Abstract
Objective:
Aging is associated with impaired insulin sensitivity and increased prevalence of type 2 diabetes. However, it remains unclear whether aging-associated insulin resistance is due to increased adiposity or other age-related factors. To address this question, we investigated the impact of aging on insulin sensitivity independently of changes in body composition.
Methods:
Cohorts of mice at 4–8 months (‘young’) and 18–27 months (‘aged’) of age exhibiting similar body composition were characterized for glucose metabolism on chow and high-fat diets. Insulin sensitivity was assessed by hyperinsulinemic-euglycemic clamp analyses. The relationship between aging and insulin resistance in humans was investigated in 1,250 non-diabetic Mexican-Americans who underwent hyperinsulinemic-euglycemic clamps.
Results:
In mice with similar body composition, age had no detrimental effect on plasma glucose and insulin levels. While aging did not diminish glucose tolerance, hyperinsulinemiceuglycemic clamps demonstrated impaired insulin sensitivity and reduced insulin clearance in aged mice on chow and high-fat diets. Consistent with results in the mouse, age remained an independent determinant of insulin resistance after adjustment for body composition in Mexican-American males.
Conclusions:
This study demonstrates that in addition to altered body composition, adiposity-independent mechanisms also contribute to aging-associated insulin resistance in mice and humans.
Keywords: aging, insulin sensitivity, insulin clearance
Introduction
The negative influence of aging on insulin sensitivity and the prevalence of type 2 diabetes (T2D) has long been recognized (1). However, the pathogenetic mechanisms responsible for the age-related deterioration of glucose metabolism remain incompletely understood (2, 3). A principal difficulty is that aging is associated with several adverse physiological changes (e.g. altered body composition, reduced physical activity, sarcopenia, inflammation), which makes it difficult to decipher the role of age-related comorbidities. For example, whereas aging-associated declines in glucose tolerance and insulin sensitivity are well documented in the mouse, previous reports are based on comparisons between young and old mice that exhibit different body compositions (4, 5, 6, 7). Thus, while the metabolic impact of age-related changes in body composition is well established (8), the potential contribution of adiposity-independent mechanisms to aging-associated insulin resistance remains unexplored.
A substantial body of evidence indicates that insulin sensitivity also declines with advanced age in humans (9, 10, 11, 12). To discriminate between the effects of age versus aging-associated changes in body composition, previous studies employed young and elderly cohorts matched for adiposity, or adjusted for this trait in population-based cohorts. Investigations in predominantly non-Hispanic white cohorts consistently failed to demonstrate an effect of aging on insulin sensitivity after adjustment for altered body composition suggesting that increased adiposity is responsible for age-associated deterioration of glucose tolerance in this population (11, 12, 13, 14, 15, 16). In contrast, in a Japanese population-based cohort, age remained positively correlated with insulin resistance even after adjusting for fat content (17). Remarkably, adjustment for adiposity revealed age-related improvement in insulin sensitivity in other cohorts with predominantly African-American participants (18, 19, 20). Taken together, these observations suggest that genetic and/or other ethnicity-specific determinants may play a role in age-related changes in insulin action and raise the possibility that impaired glucose homeostasis associated with aging may have different etiologies in different populations.
Based on the established role of increased adiposity in impaired insulin sensitivity, we hypothesized that insulin resistance associated with aging is due to age-related changes in body composition. In the present study, we explored this hypothesis in mouse and human subjects. In mice, we assessed insulin sensitivity in young and aged animals exhibiting similar body composition. In human studies, we addressed this question in Mexican-Americans, a population in which the metabolic impact of aging has not been investigated before despite strong predisposition to diabetes (21).
Methods
Animals
Young and aged male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 2–3 and 12 months of age, respectively, and maintained at Cedars-Sinai Medical Center until analysis at ages indicated in the figure legends. We used several approaches to ensure that only apparently healthy aged mice were included in our study. First, body weights were measured biweekly and mice showing weight loss at two successive time points were excluded from phenotyping. Second, complete blood cell count was evaluated with a HEMAVET 950 FS counter (Drew Scientific, FL) to identify and exclude mice with lymphomas, a prevalent neoplasm in aging C57BL/6 mice (22). Finally, after phenotypic characterization, aged mice underwent necropsy and mice with visible tumors and enlarged organs were excluded from analysis. Animals were maintained under specific pathogen-free (SPF) conditions on a 14-h light/10-h dark cycle and fed ad libitum Laboratory Rodent Diet 5053 (LabDiet) or a high-fat (HF) diet (D12492; 60 kcal% from lard; Research Diets) for 9 weeks with free access to water. Experimental procedures were approved by the Institutional Animal Care and Use Committees at Cedars-Sinai Medical Center, Western University, University of Massachusetts and Vanderbilt University.
Mouse phenotyping
Body composition was determined by quantitative magnetic resonance analysis (EchoMRI, TX). Blood glucose concentrations were measured with a OneTouch portable glucose meter (LifeScan, CA). Plasma insulin and C-peptide were assayed by ELISA (ALPCO, NH). In glucose tolerance tests (GTT), overnight fasted mice were retro-orbitally injected with 1.5 g/kg (chow diet) or 1 g/kg (HF diet) glucose followed by blood glucose measurements. Insulin tolerance tests (ITT) were performed in ad libitum fed mice by retro-orbital injection of 0.8 U/kg (chow diet) or 1.2 U/kg (HF diet) insulin followed by blood glucose measurements. Hyperinsulinemic-euglycemic clamping of mice was performed at the National Mouse Metabolic Phenotyping Centers (MMPC) at the University of Massachusetts Medical School and Vanderbilt University (Supplemental Material). The metabolic clearance rate of insulin (MCRI) was calculated as insulin infusion rate divided by the mean plasma insulin concentration at steady state (23). The calculation of MCRI assumes that endogenous insulin secretion is completely suppressed in the setting of hyperinsulinemic clamp, as has been demonstrated in several species (24, 25, 26).
Human study subjects
The current study was conducted in 1,250 non-diabetic subjects from two independent family-based cohorts recruited from the Los Angeles area, the Hypertension-Insulin Resistance (HTNIR) and the Mexican-American Coronary Artery Disease (MACAD) cohorts. HTN-IR consists of Los Angeles Hispanic-American families ascertained via a proband with essential hypertension (27). MACAD participants were drawn from adult offspring of probands with coronary artery disease and their spouses (28). Participants were free of major medical illness and none were taking glucocorticoids or antihyperglycemic agents that could affect glucose homeostasis when they were phenotyped. All subjects gave written informed consent prior to participation.
Human phenotyping
We studied 579 subjects from HTN-IR and 671 MACAD participants who had undergone phenotyping with the hyperinsulinemic-euglycemic clamp as described previously (29). The glucose infusion rate (M value) during the last 30 minutes of steady-state glucose and insulin levels during the clamp reflects glucose uptake by all tissues of the body (mainly insulin-mediated glucose uptake in muscle) and is directly correlated with tissue insulin sensitivity (30). The insulin sensitivity index (M/I) was calculated as M divided by the steady state plasma insulin level (I). The MCRI was calculated as the insulin infusion rate divided by the insulin concentration during the steady state of the euglycemic clamp, as previously described (30, 31). Body adiposity index (BAI) was calculated as (hip circumference in centimeters)/(height in meters)1.5-18) (32). BMI was calculated as weight in kilograms divided by height in meters squared. Fasting plasma C-reactive protein (CRP) concentration was measured as a proxy of inflammation. Physical activity was assessed as described in Supplemental Information. All studies were approved by Human Subjects Protection Institutional Review Boards at UCLA, the University of Southern California and Cedars-Sinai Medical Center.
Statistical analysis
In the mouse data, normally distributed values are expressed as mean ± SEM and non-normally distributed values are represented by the median and interquartile range (IQR). Comparisons between two groups were performed by Student’s t-test or Mann-Whitney U test. GTT and ITT results were analyzed by 2-way repeated measures ANOVA and Holm-Sidak post hoc tests. ANOVA and correlation analyses of non-normally distributed variables were performed after transformation or on ranks. Outlier data points were defined as those falling outside 2xIQR. Based on this criterion, 2 extreme values were excluded from analysis in the entire study (1 young and 1 aged mouse in Figure 2G). Differences at p<0.05 were considered statistically significant.
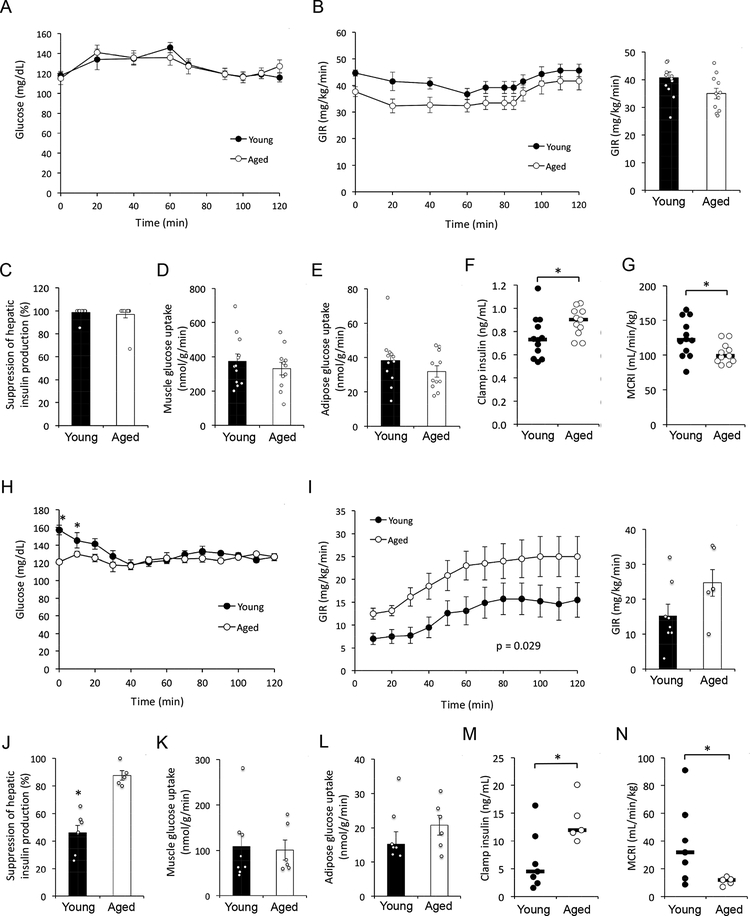
Figure 2 –
(A-G) Hyperinsulinemic-euglycemic clamp results in young (n = 12; 8 months of age) and aged (n = 11; 27 months of age) male C57BL/6J mice on chow diet. (H-N) Hyperinsulinemic-euglycemic clamp results in young (n = 7; 6 months of age) and aged (n = 5; 24 months of age) diet-induced obese male C57BL/6J mice. (A and H) Blood glucose levels. (B and I) Glucose infusion rate (GIR) during the clamp (left panel) and at steady state (80–120 min; right panel). P value corresponds to the effect of ‘Age’ factor in 2-way repeated measures ANOVA. (C and J) Suppression of hepatic glucose production. (D and K) Skeletal muscle glucose uptake. (E and L) Adipose tissue glucose uptake. (F and M) Plasma insulin levels during the final 20 min (100–120 min) of the clamp. (G and N) Metabolic clearance rate of insulin (MCRI) during the final 20 min of the clamp. (A-E and H-L) Data are presented as mean ± SEM. *, p < 0.05; comparison by Student’s t-test. (F-G and M-N) Horizontal bars represent median. *, p < 0.05; comparison by Mann-Whitney U test.
In the human data, log-transformed (BMI, CRP, fasting glucose, fasting insulin) or square root‐transformed (M value, M/I, MCRI, PAS) trait values were used to normalize the distribution for statistical analyses. Generalized estimating equations (GEE) were used to assess the relationships between pairs of traits (univariate analyses) or joint effects of multiple traits (multivariate analyses) on M, M/I, or MCRI, adjusting for familial relationships. The weighted GEE1 (35) was computed assuming an exchangeable correlation structure and using the sandwich estimator of the variance to account for familial correlation present in family data. GEE was used to derive standardized regression coefficients, which in any one regression equation are measured on the same scale, with a mean of zero and a standard deviation of one. They are then directly comparable to one another, with the largest coefficient indicating which independent variable has the greatest association with the dependent variable.
Results
Aging reduces insulin sensitivity and clearance in lean mice independent of body composition
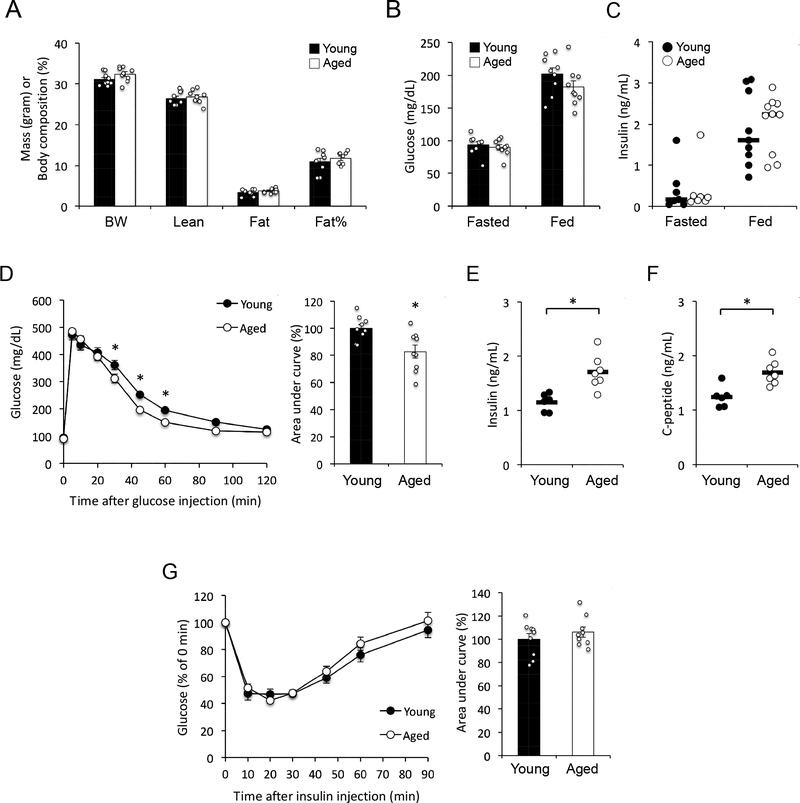
Several previous studies investigating the metabolic impact of aging documented reduced glucose tolerance and insulin resistance in old mice (14–26 months of age) relative to young (1–3 months) mice (4, 5, 6, 7). However, as body fat content increases during the first few months of life, these studies were confounded with different body compositions in old vs young mice (4, 5, 6, 7). Thus, the potential adiposity-independent metabolic effects of aging remain unknown. As body weight and adiposity reach a plateau in mature adult (~4 months) mice (36, 37), we reasoned that using mouse cohorts beyond this age would allow metabolic characterization without the confounding effects of altered body composition. Indeed, mature adult C57BL/6J mice at 4–5 months of age (from here on referred to as ‘young’) exhibited body weight, lean mass, fat mass and percent fat content that were indistinguishable from 18–21-month-old (‘aged’) mice (Fig. 1A). Furthermore, young and aged mice maintained similar fasting and ad libitum fed glucose and insulin levels (Fig. 1B and 1C). Intravenous glucose tolerance tests (GTT) revealed that aged mice were slightly, but significantly more glucose tolerant than young mice (Fig. 1D). This was due, at least in part, to increased glucose-stimulated insulin secretion in aged mice, as both insulin and C-peptide levels were elevated in this group 15 min after glucose injection (Fig. 1E and 1F), consistent with previous studies (38). In insulin tolerance tests (ITT), young and aged mice demonstrated similar glucose response to intravenously-injected insulin (Fig. 1G). To confirm these results, we characterized a second group of young (6-month-old) and aged (25-month-old) mice with similar body composition (Fig. S1A). Consistent with results obtained in the initial cohort, aged mice exhibited improved glucose tolerance (Fig. S1B), but unchanged insulin tolerance (Fig. S1C) relative to young mice.
Figure 1 –
(A) Body weight (BW) and composition of young (4–5 months) and aged (18–21 months) mice (n = 9 mice per group). Vertical axis shows mass in grams for BW, Lean and Fat, or body composition as percentage of fat for Fat%. (B) Blood glucose levels in overnight fasted and ad libitum fed mice (n = 9 mice per group). (C) Plasma insulin levels in overnight fasted and ad libitum fed mice (horizontal bars represent median, n = 7 fasted mice per group and 9–10 fed mice per group). (D) Glucose tolerance test (n = 9 mice per group). *, p < 0.05; comparison by 2-way repeated measures ANOVA (left panel) or Student’s t test (right panel). (E) Plasma insulin levels 15 minutes after glucose injection (horizontal bars represent mean, n = 6 young + 7 aged mice). *, p < 0.05; comparison by Student’s t-test. (F) Plasma C-peptide levels 15 minutes after glucose injection (horizontal bars indicate mean, n = 6 young + 7 aged mice). *, p < 0.05; comparison by Student’s t-test. (G) Insulin tolerance test (n = 9 mice per group). Data are presented as mean ± SEM unless stated otherwise.
We further investigated the metabolic impact of aging by assessing glucose homeostasis in a third cohort of young (8-month-old) and aged (27-month-old) male mice by hyperinsulinemiceuglycemic clamp analyses. Body weight and composition, fasting plasma glucose and insulin levels, and the rate of hepatic glucose production were indistinguishable between young and aged mice in the basal state (Table S1). During the clamp, no significant differences were observed in steady-state glucose levels, glucose infusion rate (GIR), whole body glucose turnover, suppression of hepatic glucose production, and glucose uptake into muscle and adipose tissue (Fig. 2A–E, Table S1). However, despite identical rates of insulin infusion, aged mice exhibited elevated plasma insulin levels (Fig. 2F) and a lower rate of insulin clearance from the circulation (Fig. 2G). Based on the observation of similar insulin action despite higher insulin levels in aged animals, we conclude that aging is associated with increased hepatic, muscle and adipose insulin resistance in male mice independent of changes in adiposity.
Aging reduces insulin sensitivity and clearance in diet-induced obese mice independent of body composition
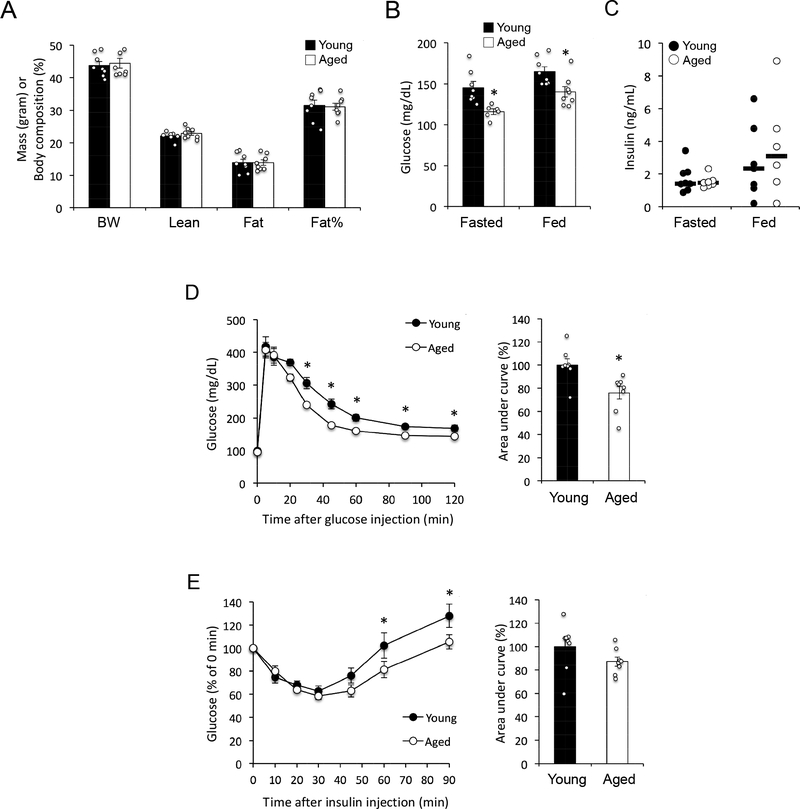
The results in lean mice prompted us to investigate whether diet-induced metabolic stress may exacerbate aging-related insulin resistance. To test this hypothesis, we assessed the impact of aging on glucose homeostasis in the context of diet-induced obesity by characterizing young (6-month-old) and aged (24-month-old) male C57BL/6J mice fed a HF diet (60 kcal% from fat) for 9 weeks. At the time of metabolic analysis young and aged mice had similar body weights and composition (Fig. 3A). Unexpectedly, aged mice maintained lower fasting and fed glucose levels (Fig. 3B) despite similar plasma insulin concentrations (Fig. 3C). Consistent with results obtained in lean mice, obese aged mice also demonstrated improved glucose tolerance relative to young animals (Fig. 3D). Furthermore, assessment of insulin tolerance by ITT revealed that exogenous insulin had a greater glucose-suppressing effect in aged mice at later time-points of the assay, although the difference in areas under the curve did not reach statistical significance (Fig. 3E).
Figure 3 –
(A) Body weight (BW) and composition of young (6 months) and aged (24 months) mice (n = 8 mice per group) after 9 weeks of HF diet feeding. Vertical axis shows mass in grams for BW, Lean and Fat, or body composition as percentage of fat for Fat%. (B) Blood glucose levels in 5-hour fasted (n = 8 young + 6 aged mice) and ad libitum fed (n = 8 mice per group) mice. *, p < 0.05; comparison by Student’s t-test. (C) Plasma insulin levels in 5-h fasted (n = 8 young + 7 aged mice) and ad libitum fed mice (n = 7 young + 6 aged mice). Horizontal bars represent median. (D) Glucose tolerance test (n = 8 mice per group). *, p < 0.05; comparison by 2-way repeated measures ANOVA (left panel) or Student’s t test (right panel). (E) Insulin tolerance test (n = 8 mice per group). *, p < 0.05; comparison by 2-way repeated measures ANOVA.
To evaluate insulin sensitivity directly, we performed hyperinsulinemic-euglycemic clamp experiments. Under basal conditions, body weight and composition, insulin levels and the rate of hepatic glucose production were similar in young and aged mice, whereas the latter exhibited significantly lower fasting glucose levels (Table S2). During the clamp, steady-state glucose levels and GIR were similar in obese young and aged mice (Fig. 2H–I, Table S2). However, obese aged mice maintained significantly higher insulin levels (Fig. 2M) and demonstrated reduced insulin clearance (Fig. 2N), consistent with results obtained in lean mice. Despite elevated plasma insulin concentration in aged mice, whole body glucose turnover and glucose uptake in muscle and adipose were similar between the groups. whereas insulin-mediated suppression of hepatic glucose production was significantly greater in aged mice (Fig. 2J–L, Table S2,). Taken together, our results indicate that in the metabolic context of diet-induced obesity, aging is associated with improved glucose tolerance, reduced insulin clearance, and impaired muscle and adipose insulin sensitivity in male mice.
Aging reduces insulin sensitivity in Mexican-American males independent of body composition and inflammation
To explore the relationship between age, insulin resistance, insulin clearance and adiposity in humans, we performed correlation analyses in a Mexican-American cohort of 1,250 participants free of diabetes and overt metabolic disease. The age range of the cohort is 7 decades (16–87 years) and insulin sensitivity has been assessed by hyperinsulinemic-euglycemic clamp. Univariate correlation analyses revealed aging-associated impairment in glucose homeostasis including elevated fasting plasma glucose levels, reduced insulin sensitivity (M and M/I) and decreased MCRI (Table 1). To assess body composition, we used BMI and BAI, an alternative surrogate measure of fat content more closely tracking percent fat content in Mexican-Americans (32). Both BMI and BAI exhibited strong positive correlations with age indicating fat accumulation during aging in this population (Table 1). Similarly, aging was also associated with increased plasma CRP level, a measure of systemic inflammation (Table 1). Physical activity showed no correlation with age in this cohort (Table 1).
Table 1.
Clinical characteristics and their correlation with age in Mexican-Americans
| n | Median | IQR | r | p | |
|---|---|---|---|---|---|
| Age [years] | 1250 | 34 | 15 | ||
| BMI [kg/m2] | 1250 | 28.2 | 6.5 | 0.188 | <0.0001 |
| BAI [%] | 1250 | 31.8 | 8.7 | 0.199 | <0.0001 |
| Fasting plasma glucose [mg/dl] | 1246 | 92.0 | 12.0 | 0.213 | <0.0001 |
| Fasting plasma insulin [μU/ml] | 1194 | 12.0 | 8.2 | 0.017 | 0.568 |
| M [mg/min/m2] | 1250 | 223 | 150 | −0.205 | <0.0001 |
| M/I [mg × ml/min/m2/mU] | 1191 | 1.72 | 1.43 | −0.192 | <0.0001 |
| MCRI [ml/m2/min] | 1191 | 459 | 135 | −0.062 | 0.020 |
| CRP [mg/l] | 960 | 1.45 | 2.33 | 0.175 | <0.0001 |
| PAS [kcal/kg/week] | 636 | 174 | 161 | −0.059 | 0.140 |
The cohort consists of 42.8% males and 57.2% females. IQR, interquartile range; BMI, body mass index; BAI, body adiposity index; M, glucose disposal rate in the final 30 min of euglycemic clamp normalized by body surface area; M/I, M value divided by steady-state insulin concentration during the clamp; MCRI, metabolic clearance rate of insulin; CRP, C-reactive protein; PAS, physical activity score. p<0.05 values appear in bold.
Sex-specific analysis demonstrated significant negative correlations between age and insulin action in both males and females with notably weaker effects in the latter (Table 2, Fig. S2). To separate the contributions of age from other factors affecting insulin action, we performed multivariate correlation analyses with measures of insulin sensitivity as dependent variables and age, body composition and plasma CRP concentration as independent variables. In males, age remained strongly correlated with insulin resistance even after adjustment for changes in body composition and CRP (Table 2). In contrast, controlling for adiposity reduced or abolished the correlation between age and insulin action in females, depending on the measure of insulin sensitivity used (Table 2). As expected, BMI/BAI and CRP levels showed strong negative correlations with measures of insulin sensitivity independent of the effect of age in both sexes (Fig. S2, Tables 3 and 4). Taken together, these results demonstrate an age-dependent decline of insulin sensitivity in Mexican-American males and females. In females, this is principally due to aging-associated increases in fat mass, whereas in males age remains an independent determinant of insulin resistance even after adjusting for measures of body composition and inflammation.
Table 2.
Multivariate correlations between insulin sensitivity and age in Mexican-Americans
| Males | Females | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | M/I | M | M/I | |||||||||
| Independent variables | n | β | p | n | β | p | n | β | p | n | β | p |
| Age | 535 | −0.279 | <0.0001 | 505 | −0.260 | <0.0001 | 715 | −0.140 | <0.0001 | 686 | −0.133 | <0.001 |
| Age, BMI | 535 | −0.205 | <0.0001 | 505 | −0.186 | <0.0001 | 715 | −0.065 | 0.044 | 686 | −0.038 | 0.241 |
| Age, BAI | 535 | −0.221 | <0.0001 | 505 | −0.197 | <0.0001 | 715 | −0.054 | 0.116 | 686 | −0.031 | 0.373 |
| Age, BMI, CRP | 405 | −0.221 | <0.0001 | 378 | −0.200 | <0.0001 | 555 | −0.042 | 0.271 | 531 | −0.026 | 0.502 |
| Age, BAI, CRP | 405 | −0.232 | <0.0001 | 378 | −0.203 | <0.0001 | 555 | −0.030 | 0.445 | 531 | −0.015 | 0.718 |
M and M/I are dependent variables; Age and measures of body composition are independent variables. Standardized coefficients (β) and p values for Age are shown. M and M/I were square root-, whereas BMI and CRP were log-transformed before analysis. n, number of subjects. p<0.05 values appear in bold.
Table 3.
Multivariate correlations between insulin sensitivity and body composition in Mexican-Americans
| Males | Females | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | M/I | M | M/I | |||||||||
| Independent variables | n | β | p | n | β | p | n | β | p | n | β | p |
| BMI | 535 | −0.486 | <0.0001 | 505 | −0.483 | <0.0001 | 715 | −0.433 | <0.0001 | 686 | −0.470 | <0.0001 |
| BAI | 535 | −0.389 | <0.0001 | 505 | −0.421 | <0.0001 | 715 | −0.367 | <0.0001 | 686 | −0.391 | <0.0001 |
| Age, BMI | 535 | −0.453 | <0.0001 | 505 | −0.456 | <0.0001 | 715 | −0.421 | <0.0001 | 686 | −0.461 | <0.0001 |
| Age, BAI | 535 | −0.353 | <0.0001 | 505 | −0.392 | <0.0001 | 715 | −0.353 | <0.0001 | 686 | −0.382 | <0.0001 |
| Age, BMI, CRP | 405 | −0.428 | <0.0001 | 378 | −0.417 | <0.0001 | 555 | −0.331 | <0.0001 | 531 | −0.415 | <0.0001 |
| Age, BAI CRP | 405 | −0.338 | <0.0001 | 378 | −0.369 | <0.0001 | 555 | −0.231 | <0.0001 | 531 | −0.288 | <0.0001 |
M and M/I are dependent variables; Age, measures of body composition and CRP are independent variables. Standardized coefficients (β) and p values for measures of body composition (BMI or BAI) are shown. M and M/I were square root transformed, and BMI and CRP were log-transformed, before analysis. n, number of subjects. p<0.05 appear in bold.
Table 4.
Multivariate correlations between insulin sensitivity and CRP in Mexican-Americans
| Males | Females | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | M/I | M | M/I | |||||||||
| Independent variables | n | β | p | n | β | p | n | β | p | n | β | p |
| CRP | 405 | −0.234 | <0.0001 | 378 | −0.217 | <0.0001 | 555 | −0.352 | <0.0001 | 531 | −0.333 | <0.0001 |
| Age, CRP | 405 | −0.183 | <0.0001 | 378 | −0.174 | <0.001 | 555 | −0.337 | <0.0001 | 531 | −0.318 | <0.0001 |
| Age, BMI, CRP | 405 | −0.054 | 0.184 | 378 | −0.048 | 0.290 | 555 | −0.172 | <0.0001 | 531 | −0.112 | 0.012 |
| Age, BAI, CRP | 405 | −0.102 | 0.014 | 378 | −0.081 | 0.089 | 555 | −0.244 | <0.0001 | 531 | −0.202 | <0.0001 |
M and M/I are dependent variables; Age, measures of body composition and CRP are independent variables. Standardized coefficients (β) and p values for CRP are shown. M and M/I were square root transformed, and BMI and CRP were log-transformed before analysis. n, number of subjects. p<0.05 appear in bold.
Sex-specific analysis of insulin clearance revealed that the age-related decline observed in the full cohort (Table 1) was exclusively due to males, as significant correlation between age and MCRI could not be detected in females (Table 5). Furthermore, multivariate analysis indicated that the aging-associated decrease of insulin clearance in males was due to increased body fat content (Table 5).
Table 5.
Multivariate correlations between insulin clearance and age in Mexican-Americans
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Independent variables | n | β | p | n | β | p |
| Age | 505 | −0.104 | 0.008 | 686 | −0.033 | 0.335 |
| Age, BMI | 505 | −0.068 | 0.112 | 686 | 0.029 | 0.422 |
| Age, BAI | 505 | −0.069 | 0.107 | 686 | 0.023 | 0.527 |
| Age, BMI, CRP | 378 | −0.089 | 0.083 | 531 | 0.019 | 0.638 |
| Age, BAI, CRP | 378 | −0.086 | 0.091 | 531 | 0.020 | 0.622 |
MCRI is the dependent variable; Age and measures of body composition are independent variables. Standardized coefficients (β) and p values for Age are shown. MCRI was square root-, BMI and CRP were log-transformed before analysis. n, number of subjects. p<0.05 values appear in bold.
Discussion
The association of aging with impaired insulin resistance has been well established in mice (4, 5, 6, 7). However, as body composition changes with normal aging, previous studies have been unable to disentangle the effects of adiposity from adiposity-independent mechanisms. In the present study, we addressed this problem by characterizing mice of different age, but similar body composition. We took advantage of the fact that body weight and fat content in mice reach a plateau at the mature adult stage (~4 months) and remain unchanged thereafter (36, 37). Thus, using ‘young’ mouse cohorts at >4 months of age allowed us to investigate the adiposity-independent effects of aging on glucose metabolism. Our use of aged mice was guided by two considerations. First, we used cohorts spanning a wide range of ages (18–27 months) for primary and replication experiments to allow generalization of the results to all mice of advanced age. Moreover, to ensure that our results reflect the effects of healthy aging and not age-related diseases, a multipronged approach (see Methods) was employed to identify and eliminate sick mice from experimental cohorts.
When mice with similar body compositions were compared, advanced age had no detrimental impact on static (i.e. plasma glucose and insulin levels) or dynamic measures (i.e. GTT, ITT) of glucose metabolism. In fact, we found that aged mice exhibited slightly improved glucose tolerance, an observation that was replicated in three independent mouse cohorts on two different diets. While this observation was initially unexpected, improved glucose tolerance in aging C57BL/6J mice has also been reported previously, consistent with our results (38). Importantly and similar to the present work, Leiter et al. used ‘young’ mice at the relatively advanced age of 4.5- to 5 months, which likely diminished differences in body composition between young and old mice, although adiposity was not directly assessed in the earlier study (38). It has also been demonstrated that aging is associated with increased pancreatic islet area and insulin content, whereas β-cell sensitivity to glucose remained unaffected (38). These results are consistent with our observation of elevated C-peptide levels after glucose administration in aged mice and suggest that elevated glucose-stimulated insulin secretion contributes to their improved glucose tolerance. Another factor likely contributing to improved glucose tolerance in aged mice is reduced insulin clearance, which we demonstrated in hyperinsulinemic clamp experiments. Thus, we hypothesize that the interaction between increased production and reduced clearance of insulin may explain the apparent over-compensation for insulin resistance in aged mice. In conclusion, while glucose intolerance is a hallmark of aging (6, 39), our results demonstrate that this is not an inevitable consequence of advanced age, but most likely due to increased adiposity associated with aging in C57BL/6J mice.
We assessed the impact of aging on insulin sensitivity by hyperinsulinemic-euglycemic clamps in young and aged mice of similar body compositions. Despite similar rates of insulin infusion, aged mice exhibited significantly higher plasma insulin concentration during the clamp. This observation held true for both lean mice maintained on a normal chow diet and diet-induced obese mice on a HF-diet. In lean mice, measures of whole-body and tissue insulin action were similar in young and aged animals. Together with elevated insulin levels in the latter, these data demonstrate that aging has adiposity-independent effects on insulin sensitivity in C57BL/6J mice.
We initially hypothesized that the metabolic stress associated with HF diet feeding would exacerbate aging-related impairments in glucose homeostasis. However, our results do not support this hypothesis. In fact, obese aged mice maintained lower basal glucose levels and exhibited improved glucose tolerance relative to their young counterparts. Furthermore, we unexpectedly found that aging impacted tissues differently in diet-induced obese mice. Consistent with observations in lean mice, despite elevated clamp insulin levels, insulin action was similar in muscle and adipose tissues of young and aged animals indicating relative insulin resistance in the latter. In contrast, suppression of hepatic glucose production was substantially stronger in obese aged versus young mice. Although the concurrent elevation of insulin levels complicates the interpretation of this observation, our results suggest that hepatic insulin sensitivity may not be adversely impacted by age in the context of diet-induced obesity. Consistent with this conclusion, aging has also been associated with improved hepatic insulin action in humans in a handful of studies where hepatic glucose production was determined (18, 40).
Most previous human studies focusing predominantly on non-Hispanic white populations led to the consensus view that aging-associated insulin resistance is due to age-related increase in adiposity (11, 12, 13, 14, 15, 16, 18). In the current study, we addressed this issue in a cohort of Mexican-American subjects. Consistent with previous reports, insulin sensitivity declined with age in both sexes. However, the relative contribution of increased adiposity to aging-associated insulin resistance was different between the sexes. In Mexican-American females, diminished insulin action was exclusively due to increased adiposity, in line with results in Caucasians (11, 12, 13, 14, 15, 16, 18). In contrast, while elevated adiposity was a major determinant of declining insulin action in males, too, aging also contributed to insulin resistance independent of changes in body composition. A similar conclusion was reached in a recent study in Japanese subjects (17). In addition to adiposity, we also considered age-related changes in physical activity and inflammation as potential mechanisms underlying aging-associated insulin resistance in Mexican-American males. A role for altered physical activity could be excluded, as the physical activity score did not change with age in this cohort. Moreover, although increased CRP levels were associated with aging, adjusting for CRP failed to diminish the correlation between age and insulin resistance. Taken together, these results demonstrate that in addition to the established role of increased adiposity in insulin resistance, aging also impairs insulin sensitivity through mechanisms that are independent of body composition, inflammation and physical activity. Furthermore, our study in Mexican-Americans highlights the previously underappreciated role of sex and ethnicity in aging-associated insulin resistance.
Previous studies investigating the impact of aging on insulin clearance yielded mixed results (11, 41, 42, 43). Here we report consistent age-related declines in the rate of insulin removal from the circulation in mouse and human subjects. Nonetheless, the underlying mechanisms appear to be different in the two species. Whereas increased adiposity is the main driver of reduced insulin turnover in aging Mexican-Americans, diminished insulin clearance in aged C57BL/6J mice is independent of body composition. This observation is consistent with the recent demonstration of age-related loss of hepatic endothelial fenestrations and diminished insulin uptake associated with reduced insulin clearance in aged mice and rats (44). Our results also raise the question whether age-related changes in insulin clearance are the consequence of insulin resistance or other aspects of aging. As aging is associated with impaired insulin sensitivity in both Mexican-Americans and the mouse model we used, further studies will be required to discriminate between these possibilities.
Although we conclusively demonstrated the contribution of adiposity-independent processes in the development of aging-associated insulin resistance, a limitation of our mouse studies is that the underlying mechanisms have not been identified. Decreased physical activity, increased systemic inflammation, elevated intramuscular lipid content and altered body fat distribution (i.e. visceral vs subcutaneous) represent potential mechanisms to account for reduced insulin sensitivity in aged mice and will need to be investigated in future studies. Nonetheless, the role of physical activity and inflammation was addressed in our human study, which suggested that neither of these factors explain the effect of aging on insulin resistance. Evaluation of additional mechanisms awaits further human studies. Our results in the Mexican-American cohort highlight the impact of sex on insulin resistance, as age was an adiposity-independent contributor to this trait only in men, but not women. As our animal study was limited to the characterization of male C57BL/6J mice, future work involving females and additional mouse strains is warranted to further investigate the role of sex and genetic determinants in age-related changes in insulin sensitivity.
In summary, the present study provides evidence in mouse and human populations that aging impairs insulin sensitivity independently of altered body composition. Based on the analysis of a Mexican-American cohort, this conclusion contrasts with previous results obtained in non-Hispanic white populations and suggests a role for genetic factors in age-related metabolic dysfunction. Consistent with a previous report (45), an intriguing possibility raised by the present study is that the mechanisms of obesity- and aging-associated insulin resistance may be distinct, which may have therapeutic implications for the treatment of T2D in the aging population. As demonstrated here, male C57BL/6J mice represent a suitable animal model to investigate the molecular mechanisms underlying adiposity-independent effects of aging on insulin resistance.
Supplementary Material
Importance of this study:
Aging is associated with increased adiposity and impaired insulin sensitivity.
Whereas body composition is a known determinant of insulin sensitivity, it remains unclear whether adiposity-independent factors also contribute to aging-associated insulin resistance.
This study demonstrates the contribution of adiposity-independent factors in the development of aging-associated insulin resistance in mice and humans.
Acknowledgements
The authors thank Drs. Maura Rossetti, Xiao Z. Shen, Tuantuan Zhao, Shuang Chen and Sujin Suk for their contributions to this project.
Funding: This study was supported by National Institutes of Health grants R01-HL088457, R01-DK079888, R01-HL67974, P30-DK063491, P50-HL55005, M01-RR000425, M01-RR000043, UL1-TR000124, 5U2C-DK093000, U2C-DK059637 and P30-DK020593.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosure: The authors declare no conflict of interest.
References
- 1.Halter JB, Musi N, McFarland Horne F, Crandall JP, Goldberg A, Harkless L, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes 2014;63: 2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson MB. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism 1979;28: 688–705. [DOI] [PubMed] [Google Scholar]
- 3.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab 2003;284: E7–12. [DOI] [PubMed] [Google Scholar]
- 4.Bailey CJ, Flatt PR. Hormonal control of glucose homeostasis during development and ageing in mice. Metabolism 1982;31: 238–246. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Rodriguez A, Mas-Gutierrez JA, Mirasierra M, Fernandez-Perez A, Lee YJ, Ko HJ, et al. Essential role of protein tyrosine phosphatase 1B in obesity-induced inflammation and peripheral insulin resistance during aging. Aging Cell 2012;11: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houtkooper RH, Argmann C, Houten SM, Canto C, Jeninga EH, Andreux PA, et al. The metabolic footprint of aging in mice. Sci Rep 2011;1: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropelle ER, Pauli JR, Cintra DE, da Silva AS, De Souza CT, Guadagnini D, et al. Targeted disruption of inducible nitric oxide synthase protects against aging, S-nitrosation, and insulin resistance in muscle of male mice. Diabetes 2013;62: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes 2012;61: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300: 1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52: 1888–1896. [DOI] [PubMed] [Google Scholar]
- 11.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003;52: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 12.Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 2006;55: 2001–2014. [DOI] [PubMed] [Google Scholar]
- 13.Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J Clin Endocrinol Metab 1992;75: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 14.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR). Diabetes 1996;45: 947–953. [DOI] [PubMed] [Google Scholar]
- 15.Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 2010;59: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utzschneider KM, Carr DB, Hull RL, Kodama K, Shofer JB, Retzlaff BM, et al. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. Diabetes 2004;53: 2867–2872. [DOI] [PubMed] [Google Scholar]
- 17.Oya J, Nakagami T, Yamamoto Y, Fukushima S, Takeda M, Endo Y, et al. Effects of age on insulin resistance and secretion in subjects without diabetes. Intern Med 2014;53: 941–947. [DOI] [PubMed] [Google Scholar]
- 18.Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, Distelmaier K, et al. Predictors of Whole-Body Insulin Sensitivity Across Ages and Adiposity in Adult Humans. J Clin Endocrinol Metab 2016;101: 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandler-Laney PC, Phadke RP, Granger WM, Fernandez JR, Munoz JA, Man CD, et al. Age-related changes in insulin sensitivity and beta-cell function among European-American and African-American women. Obesity (Silver Spring) 2011;19: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter GR, Chandler-Laney PC, Brock DW, Lara-Castro C, Fernandez JR, Gower BA. Fat distribution, aerobic fitness, blood lipids, and insulin sensitivity in African-American and European-American women. Obesity (Silver Spring) 2010;18: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 2013;13: 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol 1995;23: 570–582. [DOI] [PubMed] [Google Scholar]
- 23.Ader M, Stefanovski D, Kim SP, Richey JM, Ionut V, Catalano KJ, et al. Hepatic insulin clearance is the primary determinant of insulin sensitivity in the normal dog. Obesity (Silver Spring) 2014;22: 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, et al. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight 2017;2: e91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farmer TD, Jenkins EC, O’Brien TP, McCoy GA, Havlik AE, Nass ER, et al. Comparison of the physiological relevance of systemic vs. portal insulin delivery to evaluate whole body glucose flux during an insulin clamp. Am J Physiol Endocrinol Metab 2015;308: E206–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liljenquist JE, Horwitz DL, Jennings AS, Chiasson JL, Keller U, Rubenstein AH. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes 1978;27: 563–570. [DOI] [PubMed] [Google Scholar]
- 27.Xiang AH, Azen SP, Raffel LJ, Tan S, Cheng LS, Diaz J, et al. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in hispanic families with a hypertensive proband. Circulation 2001;103: 78–83. [DOI] [PubMed] [Google Scholar]
- 28.Goodarzi MO, Guo X, Taylor KD, Quinones MJ, Samayoa C, Yang H, et al. Determination and use of haplotypes: ethnic comparison and association of the lipoprotein lipase gene and coronary artery disease in Mexican-Americans. Genet Med 2003;5: 322–327. [DOI] [PubMed] [Google Scholar]
- 29.Labadzhyan A, Cui J, Peterfy M, Guo X, Chen YI, Hsueh WA, et al. Insulin Clearance Is Associated with Hepatic Lipase Activity and Lipid and Adiposity Traits in Mexican Americans. PLoS One 2016;11: e0166263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237: E214–223. [DOI] [PubMed] [Google Scholar]
- 31.Goodarzi MO, Taylor KD, Guo X, Quinones MJ, Cui J, Li X, et al. Variation in the gene for muscle-specific AMP deaminase is associated with insulin clearance, a highly heritable trait. Diabetes 2005;54: 1222–1227. [DOI] [PubMed] [Google Scholar]
- 32.Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, et al. A better index of body adiposity. Obesity (Silver Spring) 2011;19: 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein JH, Morgenstern H, Kellenberg J, Kalish T, Donovan J, Inadomi J, et al. Validation of a new physical activity questionnaire for a sedentary population. Dig Dis Sci 2011;56: 2678–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25: 71–80. [DOI] [PubMed] [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42: 121–130. [PubMed] [Google Scholar]
- 36.Lessard-Beaudoin M, Laroche M, Demers MJ, Grenier G, Graham RK. Characterization of age-associated changes in peripheral organ and brain region weights in C57BL/6 mice. Exp Gerontol 2015;63: 27–34. [DOI] [PubMed] [Google Scholar]
- 37.Fischer KE, Hoffman JM, Sloane LB, Gelfond JA, Soto VY, Richardson AG, et al. A cross-sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated. Aging (Albany NY) 2016;8: 2370–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leiter EH, Premdas F, Harrison DE, Lipson LG. Aging and glucose homeostasis in C57BL/6J male mice. FASEB J 1988;2: 2807–2811. [DOI] [PubMed] [Google Scholar]
- 39.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010;3: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broughton DL, James OW, Alberti KG, Taylor R. Peripheral and hepatic insulin sensitivity in healthy elderly human subjects. Eur J Clin Invest 1991;21: 13–21. [DOI] [PubMed] [Google Scholar]
- 41.Ahren B, Pacini G. Age-related reduction in glucose elimination is accompanied by reduced glucose effectiveness and increased hepatic insulin extraction in man. J Clin Endocrinol Metab 1998;83: 3350–3356. [DOI] [PubMed] [Google Scholar]
- 42.Minaker KL, Rowe JW, Tonino R, Pallotta JA. Influence of age on clearance of insulin in man. Diabetes 1982;31: 851–855. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzo C, Hanley AJ, Wagenknecht LE, Rewers MJ, Stefanovski D, Goodarzi MO, et al. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the insulin Resistance Atherosclerosis study. Diabetes Care 2013;36: 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamad M, Mitchell SJ, Wu LE, White MY, Cordwell SJ, Mach J, et al. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell 2016;15: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 2015;528: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.