Abstract
It has been fifty years since the dust mite was first appreciated to be a major source of allergen in house dust, and by extension a key trigger of allergic respiratory disease. Since that time a number of protein allergens have been identified and characterized, mainly from mite faeces, and standardized mite extracts and IgE assays have been developed. Insights into the lifecycle of dust mites and aspects of mite allergen biology have shed light on the mechanisms that lead to respiratory disease and to the development of interventions that can minimize dust mite allergen exposure. It is now clear that dust mite allergy is a key contributor to asthma in many parts of the world, and that long-term avoidance can be effective for preventing sensitization and minimizing the development and severity of respiratory disease. Here we discuss the evidence linking dust mites with respiratory disease, outline studies that support the efficacy of home environmental interventions, and highlight practical methods that have been shown to be effective as part of a multi-faceted approach to dust mite avoidance.
Keywords: house dust mite, allergic asthma, allergen avoidance, allergen particles
BACKGROUND ON DUST MITE AVOIDANCE
Although the importance of house dust as an allergen source was recognized as early as the 1920’s and several immunochemists had searched for the culprit allergen, the most important source of house dust allergens was not recognized until 19671,2. The breakthrough came from the microscopic identification of dust mites crawling in house dust samples obtained from damp houses in the Netherlands. This led rapidly to the development of techniques for growing these Acarids which made it possible to produce dust mite extracts both for skin testing and for the RAST assays3–5. The availability of dust mite extracts provided evidence that dust mite sensitization was strongly associated with asthma in many or most areas of the temperate world6. The impact of these findings was dramatic because this was the first well defined perennial allergen that could not be identified visually7. The non-seasonal nature of exposure led to a debate about causality and the impact of dust mite avoidance measures has played an important part in those ongoing arguments8.
The first purification of a dust mite allergen was dependent on a ‘gift’ of 400 grams of spent dust mite culture medium which contained dead mites, eggs and mite faeces. This allergen, which was initially called F4P1 and subsequently Der p 1, led directly to the development of a radioimmunoassay to measure the presence of mite allergen in dust samples and also in samples collected from the air9 [see also www.allergen.org]. Detailed studies on the source of the allergen focused on mite faeces10. The evidence that these particles became airborne and were the major form in which mite allergen accumulated in mite cultures provided important information about the form of exposure. However, it also challenged the prevailing consensus that particles needed to be 5 μm or smaller in order to enter the lungs. The problem was that the mite faeces, normally 20–30 μm in diameter and encased in a peritrophic membrane that prevents them from breaking up, were ‘too large’10,11. The view that particles needed to be small came from: i) the size of nebulized droplets used for bronchial challenge, i.e. 2 μm diameter, ii) from the size of occupational dust particles that were associated with progressive lung damage, and iii) from some evidence relating to ragweed particles12. There have been two major studies on the size of particles entering the lungs and both reached the same conclusion13,14. There is a progressive fall in the % of particles entering the peripheral lung with increasing size but that even with sizes ≥20 μm 10% will still enter the bronchial tree. In addition, it is clear that the entry of larger particles is inversely related to the velocity of air entering the mouth. Interestingly, a more recent study shows that nebulized large particle dust mite allergen (median 9.7 μm) induced bronchial hyperreactivity (BHR) at a lower concentration than smaller particles (median 1.1 μm)15. Thus under conditions of gentle breathing a significant proportion of large particles will enter the bronchi and those particles can contribute to progressive inflammation of the lungs16. The notion that allergen size and sensitization patterns could contribute to differences in involvement of small or large airway disease is an interesting but inadequately studied idea17.
Many authors talk about typical symptoms for dust mite allergy, but when it comes to asthma this phenomenon doesn’t exist. For example, very few mite allergic patients report rapid onset of symptoms after entering an undisturbed house infested with mites. There are multiple, non-mutually exclusive explanations to consider. The first relates to the large particle size of mite faeces and the fact that very little mite allergen stays airborne in a house for more than 10–20 minutes11. Natural exposure involvesa small number of faecal particles entering the lungs per day which do not produce noticeable symptoms or changes in lung function at the time of exposure10,16. Thus, airway inflammation results from chronic exposure to small numbers of relatively large particles. This is in contrast to bronchial challenge with nebulized droplets which involves inhaling ~108 droplets of about 2 μm diameter over 2–5 minutes resulting in a measureable decrease in lung function within 20 minutes. Another explanation relates to evidence that the inflammatory response to dust mite allergen involves non-IgE-mediated mechanisms, including T cells and innate immune cells18–20 Taken together then we have the enigma of the allergen which is most strongly associated with asthma being invisible, lacking clear-cut seasonality and rarely giving rise to respiratory symptoms at the time of exposure (See Ref 21).
Notably it was Roger Altounyan who recognized and reported that the recovery from allergen-induced non-specific BHR took months22. This phenomenon, which was first reported in relation to the grass pollen season, was subsequently confirmed among children who had improved symptoms and BHR after moving to high altitude Sanatoria in Switzerland, Italy or France23–25. The ability of prolonged avoidance to reverse BHR among mite allergic asthmatics was also observed in subjects who lived in a mite-free hospital room in London for 3–9 months26. The importance of this background to any discussion of dust mite avoidance includes: i) that the patients clinical history will not give simple evidence about the importance of mite exposure, ii) that ideally you need evidence about allergen levels in the patient’s house or at least in the community where the patient lives, and iii) it is clear that the process of recovery from prolonged allergen exposure takes months not days.
Types of Avoidance
Once it was possible to measure the concentration of relevant allergens in dust samples, it became clear that there were multiple effects of exposure. For those already sensitized and with asthma the benefit of avoidance was demonstrated in the European children that moved to the high altitude sanatoria23–25 and the adult asthmatics in London that improved with long-term hospitalization in a mite-free environment26. The target of this form of intervention, typically called tertiary avoidance, was to decrease exposure of an allergic and symptomatic patient. Subsequently many other intervention studies have proven successful for tertiary prevention, though this has not been universally true27,28. Indeed, the utility of tertiary avoidance has been the subject of much controversy, stemming in part from results of recent meta-analyses that did not demonstrate benefit29,30. Our view is that interventions that have achieved their primary objective, i.e. reducing mite exposure, have consistently demonstrated benefit31. Notably this was also the view of the 2013 Joint Task Force32.
An alternative question was whether it was possible to modify exposure in childhood sufficiently to prevent sensitization in the first place, i.e. primary avoidance. The strongest evidence that this form of primary avoidance should be possible came from studies on children raised in mite-free environments. For example, dust mite sensitization is not relevant to asthma among children raised in Los Alamos, New Mexico or in the Norbotten area of Northern Sweden33,34. In each case, there is good evidence that none of the homes in those areas contain significant mite allergen35,36.
Central to any avoidance proposal is the assumption that we know the relevant site of exposure and concentration of allergen that leads to sensitization or symptoms. Strikingly, the answer to this question may be different for primary avoidance than for secondary or tertiary avoidance. If periods of exposure of only a week or relatively low concentrations of allergen are sufficient to initiate sensitization, then primary avoidance may be impossible in a town where many other houses have high levels of dust mites37,38. Equally, avoidance of a cat in the home may not be an effective method of preventing cat sensitization if cat allergen is nonetheless present in homes or schools because it has been transferred from other homes36. Thus the real difference between Kiruna in Sweden and Manchester in the UK, or between Los Alamos, New Mexico and Charlottesville, Virginia in the United States may be community prevalence of dust mite allergens. Taken together these findings suggest that household interventions are more likely to control or minimize allergic disease rather than prevent sensitization itself.
THE PROCESS OF DUST MITE AVOIDANCE
There are many areas of the world where exposure to dust mite allergens in homes is almost inevitable. In these areas sensitization to dust mite, as judged by skin tests or serum IgE antibodies in a patient with relevant symptoms, is sufficient to recommend simple measures to decrease exposure. Any proposal to a patient should include a series of priorities (Table I). Clearly, exposure can occur anywhere in the home, at school or in the work place39. However, the bedroom is the primary focus both because we all spend a large proportion of our lives in bed, and also because it is easier to achieve major changes in exposure in the bedroom. While it is important to have an overall plan, there are specific parts of the process that are worth considering separately: These include: i) the materials used for covering pillows and mattresses, ii) humidity control, iii) room air cleaners, iV) carpets, and v) vacuum cleaners. It is clear that the odds of successful avoidance are much higher with multi-faceted approaches.
Table I.
Priorities in the Approach to Decreasing Dust Mite Exposure
| I. Bedroom |
|
| II. Whole House |
|
| III. Specific questions beyond the bedroom |
|
Mattress Casings and Laundering
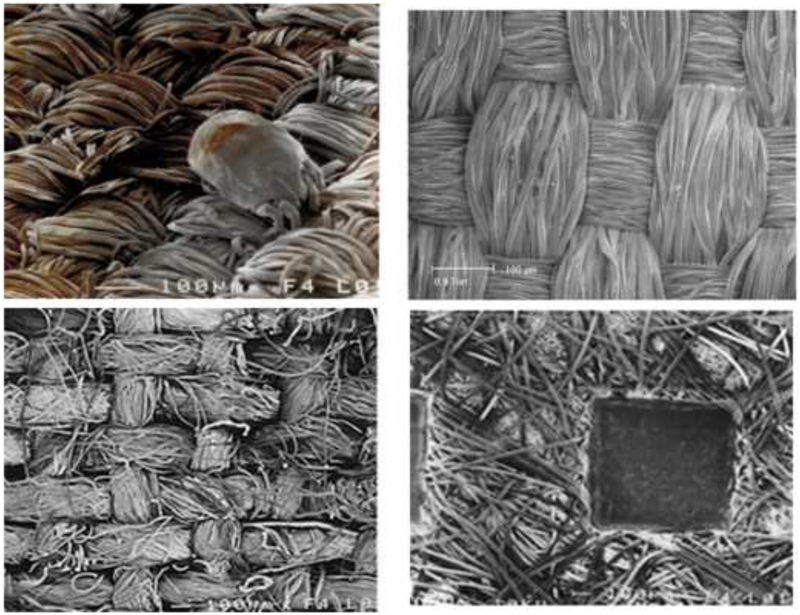
Thinking about how to decrease exposure in a bedroom started almost as soon as mites were identified as a major problem. Initially, plastic was the only fabric that would block mites though there were concerns about encasing a mattress in a non-breathing cover. In fact, there do not seem to be significant problems with using a plastic cover provided that the mattress is dry and there is a washable mattress pad on top of the plastic. Plastic covers on pillows, however, are generally unacceptable because they increase sweating. The materials used for barriers have progressed from plastic to various semipermeable fabrics which were less uncomfortable. However, a major break-through occurred around 2000 when the demand for type-writer ribbon reached a new low and manufacturers of fine woven fabrics were in search of a new market. At about the same time as the fine woven fabrics appeared, a variety of unwoven fabrics were also introduced for mite avoidance. The distinction between these fabrics is important [Fig 1]. Testing either of these fabrics with a forced air flow has been shown to prevent the passage of mite faeces40. However, when tested with live mites it became clear that mites could penetrate through the unwoven fabrics41. In addition debris from the mites gets into these fabrics and is very hard to remove. Perhaps most important, the unwoven fabrics do not withstand repeated washing. Overall, it is clear that the fine woven fabrics are the correct material for pillow cases and for covers on duvets or comforters. All other materials on top of the mattress should be suitable for regular washing. Although long-standing recommendations have emphasized washing with a hot cycle, a warm cycle achieves nearly the same effect of removing the majority of mite allergen without the risk of scalding42. A warm cycle may be somewhat less helpful for eliminating mites themselves, especially if using a front-loading machine, although this is less important if using a clothes dryer which can kill virtually all mites42–44.
Fig 1.
Scanning electron microscope images of four fabrics: (A) dust mite on fine woven fabric, (B) higher resolution image of fine woven fabric, (C) cotton sheet with large pores, and (D) nonwoven material showing heat welding.
Humidity Control
Dust mites rely on ambient moisture for growth and reproduction, although they can survive for prolonged periods in low humidity environments45. This contributes to the explanation that there can be seasonal variation in both dust mite burden and mite-related respiratory symptoms, and argues for a potential role of dehumidification as an avoidance measure46,47. A relative humidity of 45–50% is typically considered the threshold to achieve control48. Likely related to the fact that even brief periods of elevated humidity are sufficient for dust mite survival and reproduction, the results from controlled trials focusing on dehumidification have been decidedly mixed48–50. An additional note is that arid or semi-arid environments which are not typically considered to be areas with major dust mite problems, can in fact harbor significant dust mite burden under certain conditions, e.g. in homes that use evaporative coolers51–53.
Air Cleaners
There are many possible methods of cleaning the air within a house including a wide variety of electrostatic cleaners. However, most or all types of electrostatic cleaners produce ozone. In addition, charged particles stick to the walls of the room. While there are designs of electrostatic cleaners where the charged particles stick to plates within the cleaner (which can be cleaned regularly or sampled to measure exposure), unfortunately these designs are not widely available54. The major alternative is to use filters where the particles are captured physically in the filter. In this case, the best approach is to use a High Efficiency Particulate Air [HEPA] filter. Interestingly, these cleaners were first developed in 1942 as part of the program to remove Uranium particles from the air at Oak Ridge in Tennessee. This led to the development of these filters which consist of a large area of folded paper which allows passage of air and removes 99.7% of particles as small as 0.1 microns. This effectively excludes particles as small as tobacco smoke. However, an important problem with room air cleaners is to ensure that the flow of clean air coming out of the cleaner does not disturb dust on the floor. The most obvious example is a carpet, particularly in a house with a cat. A HEPA filter placed on a carpet in a house with a cat will, in some circumstances, disturb more cat allergen from the carpet than the filter removes from the air55. This emphasizes how important it is to educate patients or provide educational material. There is modest but mixed evidence that HEPA filters can reduce respiratory symptoms in atopic subjects, which argues they may be helpful as part of multi-faceted avoidance strategies56,57.
Carpets, Furniture, Drapes, etc.
Multiple studies have clearly shown that in addition to mattresses, carpet and upholstered materials are important sources of dust mite allergen58,59. The situation with carpets is fascinating because the problems involved with maintaining carpets in a hot climate were well recognized by 1900. At that time, the carpets in all ‘properly’ run [i.e. affluent] homes were taken up in the summer and put into storage. This of course is impossible with fitted carpets. Further, even with modern vacuums it is impossible to get all the debris and dust mite allergen out of a carpet60. Thus, humidity control should be part of any strategy that addresses the reservoir of allergen in carpet and upholstery. Otherwise carpets should be kept to a minimum, and taken up to be cleaned and dried in the sun. For all other sites of possible mite growth the general principle is to keep upholstered material to a minimum if humidity cannot be controlled.
Vacuum Cleaners
Ability to clean Carpets:
One of the major arguments for vacuum cleaners when they were first introduced was that they could clean a carpet without taking it up. A good vacuum is indeed a major improvement on brushing a carpet with a broom. However, vacuum cleaners cannot prevent accumulation of debris including human skin scales (i.e. mite food) in a carpet. Equally, live mites are not removed from a carpet by a vacuum cleaner, presumably because mites have elegant foot pads that allow them to hold on to any surface61,62 [Fig 2]. Steam cleaning would appear to offer a solution; however, the debris in a carpet also creates limits for steam cleaners. If the steam goes deep into a carpet, it can bring out colored material from deep in the carpet which can appear as a stain. For this reason, the steam cleaners are designed not to go into the debris that accumulates deep in the carpet. Indeed, interventions adding steam cleaning to frequent vacuuming with a HEPA filter-equipped vacuum had modest additional benefit to mite allergen reduction, though vacuuming alone did lead to significant decrease in allergen63,64.
Fig 2.
High resolution electron microscopy image of dust mite.
Disturbance of dust in the home:
Many forms of house work can disturb dust and this increases exposure10. Simply moving furniture, throw pillows, drapes or bedding can make mite allergens airborne. However, vacuum cleaners are particularly effective at disturbing dust65. These cleaners disturb dust at the front end and also with the outflow of air that has been through the fans and the cleaner bag. This disturbance is such that it has been recommended that patients not only wear a mask during cleaning but also leave the room for 20 minutes afterwards. It is possible to test elements of these cleaners66 and thus we know that good quality paper bags prevent most of the allergen containing particles coming through. Double thickness paper bags are particularly effective and in addition many of the filters are effective. However, vacuum cleaners do not have industry standards for the filtration of air coming through the bag. Furthermore, the companies change features of the models frequently, so that detailed research on models soon becomes out of date67.
CONTROLLED TRIALS OF DUST MITE AVOIDANCE
Published controlled trials of mite avoidance include many where the design clearly undermines the likelihood of a successful outcome. The most obvious problems are studies that i) did not produce a significant decrease in mite allergens68, ii) included such aggressive cleaning of the blankets, sheets, etc. in both control and intervention arms that it was very unlikely that a mattress cover would produce a significant change in exposure69, iii) may have enrolled patients with multiple sensitivities without addressing the allergens other than dust mites70, and iv) enrolled patients with relatively well-controlled disease or minimal dust mite allergen exposure at baseline71. We would highlight several previous reviews that have addressed this topic in detail27,28,31,67,72.
Obviously, there are many aspects of the recommended avoidance measures that cannot be controlled. Nonetheless successful trials have been carried out with the main intervention related to beds. We would like to focus on a group of studies that have achieved a significant decrease in exposure and maintained it for at least one year73–77 [Table 2]. The successful controlled trials primarily used physical barriers but they also included: a treatment with heat to kill mites, the added use of tannic acid to denature mite allergens, and a complex trial using measures to control animal dander, and cockroach as well as dust mites76. The outcomes include peak expiratory flow rates, BHR and in the most recent study, exacerbations requiring a hospital visit77. The central feature of all but one of the studies was that they documented prolonged decrease in mite allergen exposure. Perhaps the most interesting study is also the most recent. In this study, Murray, et al enrolled children in the northern part of the UK with a history of acute wheezing episodes requiring a visit to hospital, and did a controlled trial of avoidance that was effectively blinded77. The outcome was a significant decrease in exacerbations over the next year.
Table II.
Successful Trials of Dust Mite Avoidance in the Management of Asthma
| Authors | Length | Methods | Participants | Decrease in Mite | Outcome |
|---|---|---|---|---|---|
| Walshaw & Evans, 1986 (70) | 1 year | Physical Barriers | 22/10 | ++ | PEFR/BHR |
| Ehnert, et al, 1992 (71) | 1 year | Physical Barriers | 8/16 | ++ | PEFR/BHR |
| Htut, et al, 2001 (72) | 1 year | Heat Treatment | 10/10/10 | ++ | BHR |
| Morgan, et al, 2004 (73) | 1 year | Multiple Measures | 425/441 | ++ | Symptoms |
| Murray, et al, 2017 (74) | 1 year | Physical Barriers | 146/138 | nd | Acute Exacerbations |
CHANGES THAT COULD HAVE INFLUENCED THE RELEVANCE OF DUST MITE AVOIDANCE
Education:
When dust mites were first recognized scientifically as an important cause of allergic diseases, there was a major job to be done in educating the public about the existence of these invisible spider-like animals in house dust. At that stage, dust mite skin tests played an important role in explaining the importance of mites and the relevance of decreasing exposure. Today, there is extensive public awareness of dust mites and patients have no difficulty gaining information about the techniques of avoidance (Google query: ‘Dust Mite Avoidance’). Thus today the major problem with education may be trying to control the accuracy of the information that is available on-line. However, this is part of a much larger problem about the future of medical education.
Changes in housing:
Over the same period of time that asthma became common, i.e. 1960–2000, there have also been major changes in housing. In the UK, the main change was in central heating which can keep houses dry in the winter. In the USA, the main change was the adoption of central air-conditioning; which made it possible to control indoor humidity in areas where the water content of the outdoor air, especially in the summer, is extremely high. Overall there have been major decreases in the humidity of homes with a consequent decrease in the number of homes with optimal conditions for mite growth. Evidence that changes in the design and management of houses have decreased exposure to dust mites is not available for the USA. In Europe some estimates suggest that changes in public awareness and in management of homes have decreased exposure by as much as tenfold (R. Aalberse, personal communication). By contrast, in many areas of the developed or developing world mite allergens and mite sensitization still dominate the risk of asthma78,79.
Pharmacologic therapies:
A further major change has occurred in the management of asthma, which has been revolutionized by the availability of inhaled steroids. In particular, combination therapy with steroids and long acting beta-2-agonists (LABA) has been very effective. This treatment has been adopted widely for the treatment of bronchial inflammation in patients with mild or moderate allergic asthma, i.e. those patients who are predominantly allergic to common indoor allergens. The availability of these inhaled medicines has already decreased the number of patients who are being referred to specialists. Seeing this change positively it may mean that the relevant site for education about allergen avoidance has moved or should move into primary-care. Regardless, there is still a major role for avoidance, for measures such as anti-IgE treatment directed at the effects of allergen exposure80 and arguably for immunotherapy as well32.
Funding:
TPM: AI-205656
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vannier W, Campbell D. A starch block electrophoresis study of aqueous house dust extracts. J Allergy. 1961;32:36–54. [DOI] [PubMed] [Google Scholar]
- 2.Spieksma F, Dieges P. The history of the finding of the house dust mite. JACI. 2004;113(3):573–576. [DOI] [PubMed] [Google Scholar]
- 3.Smith J, Disney M, Williams J, Goels Z. Clinical significance of skin reactions to mite extracts in children with asthma. Br Med J. 1969;21(2):723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto T, Oshima S, Ishizaki T, Sato SH. Allergenic identity between the common floor mite (Dermatophagoides farinae Hughes, 1961) and house dust as a causative antigen in bronchial asthma. J Allergy. 1968;42(1):14–28. [DOI] [PubMed] [Google Scholar]
- 5.Wide L, Bennich H, Johansson S. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet 1967;2(7526):1105–1107. [DOI] [PubMed] [Google Scholar]
- 6.Dust mite allergens and asthma: a worldwide problem. International Workshop report. Bull World Health Organ. 1988;66(6):769–780. [PMC free article] [PubMed] [Google Scholar]
- 7.Dust mite allergens and asthma -- a worldwide problem. JACI. 1989;83(2 Pt 1):416–427. [DOI] [PubMed] [Google Scholar]
- 8.Platts-Mills TA, Chapman MD. Dust mites: immunology, allergic disease, and environmental control. J Allergy Clin Immunol. 1987;80(6):755–775. [DOI] [PubMed] [Google Scholar]
- 9.Chapman MD, Platts-Mills TA. Purification and characterization of the major allergen from Dermatophagoides pteronyssinus-antigen P1. J Immunol. 1980;125(2):587–592. [PubMed] [Google Scholar]
- 10.Tovey ER, Chapman MD, Wells CW, Platts-Mills TA. The distribution of dust mite allergen in the houses of patients with asthma. Am Rev Respir Dis. 1981;124(5):630–635. [DOI] [PubMed] [Google Scholar]
- 11.Platts-Mills TA, Heymann PW, Longbottom JL, Wilkins SR. Airborne allergens associated with asthma: particle sizes carrying dust mite and rat allergens measured with a cascade impactor. J Allergy Clin Immunol. 1986;77(6):850–857. [DOI] [PubMed] [Google Scholar]
- 12.Busse W, Reed C, Hoehne J. Where is the allergic reaction in ragweed asthma? JACI. 1972;50(5):289–293. [DOI] [PubMed] [Google Scholar]
- 13.Svartengren M, Falk R, Linnman L, Philipson K, Camner P. Deposition of large particles in human lung. Exp Lung Res. 1987;12(1):75–88. [DOI] [PubMed] [Google Scholar]
- 14.Task Group on Lung Dynamics. Health Physics. 1966;12:173. [PubMed] [Google Scholar]
- 15.Casset A, Purohit A, Birba E, et al. Bronchial challenge test in asthmatics sensitized to mites: Role of particle size in bronchial response. J Aerosol Med. 2007;20(4):509–518. [DOI] [PubMed] [Google Scholar]
- 16.Platts-Mills TAE, Mitchell EB, Tovey ER, Chapman MD, Wilkins SR. Airborne allergen exposure, allergen avoidance and bronchial hyperreactivity In: Kay AB, Austen KF, Lichtenstein LM, editors. Asthma: physiology, immunopharmacology and treatment, Third International Symposium. London: Academic Press; 1984:297–314. [Google Scholar]
- 17.Schiphof-Godart L, van der Wiel E, Ten Hacken NH, van den Berge M, Postma DS, van der Molen T. Development of a tool to recognize small airways dysfunction in asthma (SADT). Health Qual Life Outcomes. 2014;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawle FC, Mitchell EB, Platts-Mills TA. T cell responses to the major allergen from the house dust mite Dermatophagoides pteronyssinus, Antigen P1: comparison of patients with asthma, atopic dermatitis, and perennial rhinitis. J Immunol. 1984;133(1):195–201. [PubMed] [Google Scholar]
- 19.Dullaers M, Schuijs MJ, Willart M, et al. House dust mite-driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol. 2017;140(1):76–88e77. [DOI] [PubMed] [Google Scholar]
- 20.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009;123(3):612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodfolk J, Commins S, Schuyler A, Erwin E, Platts-Mills T. Allergens, soures, particles, and molelcules: Why do we make IgE responses? Allergol Int. 2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards I, Jackson D, Altounyan R. Airway hyperreactivity and its relationship to sodium cromoglycate. Agents Actions Suppl 16983;13:51–53. [PubMed] [Google Scholar]
- 23.Kerrebijn K Endogenous factors in childhood CNSLD: methodological aspects in population studies. The Netherlands: Royal Vangorcum Assesn. 1970:38–48. [Google Scholar]
- 24.Piacentini G, Martinati L, Mingoni S, Boner A. Influence of allergen avoidance on the eosinophil phase of airway inflammation in children with allergic asthma. JACI. 1996;97(5):1079–1084. [DOI] [PubMed] [Google Scholar]
- 25.Charpin D, Birnbaum J, Haddi E, et al. Altitude and allergy to house-dust mites. A paradigm of the influence of environmental exposure on allergic sensitization. Am Rev Respir Dis. 1991;143(5 Pt 1):983–986. [DOI] [PubMed] [Google Scholar]
- 26.Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR. Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet. 1982;2(8300):675–678. [DOI] [PubMed] [Google Scholar]
- 27.Custovic A, Simpson A, Chapman MD, Woodcock A. Allergen avoidance in the treatment of asthma and atopic disorders. Thorax. 1998;53(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks GB. House dust mite exposure as a risk factor for asthma: benefits of avoidance. Allergy. 1998;53(48 Suppl):108–114. [DOI] [PubMed] [Google Scholar]
- 29.Gotzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2004(4):CD001187. [DOI] [PubMed] [Google Scholar]
- 30.Gotzsche PC, Johansen HK. House dust mite control measures for asthma: systematic review. Allergy. 2008;63(6):646–659. [DOI] [PubMed] [Google Scholar]
- 31.Platts-Mills TA. Allergen avoidance in the treatment of asthma: problems with the metaanalyses. J Allergy Clin Immunol. 2008;122(4):694–696. [DOI] [PubMed] [Google Scholar]
- 32.Portnoy J, Miller JD, Williams PB, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013;111(6):465–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sporik R, Ingram JM, Price W, Sussman JH, Honsinger RW, Platts-Mills TA. Association of asthma with serum IgE and skin test reactivity to allergens among children living at high altitude. Tickling the dragon’s breath. Am J Respir Crit Care Med. 1995;151(5):1388–1392. [DOI] [PubMed] [Google Scholar]
- 34.Perzanowski M, Ronmark E, James H, et al. Relevance of Specific IgE Antibody Titer to the Prevalenance, Severity and Persistence of Asthma among 19-year-olds in Northern Sweden. JACI 2016. 138(6):1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram JM, Sporik R, Rose G, Honsinger R, Chapman MD, Platts-Mills TA. Quantitative assessment of exposure to dog (Can f 1) and cat (Fel d 1) allergens: relation to sensitization and asthma among children living in Los Alamos, New Mexico. J Allergy Clin Immunol. 1995;96(4):449–456. [DOI] [PubMed] [Google Scholar]
- 36.Perzanowski MS, Ronmark E, Nold B, Lundback B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103(6):1018–1024. [DOI] [PubMed] [Google Scholar]
- 37.Woodcock A, Lowe L, Murray C, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004;17(4):433–439. [DOI] [PubMed] [Google Scholar]
- 38.Warner AM, Bjorksten B, Munir AK, Moller C, Schou C, Kjellman NI. Childhood asthma and exposure to indoor allergens: low mite levels are associated with sensitivity. Pediatr Allergy Immunol. 1996;7(2):61–67. [DOI] [PubMed] [Google Scholar]
- 39.Custovic A, Taggart SC, Woodcock A. House dust mite and cat allergen in different indoor environments. Clin Exp Allergy. 1994;24(12):1164–1168. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan JW, McLaughlin TE, Perzanowski MS, Platts-Mills TA. Evaluation of materials used for bedding encasement: effect of pore size in blocking cat and dust mite allergen. J Allergy Clin Immunol. 1999;103(2 Pt 1):227–231. [DOI] [PubMed] [Google Scholar]
- 41.Miller JD, Naccara L, Satinover S, Platts-Mills TA. Nonwoven in contrast to woven mattress encasings accumulate mite and cat allergen. J Allergy Clin Immunol. 2007;120(4):977–979. [DOI] [PubMed] [Google Scholar]
- 42.Choi SY, Lee IY, Sohn JH, et al. Optimal conditions for the removal of house dust mite, dog dander, and pollen allergens using mechanical laundry. Ann Allergy Asthma Immunol. 2008;100(6):583–588. [DOI] [PubMed] [Google Scholar]
- 43.Miller JD. Difference in Mite Survival in Blankets Washed in Top-Loading Vs. Front-Loading Washing Machines. J Allergy Clin Immun. 2017;139(2):Ab119–Ab119. [Google Scholar]
- 44.Mason K, Riley G, Siebers R, Crane J, Fitzharris P. Hot tumble drying and mite survival in duvets. J Allergy Clin Immunol. 1999;104(2 Pt 1):499–500. [DOI] [PubMed] [Google Scholar]
- 45.Arlian LG. Water balance and humidity requirements of house dust mites. Exp Appl Acarol. 1992;16(1–2):15–35. [DOI] [PubMed] [Google Scholar]
- 46.Platts-Mills TA, Hayden ML, Chapman MD, Wilkins SR. Seasonal variation in dust mite and grass-pollen allergens in dust from the houses of patients with asthma. J Allergy Clin Immunol. 1987;79(5):781–791. [DOI] [PubMed] [Google Scholar]
- 47.Hervas D, Pons J, Mila J, Matamoros N, Hervas JA, Garcia-Marcos L. Specific IgE levels to Dermatophagoides pteronyssinus are associated with meteorological factors. Int Arch Allergy Immunol. 2013;160(4):383–386. [DOI] [PubMed] [Google Scholar]
- 48.Arlian LG, Neal JS, Vyszenski-Moher DL. Reducing relative humidity to control the house dust mite Dermatophagoides farinae. J Allergy Clin Immunol. 1999;104(4 Pt 1):852–856. [DOI] [PubMed] [Google Scholar]
- 49.Arlian LG, Neal JS, Morgan MS, Vyszenski-Moher DL, Rapp CM, Alexander AK. Reducing relative humidity is a practical way to control dust mites and their allergens in homes in temperate climates. J Allergy Clin Immunol. 2001;107(1):99–104. [DOI] [PubMed] [Google Scholar]
- 50.Singh M, Jaiswal N. Dehumidifiers for chronic asthma. Cochrane Database Syst Rev. 2013(6):CD003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellingson AR, LeDoux RA, Vedanthan PK, Weber RW. The prevalence of Dermatophagoides mite allergen in Colorado homes utilizing central evaporative coolers. J Allergy Clin Immunol. 1995;96(4):473–479. [DOI] [PubMed] [Google Scholar]
- 52.Prasad C, Hogan MB, Peele K, Wilson NW. Effect of evaporative coolers on skin test reactivity to dust mites and molds in a desert environment. Allergy Asthma Proc. 2009;30(6):624–627. [DOI] [PubMed] [Google Scholar]
- 53.Johnston JD, Barney TP, Crandall JH, et al. Prevalence of house dust mite allergens in low-income homes with evaporative coolers in a semiarid climate. Arch Environ Occup Health. 2017:1–4. [DOI] [PubMed] [Google Scholar]
- 54.Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TA. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin Exp Allergy. 2003;33(7):986–991. [DOI] [PubMed] [Google Scholar]
- 55.Luczynska CM, Li Y, Chapman MD, Platts-Mills TA. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990;141(2):361–367. [DOI] [PubMed] [Google Scholar]
- 56.Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990;85(6):1050–1057. [DOI] [PubMed] [Google Scholar]
- 57.Antonicelli L, Bilo MB, Pucci S, Schou C, Bonifazi F. Efficacy of an air-cleaning device equipped with a high efficiency particulate air filter in house dust mite respiratory allergy. Allergy. 1991;46(8):594–600. [DOI] [PubMed] [Google Scholar]
- 58.Van Strien RT, Verhoeff AP, Brunekreef B, Van Wijnen JH. Mite antigen in house dust: relationship with different housing characteristics in The Netherlands. Clin Exp Allergy. 1994;24(9):843–853. [DOI] [PubMed] [Google Scholar]
- 59.Sidenius KE, Hallas TE, Brygge T, Poulsen LK, Mosbech H. House dust mites and their allergens at selected locations in the homes of house dust mite-allergic patients. Clin Exp Allergy. 2002;32(9):1299–1304. [DOI] [PubMed] [Google Scholar]
- 60.Sercombe JK, Liu-Brennan D, Causer SM, Tovey ER. The vertical distribution of house dust mite allergen in carpet and the effect of dry vacuum cleaning. Int J Hyg Environ Health. 2007;210(1):43–50. [DOI] [PubMed] [Google Scholar]
- 61.de Boer R The control of house dust mite allergens in rugs. J Allergy Clin Immunol. 1990;86(5):808–814. [DOI] [PubMed] [Google Scholar]
- 62.Wickman M, Paues S, Emenius G. Reduction of the mite-allergen reservoir within mattresses by vacuum-cleaning. A comparison of three vacuum-cleaning systems. Allergy. 1997;52(11):1123–1127. [DOI] [PubMed] [Google Scholar]
- 63.Vojta PJ, Randels SP, Stout J, et al. Effects of physical interventions on house dust mite allergen levels in carpet, bed, and upholstery dust in low-income, urban homes. Environ Health Perspect. 2001;109(8):815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu CH, Yiin LM, Tina Fan ZH, Rhoads GG. Evaluation of HEPA vacuum cleaning and dry steam cleaning in reducing levels of polycyclic aromatic hydrocarbons and house dust mite allergens in carpets. J Environ Monit. 2009;11(1):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gore RB, Durrell B, Bishop S, Curbishley L, Woodcock A, Custovic A. High-efficiency vacuum cleaners increase personal mite allergen exposure, but only slightly. Allergy. 2006;61(1):119–123. [DOI] [PubMed] [Google Scholar]
- 66.Vaughan JW, Woodfolk JA, Platts-Mills TA. Assessment of vacuum cleaners and vacuum cleaner bags recommended for allergic subjects. J Allergy Clin Immunol. 1999;104(5):1079–1083. [DOI] [PubMed] [Google Scholar]
- 67.Platts-Mills TA, Vaughan JW, Carter MC, Woodfolk JA. The role of intervention in established allergy: avoidance of indoor allergens in the treatment of chronic allergic disease. J Allergy Clin Immunol. 2000;106(5):787–804. [DOI] [PubMed] [Google Scholar]
- 68.Woodcock A, Forster L, Matthews E, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med. 2003;349(3):225–236. [DOI] [PubMed] [Google Scholar]
- 69.Terreehorst I, Hak E, Oosting AJ, et al. Evaluation of impermeable covers for bedding in patients with allergic rhinitis. N Engl J Med. 2003;349(3):237–246. [DOI] [PubMed] [Google Scholar]
- 70.Rijssenbeek-Nouwens LH, Oosting AJ, de Bruin-Weller MS, Bregman I, de Monchy JG, Postma DS. Clinical evaluation of the effect of anti-allergic mattress covers in patients with moderate to severe asthma and house dust mite allergy: a randomised double blind placebo controlled study. Thorax. 2002;57(9):784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Vries MP, van den Bemt L, Aretz K, et al. House dust mite allergen avoidance and selfmanagement in allergic patients with asthma: randomised controlled trial. Br J Gen Pract. 2007;57(536):184–190. [PMC free article] [PubMed] [Google Scholar]
- 72.Colloff MJ. Dust Mites. Dust Mites. 2009:1–408. [Google Scholar]
- 73.Walshaw M, Evans C. Allergen avoidance in house dust mite sensitive adult asthma. Q J Med. 1986;58(226):199–215. [PubMed] [Google Scholar]
- 74.Ehnert B, Lau-Schadendort S, Weber A. Reducing domestic exposure to dust mite allergen reduces bronchial hyperreactivity in sensitive children with asthma. JACI. 1992;90:135–138. [DOI] [PubMed] [Google Scholar]
- 75.Htut T, Higenbottam T, Gill G. Eradication of house dust mite from homes of atopic asthmatic subjects: a double-blind trial. JACI. 2001;107:55–60. [DOI] [PubMed] [Google Scholar]
- 76.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. [DOI] [PubMed] [Google Scholar]
- 77.Murray C, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children: A randomised trial of mite impermeable bedcovers. AJRCCM. 2017;196(2):150–158. [DOI] [PubMed] [Google Scholar]
- 78.Erwin EA, Wickens K, Custis NJ, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol. 2005;115(1):74–79. [DOI] [PubMed] [Google Scholar]
- 79.Soto-Quiros M, Avila L, Platts-Mills TA, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. The Journal of allergy and clinical immunology. 2012;129(6):1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]