Abstract
Purpose of review:
Age-period-cohort (APC) models simultaneously estimate the effects of age – biological process of aging; time period – secular trends that occur in all ages simultaneously; and birth cohort – variation among those born around the same year or from one generation to the next. APC models inform understanding of cancer etiology, natural history, and disparities. We reviewed findings from recent studies (published 2008–2018) examining age, period, and cohort effects and summarized trends in age-standardized rates and age-specific rates by birth cohort. We also described prevalence of cancer risk factors by time period and birth cohort, including obesity, current smoking, human papilloma virus (HPV), and hepatitis C virus (HCV).
Recent findings:
Studies (n=29) used a variety of descriptive analyses and statistical models to document age, period, and cohort trends in cancer-related outcomes. Cohort effects predominated, particularly in breast, bladder, and colorectal cancers, whereas period effects were more variable. No effect of time period was observed in studies of breast, bladder, and oral cavity cancers. Age-specific prevalence of obesity, current smoking, HPV, and HCV also varied by birth cohort, which generally paralleled cancer incidence and mortality rates.
Summary:
We observed strong cohort effects across multiple cancer types and less consistent evidence supporting the effect of time period. Birth cohort effects point to exposures early in life – or accumulated across the life course – that increase risk of cancer. Birth cohort effects also illustrate the importance of reconsidering the timing and duration of well-established risk factors to identify periods of exposure conferring the greatest risk.
Keywords: Incidence, time factors, SEER program, risk factors, age factors
Introduction
Cancer registries across the world monitor incidence and mortality rates to assess the distribution of disease, often informing our understanding of cancer etiology, natural history, and disparities. Age-period-cohort (APC) models provide additional and useful insight by documenting change in cancer incidence and mortality over time that may be attributable to age, time period of observation, and birth cohort.1 Models simultaneously estimate the effects of age – biological process of aging; time period – secular trends that occur in all ages simultaneously; and birth cohort – variation among those born in or around the same year or from one generation to the next. Epidemiologic studies using APC models have improved our understanding of the burden and etiology of several cancers. For example, birth cohort effects evidenced in lung cancer2,3 point to younger age at smoking initiation and longer duration of smoking as important risk factors.
Although linear APC models have been limited by the identification problem (i.e., age, period, and cohort variables may be perfectly collinear), methodologic advances have provided several new APC models useful in cancer research, and that extend beyond the conventional, linear approach. We review recent studies estimating the differential contributions of age, period, and cohort to cancer incidence and mortality. We also estimated prevalence of cancer risk factors by time period and birth cohort.
Methods
We reviewed findings from studies examining age, period, and cohort effects published between 2008 and 2018. For each study, we described cancer type, geographic location, data source, and statistical methods. We also summarized temporal trends in age-standardized rates and age-specific rates by birth cohort.
Prevalence of Cancer Risk Factors
We described trends in the prevalence of cancer risk factors by time period and birth cohort, including obesity, current smoking, human papilloma virus (HPV), and hepatitis C virus (HCV). We obtained prevalence estimates from the National Health and Nutrition Examination Survey (NHANES) from 1999 through 2016 (approximately 45,000 adults age ≥18 years). NHANES includes a standardized physical examination, where trained health technicians collect a complete set of anthropometric and laboratory measures from survey participants.
Obesity.
Body weight and height were measured in mobile examination centers using standardized procedures and equipment. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2), and then rounded to one decimal place. Consistent with national guidelines, we defined obesity as BMI ≥ 30 kg/m2.
Current smoking.
We defined current smoking by combining responses to two survey questions: 1) “Have you smoked at least 100 cigarettes in your entire life?”; and 2) “Do you now smoke cigarettes?” Interview questions are asked in the home by trained interviewers using the Computer-Assisted Personal Interviewing (CAPI) system. Participants who replied “yes” to the first question and reported now smoking “every day” or “some days” were considered current smokers.
Hepatitis C virus.
NHANES participants’ serum specimens are tested for antibodies to HCV (anti-HCV) using VITROS Anti-HCV chemiluminescence assay (CIA). Supplemental recombinant immunoblot assays (RIBA) (Chiron RIBA 3.0 Strip Immunoblot Assay) are performed on all repeatedly positive specimens by CIA testing. Specimens with a positive RIBA results are reported as confirmed positive for antibody to HCV. Because the confirmed anti-HCV test was discontinued by the manufacturer in 2012, and subsequently no longer used in NHANES, we estimated prevalence of anti-HCV through 2012 only.
Human papilloma virus.
Women (ages 18–59 years) participating in NHANES provide self-collected vaginal swabs, which are then analyzed for 37 HPV genotypes using the Roche Linear Array Assay. We estimated prevalence of all high-risk HPV (ref) genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68), as well as prevalence of genotypes 16 and 18 (targeted in all vaccines).
For each risk factor, we report temporal trends in prevalence, as well as age-specific prevalence by birth cohort, separately for men and women. All prevalence estimates are weighted to account for survey sampling and nonresponse.
Results
Age, period, and cohort effects and cancer incidence and mortality
Table 1 summarizes findings from 29 studies of cancer incidence and/or mortality of breast, liver, gastric, bladder, bone, esophageal, oral cavity, lung, colorectal, pancreatic, and prostate cancers and leukemia. Most studies were conducted in the U.S., although about one-third were conducted internationally, including Canada, Norway, China, Argentina, Mexico, France, Japan, and Spain. A variety of statistical methods were used across studies (Table 2). Findings relevant to each cancer type are described below.
Table 1.
Characteristics of and relevant findings from studies examining age, period, and cohort effects in cancer incidence and mortality
| Author (year) | Cancer type | Geographic location | Data source, years | Method | Relevant findings |
|---|---|---|---|---|---|
| Altekruse (2009) | Liver | U.S. | SEER 9, 1975–2015; SEER 13, 1992–2015 | Not reported |
|
| Anderson (2010) | Gastric | U.S. | SEER 9, 1973–1991; SEER 13, 1992–1999; SEER 17, 2000–2006 | Estimable function approach |
|
| Andreassen (2016) | Bladder | Norway | Cancer Registry of Norway, 1981–2014 | Estimable function approach |
|
| Anfinsen (2011) | Bone1 | U.S. | SEER 9, 1976–2005 | Estimable function approach |
|
| Arnold (2017) | Esophagus | Multiple2 | Cancer Incidence in Five Continents, 1988–2007 | Not reported |
|
| Bao (2016) | All | China (Shanghai) | Shanghai Cancer Registry, 1973–2010 | Estimable function approach |
|
| Chaturvedi (2008) | Oral cavity | U.S. | SEER 9, 1973–2004 | Estimable function approach |
|
| Chaturvedi (2013) | Oral cavity | Multiple3 | Cancer Incidence in Five Continents, 1983–2002 | Estimable function approach |
|
| Franco-Marina (2009) | Breast | Mexico | National Institute of Geography and Statistics, 1980–2005 | Estimable function approach |
|
| Franco-Marina (2015) | Breast | Multiple4 | Cancer Incidence in Five Continents, 1988–2007 | Estimable function approach |
|
| Gangnon (2015) | Breast | U.S. | SEER 9, 1975–2010 | Estimable function approach |
|
| Gilhodes (2015) | Lung, oral cavity, esophagus5 | France | Regional cancer registries, 1982–2010 | Estimable function approach |
|
| Ito (2011) | All | Japan | Osaka Cancer Registry, 1968–2007 | Estimable function approach |
|
| Jemal (2012) | Lung6 | U.S. | National Center for Health Statistics, 1973–2007 | Estimable function approach |
|
| Jemal (2018) | Lung | U.S. | NAACCR, 1995–2014 | Estimable function approach |
|
| Lopez-Abente (2010) | Colorectal | Spain | European Network of Cancer Registries, 1975–2004 | Estimable function approach |
|
| Ma (2013) | Pancreas | U.S. | National Center for Health Statistics, 1970–2009 | Estimable function approach |
|
| Murphy (2017) | Esophagus | U.S. | SEER 9, 1973–2012 | Hierarchical model |
|
| Niclis (2011) | Prostate | Argentina | Cordoba Ministry of Health, 1986–2006 | Estimable function approach |
|
| Petrick (2016) | Liver7 | U.S. | SEER 18, 1992–2012 | Estimable function approach |
|
| Pocobelli (2008) | Liver | Canada | Canadian Cancer Registry, 1976–2000 | Estimable function approach |
|
| Pou (2011) | Bladder | Argentina | Cordoba Ministry of Health, 1986–2006 | Estimable function approach |
|
| Rosenberg (2012) | Leukemia8 | U.S. | SEER 13, 1992–2009; SEER 18, 2000–2009 | Estimable function approach |
|
| Siegel (2017) | Colorectal | U.S. | SEER 9, 1974–2013 | NCI web tool |
|
| van Steenbergen (2009) | Colorectal | Netherlands | Eindhoven Cancer Registry, 1970–2006 | Estimable function approach |
|
| Viel (2011) | Breast | France | Doubs Cancer Registry, 1987–2003 | Estimable function approach |
|
| Wang (2015) | Breast | Multiple9 | WHO Mortality Database and Cancer Statistic Registries, 1953–2012 | Intrinsic estimator |
|
| Yan (2015) | Liver | U.S. | SEER 18, 2003–2011 | Not reported |
|
| Yang (2013) | Bladder, kidney | China (Shanghai) | Shanghai Cancer Registry, 1973–2005 | Intrinsic estimator |
|
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; NR, not reported; HPV, human papillomavirus; WHO, World Health Organization; NAACCR, North American Association of Central
women, rates of kidney cancer Cancer Registries; IE, Intrinsic Estimator; CML, chronic myeloid leukemia; CLL, chronic lymphocytic declined among birth cohorts before leukemia; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia
Bone cancer includes osteosarcoma, Ewing sarcoma, and chondrosarcoma
U.S., Canada, Japan, Australia, Denmark, U.K., France, Netherlands, Croatia, Italy, Spain, Slovakia
India, Japan, Philippines, Singapore, Thailand, Australia, Austria, Denmark, Estonia, France, Italy, Netherlands, Poland, Slovakia, Spain, Switzerland, U.K., Canada, U.S., Brazil, Colombia, Costa Rica, Ecuador
Brazil, Colombia, Ecuador, Costa Rica, Manitoba, Canada, U.S.
Gilhodes (2011) only included young adults age 20–44 years
Jemal (2012) estimated trends among women only
Petrick (2016) reported projected (vs. observed) incidence rates by time period
Leukemia included chronic myeloid leukemia, chronic lymphocytic leukemia, acute myeloid leukemia, and acute lymphoblastic leukemia
China, South Korea, Japan, U.S.
Table 2.
Methods used to estimate age, period, and cohort effect
| Method | Description |
|---|---|
| Linear model60 |
|
| Coefficient-constraints approach60 |
|
| Estimable function approach57–59 |
|
| Intrinsic estimator62,63 |
|
| Hierarchical model |
|
| NCI web tool64 |
|
NOTE: Coefficient-constraints and estimable function approaches are two approaches within the linear model framework; intrinsic estimator is a specific example of an estimable function
Breast.
In studies of breast cancer, we noted variable period and cohort effects across studies by geographic region. For example, age-standardized incidence rates declined from 1980 through to 2010 in the U.S.4 but increased in other countries during the same period.5,6 Despite these differences, most studies of breast cancer show a stronger cohort effect than period effect, which may be due to estrogen-related or reproductive risk factors (e.g., age at menarche, breastfeeding patterns, number and age childbirth).7
Liver.
Worldwide, age-standardized incidence of liver cancer has increased dramatically since the late 1970s,8–11 primarily driven by increases in hepatocellular carcinoma. Age-specific incidence has also increased across successive birth cohorts through the 1960 birth cohort8 and subsequently declined.9 Chronic HCV infection, a common blood-borne infection, increases risk of liver cancer,12,13 and about 75% of adults infected with HCV in the U.S. are baby boomers (born between 1945 and 1965). Baby boomers likely became infected from contaminated blood and blood products before widespread screening began in the early 1990s.14
Colorectal.
Age-standardized incidence and mortality rates of colorectal cancer have decreased in the U.S. since the mid-1980s,15 with particularly dramatic declines among older adults (age ≥50 years). In contrast, incidence increased in Spain16 and the Netherlands17 during the same period. Mortality rates decreased in all three geographic regions starting around 1990. Notably, age-specific incidence rates have increased among U.S. birth cohorts after 1950.15
Lung.
Two U.S. studies of lung cancer incidence show age-specific incidence and mortality rates declined starting in the 1990s.18,19 Incidence and mortality rates have also declined across successive birth cohorts after about the 1930 birth cohort, which parallels dramatic declines in the prevalence of smoking by cohort.20,21 However, incidence increased among women born in 1950 to 1960, and in certain age groups, incidence rates in women have surpassed those of men.19 Because the prevalence of smoking has decreased among women born in the 1950s and 60s, this increase in incidence is likely not due to smoking patterns or tobacco exposure.
Bladder.
Bladder cancer incidence and mortality varied by period and cohort, and across geographic region. For example, age-standardized incidence rates increased in Norway in the 1980s and then stabilized,22 but incidence increased in China from 1973 to 2005.23 Mortality rates in Argentina declined among men after 1986 and among women after 1996.24 Cohort trends appeared more consistent, and age-specific rates were generally higher among birth cohorts born in the early 1900s.22,23 Smoking causes about half of all bladder cancers,25,26 and the consistent declines across birth cohorts may be due to simultaneous declines in smoking prevalence by birth cohort.20,21
Oral cavity.
In the U.S.27 and economically developed countries,28,29 age-standardized incidence rates of HPV-related oral cancers (e.g., oropharyngeal cancers – tonsils, tonsillar crypt, base of tongue) increased from the early 1980s through to the most recent time period, particularly among men. Age-specific incidence generally increased across successive birth cohorts starting with persons born in 1930. HPV is the most common sexually transmitted infection and the leading cause of oropharyngeal cancers (thought to cause 70% in U.S.30,31). Oral HPV is transmitted via oral sex, and changes in sexual behavior32,33 in the 1960s may explain rising incidence in more recent birth cohorts.
Gastric.
Across all racial/ethnic groups in the U.S., age-standardized incidence rates of gastric cancer declined starting in the late 1970s.34 Age-specific incidence rates declined among whites through about the 1950 birth cohort and then subsequently increased among more recent cohorts. The majority of gastric cancers are attributable to chronic infection with Helicobacter pylori,35 commonly acquired in childhood. In the U.S., prevalence of H. pylori has decreased dramatically across birth cohorts,36 likely due to improvements in sanitation and increased antibiotic use.37 Lower gastric cancer incidence rates among older birth cohorts may reflect decreased H. pylori infection in their childhood, but reasons for increasing rates in younger birth cohorts remain unclear.
Esophagus.
Starting in about 1985, age-standardized incidence rates of esophageal adenocarcinoma exceeded rates of squamous cell carcinoma worldwide.38 Rates of squamous cell carcinoma steadily declined during this same period.39 In the U.S., age-specific incidence rates increased from about 1990 to 2012 and across successive birth cohorts born from 1885 to 1950.40 Increasing rates of esophageal adenocarcinoma have been attributed to increasing prevalence of risk factors, such as obesity41 and gastroesophageal reflux disease.42,43 Our prior work shows a stronger period effect than cohort effect, largely explained by temporal trends in obesity.40 Although the increase in esophageal adenocarcinoma has slowed in recent years, it remains one of the few cancers in the U.S. with a rising incidence.44
Pancreas.
Age-standardized mortality rates of pancreatic cancer in the U.S. have varied by race/ethnicity and sex.45 For example, among white men, rates decreased from 1970 to 1995 but increased among white women during the same period. Rates also increased among black men and women from 1970 to 1989, and then subsequently declined through to 2009. Cohort effects appeared stronger in men than women, and age-specific mortality rates decreased among white and black men after the birth cohort born in 1910. Racial differences in the prevalence of risk factors, such as smoking, diabetes, and obesity,46,47 which also differ by sex, may contribute to these observed trends.
Leukemia.
Because leukemia comprises a heterogeneous group of cancer, period and cohort trends differ according to subtype. Age-standardized incidence rates of chronic myeloid leukemia (CML) and chronic lymphocytic leukemia (CLL) decreased from 1992 to 2009.48 However, rates of acute lymphoblastic leukemia (ALL), most common among children and older adults, increased during the same period. Age-specific incidence rates of ALL have also increased across successive birth cohorts, starting with persons born in the mid-1940s.
Bone.
Similarly, bone cancer comprises a diverse group of cancers, including osteosarcoma, Ewing sarcoma, and chondrosarcoma. Age-standardized incidence rates of bone cancer have been generally stable since the late 1970s.49 Notably, age-specific incidence rates of osteosarcoma declined in successive birth cohorts born between 1905 and 1934.
In studies of common cancer types (Table 3), period and cohort effects were assessed descriptively and in statistical models. Most studies described trends in one dimension (e.g., period changes in rates for all ages combined) or two dimensions (e.g., cohort changes in age- specific rates). Fewer studies reported results of statistical models estimating the independent effects of age, period, and cohort. In these studies, cohort effects predominated, particularly in breast, bladder, and colorectal cancers, whereas period effects were more variable. No effect of time period was observed in studies of breast, bladder, and oral cavity cancers.
Table 3.
Analytic methods and findings across studies of common cancers
| Analytic method | ||||||
|---|---|---|---|---|---|---|
| Descriptive analysis | Statistical models | |||||
| Period | Age x Period | Age x Cohort | Age | Period | Cohort | |
| Breast (n=5) | ++ | ++ | ++ | ++ | ++* | ++ |
| Lung (n=3) | ++ | + | + | |||
| Liver (n=4) | + | + | ++ | + | + | |
| Bladder (n=3) | ++ | + | + | ++ | +* | ++ |
| Oral cavity (n=3) | ++ | + | ++ | * | + | |
| Esophageal (n=3) | + | + | + | + | ++ | ++ |
| Colorectal (n=3) | + | + | ++ | ++ | ++ | ++ |
+ reported association in at least one study;
++ reported association in two or more studies;
* null findings in at least one study
NOTE: Cancer types for which we identified only one study in our review are not included in the table
Prevalence of cancer risk factors by period and cohort
Obesity.
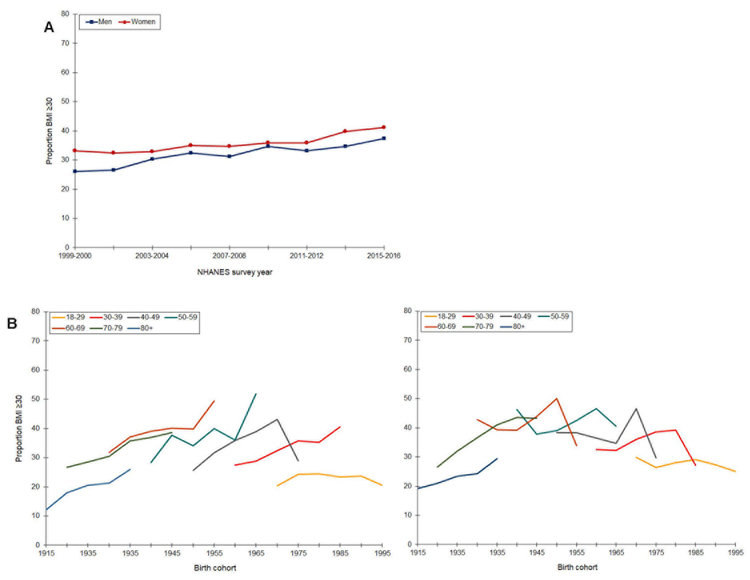
Prevalence of obesity (BMI ≥ 30 kg/m2) increased from 1999 to 2016, and prevalence was higher among women than men in all survey years (Figure 1). Among men, prevalence ranged from 27 to 32%, and among women, from 32 to 39%. Age-specific prevalence across birth cohorts also differed by sex. Except for the youngest age group (18–29 years), obesity increased among men in all age groups and across successive birth cohorts, with particularly steep increases from the 1930 though 1955 birth cohorts. Prevalence was highest among men age 60–69 years. In contrast, age-specific prevalence of obesity generally declined across successive birth cohorts among women. Obesity increased slightly among women born in 1915 through 1935, and subsequently remained stable or decreased.
Figure 1.
Prevalence of obesity by time period (A) and age-specific prevalence by birth cohort (B, men; C, women), National Health and Nutrition Examination Survey, Continuous Cycles, 1999 – 2016
Current smoking.
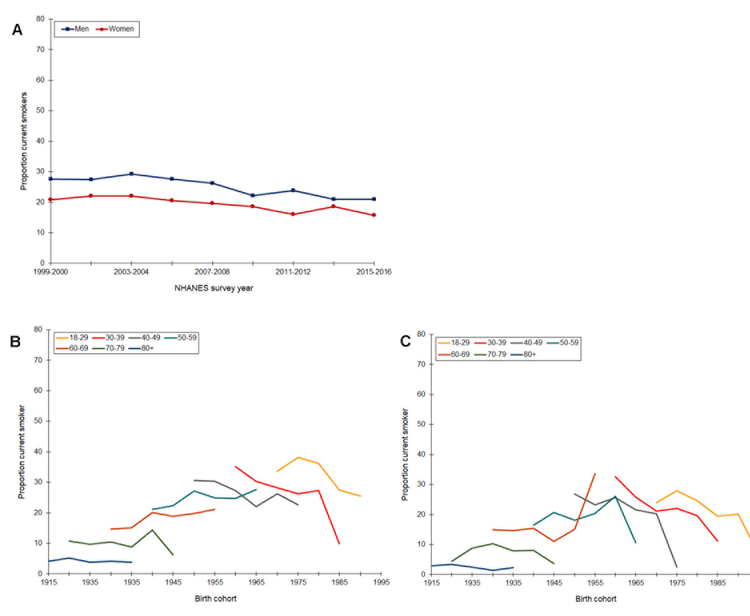
As shown in Figure 2, prevalence of smoking declined slowly from 1999 through 2016 and remained consistently higher among men (range 21 – 29%) than women (range 16 – 22%). Age-specific prevalence of smoking was highest among 18–29 year-old men born in the 1970s. Prevalence remained stable in all age groups for birth cohorts born from 1915 to 1940 but then decreased across successive birth cohorts. Among women, starting with the 1955 birth cohort, there were sharp declines in smoking in all age groups through the 1995 birth cohort.
Figure 2.
Prevalence of current smoking by time period (A) and age-specific prevalence by birth cohort (B, men; C, women), National Health and Nutrition Examination Survey, Continuous Cycles, 1999 – 2016
Hepatitis C virus.
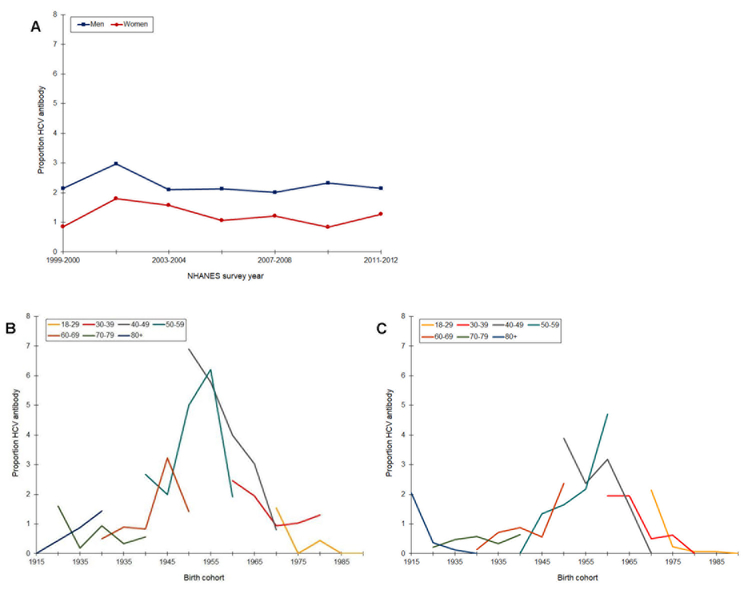
After an increase in prevalence from 1999 to 2002, anti-HCV remained stable through 2012 (Figure 3). Prevalence was consistently higher among men than women, and in both groups remained low, around 2%. Cohort trends in anti-HCV appeared much more prominent. In men and women, there were sharp increases in age-specific prevalence across the 1945 to 1960 birth cohorts. Prevalence was highest among 40- and 50-year olds born in 1955. Starting with persons born around 1960, prevalence declined in all age groups.
Figure 3.
Prevalence of Hepatitis C antibody by time period (A) and age-specific prevalence by birth cohort (B, men; C, women), National Health and Nutrition Examination Survey, Continuous Cycles, 1999 – 2012
Human papillomavirus (women only, ages 18–59 years).
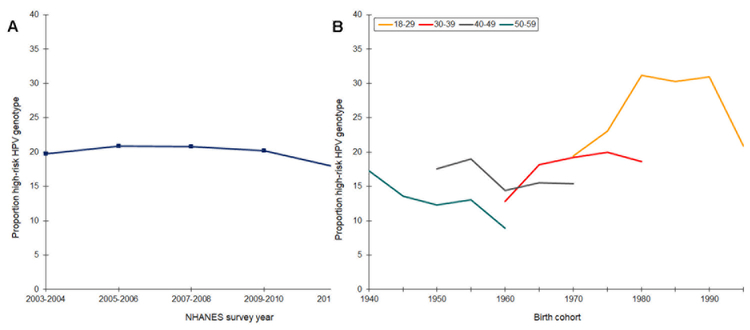
Prevalence of high-risk HPV hovered around 20% from 2003 to 2010 and then declined slightly to 17% in 2013–14 (Figure 4). We observed a similar pattern, although of smaller magnitude, for HPV genotypes 16 and 18 (not shown). Age-specific prevalence decreased among 40- and 50-years olds born in 1940 to 1960. Subsequently, and starting with women born around 1965, prevalence increased through to the 1980 birth cohort. There were hints of declines among women born in the 1980s, and prevalence decreased dramatically among the youngest birth cohort. Across all birth cohorts, high-risk HPV was highest in 18–29 year olds.
Figure 4.
Prevalence of high-risk HPV genotypes among women (ages 18–39 years) by time period (A) and age-specific prevalence by birth cohort (B), National Health and Nutrition Examination Survey, Continuous Cycles, 2003 – 2014
Conclusion
APC methods identify and quantify variation in cancer incidence and mortality associated with age, time period, and birth cohort. Across 29 studies of multiple cancer types, we observed stronger birth cohort effects than period effects. Birth cohort effects point to exposures early in life – or accumulated across the life course – that increase risk of cancer. Birth cohort effects also illustrate the importance of reconsidering the timing and duration of well-established risk factors to identify periods of exposure conferring the greatest risk. For example, obesity, associated with increased risk of several cancers,50 may contribute to some of the observed increases in incidence. Measuring obesity during windows of growth and development (e.g., birthweight51 or childhood obesity52) may advance our understanding of its role in carcinogenesis and identify vulnerable periods of exposure that matter most.
We found less consistent evidence supporting the effect of time period, which also differed by geographic region (i.e., temporal trends were not consistent across the globe). These regional differences may point to the influence of screening or diagnostic practices, such as mammography screening. Indeed, studies of breast cancer incidence and mortality showed the greatest variations in period effects, and in some regions, there was no period effect. Economically developed countries have adopted screening mammography guidelines at various time points (e.g., early 1980s in the U.S.,53 early 1990s in the U.K.54,55), and the U.S. was among the early adopters. This may explain why mortality rates declined in earlier time periods in the U.S. but not in other regions. Differences in period effects or temporal trends across geographic region may also underscore differences in the timing of risk factor prevalence. For example, HCV became prevalent in Asian countries before it did in the U.S., which parallels trends in liver cancer incidence between the two regions.
Trends in cancer incidence and mortality generally paralleled prevalence of risk factors. For example, prevalence of anti-HCV was highest among birth cohorts born between 1945 and 1960, and incidence rates of liver cancer were highest among these cohorts.8,9,11 Declines in the prevalence of smoking by birth cohort also mirrored age-specific rates of lung,18,19,29 bladder,22–24 and pancreatic45 cancer incidence and mortality. Although there were some exceptions, rates of these cancers often declined in birth cohorts with lower prevalence of smoking. Meanwhile, prevalence of obesity has increased by time period and birth cohort, which may contribute to recent observations that the incidence of gastric34 and colorectal15 cancer has increased among younger adults.
Studies used a variety of descriptive analyses and statistical models to track age, period, and cohort effects. Nearly all included one-dimensional or summary indices to describe period variation in overall incidence or mortality rates. Although useful for understanding cancer burden, these indices may be less relevant to APC analysis because they: 1) only describe variation in rates attributable to factors during the period of cancer diagnosis or death, ignoring different trends at different ages; and 2) are sensitive to the choice of standard population (i.e., for age-standardizing), which may not capture recent changes in population structure due to aging. Other studies used two-dimensional graphical displays, which improve upon summary indices by providing information on age-specific change. For example, many studies described or displayed age-specific trends across birth cohorts. Two-dimensional trends are helpful for qualitative impressions about patterns for each age group but not for quantitative assessment of the source of change.
Fewer studies reported results of statistical models, and of those that did, most used linear models and an estimable function approach. Estimable functions, such as deviation, curvature, and drift, are used to derive estimates.56–59 For example, many ascribe net drift (annual percent change of the expected age-standardized rates) to overall log-linear trends by time period and birth cohort. These approaches generally use constraints to resolve the identification problem inevitable in linear models, raising two important limitations. First, different constraints yield different estimates but identical or similar model fit. Second, effect estimates are sensitive to the choice of the identifying constraints and require a priori information, which rarely exists. These limitations also make it challenging to compare findings across studies. While we recommend researchers present results of linear models in conjunction with a detailed descriptive analysis1 and use caution in interpretation of these results, we also suggest using hierarchical APC models. Hierarchical models not only address the problems inherent in linear models, but they also offer the additional advantage of including covariates or risk factors to test explanatory hypothesis about the underlying mechanisms for observed age, period, and cohort trends.
In summary, APC models track changes in cancer incidence and mortality over time that may be attributable to age, time period of observation, and birth cohort. We observed strong cohort effects across 29 studies of various cancer types, highlighting the importance of early life exposures that may promote biological pathways initiating carcinogenesis in adulthood. We also observed variations in cancer risk factors (e.g., obesity, smoking) by time period and birth cohort, which paralleled trends in cancer incidence and mortality.
Acknowledgments
Grant support:
This work was supported by the National Cancer Institute (P30CA142543) and National Center for Advancing Translational Sciences (KL2TR001103 to Dr. Murphy) at the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Caitlin C. Murphy and Yang Claire Yang each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Yang Y, Land KC. Age-period-cohort analysis: New models, methods, and empirical applications Chapman and Hall/CRC; 2016.Explores statistical models, methods, and research designs that can be used in age-period-cohort analysis, as well as new methods that tackle the “identification problem.” Compares new and existing models and methods and provides useful guidelines on how to conduct age-period-cohort analysis. Software and programs to estimate the models are available on the book’s web page: http://yangclaireyang.web.unc.edu/age-period-cohort-analysis-new-models-methods-and-empirical-applications/
- 2.Jemal A, Travis WD, Tarone RE, Travis L, Devesa SS. Lung cancer rates convergence in young men and women in the United States: analysis by birth cohort and histologic type. International journal of cancer 2003;105(1):101–107. [DOI] [PubMed] [Google Scholar]
- 3.Bray FI, Weiderpass E. Lung cancer mortality trends in 36 European countries: secular trends and birth cohort patterns by sex and region 1970–2007. International journal of cancer 2010;126(6):1454–1466. [DOI] [PubMed] [Google Scholar]
- 4.Gangnon RE, Sprague BL, Stout NK, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2015;24(6):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco-Marina F, Lopez-Carrillo L, Keating NL, Arreola-Ornelas H, Marie Knaul F. Breast cancer age at diagnosis patterns in four Latin American Populations: A comparison with North American countries. Cancer epidemiology 2015;39(6):831–837. [DOI] [PubMed] [Google Scholar]
- 6.Viel JF, Rymzhanova R, Fournier E, Danzon A. Trends in invasive breast cancer incidence among French women not exposed to organized mammography screening: an age-period-cohort analysis. Cancer epidemiology 2011;35(6):521–525. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiologic reviews 1993;15(1):36–47. [DOI] [PubMed] [Google Scholar]
- 8.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27(9):1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(15):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pocobelli G, Cook LS, Brant R, Lee SS. Hepatocellular carcinoma incidence trends in Canada: analysis by birth cohort and period of diagnosis. Liver international : official journal of the International Association for the Study of the Liver 2008;28(9):1272–1279. [DOI] [PubMed] [Google Scholar]
- 11.Yan M, Ha J, Aguilar M, et al. Birth cohort-specific disparities in hepatocellular carcinoma stage at diagnosis, treatment, and long-term survival. Journal of hepatology 2016;64(2):326–332. [DOI] [PubMed] [Google Scholar]
- 12.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology 2006;45(4):529–538. [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Archives of Internal Medicine 2000;160(21):3227–3230. [DOI] [PubMed] [Google Scholar]
- 14.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, national health and nutrition examination survey 2003 to 2010. Annals of internal medicine 2014;160(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. Journal of the National Cancer Institute 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Abente G, Ardanaz E, Torrella-Ramos A, Mateos A, Delgado-Sanz C, Chirlaque MD. Changes in colorectal cancer incidence and mortality trends in Spain. Annals of oncology : official journal of the European Society for Medical Oncology 2010;21 Suppl 3:iii76–82. [DOI] [PubMed] [Google Scholar]
- 17.van Steenbergen LN, Lemmens VE, Louwman MJ, Straathof JW, Coebergh JW. Increasing incidence and decreasing mortality of colorectal cancer due to marked cohort effects in southern Netherlands. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2009;18(2):145–152. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Ma J, Rosenberg PS, Siegel R, Anderson WF. Increasing lung cancer death rates among young women in southern and midwestern States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30(22):2739–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. The New England journal of medicine 2018;378(21):1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900–1980. Journal of the National Cancer Institute 1983;71(3):473–479. [PubMed] [Google Scholar]
- 21.Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. American journal of public health 1996;86(2):231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreassen BK, Aagnes B, Gislefoss R, Andreassen M, Wahlqvist R. Incidence and Survival of urothelial carcinoma of the urinary bladder in Norway 1981–2014. BMC cancer 2016;16(1):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Xie L, Zheng J-L, Tan Y-T, Zhang W, Xiang Y-B. Incidence trends of urinary bladder and kidney cancers in urban Shanghai, 1973–2005. PloS one 2013;8(12):e82430.Used intrinsic estimator to estimate age, period, and cohort effects contributing to incidence of urinary bladder and kidney cancer. Intrinsic estimator offers an alternative approach to conventional age-period-cohort methods.
- 24.Pou SA, Osella AR, Diaz Mdel P. Bladder cancer mortality trends and patterns in Cordoba, Argentina (1986–2006). Cancer causes & control : CCC 2011;22(3):407–415. [DOI] [PubMed] [Google Scholar]
- 25.Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: A pooled analysis of 11 case‐control studies. International Journal of Cancer 2000;86(2):289–294. [DOI] [PubMed] [Google Scholar]
- 26.Brennan P, Bogillot O, Greiser E, et al. The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes & Control 2001;12(5):411–417. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26(4):612–619. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. Journal of clinical oncology 2013;31(36):4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilhodes J, Belot A, Bouvier AM, et al. Incidence of major smoking-related cancers: trends among adults aged 20–44 in France from 1982 to 2012. Cancer epidemiology 2015;39(5):707–713. [DOI] [PubMed] [Google Scholar]
- 30.Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine 2006;24:S11–S25. [DOI] [PubMed] [Google Scholar]
- 31.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral oncology 2014;50(5):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Hariri S, Bradley H, Gottlieb SL, Leichliter JS, Markowitz LE. Trends and patterns of sexual behaviors among adolescents and adults aged 14 to 59 years, United States. Sexually transmitted diseases 2015;42(1):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner CF, Danella RD, Rogers SM. Sexual behavior in the United States, 1930–1990. Sexually transmitted diseases 1995;22(3):173–190. [DOI] [PubMed] [Google Scholar]
- 34.Anderson WF, Camargo MC, Fraumeni JF, Jr., Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. Jama 2010;303(17):1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Reviews Cancer 2002;2(1):28. [DOI] [PubMed] [Google Scholar]
- 36.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. American journal of epidemiology 2011;175(1):54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roosendaal R, Kuipers EJ, Buitenwerf J, et al. Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. American Journal of Gastroenterology 1997;92(9). [PubMed] [Google Scholar]
- 38.Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. The American journal of gastroenterology 2017;112(8):1247–1255. [DOI] [PubMed] [Google Scholar]
- 39.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995. International journal of cancer Journal international du cancer 2002;99(6):860–868. [DOI] [PubMed] [Google Scholar]
- 40.Murphy CC, Yang YC, Shaheen NJ, Hofstetter WL, Sandler RS. An age-period-cohort analysis of obesity and incident esophageal adenocarcinoma among white males. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 2017;30(3):1–8.Uses hierarchical age-period-cohort model to estimate changes in incidence of esophageal adenocarcinoma among white men. Includes obesity as a fixed-level covariates to test explanatory hypotheses about obesity as a risk factor that contributes to the observed trends.
- 41.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. Jama 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dent J, El-Serag H, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005;54(5):710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastrooesophageal reflux disease: a systematic review. Gut 2013:gutjnl-2012–304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA: a cancer journal for clinicians 2012;62(2):118–128. [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970–2009. Journal of the National Cancer Institute 2013;105(22):1694–1700. [DOI] [PubMed] [Google Scholar]
- 46.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nature Reviews Gastroenterology and Hepatology 2009;6(12):699. [DOI] [PubMed] [Google Scholar]
- 47.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best practice & research Clinical gastroenterology 2006;20(2):197–209. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg PS, Wilson KL, Anderson WF. Are incidence rates of adult leukemia in the United States significantly associated with birth cohort? Cancer epidemiology, biomarkers & prevention a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2012;21(12):2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anfinsen KP, Devesa SS, Bray F, et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005). Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2011;20(8):1770–1777. [DOI] [PubMed] [Google Scholar]
- 50.Calle EE, Thun MJ. Obesity and cancer. Oncogene 2004;23(38):6365. [DOI] [PubMed] [Google Scholar]
- 51.Smith NR, Jensen BW, Zimmermann E, Gamborg M, Sorensen TI, Baker JL. Associations between birth weight and colon and rectal cancer risk in adulthood. Cancer epidemiology 2016;42:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in u.s. Women and men-results from two large cohort studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2015;24(4):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Society AC, Eddy DM. Guidelines for the cancer-related checkup: recommendations and rationale. American Cancer Society; 1980. [Google Scholar]
- 54.Biesheuvel C, Weigel S, Heindel W. Mammography Screening: Evidence, History and Current Practice in Germany and Other European Countries. Breast Care 2011;6(2):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United Kingdom. Jama 2003;290(16):2129–2137. [DOI] [PubMed] [Google Scholar]
- 56.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annual review of public health 1991;12(1):425–457. [DOI] [PubMed] [Google Scholar]
- 57.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age–period and age–cohort models. Statistics in medicine 1987;6(4):449–467. [DOI] [PubMed] [Google Scholar]
- 58.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: age–period–cohort models. Statistics in medicine 1987;6(4):469–481. [DOI] [PubMed] [Google Scholar]
- 59.Carstensen B Age–period–cohort models for the Lexis diagram. Statistics in medicine 2007;26(15):3018–3045. [DOI] [PubMed] [Google Scholar]
- 60.Fienberg SE, Mason WM. Specification and implementation of age, period and cohort models In: Cohort analysis in social research Springer; 1985:45–88.Provides overview of age-period-cohort models, with a focus on linear models.
- 61.Kupper LL, Janis JM, Karmous A, Greenberg BG. Statistical age-period-cohort analysis: a review and critique. Journal of chronic diseases 1985;38(10):811–830. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Fu WJ, Land KC. 2. A methodological comparison of age-period-cohort models: The intrinsic estimator and conventional generalized linear models. Sociological methodology 2004;34(1):75–110. [Google Scholar]
- 63.Yang Y, Schulhofer-Wohl S, Fu WJ, Land KC. The intrinsic estimator for age-period-cohort analysis: What it is and how to use it. American Journal of Sociology 2008;113(6):1697–1736. [Google Scholar]
- 64.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2014;23(11):2296–2302.Publicly available web tool that allows researchers to upload a dataset and derive estimates for age, period, and cohort effects