Abstract
Visual deprivation induces a rapid increase in visual cortex excitability that may result in better consolidation of spatial memory in animals and in lower visual recognition thresholds in humans. γ-Aminobutyric acid (GABA)ergic, N-methyl-d-aspartate (NMDA), and cholinergic receptors are thought to be involved in visual cortex plasticity in animal studies. Here, we used a pharmacological approach and found that lorazepam (which enhances GABAA receptor function by acting as a positive allosteric modulator), dextrometorphan (NMDA receptor antagonist), and scopolamine (muscarinic receptor antagonist) blocked rapid plastic changes associated with light deprivation. These findings suggest the involvement of GABA, NMDA, and cholinergic receptors in rapid experience-dependent plasticity in the human visual cortex.
Transcranial magnetic stimulation (TMS) delivered to occipital sites (1, 2) can elicit phosphenes, originating close to the cortical surface (3). The minimum TMS intensity required to elicit phosphenes is defined as phosphene threshold (PT) and represents a measure of visual cortex excitability (4, 5). Light deprivation in humans results in a rapid increase in visual cortex excitability, expressed as decreased PTs to TMS of occipital regions and enhanced activation in response to incoming visual input, as measured by functional magnetic resonance imaging (6). Behaviorally, visual deprivation leads to improved consolidation of spatial memory in animals (7, 8) and to lower visual recognition thresholds in humans (9). Although the mechanisms underlying this rapid process are unknown, they may include long-term potentiation (LTP), long-term depression (LTD; see ref. 10), and changes in the balance of cortical inhibition and excitation (11). Here, we hypothesized that pharmacological manipulations that interfere with synaptic plasticity would block rapid adaptation in human visual cortex to light deprivation (6). Using this approach already provided insight into the mechanisms of plasticity associated with deafferentation (12) and motor training (13) in intact humans.
We studied the effects of administration of lorazepam (LZP), a short-acting benzodiazepine that acts as a positive allosteric modulator of γ-aminobutyric acid type A (GABAA) receptors (14); dextrometorphan (DM), a drug that blocks N-methyl-d aspartate (NMDA) receptors, required for LTP and experience-dependent plasticity (15–18) during early development; scopolamine (SCO), a muscarinic receptor antagonist (19, 20); and lamotrigine (LTG), an antiepileptic drug that blocks voltage-gated Na+ and Ca2+ channels (21, 22) without affecting LTP (23). The predicted suppressive effects of a drug would point to the involvement of specific mechanisms of plasticity.
Design and Methods
Subjects.
We studied six healthy normal volunteers with no history of visual deficits or neurological abnormalities (all males, mean age ± SEM = 30.0 ± 2.2 years). The protocol was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board. All subjects gave their written informed consent and were naïve to the experimental purposes.
Phosphene Threshold Measurements.
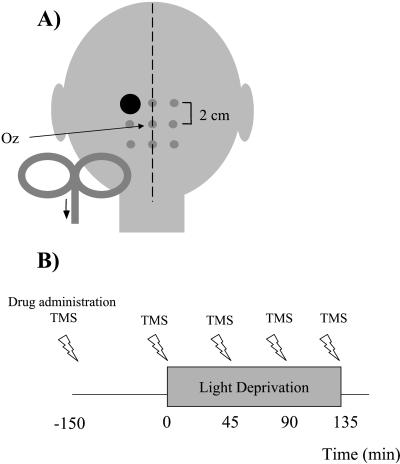
All experiments were conducted in a dark room (residual luminance in the room near zero) with subjects' eyes blindfolded to accomplish total darkness. Subjects wore a cotton swimmer's cap with a grid of 3 × 3 points centered over Oz (International 10/20 system). Stimulation points were 2 cm apart (Fig. 1A). A Cadwell high-speed magnetic stimulator (Cadwell Laboratories, Kennewick, WA), connected to a 7.5-cm figure-eight-shaped water-cooled coil (coil orientation parallel to the midline and current flowing in the cranio-caudal direction) was used to deliver magnetic stimuli (24). Pairs of TMS stimuli of equal intensity separated by a 50-ms interstimulus interval (25) were delivered to all scalp positions. TMS applied to the occipital cortex of subjects who keep their eyes closed can elicit phosphenes (flashes/spots of light in the absence of visual stimuli) (1, 2, 25–28). Subjects were asked to describe the shape, color, brightness (on an arbitrary scale of 1–5, 5 the brightest phosphene), and location of the phosphenes in the visual field as being displayed on the face of an imaginary clock in front of them. In a first step, TMS was delivered at a constant intensity of 80% of the maximal stimulator's output over all grid points to identify the position that elicited the brightest phosphenes (optimal position). PTs, defined as the minimum stimulus intensity able to elicit phosphenes in 6 of 10 consecutive trials, were determined at this position on the grid. To determine PT, stimulation intensities started at 35% maximum stimulator output and were increased in 1% steps until the definition was met. Stimulation was performed every 10 s. Participating subjects had been screened before and all fulfilled the following inclusion criteria: reported phosphenes contralateral to the stimulated position; no reports of phosphenes after stimulation of control positions (P3 and P4) of the International 10–20 system (29) or after sham stimulation with the coil tilted away from the head; reproducible PTs across at least two sessions; and reproducible PT changes after light deprivation in at least two drug-free sessions. These criteria have been described elsewhere in detail (6).
Figure 1.
Schematic presentation of the experimental settings. A 3 × 3 grid was centered over Oz (International 10–20 system). (A) Example of the optimal scalp position (large dot) stimulated to evoke phosphenes in one subject (subject FB) with electrical current flowing in cranio-caudal direction in the handle of the coil. (B) Schematic presentation of the order of TMS measurements and drug administration.
Light-Deprivation Paradigm and Pharmacological Interventions.
Light deprivation started 2.5 h after intake of a single dose of each drug or 36 h after the onset of sleep deprivation (SLD) and was maintained for 135 min. PTs were determined before, 2.5 h after intake of each drug, and at 45-min intervals thereafter during the light-deprivation period (Fig. 1B). Subjects participated in six sessions in a pseudorandomized design under the effects of LZP, DM, SCO, LTG, and SLD, and had a control, no-treatment (drug-naïve) session. Each session took place on a different day and a minimum 7-day washout period between sessions was used to prevent drug interactions. The purpose of the SLD session was to simulate the sedative effects of some of the drugs that we used. The order of drug applications was counterbalanced across subjects. The subjects and the investigator who performed the statistical analysis were blinded to the drug taken.
Each subject received one of the following treatments. (i) LZP (0.038 mg/kg), a short-acting benzodiazepine that at this dose produces functional potentiation of GABAA receptors through positive allosteric modulation (30). By the time testing began, blood levels are known to be in the therapeutic range (above 16 ng/ml) and remain stable for 3–5 h (31). (ii) DM (2 mg/kg), a potent noncompetitive NMDA receptor antagonist (32) shown at this dose to induce brain concentrations in humans (33) similar to those that elicit NMDA receptor block in vitro (32, 34). DM is rapidly metabolized to dextrorphan, a similarly active compound (35), and brain tissue DM concentration is much higher than that present in blood (36). (iii) SCO (1.5-mg dermal patch behind the ear), which is a muscarinic receptor antagonist (19, 20). Plasma concentrations during the experiment reach >50 pg/ml (36), a threshold value required for therapeutic effects such as prevention of motion sickness (37). (iv) LTG (200 mg), which is an antiepileptic drug affecting voltage-gated Na+ and Ca2+ channels (21, 22). Transient side effects included drowsiness after LZP and SLD and mild nausea after DM administration.
Statistical Analysis.
The effects of each individual drug and SLD on PTs were analyzed by using an ANOVA model with main factors intervention and light-deprivation time (significance level, P < 0.05). A similar ANOVA model with main factor light deprivation time was used to compare the effects of each drug and SLD with the drug-naïve session. The significance level was set to P < 0.01 to compensate for multiple comparisons. Additionally, changes in PTs at each time interval during light deprivation (time 45–135 min, Fig. 1B) were compared with the values obtained at baseline (time 0, Fig. 1B) using separate repeated-measures ANOVAs (main factor light deprivation time; significance level, P < 0.05) for each intervention. PTs before and after drug intake (without light deprivation, time −150 min and time 0, Fig. 1B) were compared by using Wilcoxon rank tests. The significance level was set at P < 0.05.
Results
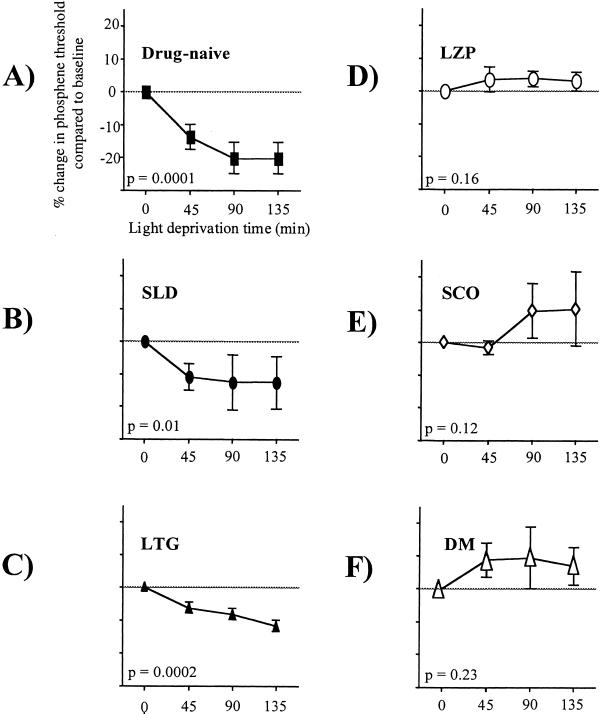
By inclusion criteria, subjects in this study reported reproducible phosphenes contralateral to the stimulation site across sessions, had a reproducible baseline PT, and experienced reproducible decreases in PT with light deprivation in drug-naïve situations. Additionally, they were naïve to the experimental purposes and blind to the drug they took. Overall, there was a significant effect of intervention and a significant interaction between intervention and light deprivation time (P = 0.02, F = 3.8 and P = 0.002, F = 3.0 respectively, repeated-measures ANOVA with main factors intervention and deprivation time). In the drug-naïve condition, light deprivation induced a significant reduction in PTs to TMS, similar to previously reported results (6) (mean decrease in PT ± SE = 20 ± 4.8%, P = 0.0001, F = 19.6, repeated-measures ANOVA with main factor deprivation time) (Fig. 2A). Pretreatment with LZP, SCO, and DM blocked the decrease in PT identified in the drug-naïve condition (P = 0.0001, F = 25.6, P = 0.0001, F = 14.2, and P = 0.004, F = 7.6, respectively), whereas SLD and LTG did not (P = 0.38, F = 1.1, and P = 0.2, F = 1.8, respectively).
Figure 2.
Changes in PT relative to baseline (time 0) during 135 min of light deprivation. Light deprivation induced a decrease in PTs in the drug-naïve condition (A), under SLD (B), and after intake of LTG (C). LZP (D), DM (E), and SCO (F) blocked this effect. Separate repeated-measures ANOVA models for each condition with main-factor light-deprivation time. Error bars indicate SE.
When the effects of each intervention were analyzed separately (see Design and Methods), PTs decreased significantly after light deprivation in SLD and LTG conditions (P = 0.01, F = 4.8, and P = 0.0002, F = 15.1, respectively; Fig. 2 B and C). After pretreatment with LZP, SCO and DM, light-deprivation induced no significant change in PT (P = 0.16, F = 2.1, P = 0.12, F = 2.3, and P = 0.23, F = 1.7, respectively; Fig. 2 D–F).
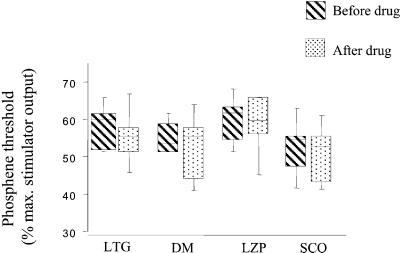
Intake of LZP, SCO, DM, and LTG in the absence of light deprivation did not modify PT (P > 0.06, Wilcoxon rank tests; Fig. 3).
Figure 3.
PTs (expressed as percentage of maximum stimulator output) before and 2.5 h after intake of a single dose of each drug in the absence of light deprivation (time −150 min and 0, Fig. 1B). None of the drugs induced a significant change in PT in the absence of light deprivation. Box limits indicate 25th and 75th percentiles. The line shows the median. The whiskers indicate the data range.
At the beginning of each stimulation period, the subjects were asked to rate the level of drowsiness on a 0–100 analog scale (0 = no drowsiness at all). LZP and SLD induced similar levels of drowsiness above those reported in the drug-naïve, LTG, SCO, and DM conditions. Overall, there was a slight insignificant increase in the level of drowsiness during the light deprivation period in all conditions.
Discussion
The mechanisms underlying short-term (within hours) changes in visual cortex function after light deprivation are incompletely understood. The present study demonstrated that single doses of the benzodiazepine agent LZP, the NMDA receptor antagonist DM, and the muscarinic receptor antagonist SCO, block light-deprivation-induced rapid changes in visual cortical excitability. In contrast to these suppressive effects, LTG, a drug that blocks voltage-gated Na+ and Ca2+ channels (21) without influencing LTP (23), had no significant effects. These results could not be explained by global influences on visual cortical excitability, because none of the drugs modified PTs in the absence of light deprivation. Similarly, drug-induced drowsiness (especially by LZP) could not explain these results, because sleep deprivation induced similar drowsiness, but unlike LZP, failed to block the light-deprivation-induced decrease in PT.
Our results are consistent with the involvement of at least three mechanisms in this form of short-term cortical adaptation. The blocking of cortical excitability changes by LZP and DM suggests the involvement of GABAergic neurotransmission and NMDA receptor function, a contention supported by the finding of experience-dependent plasticity in NMDA receptors within just 1 h of the onset of ambient light modifications (38). Additionally, synaptic plasticity in the visual cortex requires activation of NMDA receptors (15, 16) and is favored by reduced concomitant GABAergic inhibition (15). We also found that SCO, a muscarinic acetylcholine (ACh) receptor antagonist (19) that can inhibit synaptic plasticity in vitro (39–41), blocked cortical excitability changes elicited by light deprivation. ACh is a neurotransmitter that closes potassium channels so that the action potential is broadened, allowing the NMDA channels to open and trigger LTP (42). These results are consistent with previous work underlining the link between muscarinic cholinergic transmission and adaptive processes in the human visual system (23, 44).
Short-term changes in cortical organization also follow deafferentation in other sensory systems (44–47). For example, permanent denervation of the flying fox thumb results in changes in finger receptive fields within 1 min (48) and in the motor domain, transection of the facial nerve that innervates the rat's vibrissa leads to remapping of primary motor cortex representations within 1 h (49). More information is available on the long-term effects of visual deprivation that leads to substantial cortical reorganization (50). The mechanisms underlying these changes in visual cortex function in adult animals include those known to subserve synaptic plasticity, including LTP and LTD (51), increased dendritic branching (52), increased axonal collaterals in horizontal pathways (53), and the generation of new synapses (54). Previous studies in animal models demonstrated decreased levels of GABA (55), GABA receptors (56), or glutamic acid decarboxylase (55, 57) after eye removal, intravitreal tetrodotoxin injection, or eyelid suture. However, these changes have been documented no earlier than several days after the lesions. NMDA (15–18) and muscarinic ACh receptors also participate in regulating visual cortex plasticity (43). In the somatosensory system, depletion of the cholinergic projections to the cortex or application of atropine (an ACh antagonist) blocks cortical plasticity (58). Overall, previous studies indicate the involvement of GABAergic inhibition and NMDA and muscarinic receptors (all required for LTP) in regulating long-term visual plasticity as well.
In summary, our findings suggest the involvement of GABAergic inhibition, NMDA receptor activation, and cholinergic transmission as operating in rapid, experience-dependent plasticity in the human visual cortex.
Acknowledgments
We are grateful to Drs. M. Hallett and S. P. Wise for their comments on the manuscript and to D. Schoenberg, M.S., for skillful editing. This work was supported by Deutsche Forschungsgemeinschaft Grant Bo 1576/1-2 (to B.B.).
Abbreviations
- ACh
acetylcholine
- DM
dextrometorphan
- GABA
γ-aminobutyric acid
- LTD
long-term depression
- LTG
lamotrigine
- LTP
long-term potentiation
- LZP
lorazepam
- NMDA
N-methyl-d-aspartate
- PT
phosphene threshold
- SCO
scopolamine
- SLD
sleep deprivation
- TMS
transcranial magnetic stimulation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Marg E, Rudiak D. Optom Vis Sci. 1994;71:301–311. doi: 10.1097/00006324-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Meyer B U, Diehl R, Steinmetz H, Britton T C, Benecke R. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:121–134. [PubMed] [Google Scholar]
- 3.Epstein C M, Verson R, Zangaladze A. J Clin Neurophysiol. 1996;13:247–252. doi: 10.1097/00004691-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Afra J, Mascia A, Gerard P, Maertens de Noordhout A, Schoenen J. Ann Neurol. 1998;44:209–215. doi: 10.1002/ana.410440211. [DOI] [PubMed] [Google Scholar]
- 5.Aurora S K, Ahmad B K, Welch K M, Bhardhwaj P, Ramadan N M. Neurology. 1998;50:1111–1114. doi: 10.1212/wnl.50.4.1111. [DOI] [PubMed] [Google Scholar]
- 6.Boroojerdi B, Bushara K O, Corwell B, Immisch I, Battaglia F, Muellbacher W, Cohen L G. Cereb Cortex. 2000;10:529–534. doi: 10.1093/cercor/10.5.529. [DOI] [PubMed] [Google Scholar]
- 7.Grimm V E, Samuel D. Int J Neurosci. 1976;7:1–7. doi: 10.3109/00207457609147193. [DOI] [PubMed] [Google Scholar]
- 8.Worsham R W, D'Amato M R. J Exp Psychol. 1973;99:99–105. doi: 10.1037/h0034770. [DOI] [PubMed] [Google Scholar]
- 9.Suedfeld P. Am Sci. 1975;63:60–69. [PubMed] [Google Scholar]
- 10.Gilbert C D. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- 11.Pettet M W, Gilbert C D. Proc Natl Acad Sci USA. 1992;89:8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziemann U, Hallett M, Cohen L G. J Neurosci. 1998;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buetefisch C M, Davis B C, Wise S P, Kopylev L, Classen J, Cohen L G. Proc Natl Acad Sci USA. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. . (First Published March 14, 2000; 10.1073/pnas.050350297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald R L. Benzodiazepines: Mechanisms of Action. New York: Raven; 1995. [Google Scholar]
- 15.Artola A, Singer W. Nature (London) 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- 16.Bear M F. J Physiol (Paris) 1996;90:223–227. doi: 10.1016/s0928-4257(97)81428-3. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood A, Dudek S M, Gold J T, Aizenman C D, Bear M F. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 18.Roberts E B, Meredith M A, Ramoa A S. J Neurophysiol. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 19.La Rovere M T, De Ferrari G M. Drugs. 1995;50:769–776. doi: 10.2165/00003495-199550050-00001. [DOI] [PubMed] [Google Scholar]
- 20.Frey K A, Koeppe R A, Mulholland G K, Jewett D, Hichwa R, Ehrenkaufer R L, Carey J E, Wieland D M, Kuhl D E, Agranoff B W. J Cereb Blood Flow Metab. 1992;12:147–154. doi: 10.1038/jcbfm.1992.18. [DOI] [PubMed] [Google Scholar]
- 21.Leach J P, Brodie M J. Seizure. 1995;4:5–17. doi: 10.1016/s1059-1311(05)80074-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang S J, Huang C C, Hsu K S, Tsai J J, Gean P W. NeuroReport. 1996;7:3037–3040. doi: 10.1097/00001756-199611250-00048. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Z Q, Stringer J L. Epilepsy Res. 1997;27:187–194. doi: 10.1016/s0920-1211(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 24.Chen R, Gerloff C, Hallett M, Cohen L G. Ann Neurol. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- 25.Ray P G, Meador K J, Epstein C M, Loring D W, Day L J. J Clin Neurophysiol. 1998;15:351–357. doi: 10.1097/00004691-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Amassian V E, Cracco R Q, Maccabee P J, Cracco J B, Rudell A P, Eberle L. J Clin Neurophysiol. 1998;15:288–304. doi: 10.1097/00004691-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Kammer T. Neuropsychologia. 1999;37:191–198. doi: 10.1016/s0028-3932(98)00093-1. [DOI] [PubMed] [Google Scholar]
- 28.Cowey A, Walsh V. NeuroReport. 2000;11:3269–3273. doi: 10.1097/00001756-200009280-00044. [DOI] [PubMed] [Google Scholar]
- 29.Laciga Z. Cesk Neurol. 1968;31:236–238. [PubMed] [Google Scholar]
- 30.Sybirska E, Seibyl J P, Bremner J D, Baldwin R M, al-Tikriti M S, Bradberry C, Malison R T, Zea-Ponce Y, Zoghbi S, During M, et al. Neuropharmacology. 1993;32:671–680. doi: 10.1016/0028-3908(93)90080-m. [DOI] [PubMed] [Google Scholar]
- 31.Greenblatt D J, Scavone J M, Harmatz J S, Engelhardt N, Shader R I. Clin Pharmacol Ther. 1993;53:577–584. doi: 10.1038/clpt.1993.73. [DOI] [PubMed] [Google Scholar]
- 32.Wong B Y, Coulter D A, Choi D W, Prince D A. Neurosci Lett. 1988;85:261–266. doi: 10.1016/0304-3940(88)90362-x. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg G K, Bell T E, Yenari M A. J Neurosurg. 1996;84:860–866. doi: 10.3171/jns.1996.84.5.0860. [DOI] [PubMed] [Google Scholar]
- 34.Apland J P, Braitman D J. Brain Res. 1990;529:277–285. doi: 10.1016/0006-8993(90)90838-3. [DOI] [PubMed] [Google Scholar]
- 35.Hollander D, Pradas J, Kaplan R, McLeod H L, Evans W E, Munsat T L. Ann Neurol. 1994;36:920–924. doi: 10.1002/ana.410360619. [DOI] [PubMed] [Google Scholar]
- 36.Nachum Z, Shahal B, Shupak A, Spitzer O, Gonen A, Beiran I, Lavon H, Eynan M, Dachir S, Levy A. J Pharmacol Exp Ther. 2001;296:121–123. [PubMed] [Google Scholar]
- 37.Norfleet W T, Degioanni J J, Calkins D S, Reschke M F, Bungo M W, Kutyna F A, Homick J L. Aviat Space Environ Med. 1992;63:46–51. [PubMed] [Google Scholar]
- 38.Quinlan E M, Philpot B D, Huganir R L, Bear M F. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe Y, Ikegaya Y, Saito H, Abe K. Neurosci Res. 1995;21:317–322. doi: 10.1016/0168-0102(94)00867-f. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Sakurai M, Hayashi S. Neurosci Lett. 1989;98:179–183. doi: 10.1016/0304-3940(89)90506-5. [DOI] [PubMed] [Google Scholar]
- 41.Calabresi P, Centonze D, Gubellini P, Bernardi G. Neuropharmacology. 1999;38:323–326. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- 42.Metherate R, Tremblay N, Dykes R W. J Neurophysiol. 1988;59:1253–1276. doi: 10.1152/jn.1988.59.4.1253. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Jia W, Gu Q, Cynader M. Brain Res Dev Brain Res. 1994;79:63–71. doi: 10.1016/0165-3806(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 44.Sanes J N, Suner S, Lando J F, Donoghue J P. Proc Natl Acad Sci USA. 1988;85:2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garraghty P E, Kaas J H. Proc Natl Acad Sci USA. 1991;88:6976–6980. doi: 10.1073/pnas.88.16.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolarik R C, Rasey S K, Wall J T. J Neurosci. 1994;14:4269–4288. doi: 10.1523/JNEUROSCI.14-07-04269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva A C, Rasey S K, Wu X, Wall J T. J Comp Neurol. 1996;366:700–716. doi: 10.1002/(SICI)1096-9861(19960318)366:4<700::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Calford M B, Tweedale R. J Neurophysiol. 1991;65:178–187. doi: 10.1152/jn.1991.65.2.178. [DOI] [PubMed] [Google Scholar]
- 49.Donoghue J P, Suner S, Sanes J N. Exp Brain Res. 1990;79:492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- 50.Wiesel T N, Hubel D H. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch J A, Gilbert C D. J Physiol (London) 1993;461:247–262. doi: 10.1113/jphysiol.1993.sp019512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Withers G S, Greenough W T. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- 53.Darian-Smith C, Gilbert C D. Nature (London) 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 54.Andersen P, Soleng A F. Brain Res Brain Res Rev. 1998;26:353–359. doi: 10.1016/s0165-0173(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 55.Hendry S H, Jones E G. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 56.Hendry S H, Fuchs J, deBlas A L, Jones E G. J Neurosci. 1990;10:2438–2450. doi: 10.1523/JNEUROSCI.10-07-02438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arckens L, Eysel U T, Vanderhaeghen J J, Orban G A, Vandesande F. Neuroscience. 1998;83:381–391. doi: 10.1016/s0306-4522(97)00422-3. [DOI] [PubMed] [Google Scholar]
- 58.Sachdev R N, Lu S M, Wiley R G, Ebner F F. J Neurophysiol. 1998;79:3216–3228. doi: 10.1152/jn.1998.79.6.3216. [DOI] [PubMed] [Google Scholar]