Abstract
Background:
Attrition and treatment adherence are notorious challenges in pediatric obesity interventions.
Objective:
To evaluate if brief, pre-treatment motivational interviewing (MI) can improve retention (at baseline, post- and follow-up assessment) and adherence (i.e., attendance) in a parent-exclusive pediatric obesity intervention.
Methods:
MI was implemented with parents as an adjunct to a larger randomized controlled trial of NOURISH+ (Nourishing Our Understanding of Role modeling to Improve Support and Health), a parent intervention for children with overweight ages 5–11 years. Parents (N=112) were randomized to receive 2 MI sessions (one telephone; one in-person) or reminder calls.
Results:
Parents (91% female; 52% African American) who completed 1 telephone MI session were more likely to attend baseline (74%) compared with parents who received reminder calls only (53%, p<.001). After a second MI session, there were no group differences in treatment initiation (p>.05). Treatment attendance, post or 4-month follow-up assessment completion did not differ between conditions (p>.05).
Conclusion:
One MI session implemented prior to treatment can improve baseline attendance; a second MI session did not enhance these effects. A single-session, telephone-based MI pre-treatment might be a cost and time-effective strategy to enhance recruitment efforts. Further strategies to address retention and treatment attendance are needed.
Keywords: motivational interviewing, pediatric obesity, attrition, parents, treatment adherence, engagement
Introduction
Over one-third of children in the U.S. have overweight or obesity, with a higher obesity burden among racial and ethnic minorities.1 Family-based behavioral weight loss interventions are the mainstay of treatment;2 yet, poor engagement (i.e., a term encompassing treatment initiation, retention, and adherence)3 is a notorious challenge. Indeed, low treatment adherence and high attrition (up to 75%) are particular concerns when working with underserved populations, reducing intervention effectiveness.4,5 Innovative strategies to enhance engagement in pediatric obesity treatment are urgently needed.5
Low treatment engagement negatively impacts intervention effectiveness, reduces generalizability of findings, and increases costs. Further, families who discontinue treatment are less likely to experience weight-related benefits and are more likely to perceive that treatment is ineffective, reducing the likelihood of future reengagement.6 Many of the factors associated with low engagement are static at study onset (e.g., participants’ race/ethnicity, transportation, and study location and time of day) and should be considered during study design to minimize their impact when possible.7 For example, addressing practical barriers (e.g., reimbursing parking or transportation costs), making repeated contact via multiple methods to provide reminders of study visits, providing incentives for completing assessments, and implementing culturally appropriate treatments are recommended.4,5 However, there is also a need to identify and address modifiable factors (e.g., internal motivation and participant expectations) that could negatively impact treatment engagement. For example, among parents who discontinued from a tertiary care pediatric weight management program, 37% cited mismatched expectations and 39% cited family motivation as the reasons for non-return, highlighting the need to consider these variables.8
There is substantial support for the role of parents in pediatric obesity treatment.9 For younger children (≤~11 years), parent-based treatments (PBT) are demonstrated to have equal or greater reductions in child weight status compared with family based treatments (FBT).10–13 Despite their effectiveness, there might be unique barriers to engagement in PBT,14 such as parent expectations about active child involvement in treatment. This mismatch between parent expectations and program focus can negatively influence engagement.8,15 For example, Kwitowski et al.16 examined barriers to attendance in a PBT and found that parent expectations about active child involvement often were inconsistent with the intervention’s design, reducing engagement. Similarly, Boutelle and colleagues10 compared PBT to FBT for 8–12 year old children with overweight; PBT had a greater loss of participants during the early phase of treatment compared to FBT (although reasons for drop out were not reported). Addressing parent expectations early might increase engagement.
Attrition is often highest early in trials,5,10 even prior to the beginning of treatment, with non-initiation after enrollment ranging from 20–50% in some reports.7,17 For example, in a PBT for pediatric obesity, attrition between successful completion of a telephone screening (and verbalized intent to participate) to attendance at baseline sessions was ~50%, representing a significant loss of potential participants who could benefit from treatment (in addition to significant recruitment costs added to the trial).18 Of note, ambivalence about change can stall efforts to engage in the change progress, and is typically highest when considering making a behavior change;19 thus ambivalence might play a role in non-initiation or early-drop out, representing a potential early intervention target. Strategies that address internal motivation and increase engagement at the “pre-treatment” phase are needed.
Motivational Interviewing (MI) is a collaborative conversation about behavior change.19 MI engages people in exploring and resolving ambivalence via eliciting their own reasons and strategies for change in a patient-centered, empathic manner.19 Prior investigations have demonstrated that MI increased treatment engagement in multiple behavioral domains, with growing support for its use in pediatric obesity interventions.20–22 For example, MI Values was a randomized controlled trial (RCT) of MI implemented within an obesity treatment targeting primarily African American adolescents with obesity.23 In MI Values, MI participants had greater treatment adherence and retention than controls.17 Moreover, adolescents in the MI treatment arm from lower income families had better retention than those from higher income families, suggesting that MI might be a particularly effective strategy for this population, one that frequently manifests higher attrition in trials.5 There is also some evidence that MI might diffuse ambivalence about treatment participation. For example, a single MI session increased parents’ self-determined motivation to promote a healthy lifestyle for their children after receiving weight-related feedback.24 The use of MI to engage parents specifically in PBT for their children’s overweight has not been examined. The current study addressed this issue and implemented an MI intervention as a “pre-treatment” for parents of children with overweight and obesity.
The Current Study
NOURISH+MI was a pilot of an MI intervention, implemented adjunctive to a RCT of NOURISH+ (Nourishing Our Understanding of Role-modeling to Increase Support and Health),25 a culturally tailored PBT for parents of children with overweight or obesity. NOURISH+MI was designed to investigate if two brief pre-treatment MI sessions could enhance retention (at study onset [baseline], post-and follow-up assessments) and adherence (i.e., attendance at treatment sessions) in a parent-exclusive pediatric obesity intervention. It was hypothesized that parents who participated in NOURISH+MI would demonstrate better retention and treatment adherence, than parents randomized to the main trial of NOURISH+ who received reminder calls only.
Methods
Participants
Parents/caregivers were eligible for NOURISH+, and thus the adjunctive study, NOURISH+MI, under the following conditions: 1) parent age ≥18 years; 2) child age 5–11 years; 3) child BMI ≥85th percentile26; and 4) the child primarily resides in the caregiver’s home. Parents were excluded if they were non-ambulatory, non-English-speaking, pregnant, unable to participate in physical activity due to medical or other reasons, or had a medical or psychiatric diagnosis that would impair their ability to respond to assessments or participate in a group. Children participated in assessments only. More than one child per family could participate as long as each met inclusion criteria. After completing a telephone screen for eligibility, parents and children were scheduled to attend a baseline session, where eligibility was further confirmed via objective height and weight assessments, and written consent/assent and baseline assessments were completed. Study procedures were approved by the Institutional Review Board of Virginia Commonwealth University; this trial was registered with clinicaltrials.gov (NCT01912989).
Experimental Design
Screened, eligible participants were randomized at telephone screening to participate in either NOURISH+MI or the main trial of NOURISH+, using a 1 (NOURISH+MI) to 2 (main trial) ratio via a random number generator developed by the study biostatistician. Those randomized to the main trial were subsequently randomized after baseline assessments to either NOURISH+ (intervention) or Wellness (education control), resulting in a 3 group (NOURISH+MI, NOURISH+, Control) x 3 timepoint (baseline, post-test, 4-month follow-up) repeated measures design. The current study evaluated differences in engagement between NOURISH+MI and the NOURISH+ groups.
Overview of NOURISH+ and NOURISH+MI
NOURISH+MI methods, including rationale for the timing and format of MI sessions, have been described in detail elsewhere and are briefly presented here.27 NOURISH+ was a RCT of an 8-session group-based PBT (6 core group sessions with two adjunctive experiential sessions [a group cooking class and an individual dietitian visit]). This treatment targeted parents as the agent of change for their child(ren) with overweight or obesity. NOURISH+ has a foundation in Social Cognitive Theory,28 emphasizes parental role modeling for health behavior change, and is culturally sensitive for African American and lower income families; however families of all racial, ethnic, and socioeconomic backgrounds were eligible.
NOURISH+MI was designed as a brief, adjunctive MI intervention to examine the impact of MI on retention and adherence in NOURISH+. To increase internal validity, procedures for recruitment, assessments, screening, and group intervention were identical for NOURISH+MI and NOURISH+ participants (using trained interventionists, adhering to the Operations Manual, and monitoring fidelity), except for the addition of two brief MI sessions (versus reminder calls only) prior to treatment initiation in the NOURISH+MI arm. The dose of MI was selected due to prior research, which demonstrated that two sessions of MI, implemented adjunctive to an obesity treatment, were effective in enhancing engagement.17
NOURISH+MI.
MI sessions were conducted by trained interventionists (psychology doctoral trainees who were not delivering the NOURISH+ group sessions, blinded to study hypotheses). Each session was ~20–30 min in duration. Session 1 occurred over the telephone in a 2–3 week window after telephone screening and prior to baseline. Using MI, interventionists explored parents’ own reasons for enrolling in a treatment for their child’s weight, elicited change talk (and minimized sustain talk), and affirmed parents’ own reasons and strategies for change. The overall goal was to increase NOURISH+ engagement via highlighting the program’s consistency with parents’ own values and goals. Session 2 occurred in person (to be consistent with the in-person treatment format of NOURISH+) after the baseline visit, but prior to NOURISH+ treatment session 1. At this visit, parents completed a checklist to identify values that are important to them. Using MI, the interventionist developed discrepancy between parents’ values and their current family/child health behaviors, with an exploration of how participation in a PBT for their child’s weight might resolve the identified discrepancy, thus increasing motivation to attend and engage in NOURISH+. (NOURISH+MI session roadmaps are available from the first author upon request).
Treatment Fidelity.
Interventionists were trained by a clinical psychologist and member of MINT (Motivational Interviewing Network of Trainers; MKB). Practice sessions were conducted until competency was achieved, as determined both by the lead investigator and objectively, using the Motivational Interviewing Treatment Integrity Code 3.1 (MITI 3.1).29 The MITI 3.1 is a behavioral coding system used to monitor fidelity to MI. All study sessions were audiorecorded and coded for MI adherence using the MITI 3.1. Trained, blinded raters coded randomly selected 20 minute segments of each MI session; all sessions were double rated by two independent raters. Inter-rater reliabilities (using intra-class correlations [ICCs]) were assessed at study onset and throughout the study. Weekly supervision and feedback on audiorecorded sessions was provided and rigorous fidelity monitoring was conducted throughout the trial.
Measures
Demographics.
At baseline, parents reported parent and child age, gender, race, and ethnicity, and parent education and income.
Anthropometrics.
Trained, blinded staff measured parent and child height and weight using a stadiometer and digital bariatric scale, respectively. These data were used to calculate parent and child BMI (kg/m2) and were plotted on the Center for Disease Control growth charts26 to obtain children’s BMI percentile for age and sex.
Treatment Adherence and Retention.
Participants’ attendance at MI sessions (one telephone, one in-person), NOURISH+ sessions (6 group sessions, cooking class, and dietitian session), and assessments (baseline, post, 4-month follow-up) was monitored. Retention (baseline, post-test, and 4-month follow-up assessment completion); NOURISH+ treatment attendance (% of visits attended of 8 total possible visits); and NOURISH+ treatment initiation (attendance at any NOURISH+ treatment session) were examined.
Analyses
Parent and child demographics and baseline anthropometrics were examined for each group (NOURISH+, NOURISH+MI, Control) and differences examined using t-tests, Chi square analyses, or ANOVAs, as appropriate. The primary outcomes for this study were retention and treatment adherence between NOURISH+ and NOURISH+MI groups. Chi-square analyses examined differential attendance at baseline, post-test, and follow up; t-tests assessed differences in treatment attendance (% initiated treatment [any visit] and mean % NOURISH+ session attendance) between conditions (NOURISH+ and NOURISH+MI). Post-hoc analyses examined outcomes for participants who received both MI sessions, per protocol, compared with NOURISH+. Analyses were conducted in SAS v.9.3 (Cary, NC). All tests were two-tailed with p<.05 used to determine significance. A priori power analyses using PROC POWER determined that 60 individuals per group (NOURISH+ and NOURISH+MI) would have 80% power (α=0.05, two-sided) to detect a 0.21 difference in the proportion of participants completing post-intervention between groups.
Results
Participants and Recruitment
After telephone screening, 326 participants were randomized (N=112 to NOURISH+MI; N=214 to the main trial (who were subsequently randomized to NOURISH+ or Control). In the NOURISH+MI arm, 18 discontinued treatment prior to the first telephone MI session, leaving 94 participants for whom contact was attempted for completion of the first MI session. Of these, 93.6% (88/94) participated in MI session 1 prior to the baseline visit. Participants in the main trial received reminder calls only – one participant was lost to follow up. Of note, 9.9% of NOURISH+MI participants and 7.4% of NOURISH participants were ineligible at baseline (due to child BMI<85th percentile). These participants were excluded from the denominator in subsequent analyses. The second MI session was completed by 89.1% of consented, eligible participants (57/64). Both MI sessions (per protocol) were completed by 81.2% (52/64) of participants. See Figure 1 for the CONSORT diagram for the full trial and Table 1 for baseline characteristics by group. NOURISH+MI participants were mostly female (91% parents; 52% children) and African American (52% parents and children), with mean child BMI in the 97th percentile.
Figure 1.
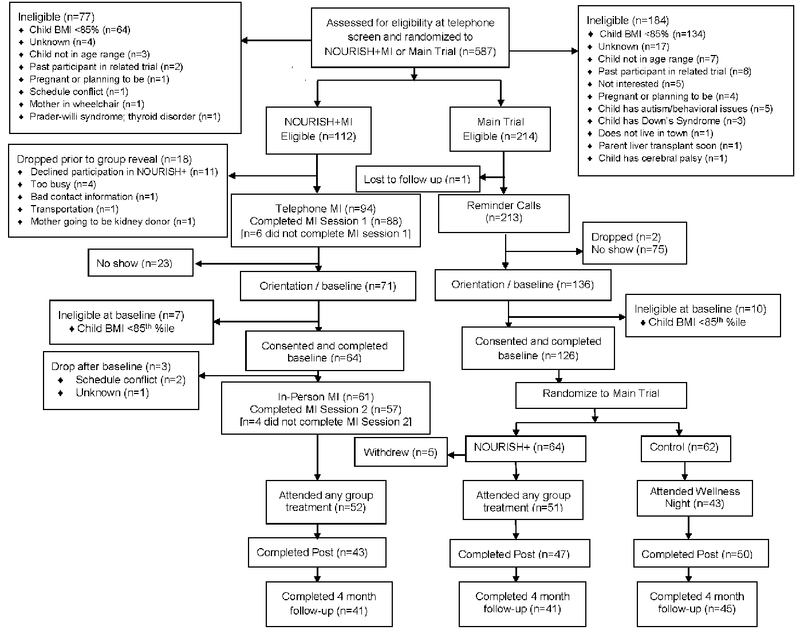
CONSORT Diagram
Table 1.
Baseline characteristics by treatment condition in the NOURISH+MI randomized controlled trial.
| Variable | NOURISH+MI N=64 (parents) N=71 (children) |
NOURISH+ N=64 (parents) N=69 (children) |
Control N=62 (parents) N=64 (children) |
p |
|---|---|---|---|---|
| Parent | n (%) or M (SD) | n (%) or M (SD) | n (%) or M (SD) | |
| Female (%) | 58 (90.6%) | 63 (98.4%) | 59 (95.2%) | .139 |
| Racea (%) | .370 | |||
| Black or African American | 33 (51.6%) | 39 (62.9%) | 41 (68.3%) | |
| White or Caucasian | 27 (42.2%) | 21 (33.9%) | 16 (26.7%) | |
| Other / More than one race | 4 (6.2%) | 2 (3.2%) | 3 (5.0%) | |
| Hispanic (%) | 2 (3.7%) | 2 (3.6%) | 2 (3.6%) | .999 |
| Age (years) | 40.7 (10.2) | 40.3 (7.8) | 38.3 (7.4) | .287 |
| BMI (kg/m2) | 35.8 (10.1) | 35.1 (9.5) | 34.4 (12.2) | .788 |
| Parent Education (%) | .508 | |||
| Less than High School Diploma | 5 (7.8%) | 2 (3.4%) | 2 (3.6%) | |
| High School Graduate | 7 (10.9%) | 6 (10.3%) | 7 (12.5%) | |
| Some College | 22 (34.4%) | 13 (22.4%) | 13 (23.2%) | |
| College Degree | 21 (32.8%) | 23 (39.7%) | 16 (28.6%) | |
| Some Graduate School | 2 (3.1%) | 3 (5.2%) | 5 (8.9%) | |
| Graduate Degree | 7 (10.9%) | 11 (19.0%) | 13 (23.2%) | |
| Parent Income (%) | .064 | |||
| <$15,000 | 12 (18.8%) | 7 (12.1%) | 16 (28.6%) | |
| $15-24,000 | 5 (7.8%) | 5 (8.6%) | 9 (16.1%) | |
| $25-34,999 | 6 (9.4%) | 8 (13.8%) | 5 (8.9%) | |
| $35,000-44,999 | 7 (10.9%) | 6 (10.3%) | 5 (8.9%) | |
| $45,000-59,999 | 9 (14.1%) | 6 (10.3%) | 3 (5.4%) | |
| $60,000-74,999 | 11 (17.2%) | 3 (5.2%) | 2 (3.6%) | |
| ≥ $75,000 | 14 (21.9%) | 23 (39.7%) | 16 (28.6%) | |
| Child | ||||
| Female (%) | 37 (52.1%) | 45 (66.2%) | 32 (50.0%) | .121 |
| Racea (%) | .038 | |||
| Black or African American | 36 (52.9%) | 45 (70.3%) | 44 (69.8%) | |
| White or Caucasian | 25 (36.7%) | 45 (70.3%) | 44 (69.8%) | |
| Other / More than one race | 7 (10.3%) | 2 (3.1%) | 8 (12.7%) | |
| Hispanic (%) | 4 (5.7%) | 3 (4.8%) | 2 (3.4%) | .834 |
| Age (years) | 9.0 (2.1) | 8.8 (1.9) | 8.8 (2.1) | .813 |
| BMI Percentile | 96.8 (4.0) | 97.1 (3.0) | 96.2 (3.8) | .325 |
Note: MI = Motivational Interviewing; BMI = Body mass index; Demographics were obtained at baseline (after the initial MI session) and are presented for the primary participating parent and all eligible children; 2 parents were permitted to attend; numbers do not always equal total N if information was missing from participant. NOURISH+MI occurred in Richmond, VA from 2013-15.
Attendance and Treatment Initiation by Group
Parents in the NOURISH+MI arm were significantly more likely to attend the baseline / orientation session (75.5%) compared with parents randomized to the main trial who received reminder calls only (63.8%, p<.001). There was no differences in the percent of parents who initiated treatment (attended ≥1 group session) in NOURISH+MI (81.2%) compared with NOURISH+ (79.7%; p=.824). Post-hoc dose analyses examined baseline attendance for NOURISH+MI participants who received both MI sessions (per protocol), and results were similar: 90.4% attended baseline; p=.114.
Mean percent attendance was not significantly different between parents randomized to NOURISH+ (61.0±30.8%) and NOURISH+MI (51.6±34.9%; t[118]=1.56, p=.122). Post-hoc dose analyses for NOURISH+MI participants who received both MI session were similar (mean attendance=55.8±33.4%; t[109]=0.85, p=.396).
Completion of post (79.7% NOURISH+ and 70.5% NOURISH+MI) and 4-month follow-up assessments (69.5% NOURISH+ and 67.2% NOURISH+MI) did not differ between conditions (p=.247, p=.789, respectively). NOURISH+MI per protocol post-hoc results were similar, with 73.1% (p=.414) retention through post and 65.4% (p=.213) through 4-month follow-up.
Treatment Fidelity
Table 2 presents fidelity to MI as measured by the MITI 3.1. Interventionists exceeded proficiency threshold, with no differences in MI adherence between interventionists or between telephone (Session 1; mean length 24.5±6.36 minutes and in person (Session 2; mean length 27.4±7.73 minutes) sessions (p>.05). Interrater reliability was strong across domains (ICCs=0.62–1.0). All sessions were double rated and ICCs remained strong (>.72).
Table 2.
Motivational Interviewing proficiency at sessions 1 and 2 compared with recommended proficiencies using the MITI 3.1a
| Mean Rating Session 1b |
Mean Rating Session 2c |
MITI 3.1 Recommended Proficiencies | ||
|---|---|---|---|---|
| MITI Domain | M (SD) | M (SD) | Basic Competency | Proficiency |
| Global Spiritd | 4.6 (0.41) | 4.6 (0.37) | 3.5 | 4 |
| Reflection:Questione | 1.6 (0.72) | 3.4 (4.22) | 1.0 | 2.0 |
| % Complex Reflectionsf | 91.0 (0.05) | 94.2 (0.05) | 40% | 50% |
| % Open Questionsg | 73.2 (0.12) | 79.4 (0.19) | 50% | 70% |
| % MI Adherenth | 100 (0.00) | 100 (0.00) | 90% | 100% |
MITI 3.1 = Motivational Interviewing Treatment Integrity Code, Version 3.1
Means represent ratings from 6 interventionists across 82 encounters for Session 1 (6 sessions were unable to be rated due to audiorecorded malfunctioning). There were no differences in adherence between interventionists (p>.05).
Means represent ratings from 5 interventionists across 53 encounters for Session 2
Global Spirit = (Evocation + Collaboration + Autonomy) / 3
Ratio = Total Reflections / Total Questions
%Complex Reflections = (Complex Reflections / Total Reflections) × 100
%Open Questions = (Open Questions / Total Questions) × 100
%MI Adherent = MI Adherent / (MI Adherent + MI Non-adherent
Discussion
Results of this pilot suggest that the addition of one brief telephone MI session, implemented prior to treatment, enhanced baseline attendance among predominately African American parents within a PBT for pediatric obesity. However, MI was insufficient to enhance retention beyond baseline or to increase treatment attendance. Indeed, although not significant, mean group attendance was lower in the MI arm (52%) compared with the main trial (61%). Nonetheless, as engagement is a significant problem in pediatric obesity treatment, the finding that one MI session enhanced baseline attendance is encouraging, and supports the potential benefit of MI as a brief, cost-effective strategy to improve initial recruitment efforts. Additional strategies to reduce attrition and increase treatment adherence, however, are needed.
This study is one of the first to examine the effect of MI on engagement within a PBT for pediatric obesity, and provides mixed support for its use. Comparison to other studies is limited given the few investigations with this population in this scientific area. Indeed, to our knowledge, only one other study investigated the effect of MI on engagement among parents of children with obesity, conducted in a tertiary care, clinic-based setting.30 Specifically, Armstrong et al. reported that MI-based text messages, sent daily for 12 weeks to parents as part of the treatment for their child’s obesity, enhanced attendance at clinic visits.30 Importantly, the intensity of the weight management treatment was much lower (monthly) in this prior investigation, compared with NOURISH+ (weekly), and the frequency and type of the adjunctive MI contact also differed substantially, which might explain the differential findings. Mixed findings have also been reported among studies targeting adolescents and young adults. Specifically, one in-person, pre-treatment MI session did not improve retention at post-testing among adolescents enrolled in an obesity treatment.31 However, a single MI session implemented prior to randomization into a behavioral weight management program for adults with obesity was associated with very high retention (96%),32 far exceeding typical results in weight management studies. Similarly, a two-session adjunctive MI intervention was associated with significantly improved treatment adherence among primarily African American adolescents enrolled in a multidisciplinary obesity treatment.17 Given these conflicting findings, and the limited investigations of MI on engagement within PBT, additional research is warranted.
In NOURISH+MI, a low telephone dose of MI minimally impacted participant burden, yet enhanced early engagement (i.e., at baseline), a time of high attrition across trials.5 Future studies might consider including MI as part of the screening process to address ambivalence upon enrollment, and not as a separate contact as implemented in the current trial, to reduce burden further and investigate effects. As noted, results did not support the benefit of an additional pre-treatment (in person) MI contact. It is possible that the initial MI session retained parents with greater ambivalence who might otherwise have dropped. Yet, this MI dose was not sufficient to overcome their continued ambivalence throughout treatment. Additional strategies that address ambivalence (such as mid-treatment booster MI sessions) are needed to enhance treatment attendance and retention beyond the baseline period. It is also possible that this second MI session, delivered in person to be consistent with NOURISH+ treatment delivery, increased participant burden by requiring an additional study visit. Although unable to be tested with the current data, MI delivery via telephone might have been preferable to any in-person, adjunctive contact. Telephone MI delivery has been demonstrated to be effective,33 and might be particularly appealing to a lower income population, a group which often faces multiple practical barriers to in-person treatment delivery.
Limitations include differential attention between groups, such that the NOURISH+MI participants had more contact with interventionists (~40 minutes total) than participants in NOURISH+ who received reminder calls only. Comparing MI to an alternative approach matched on contact (e.g., one informed by behavior change theory) or providing participants extended opportunity for questions about the treatment would further clarify the ability of MI (vs. attention alone) to enhance engagement. Further, NOURISH+MI participants learned of their treatment assignment prior to participants in the main trial, which might have positively influenced baseline attendance. Several participants discontinued from the trial prior to receiving any MI; thus those retained might have been more motivated, contributing to the effects observed. There was limited enrollment of male parents/caregivers, thus generalizability is limited to predominately female, African American parents with a child with overweight or obesity. To advance science in this area further, data on baseline attendance in both the intervention and control arms is needed. Although this study only applied MI to the treatment arm of NOURISH+, this approach can be easily adapted in future studies to apply it to all conditions at enrollment, prior to randomization. Strengths of NOURISH+MI include its design (RCT), target population of primarily African American families of low socioeconomic status, focus on parents, and emphasis on treatment integrity. NOURISH+MI contributes to the development of strategies for enhancing engagement, a major challenge in pediatric obesity research.5
Innovative strategies to improve treatment adherence and reduce attrition are critical to improve the efficacy of pediatric obesity interventions. Although it is recommended that health professionals incorporate MI into pediatric obesity treatments, more rigorous investigations are warranted, particularly among parents of children with overweight. Results suggest that a brief additional contact using MI might be a beneficial, cost and time effective approach to improve recruitment efforts within a parent intervention for pediatric overweight. Future research should include larger samples and examine how to optimize the application of MI into PBT for obesity, including investigation of the optimal dose and timing of MI, and integration of MI into (versus adjunctive to) treatment, to further understanding of the application of MI to PBT for pediatric obesity.
Acknowledgements
Funding Source: NOURISH+MI was funded by the American Heart Association 13CRP1457008 to Melanie K Bean; NOURISH+ was funded by the NIH R01HD066216 to Suzanne E Mazzeo.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to report.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017(288):1–8. [PubMed] [Google Scholar]
- 2.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009(1):CD001872. [DOI] [PubMed] [Google Scholar]
- 3.Nobles JD, Perez A, Skelton JA, Spence ND, Ball GD. The engagement pathway: A conceptual framework of engagement-related terms in weight management. Obes Res Clin Pract. 2018;12(2):133–138. [DOI] [PubMed] [Google Scholar]
- 4.Karlson CW, Rapoff MA. Attrition in randomized controlled trials for pediatric chronic conditions. J Pediatr Psychol. 2009;34(7):782–793. [DOI] [PubMed] [Google Scholar]
- 5.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obesity Reviews. 2010:e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhaliwal J, Nosworthy NM, Holt NL, et al. Attrition and the management of pediatric obesity: an integrative review. Child Obes (Print). 2014;10(6):461–473. [DOI] [PubMed] [Google Scholar]
- 7.Nobles J, Griffiths C, Pringle A, Gately P. Design programmes to maximise participant engagement: a predictive study of programme and participant characteristics associated with engagement in paediatric weight management. Int J Behav Nutr Phys Act. 2016;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampl S, Demeule M, Eneli I, et al. Parent perspectives on attrition from tertiary care pediatric weight management programs. Clin Pediatr (Phila). 2013;52(6):513–519. [DOI] [PubMed] [Google Scholar]
- 9.Faith MS, Van Horn L, Appel LJ, et al. Evaluating parents and adult caregivers as “agents of change” for treating obese children: Evidence for parent behavior change strategies and research gaps. Circulation. 2012;125(9):1186–1207. [DOI] [PubMed] [Google Scholar]
- 10.Boutelle KN, Rhee KE, Liang J, et al. Effect of attendance of the child on body weight, energy intake, and physical activity in childhood obesity treatment: a randomized clinical trial. JAMA Pediatr. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95(5):1008–1015. [DOI] [PubMed] [Google Scholar]
- 12.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. Comparison of program costs for parent-only and family-based interventions for pediatric obesity in medically underserved rural settings. J Rural Health. 2009;25(3):326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yackobovitch-Gavan M, Wolf Linhard D, Nagelberg N, et al. Intervention for childhood obesity based on parents only or parents and child compared with follow-up alone. Pediatr Obes. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Spence ND, Newton AS, Keaschuk RA, et al. Predictors of short- and long-term attrition from the parents as agents of change randomized controlled trial for managing pediatric obesity. J Pediatr Health Care. 2017;31(3):293–301. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes ET, Boles RE, Chin K, et al. Expectations for treatment in pediatric weight management and relationship to attrition. Child Obes (Print). 2017;13(2):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwitowski M, Bean MK, Mazzeo SE. An exploration of factors influencing attrition from a pediatric weight management intervention. Obes Res Clin Pract. 2017;11(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bean MK, Powell P, Quinoy A, Ingersoll K, Wickham EP 3rd, Mazzeo SE. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: results from the MI Values randomized controlled trial. Pediatr Obes. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzeo SE, Kelly NR, Stern M, et al. Parent skills training to enhance weight loss in overweight children: evaluation of NOURISH. Eat Behav. 2014;15(2):225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller W, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York: Guilford; 2013. [Google Scholar]
- 20.Resnicow K, Harris D, Wasserman R, et al. Advances in motivational interviewing for pediatric obesity: results of the Brief Motivational Interviewing to Reduce Body Mass Index Trial and future directions. Pediatr Clin North Am. 2016;63(3):539–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez M, Mullins S. Motivational interviewing and pediatric health behavior interventions. J Dev Behav Pediatr. 2008;29(5):417–428. [DOI] [PubMed] [Google Scholar]
- 22.Borrello M, Pietrabissa G, Ceccarini M, Manzoni GM, Castelnuovo G. Motivational interviewing in childhood obesity treatment. Front Psychol. 2015;6:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bean MK, Mazzeo SE, Stern M, Bowen D, Ingersoll K. A values-based motivational interviewing (MI) intervention for pediatric obesity: study design and methods for MI Values. Contemp Clin Trials. 2011;32(5):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson AM, Brown DA, Cox A, et al. Using motivational interviewing for weight feedback to parents of young children. J Paediatr Child Health. 2014;50(6):461–470. [DOI] [PubMed] [Google Scholar]
- 25.Mazzeo SE, Kelly NR, Stern M, et al. Nourishing our understanding of role modeling to improve support and health (NOURISH): Design and methods. Contemp Clin Trials. 2012;33:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. National Center for Health Statistics. Clinical Growth Charts. Available at: http://www.cdc.gov/growthcharts/. Accessed January 17, 2006 2000; [Google Scholar]
- 27.Bean MK, Jeffers AJ, Tully CB, Thornton LM, Mazzeo SE. Motivational interviewing with parents of overweight children: Study design and methods for the NOURISH+MI study. Contemp Clin Trials. 2014;37(2):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandura A Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, N.J.: Prentice-Hall; 1986. [Google Scholar]
- 29.Moyers TB, Martin JK, Manuel J, Miller WR, Ernst D. Revised Global Scales: Motivational Interviewing Treatment Integrity 3.1.1 (MITI 3.1.1). 2010. [Google Scholar]
- 30.Armstrong S, Mendelsohn A, Bennett G, Taveras EM, Kimberg A, Kemper AR. Texting motivational interviewing: a randomized controlled trial of motivational interviewing text messages designed to augment childhood obesity treatment. Child Obes (Print). 2018;14(1):4–10. [DOI] [PubMed] [Google Scholar]
- 31.Brennan L Does motivational interviewing improve retention or outcome in cognitive behaviour therapy for overweight and obese adolescents? Obes Res Clin Pract. 2016;10(4):481–486. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health Educ Res. 2005;20(4):439–447. [DOI] [PubMed] [Google Scholar]
- 33.Lin CH, Chiang SL, Heitkemper MM, et al. Effects of telephone-based motivational interviewing in lifestyle modification program on reducing metabolic risks in middle-aged and older women with metabolic syndrome: A randomized controlled trial. Int J Nurs Studies. 2016;60:12–23. [DOI] [PubMed] [Google Scholar]


