Abstract
Background:
Previous observational studies and systematic reviews have suggested that adherence to the Mediterranean diet is associated with a reduced risk of breast cancer, but have not examined associations with molecular subtypes of breast cancer. The current review examines the association with adherence to the Mediterranean diet and risk of breast cancer according to molecular subtypes.
Methods:
Bibliographic searches were conducted in PubMed and CINAHL using relevant MeSH search terms and Boolean algebra commands.
Results:
Six cohort studies and one case-control study have examined adherence with the Mediterranean diet and risk of breast cancer according to estrogen-receptor (ER) and progesterone-receptor (PR) status and human epidermal growth factor 2 (HER2) oncogene expression. Taken overall, studies suggest that the Mediterranean dietary pattern is inversely associated with breast cancer risk in postmenopausal women, and that the inverse association is somewhat stronger among ER- tumors. Although there is a suggestion that the Mediterranean diet is inversely associated with PR- tumors and with ER-/PR-/HER2- (“triple negative” tumors), results to date have been mixed and the number of studies that have examined associations with this dietary pattern among tumors characterized by multiple molecular subtypes remains small.
Conclusions:
The results of this systematic review suggest that consumption of a Mediterranean diet pattern is associated with a reduced risk of postmenopausal breast cancer, particularly among ER- tumors. Additional cohort studies that have sufficient sample sizes and long-term follow-up are warranted to identify sizeable numbers of invasive breast cancer cases, thereby allowing for characterization of the tumors by molecular subtype.
Keywords: Breast cancer, Case-control studies, Cohort studies, Epidemiology, Mediterranean diet, Molecular subtypes, Risk factors
Background
The concept of the Mediterranean diet was proposed by Keys and Grande in the 1950s [1] and refers to dietary patterns followed in olive growing areas of the Mediterranean region in parts of Spain, southern France, southern Italy, Greece, Cyprus, Crete, and parts of North Africa [2,3]. Variations in the Mediterranean diet exist as countries in the region have different diets, religions, and cultures. Diets vary between the countries that border the Mediterranean Sea and variations exist even within the same country [4]. For example, Italian diets include a high consumption of pasta and pulses (e.g., chickpeas and fava beans) are common in Greece [3]. Although several definitions of the Mediterranean diet have been proposed for use in epidemiologic studies, characteristics of this dietary pattern generally include high consumption of fruit and raw and cooked vegetables and legumes; frequent consumption of fish and seafood; high consumption of whole grain, unrefined cereals, nuts and seeds; olive oil as the main source of cooking oil and dietary lipids; low to moderate consumption of alcohol; low to moderate consumption of dairy products, eggs, and poultry; infrequent consumption of red and processed meats; and low consumption of sweets [4,5].
Several authors have noted that the analysis of dietary patterns can provide more insight into the relationship between diet and cancer because many nutrients are highly correlated and food patterns take into account synergistic effects between nutrients [4,6,7]. Conclusions about the effect of consuming a single nutrient, food group, or dietary constituent on a health outcome can be misleading. For these reasons, it is useful to examine patterns of nutrient intake such as the Mediterranean diet that express several related aspects of dietary intake concurrently [4].
Evidence from observational studies suggest that high adherence to the Mediterranean diet is associated with a reduced risk of certain types of cancer including cancer of the breast, colon, rectum, stomach, pancreas, prostate, liver, and head and neck [8]. Although several meta-analyses have been published that examined associations between adherence with the Mediterranean diet and risk of cancer of the breast and other sites [8–10], these reviews did not examine associations with molecular subtypes of breast cancer. Clarifying these associations is important for helping women and healthcare providers understand how best to reduce risk of breast cancer through dietary interventions. The goal of the current review was to examine the association of adherence to the Mediterranean diet and risk of breast cancer according to estrogen-receptor (ER) and progesterone-receptor (PR) status and human epidermal growth factor 2 (HER2) oncogene expression.
Methods
The present review is based upon bibliographic searches in PubMed and CINAHL and relevant search terms. Articles published in English from 1985 through July 31, 2017 were identified using the following MeSH search terms and Boolean algebra commands: Mediterranean diet AND (breast cancer OR breast neoplasm OR breast tumors OR mammary carcinoma OR mammary neoplasm). The searches were not limited to words appearing in the title of an article. In addition, they were not limited to studies in a particular country or geographic region of the world. The references of review articles were also reviewed [8,9,11,12]. Information obtained from bibliographic searches (title and topic of article, information in abstract, study design, and key words) was used to determine whether to retain each article identified in this way. Only studies written in English with a study arm of Mediterranean diet assessment that reported breast cancer risk in premenopausal and/or postmenopausal women according to molecular subtype were eligible for inclusion. The bibliographic searches and selection of studies were performed by two researchers (SSC and JS).
A total of 154 article citations were identified in PubMed and 35 non-duplicates in CINAHL (Figure 1). After screening the abstracts or full texts of these articles and reviewing the references of previous review articles, seven studies of adherence to the Mediterranean diet and breast cancer risk according to molecular subtype (ER, PR, HER2) met the eligibility criteria.
Figure 1:
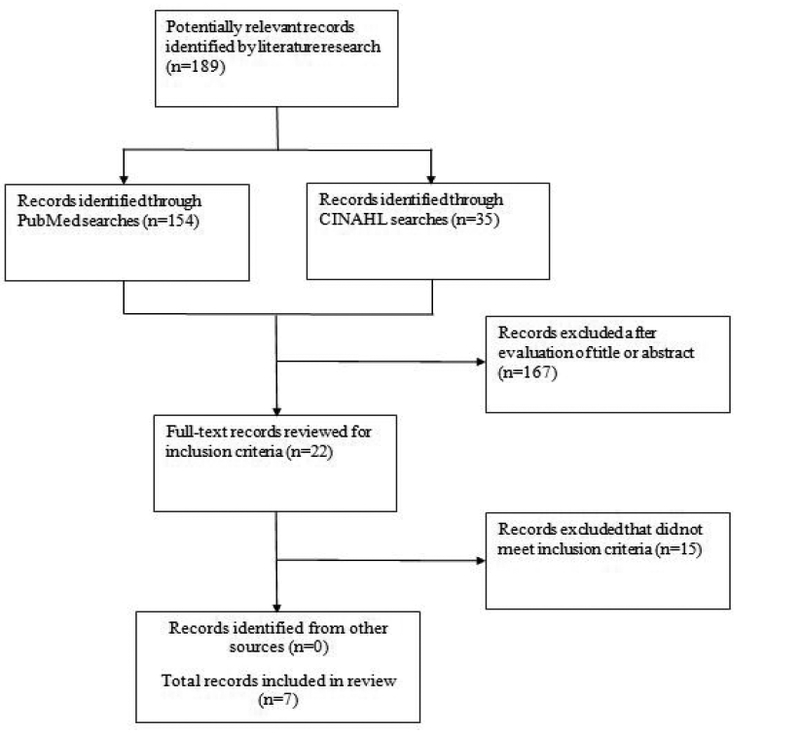
Flowchart of record selection process
Results
Six cohort studies and one case-control study have examined adherence to the Mediterranean diet and risk of breast cancer according to estrogen-receptor (ER) and progesterone-receptor (PR) status and human epidermal growth factor 2 (HER2) oncogene expression. Fung et al. [14] analyzed data from 71,058 nurses (The Nurses’ Health Study) who resided in 11 U.S. states and were followed for up to 18 years. A total of 3,580 postmenopausal incident cases of breast cancer were identified. Comparisons were made between the fifth and first quintile of dietary quality scores based on a Mediterranean diet scale proposed by Trichopoulou et al. [13] and a priori method of assessing dietary pattern. For ER+ breast cancer risk, no significant association was observed with dietary quality (Table 1). However, for ER-breast cancer risk, the adjusted hazard ratio (HR) of breast cancer incidence was 0.79 (95% confidence interval [CI] 0.60, 1.03, P for trend=0.03) comparing the fifth vs. first quintile. In cohort studies such as the one conducted by Fung et al. [14] potential sources of bias include misclassification of dietary intake.
Table 1:
Characteristics of included studies (cohort and case-control studies).
| Author | Design | Region | Follow-up duration | Sample size | Age at entry (yrs) |
Adjustment | HR/OR (95% CI) |
Menopausal status |
|---|---|---|---|---|---|---|---|---|
| Fung et al., 2006 | Cohort | USA | 18 years | 71,058 (3,580 incident breast cancer cases) | 30–55 | Age, smoking status, BMI, multivitamins, energy intake, PA, family history of breast cancer, personal history of benign breast disease, age at menopause, HRT, weight change since age 18 years |
ER+ cases HR: 1.05 (0.91, 1.18) for fifth vs. first quintile ER− cases HR: 0.79 (0.60, 1.03) for fifth vs. first quintile |
Post-meno-pausal |
| Cottet et al., 2009 | Cohort | France | 9.7 years | 65,374 (2,381 incident breast cancer cases) | 30–49 | Age, educational level, geographic area at baseline, BMI, height, family history of breast cancer, age at menarche, age at first full- term pregnancy, number of live births, HRT, history of benign breast disease or lobular carcinoma in situ, use of OCP, lifetime duration of breast feeding, frequency of Pap testing, PA, smoking, energy intake, current use of phytoestrogen supplements and vitamin/ mineral supplement |
ER+/PR+ cases HR: 0.88 (0.74, 1.05) for fourth vs. first quintile ER−/PR− cases HR: 0.78 (0.56, 1.10) for fourth vs. first quintile ER−/PR+ cases HR: 1.18 (0.58, 2.42) for fourth vs. first quintile ER+/PR− cases HR: 0.65 (0.49, 0.87) for fourth vs. first quintile |
Post-meno-pausal |
| Buckland et al., 2013 | Cohort | 10 European Countries |
11 years | 335,062 (10,225 incident breast cancer cases) | 35–70 | BMI, height, educational level, PA, smoking, menopausal status, age at menarche, OCP use, breastfeeding, age at first full-term pregnanancy, HRT, saturated fat intake, alcohol intake |
Post-menopausal women ER+ or PR+ cases HR: 0.92 (0.85, 1.01) for third vs. first tertile ER−/PR− cases HR: 0.80 (0.65, 0.99) for third vs. first tertile Pre-menopausal women ER+ or PR+ cases HR: 0.86 (0.66, 1.13) for third vs. first tertile ER−/PR− cases HR: 1.09 (0.65, 1.82) for third vs. first tertile |
Pre- and post-menopausal |
| Couto et al., 2013 | Cohort | Sweden | 16 years | 44,840 (1,278 incident breast cancer cases) | 30–49 | History of breast cancer, personal history of benign breast disease, smoking status, BMI, height, age at first birth and total number of children, educational level, age at menarche, total energy intake, consumption of beverages, potatoes, sweets, and eggs. |
Post-menopausal women ER+/PR+ cases HR: 0.97 (0.83, 1.14) for a two-point increment in the Mediter-ranean diet score ER−/PR− cases HR: 1.18 (0.86, 1.62) for a two-point increment in the Mediter-ranean diet score ER+/PR− cases HR: 0.98 (0.77, 1.25) for a two-point increment in the Mediter-ranean diet score ER−/PR+ cases HR: 3.07 (0.60, 15.75) for a two-point increment in the Mediter-ranean diet score Pre-menopausal women ER+/PR+ cases HR: 1.05 (0.93, 1.18) for a two-point increment in the Mediter-raneandiet score ER−/PR− cases HR: 1.34 (1.05, 1.71) for a two-point increment in the Mediter-raneandiet score ER+/PR− cases HR: 0.93 (0.70, 1.23) for a two-point increment in the Mediter-raneandiet score ER−/PR+ cases HR: 0.77 (0.49, 1.20) for a two-point increment in the Mediterranean diet score |
Pre- and post-menopausal |
| Castello et al., 2014 | Case-control | Spain | - - | 973 incident breast cancer cases and 973 healthy controls matched +/− 5 years on age and town of residence | - - | Total calories, alcohol consumption, BMI, PA, smoking, education, history of benign breast disease, family history of breast cancer, age at menarche, age at first delivery, menopausal status |
ER+/PR+/ HER2- cases. Dietary pattern defined a posteriori OR: 0.57 (0.40, 0.82) for fourth vs. first quartile HER2+ cases. Dietary pattern defined a posteriori OR: 0.66 (0.38, 1.13) for fourth vs. first quartile ER−/PR−/ HER2- cases. Dietary pattern defined a posteriori OR: 0.32 (0.15, 0.66) for fourth vs. first quartile ER+/PR+/HER2- cases. Dietary pattern defined a priori OR: 0.71 (0.43, 1.17) for fourth vs. first quartile HER2+ cases. Dietary pattern defined a priori OR: 0.96 (0.48, 1.94) for fourth vs. first quartile ER−/PR−/ HER2- cases. Dietary pattern defined a priori OR: 0.59 (0.21, 1.61) for fourth vs. first quartile |
Pre- and post-menopausal |
| Hirko et al. 2016 | Cohort | USA | 22 years | 100,643 | 30–35 | Energy intake, PA, parity and age at first birth, age at menarche, duration of OC use, family history of breast cancer, benign breast disease, HRT, BMI at age 18 years, weight change since age 18. |
Luminal A cases (ER+ and/or PR+ and HER2- and grade 1 or 2). HR: 1.09 (0.91, 1.30) for fifth vs. first quartile Luminal B cases (ER+ and/or PR+ and HER2+ or ER+, PR+, and HER2- with grade 3) HR: 1.02 (0.76, 1.37) for fifth vs. first quartile HER2-type cases (ER−, PR−, and HER2+). HR: 0.74 (0.42, 1.29) for fifth vs. first quartile Basal-like cases (ER−, PR−, HER2- and positive for CK 5/6 and/or EGFR) HR: 0.78 (0.49, 1.26) for fifth vs. first quartile |
Pre- and post-menopausal |
| van den Brent and Schulpen (2017) | Cohort | Netherlands | 20.3 years | 62,573 (2,321 incident breast cancer cases) | 55–69 | Age at baseline, cigarette smoking, number of cigarettes per day, duration of smoking, height, BMI, physical activity, education, family history of breast cancer, history of benign breast disease, age at menarche, parity, age at first birth, age at menopause, oral contracep-tive use, HRT, energy intake, alcohol intake. | A statistically significant inverse association was observed between Mediterr-anean diet adherence and risk of ER− breast cancer (HR = 0.60 (0.39, 0.93) for high versus low Mediterra-nean diet adherence (p-value for trend = 0.032). | Post-meno-pausal |
Abbreviations: BMI: Body mass index; CI: Confidence interval; CK 5/6; Cytokeratin 5/6; EGFR: Epidermal growth factor receptor; ER; Estrogen receptor; HER2: Human epidermal growth factor 2; HR: Hazard ratio; HRT: Hormone replacement therapy; OCP: Oral contraceptive pills; OR; Odds ratio: PA: Physical activity; PR: Progesterone receptor.
Cottet et al. [15] conducted a cohort study of 65,374 women in France (mostly teachers or their families) from the E3N-EPIC (Etude Epidemiologique aupres de Femmes de la Mutuelle Generale de l’Education Nationale) study cohort. A total of 2,381 postmenopausal invasive breast cancer cases were diagnosed during a median follow-up period of 9.7 years. Scores for dietary patterns were obtained by factor analysis which is an a posteriori approach to assessment of dietary pattern. The Mediterranean dietary pattern was negatively associated with breast cancer risk especially when tumors were ER+/PR- (adjusted HR=0.65, 95% CI 0.49, 0.87, P for trend=0.09) comparing the fourth vs. first quintile. Because the study population consisted of educated volunteers who were health conscious, the results may not be generalizable to the general population.
Buckland et al. [16] utilized a priori and a posteriori approaches to examine associations between adherence to the Mediterranean diet and risk of breast cancer among 335,062 women in 10 countries who had been enrolled in the EPIC study and followed for up to 11 years. A total of 10,225 incident breast cancer cases were identified. Adherence to the Mediterranean diet, assessed using an a priori approach, was inversely associated with postmenopausal breast cancer determined to be ER- and PR- (adjusted HR=0.80, 95% CI 0.65, 0.99, P for trend=0.043) comparing the third vs. first tertile. No associations were observed between adherence with the Mediterranean diet and risk of premenopausal breast cancer (Table 1).
Couto et al. [17] examined associations between the Mediterranean dietary pattern and risk of pre- and postmenopausal breast cancer among 44,840 women in Sweden who were followed for up to 11 years. A total of 1,278 incident breast cancer cases were identified. Dietary quality scores were based on a Mediterranean diet scale proposed by Trichopoulou et al. (2003). Adherence with a Mediterranean dietary pattern did not decrease breast cancer risk in this cohort of relatively young women, even after the cases were stratified according to ER, PR, and menopausal status (Table 1).
Castello et al. [18] examined associations between adherence with a Mediterranean diet and incident breast cancer in a hospital-based case-control study in Spain. Each case (n=973) was matched +/− 5 years with a healthy control selected from the cases’ in-law relatives, friends, neighbors, or work colleagues who resided in the same town. Principal component analysis was used to identify dietary patterns, an a posteriori approach. A higher Mediterranean pattern score was associated with a lower breast cancer risk (OR=0.56, 95% CI 0.40–0.79), comparing fourth vs. first quartiles. No statistically significant differences were observed among breast cancer molecular sub-types (p-value for heterogeneity=0.87). The protective effect of the Mediterranean dietary pattern was stronger for ER-/PR-/ HER2- (“triple negative”) tumors, with a steeper dose-response trend compared with other molecular subtypes (P-value for heterogeneity=0.04). The study is potentially limited by recall bias.
Hirko et al. [19] conducted an updated analysis of data from the Nurses’ Health Study. A total of 100,643 women were followed for 22 years. No significant associations were observed between the Mediterranean dietary pattern and risk of breast cancer by molecular subtype (Table 1). Because the study population consisted of nurses who were health conscious, the results may not be generalizable to the general population. However, survey information self-reported by nurses is likely to be more reliable and valid that information obtained from women in the general population.
van den Brent and Schulpen [20] examined associations between adherence with the Mediterranean diet and postmenopausal breast cancer risk in a cohort of 62,573 women in the Netherlands. A statistically significant inverse association was observed between Mediterranean diet adherence and risk of ER- breast cancer (HR = 0.60 (95% CI 0.39, 0.93) for high versus low Mediterranean diet adherence (p-value for trend = 0.032).
Discussion
The results of this systematic review suggest that the Mediterranean dietary pattern is inversely associated with breast cancer risk in postmenopausal women and that the inverse association is somewhat stronger among ER- tumors (Figures 2a, Figures 2b). Although there is a suggestion that the Mediterranean diet is inversely associated with PR- tumors and with ER-/PR-/HER2-(“triple negative” tumors), results to date have been mixed and the number of studies that have examined associations with this dietary pattern among tumors characterized by multiple molecular subtypes remains small.
Figure 2a:
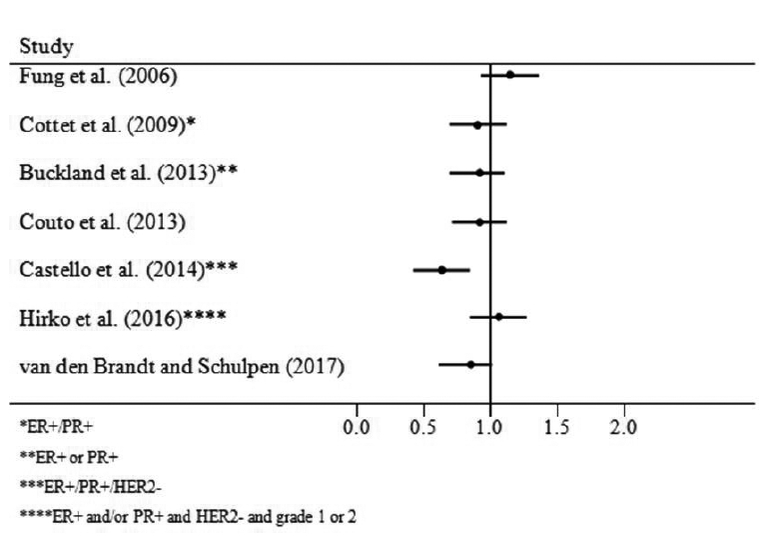
ER+ Cases, Postmenopausal
Figure 2b:
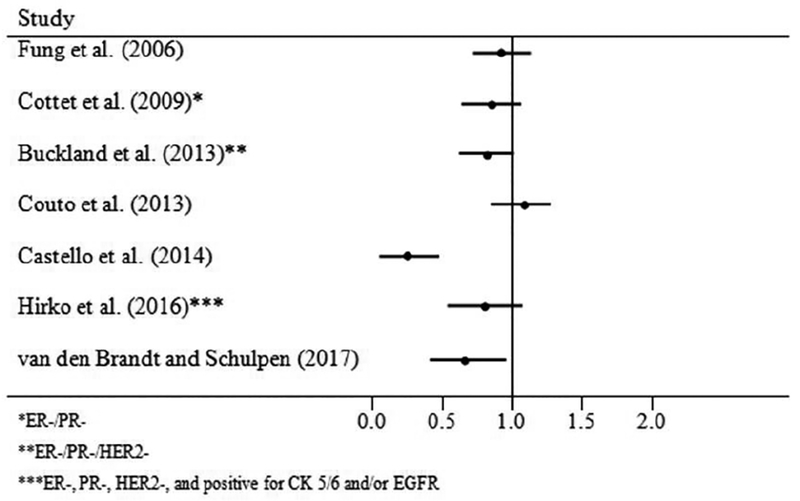
ER- Cases, Postmenopausal
Risk factors for breast cancer may differ according to the ER status of the tumor [14]. Exposure to endogenous and exogenous estrogens is one of the strongest risk factors for breast cancer and it may have less influence on ER- tumors than ER+ tumors. In ER+ tumors, the influence of dietary factors may be more difficult to detect than in ER- tumors because of the strong influence of hormonal factors [14]. Among ER- tumors, the influence of dietary factors may be greater and easier to detect [16].
A number of physiological mechanisms may explain the possible beneficial effect of the Mediterranean diet on risk of breast cancer. Plant-based foods are rich in flavonoids, carotenoids, vitamins C and E, which have antioxidant properties that can neutralize free radicals and prevent damage to DNA [16,21]. The Mediterranean diet is rich in flavonoids such as flavones, flavonols, and resveratrol which have antioxidant properties [22]. Phytoestrogens may have a role in reducing breast cancer risk as they act like estrogens in cells and may compete with estrogens in binding to estrogen receptors. Olive oil is rich in squalene which may have a tumor-inhibiting role in human mammary cells through reduction of oxidative DNA damage [23]. The favorable fatty acid profile of the Mediterranean diet may influence breast cancer risk by reducing hyperinsulinemia [22].
The Mediterranean diet has rarely been associated with breast cancer in premenopausal women. The possible differential effect by menopausal status may be due to a stronger influence of genetic factors and early life events in premenopausal breast cancer [16]. A possible protective effect of the Mediterranean diet may be more difficult to detect in premenopausal breast cancer.
Some of the studies included in this review used adaptations of a priori defined diet quality scores such as the Mediterranean Diet score [22]. Other studies used a posteriori methods to define the Mediterranean dietary pattern, such as factor analysis. This approach is quite different from using pre-assigned scores as it groups correlated food types into patterns. Patterns identified in one population can be different from those identified in other populations, which limits the transferability of population-specific patterns [24].
With respect to limitations, definitions of the Mediterranean diet and the instruments used to collect dietary information varied among the studies. The number of food items in the food frequency questionnaires varied, which might have resulted in the under representation or over representation of some food groups. Between studies, there is likely to be heterogeneity in the types of foods eaten within the Mediterranean diet, the cut-points used to define adherence, and the amounts of food intakes [16]. Caution is therefore required in comparing results across studies. In addition, the studies by Fung et al. [14] and Hirko et al. [19] were not completely independent. Potential sources of bias include misclassification of dietary exposures, residential confounding, and, in the case-control study by Castello et al. [18] recall bias. In addition, few of the studies included in this systematic review considered changes in eating habits over time. The current study did not consider whether the breast cancer risk of women who adhere to a Mediterranean diet but who live outside the Mediterranean region differs from that of women who adhere to the diet and live in the Mediterranean region.
Conclusion
In conclusion, the results of this systematic review suggest that consumption of a Mediterranean diet pattern characterized by high consumption of fruit and vegetables; frequent consumption of fish; high consumption of whole grain, unrefined cereals, nuts and seeds; olive oil as the main source of cooking oil and dietary lipids; low to moderate consumption of alcohol; and infrequent consumption of red meat and processed meats is associated with a reduced risk of postmenopausal breast cancer, particularly among ER- tumors. Additional cohort studies that have sufficient sample sizes and longitudinal follow-up are warranted to identify sizeable numbers of invasive cases of breast cancer, thereby allowing for characterization of the tumors by molecular subtype and improving risk prediction.
References
- 1.Keys A, Grande F. Dietary fat and serum cholesterol. Am J Public health 1957; 47: 1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev 1997;55 (Part 1): 383–389. [DOI] [PubMed] [Google Scholar]
- 3.Gallus S, Bosetti C, La Vecchia C. Mediterranean diet and cancer risk. Eur J Cancer 2004; 13: 447–452. [DOI] [PubMed] [Google Scholar]
- 4.Demetriou CA, Hadijsavvas A, Loizidou MA, et al. The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer 2012; 12: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadaki A, Johons L, Toumpakari Z, et al. Validation of the English version of the 14-item Mediterranean Diet Adherence Screener of the PREDIMED Study, in people at high cardiovascular risk in the UK. Nutrients 2018;10: pii: E138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessaoud F, Tretarre B, Jean-Pierre D, Gerber M. Identification of dietary patterns using two statistical approaches and their association with breast cancer risk: a case-control study in Southern France. Ann Epidemiol 2012; 22: 499–510. [DOI] [PubMed] [Google Scholar]
- 7.Cade JE, Taylor EF, Burley VJ, Greenwood DC. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr 2011; 65: 920–8. [DOI] [PubMed] [Google Scholar]
- 8.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med 2015; 4: 1933–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albuquerque RCR, Baltar V, Marchioni DML. Breast cancer and dietary patterns: a systematic review. Nutr Revs 2013; 72: 1–17. [DOI] [PubMed] [Google Scholar]
- 10.Farsinejad-Marj M, Talebi S, Ghiyasvand R, Miraghajani M. Aderence to Mediterraanean diet and risk of breast cancer in premenopausal and postmenopausal women. Arch Iran Med 2015; 18: 786–92. [PubMed] [Google Scholar]
- 11.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer 2014; 135: 1884–97. [DOI] [PubMed] [Google Scholar]
- 12.Schwingshacki L, Hoffmann G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr Nutr Rep 2016; 5: 9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl j Med 2003; 348: 2599–608. [DOI] [PubMed] [Google Scholar]
- 14.Fung TT, Hu FB, McCullough ML, et al. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr 2006; 136: 466–472. [DOI] [PubMed] [Google Scholar]
- 15.Cottet V, Touvier M, Fournier A, eta l. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol 2009; 170: 1257–67. [DOI] [PubMed] [Google Scholar]
- 16.Buckland G, Travier N, Cottet V, et al. Adherence to the Mediterranean diet and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort study. Int J Cancer 2013; 132: 2918–27. [DOI] [PubMed] [Google Scholar]
- 17.Couto E, Sandin S, Lof M, et al. Mediterranean dietary pattern and risk of breast cancer. PLOS One 2013; 8: e55374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castello A, Pollan M, Buijsse B, et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer 2014;111:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirko KA, Willett WC, Hankinson SE, et al. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat 2016; 155: 579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer 2017; 140: 2220–31. [DOI] [PubMed] [Google Scholar]
- 21.Visioli F, Grande S, Bogani P, et al. The role of antioxidants in the Mediterranean diets: focus on cancer. Eur J Cancer Prev 2004; 13: 357–43. [DOI] [PubMed] [Google Scholar]
- 22.Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D. Conformity to traditional Meditterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J Clin Nutr 2010; 92: 620–5. [DOI] [PubMed] [Google Scholar]
- 23.Warleta F, Campos M, Allouche Y, et al. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem Toxicol 2010; 8: 1092–100. [DOI] [PubMed] [Google Scholar]
- 24.Cade JE, Taylor EF, Burley VJ, Greenwood DC. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr 2011; 65: 920–28. [DOI] [PubMed] [Google Scholar]


