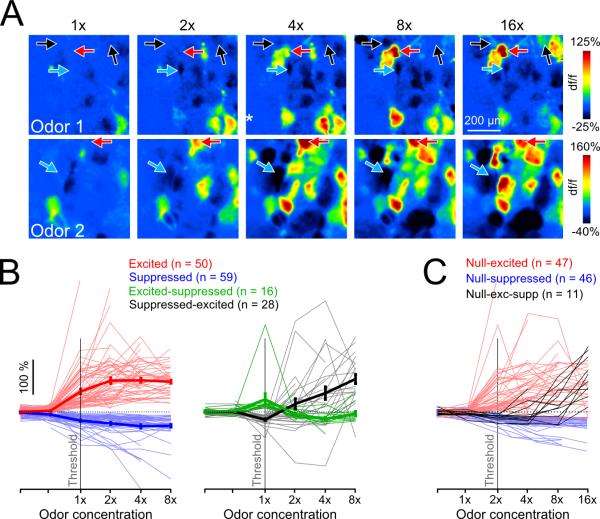
We tested the degree to which suppressive responses could be accounted for by this model by imaging responses from the same glomerulus across an up to 32-fold change in odorant concentration (typical, 16-fold). A signature of the high-sensitivity feedforward inhibition model is the transition of suppressive MT responses into excitatory responses as odorant concentration increases; this model also predicts that the opposite transition - from excitation to suppression - should be rare or not observed. Figure 5A shows examples of glomerular pcMT response patterns for a concentration series of two odorants in the same field of view. Individual glomeruli show a range of behaviors as odorant concentration increases. Overall, approximately 20% of glomeruli (18.3%; 28/153 glomeruli in 4 mice) had concentration-response functions consistent with high-sensitivity feedforward inhibition, with suppression at low odorant concentrations that switched to excitation at higher concentrations (Figure 5B, right). A smaller but still substantial fraction (10.5%; 16/153) switched polarity from excitation to suppression (Figure 5B, right). The majority of imaged glomeruli, however (71.3%), maintained their response polarity at all concentrations tested, with an approximately equal fraction showing excitatory and suppressive responses (excitation: 32.7%, suppression: 38.6%; Figure 5B, left).
Figure 5. Glomeruli show diverse patterns of pcMT excitation and suppression across odorant concentration.
A. Pseudocolor df/f maps of glomerular responses to increasing odorant concentration from two representative experiments (Odor 1, ethyl butyrate; Odor 2, methyl valerate). Arrows indicate glomeruli that transition from unresponsive to excitation at higher concentration (red), unresponsive to suppression (blue), and unresponsive to suppression and then to excitation (black).
B. Responses of all glomeruli to the same odorant across a range of concentrations. The majority of glomeruli responded with the same polarity across all concentrations (71%; 109/153), and are shown in the left plot. Glomeruli showing changes in response polarity with increasing concentration are shown in the right plot. Data points to the left of ‘1×’ represent further decreased concentrations and/or blank stimuli.
C. Responses of glomeruli exhibiting a null response to at least one low concentration of the odorant. The first suprathreshold response in nearly half of all glomeruli (47/104) was excitatory.
To ensure that we sampled concentrations across the relevant range for a glomerulus-odorant pair, we also analyzed the subset of glomeruli whose concentration-response functions were bounded by a null response on the low end and an excitatory response on the high end (Figure 5C). In 81.0% (47/58) of these glomeruli, responses transitioned from no response to excitation with no evidence of intermediate suppression (red) within the resolution of our concentration steps (0.3 log units). We additionally included the population of glomeruli whose responses are bounded below by null responses and are suppressed at all higher concentrations (46 glomeruli) (Figure 5C). Making the very conservative assumption that all of these glomeruli would switch to excitation at a high enough concentration, we still found that among this larger group, nearly half of glomeruli (47/104; 45.2%) did not display suppression at an intermediate concentration. Most glomeruli did display suppression to one or more odorants when tested against a modest odorant panel (e.g. Figure 4D, E; Supplemental Figure S4), implying that suppression is unlikely a simple function of input intensity in many glomeruli. Overall, these results do not exclude the possibility that intraglomerular circuits with differential sensitivity to input strength could contribute to glomerulus-specific suppression. At the same time, they suggest that the high-sensitivity feedforward inhibition model is unlikely to account for all – or even most –instances of odorant-evoked suppression of glomerular output.
Global versus selective interglomerular inhibition
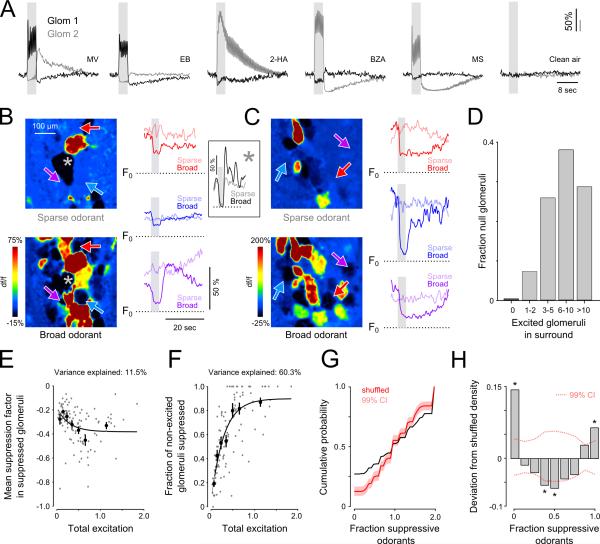
Interglomerular circuits have the potential to mediate glomerulus-specific suppression of MT cell output via lateral inhibition. Consistent with such an organization, we occasionally observed pairs of glomeruli in the same field of view that displayed apparently reciprocal response patterns to an odorant. An example of one such pair is shown in Figure 6A. Excitatory and suppressive components of the MT responses appear reciprocal in each glomerulus, showing roughly inverse responses both across odorants and across time. Such examples are suggestive of mutual inhibitory interactions between glomeruli.
Figure 6. Spatial patterns of glomerular output do not fit a model of global interglomerular inhibition.
A. Odorant-evoked responses of two glomeruli imaged in the same field of view to five odorants and clean air. Excitation in one glomerulus is accompanied by suppression in the other glomerulus for all five odorants, and vice versa. Other adjacent glomeruli (not shown) did not show such reciprocal patterns. EB, ethyl butyrate; 2-HA, 2hydroxyacetophenone.
B. Left: Map of responses to an odorant activating few (sparse odorant, pentanal; top) and many glomeruli (broad odorant, ethyl tiglate; bottom). Right: df/f traces corresponding to the three glomeruli indicated by arrows in maps. For each glomerulus, time series are shown in response to the sparse and broad odorants in light and dark colors respectively. In these examples, df/f traces represent absolute df/f relative to F0. Inset: df/f recorded in an additional glomerulus (denoted by an asterisk) that was powerfully suppressed by both odorants.
C. An additional example from a second mouse. Explanation as in (B). Sparse and broad odorants are ethyl butyrate and n-methyl piperidine.
D. Number of excited glomeruli in the surround (500 μm radius) of null. For 99.6% of null glomeruli there was at least one excited glomerulus nearby (median = 7.0; 10-90th percentile = 3-15 excited glomeruli).
E. Mean magnitude of odorant-evoked suppression across all glomeruli within a field of view plotted as a function of total excitation elicited by that odorant in the same field of view (Supplemental Experimental Procedures). Each gray point is calculated from the responses of all ROIs within a field of view to one odorant; data points were then binned to calculate averages (solid points; mean ± s.e.m.). Solid line shows sigmoidal fit to the binned means. There is only a weak relationship between mean suppression and total excitation.
F. Probability of observing suppression among all glomeruli in which excitation was not observed, plotted as a function of the total amount of excitation elicited across all glomeruli. Symbols and fit are as in (E).
G. Cumulative distribution of the fraction of non-excitatory odorants suppressing glomeruli (black line). The cumulative distribution of the same fraction from random data, in which the real responses to each odorant were repeatedly assigned to glomeruli in random order is shown in red. Shaded region represents 99% confidence intervals.
H. Difference between the probability distributions for the real and shuffled data shown in (G). Dotted lines represent 99% confidence intervals in this difference for the shuffled data. Asterisks denote bins that differed significantly from the shuffled distribution (p<0.05; with Bonferroni correction for multiple comparisons). Glomeruli are more likely to be suppressed by all odorants– or by none – more often than expected by chance.
One prevalent interglomerular circuit model involves inhibition that is broadly distributed and scales in magnitude with excitation (Banerjee et al., 2015; Cleland and Sethupathy, 2006). Alternatively, interglomerular inhibition could be selective and spread from an excited glomerulus to a sparse subset of surrounding glomeruli (Fantana et al., 2008; Migliore et al., 2010). To test these models we analyzed pcMT glomerular responses to single odorants, examining the surround of glomeruli that were unresponsive to an odorant. Surprisingly, we found that these ‘null’ glomeruli could often be found adjacent to strongly-excited glomeruli as well as to strongly-suppressed glomeruli (Figure 6B, C; top maps). Null-responsive glomeruli exhibited high degrees of spontaneous activity (Figure 6B, C) and could be suppressed (or excited) reliably by other odorants (Figure 6B,C; bottom maps and dark traces). Close appositions of null glomeruli to strongly-suppressed glomeruli indicate that null responses were not observed as a result of ubiquitous, but weak, interglomerular inhibition. Many excited glomeruli were typically observed in the surround of null glomeruli (Figure 6D; median = 7.0 excited glomeruli observed within 500 μm). Greater than one third of null glomeruli had at least one strongly suppressed glomerulus (suppression factor > 0.6) in the same field of view (34.0%; 181/532 glomeruli) and nearly all were located near significantly suppressed glomeruli in which some suppression could be detected (suppression factor > 0.25; 473/532 glomeruli).
These results contrast with predictions from a global inhibition model, in which an odorant that weakly excites only a few glomeruli should elicit relatively weak suppression in surrounding glomeruli, and a strongly excitatory odorant should elicit strong suppression in surrounding - but unexcited – glomeruli. We further evaluated this model by quantitatively analyzing the relationship between the strength of glomerular excitation and the prevalence and magnitude of suppression in glomeruli that were not excited by an odorant (see Supplemental Experimental Procedures). With scaled global inhibition, the strength of inhibition should scale with the strength of excitatory input across glomeruli, while uniformly impacting the glomerular array (Banerjee et al., 2015; Cleland and Sethupathy, 2006). However, we found that while the total observed excitation was highly predictive of the fraction of suppressed glomeruli (60.3% of variance explained by sigmoid fit), the magnitude of suppression in suppressed glomeruli was only weakly related (11.5% of variance explained) (Figure 6E, F). Importantly, the low fraction of suppressed glomeruli evoked by weaker odorants (Figure 6F) does not appear to reflect an ‘iceberg effect’ in which suppression was only detected in the more active glomeruli; spontaneous activity levels in suppressed glomeruli did not vary with the fraction of suppressed glomeruli in the field of view (Supplemental Figure S6). The lack of a strong relationship between total excitation and suppression strength, along with the commonly-observed intermingling of null-responsive, suppressed and excited glomeruli in an odorant-specific manner runs counter to predictions from a global interglomerular inhibition model.
Another consequence of nonselective interglomerular inhibition is that if suppression were detected in only a subset of non-excited glomeruli, that subset should be randomly distributed. To test this prediction, we quantified the fraction of non-excitatory odorants (out of an 8-odorant panel) that elicited suppression for each glomerulus in a field of view. Glomerulus-odorant pairs with insufficient spontaneous activity to reliably observe suppression were excluded from this analysis. To determine whether suppressed glomeruli were randomly distributed, we compared the distribution of this measure (n = 252 glomeruli, 9 OBs, 7 mice) with the distributions derived after repeatedly shuffling glomerular identity associated with the responses to each odorant (Figure 6G; see Supplemental Experimental Procedures). The empirical distribution of the fraction of non-excitatory odorants evoking suppression across all glomeruli differed significantly from the glomerulus-shuffled distribution (Kolmogorov-Smirnov test, p < 1×10−4), indicating that glomeruli are nonrandomly suppressed by odorants. Importantly, the observed and shuffled distributions differed systematically: glomeruli were much more likely than chance to be suppressed either by all or by none of the non-excitatory odorants (Figure 6H). Thus, functional inhibition across the glomerular map depends on glomerular identity, with certain glomeruli subject to more promiscuous functional inhibition than predicted by chance.
Interglomerular inhibition shapes the integration of odorant information by MT cells
The analyses thus far were based on the odorant-evoked suppression of spontaneous activity in MT cell dendrites or somata. To test the degree to which this suppression generalizes to functional inhibition of MT odorant responses – and to further test its interglomerular origin - we used binary odorant mixtures as stimuli. In this paradigm, we compared pcMT responses to each of two test odorants with their response to a binary mixture of the two odorants presented together. We specifically asked whether odorants that evoked suppressive responses when presented individually also suppressed an excitatory response to a different odorant when the two were co-presented in a binary mixture. To quantify mixture interactions we used a stringent criterion for suppression by an odorant mixture: for a given glomerulus, the response to the mixture had to be significantly smaller than that to the ‘dominant’ (i.e., strongest excitatory) odorant alone (threshold of p<0.05, t-test comparing mean excitatory and mixture response amplitudes across 4 – 16 trials). This criterion - in contrast to a comparison with the sum of the responses to each odorant (Fletcher, 2011; Gupta et al., 2015; Khan et al., 2008) – ensured that mixture suppression represented true inhibition of the response to one odorant by the addition of another, without requiring assumptions about the dynamic range or nature of additivity of inputs to the measured MT cells. To reflect this criterion and to distinguish from the suppression of spontaneous activity evoked by single odorants, we refer to such suppression as ‘mixture inhibition’.
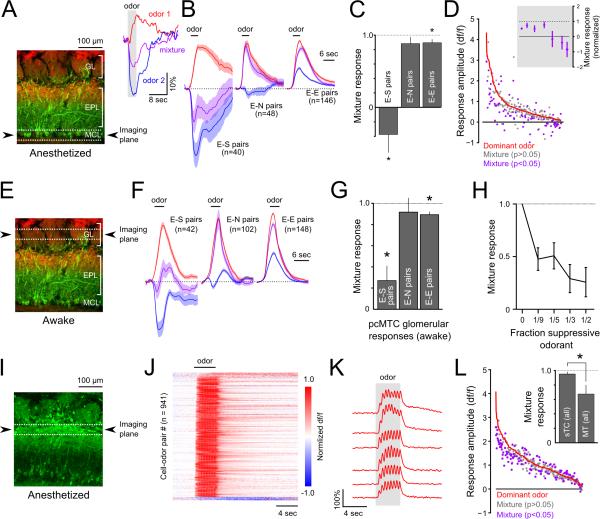
We first examined mixture interactions in pcMT somata expressing GCaMP3 (n = 189 somata, 7 mice) or GCaMP6f (122 somata, 3 mice) in anesthetized mice (Figure 7A-D). In a given pcMT cell, two odorants could elicit excitatory (E), suppressive (S), or null (N) responses in any combination when presented individually. Among E-S odorant pairs, 56% (24/40 pairs from 10 mice) showed significant mixture inhibition, with the mean response to the mixture showing net suppression below baseline activity (mean = −87% ± 34% of response to excitatory odorant). The remaining 16 E-S pairs, while not significant on a cell-by-cell basis due to across-trial variability, as a population also showed mixture inhibition, with a mean mixture response of 53 ± 32% of the excitatory response amplitude. Indeed, across all 40 E-S odorant pairs, the mean mixture response showed net suppression, equal to −31 ± 26% (mean ± SEM; p < 1×10−4, paired t-test) of the response to the excitatory odorant alone (Figure 7B,C). The magnitude of mixture inhibition was strongest for pcMT cells with weaker excitatory responses to a single odorant (Figure 7D). We saw much less evidence of mixture inhibition by null odorants, with only 6/48 (12.5%) E-N pairs showing significant inhibition and only slight inhibition of the mean excitatory response across all cell-mixture pairs (mean ± SEM, 86.4 ± 8.8%, p > 0.05; Figure 7B, C). The relative lack of mixture inhibition observed in E-N pairs suggests that there were few suppressive responses that we failed to detect and misclassified as null. E-E odorant mixtures also produced only a slight reduction in amplitude compared to the dominant odorant (Figure 7B, C; mean ± SEM, 89.7 ± 4.7%; p = 0.02). These results demonstrate that the suppression evoked by single odorants reflects inhibition that is powerful enough to suppress – and, in many cases, completely block – responses in the same MT cell to excitatory odorants.
Figure 7. Suppressive odorants inhibit excitation by other odorants in mitral but not superficial tufted cell populations.
A. Left: Reference image showing schematic of imaging plane, targeting MT cell somata, used for panels C-E. Inset: Example of an MT cell response to an excitatory odorant (red), a suppressive odorant (blue) and their binary mixture (magenta). Traces represent averages of 12 trials.
B. Time series of mean responses to the dominant odorant (red), weaker odorant (blue), and their binary mixture (magenta) when the weaker odorant was suppressive (E-S pairs), null (E-N pairs), or excitatory (E-E pairs). Time series were normalized by the dominant odorant response amplitude. Shaded areas represent 95% confidence intervals.
C. Mixture response ratios (mixture response amplitude divided by the dominant odorant response amplitude) for E-E, E-N and E-S odorant pair types. Bars indicate mean ± SEM. D. Magnitude of odorant-evoked activity in response to the dominant odor (red line) and corresponding mixture responses (points). Cell-odor pairs are rank-ordered by the dominant odorant response magnitude on the abscissa. Mixture responses significantly differing from the dominant odorant response are colored magenta. Inset: Significantly modulated responses showed suppression of the dominant odorant response, on average, regardless of dominant odorant response magnitude.
E. Reference image showing schematic of imaging plane for G-I, targeting apical dendritic tufts of MT cells in awake mice.
F. Mean time series response to the dominant odorant (red), weaker odorant (blue), and their binary mixture (magenta) when the weaker odorant was suppressive (E-S pairs), null (E-N pairs), or excitatory (E-E pairs), as in (B).
G. Mean mixture response ratios of glomerular responses for E-E, E-N and E-S odorant pairs, plotted as in (C). Bars indicate mean ± SEM.
H. Mean mixture response ratios of glomerular responses across all E-S pairs at differing concentrations of the suppressive odorant. The concentration of the dominant odorant was the same in all cases.
I. Histological section showing native GCaMP3 fluorescence in deep and superficial tufted cells in a CCK-Cre:Rosa-GCaMP3 reporter cross. Odorant responses were imaged from CCK+ somata near the border of the glomerular and external plexiform layers (dashed lines).
J. Odorant responses measured across a population of superficial tufted cells, plotted as in Figures. 1-3. Odorant responses were temporally homogenous compared to MT somata and dendritic tufts. Few suppressive responses were detected in this dataset.
K. Example responses of a group of simultaneously-imaged superficial tufted cells to an odorant (ethyl butyrate). Odorant responses were typically homogenous across different cells, limited to the period of odorant stimulation, and highly modulated by sniffing.
L. Mixture responses, plotted as in (D). Across the population, relatively little modulation by the weak odorant was detected. Inset: Across all somata and odorant pairs, sTCs displayed less mixture inhibition than MT somata. Bars indicate mean ± SEM.
We next examined mixture inhibition at the level of glomerular pcMT responses in awake mice (Figure 7E-H). As seen in pcMT somata, we observed strong mixture inhibition in E-S odorant pairs (Figure 7F, G) with a mean mixture response amplitude of 32.6 ± 11.1% of the excitatory odorant (n=46 pairs; p < 1×10−9, paired t-test) and a net reversal of polarity in 18 (of 46) E-S pairs that showed significant mixture inhibition on an individual basis (mixture response amplitude, −32.7 ± 13.3% of excitatory response). Also consistent with the somata imaging, mixture inhibition was significant, but small, in E-E glomerulus-odor pairs (88.2 ± 2.7%; n=151 pairs; p < 0.01) and not significant in E-N pairs (mean, 92.8 ± 15.0%; n=91 pairs, p = 0.11 Figure 7F, G).
We further assessed mixture inhibition by imaging glomerular responses to E-S mixtures in which the excitatory and suppressive odorants were mixed at varying ratios (n=15 odorant pairs in 4 mice). Mixture inhibition was significant even when the proportion of the suppressive odorant was lowered from 50% to 33%, 20%, and 11.1% of the total odorant concentration, while keeping the concentration of the excitatory odorant constant (Figure 7H). The magnitude of mixture suppression decreased somewhat with lower concentration of the suppressive odorant, but remained powerful at the lowest concentration tested (11.1%; 47.7 ± 10.7% of excitatory response; p<1×10−4, paired t-test). Overall, these results support the single-odorant analyses, suggesting that selective interglomerular inhibition strongly regulates odorant-evoked output from OB glomeruli and that mixture effects are not easily explained by intensity-dependent intraglomerular inhibition.
Finally, we tested for mixture inhibition in superficial tufted cells (sTCs), which are reported to be less subject to lateral inhibition than mitral or deep tufted cells (Adam et al., 2014; Fukunaga et al., 2012; Igarashi et al., 2012; Nagayama et al., 2004). Because tufted, but not mitral, cells express the peptide transmitter cholecystokinin (CCK) (Seroogy et al., 1985), we drove GCaMP3 expression in CCK-positive neurons using the CCK-IRES-Cre (CCK-Cre) and Ai38 Rosa-GCaMP3 reporter mouse strains, and imaging from somata in the superficial external plexiform layer in CCK-Cre:Rosa-GCaMP3 crosses (Figure 7I). With single-odorant stimulation, CCK-positive sTCs showed an apparent lack of suppressive responses. The lack of suppressive responses could reflect a poor ability of GCaMP3 to report suppression; however, sTC responses were also notable in their homogenous temporal response patterns (Figure 7J,K). Importantly, sTCs also showed a distinct lack of suppression by binary mixtures. Across all binary odorant mixtures that included an excitatory odorant (i.e., E-N and E-E mixtures), sTCs showed significantly less mixture inhibition than pcMTs (Figure 7L), with a mean mixture response amplitude of 93.3 ± 3.2% (SEM) of the dominant odorant response for sTCs compared to 66.3 ± 12.7% for all pcMT cells (p<0.01, t-test). This result cannot be explained by a difference in the GCaMP variant used, as GCaMP3 has been shown to faithfully follow spike rate above the detection threshold (Wachowiak et al., 2013) and mixture inhibition was apparent using GCaMP3 pcMT cells. These results also strongly argue against mixture inhibition (or suppression alone) being mediated by peripheral effects such as receptor antagonism, and strengthen the notion that sTCs are functionally distinct from both mitral and deep tufted cells in their integration of olfactory stimuli.
Discussion
Synaptic inhibition plays a fundamental role in shaping sensory responses of OB output neurons. Here, using ultrasensitive calcium reporters (Chen et al., 2013), we were able to monitor sensory-evoked excitation and suppression in OB output neurons and to map patterns of excitation and suppression to glomeruli - the functional units underlying odor coding at this level. We found that odorant-evoked inhibition potently suppresses spontaneous and odorant-evoked excitation in MT cells, that suppression of glomerular output is odorant- and glomerulus-specific, and that inhibition selectively suppresses output from relatively sparse glomerular ensembles. These results point to an unexpected specificity in the distribution of inhibition within and between glomerular modules. They also demonstrate that a fundamental feature of the input-output transformation in the OB is the emergence of odorant- and glomerulus-specific patterns of activity suppression, adding a new dimension over which changes in neural activity can represent odor information.
Imaging from the apical tufts of MT cells allowed us to monitor the activation and suppression of output from discrete glomeruli. We were able to exploit the fact that the apical tuft constitutes the lone site of sensory-evoked excitation onto MT cells, and numerous studies have demonstrated that somatic spikes reliably invade the apical tuft (Bischofberger and Jonas, 1997; Charpak et al., 2001; Debarbieux et al., 2003; Zhou et al., 2006). Thus it is unlikely that GCaMP fluorescence decreases reflect glomerular inhibition that is not associated with a suppression of spike output. Indeed, a recent study using somatic whole-cell recordings from presumed MT cells in awake animals (Kollo et al., 2014) reported a similar fraction of suppressive odorant responses (46%) as we detected from glomerular tufts in awake (36%) and anesthetized mice (pcMTs: 38%). GCaMP imaging may fail to capture more subtle impacts of inhibition having little impact on time-averaged MT spike rates, such as dendrodendritic signaling from MT cell dendrites and fine-scale temporal firing patterns of MT cells (Fukunaga et al., 2014; Najac et al., 2015; Shao et al., 2012). Nonetheless, our results strongly suggest that odorants evoke specific patterns of inhibition that alter MT cell spike output from select glomeruli.
Temporal response patterns of MT cell excitation and inhibition changed between anesthesia and wakefulness, with an increased prevalence of short-latency odor-evoked inhibition seen in the waking state and, as a result, delayed excitation in a larger fraction of pcMTs. This enhanced short-latency inhibition could explain the sparsening of MT excitatory responses reported in earlier studies in awake mice using extracellular recordings or lower-sensitivity GCaMP reporters and using response measures focused on short-latency responses (Kato et al., 2012; Rinberg et al., 2006; Wachowiak et al., 2013). Our observations also suggest that, during wakefulness, the polarity of odorant response in a given MT cell could switch from suppressed to excited with sustained sampling (i.e., sniffing) – a behavior that is a hallmark of active odor investigation.
Earlier in vivo studies have inferred the functional organization of inhibition in the OB using single OB neuron recordings and electrical or optogenetic stimulation or optical imaging from undefined neuronal populations (Banerjee et al., 2015; Fantana et al., 2008; Luo and Katz 2001; Ezeh et al. 1993). Several recent studies have proposed that inhibition is broadcast widely from an excitatory locus to surrounding glomeruli, with broad odorant selectivity that scales in magnitude with the overall strength of excitatory input (Banerjee et al., 2015; Cleland and Sethupathy, 2006; Kato et al. 2013). Feedback from piriform cortex may also mediate global inhibition (Boyd et al., 2015). Here, by directly imaging odorant-evoked suppression of MT cell output from individual glomeruli, we found strong evidence that inhibitory OB circuits impact glomerular output in a manner that involves selective inhibitory interactions between OB glomeruli rather than global or center-surround inhibition. First, suppression by single odorants was highly specific across glomeruli in a field of view, even when odorants evoked sparse excitation – a result that we could not explain by differences in spontaneous activity or an inability to detect suppression. Second, the magnitude of suppression in suppressed glomeruli scaled only weakly with total excitation among other glomeruli in the field of view (i.e., Figure 6). Third, in binary mixture experiments, suppressive odorants strongly suppressed excitatory responses from the same glomerulus, but null odorants (which excited nearby glomeruli) did not suppress excitation, nor was suppression seen in mixtures of two excitatory odorants. These results are qualitatively different from those of analogous experiments in piriform cortex, where global inhibition powerfully gates pyramidal cell excitation (Poo and Isaacson, 2009) and mixture suppression is relatively nonspecific (Stettler and Axel, 2009).
How might known inhibitory OB circuits mediate selective suppression of glomerular output? We found that suppression was shared across pcMT cells innervating a glomerulus while selectively targeting particular glomeruli, implicating inhibitory circuits within the glomerular layer. Granule cells may also mediate inhibition of select glomerular modules, and our data do not strictly rule out this possibility. However, a recent study showing that granule cell activation has minimal impact on MT membrane potential or ongoing spike rate (Fukunaga et al., 2014) suggests it is unlikely that granule cell activity could underlie the silencing of spontaneous fluorescence transients that we observed. In contrast, both intra- and interglomerular inhibitory circuits in the glomerular layer can potently suppress sensory-driven MT spiking in vivo and in OB slices (Aungst et al., 2003; Banerjee et al., 2015; Fukunaga et al., 2014; Gire and Schoppa, 2009; Shao et al., 2012).
Intraglomerular inhibition could mediate odorant-specific suppression of MT output on the basis of a differential sensitivity to input strength; models from OB slice experiments have proposed that periglomerular cells with a lower activation threshold than MT cells drive feedforward inhibition that suppresses MT cell output preferentially at low input intensities, giving way to feedforward excitation at higher input intensities (Cleland and Linster, 2012; Gire and Schoppa, 2009). We observed such concentration-dependent transitions from suppression to excitation in about 20% of cases. However, patterns of glomerular suppression were not broadly consistent with this model. In the binary mixture experiments at the level of both somata and glomeruli, co-presenting an excitatory and suppressive odorant did not lead to greater MT cell excitation as predicted from the high-sensitivity feedforward inhibition model, since presenting the two odorants together would effectively increase ligand concentration and thus input intensity to the parent glomerulus. Likewise, increasing the concentration of the suppressive odorant in the mixture led to more potent suppression rather than less suppression or excitation (e.g., Figure 7H). Thus, while high-sensitivity feedforward intraglomerular inhibition may contribute to odorant-specific patterns of glomerular excitation and suppression, in general our results suggest that MT response polarity is largely determined by odorant identity rather than relative intensity. Feedforward intraglomerular circuits may play other roles in shaping MT cell responses, such as mediating gain control or regulating sensitivity to odorant-evoked excitation; alternatively, intraglomerular inhibition may shape the temporal structure of MT cell responses relative to inhalation or across repeated samples (‘sniffs’) of odorant (Najac et al., 2015; Fukunaga et al., 2014; Shao et al., 2012; Wachowiak and Shipley, 2006).
Interglomerular inhibition mediated by short axon cells could mediate the glomerulus-wide suppression we observed. In this model, short axon cells inhibit external tufted cells located in distant glomeruli (Banerjee et al., 2015; Liu et al., 2013; Whitesell et al., 2013); external tufted cells drive excitation throughout the glomerulus, and thus their inhibition would be expected to suppress spontaneous and sensory-evoked spiking in MT cells with apical tufts in the same glomerulus (Hayar et al., 2004). How the short axon cell networks could mediate inhibition of odorant-specific glomerular ensembles is less clear, however. Short axon cells arising from a single glomerulus project widely to many neighboring glomeruli (Kiyokage et al., 2010), and focal optical stimulation of short axon cells in vivo drives widespread suppression of MT cell spiking (Banerjee et al., 2015). We found, however, that odorant stimulation led to highly selective suppression or excitation of MT output in a cohort of adajcent glomeruli. If short axon cells mediate interglomerular inhibition, a key question is how this inhibition can mediate MT cell suppression with such selectivity. One possibility is that the network of short axon cells broadcasting inhibition from one glomerulus to others is widespread but heterogeneous and relatively sparse in its innervation of surrounding glomeruli, such that each glomerulus receives short axon cell-mediated inhibition originating from a unique combination of neighbors. As a result, the strength of interglomerular inhibition targeting each glomerulus could be a combinatorial function of the strength of excitation among the cohort of surrounding glomeruli. Anatomical descriptions of short axon cell branching patterns are consistent with this model (Kiyokage et al., 2010), but higher-resolution anatomical and functional mapping studies are required to further support or reject it.
A key next step is understanding the organization of selective interglomerular inhibition with respect to odor coding space. We found evidence that this organization is nonrandom, with a subset of glomeruli being suppressed by many more odorants than predicted by chance and another subset showing little suppression. Whether this nonrandom structure is stereotyped between glomeruli representing the same odorant receptor in different individuals, reflects the odorant response properties of incoming sensory neurons (Linster et al., 2005) or is determined by odor experience remains to be determined. The ability to map activity to defined neuronal populations within OB glomeruli and, ultimately, to odorant receptor identity in vivo should enable an understanding of the functional logic by which inhibition targets specific glomerular modules.
Experimental Procedures
Details of all procedures are provided in Supplemental Information. Briefly, experiments were performed on male and female mice expressing Cre recombinase (Cre) in defined neuronal populations. The mouse strains used were: PCdh21-Cre (Nagai et al., 2005), Ai38 (Zariwala et al., 2012), and CCK-IRES-Cre (Jackson Labs). Cre-dependent expression of GCaMP3 or GCaMP6f in MT cells was achieved by crossing with the Ai38 GCaMP3 reporter line (for CCK-IRES-Cre mice) or by injection of viral vectors as described previously (Wachowiak et al., 2013). In vivo two-photon imaging was performed as described previously (Wachowiak et al., 2013) under pentobarbital or isoflurane anesthesia or in awake mice. No difference in response properties measured under the two anesthetics were observed, and so data from both conditions were pooled. To control inhalation timing, mice were tracheotomized and artificial inhalation was used to decouple breathing and odorant stimulation in anesthetized mice (Wachowiak and Cohen, 2001). For imaging in awake mice, animals were acclimated for at least two 30-minute sessions prior to data collection.
Supplementary Material
Acknowledgments
We thank T. Bozza for providing the M72-ChR2 mouse line used in the Supplemental Information and for advice on odorant panels. We also thank C. Zabawa, J. Ball and T. Rust for technical assistance as well as M. Diaz-Quesada, F. Fernandez, M. Shipley, M. Rothermel and I. Youngstrom for helpful discussions and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, M.N.E. and M.W.; Methodology, M.N.E. and M.W.; Investigation, M.N.E., K.R.H. and M.W; Data Curation, M.N.E.; Writing - Original Draft, M.N.E. and M.W; Writing - Review & Editing, M.N.E., K.R.H. and M.W; Funding Acquisition, M.N.E. and M.W.
References
- Adam Y, Livneh Y, Miyamichi K, Groysman M, Luo L, Mizrahi A. Functional transformations of odor inputs in the mouse olfactory bulb. Front Neural Circuits. 2014;8:129. doi: 10.3389/fncir.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Marbach F, Anselmi F, Koh MS, Davis MB, Garcia da Silva P, Delevich K, Oyibo HK, Gupta P, Li B, Albeanu DF. An Interglomerular Circuit Gates Glomerular Output and Implements Gain Control in the Mouse Olfactory Bulb. Neuron. 2015;87:193–207. doi: 10.1016/j.neuron.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Jonas P. Action potential propagation into the presynaptic dendrites of rat mitral cells. J Physiol. 1997;504(Pt 2):359–365. doi: 10.1111/j.1469-7793.1997.359be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Kato HK, Komiyama T, Isaacson JS. Broadcasting of cortical activity to the olfactory bulb. Cell Rep. 2015;10:1032–1039. doi: 10.1016/j.celrep.2015.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S, Mertz J, Beaurepaire E, Moreaux L, Delaney K. Odor-evoked calcium signals in dendrites of rat mitral cells. Proc Natl Acad Sci U S A. 2001;98:1230–1234. doi: 10.1073/pnas.021422798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultra-sensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Linster C. On-Center/Inhibitory-Surround Decorrelation via Intraglomerular Inhibition in the Olfactory Bulb Glomerular Layer. Front Integr Neurosci. 2012;6:5. doi: 10.3389/fnint.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 2006;7:7. doi: 10.1186/1471-2202-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbieux F, Audinat E, Charpak S. Action potential propagation in dendrites of rat mitral cells in vivo. J Neurosci. 2003;23:5553–5560. doi: 10.1523/JNEUROSCI.23-13-05553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, Hagiwara A, Bhalla US, Murthy VN, Albeanu DF. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci. 2010 doi: 10.1038/nn.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh PI, Wellis DP, Scott JW. Organization of inhibition in the rat olfactory bulb external plexiform layer. J Neurophysiol. 1993;70:263–274. doi: 10.1152/jn.1993.70.1.263. [DOI] [PubMed] [Google Scholar]
- Fantana AL, Soucy ER, Meister M. Rat olfactory bulb mitral cells receive sparse glomerular inputs. Neuron. 2008;59:802–814. doi: 10.1016/j.neuron.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Fletcher ML. Analytical processing of binary mixture information by olfactory bulb glomeruli. PLoS One. 2011;6:e29360. doi: 10.1371/journal.pone.0029360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer, Andreas T. Two Distinct Channels of Olfactory Bulb Output. Neuron. 2012;75:320–329. doi: 10.1016/j.neuron.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Fukunaga I, Herb JT, Kollo M, Boyden ES, Schaefer AT. Independent control of gamma and theta activity by distinct interneuron networks in the olfactory bulb. Nat Neurosci. 2014 doi: 10.1038/nn.3760. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci. 2009;29:13454–13464. doi: 10.1523/JNEUROSCI.2368-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Albeanu DF, Bhalla US. Olfactory bulb coding of odors, mixtures and sniffs is a linear sum of odor time profiles. Nat Neurosci. 2015 doi: 10.1038/nn.3913. advance online publication. [DOI] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: A major excitatory element that coordinates glomerular activity. J Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, Mori K. Parallel Mitral and Tufted Cell Pathways Route Distinct Odor Information to Different Targets in the Olfactory Cortex. The Journal of Neuroscience. 2012;32:7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Chu MW, Isaacson JS, Komiyama T. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron. 2012;76:962–975. doi: 10.1016/j.neuron.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Gillet SN, Peters AJ, Isaacson JS, Komiyama T. Parvalbumin-Expressing Interneurons Linearly Control Olfactory Bulb Output. Neuron. 2013 doi: 10.1016/j.neuron.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke M-T, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013;16:1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]
- Khan AG, Thattai M, Bhalla US. Odor Representations in the Rat Olfactory Bulb Change Smoothly with Morphing Stimuli. Neuron. 2008;57:571–585. doi: 10.1016/j.neuron.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Fletcher, Max L, Homma R, Yamasoba T, Nagayama S. Odorant Response Properties of Individual Neurons in an Olfactory Glomerular Module. Neuron. 2013;77:1122–1135. doi: 10.1016/j.neuron.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokage E, Pan Y-Z, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular Identity of Periglomerular and Short Axon Cells. J Neurosci. 2010;30:1185–1196. doi: 10.1523/JNEUROSCI.3497-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollo M, Schmaltz A, Abdelhamid M, Fukunaga I, Schaefer AT. 'Silent' mitral cells dominate odor responses in the olfactory bulb of awake mice. Nat Neurosci. 2014;17:1313–1315. doi: 10.1038/nn.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Sachse S, Galizia CG. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol. 2005;93:3410–3417. doi: 10.1152/jn.01285.2004. [DOI] [PubMed] [Google Scholar]
- Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory Bulb Short Axon Cell Release of GABA and Dopamine Produces a Temporally Biphasic Inhibition–Excitation Response in External Tufted Cells. The Journal of Neuroscience. 2013;33:2916–2926. doi: 10.1523/JNEUROSCI.3607-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Katz LC. Response correlation maps of neurons in the mammalian olfactory bulb. Neuron. 2001;32:1165–1179. doi: 10.1016/s0896-6273(01)00537-2. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pírez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Migliore M, Hines M, McTavish TS, Shepherd GM. Functional roles of distributed synaptic clusters in the mitral-granule cell network of the olfactory bulb. Frontiers in Integrative Neuroscience. 2010;4 doi: 10.3389/fnint.2010.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Sano H, Yokoi M. Transgenic expression of Cre recombinase in mitral/tufted cells of the olfactory bulb. genesis. 2005;43:12–16. doi: 10.1002/gene.20146. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Takahashi YK, Yoshihara Y, Mori K. Mitral and Tufted Cells Differ in the Decoding Manner of Odor Maps in the Rat Olfactory Bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- Najac M, Sanz Diez A, Kumar A, Benito N, Charpak S, De Saint Jan D. Intraglomerular lateral inhibition promotes spike timing variability in principal neurons of the olfactory bulb. J Neurosci. 2015;35:4319–4331. doi: 10.1523/JNEUROSCI.2181-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor Representations in Olfactory Cortex: “Sparse” Coding, Global Inhibition, and Oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Brunert D, Zabawa C, Díaz-Quesada M, Wachowiak M. Transgene Expression in Target-Defined Neuron Populations Mediated by Retrograde Infection with Adeno-Associated Viral Vectors. The Journal of Neuroscience. 2013;33:15195–15206. doi: 10.1523/JNEUROSCI.1618-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy KB, Brecha N, Gall C. Distribution of cholecystokinin-like immunoreactivity in the rat main olfactory bulb. J Comp Neurol. 1985;239:373–383. doi: 10.1002/cne.902390403. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic Intraglomerular Circuits Differentially Regulate Tonic and Phasic Presynaptic Inhibition of Olfactory Nerve Terminals. Journal of Neurophysiology. 2009;101:1988–2001. doi: 10.1152/jn.91116.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Liu S, Shipley MT. Intraglomerular inhibition Shapes the Strength and Temporal Structure of Glomerular Output. J Neurophysiol. 2012 doi: 10.1152/jn.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Shipley MT. Intraglomerular inhibition maintains mitral cell response contrast across input frequencies. Journal of Neurophysiology. 2013;110:2185–2191. doi: 10.1152/jn.00023.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of Odor in the Piriform Cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Tan J, Savigner A, Ma M, Luo M. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron. 2010;65:912–926. doi: 10.1016/j.neuron.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Economo MN, Díaz-Quesada M, Brunert D, Wesson DW, White JA, Rothermel M. Optical Dissection of Odor Information Processing In Vivo Using GCaMPs Expressed in Specified Cell Types of the Olfactory Bulb. The Journal of Neuroscience. 2013;33:5285–5300. doi: 10.1523/JNEUROSCI.4824-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Whitesell JD, Sorensen KA, Jarvie BC, Hentges ST, Schoppa NE. Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci. 2013;33:1552–1563. doi: 10.1523/JNEUROSCI.3410-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen T-W. A Cre-Dependent GCaMP3 Reporter Mouse for Neuronal Imaging In Vivo. The Journal of Neuroscience. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Xiong W, Zeng S, Xia A, Shepherd GM, Greer CA, Chen WR. Dendritic excitability and calcium signalling in the mitral cell distal glomerular tuft. European Journal of Neuroscience. 2006;24:1623–1632. doi: 10.1111/j.1460-9568.2006.05076.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.